Abstract
Background
The clinical benefit of cardiac resynchronization therapy (CRT) for patients with moderate-to-severely symptomatic heart failure, left ventricular systolic dysfunction, and ventricular conduction delay is established. However, some patients do not demonstrate clinical improvement following CRT. It is unclear whether systematic optimization of the programmed atrioventricular (AV) delay improves the rate of clinical response.
Methods
SMART-AV is a randomized, multicenter, double-blinded, three-armed trial that will investigate the effects of optimizing AV delay timing in heart failure patients receiving CRT + defibrillator (CRT-D) therapy. A minimum of 950 patients will be randomized in a 1:1:1 ratio using randomly permuted blocks within each center programmed to either DDD or DDDR with a lower rate of 60. The study will include echocardiographic measurements of volumes and function [e.g., left ventricular end-systolic volume (LVESV)], biochemical measurements of plasma biomarker profiles, and functional measurements (e.g., 6-minute hall walk) in CRT-D patients who are enrolled and randomized to fixed AV delay (i.e., 120 ms), AV delay determined by electrogram-based SmartDelay, or an AV delay determined by echocardiography (i.e., mitral inflow). Patients will be evaluated prior to initiation of CRT, 3 and 6 months post-implant. The primary endpoint is the relative change in LVESV at 6 months between the groups. Patient enrollment commenced in May 2008 and the study is registered at clinicaltrials.gov.
Conclusion
SMART-AV is a randomized, clinical trial designed to evaluate three different methods of AV delay optimization to determine whether systematic AV optimization is beneficial for patients receiving CRT for 6 months post-implant.
Keywords: AV delay, optimization, cardiac resynchronization therapy, biomarkers
Background
Cardiac resynchronization therapy (CRT) is indicated for patients with severe left ventricular (LV) systolic dysfunction, medically-refractory heart failure (HF) symptoms and intraventricular conduction delay.1,2 In HF patients, CRT whether with (COMPANION)3 or without (CARE-HF)4 an implantable cardiac defibrillator (ICD) reduces HF hospitalizations and prolongs survival compared with optimal medical therapy alone. Moreover, CRT improves symptoms and quality of life (QoL), increases exercise tolerance, and reduces LV dilatation.5–12 Recent studies also suggest that CRT results in decreased neurohormonal and proinflammatory biomarkers13–18 and the positive impact of markers on matrix remodeling may be associated with LV “reverse remodeling” that occurs with CRT.18,19
Achieving the optimal outcome from CRT may be dependent on proper programming of the appropriate atrioventricular (AV) delay.20,21 In fact, suboptimal AV delay programming may result in as much as a 10–15% decline in cardiac output.22,23 However, the large-scale randomized clinical trials establishing the overall efficacy of CRT have differed widely in their approach to AV optimization. The CARE-HF and MIRACLE investigators used Doppler echocardiography of transmitral flow to select the optimal AV delay,4,5,20,21,24 an approach endorsed by the American Society of Echocardiography.25 On the other hand, the COMPANION investigators used an algorithm based on the intrinsic AV interval and baseline QRS width to determine a predicted optimum programmed sensed AV delay3 and a modified version of this algorithm is available in currently available Boston Scientific CRT devices known as SmartDelay (SD; Boston Scientific, Natick, MA, USA). SD provides both paced and sensed recommendations by accounting for three inputs: intrinsic AV intervals, interventricular timing, and LV lead location. In contrast, in the CONTAK CD trial, there was no AV optimization whatsoever.10 The SD determined AV optimization; a comparison to other AV delay methods used in cardiac resynchronization therapy (SMART-AV) study was designed to compare these three alternative techniques and to assess the hypotheses that: (1) systematic AV delay optimization using echocardiography and/or the SD algorithm is superior to a fixed nominal AV delay as demonstrated by improved LV geometry after 6 months and (2) programming according to SD is noninferior to using echocardiography-determined AV delay optimization.
Methods
Study Population, and Inclusion and Exclusion Criteria
A minimum of 950 implanted and randomized patients enrolled at approximately 95 US and international centers will be enrolled and followed for 6 months post-randomization. Patients must meet current indications for CRT-D implantation [New York Heart Association (NYHA) class III or IV, left ventricular ejection fraction (LVEF) ≤35%, QRS duration ≥120 ms] and must be able to undergo a device implant and participate in all testing associated with the SMART-AV Study. Patients must be on optimal and stable pharmacologic therapy according to HF guidelines, including diuretics, β-blockers, and angiotensin converting enzyme (ACE) inhibitors or angiotensin receptor blockers for at least 30 days prior to enrollment in the study.26,27 Dosage should not decrease by 50% or increase by 100% in the 30 days prior to enrollment with the exception for a discontinued dose for up to 72 hours. Medications will remain unchanged during the study unless a change is deemed clinically necessary.
Patients who are in complete heart block or who otherwise are unable to tolerate pacing at VVI-40-RV for up to 14 days (e.g., persistent 2:1 AV block) are excluded, as are patients who have previously received CRT. Patients undergoing an upgrade of a pacemaker or ICD who are unable to tolerate pacing at VVI-40-RV for up to 14 days are also excluded. Inclusion and exclusion criteria are shown in Table I.
Table I.
Inclusion and Exclusion Criteria
| Inclusion Criteria |
| Patients who meet current indications for a BSC CRT-D device with the SmartDelay algorithm |
| Willing and capable of undergoing a device implant and participating in all testing |
| Stable optimal pharmacologic therapy including diuretics, β-blockers, and ACE inhibition |
| Expected to be in sinus rhythm at the time of implant |
| Life expectancy of more than 360 days, per physician’s discretion |
| Geographically stable and willing to comply with the required follow-up schedule |
| Age 18 or above, or of legal age to give informed consent specific to state and national law |
| Exclusion Criteria |
| Complete heart block, or who otherwise are unable to tolerate pacing at VVI-40-RV for up to 14 days (e.g., persistent 2:1 AV block) |
| Previously received cardiac resynchronization therapy |
| Undergoing an upgrade of a pacemaker or implantable cardioverter defibrillator who are unable to tolerate pacing at VVI-40-RV for up to 14 days (e.g., heart block) |
| Expected to receive a heart transplant during the course of the study |
| Cardiac surgeries or procedures planned to be performed during the course of the study |
| Currently have or who are likely to receive a tricuspid valve prosthesis (mechanical right valve) |
| Neuromuscular, orthopedic, or other noncardiac condition that prevents normal, unsupported walking (i.e., canes, crutches, or walkers) |
| Pregnant or planning to become pregnant during the study (method of assessment upon physician’s discretion) |
| Currently enrolled in another investigational study or registry that would directly impact the treatment or outcome of the current study. |
BSC = Boston Scientific.
