Abstract:
Over the years, a large number of drugs have been used in isolated perfusion of extremities or organs. To interpret the pharmacokinetics of these drugs correctly, the contributions of tissue or organ clearance and chemical degradation, respectively, to overall drug elimination from the circuit need to be identified. In support of a phase I clinical trial of isolated hepatic perfusion (IHP), delivering 5-fluorouracil (5-FU) and oxaliplatin to patients with colorectal cancer hepatic metastases, we aimed to characterize the stability of 5-FU and oxaliplatin in the IHP circuit. Stability of 5-FU and oxaliplatin was assessed in human blood, lactated Ringer infusion (LRI), and in an in vitro IHP circuit consisting of both blood and LRI. Samples were analyzed with liquid chromatography tandem mass spectrometry (5-FU) and atomic absorption spectrophotometry (oxaliplatin). 5-FU was stable under all tested in vitro conditions, but ultrafilterable platinum concentrations decreased slowly with a half-life of 85 minutes in both IHP perfusate and whole blood. The stability of 5-FU in the media containing blood is likely attributable to saturation of dihydropyrimidine dehydrogenase. The decrease of ultrafilterable platinum in blood-containing media with an 85 minutes half-life is in agreement with previous reports on oxaliplatin biotransformation. Oxaliplatin and 5-FU are sufficiently stable in the circuit for the 1-hour perfusion in ongoing and planned clinical trials.
Keywords: perfusion isolated liver 5-fluorouracil oxaliplatin
Isolated hepatic perfusion (IHP) was first described as a treatment modality for hepatic cancer more than 40 years ago when five patients were treated with nitrogen mustard infused through an IHP circuit (1). However, it took almost two decades before this treatment modality regained popularity, in part due to the technical complexity and lack of standardized assessment of responses (2).
Over the years, a large number of drugs have been used in isolated perfusion of extremities or organs (3–18). To interpret the pharmacokinetics of these drugs correctly, the contributions of tissue or organ clearance and chemical degradation, respectively, to overall drug elimination from the circuit need to be identified. The stability of various drugs has been reported in a variety of matrices, such as: infusion fluids, tissue culture medium, plasma, and whole blood. However, stability in one matrix is no guarantee for stability in another. This is illustrated by doxorubicin, which is relatively stable at 37°C in tissue culture medium, but degrades rapidly in whole blood, even at room temperature (19). It is therefore surprising that there is a paucity of data on the stability of drugs in the respective media that are used as perfusates. The stability of agents in the perfusion system may dictate the maximum effective time of perfusion because continuing to perfuse after a drug has degraded would not contribute to efficacy, but would increase the risk of complications associated with the procedure.
Oxaliplatin is a reactive platinum compound that chemically reacts with, and thereby binds to, cellular nucleophiles such as DNA and proteins, which causes tumor cell damage and, ultimately, cell death (20). Oxaliplatin can also react with extracellular nucleophiles such as plasma proteins, which results in deactivation of the platinum before it can reach the tumor cell target. Total platinum in plasma is reflective of the sum of unreacted and reacted (and consequently inactivated) platinum. Platinum that passes through a 30 KD molecular weight ultrafiltration device is referred to as ultrafilterable platinum, and is considered active platinum, that can still react with DNA and cause tumor cell toxicity. Currently, oxaliplatin is combined with 5-fluorouracil/leucovorin (5-FU/LV), in a regimen called FOLFOX), that is United States Food and Drug Administration-approved for treatment of stage III colon cancer.
5-Fluorouracil (5-FU) is an antimetabolite with several presumed mechanisms of action (21). Its main anabolite is 5-fluoro-2′-deoxyuridine-monophosphate, which irreversibly binds to and inactivates thymidylate synthase, thereby blocking cellular production of thymidine triphosphate, the rate limiting nucleotide in DNA synthesis. However, 5-FU is quickly degraded to the inactive metabolite dihydrofluorouracil by dihydropyrimidine dehydrogenase (DPD) in blood and liver. 5-FU is used to treat a wide variety of cancers, including colorectal, pancreatic, breast, esophagus, and head and neck. In support of a phase I clinical trial of IHP, delivering 5-FU and oxaliplatin to patients with colorectal cancer hepatic metastases, we aimed to characterize the stability of 5-FU and oxaliplatin in the IHP circuit.
