Abstract
Background
Health systems with limited resources may have the greatest impact on suicide if their prevention efforts target the highest-risk treatment groups during the highest-risk periods. To date, few health systems have carefully segmented their depression treatment populations by level of risk and prioritized prevention efforts on this basis.
Methods
We conducted a retrospective cohort study of 887,859 VA patients receiving depression treatment between 4/1/1999 and 9/30/2004. We calculated suicide rates for five sequential 12-week periods following treatment events that health systems could readily identify: psychiatric hospitalizations, new antidepressant starts (>6 months without fills), “other” antidepressant starts, and dose changes. Using piecewise exponential models, we examined whether rates differed across time-periods. We also examined whether suicide rates differed by age-group in these periods.
Results
Over all time periods, the suicide rate was 114/100,000 person-years (95% CI; 108,120). In the first 12-week periods, suicide rates were: 568/100,000 p-y (95% CI; 493,651) following psychiatric hospitalizations; 210/100,000 p-y (95% CI; 187, 236) following new antidepressant starts; 193/100,000 p-y (95% CI; 167, 222) following other starts; and 154/100,000 p-y (95% CI; 133, 177) following dose changes. Suicide rates remained above the base rate for 48 weeks following hospital discharge and 12 weeks following antidepressant events. Adults aged 61–80 years were at highest risk in the first 12-weeks periods
Conclusions
To have the greatest impact on suicide, health systems should prioritize prevention efforts following psychiatric hospitalizations. If resources allow, closer monitoring may also be warranted in the first 12 weeks following antidepressant starts, across all age-groups.
Suicide is an important public health concern; there were 32,637 deaths due to suicide in the US during 2005.(Center for Disease Control and Prevention, 2005) Prevention experts suggest that efforts to reduce the suicide will likely require educational interventions aimed at the general public coupled with more intensive interventions targeting high-risk populations. (Institute of Medicine, 2004) Because resources are finite, the most intensive and highest cost interventions generally must be reserved for the highest-risk populations, where risk reductions translate into greater numbers of lives saved.
Treatment guidelines for suicide prevention have typically targeted clinicians and outlined patient characteristics that increase risk and can be elicited during face-to-face evaluations. (American Psychiatric Association, 2000, 2003) As this focus suggests, clinicians traditionally have assumed most or all of the responsibility for identifying high risk individuals and working with them to reduce risks.
Most health systems have made few attempts to carefully segment their treatment populations by level of risk and to plan and to prioritize prevention efforts on this basis-- even though decisions and policies regarding clinician workload, clinical programs, and patient benefits are usually made at the health system level. Systematic identification of highest-risk groups within a health system would complement and supplement the individualized, but often ad-hoc, clinician efforts to identify vulnerable patients and reduce suicide risks, and may allow a more reasoned allocation of system resources for suicide prevention. The Department of Veterans Affairs Health system is currently making efforts to roll out prevention efforts on as system-wide basis, (Department of Veterans Affairs, Office of the Inspector General, 2007) and effectively deploying resources and implementing rational policies is a key aspect of this effort.
Focusing prevention efforts on depression treatment populations may be one way to systematically address a very high-risk group and rationally allocate prevention resources. In prior work, we reported the suicide rate for VA patients in depression treatment over a period of several years and the relative risks of suicide associated with specific patient characteristics, (Zivin et al., 2007). This work confirmed earlier reports suggesting that patients in depression treatment may be among the highest risk patient populations for suicide in most health care systems. (Simon and VonKorff, 1998).
In this paper, we examine whether there are readily identifiable high-risk periods during the course of depression treatment that might be prioritized by health systems attempting to reduce suicide. We focus on treatment periods where the literature suggests higher risks might exist and where organizational policies have been suggested to reduce suicide risks. (2006; Desai et al., 2005; Gibbons et al., 2007; Simon et al., 2006; U.S. Food and Drug Administration; U.S. Food and Drug Administration; U.S. Food and Drug Administration)
The US Food and Drug Administration (FDA)’s metaanalyses of randomized controlled trials indicated that rates of suicidal ideation and attempts are increased in treatment periods following antidepressant starts or dose changes among youth and young adults, and the agency has recommended close clinical monitoring at these times. Its most stringent recommendation has been for 7 visits to be completed the first 12 weeks following these treatment events. (U.S. Food and Drug Administration, 2004; U.S. Food and Drug Administration, 2005).
