Abstract
The coupling of dosimetry measurements and modeling represents a promising strategy for deciphering the relationship between chemical exposure and disease outcome. To support the development and implementation of biological monitoring programs, quantitative technologies for measuring xenobiotic exposure are needed. The development of portable nanotechnology-based electrochemical sensors has the potential to meet the needs for low cost, rapid, high-throughput and ultrasensitive detectors for biomonitoring an array of chemical markers. Highly selective electrochemical (EC) sensors capable of pM sensitivity, high-throughput and low sample requirements (<50uL) are discussed. These portable analytical systems have many advantages over currently available technologies, thus potentially representing the next-generation of biomonitoring analyzers. This manuscript highlights research focused on the development of field-deployable analytical instruments based on EC detection. Background information and a general overview of EC detection methods and integrated use of nanomaterials in the development of these sensors are provided. New developments in EC sensors using various types of screen-printed electrodes, integrated nanomaterials, and immunoassays are presented. Recent applications of EC sensors for assessing exposure to pesticides or detecting biomarkers of disease are highlighted to demonstrate the ability to monitor chemical metabolites, enzyme activity, or protein biomarkers of disease. In addition, future considerations and opportunities for advancing the use of EC platforms for dosimetric studies are discussed.
Keywords: biomonitoring, dosimetry, electrochemical sensors, exposure assessment
Introduction
Biological monitoring (biomonitoring) has the ability to integrate total chemical exposure to assess human dosimetry (Yantasee et al., 2007a). This includes exposure from multiple sources (i.e. air, soil, water and food residues) and multiple routes of intake (i.e. inhalation, oral and dermal). A benefit of biomonitoring is the ability to associate the internal dose of a given chemical or metabolite with a measurable effect (either tissue specific or whole body), which can then be used for risk assessment purposes (Gilbert and Sale, 2005; Christensen, 1995; Friberg and Elinder, 1993). Likewise, biomonitoring can be exploited to distinguish between internal (actual) from potential exposure. As suggested by Angerer et al. (2006) and illustrated in Figure 1, the exposure-effect continuum represents a framework for assessing chemical exposures and making both risk assessment and management decisions within epidemiological studies. In this regard, it is suggested that the most meaningful interpretation of epidemiology studies could be realized by accurately assessing chemical exposure with biological effect. However, a major impediment to conducting epidemiology studies is the lack of affordable quantitative technologies that can readily measure chemical exposure markers (biomarkers) using minimally invasive biological fluids (Weis et al., 2005). To address these limitations, inexpensive micro-analytical based sensors are needed that can accurately and precisely process small amounts of biological fluids. Ideally, these sensors can be used for parallel analyses of multiple markers or quickly adapted for detection of a broad range of biomarkers associated with chemical exposure and biological response (Liu et al., 2005; Weis et al., 2005). As reviewed by Weis et al. (2005), microsensor platforms offer great promise because they have the potential to provide rapid, accurate and quantitative detection of exposure at the level of the individual. The data generated from such devices can then be used to effectively couple environmental and personal exposure assessment in a way that enhances our ability to study the factors that affect health and disease across large populations.
Figure 1.
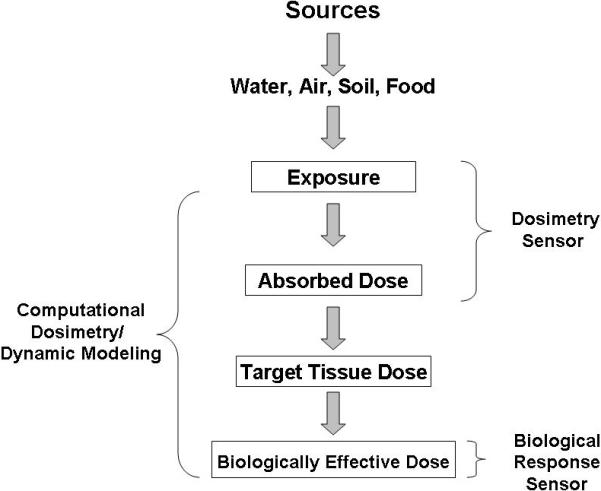
Diagram of the exposure-effect continuum relating exposure source with dosimetric and biological response. Figure adapted from Angerer et al. (2006).
The identification and quantification of target chemicals or their metabolites in biological fluids (blood, urine, and saliva) is still a cornerstone of xenobiotic metabolism research, where the analytes represent the key biological monitoring targets (Gil and Pla, 2001; Angerer et al., 2006). However, the utility of a specific analyte (e,g., chemical metabolite) for quantitative biological monitoring requires an appreciation of its pharmacokinetics; that is, a concentration associated with the rate of absorption, distribution, metabolism, and/or excretion in the relevant biological matrices (Timchalk et al., 2001; 2004a; 2004b). A strategy for the development, validation and deployment of a chemical biomonitoring platform is illustrated in Figure 2. Key criteria include identification of markers in complex matrices, such as blood, urine or saliva, validation of sensor performance, and deployment of a user-friendly platform. Validation should not only include characteristics of instrument performance (e.g., limit of detection, limit of quantification, linear performance, reproducibility, matrix effects, etc.), but the marker(s) should have positive predictive value that link chemical exposure with adverse health effects.
