Abstract
Background
Although illicit anabolic-androgenic steroid (AAS) use is widespread, the cardiac effects of long-term AAS use remain inadequately characterized. We compared cardiac parameters in weightlifters reporting long-term AAS use to those in otherwise similar weightlifters without prior AAS exposure.
Methods & Results
We performed 2-dimensional, tissue-Doppler, and speckle-tracking echocardiography to assess left ventricular (LV) ejection fraction, LV systolic strain, and conventional indices of diastolic function in long-term AAS users (n=12) and otherwise similar AAS non-users (n=7). AAS users (median [Q1,Q3] cumulative lifetime AAS exposure 468 [169–520] weeks) closely resembled non-users in age, prior duration of weightlifting, and current intensity of weight training. LV structural parameters were similar between the two groups. However, AAS users had significantly lower LV ejection fraction (50.6% [48.4, 53.6] versus 59.1% [58.0, 61.7]; p = 0.003 by Wilcoxon rank sum test, two-tailed); longitudinal strain (16.9% [14.0, 19.0] versus 21.0% [20.2, 22.9]; p = 0.004), and radial strain (38.3 [28.5, 43.7] versus 50.1 [44.3, 61.8]; p = 0.02). Ten of the 12 AAS users showed LV ejection fractions below the accepted limit of normal (≥55%). AAS users also demonstrated decreased diastolic function compared to non-users, as evidenced by a markedly lower E′ velocity (7.4 [6.8, 7.9] versus 9.9 [8.3, 10.5]; p = 0.005) and E/A ratio (0.93 [0.88, 1.39] versus 1.80 [1.48, 2.00]; p = 0.003).
Conclusions
Cardiac dysfunction in long-term AAS users appears more severe than previously reported, and may be sufficient to increase the risk of heart failure.
Keywords: myocardial contraction, mechanics, systole, diastole, anabolic androgenic steroids
INTRODUCTION
The anabolic androgenic steroids (AAS) are a family of drugs that includes the male hormone testosterone and its synthetic derivatives. More than one million American men and women have used these drugs illicitly to gain muscle and lose body fat.1–5 Long-term illicit use of supraphysiologic doses of AAS may cause adverse cardiovascular effects, but these remain poorly understood.5–7 Early work examining cardiac function in AAS users produced inconsistent results.8,9 Recent studies utilizing modern imaging techniques have found evidence of overt left ventricular (LV) diastolic dysfunction10–12 and subclinical LV systolic impairment (reduced systolic strain with normal LV ejection fraction) in AAS users.10 In addition, numerous case reports of cardiac death among AAS users suggest a causal link between AAS use and cardiovascular disease.13–19
Illicit AAS use did not become widespread in the general population until the 1980s, and thus the first large wave of long-term illicit users is only now reaching middle age.5 Therefore it has recently become both more feasible and increasingly critical to study cardiac structure and function in this population. Accordingly, we conducted a preliminary study comparing cardiac parameters in long-term male AAS-using weightlifters and age-matched male weightlifters reporting no AAS exposure.
METHODS
Study Population
AAS users and non-users were recruited by advertising in gymnasiums frequented by male weightlifters and other athletes known to have a high prevalence of AAS use, using methods to maximize the representativeness of the sample and minimize selection bias as previously described in detail.20,21 Briefly, our advertising indicated that we were conducting psychological and medical evaluations of experienced male weightlifters, but neither the study’s focus on AAS, nor the specific hypotheses being tested, were disclosed either in the advertising or in telephone screening of candidate study participants. All participants provided written informed consent before initiating the study as approved by the McLean Hospital Institutional Review Board.
The evaluation included demographic information and determination of height, weight, and body fat.22,23 Body fat was determined on the basis of six skinfold measurements with calipers, using the equations of Jackson and Pollock.22 From these data we calculated fat-free mass index, an index of muscularity developed in our laboratory and previously described in detail.23 We then elicited self-reported history of use of AAS, including specific drugs used, dosages, and durations of use, using an interview protocol described in previous studies.20,21,24 Estimated weeks of lifetime AAS use, together with average weekly AAS dose and maximum lifetime weekly AAS dose were calculated using testosterone equivalence as previously described.21,24 We also elicited a detailed history of lifetime alcohol and classical illicit substance use (i.e., cannabis, amphetamines, cocaine, opioids, etc.) using the Structured Clinical Interview for DSM-IV.25 We then elicited a history of use of other performance-enhancing drugs, such as human growth hormone, again with estimates of total lifetime exposure as previously described.20,21,24,26 Finally, we acquired traditional medical historical and exercise exposure data including lifetime duration of weightlifting, current hours per week of weightlifting, current hours of non-weightlifting exercise activity.
