Abstract
Modulation of host immunity is an important potential mechanism by which probiotics confer health benefits. This study was designed to investigate the effects of a probiotic strain, Lactobacillus casei Shirota (LcS), on immune function using human peripheral blood mononuclear cells (PBMC) in vitro. In addition, the role of monocytes in LcS-induced immunity was also explored. LcS promoted natural killer (NK) cell activity and preferentially induced expression of CD69 and CD25 on CD8+ and CD56+ subsets in the absence of any other stimulus. LcS also induced production of interleukin (IL)-1β, IL-6, tumour necrosis factor (TNF)-α, IL-12 and IL-10 in the absence of lipopolysaccharide (LPS). In the presence of LPS, LcS enhanced IL-1β production but inhibited LPS-induced IL-10 and IL-6 production, and had no further effect on TNF-α and IL-12 production. Monocyte depletion reduced significantly the impact of LcS on lymphocyte activation, cytokine production and natural killer (NK) cell activity. In conclusion, LcS activated cytotoxic lymphocytes preferentially in both the innate and specific immune systems, which suggests that LcS could potentiate the destruction of infected cells in the body. LcS also induced both proinflammatory and anti-inflammatory cytokine production in the absence of LPS, but in some cases inhibited LPS-induced cytokine production. Monocytes play an important role in LcS-induced immunological responses.
Keywords: cytokines, immunomodulation, L. casei Shirota, NK cell activity, T lymphocytes
Introduction
One of the most promising areas of development in the human nutritional field over the last two decades has been the use of probiotics and recognition of their role in human health and disease. Lactic acid-producing bacteria are the most commonly used probiotics in foods and supplements. The means by which probiotic bacteria elicit their health effects are not understood fully, but may include competitive exclusion of enteric pathogens, neutralization of dietary carcinogens, production of anti-microbial metabolites and modulation of mucosal and systemic immune function [1]. According to the currently adopted definition by the Food and Agriculture Organization/World Health Organization (FAO/WHO), probiotics are defined as ‘live microorganisms which when administered in adequate amounts confer a health benefit on the host’. However, in recognition of the increasing evidence for effects of probiotics on immune function, this definition has sometimes been extended to ‘live microorganisms, that when included in foods can influence the composition and activity of the gut microbiota, modulate the inflammatory response, improve the nonspecific intestinal barrier, and reinforce or modulate the mucosal and the systemic immune responses’[1]. It has also been suggested that the selection of an appropriate probiotic strain for its inclusion in a probiotic preparation should be made on the basis of its capacity to induce an improved gut immune response [2].
Probiotics have been shown to reduce the duration and total symptom score of the common cold in healthy volunteers [3], to decrease the duration of infection in older in-patients [4] and to reduce the incidence of influenza-like illness in healthy subjects [5]. Human intervention studies have shown that probiotics enhance innate immunity, including natural killer (NK) cell activity, phagocytic activity and respiratory burst [5–11]. However, the reported effects of probiotics on cytokine production are complex and inconsistent. Some probiotic strains, such as Lactobacillus rhamnosus GG, and a mixture of probiotic strains of L. gasseri CECT 5714 and L. coryniformis CECT 5711, appear to enhance production of the anti-inflammatory cytokine, interleukin (IL)-10, while having no effect or slightly decreasing production of the proinflammatory cytokines, IL-6, interferon (IFN)-γ and tumour necrosis factor (TNF)-α. However, a number of human studies report no effect of probiotics on the ex vivo production of cytokines [5,7,12,13]. Furthermore, there are few data on the effects of probiotics on cellular immunity, either from human studies or from in vitro experiments.
It is becoming clear that the immunomodulatory effects of probiotics are strain-dependent. L. casei Shirota (LcS), a commercial probiotic strain, increases the number of beneficial intestinal bacteria and improves the balance between beneficial and potentially harmful intestinal bacteria [14], prevents the recurrence of superficial bladder cancer and of colorectal polyps with moderate atypia [15,16], improves chronic constipation [17], enhances NK cell activity [18] and modifies allergen-induced immune responses in allergic rhinitis [19]. However, the effects of LcS on immune function are still poorly understood, particularly with respect to acquired immunity. In an in vitro study using human peripheral blood mononuclear cells (PBMCs), LcS was reported to enhance NK cell activity and induce IL-12 production [20]. In another in vitro study, heat-killed LcS stimulated IL-10, IL-12, TNF-α and IFN-γ production, promoted NK cell activity and activated CD69 expression on NK cells. The study also indicated that monocytes are important for these effects [21].
The aim of the current study was to characterize the immunomodulatory properties of LcS in an in vitro system using PBMCs, particularly with respect to T lymphocyte activation, and to determine the role of monocytes in these effects.
Materials and methods
LcS preparation
Stock cultures of LcS, isolated from the fermented milk product Yakult (Yakult Europe B.V., Almere, the Netherlands), were grown on de Man, Rogosa and Sharpe (MRS) agar (Oxoid, Hampshire, UK) for 48 h at 37°C in an anaerobic cabinet (MACS MG 1000; Don Whitley Scientific, West Yorkshire, UK) with a gas mixture of 10% H2, 10% CO2 and 80% N2 by volume, subsequently preserved in Microbank® mixed vials (ProLab Diagnostics, Neston, UK) at −80°C and kept as a stock for future use. For liquid culture, one pure colony was taken from an MRS nutrient agar plate and grown overnight in 10 ml of pre-reduced MRS broth (Oxoid) with 0·05% L-cysteine hydrochloride (Sigma, Dorset, UK) in a shaking incubator (cooled orbital incubator; Gallenkamp, Loughborough, UK) at 37°C, and 0·5 ml of the overnight culture was inoculated into another 10 ml MRS broth. The bacteria were harvested in the exponential phase, resuspended in phosphate-buffered saline (PBS; Oxoid), centrifuged twice at 1960 g (Sanyo/MSE Micro Centaur, Haverhill, USA) for 5 min and resuspended at the required concentration in RPMI-1640 (Autogen Bioclear, Wiltshire, UK) containing 0·75 mM glutamine (Autogen Bioclear).
Preparation of PBMC and monocyte-depleted PBMC (MD-PBMC)
Fasted blood samples were taken from 19 healthy adult donors aged 28–44 years in sodium heparin vacutainer tubes (Greiner Bio-One Ltd, Gloucestershire, UK). Blood was layered over an equal volume of Lympholyte (Cedarlane Laboratories Ltd, Tyne & Wear, UK) and centrifuged at 930 g for 15 min at room temperature. The plasma was removed into a sterile 15 ml tube for later use. Cells were harvested from the interface, washed once, resuspended in RPMI-1640 medium (containing glutamine) and the above steps were then repeated to achieve a lower degree of erythrocyte contamination. The pellet was finally resuspended in RPMI-1640 and the cell number was adjusted to 2 × 106 cells/ml.
For monocyte depletion experiments, monocytes were removed from PBMC using anti-CD14 magnetic beads (BD Biosciences, Oxford, UK) and the efficiency of depletion (<1·5% monocytes) was verified by flow cytometry. The MD-PBMCs were washed twice with PBS before being adjusted to 2 × 106 cells/ml.