The period following psychiatric hospitalizations has also been noted to be a high risk period for patients. (Goldacre et al., 1993) One of the most prominent organizations accrediting health care organizations in the US, the National Committee on Quality Assurance (NCQA), calls for at least one outpatient visit in the first 7 or in the first 30 days following psychiatric inpatient discharge to improve patient outcomes during this period. (2006).
To date, only a few studies have been powered to examine completed suicide in an entire treatment population, and none have examined rates of completed suicide during sequential treatment periods in an entire depression treatment population. Simon et al. reported completed suicide within all segments of the depression treatment population in the Group Health Cooperative by location of care among between 1992 and 1994, although the numbers of patients completing suicide were small.(Simon and VonKorff, 1998) Two studies have also reported completed suicide rates for VA cohorts of inpatients with a variety of diagnoses in the year following discharge or outpatients with a variety of psychiatric disorders in the year following a January outpatient visit.(Desai et al., 2008; Desai et al., 2005)
This study builds on these prior efforts by examining completed suicide during sequential time periods following salient treatment events in the entire population of patients treated for depression between between April 1, 1999 and September 30, 2004 (N=887,859 patients) in VA settings, using a longitudinal VA dataset with comprehensive diagnosis, utilization and pharmacy data and data from National Death Index (NDI). A comprehensive population-based study is most helpful in establishing priorities for suicide prevention.
We note that this also the first study to report suicide deaths following antidepressant dose changes and antidepressant starts that occur during ongoing treatment (i.e., antidepressant switches), and the largest to report suicide deaths among various patient age-groups following antidepressant changes and following hospitalizations. Given the FDA warnings emphasizing monitoring for younger but not older adults, we also examined suicide rates following antidepressant medication changes by age-group.
We discuss the implications of our study findings for health systems and policy makers that wish to rationally allocate resources to have the greatest impact on suicide deaths within their depression treatment populations.
METHODS
Study data were obtained from the VA’s National Registry for Depression (NARDEP) which was developed by the VA’s Serious Mental Illness Treatment Research and Evaluation Center (SMITREC) in Ann Arbor, Michigan. The study was approved by the Institutional Review Board of the Veterans Affairs Ann Arbor Health System.
Patient Population/Observation Days
The study sample consisted of all 887,859 patients in NARDEP who received either two depression diagnoses or a diagnosis of depression and an antidepressant fill between April 1, 1999 and September 30, 2004. Patients were excluded if they received any diagnoses of bipolar I, schizophrenia, or schizoaffective disorder during this period. Depression diagnoses were identified using the ICD-9 codes: 296.2x, 296.3x, 296.90, 296.99, 298.0, 300.4, 311, 293.83, 301.12, 309.0, or 309.1.
Observation-days for the main study analyses began on the dates of patients’ first treatment event of interest (discharge from a psychiatric hospitalization, new antidepressant start, other start, or dose change) and continued until 60 weeks following their last treatment event of interest, their date of death as indicated in the National Death Index (NDI), or the end of the study period (September 30, 2004), whichever came first.
Treatment Events: Psychiatric Hospitalizations, New Antidepressant Starts, Other Starts and Dose Changes
We ascertained dates of discharge from psychiatric hospitalizations, defined as hospitalizations with a primary or principal psychiatric discharge diagnosis of ICD-9 codes 290.x – 319.x or hospitalizations with bed section codes indicating a psychiatric stay.
Patients were considered to have received an antidepressant medication if they filled a prescription within the VA system for any of the following: amitriptyline, amoxapine, atomoxetine, bupropion, citalopram, clomipramine, desipramine, doxepin, duloxetine, escitalopram, fluoxetine, fluvoxamine, imipramine, isocarboxazid, maprotiline, mirtazapine, nefazodone, nortriptyline, paroxetine, phenelzine, protriptyline, sertraline, tranylcypromine, trazodone, trimipramine, and venlafaxine. As in prior studies, we considered trazodone, mirtazapine, amitriptyline, and nortriptyline to have been used as antidepressants rather than for other purposes only if the doses were ≥ 300 mg/day, ≥ 15 mg/day, ≥ 75 mg/day, or ≥ 25 mg, respectively.
