Abstract
Metabolic homeostasis reflects the complex output of endocrine, autonomic, and behavioral control circuits that extend throughout the central nervous system. Brain regions that control food intake and energy expenditure are privy to continuous visceral sensory feedback signals that presumably modulate appetite, satiety, digestion, and metabolism. Sensory signals from the gastrointestinal tract and associated digestive viscera are delivered to the brain primarily by vagal afferents that terminate centrally within the caudal nucleus of the solitary tract (NST), with signals subsequently relayed to higher brain regions by parallel noradrenergic and peptidergic projection pathways arising within the NST. This article begins with an overview of these ascending pathways identified in adult rats using a standard anterograde tracer microinjected into the caudal visceral sensory region of the NST, and also by immunocytochemical localization of glucagon-like peptide-1. NST projection targets identified by these two approaches are compared to the distribution of neurons that become infected after inoculating the ventral stomach wall with a neurotropic virus that transneuronally infects synaptically-linked chains of neurons in the anterograde (i.e., ascending sensory) direction. Although the focus of this article is the anatomical organization of axonal projections from the caudal visceral NST to the hypothalamus and limbic forebrain, discussion is included regarding the hypothesized role of these projections in modulating behavioral arousal and coordinating endocrine and behavioral (i.e., hypophagic) responses to stress.
Keywords: glucagon-like peptide-1, noradrenergic, hypothalamus, phaseolus vulgaris leucoagglutinin, transneuronal viral tracing
1.0 Introduction
Metabolic homeostasis reflects the complex output of endocrine, autonomic, and behavioral control circuits that extend throughout the central nervous system (CNS). Brain regions that control energy intake and expenditure are privy to continuous interoceptive feedback from the body that can modulate appetite, satiety, digestion, and metabolism. Interoceptive signals from the gastrointestinal tract and associated digestive viscera are delivered to the brain primarily by vagal afferents that terminate centrally within the medullary dorsal vagal complex (DVC), comprising the dorsal motor nucleus of the vagus (DMV), nucleus of the solitary tract (NST), and area postrema (AP) (Rinaman, 2003a). In addition to vagal inputs from gastrointestinal and other thoracic and abdominal viscera, DVC neurons receive direct and indirect interoceptive signals from olfactory, glossopharyngeal, trigeminal, facial, and spinal afferent systems. A strong topography is evident in the terminal arborizations of primary visceral afferents, with inputs from the gut terminating within the caudal medial NST (Altschuler et al., 1989; Shapiro and Miselis, 1985). In addition to synaptic inputs, the AP and a significant portion of the caudal medial NST contain fenestrated capillaries, allowing blood-borne factors (e.g., toxins, cytokines, hormones, and osmolytes) to alter local neural activity within the DVC and other brainstem targets (Cunningham et al., 1994).
A major product of integrated DVC neural activity is the modulation of autonomic vagal parasympathetic outflow to the stomach, small intestine, pancreas, and other digestive viscera (Altschuler et al., 1992). In addition, and as summarized in this review, ascending axonal projections from neurons within the caudal medial (i.e., gastrointestinal) NST target virtually every pontine, diencephalic, and telencephalic circuit node that has been implicated in the central control of energy homeostasis (Horst and Streefland, 1994), highlighting gastrointestinal interoception as a potentially critical modulator of neural circuit activity throughout the brain. Ample evidence supports the view that descending projections from hypothalamus to caudal brainstem provide critical control over the initiation and termination of food intake and feeding-related autonomic adjustments (Berthoud, 2002; Berthoud et al., 2006; Coll et al., 2007; Smith, 2000; Smith, 2003; Woods and D'Alessio, 2008; Zheng et al., 2005). Ascending projections from NST to hypothalamus are clearly involved in regulating hormone release from the anterior and posterior pituitary in response to gastrointestinal and other visceral sensory signals (Rinaman, 2007). Conversely, the influence of these ascending projections in regulating food intake is less firmly established (Luckman and Lawrence, 2003; Renner et al., 2010). Appetite and satiety are clearly modulated both by external (i.e., environmental) and internal (i.e., physiological) contexts, and, therefore, are only loosely dependent on past or current visceral sensory feedback signals.
Results from neuroanatomical studies performed primarily in rats have revealed potential pathways by which visceral sensory feedback signals can reach the hypothalamus and limbic forebrain, and thereby potentially affect the ways in which these forebrain regions control food intake. This article begins with an overview of ascending axonal projections from neurons within the caudal visceral NST to higher brain regions in adult rats. For this purpose, projections were identified using a standard anterograde tracer microinjected into the caudal NST, and also by immunocytochemical localization of glucagon-like peptide-1 (GLP-1). GLP-1-positive fibers within the brain arise exclusively from non-noradrenergic (NA) neurons within the caudal visceral NST and closely adjacent reticular formation (Larsen et al., 1997; Merchenthaler et al., 1999; Rinaman, 1999b; Vrang et al., 2007), thereby providing a clear view of ascending pathways arising from this small group of phenotypically distinct neurons. Projection pathways identified by these two approaches also are compared to the distribution of CNS neurons that become infected/labeled after inoculating the ventral stomach wall with H129, a neurotropic α-herpesvirus virus that transneuronally infects synaptically-linked chains of neurons in the anterograde direction (Rinaman and Schwartz, 2004).
The focus of this report is the anatomical organization and neurochemical phenotypes of ascending projections from the caudal gastrointestinal region of the NST in rats. Although some discussion of the hypothesized roles of these ascending projections is included where relevant, the reader is referred to several recent comprehensive reviews for more detailed information regarding the involvement of particular diencephalic and limbic forebrain regions in the central neural control of food intake and energy expenditure (Berthoud, 2002; Berthoud, 2008; Broberger, 2005; Woods and D'Alessio, 2008).
2.0 Ascending visceral pathways: standard anterograde tracing from the noradrenergic (NA) region of the caudal NST
Since most neural pathways conveying interoceptive signals from body to brain involve a synaptic relay within the NST, a description of the central projections of NST neurons effectively reveals most CNS recipients of viscerosensory information (Bailey et al., 2006; Horst et al., 1989; Horst and Streefland, 1994; Ricardo and Koh, 1978), albeit without identifying the central targets of organ-specific sensory signals. A multitude of anterograde and retrograde tract-tracing studies, performed largely in rats, have demonstrated that neurons within the caudal visceral NST1 have axons that project directly to a large number of central targets distributed across the medulla, pons, midbrain, hypothalamus, and limbic forebrain. Similarly, the present report documents the distribution of labeled axonal projections in a representative adult male Sprague-Dawley rat killed 10 days after unilateral iontophoretic injection of an anterograde neural tracer, Phaseolus vulgaris leucoagglutinin (PhAL, 2.5%) (Gerfen and Sawchenko, 1984), into the caudal visceral NST. Coronal brain sections (35 µm thick) were cut from the caudal medulla through the rostral extent of the corpus callosum, and a one-in-six series was processed for immunoperoxidase localization of PhAL. The distribution of PhAL-positive fibers was then mapped along the rostrocaudal neural axis using a light microscope equipped with a digital video camera and computerized tracing software.
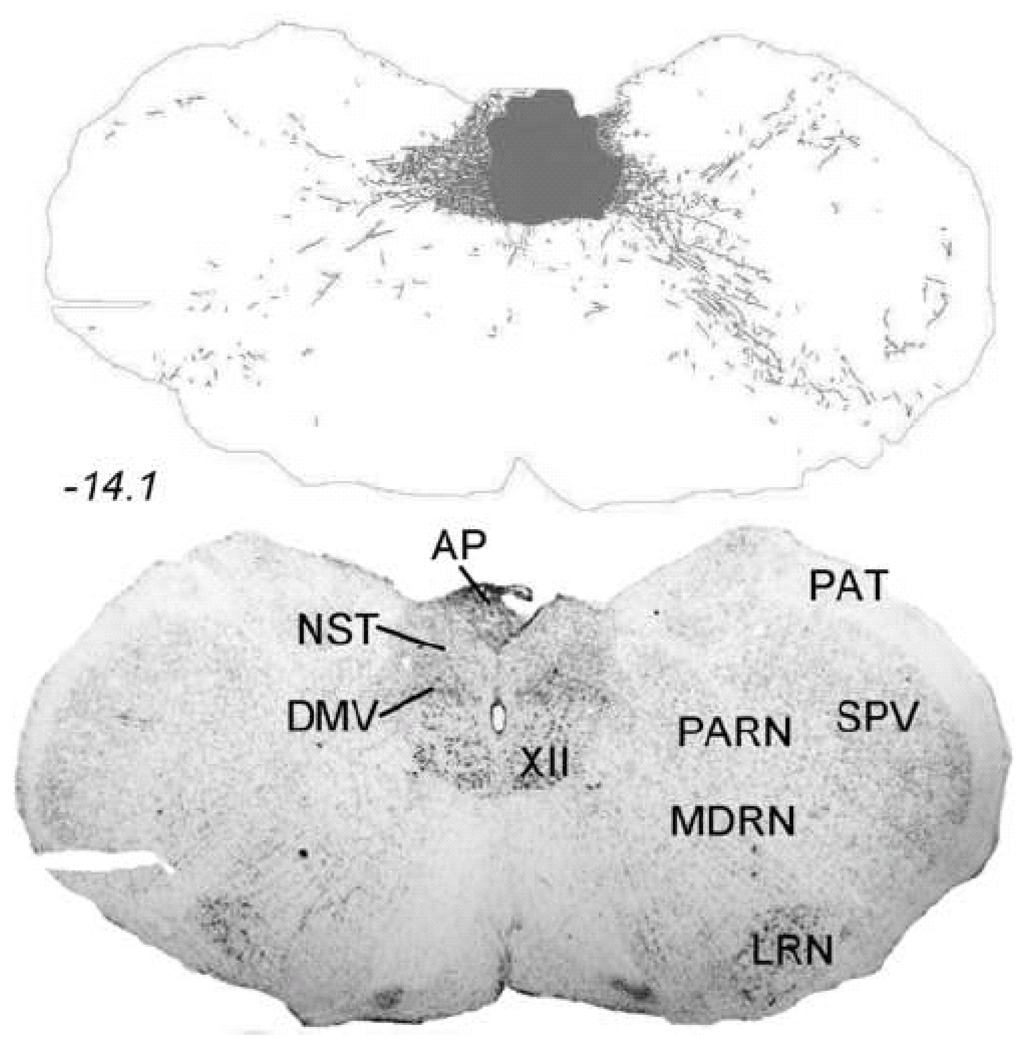
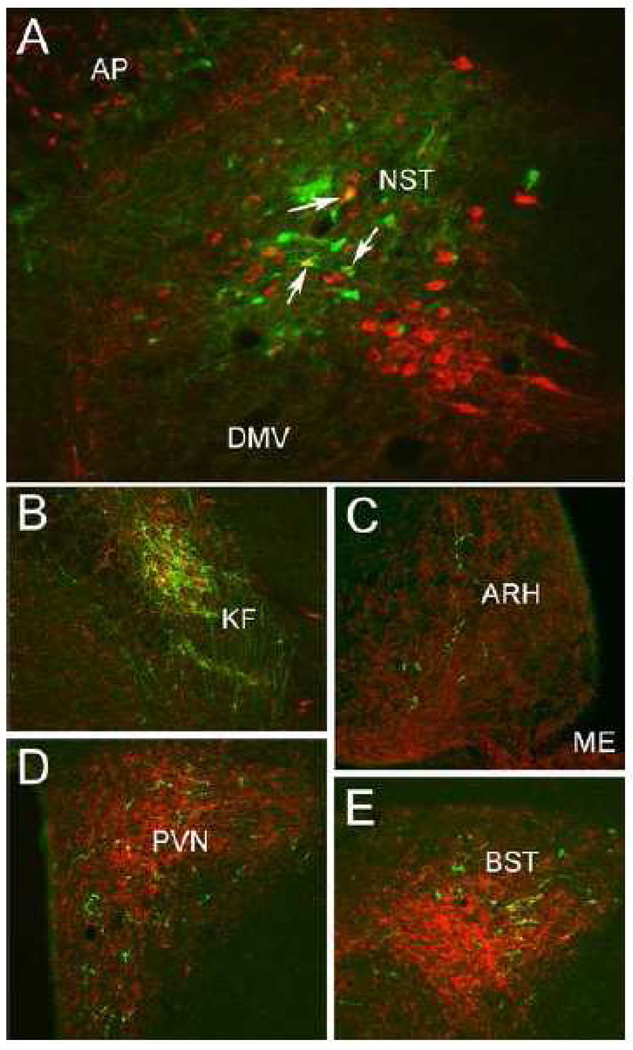
Figure 1 depicts the caudal NST-centered PhAL injection site, and PhAL-positive fibers emanating from it. Immunoperoxidase labeling was so dense within the injection site (Fig. 1, gray shaded area) that it could not be accurately traced. To more precisely localize individual neurons that took up tracer within the injection site, an alternate set of sections from the same rat was processed for dual immunofluorescent localization of PhAL and the NA synthetic enzyme, dopamine beta hydroxylase (DbH) (Fig. 2). Neurons concentrating PhAL were restricted to the medial subnucleus of the NST at the rostrocaudal level of the area postrema (AP). The injection site overlapped the NST region that contains the A2 NA cell group, and a subset of PhAL-concentrating neurons were identified as DbH-positive (Fig. 2). Dual immunofluorescence labeling confirmed that the brainstem and forebrain distribution of PhAL-positive fibers overlapped with DbH immunolabeling; a few examples are shown in Figure 2. Conversely, the injection site in this rat did not label neurons within the medial commissural NST region (adjacent to the AP) that contains aldosterone-sensitive hydroxysteroid dehydrogenase-2 (HSD2) neurons. HSD2-positive NST neurons are implicated in the central control of sodium appetite (Geerling et al., 2006a; Geerling and Loewy, 2007), and appear to project to a discrete subset of the brain regions that receive input from NA and GLP-1-positive NST neurons (Geerling and Loewy, 2006).