In vitro culture conditions
PBMC were incubated in 24-well plates in the presence of 105, 106 or 107 colony-forming units (CFU)/ml LcS and 2·5% autologous plasma for 24h at 37°C in an air/CO2 (19 : 1) atmosphere. The ratios of PBMC and LcS were 10 : 1, 1 : 1 and 1 : 10, respectively. For lymphocyte activation experiments, PBMC were incubated in the presence or absence of 2·5 µg/ml concanavalin A (ConA; Sigma) and for cytokine production they were incubated in the presence or absence of 1 µg/ml lipopolysaccharide (LPS; Sigma). At the end of the incubation, cells were stained for activation marker measurement, and supernatants were collected and stored at −20°C for later analysis of cytokine production. Non-stimulated cultures were used as negative controls. For monocyte depletion experiments, PBMCs or MD-PBMCs were incubated with LcS at 106 CFU/ml for 24 h in the presence or absence of ConA or LPS.
Lymphocyte activation analysis
Cells were removed gently from wells using cell scrapers and plastic pipettes, stained with appropriate combinations of fluorescently labelled monoclonal antibodies, including fluorescein isothiocyanate (FITC)-labelled anti-CD69 or anti-CD25 and phycoerythrin (PE)-labelled anti-CD3, anti-CD4, anti-CD8 or anti-CD56, and fixed with Cell Fix (all from BD Biosciences). The fixed cells were analysed on a Becton Dickinson FACSCalibur flow cytometer (BD Biosciences) within 24 h. The lymphocytes were gated and fluorescence data for 10 000 events were collected and analysed using Becton Dickinson CellQuest software.
Cytokine analysis
The concentrations of IL-1β, IL-6, IL-10, IL-12 (p70) and TNF-α in the supernatants of PBMC or MD-PBMC cultures were measured by commercially available enzyme-linked immunosorbent assay (ELISA) kits (BD Biosciences), according to the manufacturer's instructions.
NK cell activity
Freshly prepared PBMC and MD-PBMC or 24 h-incubated PBMC or MD-PBMC with medium or LcS at 106 CFU/ml were adjusted to a concentration of 5 × 106 cells/ml. Viable target cells (K562) were enumerated by microscopy of Trypan blue-stained cell preparations and 5 × 106 cells were collected and washed twice with PBS before incubation with carboxyfluorescein diacetate succinimidyl ester (CFDA-SE) (1 µg/ml; Sigma, Dorset, UK) for 45 min at 37°C in an air/CO2 (19 : 1) atmosphere. After incubation, the target cells were washed twice and resuspended in 1 ml of complete medium composed of RPMI-1640 medium, 0·75 mM glutamine and 10% newborn calf serum (Sigma, Dorset, UK). PBMC or MD-PBMC were incubated with CFDA-SE-labelled target cells for 2 h at 37°C in an air/CO2 (19 : 1) atmosphere at effector to target cell ratios of 100 : 1, 50 : 1, 25 : 1 and 12·5 : 1. Twenty microlitres of propidium iodide (PI) (Sigma) at 1 mg/ml were added to the samples prior to analysis on the flow cytometer. The results were expressed as the percentage lysis of the target cells.
Statistics
All the values are presented as mean and standard deviation. All data were analysed using spss version 15·0. Significant differences among treatments were evaluated by two-way analysis of variance (anova) or Student's t-test when applicable. The criterion for statistical significance was defined as P < 0·05.
Results
LcS induces CD69 expression on CD4+ and CD8+ lymphocytes and CD25 expression on CD8+ lymphocytes
Approximately 2·5% of unstimulated lymphocytes expressed CD69 (Fig. 1a). In the absence of ConA, LcS increased CD69 expression by lymphocytes, with a maximal effect at 106 CFU/ml, representing a ratio of PBMC and LcS 1 : 1 (P < 0·001) compared with unstimulated cultures (Fig. 1a). LcS-induced CD69 expression by CD8+ lymphocytes was particularly marked, although CD4+ cells were also stimulated by LcS (Fig. 1b). LcS alone also stimulated expression of CD25 (Fig. 1c). In this case, however, LcS increased expression of CD25 by CD8+ cells significantly, but not by CD4+ cells (Fig. 1d), suggesting preferential activation of the CD8+ subset.
Fig. 1.
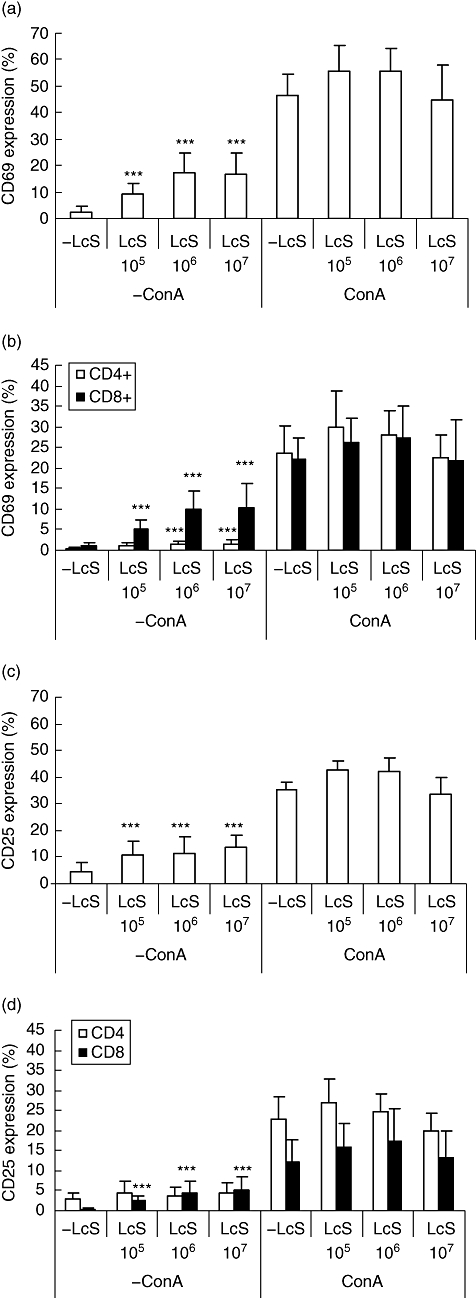
Effect of Lactobacillus casei Shirota (LcS) on expression of CD69 and CD25 by lymphocytes. Peripheral blood mononuclear cells (PBMCs) were cultured with live L. casei Shirota at 105, 106 or 107 colony-forming units (CFU)/ml in the presence or absence of concanavalin A (ConA) (2·5 µg/ml). Non-stimulated cultures were shown as negative control. Each bar represents the mean of 6 to 19 blood donors and the error bars represent standard deviation [49]. *P < 0·05, **P < 0·01, ***P < 0·001 compared with non-stimulated cultures.
In the presence of ConA, LcS had no further effect on expression of CD69 or CD25 compared with those cultures stimulated by ConA alone (Fig. 1). At suboptimal ConA concentrations (1·25 and 0·625 µg/ml), there was a tendency for LcS to enhance mitogen-stimulated CD69 and CD25 expression compared with ConA alone, but the effects did not reach statistical significance (data not shown).