Figure 2.
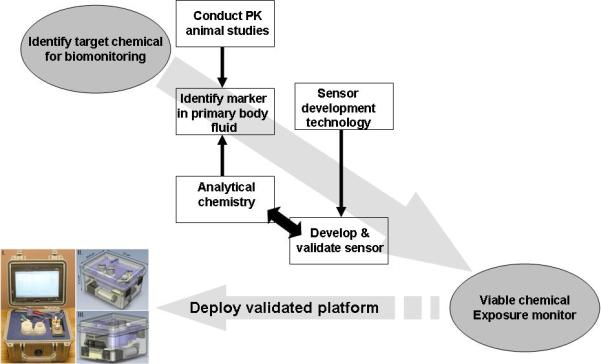
Strategy for the development, validation and deployment of biological monitoring sensor platforms.
This review is focused on the development and validation of portable electrochemical sensors that incorporate nanomaterials as either a signal transducer or as an electroactive species for indirect detection of analyte. Given the sensitivity, flexibility, and miniaturization capabilities, these sensors have the potential to become the next-generation of field-deployable analytical instruments. Our intent is to: 1) provide a general overview of electrochemical (EC) terminology, detection methods and integrated use of nanomaterials in the development of EC-based microanalytical instrumentation; 2) highlight recent developments using EC sensors for biomonitoring; 3) illustrate recent applications of nanotechnology-based EC sensors for detection and quantification of biomarkers of exposure or disease; and 4) discuss future considerations and opportunities for advancing the use of EC sensors for dosimetric studies.
Methods
Review of General Terms and Overview of Electrochemical Sensors
Electrochemical measurements are based on detection or transport of charge across an electrode. Chemical species, such as molecular ions, are referred to as electroactive species if they can either be oxidized (lose electrons) or reduced (gain electrons) at an electrode surface through the movement of electrons. A detector that measures current when an electroactive solute contacts a working electrode held at a fixed potential with respect to a reference electrode is known as an amperometric detector; whereas measurement of current that develops as a function of variable potential is known as voltammetry. In voltammetry, a variable potential excitation signal is impressed upon a solution through an electrode to generate a characteristic signal at the electrode, known as waveforms and expressed as potential per unit time. Amperometric and voltammetric detectors both generate quantitative information of electrode current versus time, but voltammetry has the added advantage of providing characteristic current response information for reversible reactions by pulsing an applied potential between high and low values. If the added potential is pulsed with short time intervals between high and low values, the current that flows back and forth through the electrode can be measured during the lifetime of the pulse. As the potential is pulsed in voltammetry between high and low voltage values, the analyte (or product) at the surface of the electrode can undergo a corresponding reduction or oxidation and generate positive or negative current flow. A plot of the difference between the changing current flows (Δi = ihighV – ilowV) versus potential results in a voltammogram that is characteristic for the electroactive species (Skoog and Leary, 1992). When the potential is applied in a staircase fashion a waveform is produced (potential per unit time) and the technique is known as square-wave voltammetry. In stripping analysis, the solute is preconcentrated (electrodeposited) at the surface of the electrode using a constant applied potential prior to application of a stripping potential (stripping voltammetry) to redissolve the material from the electrode (Figure 3). If the stripping potential is applied as a square wave, the technique is known as square wave stripping voltammetry. Classical amperometric and voltammetric EC methods have been attractive as analytical techniques because they offer detection limits on the order of 10−7−10−8 M, while stripping analysis preconcentrates solutes to achieve limits down to 10−10−10−11 M (Bard and Faulkner, 1980). Since many analytes are electroactive, EC methods are the most widely used alternatives to atomic and/or mass spectroscopic techniques for trace detection of analytes.
Figure 3.
