Abstract
In our previous studies we have shown that insulin receptor (IR) activation leads to the activation of phosphoinositide 3-kinase (PI3K) and Akt activation in rod photoreceptors. This pathway is functionally important for photoreceptor survival as deletion of IR and one of the isoforms of Akt (Akt2) resulted in stress-induced photoreceptor degeneration. However, the molecular mechanism of this degeneration is not known. Akt signaling is known to be regulated by the serine/threonine phosphatases, PHLPP and PHLPPL. In this study, we characterized these two phosphatases in the retina and examined the role of IR, PI3K, and Akt signaling on the activity of PHLPP and PHLPPL. Most of the studies published on PHLPP and PHLPPL are directed towards Akt dephosphorylation, however, there are no studies available to date on how the enzyme activities of these phosphatases are regulated. We made a novel finding in this study that both PHLPP and PHLPPL activities were significantly decreased in the presence of insulin ex vivo. The insulin-induced decrease of phosphatase activities were PI3K-dependent as pretreatment of ex vivo retinal cultures with LY294002 significantly reversed the insulin-induced inhibition. It has been shown previously that PHLPP and PHLPPL regulate the dephosphorylation of Akt isoforms, and our results demonstrate for the first time that retinal PHLPP and PHLPPL activities are under the control of the IR-activated PI3K/Akt pathway.
Keywords: Insulin Receptor, phosphatases, Retina, Protein kinase B, Akt isoforms, PHLPP, PHLPPL, Phosphoinositides, Membrane binding, Cell survival
INTRODUCTION
Akt (serine/threonine protein kinase B) is an important kinase that is activated by a variety of growth factors and insulin (Marte and Downward 1997;Galetic et al. 1999;Lawlor and Alessi 2001). These factors activate phosphoinositide-3-kinase (PI3K), which in turn leads to the generation of the lipid second messengers phosphoinositide-3,4,5-trisphosphate (PI-3,4,5-P3) and phosphoinositide-3,4-bisphosphate (PI-3,4-P2). These lipid second messengers recruit Akt to the membrane by engaging its PH domain. Once localized on the membrane, Akt is phosphorylated on two sites, the activation loop (Thr 308) and the hydrophobic motif (Ser 473) (Manning and Cantley 2007;Fayard et al. 2005;Rajala 2010). Phosphorylation at the activation domain is mediated by phosphoinositide dependent kinase-1 (PDK1) (Alessi et al. 1998) and at the hydrophobic motif by mTOR (Hresko and Mueckler 2005). Akt is said to be fully activated when it is phosphorylated on both sites, after which it dissociates from the membrane and phosphorylates many substrates in the cytoplasm and nucleus. Thus, activated Akt plays an important role in the regulation of metabolism, apoptosis, cell cycle, and transcription of various genes (New et al. 2007;Parcellier et al. 2008).
We have previously shown that rod photoreceptors express all three Akt isoforms (Li et al. 2007). There is no functional redundancy between these isoforms in the retina, because specific Akt isoforms are activated under certain conditions (Li et al. 2007). For example, knocking down Akt2 in photoreceptors causes the photoreceptors to become more susceptible to light-induced photoreceptor degeneration, whereas knocking down Akt1 does not affect photoreceptors during light stress (Li et al. 2007). Insulin treatment of retinal explants results in specific activation of Akt1 and Akt3, but not of Akt2 (Reiter et al. 2003;Li et al. 2007).
Recently, two proteins PHLPP and PHLPPL have been discovered that can directly dephosphorylate Akt at the serine 473 residue and terminate downstream Akt signaling (Brognard et al. 2007;Gao et al. 2005). These two proteins show specificity regarding which Akt isoforms they dephosphorylate; PHLPP selectively dephosphorylates Akt2 and Akt3 and PHLPPL selectively dephosphorylates Akt1 and Akt3 (Brognard et al. 2007;Gao et al. 2005).The specificity shown by PHLPP and PHLPPL is due to the specific interactions between them and Akt. PHLPP interacts only with Akt2 and Akt3 and PHLPPL interacts only with Akt1 and Akt3 (Brognard et al. 2007;Gao et al. 2005). These observations led to the hypothesis that the Akt isoforms might be regulated post-translationally by PHLPP and PHLPPL. Since neither enzyme has been studied in the retina or photoreceptors, we examined the expression and activities of PHLPP and PHLPPL in the rodent retina.
In this paper, we show that both phosphatases are present in the retina. PHLPP encodes two protein products PHLPP-α and PHLPP-β of approximated masses of 140 kD and 190 kD, while PHLPPL encodes a single 150 kD protein product. PHLPP (both isoforms) was detected in the rat retina. PHLPPL was detected both in the inner and outer segments of the photoreceptors, but is enriched in the inner segments. We also show that both phosphatases are active in the retina and that the addition of insulin to retinal ex vivo cultures resulted in the inhibition of phosphatase activities of both PHLPP and PHLPPL. Our studies also indicate that the inhibition of PHLPP and PHLPPL activities are PI3K and Akt-dependent. These studies demonstrate for first time that insulin receptor activation regulates the activity of PHLPP and PHLPPL in the retina.
EXPERIMENTAL PROCEDURES
Materials
Akt and pAkt (Ser473) antibodies were obtained from Cell Signaling (Danvers, MA). PHLPP and PHLPPL polyclonal antibodies were obtained from Novus Biologicals (Littleton, CO). Transducin alpha subunit antibody (Tα) was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Actin and rhodopsin kinase antibodies were obtained from Affinity BioReagents (Golden, CO). Arrestin antibody was a kind gift from Dr. Paul Hargrave (University of Florida, Gainesville). Anti-opsin (RD14) antibody was a kind gift from Dr. Robert Molday. All other chemicals were purchased from Sigma (St. Louis, MO).
Animals
All animal work was in strict accordance with the NIH Guide for the Care and Use of Laboratory Animals and the Association for Research in Vision and Ophthalmology on the Use of Animals in Vision Research. All protocols were approved by the IACUC at the University of Oklahoma Health Sciences Center and the Dean A. McGee Eye Institute. All the rats used in the experiment were 6–8 weeks old Sprague-Dawley (Harlan Sera-Lab, Indianapolis, IN) that were born and raised in our vivarium under dim cyclic light (5 lux, 12 h on/off, 7 a.m. to 7 p.m.) prior to experimentation.
Ex vivo retinal organ cultures
For insulin treatment of retinal explants, rats were dark-adapted overnight, killed the next day, and retinas removed and placed in Dulbecco’s modified Eagle’s medium (Invitrogen, Carlsbad, CA). One µM insulin (final concentration) or equal volume of PBS was added and the retinas were incubated at 37 °C for 5 minutes. To inhibit PI3K activity, retinal explants were incubated in 100 µM LY294002 (final concentration) or equal volume of DMSO at 37 °C for 1 hour before insulin treatment or with 200 µM AKTX inhibitor for 2 hours prior to insulin treatment.
Preparation of rat rod outer segments
Rats were killed and ROS were prepared using discontinuous sucrose gradient centrifugation procedure as described previously (Rajala et al. 2002). Briefly, the rat retinas were homogenized in 4 ml of 47% sucrose in buffer A [10 mM Tris (pH 7.4), 100 mM NaCl, 1 mM EDTA, and 1 mM PMSF]. The homogenates were transferred to a 15 ml centrifuge tube and sequentially overlaid with 4 ml of 42%, 37%, 32% sucrose dissolved in buffer A. The discontinuous gradient was spun at 82,000 g for 1 hr. ROS membrane material in the 37%–32% interface was removed and diluted in buffer B [10 mM Tris-HCl (pH 7.4), 100 mM NaCl, and 1 mM EDTA] and centrifuged at 27,000 g for 30 min. The pelleted ROS was resuspended in buffer B and frozen at −20 °C. The non-ROS band at the 37%–42% sucrose interface (called Band II), which contains membranes from other retinal cells and the inner segments of photoreceptors, was also collected and frozen at −20 °C. Protein concentration was determined by BCA reagent (Rockford, IL).
Preparation of sealed outer segments
Rats were killed and retinas were gently homogenized in buffer C [20 mM Tris (pH 7.4), 100 mM NaCl, 2 mM MgCl2, 0.1 mM EDTA, in 20% sucrose]. The solution was spun at 8000 g for 20 min and the supernatant containing crude ROS was loaded on the bottom of a continuous sucrose gradient (25%–50%) in buffer D [10 mM Tris (pH 7.4), 100 mM NaCl and 2 mM MgCl2]. This mixture was centrifuged at 80,000 g for 2 hrs. The top band contains broken outer segments and second band contains intact outer segments. The third band contains membranes from other retinal cells and the inner segments of photoreceptors. The three bands were collected and diluted in buffer E [20 mM Tris (pH 7.4) and 100 mM NaCl in 10% sucrose] and spun for 20 min at 10,000 g. The fractions were stored at −20 °C. Protein concentration was determined by BCA reagent (Rockford, IL).