LcS induces cytokine production by PBMC and whole blood cultures
PBMC were exposed to LcS at 105, 106 or 107 CFU/ml in the presence or absence of LPS, and the results showed that LcS at 106 CFU/ml had the maximum effect on induction of the cytokine production (data not shown). Figure 2 shows cytokine production induced by LcS at 106 CFU/ml, in the presence or absence of LPS. In the absence of LPS, LcS strongly induced production of IL-1β by PBMC (P < 0·001) compared with non-stimulated cultures. Stimulation with LPS also induced IL-1β production, but to a significantly lesser degree than LcS (P < 0·05). When PBMC were exposed to both LPS and LcS the IL-1β-inducing effects were additive, in that LcS enhanced IL-1β production by LPS-stimulated cells significantly (P < 0·05).
Fig. 2.
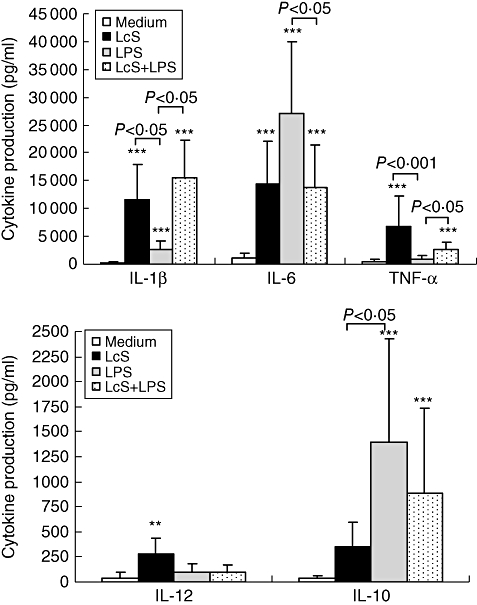
Effect of Lactobacillus casei Shirota (LcS) on production of cytokines by peripheral blood mononuclear cells (PBMCs) in the presence or absence of lipopolysaccharide (LPS). PBMCs were cultured with live LcS at 106 colony-forming units (CFU)/ml for 24 h in the presence or absence LPS (1 µg/ml). Non-stimulated cultures were shown as negative control. Each bar represents the mean of 6 to 12 blood donors and the error bars represent standard deviation [49]. *P < 0·05, **P < 0·01,***P < 0·001 compared with non-stimulated cultures.
In the absence of LPS, LcS induced production of IL-6 by PBMC significantly (P < 0·001) compared with non-stimulated cultures. However, induction of IL-6 by LPS was greater than that by LcS (P = 0·09; Fig. 2). When PBMC were exposed to both LcS and LPS, IL-6 production was reduced significantly compared with LPS alone, suggesting that although LcS itself induced IL-6 production, it inhibited LPS-induced IL-6 production.
The effects of LcS on TNF-α production were similar to those on production of IL-1β. LcS alone strongly induced production of TNF-α by PBMC, and also induced greater TNF-α production by LPS-stimulated PBMC cultures than LPS alone (P < 0·05). LPS alone had no effect on induction of TNF-α.
LcS induced IL-12 production by PBMC (P < 0·01) compared with unstimulated cultures, but had no further effect on LPS-treated cultures. The results also suggested that LPS was unable to induce IL-12.
LcS alone induced IL-10 production significantly in PBMC cultures (P < 0·001) compared with non-stimulated cultures, but to a lesser extent than LPS (P < 0·05). Similar to IL-6 production, LcS modulated the effect of LPS-induced IL-10 production. Although not significant at 106 CFU/ml, LcS at 107 CFU/ml reduced IL-10 production significantly by LPS-stimulated PMBC compared with LPS alone (data not shown).
In summary, production of IL-1β, IL-6, IL-10, IL-12 and TNF-α by unstimulated PBMC was very low. When stimulated by LcS alone, production of all cytokines analysed was induced, but to different extents. Conversely, LPS induced production of IL-1β, IL-6 and IL-10, but not TNF-α or IL-12. When PBMC were exposed to both LcS and LPS, production of IL-1β and TNF-α was enhanced, but that of IL-6 and IL-10 was inhibited, and there was no effect on IL-12 production compared with cytokines induced by LPS alone.
In addition to assessing the effects of LcS on cytokine production, a secondary aim of the study was to verify whether PBMC samples can be replaced by whole blood samples for cytokine measurement. Table 1 shows the effects of LcS on cytokine production measured in the supernatants of both PBMC and whole blood cultures. Whole blood cultures produced very similar results, and levels of cytokines produced by PBMC and whole blood were correlated highly (P < 0·01, r = 0·69–0·83, data not shown), suggesting that whole blood cultures could be employed for these types of experiments.
Table 1.
Cytokine production by peripheral blood mononuclear cells (PBMC) and whole blood cultures.
| Cytokine production (pg/ml) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| −LPS |
LPS |
||||||||
| Cytokines | Cultures | −LcS | LcS105 | LcS106 | LcS107 | −LcS | LcS105 | LcS106 | LcS107 |
| TNF-α | PBMC | 336·94 ± 639·32 | 3 229·18 ± 1 748·03 | 7 624·68 ± 6 445·59 | 5 052·09 ± 4 320·58 | 830·88 ± 633·70 | 2 412·14 ± 933·49 | 2 529·82 ± 1 423·40 | 2 295·21 ± 1 602·16 |
| Whole blood | 0 ± 7·69 | 581·68 ± 369·57 | 1 520·11 ± 890·06 | 912·41 ± 328·26 | 430·04 ± 126·26 | 554·85 ± 175·66 | 745·85 ± 349·46 | 439·31 ± 139·83 | |
| IL-1β | PBMC | 265·92 ± 245·54 | 5 426·13 ± 3 294·24 | 15 768·87 ± 7 837·44 | 13 574·36 ± 7 420·27 | 3 630·13 ± 1 518·16 | 8 456·79 ± 3 618·13 | 15 450·98 ± 6 756·7 | 13 837·80 ± 6 452·38 |
| Whole blood | 0 ± 30·88 | 279·16 ± 184·39 | 1 491·46 ± 459·71 | 2 240·31 ± 825·11 | 1 028·43 ± 424·33 | 1 424·44 ± 387·77 | 2 500·89 ± 782·03 | 2 666·67 ± 799·76 | |
| IL-6 | PBMC | 1027·36 ± 1260·59 | 17 130·94 ± 7 704·93 | 13 246·97 ± 7 608·38 | 7 895·36 ± 3 128·75 | 20 523·39 ± 7 664·50 | 25 572·54 ± 11 198·91 | 13 771·86 ± 7 608·80 | 7 213·60 ± 2 417·80 |
| Whole blood | 53·05 ± 68·82 | 818·04 ± 517·75 | 1 860·72 ± 965·38 | 728·20 ± 229·01 | 4 433·98 ± 1 418·17 | 5 155·58 ± 2 088·81 | 4 304·88 ± 1 885·20 | 1 490·41 ± 664·85 | |
| IL-10 | PBMC | 46·12 ± 23·77 | 594·06 ± 491·96 | 525·54 ± 568·90 | 131·17 ± 87·13 | 964·33 ± 630·68 | 1 461·81 ± 1 218·84 | 883·24 ± 855·63 | 278·86 ± 220·46 |
| Whole blood | 12·53 ± 32·40 | 45·96 ± 54·01 | 93·47 ± 92·99 | 56·65 ± 36·69 | 442·97 ± 240·21 | 416·38 ± 242·87 | 305·58 ± 199·57 | 115·15 ± 66·81 | |
| IL-12 | PBMC | 62·80 ± 85·14 | 84·15 ± 56·01 | 210·40 ± 192·57 | 180·44 ± 160·12 | 69·00 ± 91·19 | 61·44 ± 100·44 | 92·49 ± 80·70 | 106·56 ± 116·22 |
| Whole blood | 14·64 ± 10·99 | 29·08 ± 23·24 | 85·24 ± 99·67 | 11·61 ± 7·70 | 15·65 ± 10·62 | 18·84 ± 13·48 | 30·20 ± 33·35 | 7·72 ± 7·21 | |
Cytokine production was measured in the supernatants of PBMC and whole blood cultures incubated with three different L. casei Shirota (LcS) concentrations, 105, 106 and 107 colony-forming units (CFU)/ml, in the presence or absence of lipopolysaccharide (LPS) (1 µg/ml). The values represent mean ± standard deviation (s.d.) of six volunteers. IL, interleukin; TNF, tumour necrosis factor.