Study Design
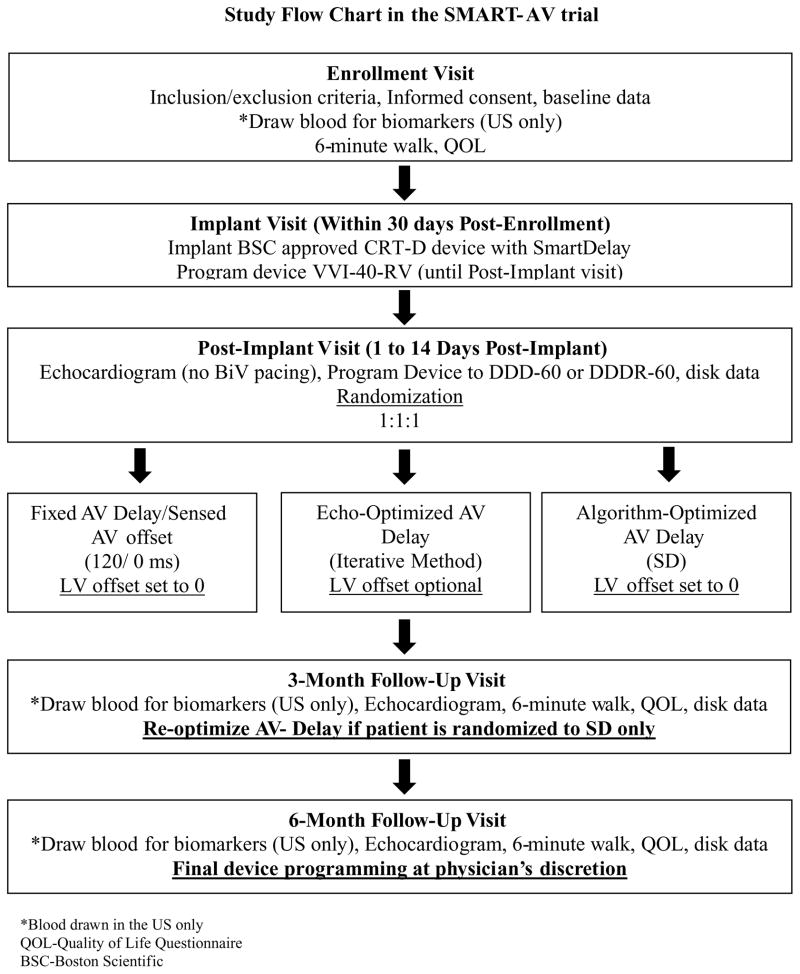
Patients will be implanted with a commercially available Boston Scientific CRT defibrillator (CRT-D) device with the SD algorithm (i.e., LIVIAN®, COGNIS®). Upon approval, future generations of Boston Scientific CRT-D device families with the SD algorithm may be included in the study. Any compatible right atrial (RA), right ventricular (RV) defibrillation and LV leads may be used (lead systems may include other manufacturers’ leads). Patients will be programmed to VVI-40-RV at implant. At 1–14 days following device implantation, patients will undergo baseline echocardiographic imaging and will then be randomized in a 1:1:1 ratio using randomly permuted blocks within each center. Following the baseline echocardiogram, patients will be programmed at the physician’s discretion to either DDD or DDDR with a lower rate of 60. Required device programming is shown in Table II with all other programming determined by the physician. This study will compare chronic changes in structural, biochemical, and functional outcomes in patients randomized to have their AV delay set at 120 ms, set using the SD algorithm, or set as determined by echocardiography (Fig. 1). Following randomization, patients will undergo echocardiographic imaging after 3 and 6 months. Patients allocated to the SD arm will undergo re-optimization using the SD algorithm after 3 months. Additional data will be collected as outlined in Table III. Patients will be blinded as to their treatment assignment and all patients will undergo the same measurement protocols at each visit, thus mitigating any difference in placebo effect between the three arms. Moreover, in order to minimize biasing study results, physicians are strongly encouraged not to change the protocol-specified programming requirements. If a programming crossover is medically necessary, the sites must obtain approval from the SMART-AV study National Primary investigator or an electrophysiologist member of the steering committee. In the case of a medical emergency where programming changes or programming crossovers occur, a member of the SMART-AV study team must be informed as soon as possible after the occurrence.
Table II.
Required Device Programming
| Parameter | Required Setting |
|---|---|
| * Pacing mode | DDD or DDDR |
| # LRL (lower rate limit) | 60 |
| ATR (atrial tachy response) | ON at 170 beats/min |
| Rate hysteresis | OFF |
| Dynamic atrioventricular (AV) delay | OFF |
| † AV delay | Fixed arm: 120 ms SmartDelay arm: per algorithm Echocardiogram arm: per iterative method |
| ‡ Left ventricular (LV) offset | Fixed arm: 0 SmartDelay arm: 0 Echocardiogram arm: recommend 0 |
Set after the baseline echocardiogram (device programmed to VVI-40-RV) at the post-implant.
Set after the baseline echocardiogram (device programmed to VVI-40-RV) at the post-implant.
Only patients randomized to the SmartDelay arm will have their AV delay settings re-optimized at the 3-month follow-up visit.
For the echocardiogram arm only, after the AV delay is determined, an LV offset other than 0 may be programmed.
Figure 1.
Study flow chart in the SMART-AV trial.
Table III.
Patient Data Collection Schedule and Follow-Up Overview
| Enrollment | Implant | Post-Implant Visit | 3-month Follow-Up Visit | 6-month Follow-Up Visit | Unscheduled Visit | |
|---|---|---|---|---|---|---|
| Patient informed consent form | √ | |||||
| Inclusion/exclusion | √ | |||||
| Clinical history | √ | |||||
| Implanted device data | √ | |||||
| ECG and EGM measurements | √ | √ | √ | |||
| Current clinical information | √ | √ | √ | √ | ||
| Current cardiovascular medications | √ | √ | √ | |||
| Echocardiogram w/TDI | √ | √ | √ | |||
| AV delay setting | √ | √ | √ | √ | ||
| QoL questionnaire | √ | √ | √ | |||
| Patient Data Disk | √ | √ | √ | √ | √ | |
| Blood draw (US only) | √ | √ | √ | |||
| 6-minute walk test | √ | √ | √ | |||
| Optional data collection | ||||||
| Chest x-ray | √ |
Deviations, adverse events, HF events, hospitalizations, and patient withdrawal information also will be collected if applicable. EGM = electrogram; TDI = tissue Doppler imaging.