A new antidepressant start was defined as a fill of an antidepressant medication that occurred after a “clean period” of ≥ 6 months without any antidepressant fills. Other antidepressant starts were fills that occurred during ongoing treatment, either without a clean period or with a short clean period (< 6 months). Otherstarts included: 1) antidepressant switches, defined as the discontinuation of one antidepressant followed by the initiation of a second in ≤ 30 days; 2) combination treatment, the addition of a second antidepressant to ongoing treatment with the first agent, and 3) and other starts with < 6 months free from all antidepressant use. We did not include “restarts” of the same antidepressant medication within a 6 month period as an “other start” of interest, as this might be due to short gaps in use due to incomplete adherence or to a restart of a previously tolerated medication. Antidepressant starts that occurred in the 6 months prior to cohort entry were all considered to be other starts and used to categorize the initial days following cohort entry, as described below. A significant change in antidepressant dose was defined as a ≥ 50% difference in total dose between two consecutive fills of a specified antidepressant occurring within 6 months. Because we had access only to data on outpatient medication fills (including discharge medications from inpatient stays), we identified new and other antidepressant starts that occurred during inpatient stays at the time of the patient’s discharge, by comparing pre and post hospital medications.
Risk Periods Following Treatment Events
We classified patient observation days into discrete “risk periods” based on their proximity to treatment events of interest occurring during the study period or, for patients newly entering the cohort, by proximity to events in the 6 months prior to study entry. Patient-days were classified by whether they fell within one of five sequential 12-week periods following each type of treatment event (new antidepressant starts, other starts, dose changes, or discharges from inpatient psychiatric stays). Days were classified as being within: 1) 1–84 days (12 weeks) following the treatment event, 2) 85–168 days (13–24 weeks) following the event, 3) 169–252 days (25–36 weeks) following the event, 4) 253–336 days (37–48 weeks) following the event, or 5) 337–420 days (49–60 weeks) following the event.
In our primary analyses, when a patient was observed to have a second occurrence of a specific treatment event of interest (e.g., a second “new antidepressant start” following a first “new antidepressant start”), observation days that occurred subsequent to the second event were “reset” and classified based on proximity to the second event. We also constructed a variable for the cumulative number of specific treatment events to date (1, 2, ≥3), allowing us to take into account the effect of additional occurrences of specific events in the analyses. In these analyses which considered each event separately, a patient who was discharged from the hospital AND who also had a new antidepressant start would have contributed exposure time to analyses that examined suicide rates following psychiatric hospitalizations and to separate analyses examining suicide rates following new antidepressant starts. However, the vast majority of antidepressant starts occurred on an outpatient basis.
For sensitivity analyses, we constructed several additional variables. First, we constructed a variable that categorized observation days only in relation to the first occurrence of a treatment event, regardless of whether there were additional occurrences of the same type of event. Secondly, recognizing that specific treatment events (such as a new antidepressant start) may be followed by other treatment events of interest (a dosage change), we constructed a variable that allowed us to examine whether the recency of “any treatment event” corresponded with higher suicide risks. For this variable, observation days were categorized based on the time from the date of the most recent occurrence of “any antidepressant treatment event” (new start, other start, or dose change) or from the most recent occurrence of “any treatment event” (new antidepressant start, other start, dose change, or psychiatric hospitalization). A patient who was discharged from the hospital AND had a new antidepressant start identified upon discharge would have contributed time in the 5 sequential 12 week periods following this common date of “any treatment event”.
Suicides
To identify suicides, we submitted National Death Index Plus queries for cohort patients who had a date of death in the VA Beneficiary Identification and Records Locator System (BIRLS) Death File during the study period. The NDI is considered the “gold standard” of US mortality databases.(Cowper et al., 2002) BIRLS data have a sensitivity of 87%–96.5% and a specificity of 94% for deaths when compared to NDI data.(Cowper et al., 2002; Dominitz et al., 2001) Because BIRLS data may be less sensitive when patients are seen only as outpatients or do not have a service-connected disability, we also initiated NDI searches for cohort patients who did not use VA services in the year following the study period, even if there was no date of death in VA data. This process resulted in a comprehensive assessment of death among cohort patients.