Role of monocytes in LcS-induced immunomodulation
To study the role of monocytes in LcS-induced immunomodulation, the effects of LcS at 106 CFU/ml on expression of activation markers and cytokine production of PBMC and monocyte-depleted PBMC (MD-PBMC) were compared. In this experiment, activation markers were examined on CD3+, CD4+, CD8+ and CD56+ subsets of lymphocytes in PBMC and MD-PBMC. LcS stimulated a significant increase in CD69 expression on all the above subsets in PBMC (P < 0·05). Following depletion of monocytes, LcS still increased the expression of CD69 on CD3+, CD8+ and CD56+ subsets relative to no stimulus, but did not increase expression on CD4+ T cells (Fig. 3a). However, the degree of induction of CD69 by LcS on MD-PBMC was significantly lower than that on PBMC (Fig. 3a). LcS induced CD25 expression only on CD8+ and CD56+ subsets in PBMC, and also MD-PBMC, but expression of this activation marker was reduced significantly by monocyte depletion (Fig. 3b).
Fig. 3.
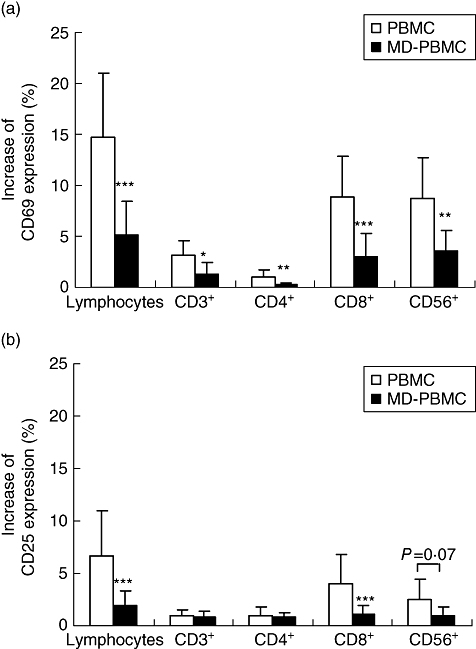
Effect of monocyte depletion on Lactobacillus casei Shirota (LcS)-induced CD69 and CD25 expression by peripheral blood mononuclear cells (PBMCs) and monocyte-depleted (MD)-PBMCs. (a) CD69 expression; (b) CD25 expression. PBMCs or MD-PBMCs were incubated with medium or live LcS at 106 colony-forming units (CFU)/ml for 24 h. Data are shown as mean ± standard deviation of 6 to 19 subjects. *P < 0·05, **P < 0·01, ***P < 0·001 compared between PBMC and MD-PBMC cultures.
Monocyte depletion resulted in a significant decrease in the production of IL-1β, IL-6, TNF-α and IL-10, and a trend towards a decrease in IL-12 production induced by LcS (Fig. 4), indicating that monocytes play an important role in LcS-induced cytokine production. LcS-induced production of IL-1β, IL-6, TNF-α and IL-12 was not inhibited entirely by monocyte depletion, whereas IL-10 production was inhibited totally compared with the unstimulated cultures (Fig. 4). For cells stimulated by LPS, monocyte depletion resulted in a significant decrease in production of IL-1β, IL-6 and IL-10 compared with PBMC (Fig. 4). Similar to the effect of LcS, monocyte depletion totally inhibited IL-10 production induced by LPS.
Fig. 4.
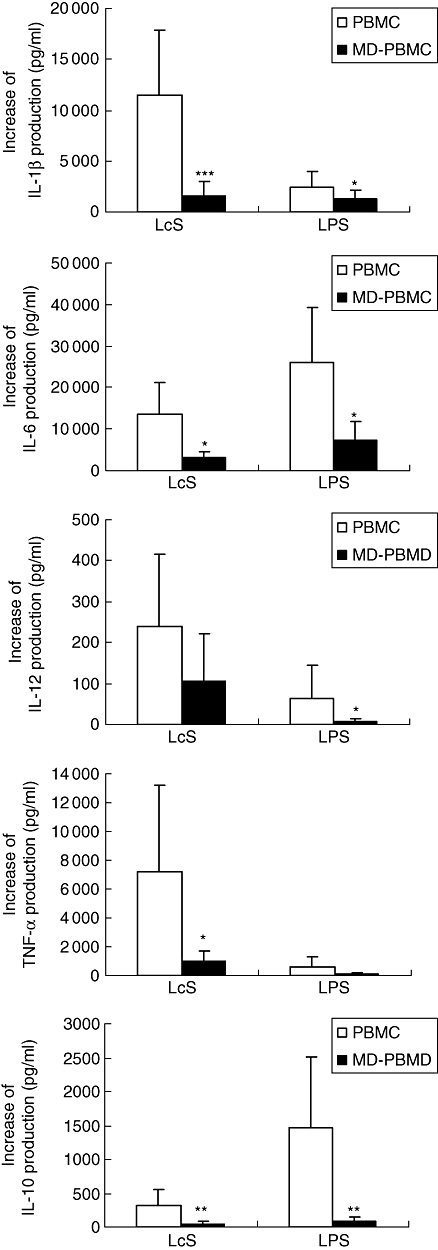
Effect of monocyte depletion on Lactobacillus casei Shirota (LcS)- and lipopolysaccharide (LPS)-induced cytokine production. Peripheral blood mononuclear cells (PBMCs) or monocyte-depleted (MD)-PBMCs were incubated with live LcS at 106 colony-forming units (CFU)/ml or LPS at 1 µg/ml for 24 h. Increase of cytokine productions of interleukin (IL)-1β, IL-6, tumour necrosis factor (TNF)-α, IL-12 and IL-10 in the supernatants of the cultures were measured by enzyme-linked immunosorbent assay. Data are shown as mean ± standard deviation of 6 to 14 subjects. *P < 0·05, **P < 0·01, ***P < 0·001 compared between PBMC and MD-PBMC cultures.