Figure 1.
Iontophoretic PhAL injection site within the caudal DVC in an adult male Sprague-Dawley rat. The dark gray shaded area in the upper panel depicts the region of the tracer injection site, which contained PhAL immunoperoxidase labeling that was too dense to accurately draw. See Figure 2 for immunofluorescence labeling of PhAL-concentrating neurons in an adjacent tissue section. Labeled fibers throughout the rest of the section in the upper panel (and in Figures 3–10) arise from PhAL-concentrating neurons located within the injection site. The lower panel is a nearby Nissl-stained tissue section from the same rat. The approximate rostro-caudal level of each section (relative to bregma, in mm) is indicated, based on a standard rat brain atlas (Swanson, 2004). See Table 1 for abbreviations.
Figure 2.
Dual immunofluorescent localization of PhAL (green) and the noradrenergic synthetic enzyme, DbH (red). A: Individual NST neurons concentrating PhAL (green) within the iontophoretic tracer injection site (see Figure 1). A subset of these PhAL-positive neurons are DbH-positive (arrows point out 3 examples). B: PhAL-labeled fibers within the KF subregion of the lateral parabrachial nucleus. C: PhAL-labeled fibers within the hypothalamic ARH. D: PhAL-labeled fibers within the PVN. E: PhAL-labeled fibers within the BST. Note that each photomicrograph depicts PhAL and DbH immunofluorescent labeling photographed at only one focal plane through the section. See Table 1 for abbreviations.
The large majority of NST neurons that project to the hypothalamus and limbic forebrain are NA neurons of the overlapping A2/C2 cell groups (Sawchenko and Swanson, 1981; Sawchenko and Swanson, 1982a; Sawchenko and Swanson, 1982b), with remaining projection neurons primarily comprising smaller and separate populations of HSD2- and GLP-1-positive neurons (Geerling et al., 2006b; Larsen et al., 1997). NA projections from the caudal NST to higher brain regions are probably mostly glutamatergic, based on extensive colocalization of tyrosine hydroxylase (the rate-limiting enzyme for catecholamine synthesis) and DNPI, the rat homolog of VGLUT2 (Stornetta et al., 2002). Despite a long scientific history supporting the involvement of central NA signaling in the central control of food intake and energy expenditure (Leibowitz et al., 1988; Ritter et al., 1975), it still is not clear whether or how NA inputs to the hypothalamus are involved in day-to-day regulation of energy balance. Conversely, there is ample evidence that NA inputs are invoved in hormonal and behavioral arousal responses to visceral stimuli. Medullary NA inputs to the hypothalamus provide critical control over the activity of stress-responsive corticotropin releasing hormone (CRH)-containing neurons within the PVH, at the apex of the HPA axis (Al-Damluji, 1988; Alonso et al., 1986; Banihashemi and Rinaman, 2006; Bienkowski and Rinaman, 2008; Gaillet et al., 1991; Kiss and Aguilera, 1992; Liposits et al., 1986; Rinaman, 2007). NA terminals also synapse directly onto thyrotropin releasing-hormone-positive neurons within the PVH (Füzesi et al., 2009), implicating NA pathways from the NST in metabolic responses to visceral stimuli. The results of phenotypically-specific lesioning experiments have demonstrated that NA inputs to the PVH are critical for the ability of systemic cholecystokinin-8 (CCK), lipopolysaccharide, lithium chloride, or yohimbine to activate Fos expression in PVH neurons, including CRH-positive neurons (Banihashemi and Rinaman, 2006; Bienkowski and Rinaman, 2008; Rinaman, 2003b; Rinaman and Dzmura, 2007). Interestingly, however, NA inputs to the PVH are unnecessary for the ability of CCK to inhibit food intake (Ritter et al., 2001). Indeed, the entire forebrain appears to be unnecessary for CCK-induced hypophagia (Grill and Smith, 1988). Although glucoprivic feeding induced by systemic 2-deoxyglucose is abolished in rats after bilateral destruction of NA inputs to the PVH (Ritter et al., 2001), it is unclear whether the same ascending pathways are important for the control of food intake under non-stressful, physiological conditions. Instead, it seems that ascending NA projections from the caudal NST may be recruited primarily during situations of real or perceived homeostatic challenge. As a case in point, experimental evidence supports the view that central prolactin releasing peptide (PrRP) signaling is involved in stress-related hypophagia (Lawrence et al., 2000; Lawrence et al., 2002; Lawrence et al., 2004), and PrRP is co-expressed by a subset of NA neurons within the NST that project to hypothalamic and limbic forebrain targets, including the PVH, paraventricular thalamic nucleus, DMH, medial preoptic area, peri-VMH, and BST (Renner et al., 2010; Yano et al., 2001).
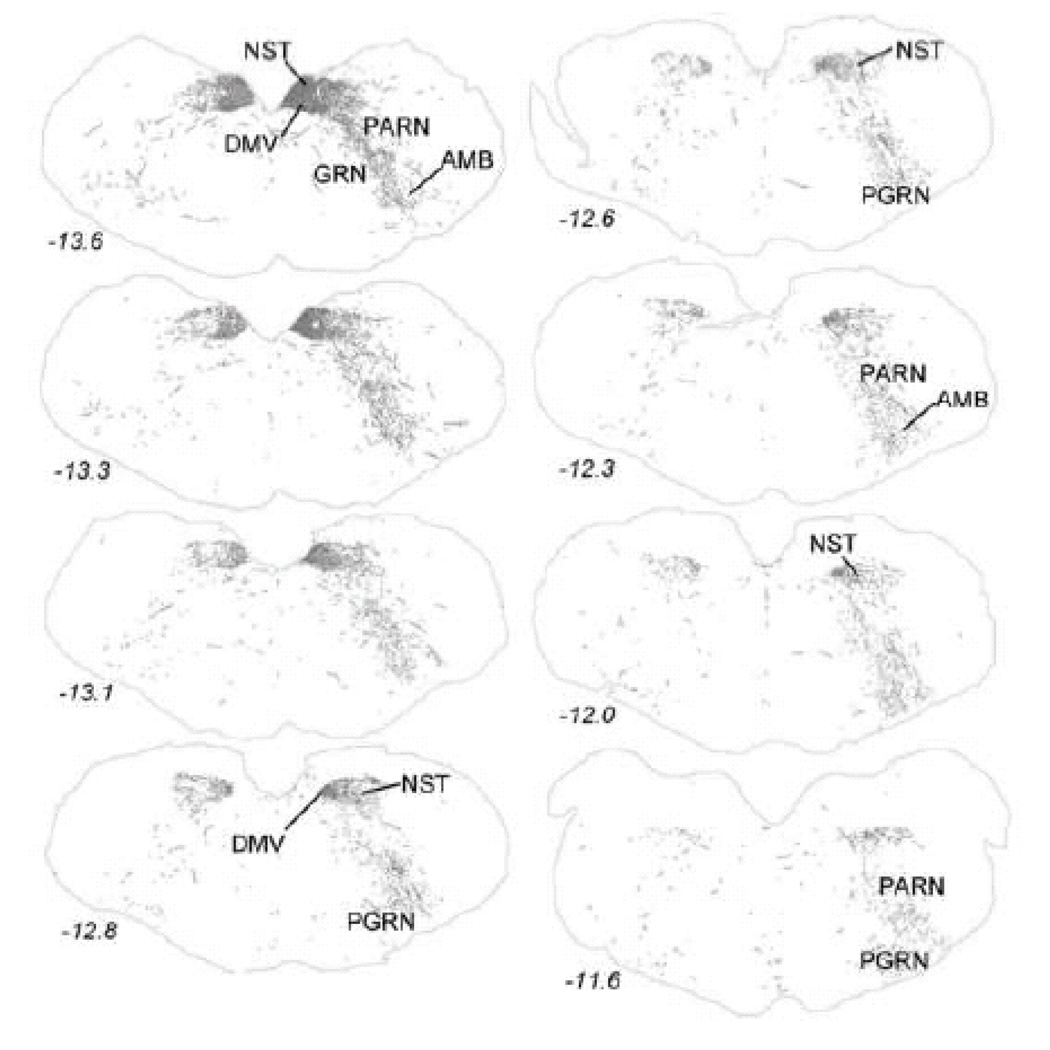
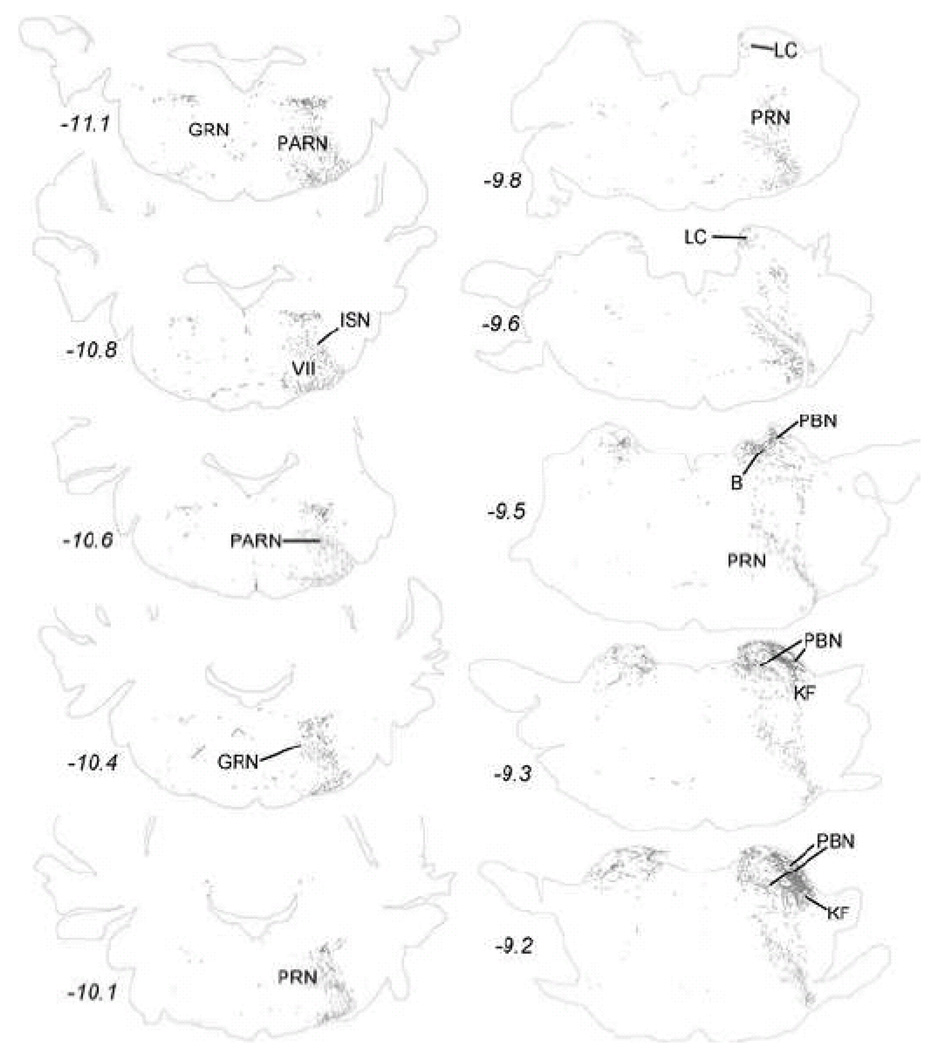
The distribution of PhAL-positive fibers reveals that neurons within the caudal visceral NST project both contralaterally and ipsilaterally, although ipsilateral projections are more prominent (Figs. 3–10). Table 2 lists most of the brain regions that contained PhAL-positive fibers in this experimental case. The reader also is referred to similar anterograde tracing results reported earlier in adult rats (Horst et al., 1989; Horst and Streefland, 1994). Within the medulla, axons arising from neurons within the caudal visceral NST densely occupy the rostral gustatory NST (Fig. 3). Labeled axons also pass through the dorsal- and ventrolateral reticular formation (Figs. 3–4) while generally avoiding more medial regions of the medulla and pons. Within the pons, PhAL-positive fibers occupy the locus coeruleus (LC) and subjacent Barrington’s nucleus (B; Fig. 4). Caudal visceral NST inputs to the medial and lateral parabrachial nuclei (PBN), including the Kölliker-Fuse (KF) subnucleus, are especially dense (Fig. 2B, Fig. 4–Fig. 5) (for more detail, see (Karimnamazi et al., 2002)). The PBN has at least 12 distinct subnuclei, some of which project to central targets that do not receive direct input from the NST (Fulwiler and Saper, 1984; Herbert et al., 1990; Moga et al., 1990; Saper and Loewy, 1980). For example, NST inputs to the internal lateral PBN, which provides a diffuse input to the intralaminar thalamic nuclei, may be involved in arousal responses to gastrointestinal and other visceral stimuli, while NST inputs to the external medial PBN may contribute to conscious appreciation of visceral sensation via thalamic relays to visceral cortex.