We first identified deaths due to suicide using ICD codes in the NDI data file that specified suicide (ICD-10 codes X60–X84, Y87.0). Because underreporting is a concern, in sensitivity analyses we used a broader definition of suicide, assuming that deaths due to “events of undetermined intent” (ICD-10 codes Y10–34, Y87.2, Y89.9) were also suicides.(Speechley and Stavraky, 1991)
Data Analyses
We completed descriptive statistics for the patient sample, using frequencies or means as appropriate. Suicide rates were calculated based on the number of suicides observed and the person-years of observation for each of the five sequential 12-week periods following hospital discharge or antidepressant treatment events. For time periods following each type of treatment event, rates were calculated across the combined occurrences of this event.
To test if the suicide rates differed significantly across the sequential 12-week periods following treatment events, we used piecewise exponential models that allowed suicide risks to vary across time periods.19 A Poisson regression model was used to fit the piecewise exponential models, 20 with generalized estimating equations to allow for correlation within patients when multiple episodes of treatment events were included in the analyses.21 Relative risks were calculated after adjusting for patient age, gender, race, ethnicity, marital status, diagnosis of a substance use disorder, post traumatic stress disorder, and service connection. Data were entered by intervals of 12-week periods for time-fixed covariates of gender, marital status, race/ethnicity, comorbid PTSD and substance use and for time-varying covariates such as psychiatric hospitalization status. The models also adjusted for the cumulative numbers of the specific treatment event occurrences to date (1, 2, ≥3).
Models that examined suicide risks following each antidepressant event type also included a time-varying covariate for psychiatric hospitalization, to adjust for potentially different suicide risks associated with a prior hospitalization. The log-likelihood ratio test was used to test for the overall differences in suicide risks due to time-periods. When suicide risks between sequential time periods were compared, we adjusted p-values to correct for multiple comparisons and protect the overall alpha level at 0.05.
We also used the likelihood ratio test to determine whether there significant differences in suicide rates across age-groups in treatment periods following each type of treatment event.
Sensitivity Analysis
In sensitivity analyses, we compared suicide risks during sequential treatment periods, following an approach used in prior work in which observation days were categorized only from the first occurrence of a specific treatment event (such as a new antidepressant start).22,23 We also conducted analyses in which we examined suicide risks in time periods following “any treatment event” and “any antidepressant treatment event”.
RESULTS
Patient Sample and Observation Days
The characteristics of the patient cohort (N=887,857) are outlined in Table 1. The population had a mean age of 58.6 years and was predominantly male (92%). There were 433,086,931 observation-days (1,185,727 patient-years) during the study period.
Table 1.
Characteristics of Patient Cohort
| Patient Characteristics | N=887,859 |
|---|---|
| Age, Mean (SD) | 58.6 (SD 14.4) |
| Gender, N(%) | |
| Male | 815,917 (91.9%) |
| Female | 71,942 (8.1%) |
| Race/Ethnicity, N (%) | |
| White | 678,829 (76.5%) |
| Black | 110,125 (12.4%) |
| Other (Asian, Am Native, Pacific Islander, Multiracial) | 18,966 (2.1%) |
| Unknown | 79,939 (9.0%) |
| Hispanic Ethnicity,* N(%) | 41,457 (4.7%) |
Hispanic ethnicity is recorded separately from race.
Psychiatric Hospitalizations, Antidepressant Starts and Dose Changes
A total of 182,518 psychiatric hospitalizations occurred during the observation period. Approximately 10% of patients had one or more and 4% had two or more hospitalizations during the study period.
Across the entire observation period, there were 654,814 new antidepressant starts, 431,771 other antidepressant starts, and 695,409 dosage changes. Approximately 81% of patients had a new antidepressant start, other start, or a dosage change during the study period. Sixty-one percent had one or more new antidepressant starts and 30% had one or more “other” antidepressant starts during ongoing treatment. Approximately 38% had one or more changes in antidepressant dose of ≥ 50%.