Echocardiography
Transthoracic echocardiography was performed using a commercially available system (Vivid-I, GE Healthcare, Milwaukee, Wisconsin). Subjects were studied after >1 hour of rest. Images were obtained using 2-dimensional (frame rate 25 to 75/second) Doppler, and color-tissue Doppler (frame rate > 100/second) imaging and were acquired from standard parasternal and apical views. All data were stored digitally and subsequently analyzed offline (EchoPac, version 6.5, GE Healthcare). Standard measurements were performed in accordance with current guidelines.27 LV ejection fraction was calculated using the modified Simpson (bi-plane) technique with a value < 55% considered abnormal.27 LV mass was calculated using the area-length method, and a body-surface-indexed LV mass >103 g/m2 was used to define LV hypertrophy.27 Tissue velocity measurements were obtained off-line from colorized 2-dimensional images. Peak systolic strain was measured from the apical 4-chamber view (longitudinal) and the parasternal short axis view (radial) using speckle tracking analysis; reported values represent average strain in the 6 wall segments designated by the processing software (EchoPac, version 6.5, GE Healthcare). Reported tissue velocity and strain values are the average of 3 consecutive cardiac cycles.
Measurement Variability
Two investigators, blinded to each other’s measurements, independently measured LV mass, tissue velocity, and strain in 10 of the 19 study participants. Interobserver variability was quantified using the intraclass correlation coefficient. The intraclass correlation coefficient, the ratio of between-subject to total variability, reflects both within-subject and between-subject variability. (Appendix 1)
Appendix 1.
Interobserver variability of LV structural and functional parameters
| Cardiac Parameter | Intraclass correlation coefficient |
|---|---|
| LV Mass | 0.98 |
| LV Ejection Fraction | 0.98 |
| Peak Early Diastolic Tissue Velocity | 0.98 |
| LV Longitudinal Strain | 0.96 |
| LV Radial Strain | 0.96 |
Statistical Analyses
Differences between groups were assessed using the non-parametric Wilcoxon rank sum test as sample sizes were too small to permit an assumption of normality. Differences in proportions were assessed by Fisher’s exact test. Group-wise comparisons of cardiac parameters were assessed using linear regression on ranked data to adjust for body surface area and hours of training per week. Linear regression with ranked data was also used to examine the relationships between AAS exposure measures (lifetime weeks of use, estimated total lifetime dose of AAS, and maximum weekly dose of AAS) and cardiac parameters. Alpha was set at 0.01, 2-tailed, to control in part for multiple comparisons.
RESULTS
Study Participants
We studied 12 AAS users and 7 non-users. Although blood pressure and exercise capacity were not measured at the time of evaluation, none of the participants had a history of hypertension, atherosclerotic vascular disease, heart failure, or exercise intolerance. AAS users reported taking median (Q1, Q3) weekly doses of 675 (513, 950) mg of testosterone equivalent for 468 (169, 520) lifetime weeks. The groups were similar in age, prior duration of weightlifting, current hours per week of weight training and other intense athletic activity, body mass index (BMI), and body surface area, but AAS users were significantly more muscular than non-users as measured by fat-free mass index, as expected (Table 1). In other words, the AAS users had a similar BMI to non-users because the former group had more muscle but less body fat. Four of the 12 AAS users were currently taking supraphysiologic doses of AAS at the time of evaluation; three were currently taking only physiologic doses of testosterone at 50–100 mg per week; one had discontinued a course of supraphysiologic AAS three weeks prior to evaluation; and the remaining four had not used AAS for at least six months prior to the evaluation.
Table 1.