Figure 3.
Anterogradely transported PhAL from the caudal DVC (see Figures 1 and 2A for injection site) to more rostral regions of the medulla. The approximate rostro-caudal level of each section (relative to bregma, in mm) is indicated, based on a standard rat brain atlas (Swanson, 2004). Upper left = caudal, lower right = rostral. See Table 1 for abbreviations.
Figure 10.
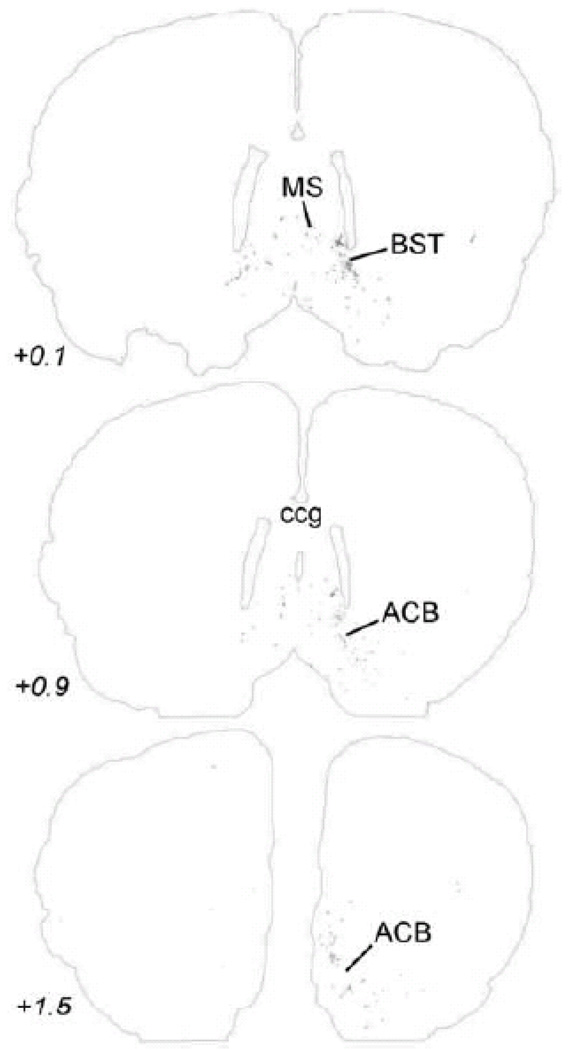
Anterogradely transported PhAL from the caudal DVC (see Figures 1 and 2A for injection site) to more rostral regions of the bed nucleus of the stria terminalis and nucleus accumbens. The approximate rostro-caudal level of each section (relative to bregma, in mm) is indicated, based on a standard rat brain atlas (Swanson, 2004). Top = caudal, bottom = rostral. See Table 1 for abbreviations.
Table 2.
Central targets of axonal projections from the caudal visceral NST identified in adult rats using three different approaches. See Table 1 for abbreviations. +, labeling present; ++, labeling moderate; +++, labeling dense.
|
CNS Division NUCLEUS |
PhAL- labeled axons from caudal medial NST |
GLP-1- immunopositive fibers |
H129 transported from stomach wall |
|
|---|---|---|---|---|
| Medulla | ||||
| DMV | +++ | + | +++ | |
| cNST | +++ | + | +++ | |
| rNST | +++ | + | + | |
| PAT | + | + | ++ | |
| RO | + | ++ | + | |
| RPA | + | + | + | |
| MDRN | ++ | + | + | |
| PARN | ++ | + | ++ | |
| SPV | + | + | + | |
| AMB | ++ | ++ | ++ | |
| PGRN | ++ | + | + | |
| ISN | + | + | + | |
| GRN | ++ | + | + | |
| PRN | ++ | + | + | |
| VII | + | + | + | |
| Pons | ||||
| LC | + | + | + | |
| subLC | + | ++ | ++ | |
| B | ++ | ++ | + | |
| PRN | ++ | + | + | |
| PBN | +++ | +++ | ++ | |
| KF | +++ | +++ | ++ | |
| Midbrain | ||||
| PAG | ++ | + | + | |
| MRN | + | + | + | |
| DR | + | + | + | |
| VTA | + | + | + | |
| Diencephalon | ||||
| thalamus | ||||
| IMD | + | + | ||
| CM | + | + | ||
| MDm | ++ | + | ||
| PVT | ++ | +++ | + | |
| hypothalamus | ||||
| PH | + | + | + | |
| LHA | ++ | + | ++ | |
| PVp | + | ++ | + | |
| TU | + | + | + | |
| TM | + | ++ | + | |
| DMH | +++ | +++ | + | |
| ARH | + | + | + | |
| PVH | +++ | +++ | ++ | |
| SO | + | ++ | ||
| ZI | + | + | ||
| RC | ++ | ++ | ||
| PVpo | + | ++ | + | |
| MEPO | + | ++ | + | |
| MPO | + | + | + | |
| LPO | ++ | |||
| SFO | ++ | |||
| ME | + | ++ | ||
| OVLT | + | ++ | ||
| Telencephalon | ||||
| subcortical | ||||
| CEA | ++ | + | ++ | |
| SI | ++ | ++ | + | |
| dlBST | ++ | ++ | ++ | |
| vlBST | +++ | +++ | + | |
| MS | + | + | + | |
| LS | + | + | ||
| ACB | + | + | ||
| cortical | ||||
| PERI | + | |||
| AI | + | |||
Figure 4.
Anterogradely transported PhAL from the caudal DVC (see Figures 1 and 2A for injection site) to the pons. The approximate rostro-caudal locations of each section relative to bregma are indicated, based on a standard rat brain atlas (Swanson, 2004). Upper left = most caudal, lower right = most rostral. See Table 1 for abbreviations.
Figure 5.
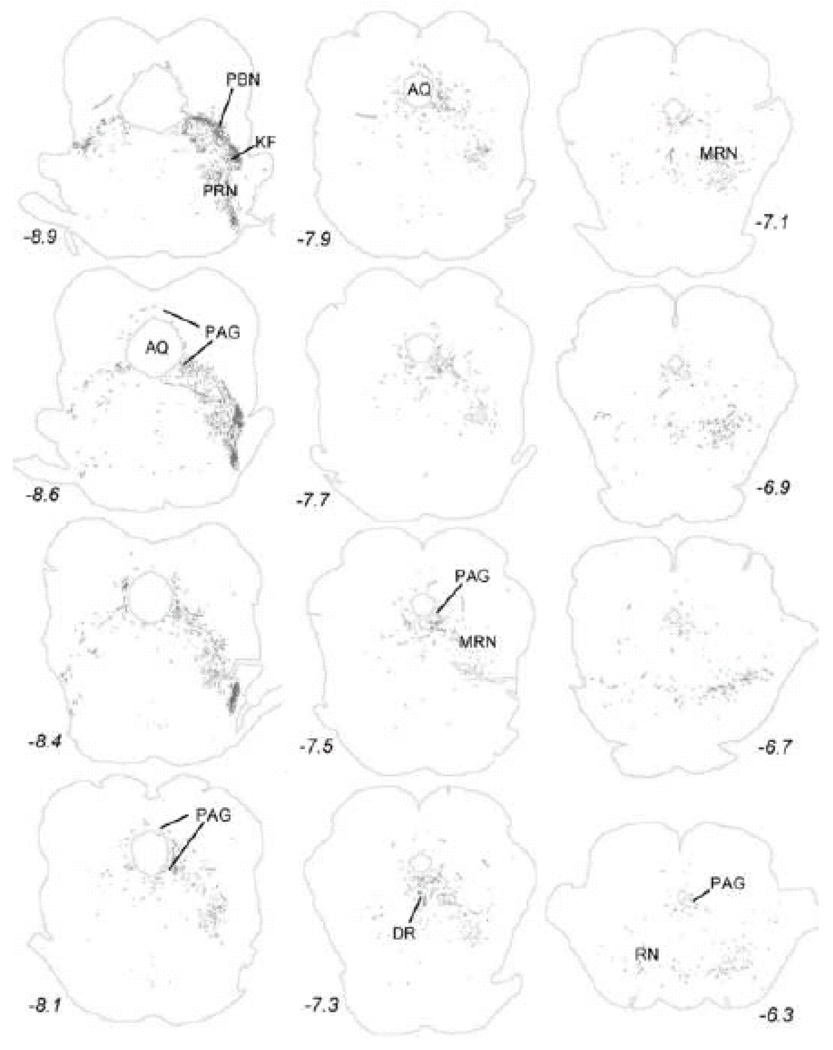
Anterogradely transported PhAL from the caudal DVC (see Figures 1 and 2A for injection site) to more rostral regions of the pons and midbrain. The approximate rostro-caudal level of each section (relative to bregma, in mm) is indicated, based on a standard rat brain atlas (Swanson, 2004). Upper left = caudal, lower right = rostral. See Table 1 for abbreviations.