Suicide Rates
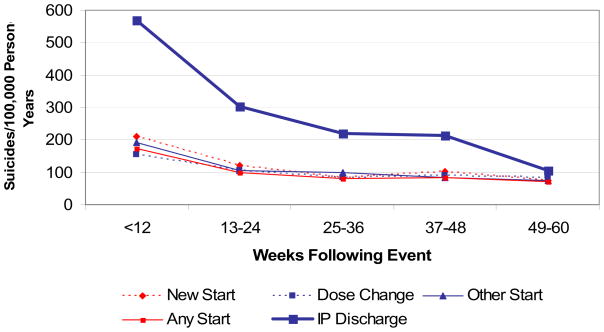
Table 2 presents the number of suicides and the suicide rates during the 60 weeks following any treatment event of interest. The table also presents suicide rates in the five sequential 12-week periods following psychiatric hospitalization, new antidepressant starts, other antidepressant starts, and dose changes. Figure 1 presents suicide rates graphically.
Table 2.
Suicides Rates per 100,000 person-years*
| Period Definition | Suicides* N | Observation Days | Suicide Rate (95% CI) |
|---|---|---|---|
| Observation period—from first treatment event until 60 weeks following the last treatment event, date of death, or end of study | 1346 | 433,086,931 | 113.5 (107.5, 119.7) |
| Suicides following discharge from psychiatric hospital stay | |||
| occurring within 12 weeks | 206 | 13,254,665 | 567.7 (492.8, 650.7) |
| occurring in the 13–24 weeks | 89 | 10,698,244 | 303.9 (244.0, 373.9) |
| occurring within 25–36 weeks | 54 | 8,992,479 | 219.3 (164.8, 286.2) |
| occurring within 37–48 weeks | 45 | 7,672,957 | 214.2 (156.2, 286.6) |
| occurring within 49–60 weeks | 19 | 6,647,370 | 104.4 (62.9, 163.0) |
| Suicides following new antidepressant start | |||
| occurring within 12 weeks | 298 | 51,725,955 | 210.4 (187.2, 235.7) |
| occurring within 13–24 weeks | 159 | 47,879,932 | 121.3 (103.2, 141.7) |
| occurring within 25–36 weeks | 102 | 44,035,199 | 84.6 (69.0, 102.7) |
| occurring within 37–48 weeks | 112 | 39,728,326 | 103.0 (84.8, 123.9) |
| occurring within 49–60 weeks | 73 | 35,223,796 | 75.7 (59.3, 95.2) |
| Suicides following other antidepressant start | |||
| occurring within 12 weeks | 197 | 37,262,981 | 193.1 (167.1, 222.0) |
| occurring within 13–24 weeks | 109 | 37,441,574 | 106.3 (87.3, 128.3) |
| occurring within 25–36 weeks | 105 | 39,021,574 | 98.3 (80.4, 119.0) |
| occurring within 37–48 weeks | 78 | 34,014,782 | 83.8 (66.2, 104.5) |
| occurring within 49–60 weeks | 60 | 29,654,378 | 73.9 (56.4, 95.1) |
| Suicides following antidepressant dose change | |||
| occurring within 12 weeks | 203 | 48,185,027 | 153.9 (133.4, 176.6) |
| occurring within 13–24 weeks | 107 | 37,300,830 | 104.8 (85.7, 126.6) |
| occurring within 25–36 weeks | 69 | 30,696,799 | 82.1 (63.9, 103.9) |
| occurring within 37–48 weeks | 64 | 25,805,156 | 90.6 (69.8, 115.7) |
| occurring within 49–60 weeks | 50 | 21,889,758 | 83.4 (61.9, 110.0) |
Based on strict definition of suicides (ICD-10 codes X60–X84, Y87.0)
Figure 1. Suicide Rates Following Treatment Events.
* Please see Table 2 for 95% confidence intervals for suicide rates in each time period following the above treatment events.
When we used a strict definition of suicide (requiring explicit ICD codes for suicide), the suicide rate during the entire study period was 114/100,000 person-years (95% CI; 108, 120/100,000 person-years). Using a broader definition that included deaths due to “events of undetermined intent”, the suicide rate was 128/100,000 person-years (95% CI; 122, 135/100,000 person-years).
As displayed in Figure 1, suicide rates were highest in the first 12 weeks following psychiatric hospitalizations at 568 (95% CI; 493, 651) per 100,000 person-years. Suicide rates following hospital discharge declined markedly in subsequent time periods but remained above the base rate through 48 weeks. There were similar, less dramatic declines in suicide rates following new antidepressant starts, other starts, and other dose changes. In the first 12 weeks following new antidepressant starts, the suicide rate per 100,000 person years was 210 (95% CI; 187, 236). The suicide rate declined significantly in the second 12 week periods to 121 (95% CI; 187, 236) per 100,000 person-years.