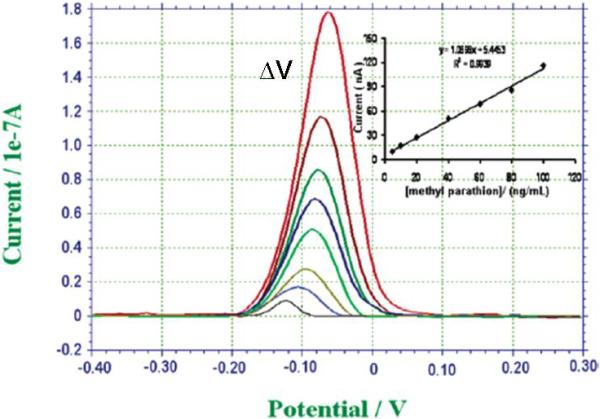
Stripping voltammograms, current versus applied potential, of increasing methyl-parathion (pesticide) concentration, from bottom to top, 5, 10, 20, 40, 60, 80, 100, and 200 ng mL−1. The inset shows the calibration curve. (Adapted from Liu and Lin, 2005)
EC methods are also more amenable to the development of portable instrumentation because of their simplicity, low power requirements, and ability to be miniaturized. Techniques that are based on manipulation of a liquid sample have been particularly fruitful for detection of toxic chemicals, these include flow-injection analysis and sequential-injection analysis using amperometric detection (Gilbert and Sale, 2005; Neufeld et al., 2000; Sole and Alegret, 2001; Xu et al., 2007), and micro-electrical mechanical systems (Chen et al., 2006a; Wang et al., 2001). In each of these injection methods, samples are injected into flow cells and transported by a carrier solution and reacted with select substrates that can be electrochemically detected (Figure 4). By using a calibration curve, analyte concentration can be calculated from the detector response. Flow-injection analysis with amperometric detection, for example, offers the possibility of real time and continuous-flow detection of toxic compounds within environmental or biological samples, using very small volumes (< 20uL) or high-throughput analysis of numerous samples (3600 injections/h) (Wang and Li, 1990; Liu et al., 2005; Liu and Lin, 2006). Microfabrication technology has been used to develop whole-sale separations and detection on a single micro-electrical mechanical systems microchip. Most fabrication processes involve photolithography, wet etching, laser ablation, or injection-molding to form microchannels, valves, and interconnects on silicon, glass or polymer substrates (Manz et al., 1991, Belmont et al., 1996, Chen et al., 2006a). Fluid flow or analyte detection is then carried out by electrical methods. Amperometric and conductivity detection have used micro-electrical mechanical devices, for example, for the detection of various residues of chemical nerve agents (Wang et al., 2001), nitroaromatic compounds (Wang et al., 2002), and heavy metal ions (Collins and Lu, 2001; Dabek-Zlotorzynska et al., 2003).
Figure 4.
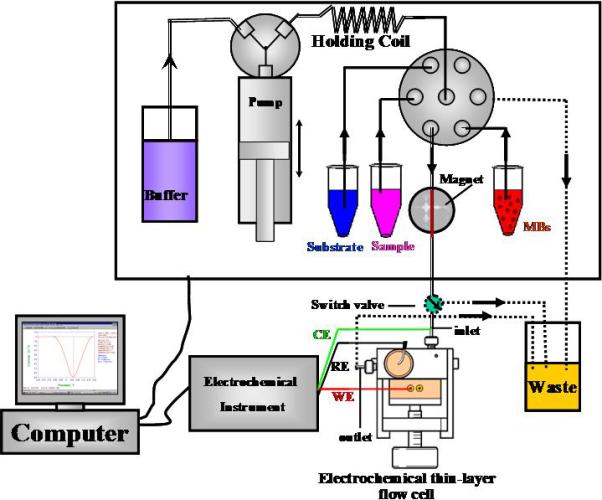
Schematic of sequential injection/electrochemical immunoassay for quantification of 3,5,6-trichloro-2-pyridinol (TCP). The computer controlled sequential-injection analysis system (MicroSIA, FIAlab Instruments Inc., WA) includes six-port selection valve for delivering sample and reagents, a thin-layer cross-flow cell (MF-1095, Bioanalytical system Inc., West Lafayette, IN) that contains a glassy carbon electrode, a Ag/AgCl reference electrode and electrochemical analyzer voltammetric detection of electroactive species (Model CHI 660, CH Instruments Inc., TX). Figure adapted from Liu et al. (2005) with permission.
Even with the tremendous inventiveness exhibited by numerous researchers, the application of EC methods has been limited because of the need for electrodes with unique electrical and selective properties. However, new developments in material science have now produced a broad range of technologies that are being adapted to increase the utility of EC sensors.
Modern Advances in the Development of Electrochemical Sensors
Recent advances in printing technology and materials science now allows greater flexibility in producing extremely inexpensive, reliable, and selective electrodes for detection of specific analytes. These advances can be grouped into three EC technologies: screen-printed electrodes, integrated nanomaterials, and EC immunoassays. Hybrid devices that incorporate the advantages of each of these new technologies are also being developed. In general, modern advances in the development of low cost and selective EC sensors offer a greater range of applications for analysis of chemical exposure for environmental or biological monitoring. These systems are suitable for the determination of chemicals, metabolites, proteins, metals, inorganic ions, organic compounds and other biological molecules in a variety of biological matrices.