Characteristics of AAS Users and AAS Non-Users
| Characteristic | AAS Users (n = 12) | AAS Non-Users (n = 7) | p |
|---|---|---|---|
| Age (years) | 40.0 (35.8, 43.6) | 40.5 (37.9, 44.9) | 0.55 |
| Years of regular weightlifting | 17.5 (12.3, 20.4) | 15.0 (13.0, 22.0) | 0.97 |
| Hours of exercise per week* | 5.5 (4.0, 7.4) | 4.0 (3.0, 15.0) | 0.67 |
| Body mass index (kg/m2) | 30.3 (28.5, 32.1) | 28.4 (26.9, 31.8) | 0.35 |
| Body surface area (m2) | 2.16 (2.02, 2.22) | 2.02 (1.91, 2.26) | 0.67 |
| Fat-free mass index (kg/m2) | 26.6 (24.5, 27.9) | 24.0 (22.7, 25.2) | 0.01 |
Data are presented as median (Q1, Q3)
All p values are from Wilcoxon rank-sum test, two-tailed
Hours of weight training and other intense athletic activity
AAS, anabolic-androgenic steroids; kg/m2, kilograms per meter squared.
Six (50%) of the AAS users, but none of the non-users, reported a past history of opioid, cocaine, or alcohol dependence. None of the participants reported amphetamine dependence. Two (17%) AAS users, but none of the non-users, reported cannabis dependence, one in the past and one current. None of the participants reported a history of cigarette use. Nine (75%) of the AAS users, but none of the non-users, reported at least some use of human growth hormone, and six of these reported human growth hormone use for more than 3 months. We assessed for possible cardiac effects of these other forms of drug use in exploratory analyses as described below.
Cardiac Structure and Function
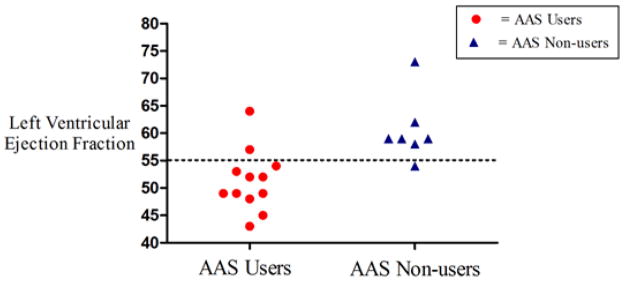
All LV structural parameters were similar between groups (Table 2). Six (50%) AAS users and 5 (71%) non-users met criteria for LV hypertrophy (p = 0.63). However, AAS users differed significantly from non-users in several complementary and independently derived indices of systolic function (Table 2). LV ejection fraction was abnormal (≤ 55%) in 10 (83%) AAS users but only one (14%) non-user, who had an LV ejection fraction of 54% (p = 0.003; Figure 1). Relative systolic dysfunction was further evidenced by significantly lower LV peak systolic strain among AAS users.
Table 2.
Left Ventricular Structure and Function in AAS Users and AAS Non-Users
| Measure | AAS Users (n = 12) | AAS Non-Users (n = 7) | p |
|---|---|---|---|
| Structural parameters | |||
| Posterior wall thickness (mm) | 11.1 (10.3, 1.9) | 11.0 (10.2, 11.0) | 0.31 |
| Interventricular septal thickness (mm) | 10.8 (9.1, 11.7) | 10.9 (10.3, 11.6) | 0.67 |
| End-diastolic diameter (mm) | 49.3 (47.8, 52.5) | 49.1 (44.5, 50.4) | 0.45 |
| Mass (grams) | 230 (195, 270) | 237 (210, 243) | 0.83 |
| Systolic function parameters | |||
| Ejection fraction (%) | 50.6 (48.4, 53.6) | 59.1 (58.0, 61.7) | 0.003 |
| Longitudinal strain (%) | 16.9 (14.0, 19.0) | 21.0 (20.2, 21.9) | 0.004 |
| Radial strain (%) | 38.3 (28.5, 43.7) | 50.1 (44.3, 61.8) | 0.02 |
| Peak systolic tissue velocity, cm/sec* | 6.2 (5.3, 7.0) | 6.3 (5.8, 6.8) | 0.50 |
| Diastolic function parameters | |||
| Trans-mitral E-wave, cm/sec | 64.0 (55.5, 78.5) | 77.0 (67.0, 86.0) | 0.05 |
| Trans-mitral A-wave, cm/sec | 61.5 (55.3, 65.8) | 43.0 (36.0, 54.0) | 0.02 |
| E/A ratio | 0.93 (0.88, 1.39) | 1.80 (1.48, 2.00) | 0.003 |
| Peak early diastolic tissue velocity, cm/sec | 7.4 (6.8, 7.9) | 9.9 (8.3, 10.5) | 0.005 |
| Peak late diastolic tissue velocity, cm/sec | 5.1 (4.1, 5.7) | 5.6 (4.4, 7.4) | 0.25 |
Data are presented as median (Q1, Q3)
All p values are from Wilcoxon rank-sum test, two-tailed
Measured at basal lateral segment of the left ventricle.
