Abstract
Model-based cluster analysis is a new clustering procedure to investigate population heterogeneity utilizing finite mixture multivariate normal densities. It is an inferentially based, statistically principled procedure that allows comparison of non-nested models using the Bayesian Information Criterion (BIC) to compare multiple models and identify the optimum number of clusters. The current study clustered 36 young men and women based on their baseline heart rate (HR) and HR variability (HRV), chronic alcohol use, and reasons for drinking. Two cluster groups were identified and labeled High Alcohol Risk and Normative groups. Compared to the Normative group, individuals in the High Alcohol Risk group had higher levels of alcohol use and more strongly endorsed disinhibition and suppression reasons for use. The High Alcohol Risk group showed significant HRV changes in response to positive and negative emotional and appetitive picture cues, compared to neutral cues. In contrast, the Normative group showed a significant HRV change only to negative cues. Findings suggest that the individuals with autonomic self-regulatory difficulties may be more susceptible to heavy alcohol use and use alcohol for emotional regulation.
Keywords: Model-based Cluster Analysis, Mixture Model, Emotional Regulation, Heart Rate Variability (HRV), Alcohol Use
Finding or classifying groups of individuals who are similar to one another within a group but sufficiently different from those of other groups has been a key interest in the behavioral sciences. Cluster analysis is a widely used approach for this purpose. It is empirically driven and exploratory in the sense that the number of groups and nature of the groups are unknown in advance (Everitt, Landau, & Leese, 2001; Webb, 2002). In the field of developmental psychology, a number of influential studies have found clusters of individuals based on their early temperamental and behavioral styles, and characterized their long-term patterns of behavioral outcomes, including the classic work on three temperamental styles from the New York Longitudinal Study (easy, difficult, slow to warm up; Thomas, Chess & Birch, 1968) and the more contemporary work from the Dunedin Multidisciplinary Health and Development (undercontrolled, inhibited, well-adjusted; Caspi, 2000; Caspi & Silva, 1995).
Furthermore, recent literature has increasingly postulated that qualitatively heterogeneous subpopulations exist in developmental pathways, and heterogeneous causal processes exist for subpopulations. Evidence of heterogeneity consisting of normative as well as atypical processes can be found in many behaviors, including depressive symptoms, aggressive behaviors, and substance use behaviors (for a brief review, see Mun, Windle, & Schainker, 2008). In addition, identifying heterogeneous subpopulations can be useful for determining individuals who may be at different stages of development. For example, in the domain of cognitive stage development, Dolan, Jansen, and Van Der Maas (2004) analyzed 101 children for subpopulations based on their conservation computer task performance on four occasions during the transition from the preoperational stage to the concrete operational stage to identify correlates associated with on-time and off-time developmental transitions. Therefore, identifying subgroups of individuals is an important developmental research issue that can shed light on different processes involved in the ontogeny of many behaviors within the person-oriented and pattern-oriented approaches (Bergman & Magnusson, 1997; Magnusson, 1998, 2000; von Eye & Bergman, 2003).
Cluster Analysis
Before the advent of computers, cluster analysis was usually performed in a subjective manner by relying on the educated judgments based on similarity and dissimilarity of objects (e.g., Linnaeus' 18th century hierarchical classifications of animals and plants). With the coming of computers, empirical, data-driven cluster analysis became possible utilizing a number of clustering algorithms that compute the distance or similarity matrix between objects and find a solution that minimizes the within-cluster variation and/or maximizes the between-cluster variation. Nonetheless, due to its exploratory nature and its lack of an objective fit measure, cluster analysis continued to be viewed as rather arbitrary and subjective. This point is illustrated in the classic text on cluster analysis by Kaufman and Rousseeuw (1990) where cluster analysis is described as “the art of finding groups in data” (p. 1). With the aid of expanding computing capability, however, it is now possible to utilize a statistical model (e.g., normal mixture densities) for cluster analysis with an objective model selection tool for applied research.
Model-based Cluster Analysis
The current paper aims to introduce and illustrate model-based cluster analysis1 (Fraley & Raftery, 1998; Raftery & Dean, 2006) as an alternative to existing heuristic clustering algorithms, such as hierarchical agglomerative (e.g., Ward's clustering algorithm, single or nearest neighbor linkage, complete or furthest neighbor linkage, average linkage, centroid linkage, median linkage) and non-hierarchical (e.g., K-means clustering algorithm) clustering methods, to be used within the person-oriented and pattern-oriented perspective. Heuristic clustering algorithms are limited by the lack of a statistical fit measure to determine the adequacy of the number of clusters (Dumenci & Windle, 2001; Tonidandel & Overall, 2004), and their susceptibility to influence by the order of data input or starting values (Steinley, 2003, 2006; van der Kloot, Spaans, & Heiser, 2005). The validity of cluster solutions has been questioned because cluster analysis is capable of producing clusters even when there are no true clusters (von Eye & Bergman, 2003) and also because the number of valid clusters is often difficult to establish. A number of methods to remedy this weakness have been suggested, including resampling (Tonidandel & Overall, 2004), and utilizing a split-sample replication criterion (Overall & Magee, 1992), and fusion coefficient levels (Mojena, 1977; cf. Webb, 2002). However, these methods would be difficult for many applied researchers to implement.
In addition, each heuristic clustering method has a unique tendency to favor a certain type of solution. For instance, Ward's method or K-means clustering, by far the most commonly used methods, tend to result in spherical clusters of the same size (see Celeux & Govaert, 1992; Everitt et al., 2001; von Eye, Mun, & Indurkhya, 2004; Yeung, Fraley, Murua, Raftery, & Ruzzo, 2001), and are susceptible to over-extraction of clusters. It is generally known that a tradeoff exists between the complexity of a model (i.e., the number of estimated parameters) and the number of clusters extracted. That is, a small number of clusters may adequately represent the observed data for a more complex model, whereas a large number of clusters may be needed for a simple model (Yeung et al., 2001; for an empirical demonstration applied to latent class growth trajectory analysis, see Bauer & Curran, 2003, 2004). Therefore, it is critical to have a statistical fit measure that penalizes for complexity and rewards for parsimony when comparing different models that vary in the number of extracted clusters (e.g., Bayesian Information Criterion). This would eliminate the ambiguity associated with relying on subjective criteria (Fraley & Raftery, 1998; Raftery & Dean, 2006).
Model-based cluster analysis is one such alternative that is an inferentially based, statistically principled procedure that allows comparison of non-nested models (Raftery & Dean, 2006; Mun et al., 2008, and Appendices A and B for details). Model-based cluster analysis is based on the assumption that the observed data come from a population consisting of several subpopulations. In model-based clustering each of the subpopulations is modeled separately and the overall population is modeled as a mixture or weighted sum of these subpopulations, using finite mixture models (Raftery & Dean, 2006). The general form of a finite mixture model with k subpopulations or groups is
where πk is the proportion of the population in the kth group and fk(.) is the probability density function for the kth group. The subpopulations are modeled by members of the same parametric density family (e.g., Gaussian or t distribution), and in the case of the Gaussian density family, the finite mixture model can be written as
where ϕϕk is the parameter vector for the kth group. The mixture model then can be used to partition the data into clusters if the posterior probability that a case belongs to group k is greater than the posterior probabilities it belongs to any other group.
The observed covariance matrix can be decomposed (Banfield & Raftery, 1993) as
where λk parameterizes the largest eigenvalue of Σk and indicates the volume of the kth cluster; Dk is the matrix of eigenvectors of λkand controls the orientation of that cluster; and Ak is a diagonal matrix with the eigenvalues that represents the shape of that cluster (see Figure 1 for a graphic example of volume, orientation, and shape). By parameterizing the volume, orientation, or shape elements of the covariance matrix, a number of models are possible, ranging from the simple models to the least parsimonious model where all elements are allowed to be different across all clusters (clusters of different volumes and shapes with varying orientation).
Figure 1. Graphic illustration of volume, shape, and orientation in two-dimensional space.
The solid lines indicate ellipsoidal clusters and encircled dotted lines indicate orientation. This two-cluster figure is an example of a cluster model with equal ellipsoidal shape, unequal volume, and varying orientation.
In sum, model-based clustering explicitly targets the identification of unobserved heterogeneity in a population based on the observed data (Everitt, 2005; Everitt & Hand, 1981; McLachlan & Peel, 2000) and utilizes the expectation-maximization (EM) algorithm2 for maximum-likelihood (ML) estimation. The tasks of clustering become that of estimating (1) the parameters of the assumed mixture (i.e., nature of clusters such as their respective means, covariances) and (2) the posterior probabilities of cluster membership (Banfield & Raftery, 1993; Everitt et al., 2001) by maximizing a log-likelihood function. The convergence (and estimation) is achieved when iterations between E-steps and M-steps result in two successive likelihood values of parameters that differ less than specified in an a priori set threshold. The cluster membership for each case is then determined by assigning each case to the cluster with the highest posterior probability.
The current paper implements model-based cluster analysis using the mclust program developed by Fraley and Raftery (1998, 1999, 2002a, 2002b, 2003) and designed for S-PLUS software program (version 6 or higher; Insightful Corporation, 1988-2006) and the R language (R Development Core Team, 2006, available gratis at http://www.r-project.org/) to explore heterogeneous groups in data. A total of ten models are analyzed simultaneously by the mclust software for one through nine clusters (this default can be increased or decreased), and each model is compared against others using the Bayesian Information Criterion (BIC). If the best fitting model indicates one cluster, then the dataset is multivariate normal and does not contain a mixture of heterogeneous subpopulations. The use of model-based cluster analysis has emerged in recent years in psychology-related empirical research (e.g., Hicks, Markon, Patrick, Krueger, & Newman, 2004; Mun et al., 2008; Skeem, Johansson, Andershed, Kerr, & Louden, 2007). The current study, however, is one of the first papers to characterize this method in the context of more traditional cluster analysis approaches, structural equation models, and emerging finite mixture models, and to detail its use as a new alternative method for the person-oriented and pattern-oriented approaches used by developmental researchers (see also Mun et al., 2008).
To illustrate this method, we use objective indices of autonomic nervous system regulation from a controlled experiment to explore how individual differences in one's ability to regulate emotional arousal may be related to development of risky alcohol use patterns. By doing so, we aim to demonstrate that model-based cluster analysis can be used to identify individuals of distinctive profiles in an experimental design with a small sample, to estimate the proportion of the identified subpopulations, and to relate the probability of membership in the cluster groups to other psychosocial characteristics.