Relative Suicide Risks
The piecewise exponential models indicated that time-period was significantly associated with suicide following all treatment events (p < 0.001). The relative risks for the first compared to the second 12 week periods: were 1.9 (95% CI= 1.5, 2.4) following psychiatric hospitalizations; 1.8 (95% CI= 1.5, 2.1) following new antidepressant starts, 1.8 (95% CI= 1.4, 2.3) following other antidepressant starts, and 1.4 (95% CI=1.1, 1.8) following dose changes. There were also significant risk reductions between the first and second 12 week periods following “any antidepressant regimen change” (RR=1.8, 95% CI=1.5, 2.1) and “any treatment event” (RR=1.8; 95% CI=1.6, 2.1).
Age-Group and Suicide Risks During Treatment Periods
Age-group was not significantly associated with suicide rates in the first 12 weeks following new antidepressant starts, other starts or dose changes when time periods following each type of antidepressant event was considered separately. However, age-group was significantly associated with suicide in the first 12 weeks following “any” antidepressant regimen change and was also significantly associated with suicide in periods following psychiatric hospitalizations. Tables 3 and 4 present suicide rates by age-group in the first 12 weeks following any antidepressant treatment event and following psychiatric hospitalizations. Adults aged 61–80 years were at highest risk for suicide during these time periods.
Table 3.
Suicide Rates by Age-Group In First 12 Weeks following New AD Starts, Other AD Starts, or AD Dose Changes
| Age Group | Suicides N | Observation Days | Suicide Rate* per 100,000 |
|---|---|---|---|
| <=30 | 15 | 3,122,987 | 175.4 |
| 31–40 | 45 | 9,354,056 | 175.7 |
| 41–50 | 126 | 24,646,914 | 186.7 |
| 51–60 | 156 | 43,697,385 | 130.4 |
| 61–70 | 96 | 17,805,186 | 196.9 |
| 71–80 | 114 | 18,846,385 | 220.9 |
| ≥81 | 32 | 6,332,859 | 184.6 |
Suicide rates differed by age group (p = 0.001)
Table 4.
Suicide Rates by Age-Group Following in First 12 Weeks Following Inpatient Psychiatric Discharges
| Age Group | Suicides N | Observation Days | Suicide Rate per 100,000 |
|---|---|---|---|
| <=30 | 6 | 325,402 | 673.5 |
| 31–40 | 21 | 1,417,156 | 541.2 |
| 41–50 | 57 | 4,552,002 | 457.4 |
| 51–60 | 70 | 4,909,817 | 520.7 |
| 61–70 | 31 | 917,004 | 1234.8 |
| 71–80 | 16 | 802,477 | 728.2 |
| ≥81 | 5 | 330,807 | 552.1 |
Suicide rates differed by age group (p = 0.0038)
DISCUSSION
Health systems should considering segmenting their depression treatment populations by levels of suicide risk to most usefully deploy limited resources for suicide prevention. Our data suggests that health systems might have the most impact on suicide if they first allocated resources for prevention efforts for depressed patients recently discharged from inpatient psychiatric settings.
In the first 12 weeks following inpatient discharge, suicide rates were 568/100,000 person years, or approximately 5 times the overall base rate in this active treatment population. Although all patients are carefully assessed for suicidal behaviors or intent prior to hospital discharge, the post-hospitalization period remains a time of considerable instability and risk.(Goldacre et al., 1993) Frequent reassessments, support, and a firm connection to outpatient services may be essential in decreasing risks at these times. (American Psychiatric Association, 2003) Currently, widely used monitors, such as those in the NCQA Health Employment Data and Information Set (HEDIS), call for one outpatient visit in the first 7 and the first 30 days following hospital discharge. This rather non-intensive level of monitoring may not be sufficient to reduce risks among this extremely vulnerable population. Treatment guidelines and health policies may need to further emphasize the importance of close follow-up at this time and the need to remain vigilant for several months. Structured transition programs may be advisable. Further research is needed to demonstrate the potential benefits of increased clinical contacts on suicide risks at this critical juncture.
