Our results showed that both use of gradual increase of power levels and restarting the generator after the equipment reached initial impedance level will increase ex vivo liver ablation.
Abstract
Purpose:
To compare an algorithm of gradually ramped-up power to a full-power-level technique to determine which technical parameters maximized tissue coagulation by using a saline-perfused electrode.
Materials and Methods:
Institutional review board approval was not necessary and animal committee approval was unnecessary because an ex vivo bovine liver model was used and the animals were not specifically killed for this study. This four-part experiment utilized multiple ablations of ex vivo bovine liver with a standard radiofrequency (RF) generator and an internally cooled needle. First, 10 RF ablations were performed at 20–60 W for 12 minutes. Second, ablation volumes obtained from an algorithm of eight ablations performed at 50 W were compared with those obtained from an algorithm of eight ablations that were gradually ramped-up to 50 W, until full impedance. Third, volumes obtained from 10 ablations performed at impedance control power levels were compared with those obtained from 10 ablations performed with a gradual ramp-up of power that started at 50 W, terminating at full impedance. Last, the third part was repeated, but with 11 ablations continuing past full impedance for 12 minutes each.
Results:
In the first part, maximum measurements of tissue coagulation seemed to plateau from 40 to 60 W. The second part produced significantly larger measurements of tissue coagulation than did the use of a constant power level of 50 W. The third and final parts produced larger measurements of tissue coagulation than did utilizing full power for 12 minutes. Larger measurements and volumes were obtained from repeat ablations after the generator reached impedance level than were obtained from ablations stopped at maximum impedance.
Conclusion:
A gradual ramp-up of power and repeating ablations after power impedance level is reached are the two methods that increased tissue ablation in this ex vivo experiment.
© RSNA, 2010
Supplemental material: http://radiology.rsna.org/lookup/suppl/doi:10.1148/radiol.09090662/-/DC1
Introduction
Radiofrequency (RF) ablation was first described as a possible method for percutaneous tissue ablation in ex vivo liver in 1990 (1). Since that time, there have been refinements of the technique, including increased RF generator capacity, different needle designs, and different coagulation algorithms, all with the goal of increasing the volume of tissue coagulation. One design included contained perfusion of the needle tip with cooled saline (2). By using this design, it was observed by Goldberg et al (3) that “perfusion of RF electrodes with chilled saline allows for increased power deposition without tissue charring,” and thus increased tissue coagulation. Different algorithms were used, including the use of pulsed energy at maximum power, and stopping treatment at 12 minutes (3). This experiment was carried over to clinical practice and comprised a pulsed algorithm that used impedance control for RF ablation of liver lesions by using a cooled-tip electrode and a power generator (Cool-Tip RF System; Covidien, Boulder, Colo): early experiments performed with an algorithm by using that equipment advocated a method of maximum current applied with a pulsed-energy delivery for 12 minutes or until generator reached full impedance as the optimum technique for tissue ablation (3–5). This has become the standard protocol for the cooled-tip needle and RF generator. For expandable, non–saline-perfused electrodes, there are treatment algorithms used as guidelines that start at lower wattages and increase (or ramp up) to midlevel power settings (6,7). However, this approach has not been used with cooled-tip needles. Thus, this ex vivo experiment was performed to compare an algorithm of gradual ramp-up of power with constant power versus pulsed energy techniques to determine what technical parameters can maximize tissue coagulation by using an internally cooled electrode. In clinical practice, we noted that during the full-power technique, a popping sound frequently preceded a foreshortened ablation. We also observed that for the ablations without the popping sound, we could achieve prolonged ablation times and larger ablations. It is possible that the rapid heating of tissues results in the production of gas that limits tissue coagulation. We hypothesized that an algorithm that used a gradual ramp-up of power will result in longer ablation times and larger volumes of tissue coagulation as compared with an algorithm that used full power alone.
Materials and Methods
No institutional review board approval was necessary, as no human subjects participated in this study. Furthermore, no animal committee approval was necessary, as we used an ex vivo bovine liver model and the animals were not specifically sacrificed for our study.
An RF generator (CC1; Covidien, Boulder, Colo) capable of producing 200 W was used with a 17-gauge needle (Covidien) with a 2-cm exposed-tip internally cooled electrode. A 2-cm exposed tip was chosen over a 3-cm exposed tip to decrease the volume of ablation, thus limiting the amount of liver needed for our experiment. Lesions were created in fresh room-temperature bovine livers obtained from the local butcher. The room-temperature livers were immersed in sterile normal saline and a 10-cm2 grounding pad was placed at least 30 cm away from the electrode. The electrodes were placed 3 cm in the livers. A mechanical pump was used to perfuse the electrode with 0°C saline at a rate of 110 mL/min prior to each ablation.