Primary Objective
The primary objective will evaluate the absolute pairwise changes in left ventricular end-systolic volume (LVESV) between randomized AV optimization groups from baseline to 6 months as determined by a echocardiographic core laboratory. Two-dimensional echocardiography-derived end-systolic and end-diastolic diameters and volumes have been reported to significantly decrease following CRT as an indication of beneficial LV reverse remodeling at early follow-up.5,11,28 Two-dimensional-derived LV volumes will be determined in the apical four- and two-chamber views by the biplane method of discs. All study sites are required to submit a pre-study echocardiogram including all of the study protocol required images to the independent echocardiographic core laboratory for certification, prior to participation in the study. The echocardiographic core laboratory will be blinded as to the patient’s treatment assignment.
Secondary Objectives
The secondary objectives of this trial, grouped within structural and functional headings evaluating the effects of individual AV optimization on the changes from baseline to 6 months, are: structural measures including echocardiography-determined LVEF and left ventricular end-diastolic volume (LVEDV) and functional measures including functional capacity as measured by the distance achieved during a 6-minute hall walk test, NYHA functional class (as assessed by an observer blinded to the patient’s treatment assignment), and QOL score. Secondary endpoints in order of analysis and the noninferiority margins in brackets are 6-minute hall walk (12 m), LVEF (5%), NYHA class (10% for the difference in percentage patients with a NYHA change of at least one class), LVEDV (8 mL), and QOL (5 points).29–34
Tertiary Objectives or Monitored parameters
The tertiary endpoints of this trial include, but are not limited to: evaluating the effects of individual AV optimization on the changes from baseline to 6 months in physical activity as determined by device-based activity log, plasma biomarker profiles (determined by a blinded core laboratory) such as inflammatory (e.g., IL-6, c-reactive protein), extracellular (e.g., TIMP-1 and MMP-9), excitation–contraction (e.g., Connexin-43, Dihydropyridine receptor), and neurohumoral (e.g., BNP/Nt-proBNP, endothelin), HF events, HF hospitalizations, cardiovascular medications, development of atrial fibrillation, cardiac output, left atrial size, time required to perform individual AV optimization, AV delay duration, and crossovers by arm.
Statistical Considerations
Sample Size Calculation
The required sample size is based on the primary endpoint of the study; change in systolic function as measured by LVESV at 6 months after CRT. Considering a change in LVESV of 15 mL between groups for the smallest clinically meaningful difference, a total of 759 patients (253 per group) are required to obtain at least 80% power. This calculation used the formula for a two-sample t-test at an alpha level of 0.05, assuming a standard deviation of 60 mL, a difference of 15 mL in LVESV between the fixed nominal and optimized groups, and no difference between the SD and echo-optimized groups. These parameters are based on results from the MIRACLE,5 MIRACLE ICD,12 MIRACLE ICD II,35 and CARE-HF4 studies. In these studies, LVESV decreased between 20 and 40 mL during a follow-up of 3–18 months among the patients receiving CRT. Therefore, using 30 mL as the median difference across the published trials, we then assumed half of the CRT effect (15 mL) would be possible with AV delay optimization over the 6-month period while still being clinically relevant. Calculations based on published results yielded a standard deviation for change in LVESV at 6 months between approximately 40 and 100 mL. To account for patient attrition and potential echocardiogram data quality issues, approximately 25% more patients will be enrolled for a total of 948 patients (approximately 950 patients), with 316 per arm.
Several smaller nonrandomized studies have observed small differences in LVESV between optimized and nonoptimized patients.36,37 If the true difference in LVESV between optimized and nonoptimized patients is in the range of 11–13 mL, and the assumed standard deviation is correct, our planned minimum sample size of 759 patients would provide between 54% and 68% power to detect such differences. If data are available for 900 of the total planned 948 enrollments, power to detect such differences will be between 68% and 78%.
Mid-Trial Sample Size Reassessment and Interim Monitoring
When approximately 75 patients (25 per arm) have 6-month results for the primary endpoint, the sample size assumptions of variability will be re-assessed in a blinded fashion. This will involve treating the initial data as an “internal pilot study” following the method outlined in Proschan et al.38 If the observed “lumped” standard deviation (the standard deviation calculated over all available observations, irrespective of the treatment group) of the primary endpoint is substantially different than what was planned, the study may be terminated for futility or the sample size may be increased to maintain adequate power. As a guideline, if the observed standard deviation is larger than 70 mL, the power for the originally calculated sample size will be reduced to less than 67%; thus, an inflation in sample size would be considered justified in order to maintain adequate power in light of the larger variability. No efficacy analysis (e.g., comparisons of mean changes between the treatment groups) will be performed at this time to avoid the possible introduction of bias or inflation of type I error. Blinding of results will be maintained until study termination to reduce the possibility of bias. The sample size reassessment will be performed by the study statistician and only the power for the originally planned treatment difference using the observed standard deviation may be released to the Steering Committee and study sponsor.
Data Analyses
Descriptive statistics used to describe treatment groups will include means/medians, standard deviations, and two-sided 95% confidence intervals. Comparisons of groups will employ χ2 or exact permutation tests for categorical variables, linear models for continuous variables, and proportional hazards models for time-to-event data. Changes in outcomes will be assessed with linear models, adjusting for baseline values.
Primary Analyses
The primary comparison of the randomized groups will be made with a general linear model, comparing the pairwise changes in LVESV from baseline to 6 months. The model will be adjusted for each patient’s baseline LVESV. If the assumptions of the linear model are not met, transformations of the change in LVESV will be employed. The required sample size was conservatively determined based on comparisons of LVESV measured at 6 months between groups, and the method of analysis for the primary endpoint described here will provide additional power. A two-sided test for superiority at an alpha level of 0.05 will be performed for the comparison of SD to the fixed AV delay and echo-based optimization to the fixed AV delay. Upon successfully meeting the superiority endpoint for the comparison of SD to the fixed AV delay, a noninferiority test at an alpha level of 0.05 will be performed to compare SD to echo-based optimization. If the noninferiority test is significant, a superiority test will also be carried out at an alpha level of 0.05. Sensitivity analyses (e.g., multiple imputations) may be used to investigate the effect of missing data on the primary comparisons.
Patients who are withdrawn post-randomization, withdraw consent post-randomization, or are lost to follow-up post-randomization will be analyzed according to the intention to treat the principle. This means that the analysis will include all data from the study patients in the groups to which they were randomized even if they never received the treatment. In order to minimize biasing study results, physicians are strongly encouraged not to change from the protocol-specified programming requirements. Patients not implanted will be withdrawn from the study, and will not be counted in the final analyses.