Screen-Printed Electrodes
Various types of carbon-based, plastic, and ceramic materials are being coated with different doping agents to enhance electron transfer or coated with selectivity agents to capture analytes of interest using screen-printing technology (e.g., photolithography). The coated electrodes are then employed as the working electrode in voltammetric analyses (Badihi-Mossberg et al., 2007; Renedo et al., 2007). The coatings may include agents such as metals, complexing molecules, immobilized enzymes or affinity agents (e.g., antibodies). Screen-printed electrodes can be categorized according to how the electrode is modified: metal-modified, enzyme-modified, or affinity capture-modified (Renedo et al., 2007). Electrodes doped with gold or silver atoms, or coated with gold, mercury, bismuth, or nickel metal films have been used with stripping voltammetry to monitor a variety of hazardous metals and chemicals in biological fluids with detection limits of ng mL−1; enzyme-immobilized screen-printed electrodes have been used to detect enzyme activity, pesticide metabolites, phenolic compounds, heavy metals, cholesterol, and glucose in biological matrices by immobilizing an enzyme onto a sol-gel matrix or conductive polymer to ng mL−1 levels; while antibody-immobilized screen-printed electrodes have been used with amperometric and voltammetric detection of antigen in biomatrices at the pg mL−1 level (Renedo et al., 2007). For a comprehensive review of the latest applications of screen-printed electrodes, see Renedo et al. (2007).
Integrated Nanomaterials for EC Applications
The advent of nanotechnologies has led to enormous advances not only in basic science but also in detection strategies. Nanomaterials offer new platforms for developing a variety of advanced analytical technologies, including more sensitive and selective electrochemical sensors for biomonitoring. The most studied nanomaterials, carbon nanotubes, metal nanoparticles, and quantum dots have been especially targeted for developing novel biosensors (Liu and Lin, 2006; He and Toh, 2006; Liu et al., 2007a; 2007b; Pumera et al., 2007; Wu et al., 2007). Nanomaterials are now being used as signal transducers to mediate current flow or as electroactive tags to indicate the detection of analyte.
Nanomaterials are attractive because of their unique electrical, chemical and physical properties (i.e., size, composition, conductivity, magnetism, mechanical strength, light absorbing and emitting properties). Carbon nanotubes, colloid gold, quantum dots, and zirconium oxide nanoparticles have all been used for electrochemical detection of chemicals in the environment and more recently in biological samples (Kim et al., 2007; Liu and Lin, 2005; Liu and Lin, 2006; Wang et al., 2008a; Wang et al., 2008c). By utilizing nanomaterials, electrochemical biosensors have shown great promise for detection of chemical markers and biomarkers of exposure primarily because the nanomaterials are used to either capture the marker or amplify the signal associated with detection. Both of these capabilities are important for trace level detection in complex biological matrices. Two approaches are currently used for nanomaterials-based electrochemical biosensors: 1) the use of nanomaterials as the electrical signal transducer and 2) nanomaterials used as electroactive tags for the indirect detection of analyte. Carbon nanotubes and metal nanoparticles have, for example, been used to modify the electrode surface for enhancing electrochemical response due to their electrocatalytic properties (Chen et al., 2007). Additionally, silicon nanowires and conducting polymer nanowires have been used as field-effect transistors (Patolsky et al., 2006). Alternatively, some nanoparticles are used as the electroactive reporters for indirect detection of analyte and/or for signal amplification purposes; the most common of which are metal (e.g., colloid gold) and inorganic nanoparticles (e.g., Fe2O3 and CdSe quantum dots) (Authier et al., 2001; Cui et al., 2007; Wu et al., 2007; Liu et al., 2007b). Collectively, EC assays that integrate nanomaterials have been used for the detection of chemical exposure or biomarkers of disease using a variety of biological matrices (Wang et al., 2003; Liu and Lin, 2005; Wang et al., 2006a; Liu and Lin, 2006).
Carbon nanomaterials contain nanostructures that seem to be especially suitable for the development of electrochemical biosensors (Tasis et al., 2006). In particular, carbon nanotubes and carbon nanofibers possess conductivity, surface areas, chemical functionalities, and biocompatibility that make them ideal for the development of compound specific biosensors (Trojanowicz, 2006; Andreescu and Marty, 2006; Du et al., 2007 Kim et al., 2007). Carbon nanotubes are composed of graphite carbon with one or more concentric tubes. Recent studies have shown that carbon nanotubes can enhance the direct electron transfer reactions of some biomolecules, including cytochrome c, catalase, and nicotinamide adenine dinucleotide due to the unique electronic structure, electrical conductivity, and high surface area available on carbon nanotubes for redox reactions (Lin et al., 2005; Liu and Lin, 2006; Wang et al., 2006b; Wang et al., 2008a).