In the original experiments performed by other investigators with pulsed techniques, the operator manually decreased generator output to the electrode for 10–15 seconds and then increased power (5). The impedance control mode has been used in commercial equipment so that when the system detects a rise in impedance, the generator automatically cuts back power for 15 seconds before increasing from low power to high power (also called pulsed energy) (8). This is the impedance mode of the generator that was used for portions of parts 3 and 4 of the experiment. For the rest of the experiment, the manual control mode was utilized to monitor impedance to keep the power output at a constant level of 30, 40, or 50 W. Manual mode allows the operator to adjust the generator output to keep the wattage at a constant setting, such as 50 W, without using pulsed energy. This was used for the ramp-up of power experiments, such as from 20 to 30 W, and the use of constant wattage, such as 50 W, for parts 1 and 2 of the experiment. In our experiments, we define full impedance as the point when the generator automatically senses that the resistance is too high and power can no longer be deposited efficiently in the tissues. At this point, the generator turns itself off.
For all experiments, wattage rather than amperage was chosen as the variable for power setting since this was the parameter we used in our clinical practice. The initial tissue impedances were measured at the beginning of each ablation and the power output was carefully monitored throughout our experiments. After each ablation was performed, the ablated tissue was sectioned by cutting approximately 4.5 × 4 × 4-cm blocks of liver that fully encompassed the ablations. Six to eight ablations were performed in each liver, each of which weighed between 15 and 25 pounds (between 6.75 and 11.25 kg). Measurements of length and diameter of the ablated tissue were obtained after each ablation. Although this slowed down the experiment, it ensured that each ablation would not interfere with a previously ablated area. Each post-RF ablation coagulation specimen was cut along the long axis of the coagulation; thus, only the length and one diameter measurement were utilized (Fig 1). It was not possible to measure the other diameter. However, we did try to measure a separate section of tissue along the other axis in early experiments, and found that the diameters were usually symmetrical in this ex vivo experiment. Thus, the single diameter was used twice in volume calculations. The length and each diameter of the white central zone of necrosis were measured with a caliper and calibrated ruler to within 1 mm. The final tissue temperature was recorded by using the electrode at the completion of each ablation, after turning off the mechanical pump used to perfuse the electrode tip and allowing 1 minute for the temperature to stabilize. An approximation for a circular area of ablated tissue was calculated (π × radius2). The volume of an ellipsoid was also obtained (length radius × diameter radius × diameter radius × 4/3π). Each investigator performed a different experiment (J.M.B., F.J.B., S.L., W.L.M.); one investigator (J.M.B.) was present during all measurements to ensure consistency.
Figure 1:
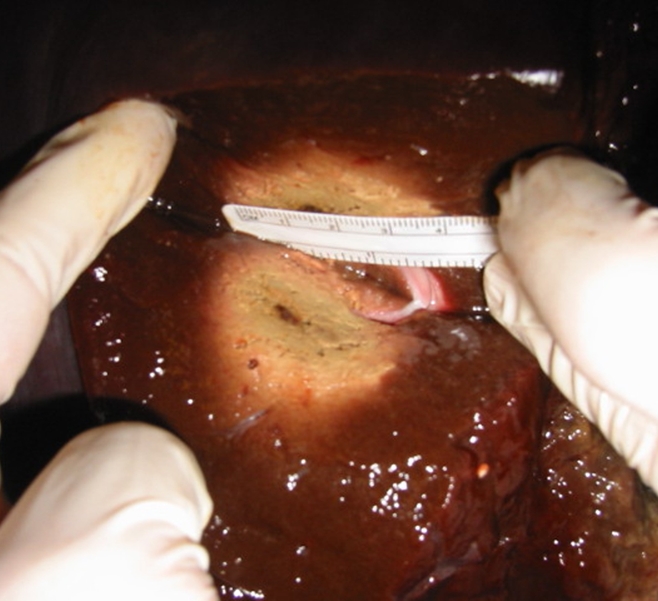
Photograph shows ex vivo liver following RF ablation with 2-cm perfused-tip electrode.
Experiment 1
Ten ablations were performed at continuous, manually controlled levels of 20, 30, 40, 50, and 60 W, for 12 minutes each. If the generator reached impedance and stopped before 12 minutes, it was restarted and continued for a total of 12 minutes. These parameters were chosen to best fit prior study designs performed with ex vivo liver to help determine a manually controlled wattage that resulted in greatest tissue coagulation (5).
Experiment 2
Eight ablations were performed at a continuous manually controlled level of 50 W, and were terminated at full impedance. Eight ablations were also performed with the ramped-up algorithm, starting at 5 W and increasing by 5 W each minute until 50 W was reached, and stopped at full impedance (5). These parameters were chosen to compare coagulation volumes obtained by using manually controlled fixed power settings with those obtained by using a gradually ramped-up algorithm.
Experiment 3
Ten ablations were performed by using a commercial pulsed algorithm set on impedance control at full power. These results were compared with those from ablations performed by using a ramped-up algorithm, in which the ablations were started at 50 W and were increased by 10 W each minute until full power was achieved. Higher-wattage settings were utilized to determine whether coagulation volume increased with higher settings, similar to the effect of using lower-wattage settings, as in experiment 2. All ablations were terminated when the generator reached maximum impedance, and thus the generator was not pulsed.