MATERIALS AND METHODS
Drugs and Reagents
Oxaliplatin for infusion (Sanofi Aventis, Bridgewater, NJ) was provided by the Division of Cancer Treatment and Diagnosis, National Cancer Institute (Bethesda, MD). 5-Fluorouracil (Abraxis Pharmaceutical Products, Schaumburg, IL) was obtained from the University of Pittsburgh Medical Center Pharmacy. Lactated Ringer’s Infusion (LRI) and Plasmalyte A were obtained from Baxter (Deerfield, IL), and control human blood was obtained from the Central Blood Bank (Pittsburgh, PA).
Isolated Perfusion
A simplified perfusion circuit was configured to specifications consistent with the chemoperfusion system designed for the clinical IHP, as described before (18). The circuit consisted almost entirely of ¼″ internal diameter (ID) tubing (Terumo X Coated, Terumo Cardiovascular Systems Corporation, Ashland, MA) that comprised the low pressure venous blood segment of the system and the arterial high pressure blood segment of the system. The exception to ¼″ ID tubing in the circuit was at the inlet of the displacement (roller type) blood pump. The tubing ID was graduated at this point from ¼″ ID to ⅜″ ID through the raceway of the roller pump. This modification is almost always incorporated in roller pump systems to enhance pump stroke volume displacement. The tubing ID was immediately reduced from ⅜″ to ¼″ ID at the point where the tubing exited the roller pump housing.
Essential perfusion components interposed in the venous side of the system included a standard hardshell venous reservoir (Terumo) and a blood sampling manifold (Terumo). Perfusion components added to the arterial side of the system included an oxygenator (Terumo SXR-1.0), a blood sampling manifold (Terumo), and a 5 μm pediatric arterial blood filter (Pall, East Hills, NY) (Figure 1). The oxygenator heat exchanger inlet and outlet water lines were connected to a heater/cooler unit (Sub-Zero, Cincinatti, OH), and the temperature was set to 39°C. No exogenous medical gases were introduced into the experimental system, which represented the only difference from the system used clinically. The blood in the circuit quickly assumed a bright red color, indicating oxygenation through the tubing. Because the circuit did not include an organ requiring oxygenation, we felt that introduction of exogenous gases was not necessary. The circuit was primed with 1 L of Plasmalyte A and de-aired. The arterial filter was isolated from the circuit with hemostats, as per normal clinical IHP protocol implemented at the University of Pittsburgh Medical Center. One liter of LRI, 500 mL of citrate-anticoagulated packed red blood cells, and 10,000 units of heparin (Abraxis, Schaumburg, IL) were added to the circuit. The perfusate was recirculated at 660 mL/min, and an 8 mL pre-dose sample of perfusate was taken from the manifold. Immediately thereafter, 68 mg (40 mg/m2 × 1.7 m2) of oxaliplatin and 340 mg (200 mg/m2 × 1.7 m2) of 5-FU were added as a bolus. These doses were calculated from dose level 1 of the associated phase I study, assuming a patient with a body surface area of 1.7 m2. After addition of the drugs, samples were taken at 0, 5, 15, 30, 45, and 60 minutes. This experiment was performed in duplicate.
Figure 1.
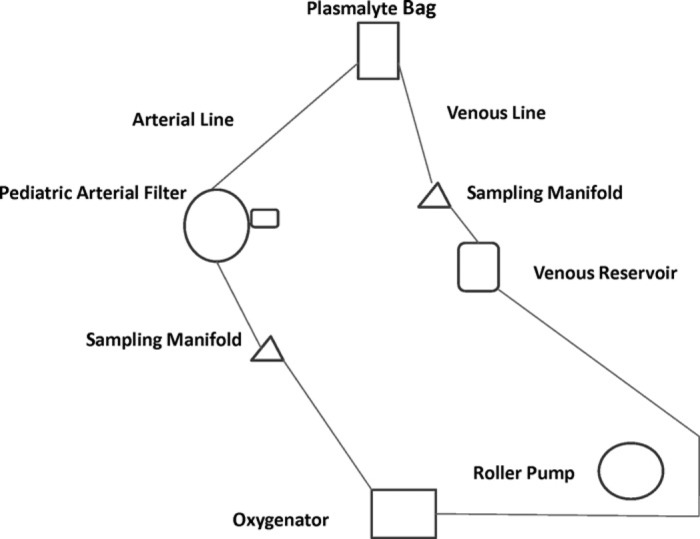
Schematic of continuous chemoperfusion circuit used in in vitro experiments.