Emotional and Behavioral Regulation and Alcohol Use
An undercontrolled temperament is thought to predispose to emotional and behavioral regulation problems that are neurobiologically based and increase the likelihood of developing an alcohol or drug use disorder (Tarter et al., 1999). The present study is focused on characterizing heterogeneity in a cognitive behavioral constellation of factors (reasons for alcohol use and the intensity of drinking) during late adolescence and emerging adulthood by identifying clusters of individuals who may share commonalities in neurophysiological processes that subserve emotional and behavioral regulation. Emotional regulation is a central feature of adjustment during childhood development (Cummings & Davies, 1996; Eisenberg & Fabes, 1992; Thompson, 1994), and attempts to regulate positive and negative emotional states are fundamental motivators of alcohol use in animals and humans at all developmental stages of use (e.g., Fromme, Stroot, & Kaplan, 1993; Koob & Le Moal, 1997, 2001; Labouvie & Bates, 2002; Wenzlaff & Wegner, 2000). Emotion regulation depends in part on the ability to adaptively adjust physiological arousal in a changing and provocative environment. Adaptive adjustments and goal-directed behavior are controlled by the central autonomic network which comprises cortical (prefrontal and anterior cingulate cortices) and subcortical (e.g., hippocampus, amygdala) brain structures that are reciprocally interconnected (Benarroch, 1997).
This network initiates cardiac adjustments to support behavioral responses to salient environmental stimuli (Thayer & Brosschot, 2005; Thayer & Lane, 2000). Autonomic nervous system regulatory functions are heritable (Singh, Larson, O'Donnell, Tsuji, Evans, & Levy, 1999), sensitive (Appelhans & Luecken, 2006), and accessible indicators of emotional regulation that should vary across divergent developmental pathways of neurobehavioral disinhibition, emotional control, and alcohol use (Doussard-Roosevelt et al., 1997; El-Sheikh, 2001; El-Sheikh & Harger, 2001; El-Sheikh, Harger, & Whitson, 2001; Katz & Gottman, 1995, 1997; Tarter, Kirisci, Habeych, Reynolds & Vanyukov, 2004). Heart rate (HR) and HR variability (HRV) are objective indices of arousal and the cardiovascular system's dynamic range of adjustment that reflect the balance of sympathetic (excitatory) and parasympathetic (inhibitory) activity available to accomplish time- and magnitude-regulated emotional responding (Appelhans & Luecken, 2006). To the extent that this system is limited in its ability to modulate arousal and support goal-directed responses, behavioral repertoires may become constricted and alcohol use may become an expedient means of regulating emotions and disinhibiting behavior.
The Current Study
The aim was to identify heterogeneous processes of emotional regulation by clustering individuals based on their baseline levels of HR and HRV, their reasons for, and extent of, alcohol use, and then examining their distinctive patterns of 0.1 Hz HRV indices during response to emotional and appetitive stimuli. HRV represents the extent to which cardiac activity can be modulated and is considered an objective index of emotionally regulated responses (for a review, see Appelhans & Luecken, 2006). We synchronized the rate of cue presentation to the resonant properties of the cardiovascular system, and used a special 0.1 Hz HRV index to maximize sensitivity of response (Vaschillo, Lehrer, Rishe, & Konstantinov, 2002; Vaschillo, Vaschillo, & Lehrer, 2006). We hypothesized that behavioral disinhibition and suppression of negative affect may become differentially salient reasons for intensive alcohol use in clusters of individuals who exhibit distinctive patterns of emotional response and regulation. While the acute and chronic effects of alcohol on changes in HR and HRV have been well documented (Bennett, Sponberg, Graham, Suomi, Higley, & DePetrillo, 2001; DePetrillo, White, Liu, Hommer, & Goldman, 1999; Koskinen, Virolainen, & Kupari, 2004; Ingjaldsson, Laberg, & Thayer, 2003; Murata, Araki, Yokoyama, Sata, Yamashita & Ono, 1994; Reed, Porges, & Newlin, 1999; Rossinen, Viitasalo, Partanen, Koskinen, Kupari, & Nieminen, 1997), it is not known whether underlying subtypes of drinkers vary systematically in terms of cardiac adjustment tendencies that may reflect adaptive versus non-adaptive emotional regulation in response to emotional and appetitive cues in the environment.
The current study features the experimental data of a small sample (N = 36) with repeated 0.1 Hz HRV indices in reaction to emotional and appetitive stimuli. Although mixture models have been used almost exclusively with large samples in the applied literature, simulation studies have shown that if the means of normal mixture components (clusters) are well separated (see Figure 1 for an example of well separated clusters), and the mixing proportion is not extreme (i.e., clusters are about equal in size; toward 0.5 in two clusters), a small sample (N = 25 or 50) can achieve reasonable levels of power for a two-component normal mixture model using the likelihood ratio test (Lo, Mendell, & Rubin, 2001; Mendell, Finch, & Thode, Jr., 1993; Mendell, Thode, Jr., & Finch, 1991; Ning & Finch, 2004).
Method
Participants
Thirty-six healthy, social drinking men and women (n = 16) between the ages of 21 and 24 years who spoke English as a first language were recruited through advertisements in a northeastern U.S. university newspaper and bulletin board postings in the university and surrounding communities. Exclusion criteria included a history of psychiatric disorder or treatment, childhood learning disability or special education, neurological conditions that precluded memory testing, medical conditions that precluded alcohol administration or confounded interpretation of HRV (e.g., diabetes, heart disease), 20% over- and- under-weight from the ideal for age and gender, consumption of < 4 standard alcohol drinks (3 drinks for women) per occasion at least twice per month in the previous year, alcohol dependence, history of treatment for a substance use disorder, regular illicit or prescription drug use, lifetime diagnosis of alcohol or drug use disorder on the part of the biological mother, and for women, pregnancy, pregnancy planning, or lactating. The majority of the participants were non-Hispanic White (61%), 22% were Asian, 6% were Hispanic White, and the remaining participants were African American and other (11%). Although we recruited in the university and surrounding communities, participants meeting the study inclusion criteria were all college students with 14.6 years of education on average (SD = 1.2). Annual family (parental) income ranged from less than $10,000 to above $100,000, with the median income category of $61,000 — $ 80,000.
Picture Cues
Emotionally valenced picture cue stimuli were from the International Affective Picture System (IAPS, Lang, Bradley, & Cuthbert, 1999). Positive and negative emotional cues were matched on standardized arousal ratings, but varied systematically in valence. Neutral pictures were of moderate valence and low arousal (Bradley, Cuthbert, & Lang, 1990). Alcohol cues were from the Normative Appetitive Picture Set (Stritzke, Patrick, & Lang, 2004), supplemented with additional stimuli developed in our lab. Marijuana and club drug picture cues were from Tapert et al. (2003), with additional stimuli developed for the current study.
Procedure
Participants were randomly assigned to an alcohol challenge, placebo challenge, or no alcohol control condition. They were individually tested in a picture cue exposure phase and a picture memory phase of the experiment (the present study involves only the cue exposure phase). Participants were asked to fast for 4 hrs following a low-fat light meal before coming to the lab. Sessions were completed between 10 a.m. and 2 p.m. to minimize circadian variations in alcohol metabolism and behavioral effects. Participants provided written informed consent, then completed substance use and related questionnaires. Blood pressure, oral temperature, and a breath estimate of blood alcohol concentration were assessed, and participants were weighed to determine alcohol dose and/or drink volume, and women completed an in-lab urine pregnancy test. Alcohol doses were mixed with an orange juice mixer in a ratio of 4 parts mixer to 1 part ethanol. The beverage was divided into three equal drinks, and each was consumed during a consecutive 5-min interval. Each participant consumed 3 volume-controlled drinks that were either 100% mixer (told no alcohol = control), mixer with 100μl ethanol float per cup and other olfactory ethanol cues (placebo), or mixer plus 95% ethanol dose to produce a target blood alcohol concentration (BAC) of 90 mg/dl (alcohol). When a BAC of ~ 60 mg/dl was reached (or after 10 min in placebo and control sessions), cue presentation began. In alcohol sessions, the cue exposure was completed during a time interval that included the peak BAC and relatively adjacent segments of the ascending and descending limbs of the blood alcohol curve. Participants received $50 in compensation for their time.
Participants were seated in a comfortable reclining chair in a dimly lit, sound attenuated room with ambient temperature between 70-75 °F. A Powerlab Acquisition System (AD Instruments, Colorado Springs, CO) was used to collect electrocardiogram (ECG) data from active electrodes on right arm and left leg and ground electrode on left arm, digitalized at a rate of 1,000 samples per second. Following electrode placement, participants completed a pre-beverage (B1) and then post-beverage (B2) low cognitive demand (“plain vanilla”) baseline task, and then began cue exposure. 180 picture cues were presented in six blocks (negative, positive, neutral, alcohol, marijuana, and polydrug); each block included 15 cues that were presented twice (order randomized within 15 cue sub-blocks) and the presentation order of blocks was randomized. Each cue was shown for 5 s on and 5 s off (white screen). During white screen, participants gave either a liking or an arousal rating (order counterbalanced). This corresponded to a presentation frequency of 0.1 Hz. This presentation rate synchronized cue presentation to the resonant properties of the cardiovascular system to maximize sensitivity of cardiovascular response (Vaschillo et al., 2002; Vaschillo et al., 2006).
The WinCPRS software program (Absolute Alien Oy, Finland) was used to analyze ECG data. Raw data were preserved for evaluation of the integrity of the recorded signal, to identify abnormal heart beats, and for artifact editing. Beat-to-beat RR intervals (RRI) were assessed from the ECG signal. Sequences of RRI were summarized in 5-min epochs (corresponding to a cue block) for analyses. Mean HR and 0.1Hz HRV (power of the RRI spectrum at the frequency of 0.1Hz) indices were calculated for each 5-min epoch. HR and HRV indices can be used to evaluate adaptive changes in response to internal or environmental challenges. Changes in time domain HR indices (e.g., Mean HR) reflect shifts in metabolic processes. Changes in frequency domain HR indices (e.g., 0.1 Hz HRV) reflect changes in sympathetic and parasympathetic activity. The 0.1 Hz HRV index is more sensitive to autonomic state change and autonomic reaction to stimuli than other indices (Vaschillo et al., 2008).
Measures
Baseline mean HR and 0.1Hz HRV indices
Baseline mean HR and 0.1Hz HRV indices were calculated pre-beverage while performing the low cognitive demand baseline task. These measures were used to tap any preexisting individual differences in heart rate and heart rate variability. Means and standard deviations of all measures used in this study are shown in Table 1.
Table 1.