Determination of PHLPP and PHLPPL activity
About 500 µg of retinal lysate was initially precleared with protein A sepharose beads (PAS) following which, 1 µg of PHLPP, PHLPPL or rabbit IgG antibody with protein A sepharose beads (PAS) was added and allowed to rock overnight at 4°C. The beads were washed 3 times in buffer F [50 mM Tris (pH 8.0), 250 mM NaCl, 5 mM EDTA, and 0.5% NP-40] and the immunoprecipitates were used to determine the phosphatase activity using pNPP as substrate as described previously (Brognard et al. 2007). Absorbance values of PHLPP or PHLPPL were subtracted from control IgG immunoprecipitated under the same conditions. The difference in absorbance was divided by time and expressed as activity/min.
Serial tangential sectioning of retina and Western blotting
Rat eyes were removed and tangentially sectioned to 10 µm sections according to previously published methods (Sokolov et al. 2002). Sections were collected in SDS sample buffer and subjected to Western blot analysis with specific primary antibodies and IRDye 800CW Anti-Rabbit or IRDye 800CW Anti-Mouse Secondary Antibody (LI-COR Biosciences, Lincoln, NE) and imaged using Li-COR Odyssey IR-fluorescent scanner (LI-COR Biosciences, Lincoln, NE).
Immunohistochemistry
Cryosections were fixed in 4% paraformaldehyde for 15 min, after which they were washed three times in PBS and quenched in 0.1% glycine for 5 minutes at RT. The sections were then permeabilized in 0.1% Triton X-100 for 15 min at RT and washed three times in PBS. Sections were blocked in 50% FBS in PBS for 1 hour at RT followed by incubation with primary antibody (1:100) in 10% FBS in PBS at 4 °C overnight. Sections were washed in PBS, followed by incubation in secondary antibody [Alexa Fluor 488 goat anti-rabbit IgG (1:300)] in 10% FBS. Sections were washed in PBS and mounted onto coverslips after applying VectorShied and visualized using Nikon epifluoresence microscope.
Exposure of Animals to Light Stress
Sprague-Dawley rats were born and raised in dim cyclic (5 lux) light. Albino rats were exposed to constant light for 3 h at an illuminance level of 5000 lux. During light exposure, animals were maintained in transparent polycarbonate cages with stainless-steel wire bar covers. Drinking water was supplied by a bottle attached to the side of the cage, so that there was no obstruction between the light and the animal, and food was placed on bedding in the bottom of the cage.
Transfections
The full-length retinal PHLPP and PHLPPL clones were obtained from Open Biosystems (Huntsville, AL). The HA-tagged Akt constructs was a kind gift from Dr. Morris Birnbaum (University of Pennsylvania). The cDNA encoding the full-length PHLPP and PHLPPL was cloned into pCDNA3 vector containing N-terminal Flag-epitope.. HEK293T cells were maintained in DMEM medium containing 10% (v/v) FBS at 37° C. Approximately 2.5 × 105 cells were seeded in each 60-mm culture dish 12–18 h before transfection. Calcium phosphate mediated DNA transfection was performed for each of the plasmids containing the cDNA of interest (Wigler et al. 1978) and cells were harvested for experiments ~48 h post-transfection. HEK cells were also transfected with PHLPP and grown for 48 hours in 10% serum media and then either serum starved overnight in media containing 0% serum or grown in regular 10% FBS containing media and the activity of PHLPP was assessed.
RESULTS
PHLPP and PHLPPL are expressed in the retina
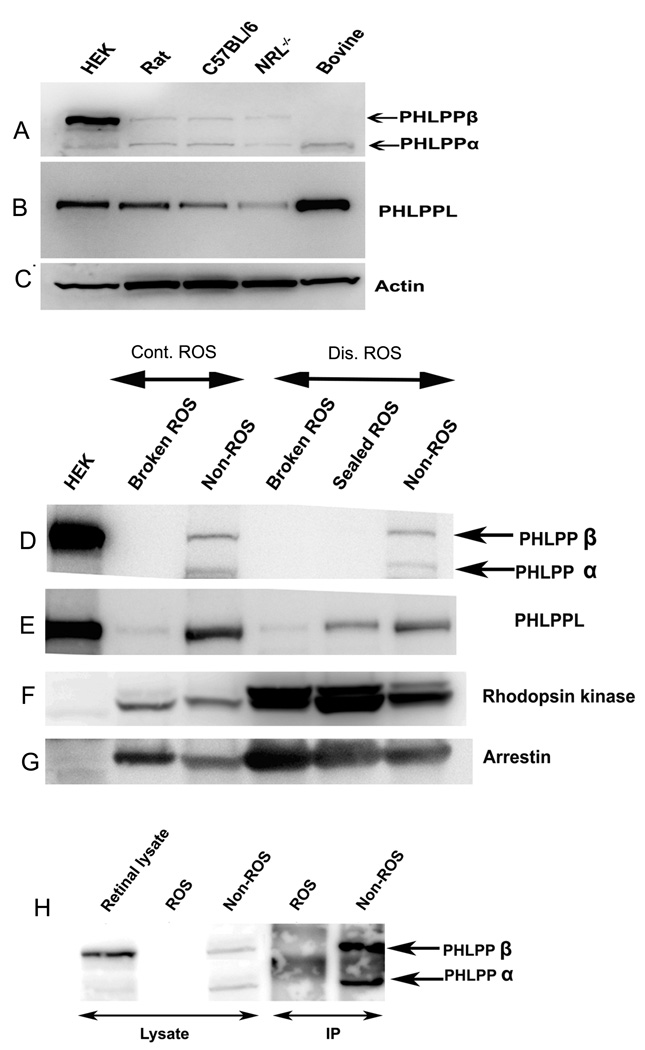
We examined the presence of PHLPP and PHLPPL in rat, mouse, and bovine retinas using HEK 293T cells, which express both proteins, as positive controls (Brognard et al. 2007;Gao et al. 2005). The results indicate the expression of both isoforms of PHLPP (α and β) in the rodent retina and only the α isoform in bovine retina. Rat, mouse and bovine retinas are rod dominant; therefore we used Nrl−/− mouse retina, which has only cone photoreceptors (Daniele et al. 2005), to show the expression of both isoforms of PHLPP in cones as well. Isoform α is about 140 kD and isoform β is 190 kD (Fig. 1A). PHLPPL is a 150 kD protein and is expressed in rat, mouse, bovine and Nrl−/− retinas (Fig. 1B). To ensure equal amount of protein in all lanes, we reprobed the blot with actin (Fig. 1C). These results suggest that PHLPP and PHLPPL are expressed in both rod and cone dominant retina.
Figure 1. Expression of PHLPP and PHLPPL in the retina.
A. PHLPPLα (140 kD) and β isoforms (190 kD) are present in the retinas of Sprague Dawley rats, C57BL/6 mouse, NRL knockout mouse, but only the α isoform is present in bovine retinas. HEK cells were used as positive control. B. PHLPPL (150 kD) is found in retinas of SD rats, C57BL/6 mouse, NRL knockout mouse, and cattle. C. Actin was used as a loading control to verify equal loading in all the retinal samples. ROS prepared from discontinuous and continuous sucrose gradient centrifugation. Thirty micrograms of retinal proteins were loaded on the gel and probed with antibodies against PHLPP, PHLPPL, rhodopsin kinase and arrestin. D. PHLPP (α or β) are not detected in broken ROS or sealed ROS membranes of discontinuous or continuous ROS preps, but is present in non-ROS membranes in both preps. E. PHLPPL is present in broken ROS membranes (both continuous and discontinuous ROS preps), sealed ROS membranes of continuous ROS preps and non-ROS membranes (both continuous and discontinuous ROS preps). F. Rhodopsin kinase, a soluble disk protein, is present enriched in sealed ROS membranes of continuous ROS preps over broken ROS membranes and non-ROS membranes of continuous or discontinues ROS preps. G. Arrestin, a membrane protein bound to rhodopsin in light, is present enriched in broken ROS membranes and sealed ROS membranes, over non-ROS membranes. H Immunoprecipitation of PHLPP from 500 µg ROS membrane preps and non-ROS membranes, proves that PHLPP (α or β isoform) is absent in ROS but present in non-ROS membranes.
Rod photoreceptors express PHLPPL
PHLPP and PHLPPL are both known to dephosphorylate Akt (Brognard et al. 2007;Gao et al. 2005). Since we previously reported the expression of Akt in rod outer segment (ROS) membranes (Li et al. 2007), we sought to determine whether PHLPP and PHLPPL are expressed in ROS prepared by discontinuous sucrose density gradient centrifugation.
The ROS and non-ROS membranes were subjected to Western blot analysis with anti-PHLPP or anti-PHLPPL antibodies. The results indicate the absence of PHLPP immunoreactivity in ROS membranes (Fig. 1D), whereas PHLPPL immunoreactivity was present in ROS membranes (Fig. 1E). Both isoforms of PHLPP (α and β) and PHLPPL were present in non-ROS fractions, which contain inner segments of the photoreceptors as well as membranes from other retinal cells (Fig. 1D and Fig. 1E). The absence of PHLPP in ROS membranes, could be due to its loss during discontinuous sucrose density gradient centrifugation, since it is a soluble protein.