In Vitro Stability
The stability of 5-FU at 37°C in whole blood and LRI, and the stability of oxaliplatin at 37°C in whole blood were also assessed. A combination of 100 μg/mL 5-FU and 20 μg/mL oxaliplatin was prepared in whole blood and in LRI. Aliquots were incubated at 37°C in a stirred water bath for 0, 5, 15, 30, 45, and 60 minutes.
Sample Handling
Blood and perfusate samples were transferred to heparinized vacutainer tubes, immediately placed on ice, and centrifuged at 1000 × g for 10 minutes. The resulting supernatants and the LRI samples were stored at −20°C, or lower, until quantitative analysis for 5-FU and total and ultrafilterable platinum content. Total platinum represents the platinum species present, in whichever form, including platinum reacted and bound to macromolecules. Ultrafilterable platinum represents low molecular weight platinum and contains platinum species that are still reactive with nucleophiles such as DNA and proteins. Ultrafiltrates of perfusate and plasma were prepared by placing 1 mL of each sample into an Amicon Centrifree micropartition device (Amicon Division, W.R. Grace, Beverly, MA) and then centrifuging those devices at 2000 × g for 20 minutes at 4°C.
Bioanalysis
Concentrations of 5-FU in perfusate plasma, blood plasma, and LRI were quantitated with a liquid chromatography tandem mass spectrometry assay previously developed in our laboratory and validated according to the most recent United States Food and Drug Administration guidelines (22). Total platinum concentrations in plasma, perfusate, and ultrafilterable platinum in their respective ultrafiltrates were assessed with a Perkin-Elmer model 1100 flameless atomic absorption spectrometer (Perkin-Elmer, Norwalk, CT) as previously described (23). Platinum concentrations were determined by comparison with a standard curve performed in an appropriate matrix and on the same day as the assay.
Pharmacokinetic Analysis
Concentration versus time data were analyzed compartmentally using the ADAPT 5 software for pharmacokinetic/ pharmacodynamic systems analysis (24), using the maximum likelihood option. The elimination rate constant (kel) was estimated by fitting a one-compartment, open, linear model to the concentration versus time data. The half-life (t1/2) was calculated as .693/kel.
Results
The stabilities of 5-FU and platinum in the in vitro isolated perfusion system, in red blood cells, and in LRI, are shown in Figure 2 and Figure 3, respectively. Total platinum and 5-FU did not decrease with time under any of the conditions, whereas ultrafilterable platinum decreased with a half-life of 85 minutes (95% confidence interval, 74–97 minutes), as would be expected upon slow binding to macromolecules present in the perfusate.
Figure 2.
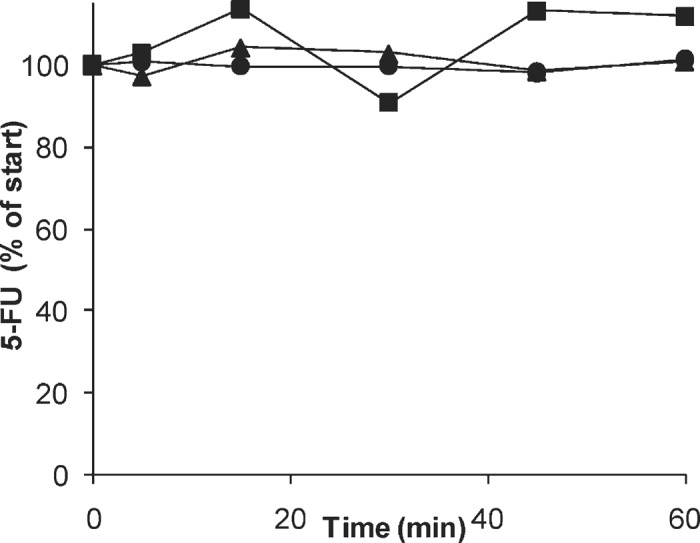
Concentrations of 5-FU in the in vitro IHP system (▪), in whole blood (▸), and in LRI (•).
Figure 3.