Experiment 4
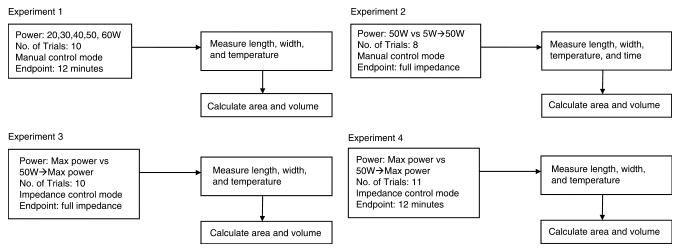
Eleven ablations were performed by using a commercial pulsed algorithm set on impedance control. These were compared with 11 ablations in which the power setting started at 50 W and was increased by 10 W each minute on impedance control. Experiment 4 was similar to experiment 3, with one significant difference: In experiment 3, ablations were terminated at full generator impedance, whereas in experiment 4, the generator was restarted and pulsed after reaching full impedance and ablations continued for 12 minutes. This continuation of the experiment was performed by pulsing the power after reaching full impedance. The generator automatically restarted for each part of the experiment, after the generator reached impedance, which was performed to determine whether larger coagulation volumes could be obtained. A flow diagram outlining the logistics of all experiments is shown in Figure 2.
Figure 2:
Flow diagram of logistics for experiments 1–4. Manual control mode allows operator to adjust generator output to keep wattage at constant setting without pulsed energy. Impedance control mode is when generator will automatically cut back on power for 15 seconds when system detects rise in impedance before delivering pulsed energy. Full impedance is when the generator automatically senses that resistance is too high and power can no longer be deposited efficiently in tissues; generator will turn itself off.
Statistical Analysis
The coagulation lengths, diameters, areas, volumes, and final temperatures of the maximum power ablations were compared by using Kruskal-Wallis tests in part 1 of the experiment and by using two-sided Wilcoxon rank-sum tests in parts 2–4. Ablation times were also compared by using two-sided Wilcoxon rank-sum tests in parts 2 and 3 of this experiment. A P value of less than .05 was considered to indicate a significant difference. Post hoc pairwise analysis in part 1 was performed by using two-sided Wilcoxon rank-sum tests with a Bonferroni correction, where a P value of .0125 (.05/4) was considered to indicate a significant difference. All analyses were performed with software (SAS, version 9.2; SAS Institute, Cary, NC).
Results
During the experiment, the initial tissue impedances ranged between 125 and 150 ohms.
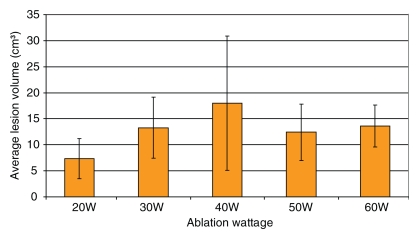
In experiment 1, we compared the results of each 12-minute ablation at 20, 30, 40, 50, and 60 W. The greatest mean coagulation length of 3.3 cm was achieved at both 40 and 60 W (Table E1 [online]). The greatest mean coagulation width and volume were achieved at 40 W and were 3.0 cm ± 0.84 (standard deviation) and 18.0 cm3 ± 13, respectively (Table E1 [online], Fig 3). By using a Kruskal-Wallis test, a significant difference was noted between each group in terms of length (P = .026), width (P = .0256), area (P = .0256), volume (P = .0155), and temperature (P < .0019). Post hoc pairwise analysis performed by using two-sided Wilcoxon rank-sum tests with a Bonferroni correction showed that there was a significant difference in temperature (P = .000195) between the 20 W (56°C ± 8) and 30 W (71°C ± 6) groups. Significant differences were seen in terms of width (P = .01), area (P = .01), volume (P = .0082), and temperature (P = .0037) between the mean values of the 20 W (width, 2.2 cm ± 0.45; area, 3.9 cm2 ± 1.7; volume, 7.3 cm3 ± 3.9; and temperature, 56°C ± 8) and 40 W (width, 3.0 cm ± 0.84; area, 7.7 cm2 ± 4.3; volume, 18 cm3 ± 13; and temperature, 69°C ± 9) groups. There was a significant difference in length (P = .0079) between the 20 W (2.7 cm ± 0.36) and 50 W (3.2 cm ± 0.44) groups. Significant differences were seen in terms of length (P = .0025), width (P = .0093), area (P = .0093), volume (P = .0015), and temperature (P = .000931) between the mean values of the 20 W (length, 2.7 cm ± 0.36; width, 2.2 cm ± 0.45; area, 3.9 cm2 ± 1.7; volume, 7.3 cm3 ± 3.9; and temperature, 56°C ± 8) and 60 W (length, 3.3 cm ± 0.42; width, 2.8 cm ± 0.34; area, 6.0 cm2 ± 1.4; volume, 13.6 cm3 ± 4.0; and temperature, 68°C ± 5) groups (Tables 1–5).