Nanoparticles such as colloidal metals (e.g., gold or silver), inorganic crystals (e.g., quantum dots), and silica are currently being used as labels, markers, or probes for detection of a wide-range of biomolecules (Guo and Wang, 2007). In most cases, these types of nanoparticles are conjugated to some biomolecule (e.g., DNA, protein, or antibody) and the biomolecule is used for identifying certain biomolecular interactions, cellular translocations, or affinity capture of an analyte of interest. The most common approaches use colloidal gold or quantum dots as the electroactive species. Quantum dots (QD) are nanoscale crystals composed of group II-VI or III-V elements (e.g. CdSe, ZnS or Fe2O3) that can either absorb light energy and emit photons at characteristic wavelengths (Jamieson, et. al., 2007; Wang et al., 2008b) or be acid solubilized to generate electroactive metal ions (Wu et al., 2007; Liu et al., 2007b). A variety of electrochemical DNA sensors, for example, have been functionalized with nanoparticles for direct and indirect detection of metal ions, proteins, RNA and DNA, with detection limits as low as zeptomoles (10−21 moles) (Authier et al., 2001; Liu and Lu, 2003; Wang et al., 2003; Drummond et al., 2003; Tansil and Gao, 2006). Nanoparticle-based electrochemical bioassays for proteins have been reviewed by Wang (2007). There are also considerable efforts to leverage nanoparticle sensitivity with antibody selectivity using EC methods, known as electrochemical immunoassays.
Electrochemical Immunoassays
Immunoassay based sensors have been developed to exploit the high degree of specificity and affinity of antibodies for specific antigens (Wu et al., 2007; Liu et al., 2007). In this particular application, a given chemical, metabolite, or modified protein can act as the antigen, and an electrochemical response linked to antibody binding with the antigen. Generally, the antibody or analyte of interest is immobilized on a membrane, magnetic bead or electrochemical transducer, and the analyte is measured via signal derived from a conjugated tag that is attached to either the antibody or analyte. Two types of immunoassays are generally employed: a competitive active site assay or the sandwich assay. In the competitive immunoassay, the analytes within a matrix compete with an analog analyte for antibody sites and the signal from the analog is used to calculate how much analyte of interest was captured. The analog is labeled or tagged in some fashion (e.g. enzyme or nanoparticle), and it is the tag that ultimately generates detectable electroactive species. In this assay, the amount of analyte present is calculated by determining the ratio of analog signal relative to the known maximum amount of signal possible for a known concentration of analog (Lin et al., 2007; Zacco et al., 2007). The second type of immunoassay utilizes a primary antibody to selectively capture the analyte from the matrix and a secondary antibody containing a signaling device to quantify the captured analyte. The two antibodies effectively sandwich the analyte in between them and the approach is referred to as a sandwich immunoassay. Conventionally, enzymes are conjugated on the secondary antibody and used to generate signal in the biological technique known as ELISA (enzyme-linked immunosorbent assay). With the addition of substrate (unrelated to the analyte) the enzyme selectively cleaves the substrate to generate signal (i.e., light, fluorescence, ions, etc). With EC assays, tags that generate electroactive species are ideal and a variety of nanomaterials are being evaluated as potential tags when conjugated to antibodies. Metal nanoparticles such as colloidal gold or CdSe quantum dots are typically used, and commercial kits are available for conjugation to antibodies (Knecht, et. al., 1986, Hainfeld, et. al. 2000, Merkoci, et. al. 2005; Jain, 2007; Wang, 2007). Based on these approaches, several immunosensors have been developed for the detection of a range of xenobiotics and protein biomarkers of disease including: the herbicide chlorsulphuron, polychlorinated biphenyls, polycyclic aromatic hydrocarbons, 2,4,6-trichloroanisole, pesticide metabolites, human myoglobin, cardiac troponin, and creatine kinase (Galve et al., 1974; Velaso-Arjona et al., 1997; Piras and Reho 2005; Renedo et al., 2007; Dong et al. 2007; Liu et al. 2005; 2006). EC immunoassays routinely achieve detection limits in the low pM range and three orders of dynamic range using <50uL of sample.
Results
EC Sensors for Biomonitoring
There have been a number of recent overviews discussing advances in the application of electrochemical sensors for industrial hygiene and environmental monitoring (Ashley, 2003; Hanrahan et al., 2004), as well as the development of personal exposure biomonitors for heavy metals (Yantasee et al., 2007a; 2007b) and ChE biosensors (Andreescu and Marty, 2006). As reviewed by Ashley, electrochemical sensors have been developed for field monitoring of a broad range of chemical contaminants including: inorganic gases and vapors (carbon monoxide, carbon dioxide, sulfur dioxide, nitric and nitric oxide), volatile organic hydrocarbons, aldehydes and ketones, heavy metals, pesticides and some persistent pollutants (Ashley, 2003). However, as noted by Weis et al. the utilization of these sensors for human studies and in particular biological monitoring has not yet been fully realized. (Weis et al., 2005).