Descriptive Statistics of the Variables Measured
| Variable | Mean (SD) | Skewness (SE) | Kurtosis (SE) | |||
|---|---|---|---|---|---|---|
| Preexisting individual characteristics used in model-based cluster analysis | ||||||
| Mean HR (beats/min) at baseline1 | 6.96 | (0.90) | .27 | (.39) | .16 | (.77) |
| 0.1 Hz HRV index (ms2/Hz)at baseline2 | 9.08 | (1.11) | −.07 | (.39) | −.12 | (.77) |
| Disinhibition reasons for drinking | .33 | (.36) | 1.16 | (.39) | .15 | (.77) |
| Suppression reasons for drinking | .23 | (.23) | 1.05 | (.39) | .52 | (.77) |
| Alcohol QFI3 | .00 | (.79) | −.34 | (.39) | −.43 | (.77) |
| 0.1 Hz HRV indices in reaction to emotional and appetitive picture cues | ||||||
| Neutral stimuli | 9.72 | (.88) | .16 | (.39) | −.76 | (.77) |
| Negative stimuli | 10.41 | (1.08) | .13 | (.39) | 1.01 | (.77) |
| Positive stimuli | 10.17 | (1.06) | −.33 | (.39) | .23 | (.77) |
| Alcohol stimuli | 9.97 | (1.11) | .35 | (.39) | −.38 | (.77) |
| Marijuana stimuli | 9.95 | (1.16) | −.05 | (.39) | −.56 | (.77) |
| Polydrug stimuli | 9.94 | (1.21) | .31 | (.39) | −.43 | (.77) |
Note. SD = Standard Deviation, SE = Standard Error.
Mean HR was divided by 10 to reduce discrepancies in the measurement unit among variables.
A 0.1 Hz HRV index was log-transformed.
Alcohol QFI (quantity × frequency index) was log-transformed and centered for each gender.
Drinking for disinhibition reasons
Drinking for disinhibition reasons was measured using the Reasons for Alcohol Use Questionnaire (Labouvie & Bates, 2002). It is an 8-item self-report measure that assesses the extent to which individuals drink for social facilitation and mood enhancement. Example items are: “to gain self-confidence and be courageous”, “to help me express feelings more freely”, “to relate better to people”, and “to feel sexy and “on the make””. A three-choice response format was used for each item, with responses ranging from 0 = not at all important to 2 = very important. Responses were summed and averaged. Internal consistency (Cronbach's α) in the current sample was .82.
Drinking for suppression reasons
Drinking for suppression reasons was measured using the Reasons for Alcohol Use Questionnaire (Labouvie & Bates, 2002). It is a 13-item self-report measure that assesses the extent to which individuals drink to reduce negative affect, cope, or reduce tension. Example items are: “to get unwanted thoughts out of my mind”, “to blank out awareness of certain feelings or desires”, and “to let me forget all my troubles.” A three-choice response format was used for each item, with responses ranging from 0 = not at all important to 2 = very important. Responses were summed and averaged. Internal consistency (Cronbach's α) in the current sample was .79.
Alcohol quantity and frequency index (QFI)
Alcohol use was assessed using a modified version of the RHHDP Alcohol and Drug Use Questionnaires (Pandina, Labouvie, & White, 1984), reliable and valid measures that have been widely used. Participants were asked about their typical alcohol use (quantity and frequency) during the last 30 days, and the index score was created by multiplying quantity by frequency. The resulting alcohol quantity and frequency index score was then log-transformed as it is the standard procedure in the literature.
Within-individual changes in 0.1 Hz HRV indices in reaction to emotional and appetitive cues
0.1 Hz HRV indices in response to six picture cue types (negative, positive, neutral, alcohol, marijuana, and polydrug) were estimated. We focused on within-individual changes in the 0.1 Hz HRV indices from the neutral block to each of the other five emotional and appetitive cue blocks (i.e., change scores by subtraction) in the current study. Participants in the alcohol and placebo experimental groups in the current sample showed decreased levels of HRV and mean HR increased for those in the alcohol group, while processing picture cues, compared to participants in the control group (Vaschillo et al., 2008). However, the mean level differences in HRV across groups did not affect within-individual changes, Fs ranging from .39 to 2.79, ns, with effect size η2 estimates ranging from .02 to .15. In other words, there were no significant group effects on intraindividual change in HRV indices in reaction to emotional and appetitive cue blocks over and above those to the neutral cue block. This was expected given that potentially different reactivity for the alcohol and placebo groups was reflected in their reactivity to the neutral block, and these differences were subsequently subtracted in the current study to create change scores. Therefore, the resulting change scores indicate intraindividual change in HRV indices in reaction to emotional and appetitive cue blocks.
Results
Model-based Cluster Analysis: Identification of Groups
Participants' baseline heart rate (HR) and HR variability (HRV), chronic alcohol use, and reasons for drinking were analyzed in model-based cluster analysis to detect if heterogeneity existed in the sample with regard to these individual background characteristics. Data were assessed for the degree to which the data fit the multivariate normal distribution both before and after cluster analysis. Model-based clustering tends to work best when the data follow the multivariate normal distribution. However, the model-based clustering methods are reasonably robust to deviations from the multivariate normal distribution (Hardin & Rocke, 2004; Yeung et al., 2001). We also examined the range and variability for each of five variables and divided the baseline mean HR by 10 to prior to cluster analysis to reduce discrepancies in measurement units across variables. Alcohol QFI was centered for each gender after the log-transformation to prevent any gender differences in alcohol consumption levels from influencing the cluster solution. Table 1 shows univariate means and standard deviations that reflect these linear transformations. Typically, variables are standardized prior to cluster analysis in heuristic hierarchical cluster methods (e.g., Ward's algorithm) because the distance measure utilized is scale-dependent. This standardization procedure is equivalent to weighting variables to be inversely proportional to the measure of variability (i.e., standard deviation). This implies that the importance of a variable in cluster analysis decreases when its variability increases, yet this may potentially weaken between-group differences on the variables that well discriminate groups (Everitt et al., 2001, pp. 48 – 52). In model-based cluster analysis, however, cluster solutions are not affected by changes in a variable's unit of measurement when dealing with normal distributions with unknown variances (Vermunt & Magidson, 2002). For these reasons, we did not standardize the five variables by each standard deviation unit. As expected, however, when all five variables were standardized, the clustering outcome remained the same.
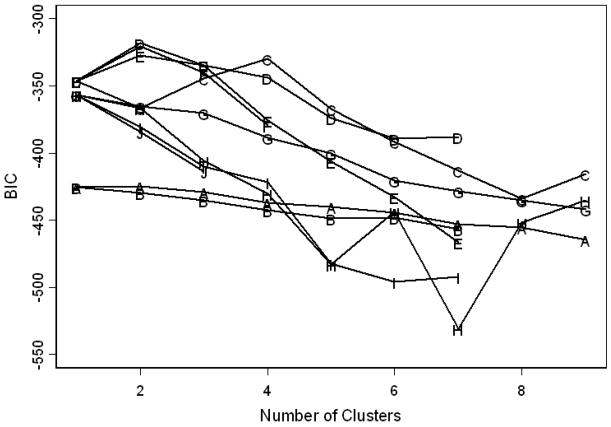
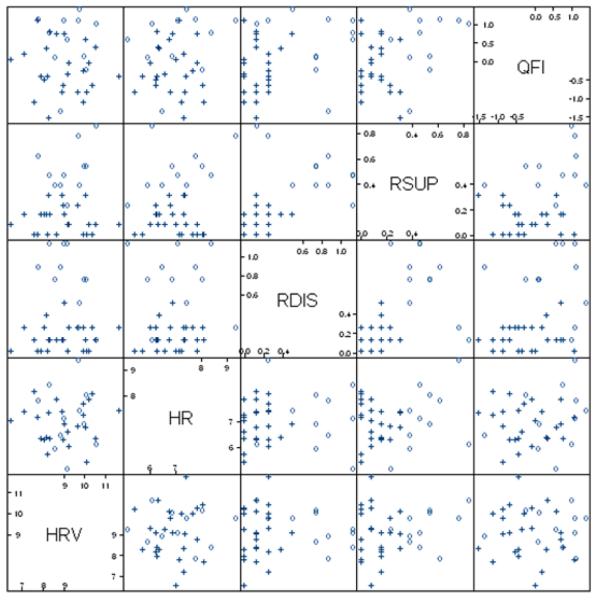
The results of the model-based cluster analysis are shown in Figure 2. The best fitting model involved a two-cluster solution with the Bayesian Information Criterion (BIC) = −318.155. This model consisted of two clusters of equal shape but different volumes. The mixing proportions were .31 (n = 11), and .69 (n = 25) for Clusters 1 and 2, respectively. The average posterior probabilities for the most likely cluster classification were .9995 and .9940 for Clusters 1 and 2, respectively. This indicates a high degree of classification certainty for the two-cluster solution. The scatter plot matrix presented in Figure 3 shows the two clusters across five variables. The two clusters are visibly separated, especially on the domains of alcohol use (QFI), and suppression and disinhibition reasons for drinking.
Figure 2. Bayesian Information Criterion (BIC) of different cluster solutions.
A = Spherical, equal volume, equal shape; B = Spherical, unequal volume, equal shape; C = Diagonal, equal volume, equal shape; D = Diagonal, unequal volume, equal shape; E = Diagonal, equal volume, varying shape; F = Diagonal, unequal volume, varying shape; G = Ellipsoidal, equal volume, equal shape, equal orientation; H = Ellipsoidal, equal volume, equal shape, varying orientation; I = Ellipsoidal, unequal volume, equal shape, varying orientation; J = Ellipsoidal, unequal volume, varying shape, varying orientation. The BIC value for D model was −318.155.
Figure 3. Scatter plot matrix showing two clusters.
The symbol “o” indicates the High Alcohol Risk group; “+” indicates the Normative group. Two clusters are visibly separated. HR = Mean heart rate, HRV = A 0.1 Hz heart rate variability index, RDIS = Disinhibition reasons for drinking, RSUP = Suppression reasons for drinking, QFI = alcohol quantity and frequency index.
We repeated the analysis for randomly selected 75% of the sample. A similar clustering model (diagonal, unequal volumes and unequal shapes) with two clusters was the best model, resulting in the BIC value = −260.371. The overlap between the full sample and the random sample was substantial as indicated by the variation of information (VI) criterion = .077. The variation of information (VI) criterion proposed by Meilă (2003, 2007) measures the amount of information lost and gained in changing from clustering “a” to clustering “b” and vice versa. Upon close examination, the two cluster solutions were similar in their mean locations on the five variables, and only one case switched his or her group. In addition, we also took steps to evaluate that multivariate normality was observed in each cluster by examining the marginal distributions (univariate normality), and also by examining variance for univariate data and generalized variance for multivariate data. Based on the additional analyses, the two cluster solution was deemed satisfactory3.