Figure 3:
Graph shows experiment 1 results. A 2-cm perfused-tip electrode was used for 12-minute ablation in ex vivo bovine liver. Each ablation was performed 10 times.
Table 1.
Summary of Post Hoc Analysis of Length for Experiment 1
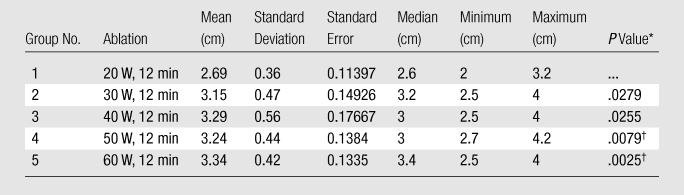
Note.—For Kruskal-Wallis comparison among all groups, P = .026 (significant). Bonferroni correction was .05/4, P = .0125.
Compared with Group 1 by using the Wilcoxon rank-sum test.
Indicates significance.
Table 5.
Summary of Post hoc Analysis of Temperature for Experiment 1
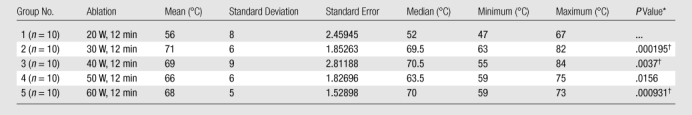
Note.—For Kruskal-Wallis comparison among all groups, P = .0019 (significant). Bonferroni correction was .05/4, P = .0125.
Compared with Group 1 by using the Wilcoxon rank-sum test.
Indicates significance.
Table 2.
Summary of Post hoc Analysis of Width for Experiment 1
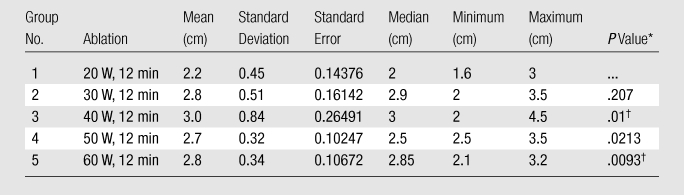
Note.—For Kruskal-Wallis comparison among all groups, P = .0256 (significant). Bonferroni correction was .05/4, P = .0125.
Compared with Group 1 by using the Wilcoxon rank-sum test.
Indicates significance.
Table 3.
Summary of Post hoc Analysis of Area for Experiment 1
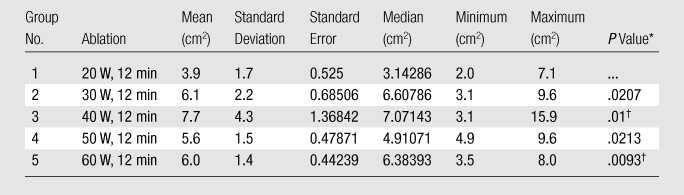
Note.—For Kruskal-Wallis comparison among all groups, P = .0256 (significant). Bonferroni correction was .05/4, P = .0125.
Compared with Group 1 by using the Wilcoxon rank-sum test.
Indicates significance.
Table 4.
Summary of Post hoc Analysis of Volume for Experiment 1
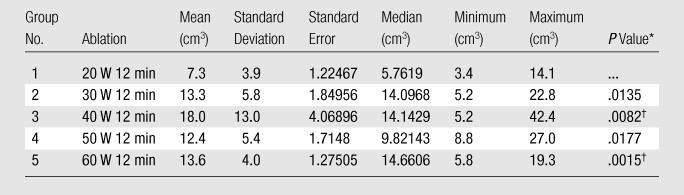
Note.—For Kruskal-Wallis comparison among all groups, P = .0155 (significant). Bonferroni correction was .05/4, P = .0125.
Compared with Group 1 by using the Wilcoxon rank-sum test.
Indicates significance.
In experiment 2 (Table E2 [online]), we found that there were significant differences in the widths (P = .023), areas (P = .023), and durations (P = .0022) of the coagulations between the mean values of the continuous-level 50-W ablations (width, 2.1 cm ± 0.27; area, 3.6 cm2 ± 0.92; and time, 4.5 min ± 1.5) and the ramped-up algorithm (width. 2.7 cm ± 0.24; area, 5.7 cm2 ± 1.0; and time, 11 min ± 1.3). There was no significance between the two groups in terms of lesion length (P = .0782). No significant difference (P = .379) was noted between the final tissue temperatures of the constant-power ablations (65°C ± 6) and the ramped-up algorithm (68°C ± 4). There were significant differences in mean volumes in the ramped-up group (volume, 13.0 cm3 ± 3.8) (Table E2 [online]) as compared with the constant-power 50-W group (volume, 6.8 cm3 ± 2.5; P = .000932).