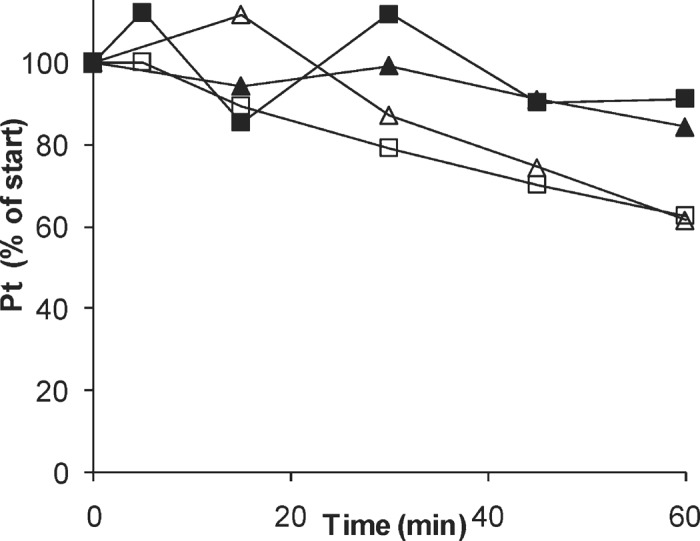
Concentrations of total platinum (filled symbols) and free platinum (open symbols) in vitro in the in vitro IHP system (squares), and in whole blood (triangles).
DISCUSSION
Colorectal cancer is one of the most common malignancies in the world. One randomized study found that of the patients with colorectal cancer, 85% had metastatic disease involving the liver and 41% had disease confined to the liver (25). First-line systemic chemotherapeutic regimens containing oxaliplatin and 5-FU produce objective response rates up to 50% (25). However, systemic side-effects of 5-FU vary greatly depending on the exposure of the systemic circulation, and include vomiting, diarrhea, stomatitis, and myelosupression (26).
Isolated regional chemotherapy can be used to improve response rates of hepatic metastases and overall patient outcome. IHP is able to isolate the liver completely from the systemic circulation, thereby allowing the tumor to be exposed to a much higher concentration of a chemotherapeutic agent. When IHP is performed well, the treatment dose is only limited by the toxicity of the drug to the liver.
5-FU has been used in isolated pelvic perfusion (27), hyperthermic pelvic isolation-perfusion (12), and IHP (28). Although these studies included pharmacokinetic studies, the non-tissue clearance was not assessed. 5-FU is stable in human plasma, but is rapidly cleared from human blood at 37°C (22,29). The lower stability of 5-FU in whole blood relative to plasma may be explained by the high expression in white blood cells of DPD, the major enzyme in 5-FU clearance (30,31). The 5-FU circuit stability after 1 hour was 112%, suggesting negligible loss of 5-FU due to the in vitro circuit components. Most likely, the apparent stability of 5-FU in the present study, while blood is known to metabolize 5-FU, is attributable to saturation of DPD at the relatively high 5-FU concentration of the perfusion solution, which was approximately 250-fold greater than the approximately .4 μg/mL Km value reported for DPD (31).
Oxaliplatin has recently been used for IHP (18). Oxaliplatin is rapidly biotransformed in plasma at 37°C with a half-life of 1.6 hours (32). The 85 minutes half-life of ultrafilterable platinum in our isolated perfusion system, which consists of a mixture of LRI and whole blood, is in line with those previously reported results. While total platinum circuit stability was close to 100% at 91%, as expected, ultrafilterable platinum circuit stability after 1 hour was 62%.
In the current study, the circuit was oxygenated by passive diffusion of oxygen through the plastic tubing, the blood was heparinized, and the temperature was kept constant, in accordance with the clinical protocol of ongoing and planned clinical trials at the University of Pittsburgh Cancer Institute. Apart from large changes in temperature, these variables are not expected to be relevant for the circuit stability of oxalplatin and 5-FU.
Characterizing the stability of drugs in the perfusion system is important for several reasons. The stability of agents in the perfusion system may dictate the maximum effective time of perfusion. Rapid in vitro degradation may prompt shortening of the procedure, because a longer procedure will not increase drug exposure or contribute to efficacy, yet would increase the risk of procedure-related complications. Alternatively, the drug dose may be divided and added to the isolated circuit as more than one administration. Furthermore, knowledge of in vitro degradation kinetics allows for estimation of the true elimination rate of drug that is attributable to the perfused tissue or organ. This may be relevant in the case of platinum-containing agents, which derive their activity from DNA adduct formation. Consequently, a decrease in ultrafilterable platinum in the clinical IHP may not be entirely due to delivery of active platinum to the tissues, but in part due to clearance by reaction with perfusate components. Our results suggest that 5-FU and oxaliplatin are sufficiently stable in the circuit to support a 1 hour perfusion procedure in the clinical trial of IHP of 5-FU and oxaliplatin. Our data support IHP as a promising approach to treating metastatic colorectal cancer and will aid in the interpretation of pharmacokinetic data obtained in future clinical trials utilizing IHP.