Consider effort has also been made to develop sensor platforms that monitor toxic metals or chemical xenobiotics in environmental applications (i.e. in air, water, or soil) (Hanrahan et al., 2004; Solé and Alegret, 2001; Sadik and Van Emon, 1996), but there is a general lack of publications that specifically focus on the detection and quantification for human biomonitoring. It is important to note that in some cases sensor systems that have been developed for environmental monitoring may be utilized with biological samples with minimum validation; but in other cases methods must be dramatically modified to instead detect a metabolic byproduct or avoid fouling of the sensor due to matrix effects. For example, a number of electrochemical based sensor platforms have been developed for the detection of the phenoxyacetic acid herbicide 2,4D in a range of environmental media (Kim et al., 2007; Halámek et al., 2001; Kröger et al., 1998). In the case of human biomonitoring, 2,4-D is readily absorbed and excreted primarily unchanged in the urine (Timchalk, 2004; Arnold and Beasley; 1989); hence, the primary issue for adapting the environmental sensor systems for human biomonitoring is to optimize performance for urine matrix effects and validate the sensor against biological samples (i.e. urine) containing 2,4-D. In other cases it may not be feasible to directly adapt environmental sensors for human biomonitoring, particularly in those situations where the environmental chemical undergoes extensive in vivo metabolism. In all cases, however, field trials will be required to validate the EC sensor performance against conventional methods. Recently, Yantasee et al. performed validation studies of nanotechnology-based capture of toxic metals from biological matrices and subsequent quantification using electrochemical methods (Yantasee et al., 2007a; 2007b). Results from a lead dosing study of rats demonstrated that the EC sensor was capable of detection limits of 0.44ppb and 0.46ppb with %RSD of 4.9 and 2.4 in 50% urine and 10% blood, respectively (Yantasee et al., 2007b). The sensor results were very similar to lead concentration values as measured by ICP MS, but EC sensor data was generated within 3 minutes per sample using 60uL of matrix while ICP MS required considerably more sample preparation.
Biomarkers of Organophosphate Pesticide Exposure
Considerable efforts are also being made to develop EC sensors that can be used for biomonitoring of pesticide biomarkers. Organophosphorus insecticides constitute a large class of chemical pesticides that are widely used (Aspelin, 1992; 1994) and have been involved in more poisoning cases than any other single class of insecticide (Al-Saleh, 1994). These chemicals have a high affinity for binding to and inhibiting the enzyme acetylcholinesterase (AChE); an enzyme specifically responsible for the destruction of the neurotransmitter acetylcholine (ACh) within nerve tissue (Wilson, 2001; Ecobichon, 2001). Since the cholinergic system is widely distributed within both the central and peripheral nervous systems, chemicals that inhibit AChE are known to produce a broad range of well characterized symptoms (for review see Savolainen, 2001). A comparison of the AChE inhibition dynamics for the interaction of ACh, and the “active” insecticide metabolite chlorpyrifos-oxon (organophosphate) with AChE is illustrated as an example in Figure 5. Both substrates have relatively high affinities for AChE and readily complex with the enzyme; however, the rates of hydrolysis and reactivation of AChE following phosphorylation of the active site will be drastically slower than for the hydrolysis of the acetylated enzyme (Ecobichon, 2001). For organophosphorus insecticide biomonitoring, sensor development has mainly focused on the measurement of ChE activity and quantification of major metabolites. Current efforts are also underway in our laboratory to detect the organophosphate chemical adducts that preferentially form on proteins (e.g. ChE) in the relevant biological matrices. Biomonitoring offers one of the best approaches for accurately assessing human dosimetry and for determining risk from chemical exposures (Friberg and Elinder, 1993; Christensen, 1995; Timchalk, 2004a; 2004b). In the case of organophosphorus insecticides, blood and urine have been the primary matrices for evaluation of both dosimetry (parent & metabolites) and ChE activity (Peoples and Knaak, 1982; Nolan et al., 1984; Chester, 1993; Timchalk et al., 2002). However, other matrices such as saliva are being investigated and may offer a single non-invasive matrix for assessing both ChE activity and dosimetry (Borzelleca and Skalsky, 1980; Ryhanen, 1983; Kousba et al., 2003; Timchalk et al., 2004a; 2007a; Henn et al., 2006).
Figure 5.
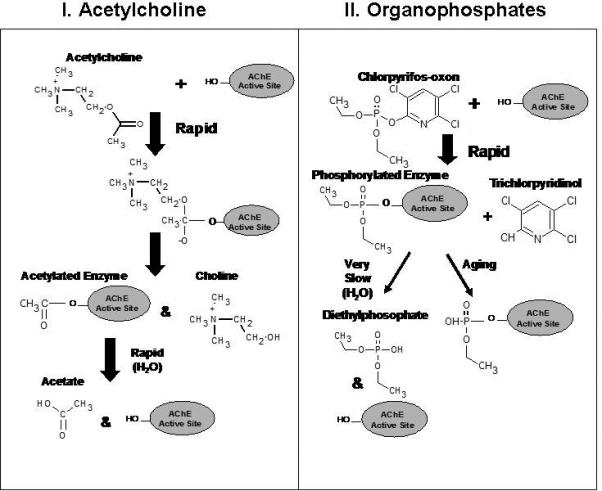
Schematic illustrating the interaction of acetylcholine (ACh) (I), and the organophosphate chlorpyrifos-oxon (II) with the active site of acetylcholinesterase (AChE).