Effect of LcS on NK cell activity
NK cell activity was assessed in PBMC and MD-PBMC before (fresh) and after 24 h incubation with medium or LcS at 106 CFU/ml. NK cell activity was higher in PBMC incubated in medium for 24h than in fresh PBMC (Fig. 5a,b). However, when PBMC were exposed to LcS at 106 CFU/ml for 24 h, NK cell activity was increased greatly compared with that in the medium-only control (Fig. 5b). NK cell activity in 24 h incubated MD-PBMC with medium was greater than that in fresh PBMC or PBMC incubated for 24 h with medium (P < 0·01). When MD-PBMC were stimulated with LcS for 24 h, NK cell activity was increased compared with MD-PBMC cultured with medium alone (P < 0·01, Fig. 5c). However, the magnitude of the increase of NK cell activity stimulated by LcS was reduced significantly by monocyte depletion. For example, at a 50 : 1 ratio of effector to target cells, the magnitude of the increase of NK cell activity by LcS was 3·14-fold in PBMC and 1·65-fold in MD-PBMC. Thus, monocyte depletion reduced NK cell activity induced by LcS.
Fig. 5.
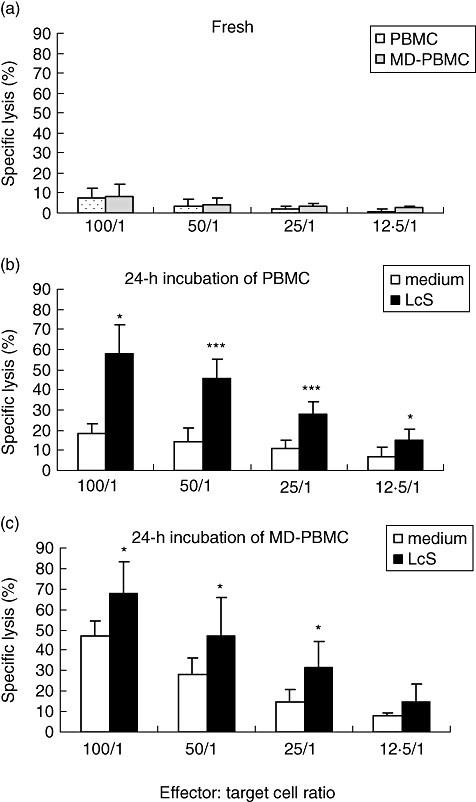
Effect of Lactobacillus casei Shirota (LcS) on natural killer (NK) cell activity in peripheral blood mononuclear cells (PBMCs) and monocyte-depleted (MD)-PBMCs. PBMCs or MD-PBMCs were incubated with medium or live LcS at 106 colony-forming units (CFU)/ml for 24 h. NK cell activity represented by percentage of specific lysis of target cells was assessed in freshly prepared PBMCs (a), in PBMCs cultured with medium or LcS (b) and in MD-PBMCs cultured with medium or LcS (c) for 24 h. Data are shown as mean ± standard deviation of five subjects. *P < 0·05, **P < 0·01, ***P < 0·001 compared with non-stimulated cultures of PBMC or MD-PBMC.
Discussion
Evidence has accumulated to suggest that probiotics confer health benefits via modulation of immune function. The present study demonstrates that the probiotic strain, LcS, promotes T cell activation, as evidenced by induction of CD69 and CD25 expression by T lymphocytes. Importantly, there was evidence of preferential activation of CD8+ T cells. LcS also enhanced CD69 and CD25 expression on the CD56+ subset, increased NK cell activity and induced cytokine production by human PBMC in vitro.
Effects of LcS on lymphocyte activation
After T cell activation via CD3/T cell receptor (TCR) or via CD2 (the alternate T cell activation pathway), the first measurable surface marker that is induced is CD69 [22]. It has been reported that quantitative flow cytometric determination of CD69 expression on T lymphocytes has several advantages over traditional lymphocyte proliferation assays and is a useful diagnostic tool for detailed assessment of T lymphocyte and subset activation [23].
CD25, the α chain of the IL-2 receptor, is a late activation marker induced during lymphoid cell activation, reaching a peak at 96 h after stimulation by phytohaemagglutinin (PHA) [24]. CD25 is expressed on activated T cells, B cells and monocytes. Formation of the high-affinity IL-2 receptor allows T cell proliferation and differentiation to be driven by IL-2 [25]. Previous studies have shown overall agreement with CD25 or CD69 and proliferation assays when a recall specific antigen was used [24].
CD25 and CD69 have been regarded generally as activation markers without discrimination of their significance for proliferation. However, Clausen [26] reported that there was discrimination between CD25 and CD69 as activation markers on cytotoxic CD56+ lymphocytes and that CD25 should be viewed as an indicator for proliferative potential, whereas CD69 is a marker for cytotoxic activity. However, further evidence is required to justify this.
Studies on the effect of LcS on cellular immunity are very limited. Our data demonstrate that LcS stimulated CD69 expression by CD3+, CD4+, CD8+ and CD56+ lymphocytes in the absence of mitogen. It also stimulated expression of CD25, but only by CD8+ and CD56+ lymphocytes. This is the first time that such an effect of LcS on T lymphocytes, and in particular CD8+ subsets, has been shown. Similar effects of other probiotic strains have been reported in a few cases. For example, Castellazzi et al.[27] demonstrated that L. paracasei I 1688, L. salivarius I 1794 and a mixture of the two strains increased the percentage of CD4+/CD25+ cells (T helper-activated regulatory T cells), CD8+/CD25+ (T suppressor/cytotoxic-activated cells) and CD16+/CD56+ (NK cells) (P < 0·05). Another in vitro experiment showed that two Lactobacillus strains, L. johnsonii La 1 and L. sakei LTH 681, increased expression of CD69 by CD8+ cells [28]. In a human trial by Meyer [29], the expression of CD69 by T lymphocytes was increased by consumption of both conventional yogurt and a probiotic product, and this activation was especially significant in the CD8+ subset, with a lesser effect on CD4+ lymphocytes. In a randomized placebo-controlled human trial [12], the effect of Saccharomyces boulardii administration was studied in healthy children aged between 6 months and 10 years who were admitted for acute diarrhoea. The patients who were supplemented with S. boulardii for 7 days showed a significant decrease in daily stool frequency and a significant increase in the percentage of CD8 lymphocytes and serum immunoglobulin A (IgA) compared with the placebo group. In a randomized, double-blind, placebo-controlled intervention study by de Vrese [3], L. gasseri PA 16/8, Bifidobacterium longum SP 07/3 and B. bifidum MF 20/5 were supplemented for 3 months in 479 healthy adults. Cellular immune parameters were evaluated in a randomly drawn subgroup of 122 volunteers before and after 14 days of supplementation. The results showed that the total symptom score, the duration of common cold episodes and days with fever during an episode were significantly lower in the probiotic group. There was also a significant increase in the percentage of cytotoxic/T suppressor cells (CD8+) after probiotic supplement. Our data are thus consistent with these studies, indicating that probiotics enhance the numbers/activation of cytotoxic lymphocytes and may enhance cytotoxic activity. It remains to be determined whether these effects are strain-dependent, or a general feature of lactobacillus and bifidobacterium species.
Several human studies have shown that probiotics, including LcS, enhance NK cell activity [5,7,8,10,13,18,30–32]. The results in the current study showed a direct enhancement of NK cell activity in human PBMC stimulated by LcS in vitro, which is in agreement with another in vitro study [21] employing heat-killed LcS and showing that both NK cell activation and activity were enhanced by LcS stimulation. These data suggest that LcS could potentiate the destruction of infected cells in the body and enhance host defence. Further research to investigate mechanisms is required and human trials are needed to confirm these immunomodulatory effects of LcS in vivo.