Secondary and Tertiary Analyses
Hypothesis tests comparing each treatment group for each secondary and tertiary endpoint will be performed. As appropriate, the same methods for the primary analysis will be used to compare the randomized groups. In addition, baseline to 3-month values will also be analyzed.
Additional secondary analyses will include the examination of the consistency of the treatment effect across clinical centers and subgroups of patients. To account for possible variations between groups of patients over time (internal pilot patients vs others) and across regions (US vs international or other international regions), random effects models will be used to assess both overall study treatment effects and group specific treatment effects. Pre-specified subgroups of patients to be examined in detail will include those with QRS widths ≤150 ms versus those with QRS widths >150 ms. These subgroups will be analyzed in a stratified fashion and tests for interaction of the randomized treatment by subgroup will be performed. It is believed a priori that optimized AV delay settings may provide greater benefit for patients with QRS widths ≤150 ms.
The consistency of the treatment effect will be compared between patients programmed to DDD versus those programmed to DDDR, and additionally for those in whom interventricular (VV) optimization is used versus those in whom VV optimization is not used. An additional analysis will compare the randomized treatment groups for the percentage of patients who are classified as improved (>15 mL reduction in LVESV), same (between ± 15 mL change in LVESV), and worsened (<15 mL reduction in LVESV).
Additional analyses will examine the role of the actual programmed AV delay setting on clinical outcomes. These analyses will include an examination of differences in the programmed AV delay between treatment groups and will also incorporate the programmed AV delay as a covariate in the treatment comparisons of the primary endpoints. The randomized groups will also be compared for other outcomes (e.g., deaths, adverse events, etc.). Unless otherwise specified, hypothesis tests will be performed at the 0.05 level, independent of each other.
Type I Error Control
A sequential ordering of comparisons (gatekeeping) will be employed to control the type I error rates in order to ensure that desired conclusions can be adequately supported. Initially, two primary superiority comparisons of this endpoint using two-sided tests, each at an alpha level of 0.05, will be run simultaneously, but independently: (1) SD versus fixed AV delay and (2) echocardiography optimized AV delay versus fixed AV delay. Only after successfully rejecting the null hypothesis for the comparison of SD versus fixed AV delay will the following noninferiority comparison using a 15-mL margin (difference) with a one-sided test at an alpha level of 0.05 be performed: SD (algorithm-optimized) versus echocardiography-determined AV delay (iterative inflow method).
Contingent on the success of both the SD versus fixed AV delay superiority test and the SD versus echocardiography-optimized AV delay noninferiority test, the primary endpoint noninferiority comparison of the SD versus echocardiography-optimized AV delay groups will be performed at the 0.05 level.
Finally, conditional on successful comparisons of groups for the primary endpoint, comparisons between the same groups will be made for the secondary endpoints. The sequential gatekeeping strategy will be employed to control the type I error rate for the secondary endpoints at a level of 0.05. If a primary endpoint comparison successfully rejects the null hypothesis, hypothesis tests comparing the same groups for the following secondary endpoints at the 0.05 level will be performed sequentially: 6-minute walk, LVEF, NYHA class, LVEDV, and QOL.
Funding Source
The SMART-AV study is sponsored by Boston Scientific.
Discussion
The clinical benefit of CRT for patients with moderate-to-severely symptomatic HF, severe left ventricular systolic dysfunction, and intraventricular conduction delay is firmly established.3–12 Nonetheless, the absolute magnitude of benefit is modest and a striking number of patients fail to improve with therapy.39 One possibility is that systematic optimization of the programmed AV delay might improve overall outcomes. However, even though many trials have shown acute benefits to AV optimization,5,6,8,20,40 only limited data exist to suggest that systematic optimization results in long-term improvements in clinical outcomes.37,40–45 On the other hand, AV optimization by echocardiography is time consuming and costly31 and it would be desirable to avoid the procedure if not beneficial or, at the very least, to replace it with a simpler yet equally effective technique.
Previous large-scale multicenter randomized clinical trials of CRT have been inconsistent in their approach to AV optimization. MIRACLE and CARE-HF used the American Society of Echocardiography-endorsed approach of using Doppler echocardiography of transmitral flow to determine optimal AV delay,4,5,20,21,24 COMPANION used an algorithm based on the intrinsic AV interval and baseline QRS width3 and in the CON-TAK CD trial,10 there was no AV optimization whatsoever. In addition to these techniques, other methods, including submaximal stress testing,46 acoustic cardiography,47,48 and a wide variety of alternative echocardiographic techniques,49,50 have also been proposed as means of AV interval optimization.
Thus, a variety of methods are used clinically for programming the AV delay, with no current consensus as to best practice. As a result, many implanters do not use either echocardiographic or electrocardiographic methods to optimize the AV interval but instead empirically program patients to a fixed AV delay interval and only optimize patients who are failing to respond to therapy. The purpose of the SMART-AV study is to determine whether systematic AV optimization using either echocardiography (Doppler assessment of transmitral inflow) or using the electrogram-based (EGM) SD approach that accounts for intrinsic intervals, interventricular timing, and lead location leads to a greater improvement in LVESV after 6 months when compared to programming a fixed AV delay of 120 ms. If systematic AV optimization using either Doppler assessment of transmitral in-flow or SD does lead to a greater improvement in LVESV after 6 months when compared to programming a fixed AV delay of 120 ms, the trial will seek to determine whether the results using SD are at least as good (noninferior) as the results of echo optimization and possibly even superior to the results of echo optimization.
A few studies have compared whether acute or chronic benefits attributed to CRT are different depending on which method of AV delay optimization is used.36,40,42,49,50 For example, the CRT-AVO study by Gold et al. studied the acute effects of the Boston Scientific proprietary algorithm now known as SD (previously known as expert ease for HF +) against other commonly used AV delay optimization methods such as AoVTI and the Ritter method as well as various fixed AV delays.40 SD was developed based on early pilot studies where hemodynamic changes (as measured by LV dP/dt max and pulse pressure) were recorded during CRT at different combinations of stimulation sites and AV delays.20,51,52 This electrogram-based method calculates sensed and paced AV delays (EGM sense AV delay and EGM pace AV delay) that provide maximum hemodynamic response based on the measurement of electrical conduction delays (i.e., AV intervals and QRS duration) to determine the optimal AV delay.40 The algorithm further accounts for LV lead location which is generally considered an important step in ensuring optimal patient response.40 The results from the CRT-AVO study demonstrated that the SD algorithm recommended a customized AV delay that increased acute hemodynamic responses in terms of % change in LV dP/dt max, as compared to fixed nominal AV delays of 100 ms, 120 ms, 140 ms, or 160 ms as well as the Ritter method and AoVTI.40 So far, however, no large-scale clinical study has directly compared different methods of AV delay optimization to determine if there are actual benefits on LV reverse remodeling that are attributed to individually optimizing the AV delay.