Model-based Cluster Analysis: Interpretation of Groups
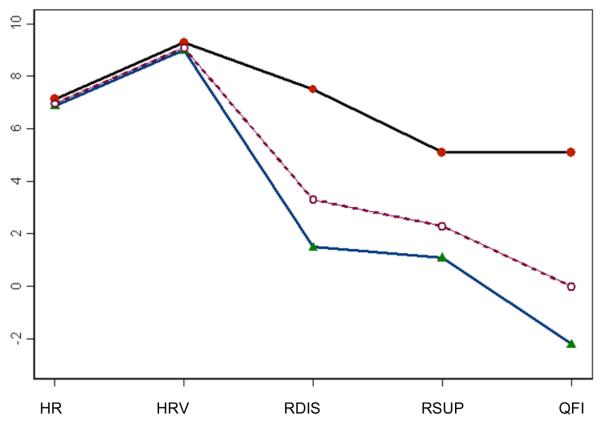
The two clusters included a High Alcohol Risk group (Cluster 1, n = 11) who reported higher levels of alcohol use, and more strongly endorsed disinhibition and negative affect suppression reasons for drinking; and a Normative group (Cluster 2, n = 25) who had lower levels of disinhibition and suppression reasons for drinking, and lower levels of alcohol use (see Table 2). There were no significant differences in mean HR and 0.1 Hz HRV indices at baseline (background state) across these two cluster groups4. Descriptive statistics for the five variables and inferential statistics across the two cluster groups are shown in Table 2. The profile plots of the five preexisting individual characteristics are shown in Figure 4.
Table 2.
Descriptive Statistics of Mean HR and HRV Indices at Baseline, Alcohol Use, and Reasons for Alcohol Use by Cluster Groups
| High Alcohol Risk (n =11) |
Normative (n = 25) |
F | η2 | |||
|---|---|---|---|---|---|---|
| Mean HR (beats/min) at baseline1 | 7.13 | (1.22) | 6.88 | (.73) | .59 | .013 |
| 0.1 Hz HRV index (ms2/Hz)at baseline2 | 9.27 | (.84) | 8.99 | (1.21) | .47 | .017 |
| Disinhibition reasons for drinking | .75 | (.34) | .15 | (.13) | 60.03* | .638 |
| Suppression reasons for drinking | .51 | (.18) | .11 | (.10) | 71.01* | .676 |
| Alcohol QFI2 | .51 | (.81) | −.22 | (.69) | 7.81* | .187 |
| Frequency of alcohol use3 | 3.42 | (2.29) | 1.60 | (1.07) | 10.86* | .319 |
| Typical drinks per occasion4 | 4.73 | (3.88) | 3.72 | (2.32) | .94 | .068 |
Note.
p < .05. Values in parenthesis indicate standard deviations.
Mean HR was divided by 10 to reduce discrepancies in the measurement unit among variables.
A 0.1 Hz HRV index and the alcohol QFI (quantity × frequency index) scores were log-transformed. Alcohol QFI was then centered for each gender.
The reported mean values of 3.42 and 1.60 for frequency of alcohol use approximately translate into drinking 3-4 days a week versus 1-2 days a week during the past month, respectively, in the two cluster groups.
Figure 4. Profile plots of HR, HRV, alcohol use, and reasons for drinking by cluster groups.
The top solid line represents the High Alcohol Risk group and the bottom solid line represents the Normative group. The dotted line in the middle represents the average. HR = Mean heart rate, HRV = A 0.1 Hz heart rate variability index, RDIS = Disinhibition reasons for drinking, RSUP = Suppression reasons for drinking, QFI = alcohol quantity and frequency index.
Changes in 0.1 Hz HRV indices in Response to Emotional and Appetitive Picture Cues
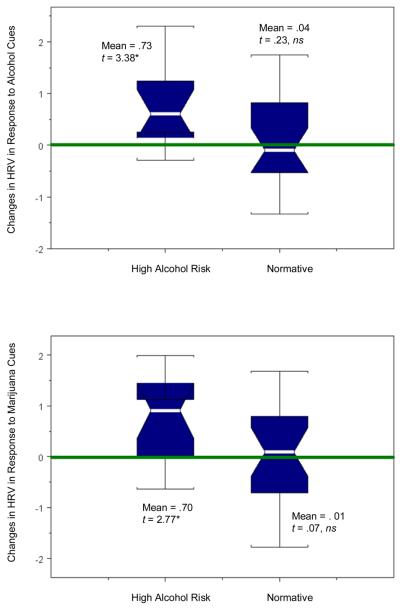
We then analyzed how individuals in the two cluster groups changed in their 0.1 Hz HRV indices in response to emotional and appetitive picture cues compared to their reaction to neutral cues. Full results on change scores in 0.1 Hz HRV indices are shown in Table 3 and key results are shown in Figure 5. In addition, original observed means and standard deviations of 0.1 Hz HRV indices in the original metric and the adjusted means and standard errors across the two cluster groups from ANCOVA results, with the 0.1 Hz index score in response to the neutral cues as a covariate, are shown in Table 4. The High Alcohol Risk group showed significant changes in 0.1 Hz HRV indices in response to all emotional and appetitive cues, and all effect size estimates exceeded .8 (large effect, Cohen, 1988, p. 40). In contrast, individuals in the Normative group showed changed levels of 0.1 Hz HRV in response only to negative cues, d = .54 (medium effect), with a slightly increased (d = .25, small effect), but nonsignificant HRV response to positive cues. For the total sample aggregated across clusters, there were statistically significant changes in 0.1 Hz HRV indices to negative and positive cues.
Table 3.
Within-individual Changes in 0.1 Hz HRV Indices in Response to Emotional and Appetitive Picture Cues Compared to Neutral Cues
| High Alcohol Risk (n = 11) |
Normative (n = 25) |
Total (N = 36) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | t | d | Mean | t | d | Mean | t | d | |
| 0.1 Hz HRV – Negative+ | 1.23 | 3.34* | 1.01 | .45 | 2.70* | .54 | .69 | 4.06* | .68 |
| 0.1 Hz HRV – Positive | .88 | 3.00* | .91 | .25 | 1.26 | .25 | .44 | 2.62* | .44 |
| 0.1 Hz HRV – Alcohol+ | .73 | 3.38* | 1.02 | .04 | .23 | .05 | .25 | 1.72 | .29 |
| 0.1 Hz HRV – Marijuana | .70 | 2.77* | .83 | .01 | .07 | .01 | .22 | 1.33 | .22 |
| 0.1 Hz HRV – Polydrug+ | .91 | 4.23* | 1.28 | −.09 | −.43 | .09 | .21 | 1.18 | .20 |
Note.
p < .05. 0.1 Hz HRV indices were log-transformed. Figure 5 illustrates Table 3 in graphic, and Table 4 shows adjusted and unadjusted mean levels of 0.1 Hz HRV indices. d = Cohen's effect size. + There were significant between-group differences in 0.1 Hz index change scores in response to Negative, Alcohol, and Polydrug picture cue blocks, t (34) = 2.27 (d = .77), 2.31 (d = .85), and 2.80 (d = 1.12) at p < .05, respectively, with the High Alcohol Risk group exhibiting significantly higher increases than the Normative group.
Figure 5. Box plots of within-individual changes in 0.1 Hz HRV indices in response to emotional and appetitive picture cues compared to neutral cues.
The notched section in the middle of the box plots gives the width of an approximate 95% confidence interval about the median, which is indicated by the white band in the middle of the notched section. The top and the bottom of the box represent the 75th and 25th percentiles of the distribution. Single lines beyond the cap of the brackets of the box plots are potential outliers. The horizontal x-axis line at y-axis = zero indicates the reference line for no within-person change in 0.1 Hz HRV. Figure 5 illustrates key results in Table 3 in graphic.
Table 4.
Unadjusted and Adjusted Means of 0.1 Hz HRV Indices in Response to Emotional and Appetitive Picture Cues
| Observed Means |
Adjusted for Reactivity to Neutral Cues |
|||||||
|---|---|---|---|---|---|---|---|---|
| High Alcohol Risk (n = 11) |
Normative (n = 25) |
High Alcohol Risk (n = 11) |
Normative (n = 25) |
|||||
| M | SD | M | SD | M | SE | M | SE | |
| 0.1 Hz HRV – Negative | 10.76 | 1.13 | 10.26 | 1.04 | 10.88 | .28 | 10.20 | .18 |
| 0.1 Hz HRV – Positive | 10.41 | 1.23 | 10.06 | .99 | 10.53 | .28 | 10.01 | .19 |
| 0.1 Hz HRV – Alcohol | 10.25 | 1.44 | 9.85 | .94 | 10.42 | .25 | 9.78 | .17 |
| 0.1 Hz HRV – Marijuana | 10.22 | 1.14 | 9.82 | 1.17 | 10.37 | .29 | 9.76 | .19 |
| 0.1 Hz HRV – Polydrug | 10.44 | 1.23 | 9.72 | 1.16 | 10.59 | .30 | 9.65 | .20 |
| 0.1 Hz HRV – Neutral | 9.52 | 1.10 | 9.81 | .78 | -- | -- | -- | -- |
Note. There were significant between-group differences in 0.1 Hz index levels in response to Negative, Alcohol, and Polydrug picture cue blocks, F (1, 33) = 4.13, 4.59, and 6.75 at p < .05, respectively, with the High Alcohol Risk group exhibiting significantly higher levels of 0.1 Hz HRV indices than the Normative group. 0.1 Hz HRV indices were log-transformed.
Relative to the Normative group, the High Alcohol Risk group appears to have low reactivity to neutral cue blocks but to exhibit relatively more arousal and reactivity to all emotional and appetitive cues (see Tables 3 and 4). Although there were no statistical differences across the two cluster groups using the original unadjusted scores, group differences in arousal and reactivity in reaction to negative, alcohol, and polydrug cue blocks surfaced when we analyzed change scores (see Table 3 and Figure 5) or analyzed change using the ANCOVA approach across the two groups (see Table 4). The High Alcohol Risk group appeared to have elevated levels of arousal and reactivity also to positive and marijuana cue blocks, compared to those of the Normative group. However, the two groups were not significantly different from each other, t(34) = 1.79, p = .08, d = .65 and t(34) =1.95, p = .06, d = .73, respectively for positive and marijuana cue blocks, due in part to the small sample5.