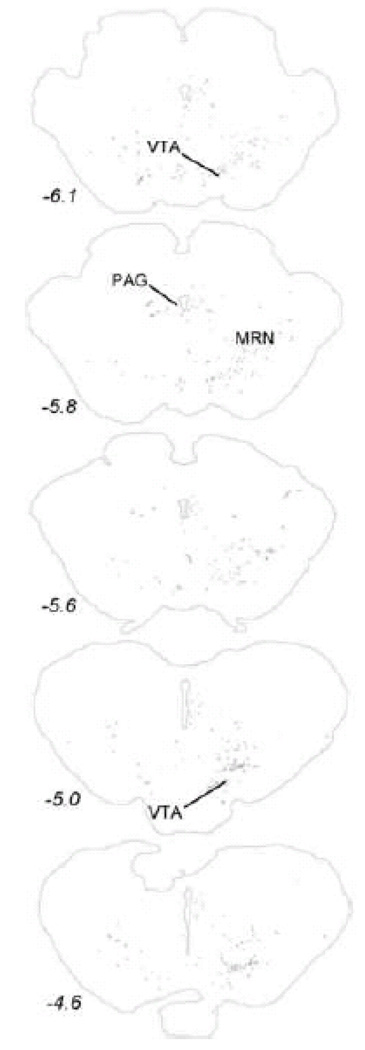
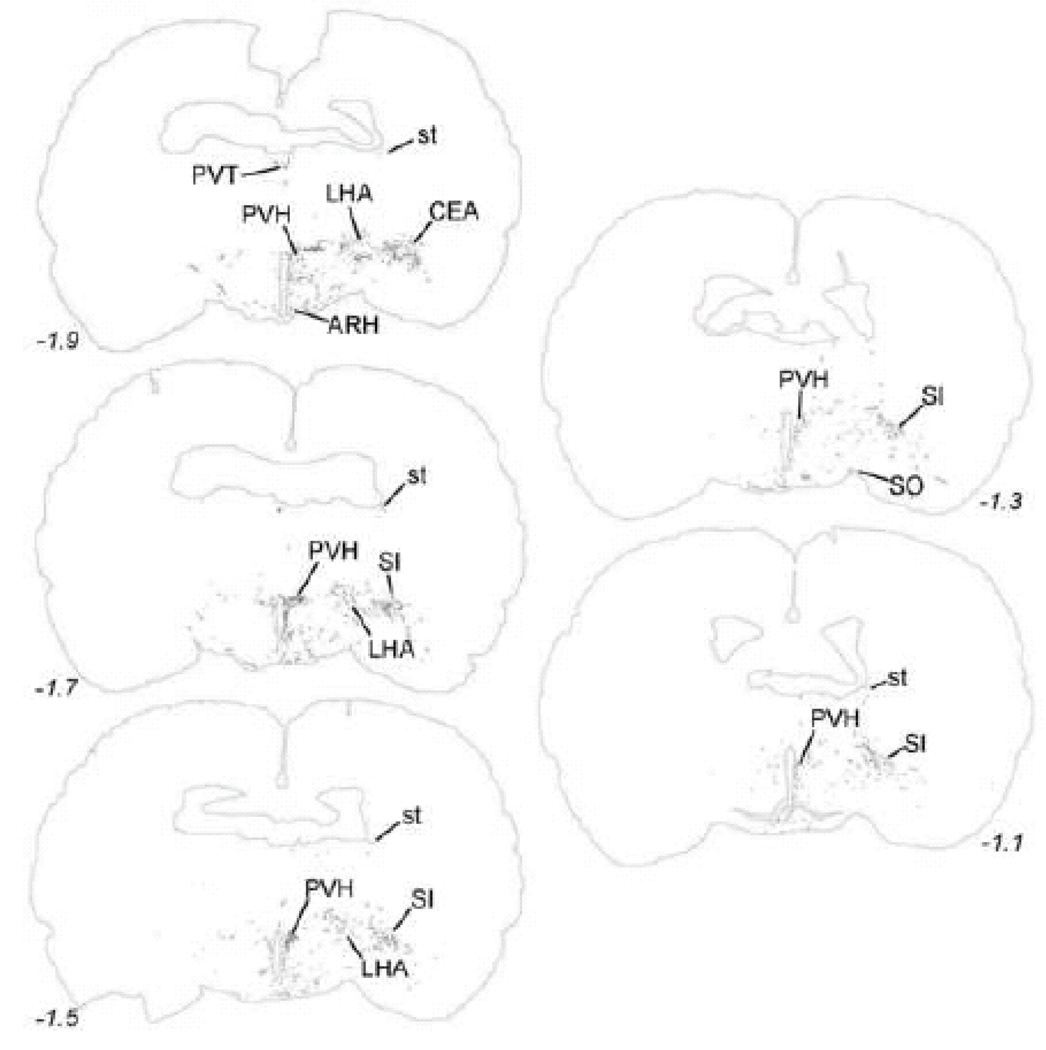
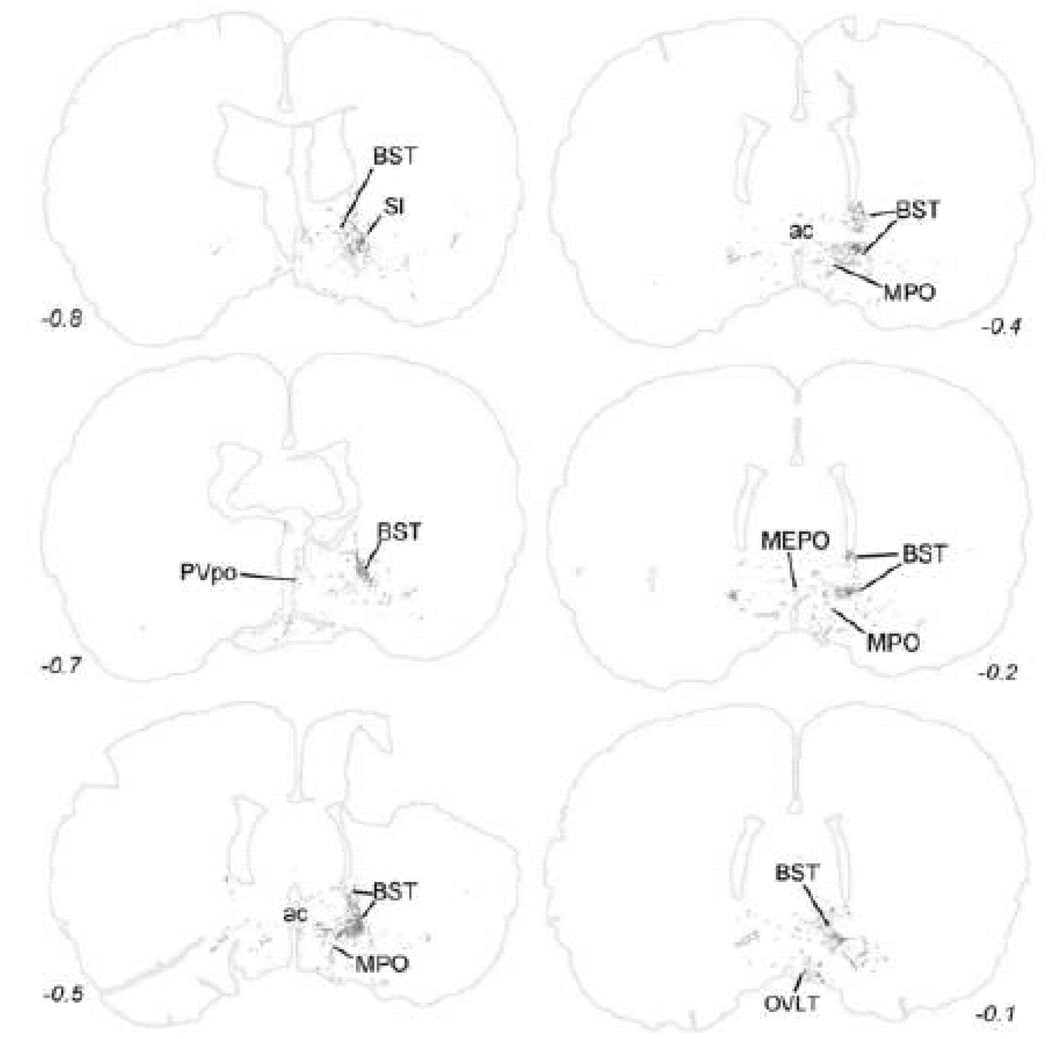
Within the midbrain, PhAL-positive fibers from the caudal visceral NST occupy the periaqueductal gray, particularly its ventral portion (Fig. 5). Labeled fibers also cluster within the serotonin-rich dorsal raphé (Fig. 5), and overlap the dopamine-rich ventral tegmental area (Fig. 6). The density of PhAL-positive fibers increases within the diencephalon (Figs. 7–8). Midline thalamic targets most notably include the paraventricular nucleus of thalamus. Hypothalamic targets include the lateral hypothalamic area (LHA), posterior hypothalamus, posterior periventricular nucleus, tuberomammillary nuclei (both dorsal and ventral), tuberal nucleus, dorsomedial nucleus, arcuate nucleus (ARH; Fig. 2C), paraventricular nucleus of the hypothalamus (PVH; including both magnocellular and parvocellular subregions; Fig. 2D), and supraoptic nucleus (SO). Interestingly, PhAL-labeled fibers tend to avoid the ventromedial hypothalamic nucleus (VMH), which has classically demonstrated roles in the central control of feeding and metabolism (King, 2006; Plata-Salaman, 1998). However, similar to neurons within the PVH and LHA (Jeanningros, 1984; Jin et al., 1993; Ueta et al., 1991), VMH neurons are activated by gastric distension via a vagal sensory pathway (Sun et al., 2006). NST inputs to VMH neurons may arrive on their distal dendrites which surround the VMH nucleus, where PhAL- (and GLP-1-)-positive fibers are present (see Fig. 12). More laterally within the subcortical telencephalon, PhAL-positive fibers cluster within the central nucleus of the amygdala and substantia innominata. More rostrally and dorsally, a small number of labeled fibers are present within the stria terminalis. Labeled fibers and varicosities also are observed within the dorsolateral horizontal limb of the diagonal band of Broca. Fibers and varicosities terminate densely within both the dorsal and ventral bed nucleus of stria terminalis (BST; Fig. 2E), and less densely within the medial and median preoptic nuclei, the organum vasculosum of the lamina terminalis, the medial septum, and the nucleus accumbens (ACB) (Figs. 9–10). The direct inputs from NST to ACB (ventral striatum) are of particular interest, given the prominent role of this limbic brain region in appetitive motivation (Kelley, 2004; Zheng et al., 2007).
Figure 6.
Anterogradely transported PhAL from the caudal DVC (see Figures 1 and 2A for injection site) to more rostral regions of the midbrain and caudal hypothalamus. The approximate rostro-caudal level of each section (relative to bregma, in mm) is indicated, based on a standard rat brain atlas (Swanson, 2004). Top = caudal, bottom = rostral. See Table 1 for abbreviations.
Figure 7.
Anterogradely transported PhAL from the caudal DVC (see Figures 1 and 2A for injection site) to the hypothalamus and amygdala. The approximate rostro-caudal level of each section (relative to bregma) is indicated, based on a standard rat brain atlas (Swanson, 2004). Upper left = caudal, lower right = rostral. See Table 1 for abbreviations.
Figure 8.
Anterogradely transported PhAL from the caudal DVC (see Figures 1 and 2A for injection site) to more rostral regions of the hypothalamus and amygdala. The approximate rostro-caudal level of each section (relative to bregma, in mm) is indicated, based on a standard rat brain atlas (Swanson, 2004). Upper left = caudal, lower right = rostral. See Table 1 for abbreviations.
Figure 12.
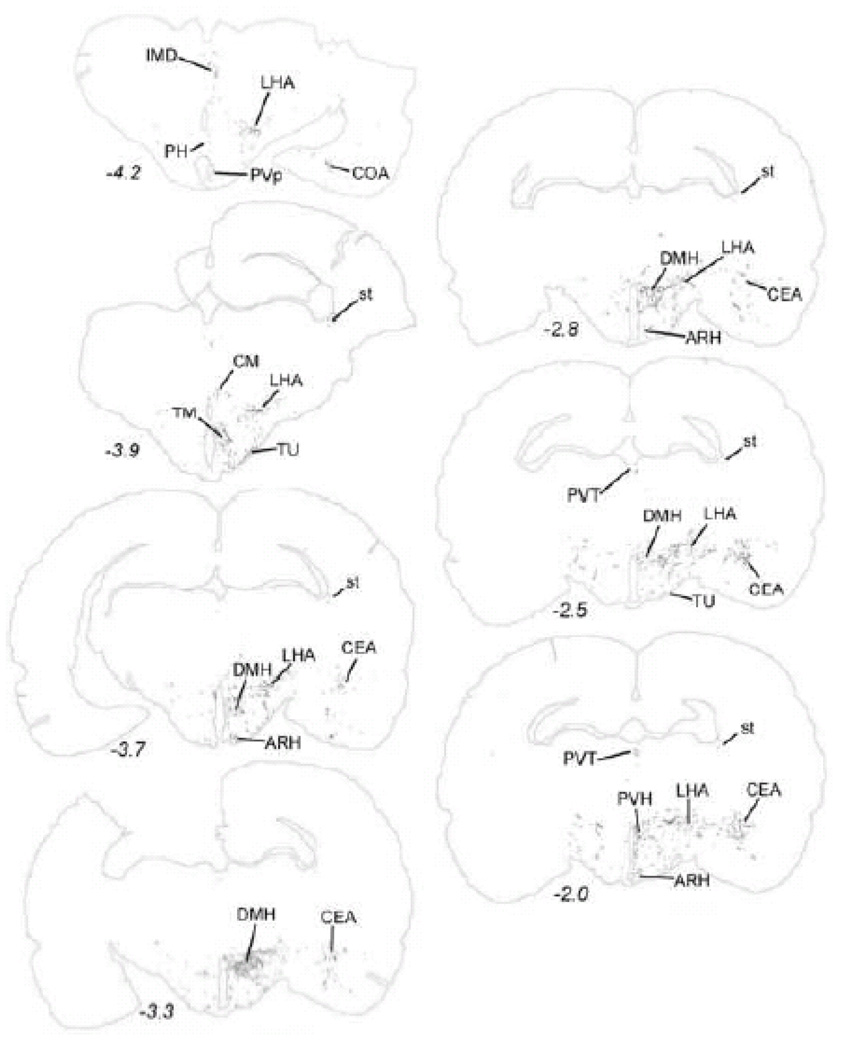
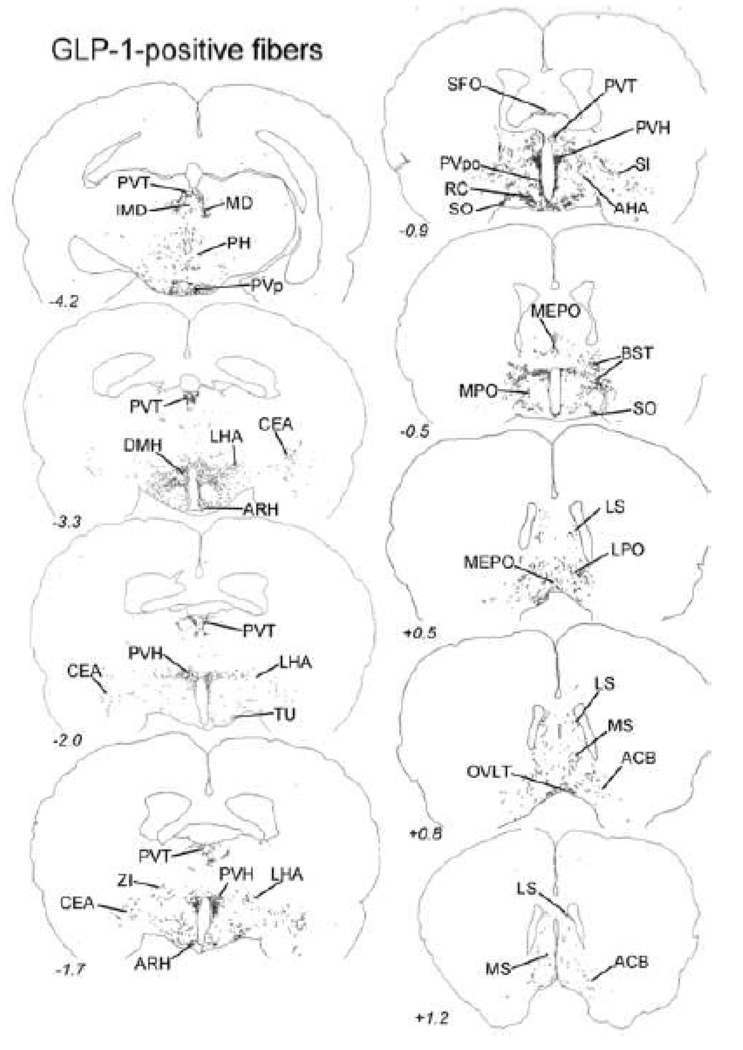
Distribution of GLP-1-immunopositive fibers within the diencephalon and limbic forebrain. The approximate rostro-caudal level of each section (relative to bregma, in mm) is indicated, based on a standard rat brain atlas (Swanson, 2004). Top = caudal, bottom = rostral. See Table 1 for abbreviations.