AAS, anabolic-androgenic steroids; kg/m2, kilograms per meter squared.
Figure 1.
Left ventricular ejection fraction in AAS users and AAS non-users – Circles represent AAS users and triangles represent AAS non-users.
Abbreviations: AAS = anabolic-androgenic steroids
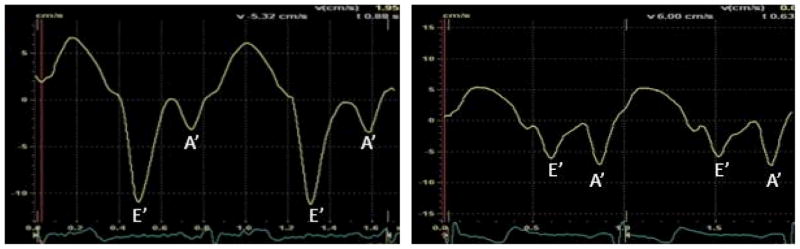
LV diastolic function also differed between the groups (Table 2). Compared to non-users, AAS users showed lower early diastolic trans-mitral blood flow velocity (E-wave) and early peak tissue velocity (E′) with preserved or increased late diastolic filling, as evidenced by late diastolic trans-mitral blood flow velocity (A-wave) and peak tissue velocity (A′). This pattern of diminished early diastolic function and enhanced late diastolic function, a hallmark of impaired LV relaxation,28 was reflected by a significantly lower ratio of early to late diastolic filling (E-wave/A-wave ratio). Representative examples of diastolic tissue velocity profiles for both groups are shown in Figure 2.
Figure 2.
Representative tissue Doppler velocity tracings - Normal diastolic profile in a non-AAS user (A) and an abnormal functional profile in a long-term AAS user (B).
Abbreviations: AAS = anabolic-androgenic steroids
Associations between AAS Use, Exercise Exposure, and Cardiac Parameters
We performed analyses adjusting 1) cardiac structural measurements for body surface area and 2) hours of weekly exercise training for all of the outcome measures in Table 2 and found that the differences between groups remained highly significant. In addition, we performed exploratory analyses testing for outlier bias by comparing groups following the exclusion of the nonuser with the highest LV ejection fraction and the nonuser with the highest E/A ratio. In both cases, all of the differences in Table 2 remained highly significant at p < 0.01.
In further exploratory analyses within the AAS-user group, we found no statistically significant associations between AAS exposure variables (lifetime weeks of exposure, total lifetime dose of AAS, and maximum weekly dose used) and the outcome measures in Table 2. We also performed an exploratory analysis excluding the 6 AAS users with past cocaine, opioid, or alcohol dependence, and compared the remaining 6 AAS users to the non-users. In this analysis, differences between the 6 AAS users with no history of other illicit drug use and the 7 non-users remained statistically significant (p < 0.05) for LV ejection fraction, longitudinal strain, peak early diastolic tissue velocity, and E/A ratio. We then performed a similar analysis excluding the 6 AAS users reporting more than 3 months of lifetime human growth hormone use. Again, group difference for LV ejection fraction, longitudinal strain, peak early diastolic tissue velocity, and E/A ratio remained similar to those found during the analyses including all 12 AAS users.
Finally, we performed exploratory analyses comparing the 7 non-users with each of the 3 subgroups of AAS users described above, namely a) the 4 users currently taking supraphysiologic doses of AAS; b) the 4 users who had not used AAS for at least 6 months; and c) the 4 remaining users who were either taking only physiologic doses of testosterone (N = 3) or who had recently stopped a course of AAS (N = 1). Each of these three individual subgroups showed similar deficits relative to the non-users on LV ejection fraction, LV systolic strain measures, and E-wave/A-wave ratio, tentatively suggesting that these deficits were not specifically associated with current AAS use or current AAS dose.