Some investigators have suggested that optimization of the VV delay may also play a role in improving the outcomes of CRT.53,54 However, in two randomized trials, VV optimization yielded no additional long-term benefit in patients who had also undergone AV optimization.32,55 Optimization of the VV timing will be optional in patients randomized to the echocardiographic optimized arm only, in order to be consistent with current clinical practice, but will not be permitted in the other two randomized arms of the trial.
It should be acknowledged that it is unlikely that there is such a thing as static or unchanging “optimum” AV delay in individual patients. Rather, it is likely that as hemodynamic conditions change, the “optimum” AV delay may change as well. In particular, it has been shown that the optimum AV delay may lengthen with time, coincident with the reduction in left ventricular volumes that accompanies successful CRT.56,57 In SMART-AV, the AV delay will be re-optimized after 3 months of follow-up in the patients in the SD arm only, to take advantage of the speed and relative simplicity of this technique. In contrast, the complexity and length of time required to perform echocardiographic AV optimization limits the ability to routinely re-optimize patients and to be consistent with current clinical practice patients in the echo arm will not be re-optimized.
While the primary purpose of this study is to perform comparative studies on different methods of AV delay in patients receiving a CRT-D, a unique feature of this study is the serial collection of plasma for the purposes of biomarker profiling. The biomarker portion of the study will examine analytes in the plasma which can be reliably quantified and potentially provide insight into the underlying pathophysiology of the natural history of LV systolic dysfunction. The overall goals of the biomarker portion of this study are two-fold. First, this study will serially collect plasma from patients presenting with significant LV dysfunction who are carefully followed with respect to clinical outcomes and LV function. Thus, this study will provide an opportunity to determine potential relationships between changes in plasma biomarker profiles to changes in LV ejection performance and dilatation. Second, and more relevant to overall study design and purpose, the collection of plasma for biomarker profiling prior to the initiation of CRT as well as at the follow-up visits will provide an opportunity for predicting which patients may optimally benefit from CRT in general, as well as those who may benefit from a particular form of AV delay programming. It must be recognized that this study will be collecting plasma, and therefore whether a genomic basis may exist with respect to optimal CRT programming and identifying optimal response candidates will not be addressed. While this biomarker portion of the study is considered exploratory, if successful, the long term clinical implications and the ability to develop effective screening algorithms for this device-based therapy are highly significant.
Conclusion and Clinical Implications
If systematic optimization does not lead to improved outcomes compared to a fixed AV delay of 120 ms then this technique could be reserved for patients who are clinical nonresponders to the therapy, saving time and effort. However, if AV delay optimization is shown to be superior to fixed nominal AV delay programming, then it will provide evidence that systematic optimization is indicated for patients undergoing CRT device implantation. In this case, if SD-based optimization is noninferior or superior to echo optimization, then this provides a simple, quick (≤ 2.5 minutes), and inexpensive means of providing optimization to these patients.
Footnotes
Conflicts of interest: Dr. Stein is currently an employee of and owns stock in Boston Scientific. In the past he has received honoraria from Boston Scientific, Medtronic, and St. Jude Medical. Dr. Ellenbogen has received honoraria from Boston Scientific, St. Jude Medical, Biotronik, Sorin Biomedical, and Medtronic and research grants from Medtronic, Boston Scientific, and St. Jude Medical. Dr. Gold has received honoraria from Boston Scientific, Medtronic, St. Jude Medical, and Biotronik and research grants from Boston Scientific, Medtronic, and St. Jude Medical. Dr. Lemke has received honoraria from Boston Scientific, Medtronic, St. Jude Medical, Biotronik, and Sorin Biomedical. Dr. Lozano has received honoraria from Boston Scientific, St. Jude Medical, and Sorin Biomedical; advisory board (Boston Scientific, Sorin Biomedical). Dr. Mittal has received honoraria from Boston Scientific, Medtronic, and St. Jude Medical; Fellowship support from Medtronic and Boston Scientific; Advisory board (Biotronik). Dr. Spinale has received honoraria from Boston Scientific. Dr. Van Eyk has no conflict of interest. Mr. Waggoner has received honoraria from Boston Scientific and St. Jude Medical. Dr. Meyer is an employee of and owns stock in Boston Scientific
References
- 1.Epstein AE, DiMarco JP, Ellenbogen KA, Estes NA, 3rd, Freedman RA, Gettes LS, Gillinov AM, et al. American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices); American Association for Thoracic Surgery; Society of Thoracic Surgeons: ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices) developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. J Am Coll Cardiol. 2008;51:1–62. doi: 10.1016/j.jacc.2008.02.032. [DOI] [PubMed] [Google Scholar]