Figure 9.
Anterogradely transported PhAL from the caudal DVC (see Figures 1 and 2A for injection site) to more rostral regions of the amygdala, substantia innominata, and bed nucleus of the stria terminalis. The approximate rostro-caudal level of each section (relative to bregma, in mm) is indicated. Upper left = caudal, lower right = rostral. See Table 1 for abbreviations.
No PhAL-positive fibers were observed within medial or lateral visceral cortex in this experimental case or in other similar PhAL tracing experiments from our laboratory, supporting previous reports that visceral sensory signals are relayed to the visceral cortices via the thalamus and other brain regions, including the LC and LHA. Wilder Penfield’s cortical stimulation studies in humans revealed subjective sensations of oropharyngeal, esophageal, and gastrointestinal sensation organized in a topographic sensory homunculus within Brodmann’s area 13, running ventrally from the tongue sensory area into the operculum and insular cortex (Penfield and Faulk, 1955). Cechetto and Saper reported a similar topographic pattern of visceral sensory responses in rats, involving regions of insular cortex that corresponded to viscerotopically organized inputs from the thalamus (Cechetto and Saper, 1987). In addition, the LC, LHA, and midline thalamic nuclei each have direct but diffuse cortical projections that likely participate in arousal and overall cortical “tone,” and each of these regions receives visceral sensory input relayed directly from the caudal visceral NST in rats (Figs. 4, 7–8). In addition to receiving direct inputs from the caudal medial NST, the basal forebrain cholinergic corticopetal system also receives inputs relayed via the nucleus paragigantocellularis and LC (Bernston et al., 1998; Berntson et al., 2003). This cholinergic system is implicated in cortical arousal, attention, and anxiety, and is considered a widespread regulatory modulator that serves to enhance or amplify cognitive processing.
3.0 Ascending projections from the caudal visceral NST: immunocytochemical localization of GLP-1
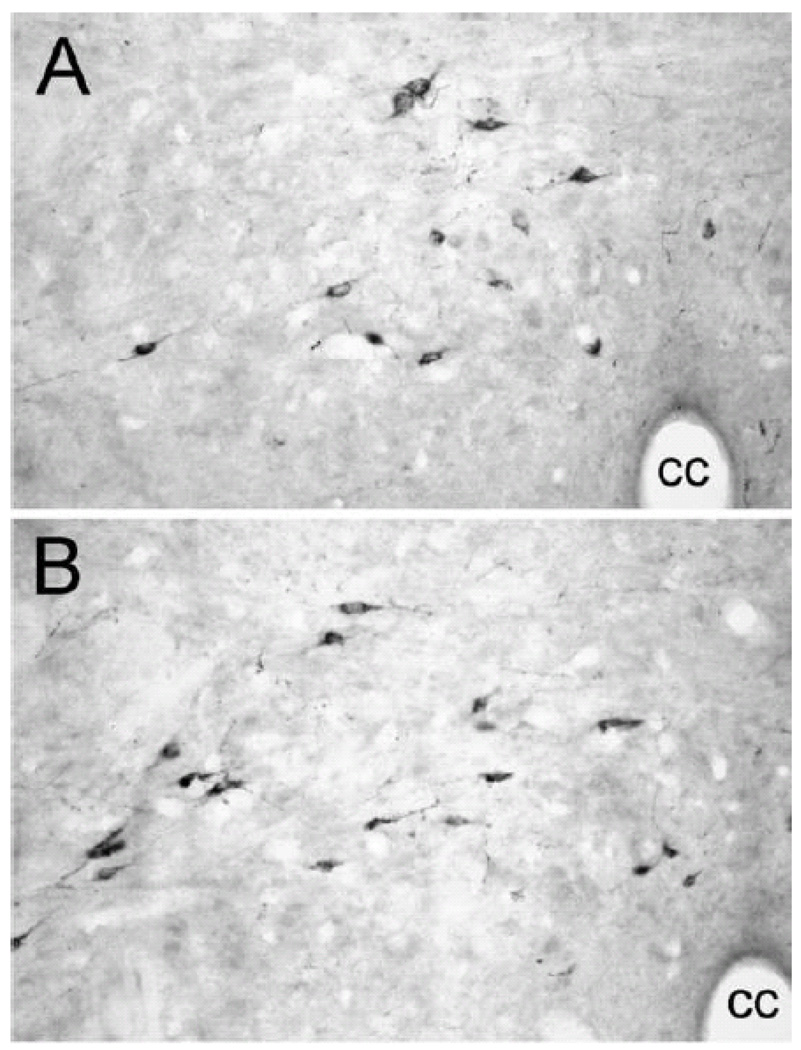
GLP-1 is expressed by a relatively small number of neurons located within the caudal visceral NST (Fig. 11) and adjacent dorsal reticular formation (Jin et al., 1988; Larsen et al., 1997; Merchenthaler et al., 1999). GLP-1-positive neurons are not adrenergic, but co-express β inhibin 1, somatostatin, and met-enkephalin (Sawchenko et al., 1990). Their ascending axonal projections largely parallel NA projections from the caudal NST. Indeed, all brain regions that contain GLP-1-positive fibers and terminals in adult rats also contain DbH-immunopositive fibers and terminals; however, the converse is not always true (personal observation), as the caudal NST is not the only source of NA fibers and terminals within the brain.
Figure 11.
GLP-1 immunoperoxidase labeling of neuron cell bodies within the caudal medullary DVC (A and B, approximately 15.0 and 14.0 mm caudal to bregma, respectively). See Table 1 for abbreviations.
Central GLP-1 signaling pathways are implicated in the central control of food intake, glucose homeostasis, and HPA axis and autonomic responses to stress (Holst, 2007; Imeryuz et al., 1997; Kinzig et al., 2003; Nakade et al., 2007; Rinaman, 1999a; Sandoval et al., 2008; Tang-Christensen et al., 2001; Turton et al., 1996). GLP-1-positive terminals robustly innervate corticotropin releasing hormone (CRH)-positive neurons within the hypothalamic PVH (Sarkar et al., 2003), the apex of the HPA stress axis, as well as oxytocin (but not vasopressin) -positive neurons within the PVH and supraoptic nucleus (Rinaman and Rothe, 2002). GLP-1 modulates the activity of hypocretin/orexin-positive (but not melanin-concentrating hormone-positive) neurons within the LHA (Acuna-Goycolea and Pol, 2004), implicating GLP-1 signaling in behavioral arousal and reward-based feeding (Borgland et al., 2009; Cason et al., 2010 (in press)). In addition to their prominence within the PVH and LHA, GLP-1 receptors also are located within the ARH, along with GLP-1-positive fibers (see Fig. 12) (Alvarez et al., 1996; Merchenthaler et al., 1999).
GLP-1-positive NST neurons that project to the hypothalamus are activated by interoceptive stimuli that stimulate the HPA stress axis and also inhibit food intake in rats (Rinaman, 1999b), and central antagonism of GLP-1 receptors is sufficient to attenuate the hypophagic effect of lithium chloride (Rinaman, 1999a; Seeley et al., 2000). It remains unclear whether GLP-1 signaling within the forebrain, brainstem, or both regions is important for the control of food intake under natural conditions. Food intake is inhibited in rats after lateral or third ventricular administration of synthetic GLP-1 or agonist, or after microinjection of GLP-1 or agonist into the PVH, LHA, DMH, or VMH (Donahey et al., 1998; Schick et al., 2003). While these observations do not constitute evidence that endogenous GLP-1 plays a role in day-to-day body energy homeostasis, the available evidence does support the view that GLP-1 signaling pathways participate in stress-related hypophagia and activation of the HPA axis.
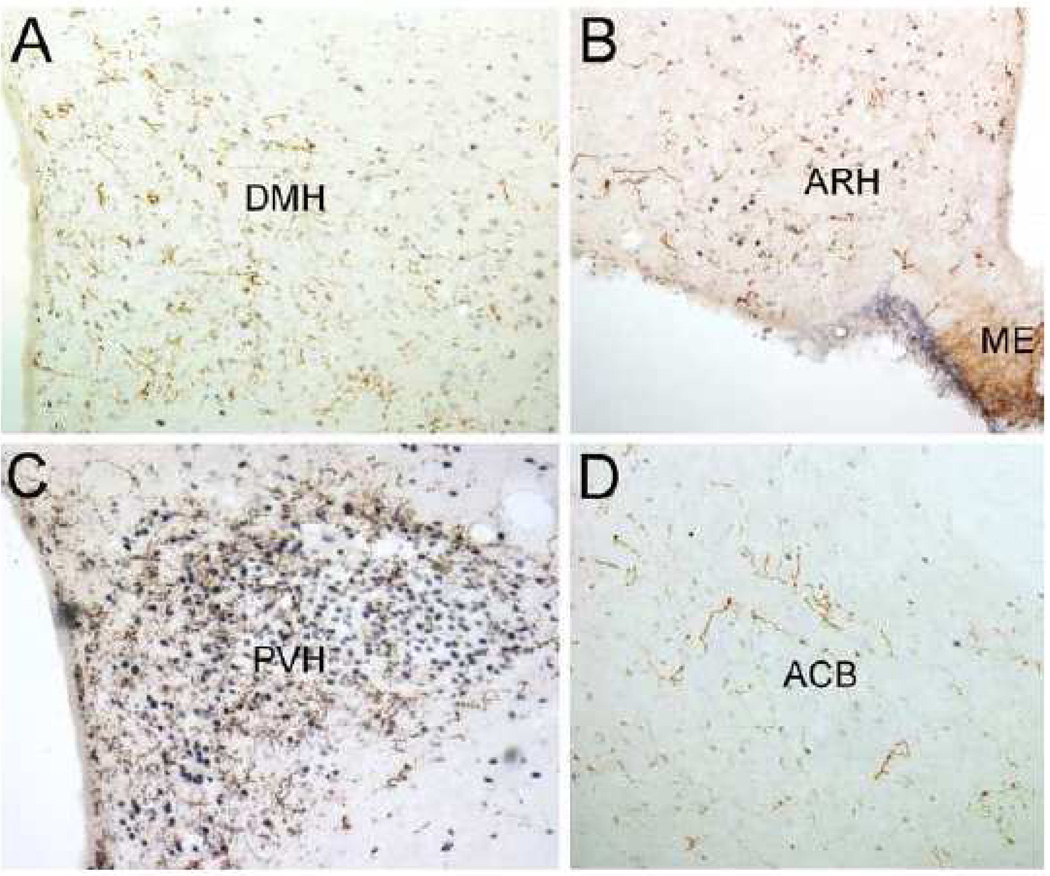
As seen in Table 2, every brain region that contained labeled fibers after PhAL injection into the caudal visceral NST also contained GLP-1-positive fibers. The distribution of GLP-1-positive fibers within the diencephalon and telencephalon is illustrated in Figure 12. As previously reported, GLP-1 positive fibers are relatively dense within the PVH (Fig. 13C), ARH (Fig. 13B), SO, DMH (Fig. 13A), PVT, and dorsal and ventral BST (Larsen et al., 1997; Merchenthaler et al., 1999; Rinaman, 1999b; Sarkar et al., 2003) (Fig. 12). GLP-1-positive fibers also occupy the ventral striatum (ACB; Fig. 13D).