Public debate has recently focused on the need for close monitoring following antidepressant starts or dose changes and our data confirm that completed suicide rates were approximately twice the base rate following antidepressant starts in VA clinical settings. In the first 12 weeks after new and other starts, suicide rates were 210 and 193 per 100,000 person-years, respectively. Antidepressant dose changes were not as potent a maker for high risk periods, with smaller elevations in completed suicide observed (154/100,000 person-years in first 12 weeks).
The FDA has recommended that children, adolescents, and young adults, be monitored very closely following antidepressant starts or dose changes, with the most stringent recommendation being for 7 visits in the first 12 weeks. (U.S. Food and Drug Administration; U.S. Food and Drug Administration) This recommendation was made because of risks that might be due to antidepressant exposure per se. The increase in completed suicide observed in clinical settings may be due in part to antidepressant exposure, but also likely reflects patients’ illness severity which prompted the antidepressant initiation or change. However, regardless of the etiology of increased risks, closer monitoring may be warranted, if resources allow. Currently, the FDA’s recommendations for monitoring following antidepressant initiation/change are more intensive than many recommendations for monitoring following hospitalization. Given the higher rates of completed suicide following hospitalization, providing close monitoring following antidepressant starts may be a second target area for health systems.
The FDA warnings, based on clinical trial data, has emphasized close monitoring after antidepressant starts or changes for adult patients 24 years and younger. However, in clinical settings younger adults do not appear to be the age-group at greatest risk for suicide after antidepressant initiation/change. Potentially because of illness severity rather than antidepressant exposure per se, patients aged 61–80 years show the highest suicide risks. Patients in these age-groups also need closer monitoring at these times, even if their increased risks are not due to medication effects.
Limitations
The VA study population during the study period consisted predominantly of men and older individuals. Thus, the specific elevations observed in suicide rates and the trajectory of risks following readily identifiable treatment events in VA treatment populations may not generalize to other treatment populations and also may change as younger veterans enter the system following conflicts in Iraq and Afghanistan. We note that the suicide risks outlined in this paper may also change if levels of medication adherence or treatment practices and care delivery change significantly in usual VA care settings.
We also relied on antidepressant prescription fills and hospitalizations within the VA as flags for high-risk periods. Some patients may have used mental health services outside of the VA system and have had antidepressant starts or psychiatric hospitalizations that were not recorded in our dataset. However, prior reports indicate that only 7–16% of VA mental health users receive care in other health systems. (Desai and Rosenheck, 2002; Desai et al., 2001) During this period, patients eligible for VA coverage often exclusively used VA pharmacies because of the VA’s generous drug benefit.(Piette and Heisler, 2004) Hospitalizations and antidepressant starts that occurred outside of the VA would also have served only to reduce the magnitude of relative risks following recorded antidepressant starts and hospital use.
Summary
Health systems with limited resources may need to first focus on the highest risk treatment periods which follow psychiatric hospitalization. Structured transition programs with regular patient contacts may be advisable at these times. If resources permit, health systems might also consider providing closer monitoring in the first 12 weeks immediately following antidepressant starts, across all adult age-groups.
Acknowledgments
This research was supported by grants from the Department of Veterans Affairs, Health Services Research and Development Service, IIR 04-211-1 and MRP 03-320 and by the National Institute of Mental Health, R01-MH078698-01. Resources were also contributed by the Serious Mental Illness Treatment, Research, and Evaluation Center, Ann Arbor, MI. The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs.
References
- Web-based Injury Statistics Query and Reporting System of the National Center for Injury Prevention and Control, Center for Disease Control and Prevention. available at http://www.cdc.gov/ncipc/wisqars.
- National Committee on Quality Assurance. State of Health Care Quality Report, Antidepressant Medication Management. available at http://www.ncqa.org/sohc2003/antidepressant_medication_manage.htm.