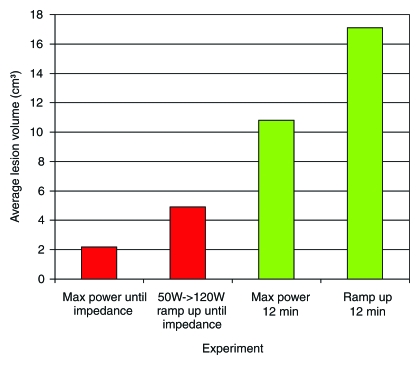
In experiment 3, we compared maximum power ablations with a 50-W ramped-up algorithm. Ablations were terminated once full impedance was reached and the generator was not restarted (Table E3 [online]). By using full power on impedance control, mean values were lesion length, 2.1 cm ± 0.39; width, 1.3 cm ± 0.41; and final temperature, 59°C ± 5 (Table E3 [online]). For the 50-W ramped-up algorithm, mean values were lesion length, 2.7 cm ± 0.48; width, 1.8 cm ± 0.35; and final temperature, 60°C ± 3 (Table E3 [online]). We found significant differences in length (P = .0112), width (P = .0105), and time (P < .0001). No significant difference was achieved for final temperature (P = .8959). For the maximum-power ablations, the mean area and volume of ablated tissue were 1.50 cm2 ± 0.95 and 2.2 cm3 ± 1.8, respectively. For the ramped-up algorithm, the mean area and volume of ablated tissue were 2.6 cm2 ± 1.1 and 4.9 cm3 ± 2.7, respectively (Fig 4). There were significant differences between the two groups in terms of area (P = .0105) and volume (P = .0055).
Figure 4:
Graph shows average ablation volumes for experiments 3 and 4, which were performed in the same fashion, except that in experiment 3 ablation was stopped after generator reached impedance. In experiment 4, generator was restarted and continued for 12 minutes.
In experiment 4, we compared the pulsed algorithm set on impedance control with a ramped-up algorithm set on impedance control starting at 50 W. In this experiment, once the generator reached impedance, it was automatically restarted in both portions of the experiment and continued for 12 minutes. The maximum power ablations resulted in mean values of lesion length, 3.1 cm ± 0.38; width, 2.5 cm ± 0.44; and final temperature, 68°C ± 4 (Table E4 [online]). In comparison, the ramped-up algorithm resulted in mean lesion length, 3.7 cm ± 0.25, width, 2.9 cm ± 0.49, and final temperature, 69°C ± 6 (Table E4 [online]). There were significant differences between the lesion lengths (P = .000352) and widths (P = .0354), but no difference was seen with regard to the final temperatures (P = .5071). Respective mean areas and volumes were 5.1 cm2 ± 1.9 and 10.8 cm3 ± 5.5 for full power, compared with 6.9 cm2 ± 2.3 and 17.1 cm3 ± 6.7 in the ramp-up treatment, with significant differences between the groups (P = .0354 and P = .0055) (Fig 4).
Discussion
Percutaneous RF ablation is a technique that was described in the literature almost 2 decades ago (1). Since that time, a number of different needles, generators, algorithms, and power settings have been utilized to treat various pathologies (9–14). This has included use of a saline-perfused electrode, while others have used multiple tines and simultaneous infusion of saline in the tissue to increase coagulation (15,16). Initial experiments with a cooled-tip saline-perfused needle demonstrated increased coagulation volumes compared with a nonperfused electrode (3,4). Further experiments demonstrated further increase of tissue ablation volumes with internally cooled needles was obtained by using pulsed techniques (5). There has been little comparative data to validate these results or to study the effects of altering other RF ablation parameters on tissue coagulation.
Part 1 of our experiment demonstrated that there was a gradual increase in lesion length, diameter, and volume until a plateau was reached between 40 and 60 W. This is in concordance with prior research demonstrating a plateau of lesion size at approximately 50 W (5). Thus, we utilized 50 W for part 2 of the experiment. Results of other studies have noted a limitation to tissue coagulation with increasing power settings in ex vivo tissue. For instance, Goldberg et al (3) noted, in ex vivo liver with no pulsed energy delivery, that “at 75 watts, these signs of tissue boiling were observed in 50% of the trials lasting longer than 9 minutes.” They also noted that “increasing the power above 50 watts did not result in larger lesions in normal liver tissue despite the fact that charring was not observed until the deposition of 67 watts of power” (3). Thus, in ex vivo liver, there is a limit to the volume of tissue coagulation achievable by means of excess power delivery. Our post hoc tests showed that the significance shown in experiment 1 is a result of differences between the 20 W group and the other groups, which suggests that there may not be a significant difference in terms of ablations at 30, 40, 50, and 60 W in ex vivo liver. Further experiments should be performed with an in vivo model to see if this really is the case. Factors such as the heat sink effect will also further decrease the power deposited in living tissue.