ACKNOWLEDGMENTS
Supported by grants R21 CA115059 and 2P30CA47904 from the National Institutes of Health. We thank the University of Pittsburgh Cancer Institute Hematology/Oncology Writing Group for constructive suggestions regarding the manuscript.
REFERENCES
- 1.Ausman RK.. Development of a technic for isolated perfusion of the liver. NY State J Med. 1961;61:3993–7. [PubMed] [Google Scholar]
- 2.Alexander HR Jr, Libutti SK.. Isolated hepatic perfusion for extensive liver metastases. London: W.B. Saunders; 2000;81b:1607–15. [Google Scholar]
- 3.Bartlett DL, Libutti SK, Figg WD, Fraker DL, Alexander HR.. Isolated hepatic perfusion for unresectable hepatic metastases from colorectal cancer. Surgery. 2001;2:176–87. [DOI] [PubMed] [Google Scholar]
- 4.Didolkar MS, Jackson AJ, Lesko LJ, et al. Pharmacokinetics of dacarbazine in the regional perfusion of extremities with melanoma. J Surg Oncol. 1996;3:148–58. [DOI] [PubMed] [Google Scholar]
- 5.Shiu MH, Knapper WH, Fortner JG, et al. Regional isolated limb perfusion of melanoma intransit metastases using mechlorethamine (nitrogen mustard). J Clin Oncol. 1986;12:1819–26. [DOI] [PubMed] [Google Scholar]
- 6.Pontes L, Lopes M, Ribeiro M, Santos JG, Azevedo MC.. Isolated limb perfusion with fotemustine after chemosensitization with dacarbazine in melanoma. Melanoma Res. 1997;5:417–9. [DOI] [PubMed] [Google Scholar]
- 7.Ariyan S, Poo WJ, Bolognia J.. Regional isolated perfusion of extremities for melanoma: A 20-year experience with drugs other than L-phenylalanine mustard. Plast Reconstr Surg. 1997;4:1023–9. [DOI] [PubMed] [Google Scholar]
- 8.Murdter TE, Linder A, Friedel G, et al. Pharmacokinetics of cyclophosphamide, adriamycin and adriamycin prodrug (HMR 1928) using an ex vivo isolated perfused human lung model (IHLP). Pneumologie. 2000;11:494–8 [in German]. [DOI] [PubMed] [Google Scholar]
- 9.Burt ME, Liu D, Abolhoda A, et al. Isolated lung perfusion for patients with unresectable metastases from sarcoma: A phase I trial. Ann Thorac Surg. 2000;5:1542–9. [DOI] [PubMed] [Google Scholar]
- 10.Arredondo MA, Chaudhuri B, Kar R, Crist KA, Thomford NR, Chaudhuri PK.. Isolated perfusion of pancreas with mitomycin C. Am J Surg. 1990;6:569–74. [DOI] [PubMed] [Google Scholar]
- 11.Huber R, Kruger I, Kuper K, Huber PM, Pichlmaier H.. Isolated hyperthermic perfusion with mitoxantrone of melphalan in malignant melanoma of the limb. Am J Surg. 1995;4:345–52. [DOI] [PubMed] [Google Scholar]
- 12.Wile AG, Stemmer EA, Andrews PA, Murphy MP, Abramson IS, Howell SB.. Pharmacokinetics of 5-fluorouracil during hyperthermic pelvic isolation-perfusion. J Clin Oncol. 1985;6:849–52. [DOI] [PubMed] [Google Scholar]
- 13.Taeger G, Grabellus F, Podleska LE, Muller S, Ruchholtz S.. Effectiveness of regional chemotherapy with TNF-alpha/melphalan in advanced soft tissue sarcoma of the extremities. Int J Hyperthermia. 2008;3:193–203. [DOI] [PubMed] [Google Scholar]
- 14.Lienard D, Eggermont AM, Koops HS, et al. Isolated limb perfusion with tumour necrosis factor-alpha and melphalan with or without interferon-gamma for the treatment of in-transit melanoma metastases: A multicentre randomized phase II study. Melanoma Res. 1999;5:491–502. [DOI] [PubMed] [Google Scholar]
- 15.Tominaga R, Nakano T, Shibata S, et al. Systemic effects of hyperthermic isolated lower limb perfusion with carboplatin and interferon-beta. Artif Organs. 2001;1:36–41. [DOI] [PubMed] [Google Scholar]