Effects of LcS on cytokine production by PBMC
Probiotics clearly modulate cytokine production and the effects appear to be strain-specific [33]. Data on the effects of LcS on cytokine production, however, are limited. The current study demonstrates that live LcS greatly induces production of IL-1β, IL-6 and TNF-α, and also significantly induces production of IL-12 and IL-10. These results are consistent with an in vitro study, which showed that heat-killed LcS stimulated human PBMC to secrete IL-12, IFN-γ, TNF-α and IL-10 [21]. In another in vitro study using murine monocyte/macrophage cell line J774A.1, LcS was shown to stimulate markedly secretion of IL-12 and TNF-α, but had no effect on IL-10 production [34]. This study also showed that live bacteria stimulated higher levels of IL-12 and TNF-α than heat-killed preparations.
In the current study, LPS (the main component of the cell wall of Gram-negative bacteria) induced greater production of IL-10 and IL-6, lower production of IL-1β than LcS, but had no significant effect on production of IL-12 or TNF-α. According to other studies employing human PBMC, Escherichia coli and LPS are weaker inducers of IL-12, TNF-α and IFN-γ, but far more potent inducers of the anti-inflammatory cytokine, IL-10, than lactic acid bacteria [34–37]. In an in vitro study, LPS was a more potent inducer of IL-10 than several live B. longum strains, but a weak inducer of TNF-α production [38,39]. In another in vitro study, LPS induced production of IL-1β, IL-10, IL-12 and, in particular, a large amount of IL-6, by human myeloid dendritic cells, but did not induce production of TNF-α[38]. On the other hand, Lactobacillus strains have been reported to induce production of IL-12, TNF-α and IFN-γ, as well as IL-10 [40,41]. In addition, our results also confirmed that PBMC samples can be replaced by whole blood samples for cytokine measurement [42].
The current study showed that LcS enhanced LPS-induced IL-1β, but inhibited LPS-induced IL-10 and IL-6 production and had no further effect on production of IL-12 or TNF-α. Interestingly, Mohamadzadeh et al.[38] showed that production of IL-12 by myeloid dendritic cells was sustained in response to three Lactobacillus species in the presence of LPS, whereas LPS-induced IL-10 production was greatly inhibited. These results indicate that the effects of probiotics on inflammatory responses might be strain-dependent, and also dependent upon host immune status. The interpretation of these effects of lactobacilli is clearly complex, but it is suggested that the literature to date supports evidence for an augmentation of innate immune defences by probiotics, coupled with an ability to regulate inflammation under some conditions [21].
It is suggested that cell-surface components of the bacteria play a central role in inducing immune responses [35,39]. An in vitro study showed that the effects of cell-surface components obtained by sonication of B. longum strains replicated the stimulation of PBMCs with live cells, indicating that these components are important determinants of the immunomodulatory activity of B. longum. On the other hand, cell-free culture supernatants of the studied strains do not tend to induce production of TNF-α, indicating that surface structures are important in determining the immunomodulatory activity of probiotic bacteria [39].
These surface structures are generally called pathogen-associated molecular patterns (PAMPs), and are recognized by pattern recognition receptors (PRRs), which include TLRs [43]. Activation of different TLRs results in induction of different cytokines. For example, TLR-4 is the key target for LPS-induced signalling which results in IL-10 production, while TLR-2 recognizes lipoprotein/lipopeptide, lipoteichoic acid, glycoinositol-phospholipids and peptidoglycan, which are present in Gram-positive and/or Gram-negative bacteria cell walls, which results in secretion of proinflammatory cytokines and anti-inflammatory IL-10 [44].
Role of monocytes on LcS-induced immunomodulation
In the current study, LcS induced preferential activation of cytotoxic cells (both CD8+ and CD56+). It has been reported that early production of IL-12 by macrophages contributes to the maturation of both NK cells and CD8+ T cells, leading to a T helper type 1 (Th1)-biased response [45]. In an in vitro study, LcS was shown to be phagocytosed by monocytes and stimulated them directly to secrete not only proinflammatory cytokines, such as IL-12 and TNF-α, but also the anti-inflammatory cytokine, IL-10 [21]. It was therefore proposed that monocytes play an important role in LcS-induced immunomodulation. The current study demonstrates that monocyte depletion reduced significantly the activation of T cells and NK cells, as well as the production of IL-1β, IL-6, TNF-α and IL-12, and totally blocked the secretion of IL-10. This suggests that monocytes are critical for the induction of IL-10 by LcS, which is supported by another study in which removal of the monocytes abrogated IL-10 production upon interaction with lactic acid bacteria [28] or LPS [46], and are important (but not critical) for the production of other cytokines and induction of activation markers. Other studies have reported that L. johnsonii induces CD25 expression directly on purified NK cells [45], and mRNA for the p35 and p40 subunits of IL-12 was still induced in monocyte-depleted cultures [46], which indicates that LcS could modify immune function via pathways not involving monocytes.
Our results show that the magnitude of the increase of NK cell activity in PBMC was greater than that in MD-PBMC after 24 h incubation with LcS, suggesting that monocytes potentiate the effect of the LcS-induced increase in NK cell activity. This is consistent with the study of Shida et al.[21], which reported that monocytes play a role in LcS-induced NK cell activity. In the current study, the greater NK cell activity in MD-PBMC than in PBMC after 24 h incubation may be due partly to a relatively higher proportion of NK cells in MD-PBMC than in PBMC (as monocyte depletion by nature will result in a higher proportion of NK cells). Moreover, some other studies support the observation that monocytes might suppress NK cell activity [46–48]. It is possible that increased NK cell activity in monocyte-depleted cultures is a consequence of the reduced/inhibited IL-10 secretion, as there is evidence that IL-10 plays a part in the regulation of NK cell function. For example, Goodier [46] found that cytolytic activity, IFN-γ production, proliferation and expansion of human peripheral blood CD3– CD56+ cells (NK cells) induced by LPS were enhanced in the presence of anti-IL-10 receptor-blocking antibodies or on the removal of CD14+ cells (monocytes) from the cultures. IL-10 was lost from the culture supernatants of CD14-depleted PBMC and rIL-10 reversed the effect of this depletion.
In conclusion, in the absence of mitogenic stimulation, LcS enhances immune function, particularly that of cytotoxic T lymphocytes (CD8+ T cells), possibly via induction of proinflammatory cytokines. This is the first time such an effect of LcS has been shown, and suggests that LcS could potentiate the destruction of infected cells in the body. Further research to investigate mechanisms is required and human trials are needed to confirm these immunomodulatory effects of LcS in vivo.
Acknowledgments
This research was sponsored by a Dorothy Hodgkin Postgraduate Award and Yakult UK.
Disclosure
None declared.