- 2.Vardas PE, Auricchio A, Blanc JJ, Daubert JC, Drexler H, Ector H, Gasparini M, et al. European Society of Cardiology; European Heart Rhythm Association: Guidelines for cardiac pacing and cardiac resynchronization therapy: The Task Force for Cardiac Pacing and Cardiac Resynchronization Therapy of the European Society of Cardiology. Developed in collaboration with the European Heart Rhythm Association. Eur Heart J. 2007;28:2256–2295. doi: 10.1093/eurheartj/ehm305. [DOI] [PubMed] [Google Scholar]
- 3.Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De Marco T, Carson P, et al. Comparison of Medical Therapy, Pacing, and De-fibrillation in Heart Failure (COMPANION) Investigators: Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140–2150. doi: 10.1056/NEJMoa032423. [DOI] [PubMed] [Google Scholar]
- 4.Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, Tavazzi L. Cardiac Resynchronization-Heart Failure (CARE-HF) Study Investigators: The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–1549. doi: 10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]
- 5.Abraham WT, Fisher WG, Smith AL, Delurgio DB, Leon AR, Loh E, Kocovic DZ, et al. MIRACLE Study Group. Multicenter InSync Randomized Clinical Evaluation. Cardiac resynchronization in chronic heart failure. N Engl J Med. 2002;346:1845–1853. doi: 10.1056/NEJMoa013168. [DOI] [PubMed] [Google Scholar]
- 6.Auricchio A, Stellbrink C, Sack S, Block M, Vogt J, Bakker P, Huth C, et al. Pacing Therapies in Congestive Heart Failure (PATH-CHF) Study Group. Long-term clinical effect of hemodynamically optimized cardiac resynchronization therapy in patients with heart failure and ventricular conduction delay. J Am Coll Cardiol. 2002;39:2026–2033. doi: 10.1016/s0735-1097(02)01895-8. [DOI] [PubMed] [Google Scholar]
- 7.Bakker PF, Meijburg HW, de Vries JW, Mower MM, Thomas AC, Hull ML, Robles De Medina EO, et al. Biventricular pacing in end-stage heart failure improves functional capacity and left ventricular function. J Interv Card Electrophysiol. 2000;4:395–404. doi: 10.1023/a:1009854417694. [DOI] [PubMed] [Google Scholar]
- 8.Cazeau S, Leclercq C, Lavergne T, Walker S, Varma C, Linde C, Garrigue S, et al. Multisite Stimulation in Cardiomyopathies (MUSTIC) Study Investigators. Effects of multisite biventricular pacing in patients with heart failure and intraventricular conduction delay. N Engl J Med. 2001;344:873–880. doi: 10.1056/NEJM200103223441202. [DOI] [PubMed] [Google Scholar]
- 9.Gras D, Leclercq C, Tang AS, Bucknall C, Luttikhuis HO, Kirstein-Pedersen A. Cardiac resynchronization therapy in advanced heart failure the multicenter InSync clinical study. Eur J Heart Fail. 2002;4:311–320. doi: 10.1016/s1388-9842(02)00018-1. [DOI] [PubMed] [Google Scholar]
- 10.Higgins SL, Hummel JD, Niazi IK, Giudici MC, Worley SJ, Saxon LA, Boehmer JP, et al. Cardiac resynchronization therapy for the treatment of heart failure in patients with intraventricular conduction delay and malignant ventricular tachyarrhythmias. J Am Coll Cardiol. 2003;42:1454–1459. doi: 10.1016/s0735-1097(03)01042-8. [DOI] [PubMed] [Google Scholar]
- 11.Linde C, Leclercq C, Rex S, Garrigue S, Lavergne T, Cazeau S, McKenna W, et al. Long-term benefits of biventricular pacing in congestive heart failure: Results from the MUltisite STimulation in cardiomyopathy (MUSTIC) study. J Am Coll Cardiol. 2002;40:111–118. doi: 10.1016/s0735-1097(02)01932-0. [DOI] [PubMed] [Google Scholar]
- 12.Young JB, Abraham WT, Smith AL, Leon AR, Lieberman R, Wilkoff B, Canby RC, et al. Multicenter InSync ICD Randomized Clinical Evaluation (MIRACLE ICD) Trial Investigators. Combined cardiac resynchronization and implantable cardioversion defibrillation in advanced chronic heart failure: The MIRACLE ICD Trial. JAMA. 2003;289:2685–2694. doi: 10.1001/jama.289.20.2685. [DOI] [PubMed] [Google Scholar]
- 13.Erol-Yilmaz A, Verberne HJ, Schrama TA, Hrudova J, De Winter RJ, Van Eck-Smit BL, De Bruin R, et al. Cardiac resynchronization induces favorable neurohumoral changes. Pacing Clin Electrophysiol. 2005;28:304–310. doi: 10.1111/j.1540-8159.2005.09508.x. [DOI] [PubMed] [Google Scholar]
- 14.Lappegard KT, Bjornstad H. Anti-inflammatory effect of cardiac resynchronization therapy. Pacing Clin Electrophysiol. 2006;29:753–758. doi: 10.1111/j.1540-8159.2006.00430.x. [DOI] [PubMed] [Google Scholar]
- 15.Sinha AM, Filzmaier K, Breithardt OA, Kunz D, Graf J, Markus KU, Hanrath P, et al. Usefulness of brain natriuretic peptide release as a surrogate marker of the efficacy of long-term cardiac resynchronization therapy in patients with heart failure. Am J Cardiol. 2003;91:755–758. doi: 10.1016/s0002-9149(02)03425-2. [DOI] [PubMed] [Google Scholar]
- 16.Theodorakis GN, Flevari P, Kroupis C, Adamopoulos S, Livanis EG, Kostopoulou A, Kolokathis F, et al. Antiinflammatory effects of cardiac resynchronization therapy in patients with chronic heart failure. Pacing Clin Electrophysiol. 2006;29:255–261. doi: 10.1111/j.1540-8159.2006.00331.x. [DOI] [PubMed] [Google Scholar]
- 17.Yu CM, Fung JW, Zhang Q, Chan CK, Chan I, Chan YS, Kong SL, et al. Improvement of serum NT-ProBNP predicts improvement in cardiac function and favorable prognosis after cardiac resynchronization therapy for heart failure. J Card Fail. 2005;11:S42–S46. doi: 10.1016/j.cardfail.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 18.Michelucci A, Ricciardi G, Sofi F, Gori AM, Pirolo F, Pieragnoli P, Giaccardi M, et al. Relation of inflammatory status to major adverse cardiac events and reverse remodeling in patients undergoing cardiac resynchronization therapy. J Card Fail. 2007;13:207–210. doi: 10.1016/j.cardfail.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 19.Yan AT, Yan RT, Spinale FG, Afzal R, Gunasinghe HR, Arnold M, Demers C, et al. Plasma matrix metalloproteinase-9 level is correlated with left ventricular volumes and ejection fraction in patients with heart failure. J Card Fail. 2006;12:514–519. doi: 10.1016/j.cardfail.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 20.Auricchio A, Stellbrink C, Block M, Sack S, Vogt J, Bakker P, Klein H, et al. Effect of pacing chamber and atrioventricular delay on acute systolic function of paced patients with congestive heart failure. The Pacing Therapies for Congestive Heart Failure Study Group. The Guidant Congestive Heart Failure Research Group. Circulation. 1999;99:2993–3001. doi: 10.1161/01.cir.99.23.2993. [DOI] [PubMed] [Google Scholar]