To determine the nature of the protein, either soluble or membrane bound, we prepared ROS by continuous sucrose gradient centrifugation, which yields sealed ROS containing soluble proteins. This method also allowed us to recover the broken ROS, which do not contain soluble proteins. To determine the yield of soluble proteins, we examined for the presence rhodopsin kinase, a soluble protein marker. Broken ROS, sealed ROS, and non-ROS fractions from continuous sucrose gradient centrifugation were subjected to Western blot analysis with anti-rhodopsin kinase antibody. There were increased amounts of rhodopsin kinase in sealed ROS compared to broken ROS and non-ROS fractions from continuous sucrose density centrifugation (Fig. 1F). We also examined the presence of arrestin, a soluble protein, whose binding to ROS membranes (binding to photoactivated rhodospin) is light-dependent (McGinnis et al. 2002). Since our continuous ROS preps were made under light conditions, we observed increased amounts of arrestin in broken ROS membranes, compared to sealed ROS membranes and non-ROS fractions prepared from continuous sucrose density gradient centrifugation (Fig. 1G). These experiments confirm that ROS prepared from continuous sucrose gradient contain soluble proteins. The broken, sealed and non-ROS fractions were subjected to Western blot analysis with anti-PHLPP and anti-PHLPPL antibodies and the results indicate the absence of PHLPP isoforms in broken as well as in sealed ROS, however, it is present in non-ROS fraction (Fig. 1D). On the other hand, PHLPPL is present in both, ROS and non-ROS fractions; however, its presence is much less in broken ROS compared to sealed and non-ROS fractions. These experiments confirm the presence of PHLPPL in ROS. The failure to detect PHLPP isoforms in ROS could be due to low levels of this protein in ROS compared to PHLPPL. To address this issue, we have immunoprecipitated the PHLPP from ROS and non-ROS fractions with anti-PHLPP antibody followed by Western blot analysis with anti-PHLPP antibody. The results indicate the absence of PHLPP in ROS; however its presence is enriched in non-ROS fractions (Fig. 1H). Collectively these experiments suggest that PHLPPL is present in ROS. Under our experimental conditions we failed to observe the presence of PHLPP in ROS.
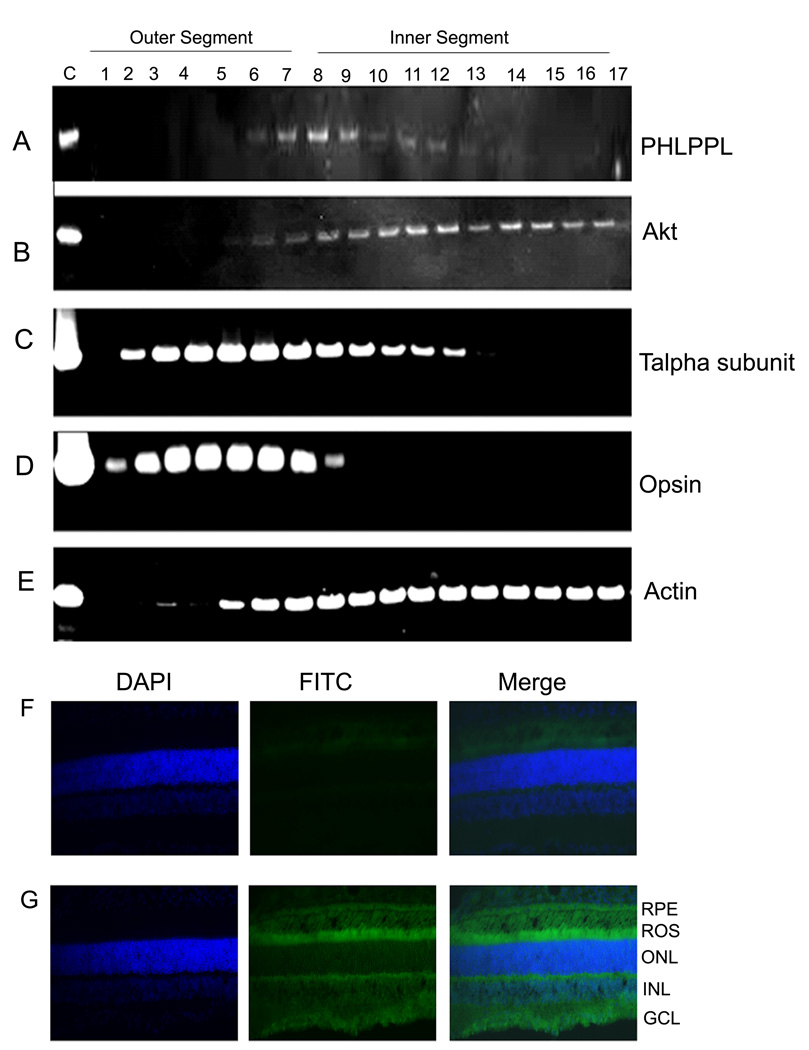
To determine the location of PHLPPL in rod photoreceptors and further confirm the absence of PHLPP from rods, we did Western blot analysis on 10 µm serial sections from light-adapted retinas. To demarcate the rod outer segments, we probed the sections with rhodopsin, which is enriched in rod outer segments (Fig. 2D); inner segment demarcation was identified by rod transducin (Fig. 2C), which is present in the inner segment in the light-adapted retina. Using a combination of the two antibodies, rhodopsin and rod transducin, we could make a prediction of rod outer segment (Fig. 2, lanes 2–7) and rod inner segments (Fig. 2, lanes 8–17). PHLPPL is detected in outer segments (Fig. 2A, lanes 6 and 7) but mostly exists in inner segments (Fig. 2A, lanes 8–13). Under our experimental conditions we failed to observe the expression of PHLPP in rod photoreceptors (data not shown). The serial sections were also probed with total Akt and showed limited presence of Akt in outer segments (Fig. 2B, lanes 6 and 7) but abundantly present in inner segments (Fig. 2B, lanes 8–17). From the serial section Western blots, we show that PHLPPL and Akt are expressed mainly in photoreceptor inner segments, which makes it possible that PHLPPL could regulate photoreceptor Akt phosphorylation. The blots were also probed with actin (Fig. 2E) to ensure that there was protein in all the fractions.
Figure 2. Determination of protein distribution throughout the photoreceptor layer of the rat retina by serial tangential cyrosectioning with Western blotting.
Western blots showing distribution of PHLPPL (A), Akt (B), and photoreceptor marker proteins, transducin alpha subunit (C), rhodopsin (D) and actin (E) in the serial sections obtained from the retinas of rat. Each line of the gel represents the protein content of a single 10 µm section into the retina starting from the outer segment tips to the end of inner segment. F. Immunohistochemistry of PHLPPL in rat retinal cryosections with no primary antibody. G. Immunohistochemistry of PHLPPL in rat retinal cryosections with PHLPPL antibody (FITC staining) shows the presence of PHLPPL in outer and inner segments.
To further confirm the presence of PHLPPL in rod photoreceptors, we did immunohistochemistry on rat retinal sections with PHLPPL antibody and detected the presence of PHLPPL mainly in rod inner segments but also in rod outer segments (Figure 1G), which is similar to the results in rat retinal serial sections. PHLPP antibody was not suitable for use for immunohistochemistry.