Experiment 2 involved comparing the constant 50 W algorithm by using an internally cooled electrode and pulsed energy with the same parameters, but with gradual increase in power up to 50 W. Our experiment demonstrated that more gradual increases of power at the lower setting yield a significantly increased diameter, length, and volume of coagulated liver as compared with the conventional algorithm of maximum RF current. However, doubling the volume of tissue coagulation for the two groups from 6.8 to 13.0 cm3 came at the expense of increasing time of ablation from 5 to 11 minutes. Similar results for volume increase versus time were seen when comparing the conventional impedance control of the pulsed energy group with the gradual ramped-up group, when starting above 50 W (as in experiment 3), and stopping once full impedance was reached. Finally, in experiment 4, where ablation continued for 12 minutes, there was an increased volume of tissue coagulation in the ramped-up group, as compared with the full-power group in impedance mode. The reason for this increased coagulation in the ramped-up group is not fully known, but could be accounted for because of several factors. As first noted by McGahan et al (1) and then redefined by Lorentzen (2), current flow that is too rapid may cause tissue desiccation and loss of free sodium and chloride in cells, which stops current flow and limits tissue coagulation. Lorentzen (2) limited this effect by designing an internally cooled needle. This decreased local tissue desiccation and limited the char around the needle, resulting in increased volume of tissue coagulation. While it seems contradictory that cooling the needle should increase tissue coagulation, this effect has been validated in clinical practice and in ex vivo and in vivo research (2,3).
Similarly, varying wattage algorithms may produce larger volumes of tissue coagulation than do consistent- or full-power techniques. For instance, Goldberg et al (5) showed that pulsed current actually delivered less power per minute than consistent full power, but created a larger volume of tissue ablation. This may seem contradictory, but since then, others studies have shown that different pulsing techniques may yield larger volumes of tissue coagulation. Pulsing energy, as noted by the manufacturer, “by operating at intervals of low output and high output, the local tissue is allowed to partially cool.” Consequently, the tissue is able to accept greater energy (17). Furthermore, Solazzo et al (18) have shown that maximum coagulation was achieved by using a 3-cm single electrode at a high-pulse power setting: on for 10–18 seconds and off for 11–20 seconds.
We found in experiment 3 that a different algorithm that used a gradual increase of energy created a larger volume of tissue necrosis than did an algorithm of maximum energy until maximum impedance was reached. Again, it seems a paradox that less-rapid heating, or a gradual ramp-up of power noted in our ex vivo experiment, would increase the volume of coagulation when compared with constant application of pulsed energy. Furthermore, experiment 4 showed that if the energy application was continued after the generator reached impedance, larger volumes of tissue ablation were obtained by restarting the generator (pulsing). The largest volume of tissue ablation was obtained in experiment 4 with a gradual increase of power until full impedance and then restarting (pulsing) the energy application. However, for an expandable nonsaline–perfused electrode, the treatment algorithms started at lower wattages and ramped up to midlevel power settings. For instance, by using a 3.0-cm expandable electrode (Leveen), treatment is started at 40 W and increased by 10 W every 30 seconds to 80 W (7,18). What is the explanation of this phenomenon at work? Perhaps the gradual increase in power leads to less-rapid tissue desiccation. We showed that gradually increasing the power allowed for longer periods of current flow and ultimately increased the volume of liver coagulation. This phenomenon may be likened to a slow cooking of tissue, in which the internal tissue is evenly heated but there is no surface charring. In contrast, rapidly heating the tissue would produce an external charring, but without even cooking occurring internally. This crude analogy is the basis for the principle of the cooled-tip needle, which prevents local charring adjacent to the needle and thus increases the volume of internal tissue coagulation. Thus, a slower cooking may limit tissue desiccation and thereby allow for continued current flow and increased ablation. Certainly, rapid deposition of power will more rapidly increase tissue coagulation but final volume of tissue coagulation may be limited when compared with the coagulation obtained from different algorithms. Our experiment showed that with more gradual ramp-up of power, there was increased tissue coagulation.
Part 4 of our experiment demonstrated that tissue ablation occurred after the generator reached impedance if the generator was automatically reset (pulsed). Therefore, why stop when the system reaches impedance? While reviewing some of the original work on this topic, we looked at how to maximize ablation in a time-efficient fashion. Goldberg et al (3) noted in their experiment that the lesion coagulation diameter increased rapidly in the first few minutes, gradually leveling off with time. Other work with ex vivo and in vivo tissue showed increased tissue coagulation for all experiments with increasing time, except for one experiment comparing in vivo liver for 12 versus 20 minutes (5). For instance, the coagulation diameter average for an in vivo muscle tissue group was approximately 7.5 cm at 12 minutes compared with 9.5 cm at 30 minutes by using a 2.5-cm clustered electrode, which was also true for ex vivo liver (5). While a 2-cm increase in coagulation diameter seems trivial, the coagulation volume increase is quite dramatic, since volume calculation is a cubic function. Thus, even if coagulation length shows minimal increase, increasing the coagulation diameter from 7.5 to 9.5 cm will create a two-fold increase in coagulation volume. Again, the manufacturer of the expandable electrode recommends continuous gradual increase of power until rolloff, and then starting at 70% of maximum power until the system rolls off one more time, for two treatment cycles (6,7). Results from other work with ex vivo liver performed by Solazzo et al (18) demonstrated that, in 12 to 20 minutes, coagulation diameter changed from 4.8 to 5.3 cm by using a 3-cm electrode with their algorithm and maximum milliamperage. By using a 2.5-cm saline-perfused cluster electrode, their coagulation diameter changed from 6.5 cm to 7.7 cm in 12 to 20 minutes. We found during the ex vivo experiment that volume increases by 92% ([13–6.8]/6.8) as time increased from 5 minutes to 11 minutes when comparing the full-power technique with the ramp-up technique in part 2 of our experiment.