Diethylphosphorothionates, for example, are one of the major sub-classes of organophosphorus insecticides, which include a number of commonly used pesticides, such as chlorpyrifos, diazinon, and parathion. A representative scheme (Figure 6) for the metabolism of chlorpyrifos shows that it undergoes CYP450-mediated oxidative desulfation or dearylation to form chlorpyrifos-oxon (the neurotoxic moiety) or 3,5,6-trichloro-2-pyridinol (TCP) and diethylthiophosphate, respectively (Chambers and Chambers, 1989; Ma and Chambers, 1994). Hepatic and extrahepatic esterases such as paraoxonase and cholinesterase effectively metabolize chlorpyrifos-oxon to form TCP and diethylphosphate. Dialkylphosphates, such as diethylphosphate and diethylthiophosphate, have long been used as general urinary biomarkers for this class of insecticides (Hardt et al., 2000; Bradman et al., 2005; CDC, 2005; Timchalk et al., 2007b). For assessing human exposure to specific organophosphorus insecticides, the metabolite containing the organic moiety, such as TCP in the case of chlorpyrifos, has been used since this is a specific biomarker found in urine (Nolan et al., 1984; Berkowitz et al., 2004; Eskenazi et al., 2004; CDC, 2005; Barr et al., 2004; Timchalk et al., 2007b).
Figure 6.
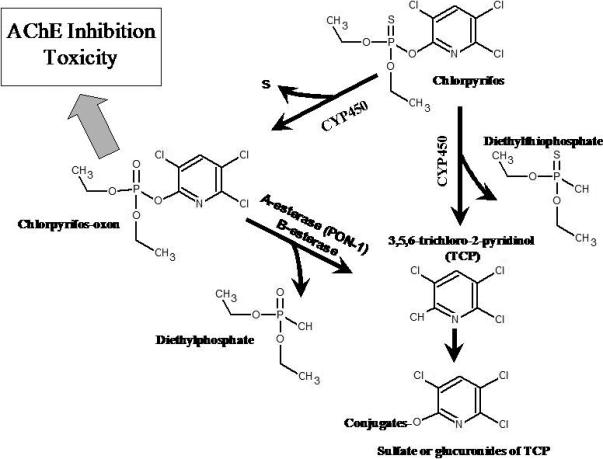
Metabolic scheme for the metabolism of chlorpyrifos and the major metabolites chlorpyrifos-oxon, trichloropyridinol (and conjugates), diethylphosphate and diethythiophosphate. Figure adapted from Timchalk et al. (2004) with permission.
In addition, the chemical reactivity and covalent binding of organophosphorus insecticides and nerve agents with blood and tissue proteins produce novel chemical adducts (known as alkylphosphorylation and simply referred to as phosphorylation) on specific matrix proteins that have the potential to be exploited as biomarkers of exposure. The most common modification is phosphorylation of cholinesterase (either acetylcholinesterase or butyrylcholinesterase) and subsequent inactivation of cholinesterase, leading to cholinergic system failure. Phosphorylated adducts have also been detected on other proteins, including carboxylesterase, neuropathy target esterase, trypsin, chymotrypsin, and human serum albumin (Ooms and van Dijk, 1966; Boter and Ooms, 1967; Ecobichon and Comeau, 1973; Johnson, 1975; Johnson and Glynn, 1995; Fonnum et al., 1985; Elhanany et al., 2001; Black et al., 1999; Peeples et al., 2005; Li et al., 2007). Biomonitoring of protein adducts extends the time interval between exposure and sampling and may be a suitable approach to detect low-level exposure. In this regard, Polhuijs et al. (1997) developed a procedure for the analysis of phosphorylated binding sites, which is based on reactivation of the phosphorylated enzyme with fluoride ions. Based on these methods, it was suggested that detection levels in the range of ∼0.01% inhibited butyrylcholinesterase should be quantifiable. This represents a detection level that is several orders of magnitude greater than what is currently possible on the basis of measuring cholinesterase activity. Thus, three different types of biomarkers of OP exposure are available which can be used to provide information as to the subclass of pesticide involved (e.g., organophosphate versus carbamate), extent of exposure, and used to determine the association with adverse health: cholinesterase activity, chemical metabolites, and phosphorylated protein.
Applications of EC sensors for Biomonitoring AChE Activity
The utility and advantage of using carbon nanotubes in EC biosensors is demonstrated below in two examples that seek to monitor biological enzyme activity as a way to assess chemical exposure. Each uses an enzyme-immobilized on a screen-printed carbon nanotube electrode but one type is for the direct detection of substrate hydrolysis products, while the other is an indirect method that relies on a redox (electron exchange) reaction to generate hydrogen peroxide (H2O2).