- American Psychiatric Association. Am J Psychiatry. Vol. 157. American Psychiatric Association; 2000. Practice guideline for the treatment of patients with major depressive disorder (revision) pp. 1–45. [PubMed] [Google Scholar]
- American Psychiatric Association. Practice guideline for the assessment and treatment of patients with suicidal behaviors. Am J Psychiatry. 2003;160:1–60. [PubMed] [Google Scholar]
- Cowper DC, Kubal JD, Maynard C, Hynes DM. A primer and comparative review of major US mortality databases. Ann Epidemiol. 2002;12:462–468. doi: 10.1016/s1047-2797(01)00285-x. [DOI] [PubMed] [Google Scholar]
- Desai MM, Rosenheck RA, Desai RA. Time trends and predictors of suicide among mental health outpatients in the department of veterans affairs. J Behav Health Serv Res. 2008;35:115–124. doi: 10.1007/s11414-007-9092-0. [DOI] [PubMed] [Google Scholar]
- Desai RA, Dausey DJ, Rosenheck RA. Mental health service delivery and suicide risk: the role of individual patient and facility factors. Am J Psychiatry. 2005;162:311–318. doi: 10.1176/appi.ajp.162.2.311. [DOI] [PubMed] [Google Scholar]
- Desai RA, Rosenheck RA. The impact of managed care on cross-system use of mental health services by veterans in Colorado. Psychiatr Serv. 2002;53:1599–1604. doi: 10.1176/appi.ps.53.12.1599. [DOI] [PubMed] [Google Scholar]
- Desai RA, Rosenheck RA, Rothbard A. Cross-system service use among VA mental health patients living in Philadelphia. Adm Policy Ment Health. 2001;28:299–309. doi: 10.1023/a:1011137630558. [DOI] [PubMed] [Google Scholar]
- Dominitz JA, Maynard C, Boyko EJ. Assessment of vital status in Department of Veterans Affairs national databases. comparison with state death certificates. Ann Epidemiol. 2001;11:286–291. doi: 10.1016/s1047-2797(01)00211-3. [DOI] [PubMed] [Google Scholar]
- Gibbons RD, Brown CH, Hur K, Marcus SM, Bhaumik DK, Mann JJ. Relationship between antidepressants and suicide attempts: an analysis of the Veterans Health Administration data sets. Am J Psychiatry. 2007;164:1044–1049. doi: 10.1176/ajp.2007.164.7.1044. [DOI] [PubMed] [Google Scholar]
- Goldacre M, Seagroatt V, Hawton K. Suicide after discharge from psychiatric inpatient care. Lancet. 1993;342:283–286. doi: 10.1016/0140-6736(93)91822-4. [DOI] [PubMed] [Google Scholar]
- Implementing VHA’s Mental Health Strategic Plan Initiatives for Suicide Prevention Report 06-03706-126. VA Office of Inspector General; Washington, DC: [Google Scholar]
- Institute of Medicine. Reducing Suicide: A National Imperative. National Academies Press; Washington, DC: 2004. [PubMed] [Google Scholar]
- Piette JD, Heisler M. Problems due to medication costs among VA and non-VA patients with chronic illnesses. Am J Manag Care. 2004;10:861–868. [PubMed] [Google Scholar]
- Simon GE, Savarino J, Operskalski B, Wang PS. Suicide risk during antidepressant treatment. Am J Psychiatry. 2006;163:41–47. doi: 10.1176/appi.ajp.163.1.41. [DOI] [PubMed] [Google Scholar]
- Simon GE, VonKorff M. Suicide mortality among patients treated for depression in an insured population. Am J Epidemiol. 1998;147:155–160. doi: 10.1093/oxfordjournals.aje.a009428. [DOI] [PubMed] [Google Scholar]
- Speechley M, Stavraky KM. The adequacy of suicide statistics for use in epidemiology and public health. Can J Public Health. 1991;82:38–42. [PubMed] [Google Scholar]
- U.S. Food and Drug Administration. Class Suicidality Labeling Language for Antidepressants. 2005 available at http://www.fda.gov/cder/drug/antidepressants/PI_template.pdf.
- U.S. Food and Drug Administration. FDA Public Health Advisory: Suicidality in Adults Being Treated with Antidepressant Medication. [Google Scholar]
- U.S. Food and Drug Administration. FDA Public Health Advisory: Suicidality in Children and Adolescents Being Treated with Antidepressant Medications. 2004 available at http://www.fda.gov/cder/drug/antidepressants/SSRIPHA200410.htm.
- Zivin K, Kim HM, McCarthy JF, Austin KL, Hoggatt KJ, Walters H, Valenstein M. Suicide mortality among individuals receiving treatment for depression in the Veterans Affairs health system: associations with patient and treatment setting characteristics. Am J Public Health. 2007;97:2193–2198. doi: 10.2105/AJPH.2007.115477. [DOI] [PMC free article] [PubMed] [Google Scholar]