In these experiments (3,5,19), does a 1-cm increase in coagulation diameter have any direct clinical application? Consider results from work by Dodd et al (19), who used an electrode that creates a 3-cm diameter coagulation to perform a satisfactory ablation of a 3.75-cm tumor. It would require five perfectly placed needles with six separate ablations to obtain satisfactory tumor coagulation (15). However, if a 0.5-cm surgical margin was used, then six perfectly overlapping ablations and a 3-cm diameter ablation would be needed to treat a tumor 2.75 cm in diameter. Furthermore, if a 1-cm surgical margin was needed, then only a 1.75-cm diameter tumor could be treated. Thus, six ablations of 12 minutes each, or 72 minutes total, would be required to adequately treat such a lesion. Alternatively, if a single ablation diameter increased from 3 cm to 4 cm by increasing time of ablation from 12 to 20 minutes, then the 2.75-cm tumor could be treated in less time (20 minutes) as compared with 72 minutes for six ablations at 12 minutes each (15,19), with a 0.625-cm safety margin around the tumor. Furthermore, when considering RF ablation, tissue coagulation itself may be a small factor in the total patient treatment time. Procedure time includes patient prep, transport, anesthesia, tumor localization, and, most importantly, needle positioning.
Limitations of this study included the fact that this is all theoretic, since coagulation zones are not perfect spheres, but are oblong (15). Additionally, all RF applications are affected by surrounding structures, such as vessels, which alter the geometry of the coagulation zone. Local effects (such as the heat sink) may cause coagulation asymmetry and not all tissue has the same rate of coagulation for a given algorithm—such cofactors did not exist in our model. Furthermore, experiment was performed in ex vivo tissue, while in clinical practice, the effects are likely to be different (ie, volume of necrosis may be quite less for clinical tissues when compared with ex vivo results). It is entirely possible that our results can only be achieved in ex vivo models, and thus, further in vivo experiment is needed to see if our findings are clinically applicable. Another potential limitation was that, in vivo, there may be longer times for equipment to reach impedance owing to local heat sink effects. Thus, in practice, maximum tissue ablation may require prolonged treatment cycles, including restarting the equipment for ablations that last longer than 12 minutes, or following maximum impedance. An additional potential weakness of our study was that measurement errors in the diameter were compounded when used to estimate volume. We may have obtained more accurate volume results by measuring all dimensions of the ablated tissue. To limit the measurement errors, we calculated ablation area and volumes, both of which showed significance in each experiment. Finally, we tested only a single exposed-tip needle design of 2 cm. However, these results will likely be of a similar trend to experiments performed with a single internally cooled needle with a 3-cm exposed tip. Additional experiments performed with a cluster electrode should also be performed by using a ramped-up algorithm to see if they also result in larger ablations.
In conclusion, in ex vivo liver tissue, the gradual ramp-up of power produces larger volumes of liver coagulation, compared with an impedance-controlled full-power technique. Additionally, restarting ablations after the equipment reaches impedance produces larger ablations when compared with stopping ablations after the equipment initially reaches impedance. By using both gradual increases of power and restarting the generator after the experiment initially reaches imedance, ex vivo liver ablation will increase.
Practical applications: The use of the ramped-up algorithm may result in an increased volume of tissue ablation with decreased procedural time in clinical practice.
Advances in Knowledge.
Gradual ramp-up of power produces larger volumes of liver coagulation compared with techniques that utilize full power.
The theory that restarting the ablation after the equipment initially reaches impedance level will produce larger volumes of liver coagulation when compared with stopping the ablation after the equipment initially reaches impedance level is validated.
Largest volume of liver coagulation is obtained by using a gradual ramp-up of power levels with a pulsed algorithm.
Implication for Patient Care.
A gradual ramp-up of power levels and prolonging ablation after the equipment reaches impedance may result in fewer electrode placements, decreased ablation time, and increased volume of tissue ablation in clinical practice.
Supplementary Material
Received April 23, 2009; revision requested June 5; revision received June 29; accepted August 10; final version accepted August 27.
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official view of the National Center for Research Resources or National Institutes of Health. Information on Re-engineering the Clinical Research Enterprise can be obtained from http://nihroadmap.nih.gov/clinicalresearch/overview-translational.asp.
Funding: This research was supported by National Center for Research Resources, National Institutes of Health (grant UL1 RR024146) and NIH Roadmap for Medical Research.