Figure 13.
GLP-1-positive fibers (brown) within the hypothalamic DMH (A), ARH (B), and PVH (C), and within the ventral striatum (D). Blue-black nuclear Fos immunolabeling is the result of lithium chloride (0.15M, 1% BW, i.p.) administration 90 min before perfusion fixation. See Table 1 for abbreviations.
4.0 Ascending gastric sensory pathways: viral transneuronal anterograde tracing
Standard anterograde and retrograde tracing techniques are useful tools with which to survey the inputs and outputs of various brain regions. However, light microscopic tracing using PhAL or other standard tracers cannot by itself demonstrate synaptic connections between labeled projection neurons and their targets. Further, PhAL tracing experiments like the one illustrated in Figures 1–10 cannot discriminate projections that carry gastrointestinal-related signals from those that carry cardiovascular or other visceral sensory information. In an attempt to isolate the postsynaptic targets of gastric-specific ascending sensory projections, we turned to a novel transneuronal anterograde tracer, the H129 strain of herpes simplex virus-1 (Dix et al., 1983). H129 undergoes anterograde transneuronal transport in cebus monkeys after inoculation of primary motor cortex (Kelly and Strick, 2003; Zemanick et al., 1991) and in mice after inoculation of tooth pulp (Barnett et al., 1995) or the vitreous body of the eye (Sun et al., 1996). We found that H129 also has utility as an anterograde transneuronal viral tracer in rats, effectively revealing CNS regions that receive relayed gastric viscerosensory input (Rinaman and Schwartz, 2004). After H129 inoculation of the ventral stomach wall in adult male Sprague-Dawley rats, H129-immunopositive cells and fibers were present within the medullary DVC (including the caudal medial NST and medial DMV), thoracic spinal dorsal root entry zone, and thoracic spinal laminas I and II. In rats with longer post-inoculation survival times, additional spinal, brainstem, diencephalic, and telencephalic regions contained H129-positive cells (summarized in Table 2). Interestingly, some of the brain regions that contained transneuronal H129 labeling do not appear to receive direct projections from the caudal visceral NST, as evidenced by the absence of both PhAL anterograde labeling and GLP-1 immunopositive fibers in those regions (Table 2). For example, H129 labeling within visceral cortices presumably arose from the thalamus, LHA, and/or other brain region that contained H129-positive neurons and projects to visceral cortex. Further, transneuronal H129 transport from the stomach wall included ascending spinal visceral pathways in addition to pathways arising from the DVC, and so some H129 labeling is likely of spinal origin.
It seems curious that relatively little transneuronal H129 infection was observed within the ventrolateral BST, a forebrain region that receives particularly dense NA (Banihashemi and Rinaman, 2006; Myers et al., 2005) and GLP-1 inputs from the caudal NST (Fig. 12). H129 labeling also was not obvious within the ACB, another direct target of NA and GLP-1-positive projections from the caudal NST. Speculatively, the absence of H129 labeling in these regions may be due to a relatively low density of synaptic contacts. Transneuronal H129 infection depends on the presence of synaptic contacts for viral transport to occur from axon terminal to postsynaptic target; therefore, H129 transneuronal tracing cannot reveal forebrain neurons that are subject to visceral sensory modulation through local “paracrine” release of neurotransmitter from axon varicosities. Indeed, fewer than 20% of NA axon terminals within the ventrolateral BST form synaptic contacts (Phelix et al., 1992), and a hallmark of NA terminals within the medial PVH, ventrolateral BST, and other forebrain regions that receive dense inputs from the A2 region of caudal NST is the predominance of axonal varicosities that do not form synaptic contacts (Balcita-Pedicino and Rinaman, 2007). An absence of synaptic contacts, however, does not indicate an absence of neural signal transmission.
5.0 Conclusion
The CNS is privy to a plethora of peripheral neural and humoral signals that reflect current digestive status and energy availability, energy needs, and energy stores. Body energy homeostasis depends on the organism’s ability to integrate and respond adaptively to these signals by modulating current and future energy intake and expenditure. Clearly, descending projections from the hypothalamus and limbic forebrain to the DVC and other caudal brainstem regions are critical in the central control of digestion and feeding. The robust anatomical representation of reciprocal projections from the caudal visceral NST to feeding-related regions of the hypothalamus and limbic forebrain supports the view that these projections also are important in the central control of food intake. However, the available evidence indicates that signals carried to the forebrain may be important primarily for arousal and coordination of physiological and behavioral (i.e., hypophagic) responses to homeostatic challenge, rather than for modulating feeding and/or energy expenditure on a day-to-day basis. Future studies should challenge this assessment to determine whether or not it is accurate, and to further reveal the physiological importance of visceral sensory signaling from gut to brain.
Table 1. Abbreviations used in text and figures.
Anatomical terminology after Swanson (Swanson, 2004).
| 3, third ventricle |
| ac, anterior commissure |
| ACB, nucleus accumbens |
| AHA, anterior hypothalamic area |
| AI, agranular insular cortex |
| AMB, nucleus ambiguus |
| AP, area postrema |
| ARH, arcuate nucleus of hypothalamus |
| B, Barrington’s nucleus |
| BST, bed nucleus of stria terminalis |
| cc, central canal |
| ccg, corpus callosum genu |
| CEA, central nucleus of amygdala |
| CM, central medial nucleus of thalamus |
| CNS, central nervous system |
| COA, cortical amygdalar nucleus |
| DbH, dopamine β hydroxylase |
| DMH, dorsomedial nucleus of hypothalamus |
| DMV, dorsal motor nucleus of the vagus |
| DR, dorsal raphé nucleus |
| DVC, dorsal vagal complex (i.e., AP, NST and DMV) |
| GLP-1, glucagon-like peptide 1 |
| GRN, gigantocellular reticular nucleus |
| HPA, hypothalamic-pituitary-adrenal |
| HSD2, 11-β-hydroxysteroid dehydrogenase type 2 |
| HSV-1, herpes simplex virus type 1 |
| IMD, intermediodorsal nucleus of thalamus |
| ISN, inferior salivatory nucleus |
| KF, Kölliker-Fuse subnucleus of PBN |
| LC, locus coeruleus |
| LHA, lateral hypothalamic area |
| LPO, lateral preoptic nucleus of hypothalamus |
| LS, lateral septum |
| MD, mediodorsal nucleus of thalamus |
| MDRN, medullary reticular nucleus |
| ME, median eminence |
| MEPO, medial preoptic nucleus of hypothalamus |
| MnPO, median preoptic nucleus of hypothalamus |
| MPO, medial preoptic area |
| MRN, midbrain reticular nucleus |
| MS, medial septum |
| NA, noradrenergic (i.e., DbH-positive) |
| NST, nucleus of the solitary tract |
| OVLT, organum vasculosum of the lamina terminalis |
| OT, oxytocin |
| PAG, periaqueductal gray |
| PARN, parvicellular reticular nucleus |
| PAT, paratrigeminal nucleus |
| PERI, perirhinal cortex |
| PGRN, paragigantocellular reticular nucleus |
| PH, posterior hypothalamus |
| PhAL, phaseolus vulgaris leucoagglutinin |
| PRN, pontine reticular nucleus |
| PrRP, prolactin-releasing peptide |
| PBN, parabrachial nucleus |
| PVH, paraventricular nucleus of hypothalamus |
| PVp, posterior periventricular nucleus of hypothalmus |
| PVpo, preoptic periventricular nucleus of hypothalamus |
| PVT, paraventricular nucleus of thalamus |
| RC, retrochiasmatic nucleus of hypothalamus |
| RN, red nucleus |
| SI, substantia innominata |
| SFO, subfornical organ |
| SO, supraoptic nucleus of hypothalamus |
| SPVC, spinal nucleus of trigeminal |
| st, stria terminalis |
| subLC, sucoeruleus |
| TM, tuberomammilary nucleus of hypothalamus |
| TU, tuberal nucleus of hypothalamus |
| VLM, ventrolateral medulla |
| VII, facial motor nucleus |
| VTA, ventral tegmental area |
| XII, hypoglossal motor nucleus |
| ZI, zona incerta |
Acknowledgements
Research supported by the National Institutes of Health (MH59911). The expert technical assistance of Victoria Maldovan Dzmura, Hana Bakalli and Vanessa Cole is gratefully acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The rostral gustatory NST gives rise to a largely distinct and more limited set of efferent projections (Norgren et al., 2003).