DISCUSSION
Results from this study are consistent with previous findings showing LV diastolic dysfunction10–12 and subclinical LV systolic impairment10 in AAS users. However, our results suggest that the cardiac impairment in long-term AAS users may be more severe than previously reported. Specifically long-term AAS users were found to have significant LV systolic dysfunction – both by relative standards (in comparison to the AAS non-users cohort) and absolute standards (as defined by current clinical practice). To our knowledge, this is the first study demonstrating an association between long-term AAS use and a clinically relevant reduction in LV ejection fraction.
There is a well-established relationship between LV dysfunction and exposure both to certain medications29 and to some drugs of abuse.30–30 Further, the prognostic importance of toxin-induced cardiac dysfunction has been well documented.33–36 Data from this study suggest that AAS, particularly when used over long durations, may be another important cause of toxin-mediated myocardial impairment. Although confirmatory data are required, results from this study suggest that AAS exposure should be a diagnostic consideration among individuals with asymptomatic LV dysfunction or incident heart failure.
Although several previous studies have reported mild cardiac dysfunction, LV dysfunction among our AAS users was more severe than previously reported. Several hypotheses might explain this discrepancy. First, our AAS users were several years older, on average, than those in two of the three most recent previous studies.10,12 Second, these previous studies selected “top-level competitive bodybuilders,”10 “squad athletes,”12 or individuals from “bodybuilding studios,”12 – thus possibly favoring healthier individuals with better cardiac function than the largely non-competitive individuals21 in our study. Finally, the specific AAS compounds and dosages typically used by our American participants may have differed from those of participants in previous studies, which were largely conducted in Europe.
It is noteworthy that our analysis revealed no significant relationship between cumulative AAS use and cardiac dysfunction. This observation suggests that AAS-associated cardiotoxicity might perhaps be only partially and unpredictably related to lifetime dose, in a manner similar to the cardiac toxicity of alcohol.37 Further work is required to determine whether cardiac function is influenced by factors such as the recency of AAS use (e.g., current vs. long past), specific AAS used (e.g., possibly more toxic oral agents5,38 versus injectable agents), duration of exposure to very high doses (e.g., ≥ 2000 mg per week), or concomitant use of other drugs (e.g., human growth hormone39,40).
There are several important limitations to this study. First, our sample might not be representative of the overall source population of long-term AAS users or comparison weightlifters. Although we used recruitment procedures designed to generate a sample that is maximally representative,20,21 it is possible that the group of AAS users in the present study is not entirely representative of the overall population. Second, our sample sizes were small and unequal. However, this limitation would likely produce false-negative findings (i.e., type II errors) rather than false-positive results due to the reduced statistical power afforded by small sample sizes. Therefore, the highly statistically significant findings in the present study, despite small sample sizes, suggest that the association between AAS use and cardiac pathology may be particularly strong (i.e., a very large effect size). Conversely, the possibility of a type II error must be considered in instances where group comparisons were not significant. For example, the lack of association between AAS exposure and cardiac dysfunction must be interpreted with caution. Future study with adequate statistical power is warranted to examine this issue. A third limitation is our reliance on participants’ self-reporting of AAS use. We recognize that errors based on self-report could have arisen if: 1.) actual AAS users denied use and were misclassified as non-users; 2.) individuals classified as non-users had unknowingly ingested supplements contaminated with actual AAS; or 3.) individuals classified as users had ingested only counterfeit black-market AAS and hence not used genuine drugs. However, each of these forms of misclassification would only narrow the differences between groups, causing us to underestimate the true association of AAS use with cardiac pathology. A fourth limitation is our use of retrospective exercise exposure assessment and the possibility that AAS-users differed from non-users with respect to exercise intensity. However, it is unlikely that this factor contributed to our observations as no prior studies have demonstrated LV dysfunction secondary to intense, sustained exercise training. Finally, the cross-sectional nature of this exploratory study does not permit definitive conclusions about the long-term clinical implications of our findings. This represents an important area of future work.