Discussion
Model-based Cluster Analysis
The current study sought to examine one aspect of the heterogeneity in emotion regulation using a mixture model-based cluster analysis approach. Model-based cluster analysis utilizing finite mixture densities can be a valuable analytic tool for research in developmental psychology for a number of reasons. First, model-based cluster analysis can be used to generate a new set of hypotheses based on salient detected patterns of cases or individuals. Since developmental psychology is an integrative discipline that strives to delineate normative as well as atypical processes, it often becomes necessary for researchers to explore data in order to generate new hypotheses for future directions, and to explore heterogeneous populations to better integrate distinctive processes into a consistent conceptual framework. Second, this method can be used as an alternative method for comparing groups when the assumption of measurement invariance is either untenable or unreasonable across different populations or longitudinal time measurements. Measurement invariance is obtained when the measurement model that relates observed variables to underlying factors is shown to be identical across subpopulations or across time. Strong measurement invariance requires equality of factor loadings, intercepts, and residual variances across measurements (Meredith, 1993). Model-based cluster analysis does not require the assumption of measurement invariance, and may be used as an alternative method. Third, model-based cluster analysis can be used to address person-oriented questions that have arisen through variable-oriented research.
Model-based cluster analysis shares a commonality with other emerging analytic techniques that utilize finite mixture densities, including latent class growth curve analysis and, more broadly, parametric factor mixture models. That is, all of these analyses are concerned with detecting heterogeneous subpopulations in data. In model-based cluster analysis, however, the individual is the focal point of analysis, while in parametric factor mixture models, the relationships among (latent) variables are typically the main focus, with latent classes serving as a moderator in the specified relationships among variables. On the one hand, the latter presents an opportunity to discover pockets of individuals who may not be easily explained by the specified relationships. For example, using the factor mixture approach in treatment efficacy research, it is possible to empirically identify those who respond differentially to the treatment. On the other hand, poorly specified relationships among variables could be masked and compensated by extracting more latent classes to fit the data, potentially resulting in misleading conclusions with this more variable-oriented approach. Model-based cluster analysis identifies individuals who are similar to one another within a cluster but sufficiently different from those of other clusters, using the same statistical model (i.e., finite mixture densities) with an objective fit measure. We propose that the model-based cluster analysis approach is an attractive option in the arsenal of powerful analytic tools at the disposal of developmental researchers.
Emotional Self-Regulation and Alcohol Use
Substantively, the current study demonstrated that psychophysiological data can be used to examine heterogeneity in an individual's response to emotional and appetitive cues in the environment, which may reflect individual differences in susceptibility to stress and vulnerability. We identified two clusters of individuals based on their background state HR and HRV indices, chronic alcohol use, and reasons for drinking. As indicated by profile plots, the two clusters were well separated in the domains of alcohol use and reasons for drinking. The High Alcohol Risk group accounted for 31% of the total sample and exhibited relatively higher levels of chronic alcohol use, and more strongly endorsed disinhibition and negative affect suppression reasons for drinking. Individuals in the Normative group accounted for 69% of the total sample and exhibited more normative and lower levels of alcohol use and reasons for drinking6.
Of the two cluster groups, the High Alcohol Risk group was highly reactive to emotional and appetitive cues as indicated by significant and large effect size changes in 0.1 Hz HRV indices. The Normative group showed a significant, moderate effect size change in HRV only to negative cues. Results suggest that behavioral disinhibition and suppression of negative affect and thoughts may have become differentially salient reasons for intensive alcohol use for the High Alcohol Risk group, which exhibited distinctive patterns of emotional response and regulation.
Findings from the present study further underscore the importance of individual differences in self-regulation in emotions, behaviors, and biological functions in understanding the etiology of maladaptive behaviors such as heavy substance use. Although temperamental deviations have long been suggested (Moffitt, 1993; Tarter & Vanyukov, 1994; Zucker, Fitzgerald, & Moses, 1995) as an early indicator of risk and/or deficits in behavioral and emotional regulation in expressing one's self in socially acceptable ways (Posner & Rothbart, 2000), few studies exist that empirically tested objective indices of emotional regulatory patterns in a controlled experiment. Furthermore, while negative mood states are thought to initiate a chain of failures to self-regulate substance use, acute changes in negative affect are likely to strain physiological systems that maintain homeostasis more in some individuals than others. This model of individual differences in emotion regulation and differential alcohol use risk has not been previously applied to adolescents and emerging adults who are at earlier stages of alcohol and drug exposure, compared to chronic heavy substance users in clinical populations. Findings from the present study suggest that the individuals with emotional self-regulatory difficulties may be more susceptible to heavy alcohol use, and this link may well be established by the early twenties. Self-regulatory difficulties may be traced to early childhood (El-Sheikh, 2005). Recent studies have revealed that marital conflict is associated with disruptions in children's biological regulation of sleep (El-Sheikh, Buckhalt, Mize, & Acebo, 2006), which, in turn, is increasingly linked with adjustment problems in childhood and adolescence, including early onset of alcohol and other drug use (Wong, Brower, Fitzgerald, & Zucker, 2004), and neurobehavioral functioning and behavior problems (Sadeh, Gruber, & Raviv, 2002), although the exact mechanisms of these associations remain unknown.
Intraindividual change and change scores
This study took the approach of examining within-individual change in reactivity using change scores rather than comparing individuals on their absolute levels in response to the emotional and appetitive cue blocks. Change scores (or difference/gain scores) have long been misconstrued as inherently unreliable (e.g., Lord, 1956; cf. Rogosa, 1988) and Cronbach and Furby (1970) influentially and provocatively challenged whether change should be measured at all (cf. Raykov, 1999). In response, Rogosa (1988, 1995) and Williams and Zimmerman (1996), among others, demonstrate that the reliability of change scores depends on the following three factors: First, the ratio of the pretest standard deviation and the posttest standard deviation (the smaller the better); second, the reliability of pretest and posttest scores (the higher the better); and third, the correlation between pretest and posttest scores (the smaller the better). The current study features highly reliable experimental measures, with relatively modest correlations ranging from .47 to .63, and the ratios of standard deviations ranging from .73 to .83. Therefore, analyzing change scores is a good analytic approach for the data in the current study. In this study, the change scores approach and the ANCOVA approach yielded the same statistical results on group differences in changes and adjusted levels.
However, one needs to consider that the change score approach tends to show biased results when the distribution is skewed, while the ANCOVA approach tends to yield biased results when individual differences at baseline are real (Cribbie & Jamieson, 2000; Jamieson, 1999). Note also that change scores can be utilized in structural equation models (e.g., Bates, Mun, Vaschillo, Vaschillo, & Udo, 2007; McArdle & Hamagami, 2001; Mun, von Eye, & White, 2009; Raykov, 1994, 1999) or in a manifest variable categorical data analysis approach (e.g., von Eye & Mun, 2007, 2008).
Directions for Future Research
The present study has several potential limitations. First, it features a sample of predominantly college students of similar age and backgrounds who do not have alcohol use disorders at this time. Studies that utilize the same design for individuals in different age groups, and with different substance use histories and other backgrounds could shed light on other relevant mechanisms involved in self-regulatory processes. Second, the present study is potentially limited by the small sample size. The feasibility of utilizing two component normal mixture models has been demonstrated in simulation studies (Lo et al, 2001; Mendell et al., 1991, 1993; Ning & Finch, 2004). However, more simulation studies under different conditions are needed to better understand the sample sizes necessary to reliably detect three or more components that differ in the mixing proportion, the distance, and the number of variables7. In addition, empirical replication studies may further buttress the findings reported in this study. Third, due to the nature of one-time data collection, it is difficult to gauge the temporal direction, that is, whether other preexisting individual vulnerabilities contribute to different patterns of emotional regulation, or difficulties in emotional regulation contribute to individual vulnerabilities to heavy alcohol use.
Despite these limitations, we believe that the current study has strengths and contributes to the literature. First, unlike many other existing clustering procedures, the new mixture clustering method used in this study is ideal for finding groups unknown in advance but where patterns are suspected. The model-based cluster analysis using the mclust program (Fraley & Raftery, 2002a, 2002b, 2003), in particular, eliminates the subjective arbitrariness one faces when selecting a clustering procedure and deciding the number of clusters, which is especially important since there generally exists a trade-off between model complexity and the number of clusters (see Bauer & Curran, 2003, 2004 for examples). Second, the current study utilized an experimental design to study emotional regulation, and revealed that substantial individual differences exist in processing emotional and appetitive environmental cues. By the ages of 21 to 24 years, it appears that some individuals may have developed alcohol use patterns to help regulate their emotional arousal.
Acknowledgments
This study was supported, in part, by grants R01 AA015248 and K02 AA00325 from the National Institute of Alcohol Abuse and Alcoholism, and grant P20 DA017552 from the National Institute of Drug Abuse. The authors thank Paul Lehrer, Steve Buyske, and anonymous reviewers for helpful suggestions, and Tomoko Udo and Bronya Vaschillo for assistance with data collection.
Appendix A
Model-based Cluster Analysis, Factor Analysis, and Principal Component Analysis A general model for confirmatory factor analysis is expressed as x = α + Λξ + ε. The covariance matrix is expressed as E[(x − μ)(x − μ′) = Λ Φ Λ′ + ϴ. If residual covariance matrix ϴ = 0 and correlation matrix among latent factors Φ = I, then factor analysis is equivalent to principal component analysis and the resulting covariance matrix is simplified to Σ = ΛΛ′. When there are p number of variables and all p components (or factors) are extracted, this covariance matrix can alternatively be expressed into Σ = DΛD′, or Σ = λDAD′, where D = n × p orthogonal matrix of eigenvectors, and Λ = λ A , p × p matrix of eigenvalues, where λ is a scalar and A is a diagonal matrix whose elements are proportional to the eigenvalues of Σ. The following three components determine the geometric features of the observed data: λ parameterizes the volume of the observation, D indicates the orientation, and A represents the shape of the observation.
When population heterogeneity is explicitly hypothesized as in model-based cluster analysis, the observed covariance matrix is decomposed into the following general form
where λk parameterizes the volume of the kth cluster, Dk indicates the orientation of that cluster, and Ak represents the shape of that cluster (Banfield & Raftery, 1993, Fraley & Raftery, 1998, 1999, 2002a, 2002b, 2003). The subscript k indicates that each component (or cluster) can have different volume, shape, and orientation. The ten models tested simultaneously using the mclust program reflect different spectral decompositions: A = Spherical, equal volume, equal shape; B = Spherical, unequal volume, equal shape; C = Diagonal, equal volume, equal shape; D = Diagonal, unequal volume, equal shape; E = Diagonal, equal volume, varying shape; F = Diagonal, unequal volume, varying shape; G = Ellipsoidal, equal volume, equal shape, equal orientation; H = Ellipsoidal, equal volume, equal shape, varying orientation; I = Ellipsoidal, unequal volume, equal shape, varying orientation; J = Ellipsoidal, unequal volume, varying shape, varying orientation.
Appendix B - Partial mclust Input File
Note: Anything following a ‘>’ sign indicates commands that are typed in the commands window. Anything following a ‘#’ sign indicates comments.