References
- Acuna-Goycolea C, van den Pol A. Glucagon-like peptide 1 excites hypocretin/orexin neurons by direct and indirect mechanisms: implications for viscera-mediated arousal. The Journal of Neuroscience. 2004;24:8141–8152. doi: 10.1523/JNEUROSCI.1607-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Damluji S. Adrenergic mechanisms in the control of corticotropin secretion. Journal of Endocrinology. 1988;119:5–14. doi: 10.1677/joe.0.1190005. [DOI] [PubMed] [Google Scholar]
- Alonso G, Szafarczyk A, Balmefrezol M, Assenmacher I. Immunocytochemical evidence of stimulatory control by the ventral noradrenergic bundle of parvicellular neurons of the paraventricular nucleus secreting corticotropin-releasing hormone and vasopressin in rats. Brain Research. 1986;397:297–307. doi: 10.1016/0006-8993(86)90631-1. [DOI] [PubMed] [Google Scholar]
- Altschuler SM, Rinaman L, Miselis RR. Viscerotopic representation of the alimentary tract in the dorsal and ventral vagal complex in the rat. In: Ritter S, Ritter RC, Barnes CD, editors. Neuroanatomy and Physiology of Abdominal Vagal Afferents. Vol. Boca Raton: CRC Press; 1992. pp. 21–54. [Google Scholar]
- Altschuler SM, Bao X, Bieger D, Hopkins DA, Miselis RR. Viscerotopic representation of the upper alimentary tract in the rat: sensory ganglia and nuclei of the solitary and spinal trigeminal tracts. Journal of Comparative Neurology. 1989;283:248–268. doi: 10.1002/cne.902830207. [DOI] [PubMed] [Google Scholar]
- Alvarez E, Roncero I, Chowen JA, Thorens B, Blazquez E. Expression of the glucagon-like peptide-1 receptor gene in rat brain. Journal of Neurochemistry. 1996;66:920–927. doi: 10.1046/j.1471-4159.1996.66030920.x. [DOI] [PubMed] [Google Scholar]
- Bailey TW, Hermes SM, Andresen MC, Aicher AA. Cranial visceral afferent pathways through the nucleus of the solitary tract to caudal ventrolateral medulla or paraventricular hypothalamus: target-specific synaptic reliability and convergence patterns. The Journal of Neuroscience. 2006;26:11893–11902. doi: 10.1523/JNEUROSCI.2044-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balcita-Pedicino JJ, Rinaman L. Noradrenergic axon terminals contact gastric pre-autonomic neurons in the paraventricular nucleus of the hypothalamus in rats. Journal of Comparative Neurology. 2007;501:608–618. doi: 10.1002/cne.21267. [DOI] [PubMed] [Google Scholar]
- Banihashemi L, Rinaman L. Noradrenergic inputs to the bed nucleus of the stria teminalis and paraventricular nucleus of the hypothalamus underlie hypothalamic-pituitary-adrenal axis but not hypophagic or conditioned avoidance responses to systemic yohimbine. The Journal of Neuroscience. 2006;26:11442–11453. doi: 10.1523/JNEUROSCI.3561-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett EM, Evans GD, Sun N, Perlman S, Cassell MD. Anterograde tracing of trigeminal afferent pathways from the murine tooth pulp to cortex using herpes simplex virus type I. The Journal of Neuroscience. 1995;15:2972–2984. doi: 10.1523/JNEUROSCI.15-04-02972.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernston GG, Sarter M, Cacioppo JT. Anxiety and cardiovascular reactivity: the basal forebrain cholinergic link. Behavioural Brain Research. 1998;94:225–248. doi: 10.1016/s0166-4328(98)00041-2. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Sarter M, Cacioppo JT. Ascending visceral regulation of cortical affective information processing. European Journal of Neuroscience. 2003;18:2103–2109. doi: 10.1046/j.1460-9568.2003.02967.x. [DOI] [PubMed] [Google Scholar]
- Berthoud H-R. Multiple neural systems controlling food intake and body weight. Neuroscience and Biobehavioral Reviews. 2002;26:393–428. doi: 10.1016/s0149-7634(02)00014-3. [DOI] [PubMed] [Google Scholar]
- Berthoud H-R, Sutton GM, Townsend RL, Patterson LM, Zheng H. Brainstem mechanisms integrating gut-derived satiety signals and descending forebrain information in the control of meal size. Physiology and Behavior. 2006;89:517–524. doi: 10.1016/j.physbeh.2006.08.018. [DOI] [PubMed] [Google Scholar]
- Berthoud H-R. Vagal and hormonal gut-brain communication: from satiation to satisfaction. Neurogastroenterology and Motility. 2008;20:64–72. doi: 10.1111/j.1365-2982.2008.01104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienkowski MS, Rinaman L. Noradrenergic inputs to the paraventricular hypothalamus contribute to hypothalamic-pituitary-adrenal axis and central Fos activation in rats after acute systemic endotoxin exposure. In Neuroscience. 2008;156:1093–1102. doi: 10.1016/j.neuroscience.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgland SL, Chang S-J, Bowers MS, Thompson JL, Vittoz N, Floresco SB, Chou J, Chen BT, Bonci A. Orexin A/hypocretin-1 selectively promotes motivation for positive reinforcers. The Journal of Neuroscience. 2009;29:11215–11225. doi: 10.1523/JNEUROSCI.6096-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broberger C. Brain regulation of food intake and appetite: molecules and networks. Journal of Internal Medicine. 2005;258:301–327. doi: 10.1111/j.1365-2796.2005.01553.x. [DOI] [PubMed] [Google Scholar]
- Cason AM, Smith RJ, Tahsili-Fahadan P, Moorman DE, Sartor GC, Astron-Jones G. Role of orexin/hypocretin in reward-seeking and addiction: implications for obesity. Physiology and Behavior. 2010 doi: 10.1016/j.physbeh.2010.03.009. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cechetto DF, Saper CB. Evidence for a viscerotopic sensory representation in the cortex and thalamus in the rat. The Journal of Comparative Neurology. 1987;262:27–45. doi: 10.1002/cne.902620104. [DOI] [PubMed] [Google Scholar]
- Coll A, Farooqi I, O'Rahilly S. The hormonal control of food intake. Cell. 2007;129:251–262. doi: 10.1016/j.cell.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham ET, Jr, Miselis RR, Sawchenko PE. The relationship of efferent projections from the area postema to vagal motor and brain stem catecholamine-containing cell groups: an axonal transport and immunohistochemical study in the rat. Neuroscience. 1994;58:635–648. doi: 10.1016/0306-4522(94)90087-6. [DOI] [PubMed] [Google Scholar]
- Dix RD, McKendall RR, Baringer JR. Comparative neurovirulence of herpes simplex type 1 strains after peripheral or intracerebral inoculation of BALB/c mice. Infection and Immunity. 1983;40:103–112. doi: 10.1128/iai.40.1.103-112.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahey JC, van Dijk G, Woods SC, Seeley RJ. Intraventricular GLP-1 reduces short- but not long-term food intake or body weight in lean and obese rats. Brain Research. 1998;779:75–83. doi: 10.1016/s0006-8993(97)01057-3. [DOI] [PubMed] [Google Scholar]
- Fulwiler CE, Saper CB. Subnuclear organization of the efferent connections of the parabrachial nucleus in the rat. Brain Research Reviews. 1984;7:229–259. doi: 10.1016/0165-0173(84)90012-2. [DOI] [PubMed] [Google Scholar]
- Füzesi T, Wittmann G, Lechan RM, Liposits Z, Fekete C. Noradrenergic innervation of hypophysiotropic thyrotropin-releasing hormone synthesizing neurons in rats. Brain Research. 2009;1294:38–44. doi: 10.1016/j.brainres.2009.07.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillet S, Lachuer J, Malaval F, Assenmacher I, Szafarczyk A. The involvement of noradrenergic ascending pathways in the stress-induced activation of ACTH and corticosterone secretions is dependent on the nature of the stressors. Experimental Brain Research. 1991;87:173–180. doi: 10.1007/BF00228518. [DOI] [PubMed] [Google Scholar]
- Geerling JC, Engeland WC, Kawata M, Loewy AD. Aldosterone target neurons in the nucleus tractus solitarius drive sodium appetite. The Journal of Neuroscience. 2006a;26:411–417. doi: 10.1523/JNEUROSCI.3115-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerling JC, Kawata M, Loewy AD. Aldosterone-sensitive neurons in the rat central nervous system. The Journal of Comparative Neurology. 2006b;494:515–527. doi: 10.1002/cne.20808. [DOI] [PubMed] [Google Scholar]
- Geerling JC, Loewy AD. Aldosterone-sensitive neurons in the nucleus of the solitary tract: efferent projections. The Journal of Comparative Neurology. 2006;497:223–250. doi: 10.1002/cne.20993. [DOI] [PubMed] [Google Scholar]
- Geerling JC, Loewy AD. Sodium deprivation and salt intake activate separate neuronal subpopulations in the nucleus of the solitary tract and the parabrachial complex. The Journal of Comparative Neurology. 2007;504:379–403. doi: 10.1002/cne.21452. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Sawchenko PE. An anterograde neuroanatomical tracing method that shows the detailed morphology of neurons, their axons and terminals: immunohistochemical localization of an axonally transported plant lectin, Phaseolus vulgaris leucoagglutinin (PHA-L) Brain Research. 1984;290:219–238. doi: 10.1016/0006-8993(84)90940-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill HJ, Smith GP. Cholecystokinin decreases sucrose intake in chronic decerebrate rats. American Journal of Physiology. 1988;253:R853–R856. doi: 10.1152/ajpregu.1988.254.6.R853. [DOI] [PubMed] [Google Scholar]
- Herbert H, Moga MM, Saper CB. Connections of the parabrachial nucleus with the nucleus of the solitary tract and the medullary reticular formation in the rat. The Journal of Comparative Neurology. 1990;293:540–580. doi: 10.1002/cne.902930404. [DOI] [PubMed] [Google Scholar]
- Holst JJ. The physiology of glucagon-like peptide 1. Physiological Reviews. 2007;87:1409–1439. doi: 10.1152/physrev.00034.2006. [DOI] [PubMed] [Google Scholar]
- Horst GJT, Boer PD, Luiten PGM, Willigen JDV. Ascending projections from the solitary tract nucleus to the hypothalamus. A Phaseolus vulgaris lectin tracing study in the rat. Neuroscience. 1989;31:785–797. doi: 10.1016/0306-4522(89)90441-7. [DOI] [PubMed] [Google Scholar]
- Horst GJT, Streefland C. Ascending projections of the solitary tract nucleus. In: Barraco IRA, editor. Nucleus of the Solitary Tract. Boca Raton: CRC Press; 1994. pp. 93–103. [Google Scholar]
- Imeryuz N, Yegen BC, Bozkurt A, Coskun T, Villanueva-Penacarrillo ML, Ulusoy NB. Glucagon-like peptide-1 inhibits gastric emptying via vagal afferent-mediated central mechanisms. American Journal of Physiology. 1997;273:G920–G927. doi: 10.1152/ajpgi.1997.273.4.G920. [DOI] [PubMed] [Google Scholar]
- Jeanningros R. Modulation of lateral hypothalamic single unit activity by gastric and intestinal distension. Journal of the Autonomic Nervous System. 1984;11:1–11. doi: 10.1016/0165-1838(84)90003-1. [DOI] [PubMed] [Google Scholar]
- Jin SL, Han VK, Simmons JG, Towle AC, Lauder JM, Lund PK. Distribution of glucagonlike peptide I (GLP-I), glucagon, and glicentin in the rat brain: an immunocytochemical study. Journal of Comparative Neurology. 1988;271:519–532. doi: 10.1002/cne.902710405. [DOI] [PubMed] [Google Scholar]
- Jin Y, Ueta Y, Kannan H, Yamashita H. Synaptic inputs from the stomach to tuberoinfundibular neurons in the paraventricular nucleus of the hypothalamus in rats. Brain Research. 1993;617:151–154. doi: 10.1016/0006-8993(93)90627-y. [DOI] [PubMed] [Google Scholar]
- Karimnamazi H, Travers SP, Travers JB. Oral and gastric input to the parabrachial nucleus of the rat. Brain Research. 2002;957:193–206. doi: 10.1016/s0006-8993(02)03438-8. [DOI] [PubMed] [Google Scholar]
- Kelley AE. Ventral striatal control of appetitive motivation: role in ingestive behavior and reward-related learning. Neuroscience and Biobehavioral Reviews. 2004;27:765–776. doi: 10.1016/j.neubiorev.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Kelly RM, Strick PL. Cerebellar loops with motor cortex and prefrontal cortex of a nonhuman primate. The Journal of Neuroscience. 2003;23:8432–8444. doi: 10.1523/JNEUROSCI.23-23-08432.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King BM. The rise, fall, and resurrection of the ventromedial hypothalamus in the regulation of feeding behavior and body weight. Physiology and Behavior. 2006;87:221–244. doi: 10.1016/j.physbeh.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Kinzig KP, D'Alessio DA, Herman JP, Sakai RR, Vahl TP, Figueredo HF, Murphy EK, Seeley RJ. CNS Glucagon-like peptide-1 receptors mediate endocrine and anxiety responses to interoceptive and psychogenic stressors. The Journal of Neuroscience. 2003;23:6163–6170. doi: 10.1523/JNEUROSCI.23-15-06163.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss A, Aguilera G. Participation of alpha1-adrenergic receptors in the secretion of hypothalamic corticotropin-releasing hormone during stress. Neuroendocrinology. 1992;56:153–160. doi: 10.1159/000126223. [DOI] [PubMed] [Google Scholar]
- Larsen PJ, Tang-Christensen M, Holst JJ, Orskov C. Distribution of glucagon-like peptide-1 and other preproglucagon-derived peptides in rat hypothalamus and brainstem. Neuroscience. 1997;77:257–270. doi: 10.1016/s0306-4522(96)00434-4. [DOI] [PubMed] [Google Scholar]