- 21.Auricchio A, Ding J, Spinelli JC, Kramer AP, Salo RW, Hoersch W, KenKnight BH, et al. Cardiac resynchronization therapy restores optimal atrioventricular mechanical timing in heart failure patients with ventricular conduction delay. J Am Coll Cardiol. 2002;39:1163–1169. doi: 10.1016/s0735-1097(02)01727-8. [DOI] [PubMed] [Google Scholar]
- 22.Inoue N, Ishikawa T, Sumita S, Nakagawa T, Kobayashi T, Matsushita K, Matsumoto K, et al. Long-term follow-up of atrioventricular delay optimization in patients with biventricular pacing. Circ J. 2005;69:201–204. doi: 10.1253/circj.69.201. [DOI] [PubMed] [Google Scholar]
- 23.Meisner JS, McQueen DM, Ishida Y, Vetter HO, Bortolotti U, Strom JA, Frater RW, et al. Effects of timing of atrial systole on LV filling and mitral valve closure: Computer and dog studies. Am J Physiol. 1985;249:H604–H619. doi: 10.1152/ajpheart.1985.249.3.H604. [DOI] [PubMed] [Google Scholar]
- 24.Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, Klein W, et al. CARE-HF study Steering Committee and Investigators. The CARE-HF study (CArdiac REsynchronisation in Heart Failure study): Rationale, design and end-points. Eur J Heart Fail. 2001;3:481–489. doi: 10.1016/s1388-9842(01)00176-3. [DOI] [PubMed] [Google Scholar]
- 25.Gorcsan J, 3rd, Abraham T, Agler DA, Bax JJ, Derumeaux G, Grimm RA, Martin R, et al. American Society of Echocardiography Dyssynchrony Writing Group. Echocardiography for cardiac resynchronization therapy: Recommendations for performance and reporting—a report from the American Society of Echocardiography Dyssynchrony Writing Group endorsed by the Heart Rhythm Society. J Am Soc Echocardiogr. 2008;21:191–213. doi: 10.1016/j.echo.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 26.Hunt SA, Baker DW, Chin MH, Cinquegrani MP, Feldman AM, Francis GS, Ganiats TG, et al. American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1995 Guidelines for the Evaluation and Management of Heart Failure); International Society for Heart and Lung Transplantation; Heart Failure Society of America: ACC/AHA Guidelines for the Evaluation and Management of Chronic Heart Failure in the Adult: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1995 Guidelines for the Evaluation and Management of Heart Failure): Developed in Collaboration With the International Society for Heart and Lung Transplantation; Endorsed by the Heart Failure Society of America. Circulation. 2001;104:2996–3007. doi: 10.1161/hc4901.102568. [DOI] [PubMed] [Google Scholar]
- 27.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, et al. American College of Cardiology; American Heart Association Task Force on Practice Guidelines; American College of Chest Physicians; International Society for Heart and Lung Transplantation; Heart Rhythm Society: ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): Developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: Endorsed by the Heart Rhythm Society. Circulation. 2005;112:154–235. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- 28.Stellbrink C, Breithardt OA, Franke A, Sack S, Bakker P, Auricchio A, Pochet T, et al. PATH-CHF (PAcing THerapies in Congestive Heart Failure) Investigators; CPI Guidant Congestive Heart Failure Research Group: Impact of cardiac resynchronization therapy using hemodynamically optimized pacing on left ventricular remodeling in patients with congestive heart failure and ventricular conduction disturbances. J Am Coll Cardiol. 2001;38:1957–1965. doi: 10.1016/s0735-1097(01)01637-0. [DOI] [PubMed] [Google Scholar]
- 29.Bristow MR, Feldman AM, Saxon LA. Heart failure management using implantable devices for ventricular resynchronization: Comparison of Medical Therapy, Pacing, and Defibrillation in Chronic Heart Failure (COMPANION) trial. COMPANION Steering Committee and COMPANION Clinical Investigators. J Card Fail. 2000;6:276–285. doi: 10.1054/jcaf.2000.9501. [DOI] [PubMed] [Google Scholar]
- 30.Guidant Corporation. Final Clinical Report Update to US Food and Drug Administration. 2001. VENTAK CHF/CONTAK CD. 9–6-2001. [Google Scholar]
- 31.St John Sutton MG, Plappert T, Abraham WT, Smith AL, DeLurgio DB, Leon AR, Loh E, et al. Multicenter InSync Randomized Clinical Evaluation (MIRACLE) Study Group. Effect of cardiac resynchronization therapy on left ventricular size and function in chronic heart failure. Circulation. 2003;107:1985–1990. doi: 10.1161/01.CIR.0000065226.24159.E9. [DOI] [PubMed] [Google Scholar]
- 32.Rao RK, Kumar UN, Schafer J, Viloria E, De Lurgio D, Foster E. Reduced ventricular volumes and improved systolic function with cardiac resynchronization therapy: A randomized trial comparing simultaneous biventricular pacing, sequential biventricular pacing, and left ventricular pacing. Circulation. 2007;115:2136–2144. doi: 10.1161/CIRCULATIONAHA.106.634444. [DOI] [PubMed] [Google Scholar]
- 33.US Food and Drug Administration. Summary of Safety and Effectiveness Data, Medtronic, P010031. 2008 Accessed from www.fda.gov. 6–26-2002.
- 34.US Food and Drug Administration. Summary of Safety and Effectiveness Data, St. Jude Medical, P030054. 2008 Accessed from www.fda.gov on June 30, 2004.