# A command to invoke the mclust library
> library (mclust)
# The new data file “mebset1” is created from the columns 4 through 8 for all cases from the existing “dumpdp” data file
> mebset1 <- dumpdp[, 4:8]
# “dpcluster” is the name of the output file for cluster results, and “mebset1” is the name of the data file to be clustered
> dpcluster <- EMclust (mebset1)
# Print cluster results on screen
> dpcluster
# Print a plot of all BIC values on screen
> plot (dpcluster)
# This summary function gives a classification as well as model parameters
> dpclssum <- summary (dpcluster, mebset1)
# Print key statistics on screen
> dpclssum
# Save the most likely cluster membership
> dpclass <- dpclssum$classification
# Save the posterior probabilities for each cluster for each individual case
> dpz <- dpclssum$z
# The most likely cluster membership and posterior probabilities for each cluster for each individual case are added to the original dataset
> mebsetall <- cbind (matrix(seq(1:36), ncol=1), dumpdp, dpclass, dpz)
# A random sample selected from the full sample with .75 selection probability and analyzed
> random1 <- mebset1 [rbinom(nrow(mebset1), 1, .75) == 1, ]
> dpclsr1 <- EMclust (random1)
> dpclsr1
> plot (dpclsr1)
> dpclsr1sum <- summary (dpclsr1, random1)
> dpclsr1sum
> dpclassr1 <- dpclsr1sum$classification
> dpz1 <- dpclsr1sum$z
# Two independent cluster groupings can be compared - An example
> compareClass(random1.class$rdclass1, random1.class$ogclass1)
Appendix C - Model-based Cluster Analysis and Other Heuristic Clustering Methods
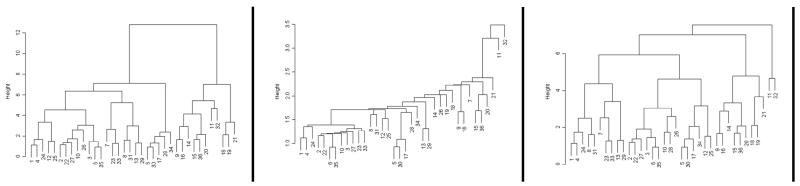
Cluster solutions from model-based cluster analysis, Ward's method (with standardized variables), and K-means produced the same clustering classification (see Table below). Average, complete, and single linkage methods resulted in similar classifications. However, they tended to result in a very small size cluster with the present data (see Figure below). Smaller VI criterion estimates in Table below indicate better convergence between any two clustering solutions.
Table.
Comparisons between Model-based Cluster Analysis Solution and Heuristic Clustering Algorithms using the VI criterion
| 2 | 3 | 4 | 5 | 6 | 7 | |
|---|---|---|---|---|---|---|
| 1. Model-based clustering | 0.000 | 0.000 | 0.331 | 0.188 | 0.193 | 0.193 |
| 2. Ward's with standardized variables1 | - | 0.000 | 0.331 | 0.188 | 0.193 | 0.193 |
| 3. K-means without standardized variables | - | 0.331 | 0.188 | 0.193 | 0.193 | |
| 4. Ward's without standardized variables1 | - | 0.188 | 0.229 | 0.229 | ||
| 5. Single linkage with standardized variables1 | - | 0.046 | 0.046 | |||
| 6. Complete linkage with standardized variables1 | - | 0.000 | ||||
| 7. Average linkage with standardized variables1 | - |
Note.
Euclidean distance was used as a dissimilarity base measure. For clustering solutions from 2 to 7, the number of clusters was set to 2.
Figure.
Ward, single linkage, and complete linkage dendrograms for the data, respectively from left to right. The height on the y-axis represents the distance at which each fusion of clusters was made.
Footnotes
Model-based cluster analysis can also be referred to as mixture cluster analysis, non-parametric factor mixture analysis (Muthén, 2006), unsupervised learning (McLachlan & Peel, 1996), or latent class cluster analysis (Vermunt & Magidson, 2000).
The EM algorithm was originally introduced by Dempster, Laird, and Rubin (1977) to provide iterative computation of the maximum likelihood estimates from incomplete data. In the context of model-based cluster analysis, it is the labels of the component density that are missing in observed data, and the EM algorithm for MLE iterates until it reaches the convergent criteria computing estimates of the posterior probabilities (i.e., probabilistic cluster membership) from the likelihood equations given initial estimates of the parameters (i.e., the nature or form of clusters such as size, orientation, etc.) of the mixture, and evaluating the likelihood equations. This is in stark contrast to a hierarchical agglomerative method where once objects are grouped into a cluster in a series from the initial N-number of clusters (each object constitutes one cluster) to a chosen k-number of clusters, they cannot be reconsidered (i.e., hard classification; also see the dendrograms in Appendix C), which is one reason why the entry order of cases in data can influence resulting cluster solutions. The same applies for hierarchical partitioning or divisive algorithms.
We additionally analyzed several heuristic clustering methods and compared their solutions with the one reported from model-based cluster analysis (see Appendix C).
When baseline HR and HRV were excluded from model-based cluster analysis, the analysis resulted in the same cluster model with two clusters. The comparisons between the two cluster solutions resulted in VI = .06 and one individual case crossing over to the other category. However, the solution came with an increased uncertainty level; therefore, we decided to include baseline mean HR and HRV in model-based cluster analysis.
Power analysis revealed that to achieve the nominal power of 0.8, 39 and 31 individuals per each group were needed to detect group differences, in reaction to positive and marijuana cue blocks, respectively.
We additionally compared the two cluster groups to see if the High Alcohol Risk group had higher levels of social reasons for drinking than the Normative group. Social reasons, the other of the three reasons factors identified by Labouvie and Bates (2002), includes items such as “It's something my friends do when we get together” and “My friends expect me to drink.” The two cluster groups did not significantly differ on the domain of social reasons for drinking, F = 4.03, η2 = .11. This suggests that reasons for use in the High Alcohol Risk group were differentially high for disinhibition and suppression, but not for social facilitation.
Ning and Finch (2004) tabulated power tables for N = 25, 50, 100, and 200 for two component mixture models and demonstrated that, with the mixing proportion ranging from .5 to .7 and the Mahalanobis distance (MD) of at least 4 between two clusters, power of .8 or above is achieved with a sample of 50. In our current study, the MD between two clusters was 4.54 using the minimum volume ellipsoid covariance estimate, and 1.98 using the observed common covariance estimate. However, N requirement to reliably detect components considerably increases if one hypothesizes a proportionately small subset of individuals (e.g., 5%) or several heterogeneous clusters. In addition, other conditions may exist that affect power estimates. For example, power for factor mixture models, in which the structure among variables is specified to hold within each of the derived latent classes, is more complex and is influenced by a number of factors, including the mixing proportion and the separation among clusters (Lubke & Neal, 2006) and model size, covariates, and class-specific parameters in factor mixture models (Lubke & Muthén, 2007).
References
- Absolute Aliens Oy . WinCPRS [computer software] Author; Turku, Finland: 1999. [Google Scholar]
- Appelhans BM, Luecken LJ. Heart rate variability as an index of regulated emotional responding. Review of General Psychology. 2006;10(3):229–240. [Google Scholar]
- Banfield JD, Raftery AE. Model-based Gaussian and non-Gaussian clustering. Biometrics. 1993;49:803–821. [Google Scholar]
- Bates ME, Mun EY, Vaschillo E, Vaschillo B, Udo T. Discovery focused hypothesis generation in the experimental study of change mechanisms [Abstract] Alcoholism: Clinical and Experimental Research. 2007;31(6):S047. [Google Scholar]
- Bauer DJ, Curran PJ. Distributional assumptions of growth mixture models: Implications for overextraction of latent trajectory classes. Psychological Methods. 2003;8(3):338–363. doi: 10.1037/1082-989X.8.3.338. [DOI] [PubMed] [Google Scholar]
- Bauer DJ, Curran PJ. The integration of continuous and discrete latent variable models: Potential problems and promising opportunities. Psychological Methods. 2004;9(1):3–29. doi: 10.1037/1082-989X.9.1.3. [DOI] [PubMed] [Google Scholar]
- Benarroch EE. Overview of the organization of the central autonomic network. In: Benarroch EE, editor. Central autonomic network: Functional organization and clinical correlations. Futura Publishing Company; Armonk, NY: 1997. pp. 3–28. [Google Scholar]
- Bennett AJ, Sponberg AC, Graham T, Suomi SJ, Higley JD, DePetrillo PB. Initial ethanol exposure results in decreased heart rate variability in ethanol-naïve rhesus monkeys. European Journal of Pharmacology. 2001;433:169–172. doi: 10.1016/s0014-2999(01)01445-5. [DOI] [PubMed] [Google Scholar]
- Bergman LR, Magnusson D. A person-oriented approach in research on developmental psychopathology. Development and Psychopathology. 1997;9:291–319. doi: 10.1017/s095457949700206x. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Cuthbert BN, Lang PJ. Startle reflex modification: Emotion or attention? Psychophysiology. 1990;27:238–248. doi: 10.1111/j.1469-8986.1990.tb01966.x. [DOI] [PubMed] [Google Scholar]
- Caspi A. The child is father of the man: Personality continuities from childhood to adulthood. Journal of Personality and Social Psychology. 2000;78:158–172. doi: 10.1037//0022-3514.78.1.158. [DOI] [PubMed] [Google Scholar]
- Caspi A, Silva PA. Temperamental qualities at age three predict personality traits in young adulthood: Longitudinal evidence from a birth cohort. Child Development. 1995;66:486–498. doi: 10.1111/j.1467-8624.1995.tb00885.x. [DOI] [PubMed] [Google Scholar]
- Celeux G, Govaert G. Comparison of the mixture and the classification maximum likelihood in cluster analysis. Journal of Statistical Computation and Simulation. 1992;47:127–146. [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. Lawrence Erlbaum Associates; Hillsdale, NJ: 1988. [Google Scholar]
- Cribbie RA, Jamieson J. Structural equation models and the regression bias for measuring correlates of change. Educational and Psychological Measurement. 2000;60:893–907. [Google Scholar]
- Cronbach LJ, Furby L. How should we measure change-or should we? Psychological Bulletin. 1970;74:68–80. [Google Scholar]