Insulin-induced inhibition of PHLPP and PHLPPL activity ex vivo
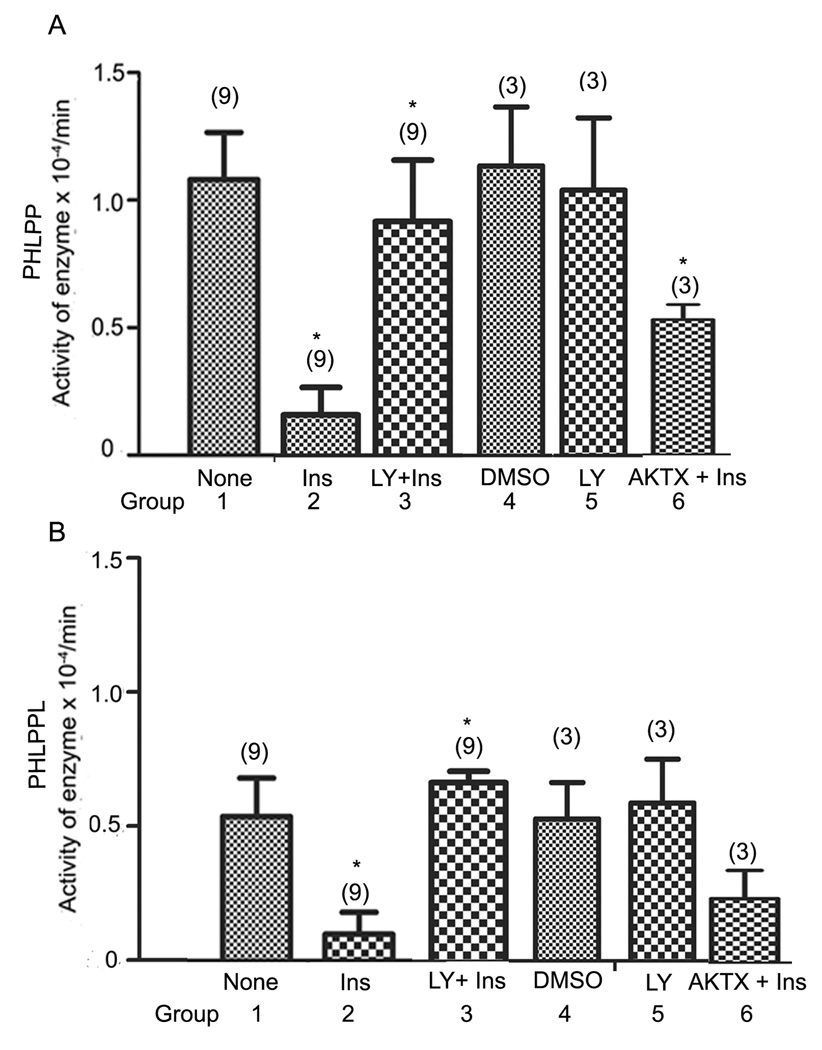
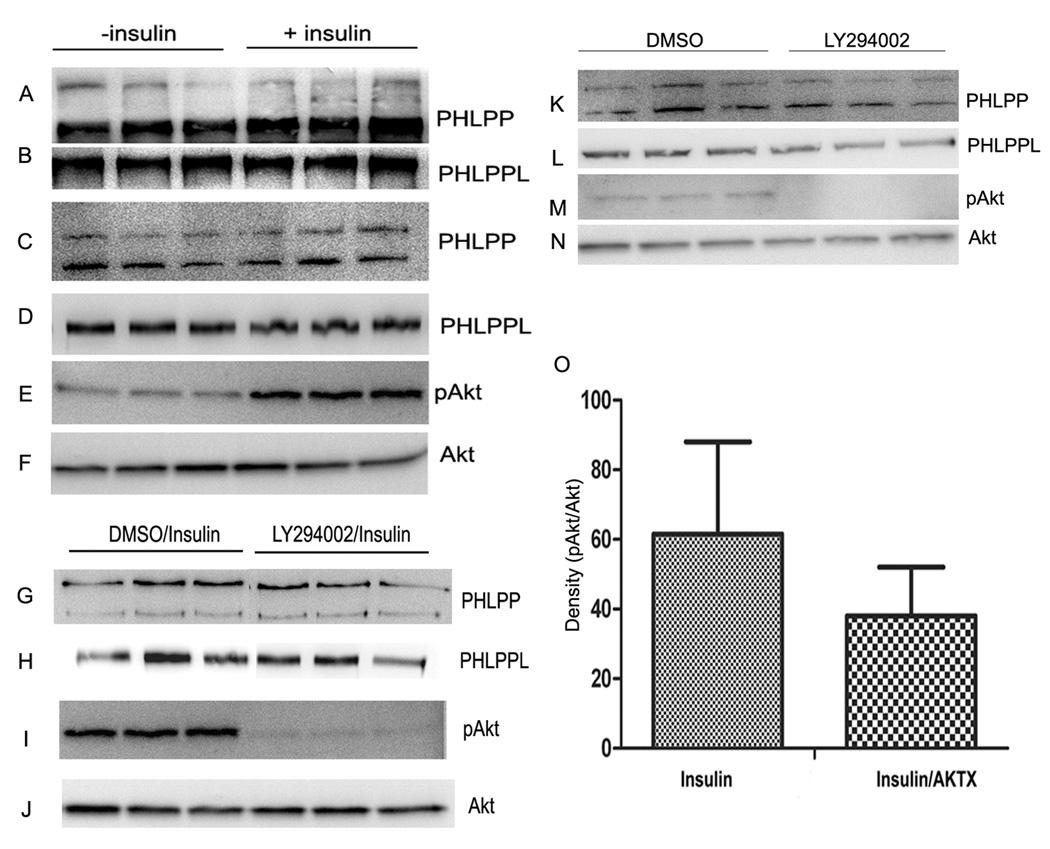
To understand the regulation of PHLPP and PHLPPL activity under conditions where pAkt levels are changed, retinal ex vivo organ cultures was stimulated with or without 1 µM insulin for 5 min at 37 °C. Retinal lysates were immunoprecipitated with anti-PHLPP and anti-PHLPPL antibodies and the phosphatase activity was measured. Retinal lysates were subjected to Western blot analysis with PHLPP and PHLPPL antibodies. The effect of insulin on phosphatase activity was determined by determining the ratio of pAkt to total Akt on Western blot with anti-pAkt and Akt antibodies, respectively. Insulin significantly inhibited both PHLPP and PHLPPL activities (Fig. 3). The observed inhibition is through insulin as we observed increased pAkt immunoreactivity in insulin-treated retinas (Fig. 4E). The blot was stripped and reprobed with anti-Akt to ensure equal amount of Akt in each lane (Fig. 4F). The PHLPP and PHLPPL levels were not altered in insulin-treated retinal lysates (Fig. 4C and D). To ensure equal amount of protein in each immunoprecipitate used for phosphatase activity, the immunoprecipitates were probed for PHLPP and PHLPPL; equal amounts of protein was found in each immunoprecipitate (Fig. 4A and B).
Figure 3. Insulin/PI3K/Akt-dependent inhibition of retinal PHLPP and PHLPPL activity.
Ex vivo retinal organ cultures were stimulated with or without 1 µM insulin for 5 min at 37° C. The phosphatase activities were also measured in the presence of LY294002 and AKTX inhibitor and the details are described in Figure 4. The retinal lysates were subjected to immunoprecipitation with anti-PHLPP and anti-PHLPPL antibodies and measured the phosphatase activity as described in the “Experimental Procedures”. Absorbance values of PHLPP or PHLPPL were subtracted substrate from control IgG immunoprecipitates under the same conditions. The difference in absorbance was divided by time and expressed as activity per min. Data are mean ± SD, sample size is indicated on top of each bar. Student t test was used to calculate the significance between the groups. The PI3K inhibitor LY294002 was dissolved in DMSO. Group 1, none; Group 2, insulin; Group 3, LY294002 + insulin; Group 4, DMSO; Group 5, LY294002; Group 6, AKTX + insulin. *Significance for PHLPP: Group I vs 2, p<0.0006; Group 2 vs 3, p<0.0.125; Group 4 vs 5, not significant; Group 2 vs 6, p<0.030. *Significance for PHLPPL: Group 1 vs 2, p<0.019; Group 2 vs 3, p<0.0014; Group 4 vs 5, not significant; Group 2 vs 6, not significant. None (no addition); Ins, insulin; DMSO, dimethyl sulfoxide; LY, LY294002; AKTX, Akt inhibitor X.
Figure 4. Insulin/PI3K/Akt-dependent inhibition of retinal PHLPP and PHLPPL activity.
Ex vivo retinal organ cultures were stimulated with or without 1 µM insulin for 5 min at 37° C. The retinal lysates were subjected to immunoprecipitation with anti-PHLPP and anti-PHLPPL antibodies and measured the phosphatase activity (Fig. 3). The immunoprecipitates were reprobed with anti-PHLPP (A) and anti-PHLPPL (B) antibodies to ensure equal amount of protein in each line. Insulin stimulated and unstimulated retinal proteins were subjected to Western blot analysis with anti-PHLPP (C), anti-PHLPPL (D), pAkt (E) and Akt (F) antibodies. Effect of insulin stimulation in the presence and absence of LY294002 on PHLPP and PHLPPL activity. Ex vivo retinal organ cultures were treated with DMSO or 100 µM LY294002 for 60 min prior to the addition of 1 µM insulin for 5 min at 37° C. The retinal lysates were subjected to immunoprecipitation with anti-PHLPP and anti-PHLPPL antibodies and measured the phosphatase activity (Fig. 3). DMSO or LY294002 treated retinal proteins were subjected to Western blot analysis with anti-PHLPP (G), anti-PHLPPL (H), pAkt (I) and Akt (J) antibodies. Effect of LY294002 on the activity of PHLPP and PHLPPL. Ex vivo retinal organ cultures were treated with DMSO or 100 µM LY294002 for 60 min at 37° C. The retinal lysates were subjected to immunoprecipitation with anti-PHLPP and anti-PHLPPL antibodies and measured the phosphatase activity (Fig. 3). DMSO or LY294002 treated retinal proteins were subjected to Western blot analysis with anti-PHLPP (K), anti-PHLPPL (L), pAkt (M) and Akt (N) antibodies. Effect of AktX inhibitor on the activity of PHLPP and PHLPPL. Ex vivo retinal organ cultures were treated with PBS or 200 µM AktX for 2 hours at 37° C and then stimulated with 1 µM Insulin. Activity was measured from 3 independent retina samples (Fig. 3). Quantitative analysis of bands of respective Western blot analyses was performed using Kodak Image Software. Densitometric analysis of immunoblots of pAkt was performed in the linear range of detection and absolute values were then normalized to total Akt (O). Data are mean ± SD. Three independent retinal samples were analyzed for every treatment.