In summary, data from the present study suggest that AAS-induced LV dysfunction may be greater than previously reported. The reductions in LV systolic function observed in this group of AAS users are of a magnitude shown to increase the risk of heart failure and sudden cardiac death in other populations.41,42 Further work is needed to confirm our findings and to determine the extent to which AAS-associated cardiac dysfunction leads to adverse clinical outcomes.
Acknowledgments
Funding Sources: Supported in part by National Institute on Drug Abuse Grant DA 016744 (to Drs. Pope, Kanayama, and Hudson).
Footnotes
Conflict of Interest Disclosures: None
References
- 1.Buckley WE, Yesalis CE, Friedl KE, Anderson WA, Streit AL, Wright JE. Estimated prevalence of anabolic steroid use among male high school seniors. Jama. 1988;260:3441–5. [PubMed] [Google Scholar]
- 2.McCabe SE, Brower KJ, West BT, Nelson TF, Wechsler H. Trends in non-medical use of anabolic steroids by U.S. college students: results from four national surveys. Drug Alcohol Depend. 2007;90:243–51. doi: 10.1016/j.drugalcdep.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gruber AJ, Pope HG., Jr Psychiatric and medical effects of anabolic-androgenic steroid use in women. Psychother Psychosom. 2000;69:19–26. doi: 10.1159/000012362. [DOI] [PubMed] [Google Scholar]
- 4.Kanayama G, Boynes M, Hudson JI, Field AE, Pope HG., Jr Anabolic steroid abuse among teenage girls: an illusory problem? Drug Alcohol Depend. 2007;88:156–62. doi: 10.1016/j.drugalcdep.2006.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kanayama G, Hudson JI, Pope HG., Jr Long-term psychiatric and medical consequences of anabolic-androgenic steroid abuse: a looming public health concern? Drug Alcohol Depend. 2008;98:1–12. doi: 10.1016/j.drugalcdep.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parssinen M, Seppala T. Steroid use and long-term health risks in former athletes. Sports Med. 2002;32:83–94. doi: 10.2165/00007256-200232020-00001. [DOI] [PubMed] [Google Scholar]
- 7.Thiblin I, Petersson A. Pharmacoepidemiology of anabolic androgenic steroids: a review. Fundam Clin Pharmacol. 2005;19:27–44. doi: 10.1111/j.1472-8206.2004.00298.x. [DOI] [PubMed] [Google Scholar]
- 8.De Piccoli B, Giada F, Benettin A, Sartori F, Piccolo E. Anabolic steroid use in body builders: an echocardiographic study of left ventricle morphology and function. Int J Sports Med. 1991;12:408–12. doi: 10.1055/s-2007-1024703. [DOI] [PubMed] [Google Scholar]
- 9.Thompson PD, Sadaniantz A, Cullinane EM, Bodziony KS, Catlin DH, Torek-Both G, Douglas PS. Left ventricular function is not impaired in weight-lifters who use anabolic steroids. J Am Coll Cardiol. 1992;19:278–82. doi: 10.1016/0735-1097(92)90478-6. [DOI] [PubMed] [Google Scholar]
- 10.D’Andrea A, Caso P, Salerno G, Scarafile R, De Corato G, Mita C, Di Salvo G, Severino S, Cuomo S, Liccardo B, Esposito N, Calabro R. Left ventricular early myocardial dysfunction after chronic misuse of anabolic androgenic steroids: a Doppler myocardial and strain imaging analysis. Br J Sports Med. 2007;41:149–55. doi: 10.1136/bjsm.2006.030171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nottin S, Nguyen LD, Terbah M, Obert P. Cardiovascular effects of androgenic anabolic steroids in male bodybuilders determined by tissue Doppler imaging. Am J Cardiol. 2006;97:912–5. doi: 10.1016/j.amjcard.2005.10.026. [DOI] [PubMed] [Google Scholar]