- 16.Pommier RF, Moseley HS, Cohen J, Huang CS, Townsend R, Fletcher WS.. Pharmacokinetics, toxicity, and short-term results of cisplatin hyperthermic isolated limb perfusion for soft-tissue sarcoma and melanoma of the extremities. Am J Surg. 1988;5:667–71. [DOI] [PubMed] [Google Scholar]
- 17.Nakayama J, Takeuchi M, Mayumi H, et al. Hyperthermic isolated limb perfusion with intra-arterial administration of carboplatin and/or interferon-beta for the treatment of malignant melanoma of the leg. J Dermatol. 1994;11:915–22. [DOI] [PubMed] [Google Scholar]
- 18.Zeh HJ III, Brown CK, Holtzman MP, et al. A phase I study of hyperthermic isolated hepatic perfusion with oxaliplatin in the treatment of unresectable liver metastases from colorectal cancer. Ann Surg Oncol. 2009;16:385–94. [DOI] [PubMed] [Google Scholar]
- 19.Laubrock N, Hempel G, Schulze-Westhoff P, Wurthwein G, Flege S, Boos J.. The stability of doxorubicin and ldarubicin in plasma and whole blood. Chromatographia. 2008;1–2:9–13. [Google Scholar]
- 20.Levi F, Metzger G, Massari C, Milano G.. Oxaliplatin: Pharmacokinetics and chronopharmacological aspects. Clin Pharmacokinet. 2000;1:1–21. [DOI] [PubMed] [Google Scholar]
- 21.Diasio RB, Harris BE.. Clinical pharmacology of 5-fluorouracil. Clin Pharmacokinet. 1989;4:215–37. [DOI] [PubMed] [Google Scholar]
- 22.Kosovec JE, Egorin MJ, Gjurich S, Beumer JH.. Quantitation of 5-fluorouracil (5-FU) in human plasma by liquid chromatography/electrospray ionization tandem mass spectrometry. Rapid Commun Mass Spectrom. 2008;2:224–30. [DOI] [PubMed] [Google Scholar]
- 23.Erkmen K, Egorin MJ, Reyno LM, Morgan R Jr, Doroshow JH.. Effects of storage on the binding of carboplatin to plasma proteins. Cancer Chemother Pharmacol. 1995;3:254–6. [DOI] [PubMed] [Google Scholar]
- 24.D’Argenio DZ, Schumitzky A.. ADAPT II User’s Guide: Pharmacokinetic/Pharmacodynamic Systems Analysis Software. Los Angeles: University of Southern California; 1997. [Google Scholar]
- 25.Grover A, Alexander HR Jr.. The past decade of experience with isolated hepatic perfusion. Oncologist. 2004;6:653–64. [DOI] [PubMed] [Google Scholar]
- 26.Abeloff MD, Armitage JO, Niederhuber JE, Kastan MB, McKenna WG.. Clinical Oncology. Philadelphia: Elsevier Churchill Livingstone; 2004:1926. [Google Scholar]
- 27.Wanebo HJ, Belliveau JF.. A pharmacokinetic model and the clinical pharmacology of cis-platinum, 5-fluorouracil and mitomycin-C in isolated pelvic perfusion. Cancer Chemother Pharmacol. 1999;5:427–34. [DOI] [PubMed] [Google Scholar]
- 28.Aigner K, Walther H, Tonn JC, et al. Isolated liver perfusion with 5-fluorouracil (5-FU) in the human. Chirurg. 1982;9:571–3 [in German]. [PubMed] [Google Scholar]
- 29.Beumer JH, Courtney J, Stocker D, et al. Highlights from: 5-Fluorouracil Drug Management Pharmacokinetics and Pharmacogenomics Workshop; Orlando, Florida, January 2007. Clin Colorectal Cancer. 2007;6:407–22. [DOI] [PubMed] [Google Scholar]
- 30.Nishimura M, Naito S.. Tissue-specific mRNA expression profiles of human phase I metabolizing enzymes except for cytochrome P450 and phase II metabolizing enzymes. Drug Metab Pharmacokinet. 2006;5:357–74. [DOI] [PubMed] [Google Scholar]
- 31.Ploylearmsaeng SA, Fuhr U, Jetter A.. How may anticancer chemotherapy with fluorouracil be individualised? Clin Pharmacokinet. 2006;6:567–92. [DOI] [PubMed] [Google Scholar]
- 32.Pendyala L, Creaven PJ.. In vitro cytotoxicity, protein binding, red blood cell partitioning, and biotransformation of oxaliplatin. Cancer Res. 1993;24:5970–6. [PubMed] [Google Scholar]