- Lawrence CB, Celsi F, Brennand J, Luckman SM. Alternative role for prolactin-releasing peptide in the regulation of food intake. Nature. 2000;3:645–646. doi: 10.1038/76597. [DOI] [PubMed] [Google Scholar]
- Lawrence CB, Ellacott KLJ, Luckman SM. PRL-releasing peptide reduces food intake and may mediate satiety signaling. Endocrinology. 2002;143:360–367. doi: 10.1210/endo.143.2.8609. [DOI] [PubMed] [Google Scholar]
- Lawrence CB, Liu Y-L, Stock MJ, Luckman SM. Anorectic actions of prolactin-releasing peptide are mediated by corticotropin-releasing hormone receptors. American Journal of Physiology Regulatory Integrative and Comparative Physiology. 2004;286:R101–R107. doi: 10.1152/ajpregu.00402.2003. [DOI] [PubMed] [Google Scholar]
- Leibowitz SF, Sladek C, Spencer L, Tempel D. Neuropeptide Y, epinephrine and norepinephrine in the paraventricular nucleus: stimulation of feeding and the release of corticosterone, vasopressin and glucose. Brain Research Bulletin. 1988;21:905–912. doi: 10.1016/0361-9230(88)90025-1. [DOI] [PubMed] [Google Scholar]
- Liposits Z, Phelix C, Paull WK. Adrenergic innervation of corticotropin releasing factor (CRF) - synthesizing neurons in the hypothalamic paraventricular nucleus of the rat. Histochemistry. 1986;84:201–205. doi: 10.1007/BF00495783. [DOI] [PubMed] [Google Scholar]
- Luckman SM, Lawrence CB. Anorectic brainstem peptides: more pieces to the puzzle. Trends in Endocrinology and Metabolism. 2003;14:60–65. doi: 10.1016/s1043-2760(02)00033-4. [DOI] [PubMed] [Google Scholar]
- Merchenthaler I, Lane M, Shughrue P. Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. Journal of Comparative Neurology. 1999;403:261–280. doi: 10.1002/(sici)1096-9861(19990111)403:2<261::aid-cne8>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Moga MM, Herbert H, Hurley KM, Yasui Y, Gray TS, Saper CB. Organization of cortical, basal forebrain, and hypothalamic afferents to the parabrachial nucleus in the rat. The Journal of Comparative Neurology. 1990;295:624–661. doi: 10.1002/cne.902950408. [DOI] [PubMed] [Google Scholar]
- Myers EA, Banihashemi L, Rinaman L. The anxiogenic drug yohimbine activates central viscerosensory circuits in rats. the Journal of Comparative Neurology. 2005;492:426–441. doi: 10.1002/cne.20727. [DOI] [PubMed] [Google Scholar]
- Nakade Y, Tsukamoto K, Iwa M, Pappas TN, Takahashi T. Glucagon-like peptide 1 accelerates colonic transit via central CRF and peripheral vagal pathways in conscious rats. Autonomic Neuroscience. 2007;131:50–56. doi: 10.1016/j.autneu.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Norgren R, Grigson PS, Hajnal A, Lundy RF., Jr Motivational modulation of taste. International Congress Series. 2003;1250:319–334. [Google Scholar]
- Penfield W, Faulk ME., Jr The insula; further observations on its function. Brain. 1955;78:445–470. doi: 10.1093/brain/78.4.445. [DOI] [PubMed] [Google Scholar]
- Phelix CF, Liposits Z, Paull WK. Monoamine innervation of bed nucleus of stria terminalis: an electron microscopic investigation. Brain Research Bulletin. 1992;28:949–965. doi: 10.1016/0361-9230(92)90218-m. [DOI] [PubMed] [Google Scholar]
- Plata-Salaman CR. Hypothalamus and the control of feeding: fifteen decades of direct association. Nutrition. 1998;14:67–70. doi: 10.1016/s0899-9007(97)00400-0. [DOI] [PubMed] [Google Scholar]
- Renner E, Szabó-Meltzer KI, Puskás N, Tóth ZE, Dobolyi A, Palkovits M. Activation of neurons in the hypothalamic dorsomedial nucleus via hypothalamic projections of the nucleus of the solitary tract following refeeding of fasted rats. European Journal of Neuroscience. 2010;31:302–314. doi: 10.1111/j.1460-9568.2009.07053.x. [DOI] [PubMed] [Google Scholar]
- Ricardo JA, Koh ET. Anatomical evidence of direct projections from the nucleus of the solitary tract to the hypothalamus, amygdala, and other forebrain structures in the rat. Brain Research. 1978;153:1–26. doi: 10.1016/0006-8993(78)91125-3. [DOI] [PubMed] [Google Scholar]
- Rinaman L. A functional role for central glucagon-like peptide-1 receptors in lithium chloride-induced anorexia. American Journal of Physiology. 1999a;277:R1537–R1540. doi: 10.1152/ajpregu.1999.277.5.R1537. [DOI] [PubMed] [Google Scholar]
- Rinaman L. Interoceptive stress activates glucagon-like peptide-1 neurons that project to the hypothalamus. American Journal of Physiology. 1999b;277:R582–R590. doi: 10.1152/ajpregu.1999.277.2.R582. [DOI] [PubMed] [Google Scholar]
- Rinaman L, Rothe EE. GLP-1 receptor signaling contributes to anorexigenic effect of centrally administered oxytocin in rats. American Journal of Physiology Integrative Regulatory Comparative Physiology. 2002;283:R99–R106. doi: 10.1152/ajpregu.00008.2002. [DOI] [PubMed] [Google Scholar]
- Rinaman L. Postnatal development of hypothalamic inputs to the dorsal vagal complex in rats. Physiology and Behavior. 2003a;79:65–70. doi: 10.1016/s0031-9384(03)00105-7. [DOI] [PubMed] [Google Scholar]
- Rinaman L. Hindbrain noradrenergic lesions attenuate anorexia and alter central cFos expression in rats after gastric viscerosensory stimulation. The Journal of Neuroscience. 2003b;23:10084–10092. doi: 10.1523/JNEUROSCI.23-31-10084.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaman L, Schwartz GJ. Anterograde transneuronal viral tracing of central viscerosensory pathways in rats. The Journal of Neuroscience. 2004;24:2782–2786. doi: 10.1523/JNEUROSCI.5329-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaman L. Visceral sensory inputs to the endocrine hypothalamus. Frontiers in Neuroendocrinology. 2007;28:50–60. doi: 10.1016/j.yfrne.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaman L, Dzmura V. Experimental dissociation of neural circuits underlying conditioned avoidance and hypophagic responses to lithium chloride. American Journal of Physiology Regulatory Integrative and Comparative Physiology. 2007;293:R1495–R1503. doi: 10.1152/ajpregu.00393.2007. [DOI] [PubMed] [Google Scholar]
- Ritter S, Wise D, Stein L. Neurochemical regulation of feeding in the rat: facilitation by alpha-noradrenergic, but not dopaminergic, receptor stimulants. Journal of Comparative and Physiological Psychology. 1975;88:778–784. doi: 10.1037/h0076402. [DOI] [PubMed] [Google Scholar]
- Ritter S, Bugarith K, Dinh TT. Immunotoxic destruction of distinct catecholamine subgroups produces selective impairment of glucoregulatory responses and neuronal activation. Journal of Comparative Neurology. 2001;432:197–216. doi: 10.1002/cne.1097. [DOI] [PubMed] [Google Scholar]
- Sandoval DA, Bagnol D, Woods SC, D'Alessio DA, Seeley RJ. Arcuate glucagon-like peptide 1 receptors regulate glucose homeostasis but not food intake. Diabetes. 2008;57:2046–2054. doi: 10.2337/db07-1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper CB, Loewy AD. Efferent connections of the parabrachial nucleus in the rat. Brain Research. 1980;197:291–317. doi: 10.1016/0006-8993(80)91117-8. [DOI] [PubMed] [Google Scholar]
- Sarkar S, Fekete C, Legradi G, Lechan RM. Glucagon like peptide-1 (7–36) amide (GLP-1) nerve terminals densely innervate corticotropin-releasing hormone neurons in the hypothalamic paraventricular nucleus. Brain Research. 2003;985:163–168. doi: 10.1016/s0006-8993(03)03117-2. [DOI] [PubMed] [Google Scholar]
- Sawchenko PE, Swanson LW. Central noradrenergic pathways for the integration of hypothalamic neuroendocrine and autonomic responses. Science. 1981;214:685–687. doi: 10.1126/science.7292008. [DOI] [PubMed] [Google Scholar]
- Sawchenko PE, Swanson LW. The organization of noradrenergic pathways from the brainstem to the paraventricular and supraoptic nuclei in the rat. Brain Research Reviews. 1982a;4:275–325. doi: 10.1016/0165-0173(82)90010-8. [DOI] [PubMed] [Google Scholar]
- Sawchenko PE, Swanson LW. Immunohistochemical identification of neurons in the paraventricular nucleus of the hypothalamus that project to the medulla or to the spinal cord in the rat. The Journal of Comparative Neurology. 1982b;205:260–272. doi: 10.1002/cne.902050306. [DOI] [PubMed] [Google Scholar]
- Sawchenko PE, Arias C, Bittencourt JC. Inhibin B, somatostatin, and enkephalin immunoreactivities coexist in caudal medullary neurons that project to the paraventricular nucleus of the hypothalamus. Journal of Comparative Neurology. 1990;291:269–280. doi: 10.1002/cne.902910209. [DOI] [PubMed] [Google Scholar]
- Schick RR, Zimmermann JP, Walde Tv, Schusdziarra V. Glucagon-like peptide 1-(7–36) amide acts at lateral and medial hypothalamic sites to suppress feeding in rats. Am J Physiol Regulatory Integrative Comp Physiol. 2003;284:R1427–R1435. doi: 10.1152/ajpregu.00479.2002. [DOI] [PubMed] [Google Scholar]
- Seeley RJ, Blake K, Rushing PA, Benoit S, Eng J, Woods SC, D'Alessio D. The role of CNS glucagon-like peptide-1 (7–36) amide receptors in mediating the visceral illness effects of lithium chloride. The Journal of Neuroscience. 2000;20:1616–1621. doi: 10.1523/JNEUROSCI.20-04-01616.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro RE, Miselis RR. The central organization of the vagus nerve innervating the stomach of the rat. Journal of Comparative Neurology. 1985;238:473–488. doi: 10.1002/cne.902380411. [DOI] [PubMed] [Google Scholar]
- Smith GP. The controls of eating: a shift from nutritional homeostasis to behavioral neuroscience. Nutrition. 2000;16:814–820. doi: 10.1016/s0899-9007(00)00457-3. [DOI] [PubMed] [Google Scholar]
- Smith GP. Feeding: control of eating. In: Adelman G, Smith BH, editors. Elsevier's Encyclopedia of Neuroscience. 3rd Edition. New York: CD-ROM; 2004. [Google Scholar]
- Stornetta RL, Sevigny CP, Guyenet PG. Vesicular glutamate transporter DNPI/VGLUT2 mRNA is present in C1 and several other groups of brainstem catecholaminergic neurons. The Journal of Comparative Neurology. 2002;444:191–206. doi: 10.1002/cne.10141. [DOI] [PubMed] [Google Scholar]
- Sun N, Cassell MD, Perlman S. Anterograde, transneuronal transport of herpes simplex virus type 1 strain H129 in the murine visual system. Journal of Virology. 1996;70:5405–5413. doi: 10.1128/jvi.70.8.5405-5413.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Tang M, Zhang J, Chen JDZ. Excitatory effects of gastric electrical stimulation on gastric distension responsive neurons in ventromedial hypothalamus (VMH) in rats. Neuroscience Research. 2006;55:451–457. doi: 10.1016/j.neures.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Swanson L. Structure of the Rat Brain. Elsevier: Amsterdam; 2004. Brain Maps III. [Google Scholar]
- Tang-Christensen M, Vrang N, Larsen PJ. Glucagon-like peptide containing pathways in the regulation of feeding behaviour. International Journal of Obesity. 2001;25:S42–S47. doi: 10.1038/sj.ijo.0801912. [DOI] [PubMed] [Google Scholar]
- Turton MD, O'Shea D, Gunn I, Beak SA, Edwards CM, Meeran K, Choi SJ, Taylor GM, Heath MM, Lambert PD, Wilding JP, Smith DM, Ghatei MA, Herbert J, Bloom SR. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature. 1996;379:69–72. doi: 10.1038/379069a0. [DOI] [PubMed] [Google Scholar]
- Ueta Y, Kannan H, Yamashita H. Gastric afferents to the paraventricular nucleus in the rat. Experimental Brain Research. 1991;84:487–494. doi: 10.1007/BF00230960. [DOI] [PubMed] [Google Scholar]
- Vrang N, Hansen M, Larsen PJ, Tang-Christensen M. Characterization of brainstem preproglucagon projections to the paraventricular and dorsomedial hypothalamic nuclei. Brain Research. 2007;1149:118–126. doi: 10.1016/j.brainres.2007.02.043. [DOI] [PubMed] [Google Scholar]
- Woods SC, D'Alessio DA. Central control of body weight and appetite. Journal of Clinical Endocrinology and Metabolism. 2008;93:S37–S50. doi: 10.1210/jc.2008-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano T, Iijima N, Kataoka Y, Hinuma S, Tanaka M, Ibata Y. Developmental expression of prolactin releasing peptide in the rat brain: localization of messenger ribonucleic acid and immunoreactive neurons. Developmental Brain Research. 2001;128:101–111. doi: 10.1016/s0165-3806(01)00148-1. [DOI] [PubMed] [Google Scholar]
- Zemanick MC, Strick PL, Dix RD. Direction of transneuronal transport of herpes simplex virus 1 in the primate motor system is strain-dependent. Proceedings of the National Academy of Science. 1991;88:8048–8051. doi: 10.1073/pnas.88.18.8048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Patterson LM, Berthoud H-R. Orexin-A projections to the caudal medulla and orexin-induced c-fos expression, food intake, and autonomic function. The Journal of Comparative Neurology. 2005;485:127–142. doi: 10.1002/cne.20515. [DOI] [PubMed] [Google Scholar]
- Zheng H, Patterson LM, Berthoud H-R. Orexin signaling in the ventral tegmental area is required for high-fat appetite induced by opioid stimulation of the nucleus accumbens. The Journal of Neuroscience. 2007;27:11075–11082. doi: 10.1523/JNEUROSCI.3542-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]