- 12.Krieg A, Scharhag J, Albers T, Kindermann W, Urhausen A. Cardiac tissue Doppler in steroid users. Int J Sports Med. 2007;28:638–43. doi: 10.1055/s-2007-964848. [DOI] [PubMed] [Google Scholar]
- 13.Di Paolo M, Agozzino M, Toni C, Luciani AB, Molendini L, Scaglione M, Inzani F, Pasotti M, Buzzi F, Arbustini E. Sudden anabolic steroid abuse-related death in athletes. Int J Cardiol. 2007;114:114–7. doi: 10.1016/j.ijcard.2005.11.033. [DOI] [PubMed] [Google Scholar]
- 14.Dickerman RD, Schaller F, Prather I, McConathy WJ. Sudden cardiac death in a 20-year-old bodybuilder using anabolic steroids. Cardiology. 1995;86:172–3. doi: 10.1159/000176867. [DOI] [PubMed] [Google Scholar]
- 15.Ferenchick GS, Adelman S. Myocardial infarction associated with anabolic steroid use in a previously healthy 37-year-old weight lifter. Am Heart J. 1992;124:507–8. doi: 10.1016/0002-8703(92)90620-b. [DOI] [PubMed] [Google Scholar]
- 16.Fineschi V, Riezzo I, Centini F, Silingardi E, Licata M, Beduschi G, Karch SB. Sudden cardiac death during anabolic steroid abuse: morphologic and toxicologic findings in two fatal cases of bodybuilders. Int J Legal Med. 2007;121:48–53. doi: 10.1007/s00414-005-0055-9. [DOI] [PubMed] [Google Scholar]
- 17.Fisher M, Appleby M, Rittoo D, Cotter L. Myocardial infarction with extensive intracoronary thrombus induced by anabolic steroids. Br J Clin Pract. 1996;50:222–3. [PubMed] [Google Scholar]
- 18.Kennedy MC, Lawrence C. Anabolic steroid abuse and cardiac death. Med J Aust. 1993;158:346–8. doi: 10.5694/j.1326-5377.1993.tb121797.x. [DOI] [PubMed] [Google Scholar]
- 19.McNutt RA, Ferenchick GS, Kirlin PC, Hamlin NJ. Acute myocardial infarction in a 22-year-old world class weight lifter using anabolic steroids. Am J Cardiol. 1988;62:164. doi: 10.1016/0002-9149(88)91390-2. [DOI] [PubMed] [Google Scholar]
- 20.Kanayama G, Pope HG, Cohane G, Hudson JI. Risk factors for anabolic-androgenic steroid use among weightlifters: a case-control study. Drug Alcohol Depend. 2003;71:77–86. doi: 10.1016/s0376-8716(03)00069-3. [DOI] [PubMed] [Google Scholar]
- 21.Kanayama G, Hudson JI, Pope HG. Demographic and psychiatric features of men with anabolic-androgenic steroid dependence: a comparative study. Drug and Alcohol Dependence. 2009;102:130–137. doi: 10.1016/j.drugalcdep.2009.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jackson AS, Pollock ML. Generalized equations for predicting body density of men. Br J Nutr. 2004;91:161–8. [PubMed] [Google Scholar]
- 23.Kouri EM, Pope HG, Jr, Katz DL, Oliva P. Fat-free mass index in users and nonusers of anabolic-androgenic steroids. Clin J Sport Med. 1995;5:223–8. doi: 10.1097/00042752-199510000-00003. [DOI] [PubMed] [Google Scholar]
- 24.Pope HG, Jr, Katz DL. Psychiatric and medical effects of anabolic-androgenic steroid use. A controlled study of 160 athletes. Arch Gen Psychiatry. 1994;51:375–82. doi: 10.1001/archpsyc.1994.03950050035004. [DOI] [PubMed] [Google Scholar]
- 25.First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV Axis I Disorders -- Patient Edition. New York: Biometrics Research Department, New York State Psychiatric Institute; 2001. [Google Scholar]
- 26.Brennan BP, Kanayama G, Hudson JI, Pope HG. Illicit human growth hormone abuse in male weightlifters. Addict Behav. 2010 doi: 10.1111/j.1521-0391.2010.00093.x. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–63. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 28.Lester SJ, Tajik AJ, Nishimura RA, Oh JK, Khandheria BK, Seward JB. Unlocking the mysteries of diastolic function: deciphering the Rosetta Stone 10 years later. J Am Coll Cardiol. 2008;51:679–89. doi: 10.1016/j.jacc.2007.09.061. [DOI] [PubMed] [Google Scholar]