References
- 1.Corthesy B, Gaskins HR, Mercenier A. Cross-talk between probiotic bacteria and the host immune system. J Nutr. 2007;137:781S–790S. doi: 10.1093/jn/137.3.781S. [DOI] [PubMed] [Google Scholar]
- 2.Galdeano CM, de LeBlanc AD, Vinderola G, Bonet AEB, Perdigon G. Proposed model: mechanisms of immunomodulation induced by probiotic bacteria. Clin Vaccine Immunol. 2007;14:485–92. doi: 10.1128/CVI.00406-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Vrese M, Winkler P, Rautenberg P, et al. Effect of Lactobacillus gasseri PA 16/8, Bifidobacterium longum SP 07/3, B-bifidum MF 20/5 on common cold episodes: a double blind, randomized, controlled trial. Clin Nutr. 2005;24:481–91. doi: 10.1016/j.clnu.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 4.Fukushima YMS, Yamano T, Kaburagi T, Iino H, Ushida K, Sato K. Improvement of nutritional status and incidence of infection in hospitalized enterally fed elderly by feeding of fermented milk containing probiotic Lactobacillus johnsonii La1(NCC533) Br J Nutr. 2007;98:969–77. doi: 10.1017/S0007114507764723. [DOI] [PubMed] [Google Scholar]
- 5.Olivares M, Diaz-Ropero MP, Sierra S, et al. Oral intake of Lactobacillus fermentum CECT5716 enhances the effects of influenza vaccination. Nutrition. 2007;23:254–60. doi: 10.1016/j.nut.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 6.Arunachalam K, Gill HS, Chandra RK. Enhancement of natural immune function by dietary consumption of Bifidobacterium lactis (HN019) Eur J Clin Nutr. 2000;54:263–7. doi: 10.1038/sj.ejcn.1600938. [DOI] [PubMed] [Google Scholar]
- 7.Bunout D, Barrera G, Hirsch S, et al. Effects of a nutritional supplement on the immune response and cytokine production in free-living Chilean elderly. J Parenter Enteral Nutr. 2004;28:348–54. doi: 10.1177/0148607104028005348. [DOI] [PubMed] [Google Scholar]
- 8.Gill HS, Rutherfurd KJ, Cross ML. Dietary probiotic supplementation enhances natural killer cell activity in the elderly: an investigation of age-related immunological changes. J Clin Immunol. 2001;21:264–71. doi: 10.1023/a:1010979225018. [DOI] [PubMed] [Google Scholar]
- 9.Klein A, Friedrich U, Vogelsang H, Jahreis G. Lactobacillus acidophilus 74-2 and Bifidobacterium animalis subsp lactis DGCC 420 modulate unspecific cellular immune response in healthy adults. Eur J Clin Nutr. 2008;62:584–93. doi: 10.1038/sj.ejcn.1602761. [DOI] [PubMed] [Google Scholar]
- 10.Sheih YH, Chiang BL, Wang LH, Liao CK, Gill HS. Systemic immunity-enhancing effects in healthy subjects following dietary consumption of the lactic acid bacterium Lactobacillus rhamnosus HN001. J Am Coll Nutr. 2001;20:149–56. doi: 10.1080/07315724.2001.10719027. [DOI] [PubMed] [Google Scholar]
- 11.Gill HS, Rutherfurd KJ. Probiotic supplementation to enhance natural immunity in the elderly: effects of a newly characterized immunostimulatory strain Lactobacillus rhamnosus HN001 (DR20 (TM)) on leucocyte phagocytosis. Nutr Res. 2001;21:183–9. [Google Scholar]
- 12.Ozkan TB, Sahin E, Erdemir G, Budak F. Effect of Saccharomyces boulardii in children with acute gastroenteritis and its relationship to the immune response. J Int Med Res. 2007;35:201–12. doi: 10.1177/147323000703500204. [DOI] [PubMed] [Google Scholar]
- 13.Takeda K, Okumura K. Effects of a fermented milk drink containing Lactobacillus casei strain shirota on the human NK-cell activity. J Nutr. 2007;137:791S–3S. doi: 10.1093/jn/137.3.791S. [DOI] [PubMed] [Google Scholar]
- 14.Matsumoto K, Takada T, Shimizu K, et al. The effects of a probiotic milk product containing Lactobacillus casei strain Shirota on the defecation frequency and the intestinal microflora of sub-optimal health state volunteers: a randomized placebo-controlled cross-over study. Biosci Microflora. 2006;25:39–48. [Google Scholar]
- 15.Aso Y, Akaza H, Kotake T, Tsukamoto T, Imai K, Naito S. Preventive effect of a Lactobacillus-casei preparation on the recurrence of superficial bladder-cancer in a double-blind trial. Eur Urol. 1995;27:104–9. doi: 10.1159/000475138. [DOI] [PubMed] [Google Scholar]
- 16.Ishikawa H, Akedo I, Otani T, et al. Randomized trial of dietary fiber and Lactobacillus casei administration for prevention of colorectal tumors. Int J Cancer. 2005;116:762–7. doi: 10.1002/ijc.21115. [DOI] [PubMed] [Google Scholar]
- 17.Koebnick C, Wagner I, Leitzmann P, Stern U, Zunft HJF. Probiotic beverage containing Lactobacillus casei Shirota improves gastrointestinal symptoms in patients with chronic constipation. Can J Gastroenterol. 2003;17:655–9. doi: 10.1155/2003/654907. [DOI] [PubMed] [Google Scholar]
- 18.Nagao F, Nakayama M, Muto T, Okumura K. Effects of a fermented milk drink containing Lactobacillus casei strain Shirota on the immune system in healthy human subjects. Biosci Biotechnol Biochem. 2000;64:2706–8. doi: 10.1271/bbb.64.2706. [DOI] [PubMed] [Google Scholar]
- 19.Ivory K, Chambers SJ, Pin C, Prieto E, Arques JL, Nicoletti C. Oral delivery of Lactobacillus casei Shirota modifies allergen-induced immune responses in allergic rhinitis. Clin Exp Allergy. 2008;38:1282–9. doi: 10.1111/j.1365-2222.2008.03025.x. [DOI] [PubMed] [Google Scholar]
- 20.Takeda K, Suzuki T, Shimada SI, Shida K, Nanno M, Okumura K. Interleukin-12 is involved in the enhancement of human natural killer cell activity by Lactobacillus casei Shirota. Clin Exp Immunol. 2006;146:109–15. doi: 10.1111/j.1365-2249.2006.03165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shida K, Suzuki T, Kiyoshima-Shibata J, Shimada S, Nanno M. Essential roles of monocytes in stimulating human peripheral blood mononuclear cells with Lactobacillus casei to produce cytokines and augment natural killer cell activity. Clin Vaccine Immunol. 2006;13:997–1003. doi: 10.1128/CVI.00076-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rutella S, Rumi C, Lucia MB, et al. Induction of CD69 antigen on normal CD4(+) and CD8(+) lymphocyte subsets and its relationship with the phenotype of responding T-cells. Cytometry. 1999;38:95–101. [PubMed] [Google Scholar]
- 23.Lindsey WB, Lowdell MW, Marti GE, et al. CD69 expression as an index of T-cell function: assay standardization, validation and use in monitoring immune recovery. Cytotherapy. 2007;9:123–32. doi: 10.1080/14653240601182838. [DOI] [PubMed] [Google Scholar]
- 24.Antas PRZ, Oliveira EB, Milagres AS, et al. Kinetics of T cell-activation molecules in response to Mycobacterium tuberculosis antigens. Mem Inst Oswaldo Cruz. 2002;97:1097–9. doi: 10.1590/s0074-02762002000800005. [DOI] [PubMed] [Google Scholar]