Insulin-induced inhibition of PHLPP and PHLPPL activities is PI3K-dependent
Retinal ex vivo organ cultures were treated with 100 µM LY294002 or equal volumes of DMSO for 1 hour at 37 °C prior to addition of 1 µM insulin for 5 minutes. PHLPP and PHLPPL activities and Akt activation were measured similar to the conditions described for Fig. 3A–F. Addition of insulin inhibited the activities of PHLPP and PHLPPL, whereas treatment of cultures with PI3K inhibitor prior to the addition of insulin resulted in a significant increase of PHLPP and PHLPPL activities (Fig. 3). The results of the Western blots indicate the activation of Akt in insulin-stimulated conditions (Fig. 4I), whereas the PI3K inhibitor blocked the activation of Akt (Fig. 4I). The total PHLPP (Fig. 4G), PHLPPL (Fig. 4H), and Akt (Fig. 4J) levels were unchanged between control and PI3K inhibitor treatments. Collectively, the data indicate that insulin-induced inhibition of PHLPP and PHLPPL activities is PI3K-dependent.
To determine whether PI3K inhibitor, LY294002 has any direct effect on PHLPP or PHLPPL activity, we treated retinal samples with 100 µM LY294002 or equal volumes of DMSO for 1 hour at 37 °C and measured the PHLPP and PHLPPL activities. There was no difference between control and inhibitor-treated activities (Fig. 3), even through we observed the inhibition of basal level of Akt phosphorylation in PI3K inhibitor-treated ex vivo cultures (Fig. 4M). The total PHLPP (Fig. 4K), PHLPPL (Fig. 4L), and Akt (Fig. 4N) levels were unchanged between control and PI3K inhibitor treatments. The results described in Fig. 4 (K–N) are not due to the direct effect of PI3K inhibitor, but rather to insulin-induced activation of PI3K.
Akt activation is sufficient to inhibit PHLPP activity
To determine if the observed inhibition of PHLPP and PHLPPL is mediated through the activation Akt, we pretreated the ex vivo organ cultures with Akt inhibitor, AKTX (200 µM) for 2 hours at 37 °C prior to insulin treatment. Retinal lysates were immunoprecipitated with anti-PHLPP and anti-PHLPPL antibodies and the phosphatase activity was measured. Inhibition of Akt activity (Fig. 4O) resulted in the significant activation of PHLPP activity compared to insulin-treated conditions (Fig. 3). We failed to observe a significant increase of PHLPPL activity. This experiment suggests that Akt activation is sufficient to inhibit the activity of PHLPP. It is possible that higher concentrations of AKTX inhibitor might be required to inhibit the activity of PHLPPL; and inhibition at higher concentrations may be non-specific.
PH domain of PHLPP and PHLPPL do not bind PI3K generated phosphoinositides
To understand if PHLPP and PHLPPL, are regulated directly by PI3K generated phosphoinositides such as PI-3, 4 diphosphate or PI-3, 4, 5 triphosphate, we cloned the PH domain of PHLPP and PHLPPL as GST-fusion proteins and tested the binding of these domains to nitrocellulose membranes containing immobilized phosphoinositides. As a positive control, we also cloned the PH domain of Akt-1 which is known to bind to PI3K generated phosphoinositides (Li et al. 2008). Our results show that the PH domains of both PHLPP and PHLPPL do not bind to PI3K generated phosphoinositides such as PI-3, 4 diphosphate or PI-3, 4, 5 triphosphate which were bound by the PH domain of Akt1 (data not shown). These results prove that because both PHLPP and PHLPPL do not directly bind PI3K generated phosphoinositides unlike Akt, which binds both the PI3K generated phosphoinositides, PHLPP and PHLPPL cannot be directly regulated by PI3K generated phosphoinositides. However, this does not rule out their indirect regulation by PI3K, by their regulation by a protein that is regulated by PI3K generated phosphoinositides.
Light stress-induced inhibition of PHLPPL activity
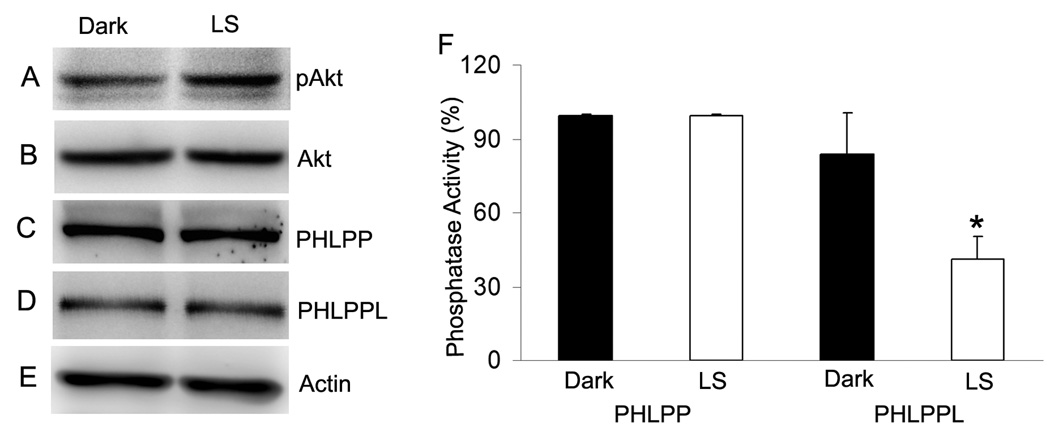
We previously reported that light stress induced the activation of IR, PI3K and Akt and the activation of this pathway is neuroprotective (Rajala et al. 2008;Li et al. 2007).To determine whether light stress induced the activation of PHLPP or PHLPPL, we have subjected albino rats to light stress for 3h at 5000 lux. At the end of light exposure, rats were killed and the retinas harvested. Control experiments were done on rats dark-adapted overnight. Retinas were lysed and an equal amount of protein was subjected to Western blot analysis with anti-pAkt, anti-Akt, anti-PHLPP anti-PHLPPL and anti-actin antibodies. The results indicate that light stress results in the activation of Akt compared to unexposed dark-adapted control (Fig. 5A). The level of Akt, PHLPP, PHLPPL and actin remains constant between control and light stress groups (Fig. 5B–E). To determine whether PHLPP or PHLPPL was activated in response to light stress, we immunoprecipitated the control and light-stressed retinal lysates with anti-PHLPP and anti-PHLPPL antibodies, and the immune complexes were used to measure the phosphatase activity. The results indicate that light stress inhibited the activity of PHLPPL but not PHLPP compared to control (Fig. 5F). These studies suggest that activation of IR/PI3K/Akt signaling induces the inhibition of PHLPPL in vivo.
Figure 5. Inhibition of PHLPPL isoform in response to light-stress.
Rats were subjected to light stress for 3h at 5000 lux. Control experiments were done on overnight dark-adapted rats. Retinas were lysed and equal amount of protein was subjected to Western blot analysis with anti-pAkt (A), anti-Akt (B), anti-PHLPP (C), anti-PHLPPL (D) and anti-actin (E) antibodies. To determine the specific isoform activation in response to light stress; we have immunoprecipitated the proteins from light stress and control samples with anti-PHLPP and anti-PHLPPL antibodies. The immune complexes were subjected to phosphatase activity (F). Data are mean ± SD, n=3, *p<0.026.