- Cummings EM, Davies P. Emotional security as a regulatory process in normal development and the development of psychopathology. Development and Psychopathology. 1996;8:123–139. [Google Scholar]
- Dempster AP, Laird NM, Rubin DB. Maximum likelihood from incomplete data via the EM algorithm. Journal of the Royal Statistical Society. 1977;39:1–38. [Google Scholar]
- DePetrillo PB, White KV, Liu M, Hommer D, Goldman D. Effects of alcohol use and gender on the dynamics of EKG time-series data. Alcoholism: Clinical and Experimental Research. 1999;23(4):745–750. [PubMed] [Google Scholar]
- Dolan BR, Jansen RJ, Van Der Maas HLJ. Constrained and unconstrained multivariate normal fixture modeling of Piagetian data. Multivariate Behavioral Research. 2004;39(1):69–98. doi: 10.1207/s15327906mbr3901_3. [DOI] [PubMed] [Google Scholar]
- Doussard-Roosevelt JA, Porges SW, Scanlon JW, Alemi B, Scanlon KB. Vagal regulation of heart rate in the prediction of developmental outcome for very low birth weight preterm infants. Child Development. 1997;68(2):173–186. [PubMed] [Google Scholar]
- Dumenci L, Windle M. Cluster analysis as a method of recovering types of intraindividual growth trajectories: A Monte Carlo study. Multivariate Behavioral Research. 2001;36(4):501–522. doi: 10.1207/S15327906MBR3604_02. [DOI] [PubMed] [Google Scholar]
- Eisenberg N, Fabes RA. Emotion, regulation, and the development of social competence. In: Clark M, editor. Emotion and social behavior: Review of personality and social psychology. Sage; Newbury Park, CA: 1992. pp. 119–150. [Google Scholar]
- El-Sheikh M. Parental drinking problems and children's adjustment: Vagal regulation and emotional reactivity as pathways and moderators of risk. Journal of Abnormal Psychology. 2001;110(4):499–515. doi: 10.1037//0021-843x.110.4.499. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M. Does poor vagal tone exacerbate child maladjustment in the context of parental problem drinking? A longitudinal examination. Journal of Abnormal Psychology. 2005;114(4):735–741. doi: 10.1037/0021-843X.114.4.735. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, Buckhalt JA, Mize J, Acebo C. Marital conflict and disruption of children's sleep. Child Development. 2006;77(1):31–43. doi: 10.1111/j.1467-8624.2006.00854.x. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, Harger J. Appraisals of marital conflict and children's adjustment, health, and physiological reactivity. Developmental Psychology. 2001;37(6):875–885. [PubMed] [Google Scholar]
- El-Sheikh M, Harger J, Whitson SM. Exposure to interparental conflict and children's adjustment and physical health: The moderating role of vagal tone. Child Development. 2001;72(6):1617–1636. doi: 10.1111/1467-8624.00369. [DOI] [PubMed] [Google Scholar]
- Everitt BS. Finite mixture distributions. In: Everitt BS, Howell D, editors. Encyclopedia of statistics in behavioral science. John Wiley & Sons; London: 2005. pp. 652–658. [Google Scholar]
- Everitt BS, Hand DJ. Finite mixture distributions. Chapman & Hall CRC; London: 1981. [Google Scholar]
- Everitt BS, Landau S, Leese M. Cluster analysis. 4th Ed. Arnold; London: 2001. [Google Scholar]
- Fraley C, Raftery AE. How many clusters? Which clustering method? Answer via model-based cluster analysis. The Computer Journal. 1998;41(8):578–588. [Google Scholar]
- Fraley C, Raftery AE. MCLUST: Software for model-based cluster analysis. Journal of Classification. 1999;16:297–206. [Google Scholar]
- Fraley C, Raftery AE. Model-based clustering, discriminant analysis, and density estimation. Journal of the American Statistical Association. 2002a;97:611–631. [Google Scholar]
- Fraley C, Raftery AE. MCLUST: Software for model-based clustering, density estimation, and discriminant analysis. Department of Statistics, University of Washington; Seattle, WA: 2002b. Technical report No. 415. [Google Scholar]
- Fraley C, Raftery AE. Enhanced software for model-based clustering, discriminant analysis, and density estimation: MCLUST. Journal of Classification. 2003;20:263–286. [Google Scholar]
- Fromme K, Stroot E, Kaplan D. Comprehensive effects of alcohol: Development and psychometric assessment of a new expectancy questionnaire. Psychological Assessment. 1993;5(1):19–26. [Google Scholar]
- Hardin J, Rocke DM. Outlier detection in the multiple cluster setting using the minimum covariance determinant estimator. Computational Statistics & Data Analysis. 2002;44:625–638. [Google Scholar]
- Hicks BM, Markon KE, Patrick CJ, Krueger RE, Newman JP. Identifying psychopathy subtypes on the basis of personality structure. Psychological Assessment. 2004;16(3):276–288. doi: 10.1037/1040-3590.16.3.276. [DOI] [PubMed] [Google Scholar]
- Ingjaldsson JT, Laberg JC, Thayer JF. Reduced heart rate variability in chronic alcohol abuse: Relationship with negative mood, chronic thought suppression, and compulsive drinking. Biological Psychiatry. 2003;54:1427–1436. doi: 10.1016/s0006-3223(02)01926-1. [DOI] [PubMed] [Google Scholar]
- Insightful Corporation . S-PLUS 8 for Windows (Version 8.0.4) [computer software] Author; Seattle, WA: 2007. [Google Scholar]
- Jamieson J. Dealing with baseline differences: Two principles and two dilemmas. International Journal of Psychopathology. 1999;31:155–161. doi: 10.1016/s0167-8760(98)00048-8. [DOI] [PubMed] [Google Scholar]
- Katz LF, Gottman JM. Vagal tone protects children from marital conflict. Development and Psychopathology. 1995;7:83–92. [Google Scholar]
- Katz LF, Gottman JM. Buffering children from marital conflict and dissolution. Journal of Clinical and Child Psychology. 1997;26(2):157–171. doi: 10.1207/s15374424jccp2602_4. [DOI] [PubMed] [Google Scholar]
- Kaufman L, Rousseeuw PJ. Finding groups in data: An introduction to cluster analysis. John Wiley & Sons, Inc.; New York: 1990. [Google Scholar]
- Koob GF, Le Moal M. Drug abuse: Hedonic homeostatic dysregulation. Science. 1997 October 3;278(5335):52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24(2):97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Koskinen P, Virolainen J, Kupari M. Acute alcohol intake decreases short-term heart rate variability in healthy subjects. Clinical Science. 1994;87:225–230. doi: 10.1042/cs0870225. [DOI] [PubMed] [Google Scholar]
- Labouvie EW, Bates ME. Reasons for alcohol use in young adulthood: Validation of a three-dimensional measure. Journal of Studies on Alcohol. 2002;63:145–155. doi: 10.15288/jsa.2002.63.145. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International Affective Picture System (IAPS): Instruction manual and affective ratings. The Center for Research in Psychophysiology, University of Florida; Gainesville, FL: 1999. Technical Report A-4. [Google Scholar]
- Lo Y, Mendell NR, Rubin DB. Testing the number of components in a normal mixture. Biometrics. 2001;88(3):767–778. [Google Scholar]
- Lord FM. The measurement of growth. Educational and Psychological Measurement. 1956;16:421–437. [Google Scholar]
- Lubke GH, Muthén B. Investigating population heterogeneity with factor mixture models. Psychological Methods. 2005;10(1):21–39. doi: 10.1037/1082-989X.10.1.21. [DOI] [PubMed] [Google Scholar]
- Lubke GH, Muthén B. Performance of factor mixture models as a function of model size, covariate effects, and class-specific parameters. Structural Equation Modeling. 2007;14(1):26–47. [Google Scholar]
- Lubke GH, Neal MC. Distinguishing between latent classes and continuous factors: Resolution by maximum likelihood. Multivariate Behavioral Research. 2006;41(4):499–532. doi: 10.1207/s15327906mbr4104_4. [DOI] [PubMed] [Google Scholar]
- Magnusson D. The logic and implications of a person-oriented approach. In: Cairns RB, Bergman LR, Kagan J, editors. Methods and models for studying the individual. Sage; Thousand Oaks, CA: 1998. pp. 33–64. [Google Scholar]
- Magnusson D. The individual as the organizing principle in psychological inquiry: A holistic approach. In: Bergman LR, Cairns RB, Nilsson L-G, Nystedt L, editors. Developmental science and the holistic approach. Lawrence Erlbaum Associates, Inc.; Mahwah, NJ: 2000. pp. 33–47. [Google Scholar]
- McArdle JJ, Hamagami F. Latent difference score structural models for linear dynamic analysis with incomplete longitudinal data. In: Collins L, Sayer A, editors. New methods for the analysis of change. American Psychological Association; Washington, DC: 2001. pp. 139–175. [Google Scholar]
- McLachlan G, Peel D. An algorithm for unsupervised learning via normal mixture models. In: Dowe DL, Korb KB, Oliver JJ, editors. Information, statistics and induction in science. World Scientific Publishing; Singapore: 1996. pp. 354–363. [Google Scholar]
- McLachlan G, Peel D. Finite mixture models. Wiley; New York: 2000. [Google Scholar]
- Meilă M. Comparing clusterings by the variation of information. In: Schölkopf B, Warmuth MK, editors. Learning theory and kernel machines: Proceedings of the 16th annual conference on computational learning theory; New York: Springer; 2003. pp. 173–187. [Google Scholar]
- Meila M. Comparing clusterings – An information based distance. Journal of Multivariate Analysis. 2007;98:873–895. [Google Scholar]
- Mendell NR, Finch SJ, Thode HC., Jr. Where is the likelihood ratio test powerful for detecting two component normal mixtures? Biometrics. 1993;49(3):907–915. [PubMed] [Google Scholar]
- Mendell NR, Thode HC, Jr., Finch SJ. The likelihood ratio test for the two-component normal mixture problem: Power and sample size analysis. Biometrics. 1991;47(3):1143–1148. [PubMed] [Google Scholar]
- Meredith W. Measurement invariance, factor analysis, and factorial invariance. Psychometrika. 1993;58:525–543. [Google Scholar]
- Moffitt TE. Adolescence-limited and life-course persistent antisocial behavior: A developmental taxonomy. Psychological Review. 1993;100:674–701. [PubMed] [Google Scholar]
- Mojena R. Hierarchical grouping methods and stopping rules: An evaluation. Computer Journal. 1977;20:359–363. [Google Scholar]