- 25.Foote MR, Nonnecke BJ, Fowler MA, Miller BL, Beitz DC, Waters WR. Effects of age and nutrition on expression of CD25, CD44, and L-selectin (CD62L) on T-cells from neonatal calves. J Dairy Sci. 2005;88:2718–29. doi: 10.3168/jds.S0022-0302(05)72951-9. [DOI] [PubMed] [Google Scholar]
- 26.Clausen J, Vergeiner B, Enk M, Petzer AL, Gastl G, Gunsilius E. Functional significance of the activation-associated receptors CD25 and CD69 on human NK-cells and NK-like T-cells. Immunobiology. 2003;207:85–93. doi: 10.1078/0171-2985-00219. [DOI] [PubMed] [Google Scholar]
- 27.Castellazzi AM, Valsecchi C, Montagna L, et al. In vitro activation of mononuclear cells by two probiotics: Lactobacillus paracasei I 1688, Lactobacillus salivarius I 1794, and their mixture (PSMIX) Immunol Invest. 2007;36:413–21. doi: 10.1080/08820130701361160. [DOI] [PubMed] [Google Scholar]
- 28.Haller D, Blum S, Bode C, Hammes WP, Schiffrin EJ. Activation of human peripheral blood mononuclear cells by nonpathogenic bacteria in vitro: evidence of NK cells as primary targets. Infect Immun. 2000;68:752–9. doi: 10.1128/iai.68.2.752-759.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meyer AL, Micksche M, Herbacek I, Elmadfa I. Daily intake of probiotic as well as conventional yogurt has a stimulating effect on cellular immunity in young healthy women. Ann Nutr Metab. 2006;50:282–9. doi: 10.1159/000091687. [DOI] [PubMed] [Google Scholar]
- 30.Olivares M, Diaz-Ropero MP, Gomez N, et al. The consumption of two new probiotic strains, Lactobacillus gasseri CECT 5714 and Lactobacillus coryniformis CECT 5711, boosts the immune system of healthy humans. Int Microbiol. 2006;9:47–52. [PubMed] [Google Scholar]
- 31.Arunachalam KGH, Chandra RK. Enhancement of natural immune function by dietary consumption of Bifidobacterium lactis HN019. Eur J Clin Nutr. 2000;54:263–7. doi: 10.1038/sj.ejcn.1600938. [DOI] [PubMed] [Google Scholar]
- 32.Zanini K, Marzotto M, Castellazzi A, Borsari A, Dellaglio F, Torriani S. The effects of fermented milks with simple and complex probiotic mixtures on the intestinal microblota and immune response of healthy adults and children. Int Dairy J. 2007;17:1332–43. [Google Scholar]
- 33.Borchers AT, Selmi C, Meyers FJ, Keen CL, Gershwin ME. Probiotics and immunity. J Gastroenterol. 2009;44:26–46. doi: 10.1007/s00535-008-2296-0. [DOI] [PubMed] [Google Scholar]
- 34.Cross ML, Ganner A, Teilab D, Fray LM. Patterns of cytokine induction by Gram-positive and Gram-negative probiotic bacteria. FEMS Immunol Med Microbiol. 2004;42:173–80. doi: 10.1016/j.femsim.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 35.Helwig U, Lammers KM, Rizzello F, et al. Lactobacilli, bifidobacteria and E-coli nissle induce pro- an anti-inflammatory cytokines in peripheral blood mononuclear cells. World J Gastroenterol. 2006;12:5978–86. doi: 10.3748/wjg.v12.i37.5978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hessle C, Hanson LA, Wold AE. Lactobacilli from human gastrointestinal mucosa are strong stimulators of IL-12 production. Clin Exp Immunol. 1999;116:276–82. doi: 10.1046/j.1365-2249.1999.00885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zeuthen LH, Christensen HR, Frokiaer H. Lactic acid bacteria inducing a weak interleukin-12 and tumor necrosis factor alpha response in human dendritic cells inhibit strongly stimulating lactic acid bacteria but act synergistically with Gram-negative bacteria. Clin Vaccine Immunol. 2006;13:365–75. doi: 10.1128/CVI.13.3.365-375.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mohamadzadeh M, Olson S, Kalina WV, et al. Lactobacilli activate human dendritic cells that skew T cells toward T helper 1 polarization. Proc Natl Acad Sci USA. 2005;102:2880–5. doi: 10.1073/pnas.0500098102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Medina M, Izquierdo E, Ennahar S, Sanz Y. Differential immunomodulatory properties of Bifidobacterium logum strains: relevance to probiotic selection and clinical applications. Clin Exp Immunol. 2007;150:531–8. doi: 10.1111/j.1365-2249.2007.03522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lammers KM, Brigidi P, Vitali B, et al. Immunomodulatory effects of probiotic bacteria DNA: IL-1 and IL-10 response in human peripheral blood mononuclear cells. FEMS Immunol Med Microbiol. 2003;38:165–72. doi: 10.1016/S0928-8244(03)00144-5. [DOI] [PubMed] [Google Scholar]
- 41.Hart AL, Lammers K, Brigidi P, et al. Modulation of human dendritic cell phenotype and function by probiotic bacteria. Gut. 2004;53:1602–9. doi: 10.1136/gut.2003.037325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yaqoob P, Newsholme EA, Calder PC. Comparison of cytokine production in cultures of whole human blood and purified mononuclear cells. Cytokine. 1999;11:600–5. doi: 10.1006/cyto.1998.0471. [DOI] [PubMed] [Google Scholar]
- 43.Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol. 2001;1:135–45. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- 44.Bauer S, Hangel D, Yu P. Immunobiology of Toll-like receptors in allergic disease. Immunobiology. 2007;212:521–33. doi: 10.1016/j.imbio.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 45.Niers LEM, Timmerman HM, Rijkers GT, et al. Identification of strong interleukin-10 inducing lactic acid bacteria which down-regulate T helper type 2 cytokines. Clin Exp Allergy. 2005;35:1481–9. doi: 10.1111/j.1365-2222.2005.02375.x. [DOI] [PubMed] [Google Scholar]
- 46.Goodier MR, Londei M. Lipopolysaccharide stimulates the proliferation of human CD56(+)CD3(-) NK cells: a regulatory role of monocytes and IL-10. J Immunol. 2000;165:139–47. doi: 10.4049/jimmunol.165.1.139. [DOI] [PubMed] [Google Scholar]
- 47.Esin S, Batoni G, Pardini M, et al. Functional characterization of human natural killer cells responding to Mycobacterium bovis bacille Calmette–Guerin. Immunology. 2004;112:143–52. doi: 10.1111/j.1365-2567.2004.01858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Higuchi K, Aoki K, Kimbara T, Hosoi N, Yamamoto T, Okada H. Suppression of natural-killer-cell activity by monocytes follwing immunotherapy for recurrent spontaneous aborters. Am J Reprod Immunol. 1995;33:221–7. doi: 10.1111/j.1600-0897.1995.tb00888.x. [DOI] [PubMed] [Google Scholar]
- 49.Berman SH, Eichelsdoerfer P, Yim D, Elmer GW, Wenner CA. Daily ingestion of a nutritional probiotic supplement enhances innate immune function in healthy adults. Nutr Res. 2006;26:454–9. [Google Scholar]