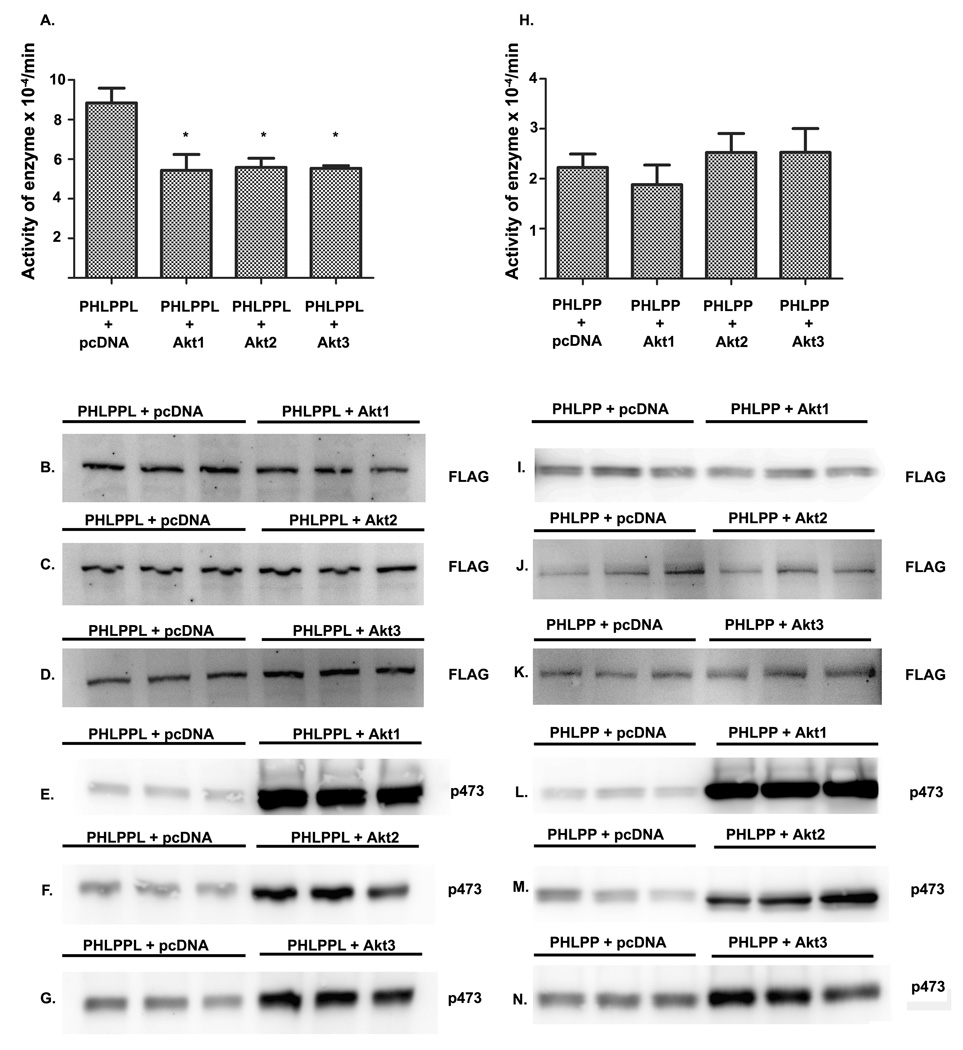
PHLPPL activity is regulated by all three Akt isoforms
To investigate if PHLPP or PHLPPL activity was modulated directly by Akt, we cloned full length α PHLPP and full length PHLPPL as FLAG tagged protein. We then co-transfected PHLPP and PHLPPL into HEK cells with pcDNA3, or each of the Akt isoforms, Akt1, Akt2 or Akt3. We immunoprecipitated PHLPP and PHLPPL from pcDNA or Akt co-transfected cells with FLAG antibody and estimated the activity in the absence and presence of Akt isoforms. Our results show a decrease (38%) in the activity of PHLPPL in the presence of Akt1, Akt2 and Akt3 isoforms (Fig. 6A). This decrease in PHLPPL activity, is not a result of decrease in protein expression when co-expressed with Akt, as shown by comparable levels of PHLPPL expression when expressed in the presence of pcDNA or each of the AKT isoforms (Fig. 6B–D). To verify transfection of the Akt isoforms, as well as increased Akt phosphorylation in HEK cells co-transfected with the individual Akt isoforms, we probed blots with pAkt antibody (p473), which showed an expected increase in p473 phosphorylation in HEK cells co-transfected with the Akt isoforms, proving that these cells had an increased quantity of pAkt because they were transfected with Akt isoforms compared to the pcDNA transfected cells (Fig. 6E–G).
Figure 6. Modulation of PHLPP or PHLPPL activity by transfection with pcDNA or different isoforms of Akt.
PHLPP or PHLPPL was transfected into HEK cells in the presence of pcDNA or different isoforms of Akt. (A) PHLPPL activity was assessed in the presence of pcDNA or different isoforms of Akt such as Akt1, Akt2 or Akt3, shows a significant decrease of PHLPPL activity in the presence of the different isoforms of Akt. Data are mean ± SD, n=3. Student t test was used to calculate the significance between the groups. *p<0.05. Expression of PHLPPL when co-expressed with pcDNA or Akt1 (B), Akt2 (C) or Akt3 (D), show comparable levels of expression in Western blots as assessed by probing with FLAG antibody. Increased expression of pAkt in HEK cells transfected with pcDNA and Akt1 (E), Akt2 (F), or Akt3 (G) is assessed on Western blots probed with p473 antibody. PHLPP activity was assessed in the presence of pcDNA or different isoforms of Akt such as Akt1, Akt2 or Akt3, shows no significant modulation of PHLPP activity in the presence of the different isoforms of Akt (H). Expression of PHLPP when co-expressed with pcDNA or Akt1 (I), Akt2 (J) or Akt3 (K), show comparable levels of expression in Western blots as assessed by probing with FLAG antibody. Increased expression of pAkt in HEK cells transfected with pcDNA and Akt1 (L), Akt2 (M), or Akt3 (N) is assessed on Western blots probed with p473 antibody.
PHLPP activity however, was not modulated by co-transfection with Akt1, Akt2 or Akt3 and the activity remained the same as pcDNA transfected cells (Fig. 6H). PHLPP was expressed to comparable levels in the pcDNA transfected cells and the cells transfected with the different isoforms of Akt. (Fig. 6I–K). To verify the increased expression of Akt in cells transfected with the different isoforms of Akt, we probed for pAkt (p473). The increased p473 phosphorylation indicated increased quantity of Akt transfected in the Akt transfected (Fig. 6L–N).
In the experiments where PHLPP or PHLPPL were transfected with pcDNA or the Akt isoforms, only PHLPPL activity was directly modulated by Akt and not PHLPP. This Akt mediated inhibition of PHLPPL activity cannot be explained by Akt mediated phosphorylation of PHLPPL because, we were unable to detect phosphorylation on PHLPPL by immunoprecipitating PHLPPL with FLAG antibody and immunoblotting with PAS antibody (data not shown).
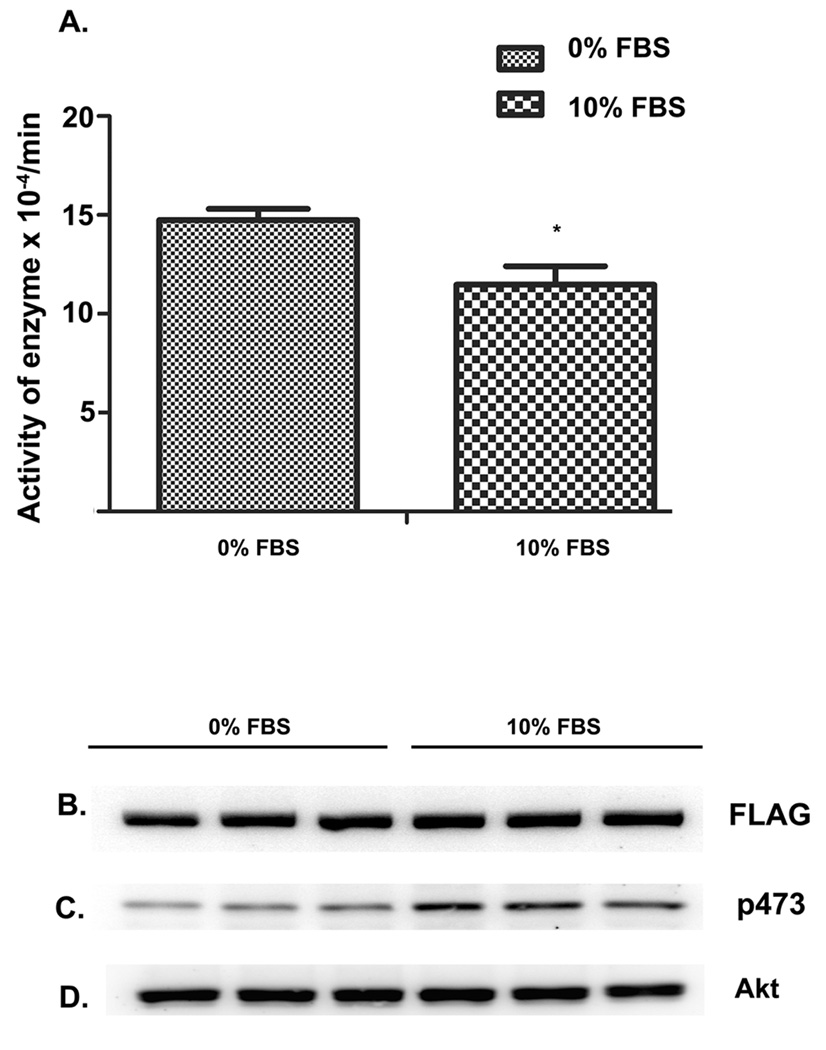
PHLPP activity was modulated under conditions that alter Akt phosphorylation, such as serum starvation of cells. When we serum starved PHLPP transfected HEK cells and compared PHLPP activity with cells grown in regular serum, we found a significant decrease of PHLPP activity in HEK cells grown in 10% serum compared to cells grown in 0% serum (Fig. 7A). This decrease in activity is not due to decrease in protein expression because we see comparable expression under conditions of 10% serum and 0% serum (Fig. 7B). To verify if the serum deprivation condition decreased pAkt, we probed the blots with p473, and saw decreased p473 phosphorylation in cells that are deprived of serum compared to cells grown under serum conditions (Fig. 7C). Finally, probing the blots with total Akt, showed equal loading of protein in all the lanes.
Figure 7. Modulation of PHLPP activity by serum starvation.