- Mun EY, von Eye A, White HR. An SEM approach for the evaluation of intervention effects using pre-post-post designs. Structural Equation Modeling. 2009;16(2):315–337. doi: 10.1080/10705510902751358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mun EY, Windle M, Schainker LM. A model-based cluster analysis approach to adolescent problem behaviors and young adult outcomes. Development and Psychopathology. 2008;20(1):291–318. doi: 10.1017/S095457940800014X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata K, Araki S, Yokoyama K, Sata F, Yamashita K, Ono Y. Autonomic neurotoxicity of alcohol assessed by heart rate variability. Journal of the Autonomic Nervous System. 1994;48(2):105–111. doi: 10.1016/0165-1838(94)90026-4. [DOI] [PubMed] [Google Scholar]
- Muthén BO. Latent variable hybrids: Overview of old and new models. In: Hancock GR, editor. Mixture models in latent variable research; Symposium presented at the University of Maryland Center for Integrated Latent Variable Research conference; College Park, Maryland. May, 2006. [Google Scholar]
- Ning Y, Finch SJ. The likelihood ratio test with the Box-Cox transformation for the normal mixture problem: Power and sample size study. Communications in Statistics Simulations and Computations. 2004;33(3):553–565. [Google Scholar]
- Overall JE, Magee KN. Replication as a rule for determining the number of clusters in hierarchical cluster analysis. Applied Psychological Measurement. 1992;16:119–128. [Google Scholar]
- Pandina RJ, Labouvie EW, White HR. Potential contributions of the life span developmental approach to study of adolescent alcohol drug use: The Rutgers Health and Human Development Project. Journal of Drug Issues. 1984;14:253–268. [Google Scholar]
- Posner MI, Rothbart MK. Developing mechanisms of self-regulation. Development and Psychopathology. 2000;12:427–441. doi: 10.1017/s0954579400003096. [DOI] [PubMed] [Google Scholar]
- Raftery AE, Dean N. Variable selection for model-based clustering. Journal of the American Statistical Association. 2006;101(473):168–178. [Google Scholar]
- Raykov T. Studying correlates and predictors of longitudinal change using structural equation modeling. Applied Psychological Measurement. 1994;18(1):63–77. [Google Scholar]
- Raykov T. Are simple change scores obsolete? An approach to studying correlates and predictors of change. Applied Psychological Measurement. 1999;23(2):120–126. [Google Scholar]
- R Development Core Team . R: A language and environment for statistical computing. Vienna, Austria: 2006. [Google Scholar]
- Reed SF, Porges SW, Newlin DB. Effect of alcohol on vagal regulation of cardiovascular function: Contributions of the polyvagal theory to the psychophysiology of alcohol. Experimental and Clinical Psychopharmacology. 1999;7:484–492. doi: 10.1037//1064-1297.7.4.484. [DOI] [PubMed] [Google Scholar]
- Rogosa DR. Myths about longitudinal research. In: Schaie KW, Campbell RT, Meredith WM, Rawlings SC, editors. Methodological issues in aging research. Springer; New York: 1988. pp. 171–209. [Google Scholar]
- Rogosa DR. Myths and methods: “Myths about longitudinal research” plus supplemental questions. In: Gottman JM, editor. The analysis of change. Lawrence Erlbaum Associates; Mahwah, NJ: 1995. pp. 3–66. [Google Scholar]
- Rossinen J, Viitasalo M, Partanen J, Koskinen P, Kupari M, Nieminen MS. Effects of acute alcohol ingestion on heart rate variability in patients with documented coronary artery disease and stable angina pectoris. American Journal of Cardiology. 1997;79:487–491. doi: 10.1016/s0002-9149(96)00790-4. [DOI] [PubMed] [Google Scholar]
- Sadeh A, Gruber R, Raviv A. Sleep, neurobehavioral functioning, and behavior problems in school-age children. Child Development. 2002;73(2):405–417. doi: 10.1111/1467-8624.00414. [DOI] [PubMed] [Google Scholar]
- Singh JP, Larson MG, O'Donnell CJ, Tsuji H, Evans JC, Levy D. Heritability of heart rate variability: The Framingham Heart Study. Circulation. 1999;99:2251–2254. doi: 10.1161/01.cir.99.17.2251. [DOI] [PubMed] [Google Scholar]
- Skeem J, Johansson P, Andershed H, Kerr M, Louden JE. Two subtypes of psychopathic violent offenders that parallel primary and secondary variants. Journal of Abnormal Psychology. 2007;116(2):395–409. doi: 10.1037/0021-843X.116.2.395. [DOI] [PubMed] [Google Scholar]
- Steinley D. Local optima in K-means clustering: What you don't know may hurt you. Psychological Methods. 2003;8(3):294–304. doi: 10.1037/1082-989X.8.3.294. [DOI] [PubMed] [Google Scholar]
- Steinley D. Profiling local optima in K-means clustering: Developing a diagnostic technique. Psychological Methods. 2006;11(2):178–192. doi: 10.1037/1082-989X.11.2.178. [DOI] [PubMed] [Google Scholar]
- Stritzke WGK, Breiner MJ, Curtin JJ, Lang AR. Assessment of substance cue reactivity: Advances in reliability, specificity, and validity. Psychology of Addictive Behaviors. 2004;18(2):148–159. doi: 10.1037/0893-164X.18.2.148. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Cheung EH, Brown GG, Frank ER, Paulus MP, Schweinsburg AD, Meloy MJ, Brown SA. Neural response to alcohol stimuli in adolescents with alcohol use disorder. Archives of General Psychiatry. 2003;60:727–735. doi: 10.1001/archpsyc.60.7.727. [DOI] [PubMed] [Google Scholar]
- Tarter RE, Kirisci L, Habeych M, Reynolds M, Vanyukov M. Neurobehavior disinhibition in childhood predisposes boys to substance use disorder by young adulthood: Direct and mediated etiologic pathways. Drug and Alcohol Dependence. 2004;73(2):121–132. doi: 10.1016/j.drugalcdep.2003.07.004. [DOI] [PubMed] [Google Scholar]
- Tarter RE, Vanyukov M. Alcoholism: A developmental disorder. Journal of Consulting and Clinical Psychology. 1994;62(6):1096–1107. doi: 10.1037//0022-006x.62.6.1096. [DOI] [PubMed] [Google Scholar]
- Tarter R, Vanyukov M, Giancola P, Dawes M, Blackson T, Mezzich A, Clark DB. Etiology of early age onset substance use disorder: a maturational perspective. Development and Psychopathology. 1999;11(4):657–683. doi: 10.1017/s0954579499002266. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Brosschot JF. Psychosomatics and psychopathology: Looking up and down from the brain. Psychoneuroendocrinology. 2005;30(10):1050–1058. doi: 10.1016/j.psyneuen.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Lane RD. A model of neurovisceral integration in emotion regulation and dysregulation. Journal of Affective Disorders. 2000;61(3):201–216. doi: 10.1016/s0165-0327(00)00338-4. [DOI] [PubMed] [Google Scholar]
- Thomas A, Chess S, Birch HG. Temperament and behavior disorders in children. New York University Press; New York: 1968. [Google Scholar]
- Thompson RA. Emotion regulation: A theme in search of definition. Monographs of the Society for Research in Child Development. 1994;59 Serial No. 240. [PubMed] [Google Scholar]
- Tonidandel S, Overall JE. Determining the number of clusters by sampling with replacement. Psychological Methods. 2004;9(2):238–249. doi: 10.1037/1082-989X.9.2.238. [DOI] [PubMed] [Google Scholar]
- van der Kloot WA, Spaans AMJ, Heiser WJ. Instability of hierarchical cluster analysis due to input order of the data: The PermuCLUSTER solution. Psychological Methods. 2005;10(4):468–476. doi: 10.1037/1082-989X.10.4.468. [DOI] [PubMed] [Google Scholar]
- Vaschillo EG, Lehrer P, Rishe N, Konstantinov M. Heart rate variability biofeedback as a method for assessing baroreflex function: A preliminary study of resonance in the cardiovascular system. Applied Psychophysiology and Biofeedback. 2002;27:1–27. doi: 10.1023/a:1014587304314. [DOI] [PubMed] [Google Scholar]
- Vaschillo EG, Vaschillo B, Lehrer P. Characteristics of resonance in heart variability stimulated by biofeedback. Applied Psychophysiology and Biofeedback. 2006;31(2):129–142. doi: 10.1007/s10484-006-9009-3. [DOI] [PubMed] [Google Scholar]
- Vaschillo EG, Bates ME, Vaschillo B, Lehrer P, Udo T, Mun EY, Ray S. Heart rate response to alcohol, placebo, and emotional picture cue challenges: Effects of 0.1 Hz stimulation. Psychophysiology. 2008;45:847–858. doi: 10.1111/j.1469-8986.2008.00673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermunt JK, Magidson J. Latent GOLD's user's guide. Statistical Innovations Inc.; Boston, MA: 2000. [Google Scholar]
- Vermunt JK, Magidson J. Latent class cluster analysis. In: Hagenaars JA, McCutcheon AL, editors. Applied latent class analysis. Cambridge University Press; Cambridge, UK: 2002. pp. 89–106. [Google Scholar]
- von Eye A, Bergman LR. Research strategies in developmental psychopathology: Dimensional identity and the person-oriented approach. Development and Psychopathology. 2003;15:553–580. doi: 10.1017/s0954579403000294. [DOI] [PubMed] [Google Scholar]
- von Eye A, Mun EY. A note on the analysis of difference patterns – Structural zeros by design. Psychology Science. 2007;49(1):14–25. [Google Scholar]
- von Eye A, Mun EY. Configural frequency analysis of longitudinal data. In: Menard S, editor. Handbook of longitudinal research: Design, measurement, and analysis across the social sciences. Elsevier; San Diego, CA: 2008. pp. 313–332. [Google Scholar]
- von Eye A, Mun EY, Indurkhya A. Typifying developmental trajectories - A decision making perspective. Psychology Science. 2004;46(1):65–98. [Google Scholar]
- Webb AR. Statistical pattern recognition. 2nd Ed. Wiley; New York: 2002. [Google Scholar]
- Wenzlaff RM, Wegner DM. Thought suppression. Annual Review of Psychology. 2000;51:59–91. doi: 10.1146/annurev.psych.51.1.59. [DOI] [PubMed] [Google Scholar]
- Williams RH, Zimmerman DW. Are simple gain scores obsolete? Applied Psychological Measurement. 1996;20:59–69. [Google Scholar]
- Wong MM, Brower KJ, Fitzgerald HE, Zucker RA. Sleep problems in early childhood and early onset of alcohol and other drug use in adolescence. Alcoholism: Clinical and Experimental Research. 2004;28(4):578–587. doi: 10.1097/01.alc.0000121651.75952.39. [DOI] [PubMed] [Google Scholar]
- Yeung KY, Fraley C, Murua A, Raftery AE, Ruzzo WL. Model-based clustering and data transformations for gene expression data. Bioinformatics. 2001;17(10):977–987. doi: 10.1093/bioinformatics/17.10.977. [DOI] [PubMed] [Google Scholar]
- Zucker RA, Fitzgerald HE, Moses HD. Emergence of alcohol problems and the severe alcoholisms: A developmental perspective on etiological theory and life course trajectory. In: Cicchetti D, Cohen D, editors. Developmental psychopathology. Vol. 2. Wiley; New York: 1995. pp. 677–711. [Google Scholar]