- 29.Yeh ET, Bickford CL. Cardiovascular complications of cancer therapy: incidence, pathogenesis, diagnosis, and management. J Am Coll Cardiol. 2009;53:2231–47. doi: 10.1016/j.jacc.2009.02.050. [DOI] [PubMed] [Google Scholar]
- 30.McKenna CJ, Codd MB, McCann HA, Sugrue DD. Alcohol consumption and idiopathic dilated cardiomyopathy: a case control study. Am Heart J. 1998;135:833–7. doi: 10.1016/s0002-8703(98)70042-0. [DOI] [PubMed] [Google Scholar]
- 31.Virmani R, Robinowitz M, Smialek JE, Smyth DF. Cardiovascular effects of cocaine: an autopsy study of 40 patients. Am Heart J. 1988;115:1068–76. doi: 10.1016/0002-8703(88)90078-6. [DOI] [PubMed] [Google Scholar]
- 32.Ren S, Tong W, Lai H, Osman NF, Pannu H, Lai S. Effect of long-term cocaine use on regional left ventricular function as determined by magnetic resonance imaging. Am J Cardiol. 2006;97:1085–8. doi: 10.1016/j.amjcard.2005.10.056. [DOI] [PubMed] [Google Scholar]
- 33.Felker GM, Thompson RE, Hare JM, Hruban RH, Clemetson DE, Howard DL, Baughman KL, Kasper EK. Underlying causes and long-term survival in patients with initially unexplained cardiomyopathy. N Engl J Med. 2000;342:1077–84. doi: 10.1056/NEJM200004133421502. [DOI] [PubMed] [Google Scholar]
- 34.Fauchier L, Babuty D, Poret P, Casset-Senon D, Autret ML, Cosnay P, Fauchier JP. Comparison of long-term outcome of alcoholic and idiopathic dilated cardiomyopathy. Eur Heart J. 2000;21:306–14. doi: 10.1053/euhj.1999.1761. [DOI] [PubMed] [Google Scholar]
- 35.Gavazzi A, De Maria R, Parolini M, Porcu M. Alcohol abuse and dilated cardiomyopathy in men. Am J Cardiol. 2000;85:1114–8. doi: 10.1016/s0002-9149(00)00706-2. [DOI] [PubMed] [Google Scholar]
- 36.Lange RA, Hillis LD. Cardiovascular complications of cocaine use. N Engl J Med. 2001;345:351–8. doi: 10.1056/NEJM200108023450507. [DOI] [PubMed] [Google Scholar]
- 37.Kupari M, Koskinen P, Suokas A. Left ventricular size, mass and function in relation to the duration and quantity of heavy drinking in alcoholics. Am J Cardiol. 1991;67:274–9. doi: 10.1016/0002-9149(91)90559-4. [DOI] [PubMed] [Google Scholar]
- 38.Lenders JW, Demacker PN, Vos JA, Jansen PL, Hoitsma AJ, van’t Laar A, Thien T. Deleterious effects of anabolic steroids on serum lipoproteins, blood pressure, and liver function in amateur body builders. Int J Sports Med. 1988;9:19–23. doi: 10.1055/s-2007-1024972. [DOI] [PubMed] [Google Scholar]
- 39.Cittadini A, Berggren A, Longobardi S, Ehrnborg C, Napoli R, Rosen T, Fazio S, Caidahl K, Bengtsson BA, Sacca L. Supraphysiological doses of GH induce rapid changes in cardiac morphology and function. J Clin Endocrinol Metab. 2002;87:1654–9. doi: 10.1210/jcem.87.4.8363. [DOI] [PubMed] [Google Scholar]
- 40.Sacca L, Napoli R, Cittadini A. Growth hormone, acromegaly, and heart failure: an intricate triangulation. Clin Endocrinol (Oxf) 2003;59:660–71. doi: 10.1046/j.1365-2265.2003.01780.x. [DOI] [PubMed] [Google Scholar]
- 41.Kannel WB, Belanger AJ. Epidemiology of heart failure. Am Heart J. 1991;121:951–7. doi: 10.1016/0002-8703(91)90225-7. [DOI] [PubMed] [Google Scholar]
- 42.Wang TJ, Evans JC, Benjamin EJ, Levy D, LeRoy EC, Vasan RS. Natural history of asymptomatic left ventricular dysfunction in the community. Circulation. 2003;108:977–82. doi: 10.1161/01.CIR.0000085166.44904.79. [DOI] [PubMed] [Google Scholar]