HEK cells were transfected with PHLPP and grown either in 10% serum or 0% serum conditions and PHLPP activity was assessed under the two conditions, shows a significant decrease of activity in 10% serum conditions compared to 0% serum. Data are mean ± SD, n=3. Student t test was used to calculate the significance between the groups. *p<0.05 (A). Expression of PHLPP was comparable in HEK cells grown in 0% serum and in 10% serum conditions (B). Phosphorylation at p473 was higher in HEK grown in 10% serum compared to 0% serum as assessed in Western blots probed with p473 antibody (C). Total Akt was comparable in HEK cells grown in 10% serum and in 0% serum conditions (D).
Our results prove that only PHLPPL can be directly modulated by Akt transfection, while PHLPP cannot be modulated by Akt directly. However, conditions that can modulate Akt levels such as the deprivation of growth factors in serum can increase PHLPP activity. Therefore, modulation of PHLPP activity is by a signaling mechanism downstream to Akt activation.
DISCUSSION
Growth factor-induced activation of Akt has been shown in transformed retinal neurons (Barber et al. 2001) and mouse models of retinal degeneration (Samardzija et al. 2006). In situ hybridization has shown that all three Akt messenger RNAs are present in different layers of the retina (Reiter et al. 2003). The Akt pathway is active in the retina and protects the photoreceptors from oxidative stress (Yu et al. 2006), light stress (Li et al. 2007), and apoptotic stimulus (Mackey et al. 2008). Inactivating the Akt pathway by using PI3K inhibitors results in photoreceptor death (Yu et al. 2004;Yu et al. 2006;Mackey et al. 2008). The retina expresses all three Akt isoforms (Akt1, Akt2 and Akt3) (Li et al. 2007;Reiter et al. 2003).
It has also been reported that only specific isoforms of Akt are activated by certain signals, such as Akt1 and Akt3 activation in retina after insulin treatment (Reiter et al. 2003;Li et al. 2008) or Ak2 activation in retina due to light stress (Li et al. 2007). The isoform specific activation of Akt cannot be explained by the inactivation of the phosphatase PTEN or SHIP2, because inactivation of these phosphatases would result in the decrease of PI-3,4,5-P3, which would decrease the pAkt levels of all isoforms. The isoform specific regulation of Akt was a mystery until the discovery of the PHLPP family of protein phosphatases.
The PHLPP family of proteins comprises of 2 members, PHLPP and PHLPPL which belong to the PP2C subfamily of phosphatases. Both proteins have a similar domain structure, which has a PH domain followed by leucine-rich repeats (LRR), a PP2C phosphatase domain, and a C-terminal PDZ domain. PHLPPβ has an additional Ras association domain (RA) preceding the PH domain at the N-terminus of the protein. The two proteins share a 63% identity in the PH domain and 58% identity in the PP2C domain. PHLPP and PHLPPL dephosphorylate serine-473 of AKT and thus terminate AKT activation. Further, it has been shown that these two phosphatases selectively dephosphorylate Akt isoforms (Brognard et al. 2007;Gao et al. 2005).
To our knowledge, there are no published studies on the role of Akt regulating enzymes such as PHLPP and PHLPPL in the retina. In this study, we show that both PHLPP and PHLPPL are present in the retina and they are expressed in rod and cone photoreceptors. PHLPP exists as 2 splice variants α (140 kD) and β (190 kD), whereas PHLPPL exists as a single variant (150 kD).
We have previously reported that only Akt2 is essential for stress-induced photoreceptor survival and maintenance, although all three Akt isoforms are present in photoreceptor cells (Li et al. 2007). We (Rajala et al. 2004) and others (Reiter et al. 2003) reported the activation of Akt through insulin receptor-PI3K pathway. Constitutive activation of Akt is important for cone survival, and, very recently it was reported that insulin deprivation leads to cone photoreceptor cell death due to nutrient starvation (Punzo et al. 2009). These studies highlight the importance of Akt activation in neuroprotection of both rods and cones. Our current study shows the expression of both PHLPP and PHLPPL in retina and our earlier studies indicate that deletion of proteins of IR-signaling pathway leads to stress-induced photoreceptor degeneration (Rajala et al. 2008). These observations led to the hypothesis that down-regulation of the activity of these phosphatases is necessary to achieve neuroprotection.
Since PHLPP and PHLPPL are able to dephosphorylate Akt, and Akt is a downstream effector of IR/PI3K pathway, we examined whether IR-signaling pathway regulates the activity of PHLPP and PHLPPL. Consistent with this hypothesis, insulin induced the inhibition of PHLPP and PHLPPL activity ex vivo. To determine whether the observed inhibition is mediated through PI3K, we measured the insulin-induced inhibition of PHLPP and PHLPP activities in the presence of PI3K inhibitor, LY294002. Both enzymes are regulated through PI3K, as inhibition of PI3K activity resulted in the activation of PHLPP and PHLPPL phosphatase activities.
The insulin-induced inhibition of PHLPP and PHLPPL activities could be mediated by two putative mechanisms. First, the generation of 3’-phosphoinositides could directly regulate the phosphatase activity. Second, the 3’-phosphoinostides could regulate Akt activation. Both PHLPP and PHLPPL have a PH domain, which could directly bind to PI3K-generated 3’-phosphoinosides and there to modulate their activity. To attest this possibility, we expressed the PH domain as a GST-fusion protein and incubated it with PIP-strips (Echelon), and did not observe any binding under experimental conditions where the PH domain of Akt1 bound to 3’-phosphoinositides. This experiment rules out the possibility of the direct involvement of 3’-phosphoinositides in the inhibition of these two phosphatases but does not rule out an indirect regulation through another protein that is modulated by PI3K generated phosphoinositides.
To address whether Akt directly regulates PHLPP and PHLPPL phosphatase activities, we cloned full length α PHLPP and PHLPPL, and transfected the phosphatases with either pcDNA or each of the Akt isoforms and estimated the activity. Activity of PHLPPL was decreased in the presence of each of the Akt isoforms compared to pcDNA, proving that Akt modulates the activity of PHLPPL.
We tested the possibility that the molecular mechanism of Akt-mediated PHLPPL inhibition may be due to selective phosphorylation of PHLPPL by Akt. However, we were unable to detect Akt mediated phosphorylation on PHLPPL in HEK samples co-transfected with Akt1, Akt2 or Akt3 by the conventional Akt substrate antibody (PAS), disproving a direct phosphorylation effect of Akt (data not shown). Therefore, the Akt mediated inhibition of PHLPPL has to be an indirect effect by an Akt activated substrate rather than a direct effect by Akt.
Our studies suggest that both PHLPP and PHLPPL activities are PI3K-depenent. In our experimental conditions, chemical inhibition of Akt activation inhibits the activity of PHLPP but not PHLPPL. It may be possible that higher concentrations of this inhibitor might be required to achieve the inhibition of PHLPPL activity. On the other hand, co-expression of all 3 Akt isoforms along with PHLPP or PHLPPL showed different results. The results indicate that all 3 Akt isoforms are able to inhibit the activity of PHLPPL but not PHLPP. The contradictory results from the ex vivo retinal organ cultures with PI3K inhibitor and lack of inhibition of PHLPP activity by overexpression of all 3 Akt isoforms suggest that that PHLPP activity could be under the control of Akt-downstream effector(s), and this effector might be expressed in retina but not HEK-293T cells. However, serum starvation of HEK293-T cells resulted in the activation of PHLPP activity suggesting an alternative pathway for the activation of PHLPP in the HEK-293T cells. Consistent with this hypothesis we failed to observe the inhibition of PHLPP activity in light stress. Our data also suggest that Akt activation may be sufficient to inhibit the activity of PHLPPL and we confirmed this hypothesis from both ex vivo retinal organ cultures and co-transfection of all 3 Akt isoforms along with PHLPPL in HEK −293T cells. Our study is functionally important as PHLPPL and Akt are expressed in rod photoreceptors and inhibition of PHLPPL activity could be neuroprotective. Based on our previous and current study suggest that the inhibition of PHLPPL activity through IR/PI3K/Akt signaling is neuroprotective. It is tempting to speculate that inactivation of Akt may be a triggering signal for the activation of PHLPPL, which would then completely inactivate Akt by dephosphorylation in retinal degeneration. Studies are underway in our laboratory to test this hypothesis.
In summary, our results demonstrate that activation of IR signaling inhibits the activities of both PHLPP and PHLPPL. Further, PHLPPL and Akt are predominantly expressed in the inner segment of the rod photoreceptor cells. Both PHLPP and PHLPPL phosphatases have been shown to dephosphorylate Akt (Brognard et al. 2007), but our studies clearly suggest the inactivation of these phosphatases by Akt activation. Akt activation is shown to be essential for the retinal neuroprotection (Li et al. 2007) and if Akt serves as a substrate for PHLPPL, one would expect to observe a retinal degeneration phenotype. Even in the presence of PHLPPL the rod photoreceptors do not undergo any retinal degeneration suggesting that PHLPP activity could be regulated in a tissue specific manner. Further studies are required to understand whether PHLPPL has any role in retinal degenerations.