- 35.Abraham WT, Young JB, León AR, Adler S, Bank AJ, Hall SA, Lieberman R, et al. Multicenter InSync ICD II Study Group: Effects of cardiac resynchronization on disease progression in patients with left ventricular systolic dysfunction, an indication for an implantable cardioverter-defibrillator, and mildly symptomatic chronic heart failure. Circulation. 2004;110:2864–2868. doi: 10.1161/01.CIR.0000146336.92331.D1. [DOI] [PubMed] [Google Scholar]
- 36.Sawhney NS, Waggoner AD, Garhwal S, Chawla MK, Osborn J, Faddis MN. Randomized prospective trial of atrioventricular delay programming for cardiac resynchronization therapy. Heart Rhythm. 2004;1:562–567. doi: 10.1016/j.hrthm.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 37.Vidal B, Sitges M, Marigliano A, Delgado V, Díaz-Infante E, Azqueta M, Tamborero D, et al. Optimizing the programation of cardiac resynchronization therapy devices in patients with heart failure and left bundle branch block. Am J Cardiol. 2007;100:1002–1006. doi: 10.1016/j.amjcard.2007.04.046. [DOI] [PubMed] [Google Scholar]
- 38.Proschan MA, Lan KK, Wittes JT. Statistical Monitoring of Clinical Trials: A Unified Approach. New York: Springer; 2006. [Google Scholar]
- 39.Stein KM. Clinical trials and response to CRT therapy. In: Ellenbogen KA, Aurrichio A, editors. Pacing to Support the Failing Heart. Hoboken, NJ: Wiley-Blackwell; 2008. pp. 130–155. [Google Scholar]
- 40.Gold MR, Niazi I, Giudici M, Leman RB, Sturdivant JL, Kim MH, Yu Y, et al. A prospective comparison of AV delay programming methods for hemodynamic optimization during cardiac resynchronization therapy. J Cardiovasc Electrophysiol. 2007;18:490–496. doi: 10.1111/j.1540-8167.2007.00770.x. [DOI] [PubMed] [Google Scholar]
- 41.Morales MA, Startari U, Panchetti L, Rossi A, Piacenti M. Atrioventricular delay optimization by doppler-derived left ventricular dP/dt improves 6-month outcome of resynchronized patients. Pacing Clin Electrophysiol. 2006;29:564–568. doi: 10.1111/j.1540-8159.2006.00402.x. [DOI] [PubMed] [Google Scholar]
- 42.Steendijk P, Tulner SA, Bax JJ, Oemrawsingh PV, Bleeker GB, van Erven L, Putter H, et al. Hemodynamic effects of long-term cardiac resynchronization therapy: Analysis by pressure-volume loops. Circulation. 2006;113:1295–1304. doi: 10.1161/CIRCULATIONAHA.105.540435. [DOI] [PubMed] [Google Scholar]
- 43.Hardt SE, Yazdi SH, Bauer A, Filusch A, Korosoglou G, Hansen A, Bekeredjian R, et al. Immediate and chronic effects of AV-delay optimization in patients with cardiac resynchronization therapy. Int J Cardiol. 2007;115:318–32g5. doi: 10.1016/j.ijcard.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 44.Tournoux FB, Alabiad C, Fan D, Chen AA, Chaput M, Heist EK, Mela T, et al. Echocardiographic measures of acute haemodynamic response after cardiac resynchronization therapy predict long-term clinical outcome. Eur Heart J. 2007;28:1143–1148. doi: 10.1093/eurheartj/ehm050. [DOI] [PubMed] [Google Scholar]
- 45.Kedia N, Ng K, Apperson-Hansen C, Wang C, Tchou P, Wilkoff BL, Grimm RA. Usefulness of atrioventricular delay optimization using Doppler assessment of mitral inflow in patients undergoing cardiac resynchronization therapy. Am J Cardiol. 2006;98:780–785. doi: 10.1016/j.amjcard.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 46.Whinnett ZI, Briscoe C, Davies JE, Willson K, Manisty CH, Davies DW, Peters NS, et al. The atrioventricular delay of cardiac resynchronization can be optimized hemodynamically during exercise and predicted from resting measurements. Heart Rhythm. 2008;5:378–386. doi: 10.1016/j.hrthm.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 47.Valzania C, Eriksson MJ, Boriani G, Gadler F. Cardiac resynchronization therapy during rest and exercise: Comparison of two optimization methods. Europace. 2008;10:1161–1169. doi: 10.1093/europace/eun216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zuber M, Toggweiler S, Quinn-Tate L, Brown L, Amkieh A, Erne P. A comparison of acoustic cardiography and echocardiography for optimizing pacemaker settings in cardiac resynchronization therapy. Pacing Clin Electrophysiol. 2008;31:802–811. doi: 10.1111/j.1540-8159.2008.01094.x. [DOI] [PubMed] [Google Scholar]
- 49.Jansen AH, Bracke FA, van Dantzig JM, Meijer A, Van Der Voort PH, Aarnoudse W, van Gelder BM, et al. Correlation of echo-Doppler optimization of atrioventricular delay in cardiac resynchronization therapy with invasive hemodynamics in patients with heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 2006;97:552–557. doi: 10.1016/j.amjcard.2005.08.076. [DOI] [PubMed] [Google Scholar]
- 50.Kerlan JE, Sawhney NS, Waggoner AD, Chawla MK, Garhwal S, Osborn JL, Faddis MN. Prospective comparison of echocardiographic atrioventricular delay optimization methods for cardiac resynchronization therapy. Heart Rhythm. 2006;3:148–154. doi: 10.1016/j.hrthm.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 51.Auricchio A, Stellbrink C, Butter C, Sack S, Vogt J, Misier AR, Böcker D, et al. Pacing Therapies in Congestive Heart Failure II Study Group; Guidant Heart Failure Research Group: Clinical efficacy of cardiac resynchronization therapy using left ventricular pacing in heart failure patients stratified by severity of ventricular conduction delay. J Am Coll Cardiol. 2003;42:2109–2116. doi: 10.1016/j.jacc.2003.04.003. [DOI] [PubMed] [Google Scholar]
- 52.Di Pede F, Gasparini G, De Piccoli B, Yu Y, Cuesta F, Raviele A. Hemodynamic effects of atrial septal pacing in cardiac resynchronization therapy patients. J Cardiovasc Electrophysiol. 2005;16:1273–1278. doi: 10.1111/j.1540-8167.2005.00246.x. [DOI] [PubMed] [Google Scholar]
- 53.Sogaard P, Egeblad H, Pedersen AK, Kim WY, Kristensen BO, Hansen PS, Mortensen PT. Sequential versus simultaneous biventricular resynchronization for severe heart failure: Evaluation by tissue Doppler imaging. Circulation. 2002;106:2078–2084. doi: 10.1161/01.cir.0000034512.90874.8e. [DOI] [PubMed] [Google Scholar]
- 54.van Gelder BM, Bracke FA, Meijer A, Lakerveld LJ, Pijls NH. Effect of optimizing the VV interval on left ventricular contractility in cardiac resynchronization therapy. Am J Cardiol. 2004;93:1500–1503. doi: 10.1016/j.amjcard.2004.02.061. [DOI] [PubMed] [Google Scholar]
- 55.Boriani G, Muller CP, Seidl KH, Grove R, Vogt J, Danschel W, Schuchert A, et al. Randomized comparison of simultaneous biventricular stimulation versus optimized interventricular delay in cardiac resynchronization therapy: The Resynchronization for the Hemodynamic Treatment for Heart Failure Management II Implantable Cardioverter Defibrillator (RHYTHM II ICD) study. Am Heart J. 2006;151:1050–1058. doi: 10.1016/j.ahj.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 56.O’Donnell D, Nadurata V, Hamer A, Kertes P, Mohammed W. Long-term variations in optimal programming of cardiac resynchronization therapy devices. Pacing Clin Electrophysiol. 2005;28:S24–S26. doi: 10.1111/j.1540-8159.2005.00070.x. [DOI] [PubMed] [Google Scholar]
- 57.Valzania C, Biffi M, Martignani C, Diemberger I, Bertini M, Ziacchi M, Bacchi L, et al. Cardiac resynchronization therapy: Variations in echo-guided optimized atrioventricular and interventricular delays during follow-up. Echocardiography. 2007;24:933–939. doi: 10.1111/j.1540-8175.2007.00491.x. [DOI] [PubMed] [Google Scholar]