Abbreviation:
- RF
- radiofrequency
References
- 1.McGahan JP, Browning PD, Brock JM, Tesluk H. Hepatic ablation using radiofrequency electrocautery. Invest Radiol 1990;25:267–270 [DOI] [PubMed] [Google Scholar]
- 2.Lorentzen T. Modified needle technique for large lesion ablation by using monopolar RF electrosurgery under US control: an in vivo study [abstr?]. Radiology 1995;197(P):200 [Google Scholar]
- 3.Goldberg SN, Gazelle GS, Solbiati L, Rittman WJ, Mueller PR. Radiofrequency tissue ablation: increased lesion diameter with a perfused electrode. Acad Radiol 1996;3:636–644 [DOI] [PubMed] [Google Scholar]
- 4.Goldberg SN, Hahn PF, Tanabe KK, et al. Percutaneous radiofrequency tissue ablation: does perfusion-mediated tissue cooling limit coagulation necrosis? J Vasc Interv Radiol 1998;9(1 pt 1):101–111 [DOI] [PubMed] [Google Scholar]
- 5.Goldberg SN, Solbiati L, Hahn PF, et al. Large-volume tissue ablation with radio frequency by using a clustered, internally cooled electrode technique: laboratory and clinical experience in liver metastases. Radiology 1998;209:371–379 [DOI] [PubMed] [Google Scholar]
- 6.Lin SM, Lin CJ, Chung HJ, Hsu CW, Peng CY. Power rolloff during interactive radiofrequency ablation can enhance necrosis when treating hepatocellular carcinoma. AJR Am J Roentgenol 2003;180:151–157 [DOI] [PubMed] [Google Scholar]
- 7.Boston Scientific Corporation The Leveen needle electrode users manual Natick, Mass: Boston Scientific Corporation, 2007;12 [Google Scholar]
- 8.Radionics, a division of Tyco Healthcare Group LP Cool-tip RF system operator’s manual. 921-60-004 Rev D Burlington, Mass: Radionics, 2000; 2–7 [Google Scholar]
- 9.Solbiati L, Ierace T, Goldberg SN, et al. Percutaneous US-guided radiofrequency tissue ablation of liver metastases: treatment and follow-up in 16 patients. Radiology 1997;202:195–203 [DOI] [PubMed] [Google Scholar]
- 10.Hall WH, McGahan JP, Link DP, deVere White RW. Combined embolization and percutaneous radiofrequency ablation of a solid renal tumor. AJR Am J Roentgenol 2000;174:1592–1594 [DOI] [PubMed] [Google Scholar]
- 11.Rosenthal DI, Alexander A, Rosenberg AE, Springfield D. Ablation of osteoid osteomas with a percutaneously placed electrode: a new procedure. Radiology 1992;183:29–33 [DOI] [PubMed] [Google Scholar]
- 12.Khatri VP, McGahan JP, Ramsamooj R, et al. A phase II trial of image-guided radiofrequency ablation of small invasive breast carcinomas: use of a saline-cooled tip electrode. Ann Surg Oncol 2007;14:1644–1652 [DOI] [PubMed] [Google Scholar]
- 13.Gervais DA, Arellano RS, McGovern FJ, McDougal WS, Mueller PR. Radiofrequency ablation of renal cell carcinoma: part 2, lessons learned with ablation of 100 tumors. AJR Am J Roentgenol 2005;185:72–80 [DOI] [PubMed] [Google Scholar]
- 14.Livraghi T, Meloni F, Di Stasi M, et al. Sustained complete response and complication rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: is resection still the treatment of choice? Hepatology 2008;47:82–89 [DOI] [PubMed] [Google Scholar]
- 15.McGahan JP, Dodd GD., 3rd Radiofrequency ablation of the liver: current status. AJR Am J Roentgenol 2001;176:3–16 [DOI] [PubMed] [Google Scholar]
- 16.Rossi S, Buscarini E, Garbagnati F, et al. Percutaneous treatment of small hepatic tumors by an expandable RF needle electrode. AJR Am J Roentgenol 1998;170:1015–1022 [DOI] [PubMed] [Google Scholar]
- 17.Shimizu A, Ishizaka H, Awata S, et al. Expansion of radiofrequency ablation volume by saturated NaCl saline injection in the area of vaporization. Acta Radiol 2009;50:61–64 [DOI] [PubMed] [Google Scholar]
- 18.Solazzo SA, Ahmed M, Liu Z, Hines-Peralta AU, Goldberg SN. High-power generator for radiofrequency ablation: larger electrodes and pulsing algorithms in bovine ex vivo and porcine in vivo settings. Radiology 2007;242:743–750 [DOI] [PubMed] [Google Scholar]
- 19.Dodd GD, 3rd, Frank MS, Aribandi M, Chopra S, Chintapalli KN. Radiofrequency thermal ablation: computer analysis of the size of the thermal injury created by overlapping ablations. AJR Am J Roentgenol 2001;177:777–782 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.