ACKNOWLEDGEMENTS
This work was supported by grants from the National Institutes of Health (EY016507; EY00871), NEI Core grant (EY12190), NCRR COBRE core modules (RR17703) and Research to Prevent Blindness, Inc. We thank Dr. Vivek K. Gupta for the critical review of this manuscript.
The abbreviations used are
- ROS
Rod outer segments
- IR
Insulin receptor
- PHLPP
PH domain and leucine rich repeat protein phosphatases
- PHLPPL
PH domain and leucine rich repeat protein phosphatase-like
- Nrl
Neural retinal leucine zipper transcription factor
REFERENCES
- Alessi DR, Kozlowski MT, Weng QP, Morrice N, Avruch J. 3-Phosphoinositide-dependent protein kinase 1 (PDK1) phosphorylates and activates the p70 S6 kinase in vivo and in vitro. Curr Biol. 1998;8:69–81. doi: 10.1016/s0960-9822(98)70037-5. [DOI] [PubMed] [Google Scholar]
- Barber AJ, Nakamura M, Wolpert EB, Reiter CE, Seigel GM, Antonetti DA, Gardner TW. Insulin rescues retinal neurons from apoptosis by a phosphatidylinositol 3-kinase/Akt-mediated mechanism that reduces the activation of caspase-3. J Biol Chem. 2001;276:32814–32821. doi: 10.1074/jbc.M104738200. [DOI] [PubMed] [Google Scholar]
- Brognard J, Sierecki E, Gao T, Newton AC. PHLPP and a second isoform, PHLPP2, differentially attenuate the amplitude of Akt signaling by regulating distinct Akt isoforms. Mol Cell. 2007;25:917–931. doi: 10.1016/j.molcel.2007.02.017. [DOI] [PubMed] [Google Scholar]
- Daniele LL, Lillo C, Lyubarsky AL, Nikonov SS, Philp N, Mears AJ, Swaroop A, Williams DS, Pugh EN., Jr Cone-like morphological, molecular, and electrophysiological features of the photoreceptors of the Nrl knockout mouse. Invest Ophthalmol Vis Sci. 2005;46:2156–2167. doi: 10.1167/iovs.04-1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fayard E, Tintignac LA, Baudry A, Hemmings BA. Protein kinase B/Akt at a glance. J Cell Sci. 2005;118:5675–5678. doi: 10.1242/jcs.02724. [DOI] [PubMed] [Google Scholar]
- Galetic I, Andjelkovic M, Meier R, Brodbeck D, Park J, Hemmings BA. Mechanism of protein kinase B activation by insulin/insulin-like growth factor-1 revealed by specific inhibitors of phosphoinositide 3-kinase--significance for diabetes and cancer. Pharmacol Ther. 1999;82:409–425. doi: 10.1016/s0163-7258(98)00071-0. [DOI] [PubMed] [Google Scholar]
- Gao T, Furnari F, Newton AC. PHLPP: a phosphatase that directly dephosphorylates Akt, promotes apoptosis, and suppresses tumor growth. Mol Cell. 2005;18:13–24. doi: 10.1016/j.molcel.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Hresko RC, Mueckler M. mTOR.RICTOR is the Ser473 kinase for Akt/protein kinase B in 3T3-L1 adipocytes. J Biol Chem. 2005;280:40406–40416. doi: 10.1074/jbc.M508361200. [DOI] [PubMed] [Google Scholar]
- Lawlor MA, Alessi DR. PKB/Akt: a key mediator of cell proliferation, survival and insulin responses? J Cell Sci. 2001;114:2903–2910. doi: 10.1242/jcs.114.16.2903. [DOI] [PubMed] [Google Scholar]
- Li G, Anderson RE, Tomita H, Adler R, Liu X, Zack DJ, Rajala RV. Nonredundant role of Akt2 for neuroprotection of rod photoreceptor cells from light-induced cell death. J Neurosci. 2007;27:203–211. doi: 10.1523/JNEUROSCI.0445-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Rajala A, Wiechmann AF, Anderson RE, Rajala RV. Activation and membrane binding of retinal protein kinase Balpha/Akt1 is regulated through light-dependent generation of phosphoinositides. J Neurochem. 2008;107:1382–1397. doi: 10.1111/j.1471-4159.2008.05707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackey AM, Sanvicens N, Groeger G, Doonan F, Wallace D, Cotter TG. Redox survival signalling in retina-derived 661W cells. Cell Death Differ. 2008;15:1291–1303. doi: 10.1038/cdd.2008.43. [DOI] [PubMed] [Google Scholar]
- Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marte BM, Downward J. PKB/Akt: connecting phosphoinositide 3-kinase to cell survival and beyond. Trends Biochem Sci. 1997;22:355–358. doi: 10.1016/s0968-0004(97)01097-9. [DOI] [PubMed] [Google Scholar]
- McGinnis JF, Matsumoto B, Whelan JP, Cao W. Cytoskeleton participation in subcellular trafficking of signal transduction proteins in rod photoreceptor cells. J Neurosci Res. 2002;67:290–297. doi: 10.1002/jnr.10120. [DOI] [PubMed] [Google Scholar]
- New DC, Wu K, Kwok AW, Wong YH. G protein-coupled receptor-induced Akt activity in cellular proliferation and apoptosis. FEBS J. 2007;274:6025–6036. doi: 10.1111/j.1742-4658.2007.06116.x. [DOI] [PubMed] [Google Scholar]
- Parcellier A, Tintignac LA, Zhuravleva E, Hemmings BA. PKB and the mitochondria: AKTing on apoptosis. Cell Signal. 2008;20:21–30. doi: 10.1016/j.cellsig.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Punzo C, Kornacker K, Cepko CL. Stimulation of the insulin/mTOR pathway delays cone death in a mouse model of retinitis pigmentosa. Nat Neurosci. 2009;12:44–52. doi: 10.1038/nn.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajala A, Tanito M, Le YZ, Kahn CR, Rajala RV. Loss of neuroprotective survival signal in mice lacking insulin receptor gene in rod photoreceptor cells. J Biol Chem. 2008;283:19781–19792. doi: 10.1074/jbc.M802374200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajala RV. Phosphoinositide 3-kinase signaling in the vertebrate retina. J Lipid Res. 2010;51:4–22. doi: 10.1194/jlr.R000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajala RV, McClellan ME, Ash JD, Anderson RE. In vivo regulation of phosphoinositide 3-kinase in retina through light-induced tyrosine phosphorylation of the insulin receptor beta-subunit. J Biol Chem. 2002;277:43319–43326. doi: 10.1074/jbc.M206355200. [DOI] [PubMed] [Google Scholar]
- Rajala RV, McClellan ME, Chan MD, Tsiokas L, Anderson RE. Interaction of the Retinal Insulin Receptor beta-Subunit with the P85 Subunit of Phosphoinositide 3-Kinase. Biochemistry. 2004;43:5637–5650. doi: 10.1021/bi035913v. [DOI] [PubMed] [Google Scholar]
- Reiter CE, Sandirasegarane L, Wolpert EB, Klinger M, Simpson IA, Barber AJ, Antonetti DA, Kester M, Gardner TW. Characterization of insulin signaling in rat retina in vivo and ex vivo. Am J Physiol Endocrinol Metab. 2003;285:E763–E774. doi: 10.1152/ajpendo.00507.2002. [DOI] [PubMed] [Google Scholar]
- Samardzija M, Wenzel A, Aufenberg S, Thiersch M, Reme C, Grimm C. Differential role of Jak-STAT signaling in retinal degenerations. FASEB J. 2006;20:2411–2413. doi: 10.1096/fj.06-5895fje. [DOI] [PubMed] [Google Scholar]
- Sokolov M, Lyubarsky AL, Strissel KJ, Savchenko AB, Govardovskii VI, Pugh EN, Jr, Arshavsky VY. Massive light-driven translocation of transducin between the two major compartments of rod cells: a novel mechanism of light adaptation. Neuron. 2002;34:95–106. doi: 10.1016/s0896-6273(02)00636-0. [DOI] [PubMed] [Google Scholar]
- Wigler M, Pellicer A, Silverstein S, Axel R. Biochemical transfer of single-copy eucaryotic genes using total cellular DNA as donor. Cell. 1978;14:725–731. doi: 10.1016/0092-8674(78)90254-4. [DOI] [PubMed] [Google Scholar]
- Yu X, Rajala RV, McGinnis JF, Li F, Anderson RE, Yan X, Li S, Elias RV, Knapp RR, Zhou X, Cao W. Involvement of insulin/phosphoinositide 3-kinase/Akt signal pathway in 17 beta-estradiol-mediated neuroprotection. J Biol Chem. 2004;279:13086–13094. doi: 10.1074/jbc.M313283200. [DOI] [PubMed] [Google Scholar]
- Yu XR, Jia GR, Gao GD, Wang SH, Han Y, Cao W. Neuroprotection of insulin against oxidative stress-induced apoptosis in cultured retinal neurons: involvement of phosphoinositide 3-kinase/Akt signal pathway. Acta Biochim Biophys Sin (Shanghai) 2006;38:241–248. doi: 10.1111/j.1745-7270.2006.00152.x. [DOI] [PubMed] [Google Scholar]