Generally, two approaches have been used for characterization of cholinesterase activity in biological matrices using carbon nanotube-based EC biosensors: either a single step system utilizing indigenous cholinesterase and appropriate substrate (Liu and Lin, 2006) or a binary method where cholinesterase is combined with choline oxidase (ChO) to generate and detect H2O2 as an electroactive end-product (Guerrieri and Palmisano, 2001; Lin et al., 2004). The cholinesterase activity in either system is monitored by measuring the oxidation or reduction current of the product of the enzymatic reaction(s).
The first example of a cholinesterase-immobilized screen-printed electrode reaction series is shown in Equations 1 and 2 (Liu and Lin, 2006). In this sensor, acetylcholinesterase (AChE) was self-assembled on top of a carbon nanotube (graphite carbon) surface and integrated with a flow injection system using amperometric monitoring for the detection of hydrolyzed acetylthiocholine substrate. The carbon nanotube acts in this case as both a convenient platform for enzyme immobilization and efficient electron transducer to monitor inhibition of the enzyme in the presence of the pesticide paraoxon. The single-step reaction involved AChE hydrolysis of a known amount of acetylthiocholine substrate:
| (1) |
The subsequent oxidation of the thiocholine (TCh) at the electrode surface gives rise to a current that constitutes a quantitative measurement of the enzymatic activity:
| (2) |
Since AChE activity is inversely related to the amount of paraoxon present in the system, the sensor can be used for quantification of inhibitor present in the matrix. This sensor was sensitive to sub pM levels (pg mL−1) of paraoxon and 20% AChE inhibition after six minutes of exposure (incubation) using 20 uL of sample.
In some cases, it may be necessary to indirectly detect enzyme inhibition by adding reagents that are more amenable for detection by EC methods. In the following binary enzyme system (Eqns 3 and 4), AChE enzymatically cleaves the neurotransmitter acetylcholine in vivo into acetate and choline, and choline is subsequently converted to an aldehyde and hydrogen peroxide (H2O2) by choline oxidase (ChO) for the amperometric detection of H2O2 (Lin et al., 2004):
| (3) |
| (4) |
For this system, the carbon nanotube-EC method produced a detection limit of 50 nM organophosphate pesticide and a sensitivity of 0.48% inhibition/uM enzyme (Lin et al., 2004).
In both of the carbon nanotube sensor examples, the presence of any chemical inhibitor of AChE, such as an organophosphate pesticide, would lead to lower current measurements. A comparison with normal activity levels could then be used to calculate the level of chemical exposure. The sensitivity of these types of sensors depends considerably on the chosen method of enzyme immobilization, transducer material employed, rate and number of chemical reaction(s), and sensitivity for a given electroactive species. Carbon nanotube detection of the single-step production of thiocholine, for example, was much better than the binary production of H2O2 by ∼60-fold. In both cases, use of a carbon nanotube electrode generated much greater S/N responses compared with an unmodified carbon screen-printed electrode, indicating a catalytic advantage. This advantage is attributed to the large surface area of the carbon nanotube available for redox reactions.
Applications of EC Immunosensors for Biomonitoring Metabolites or Protein Markers
Direct monitoring of key chemical or protein markers associated with a specific pesticide or disease offers the ability to quickly assess individuals. Competitive and sandwich-type EC immunoassays are being developed in our laboratories to provide the selectivity and sensitivity required for personalized biomonitoring. In addition, portable EC sensor platforms are being developed to integrate the EC sensor within an automated sample processor (Liu et al. 2005; 2006; Wu et al., 2007; Liu et al. 2007b).
Two types of competitive binding EC immunoassays for the indirect detection of metabolite are being optimized using either an enzyme-tagged analog for detection of H2O2 (Liu et al. 2005; 2006) or a quantum dot-tagged analog for detection of acid hydrolyzed Cd ions (Wu et al., 2007; Liu et al. 2007b).
A competitive EC immunoassay for the quantification of 3,5,6-trichloro-2-pyridinol (TCP), a metabolite marker for the insecticide chlorpyrifos, was recently developed (Liu et al., 2005; 2006). In this case, anti-TCP antibody was immobilized on magnetic particles to capture TCP, followed by the addition of sample and horseradish-peroxidase (HRP) enzyme-tagged TCP (analog), then addition of the HRP substrate TMB (3,3’,5,5’-tetramethyl-benzidine dihydrochloride) to generate electroactive H2O2 (Figure 7) (Liu et al., 2005). Using a sequential-injection analysis system to automatically deliver reagents and sample (Figure 4), antibody coated magnetic beads (TCP-AB-MBs) were first pumped to a specific zone of the ‘reactor tube’, where a magnetic field was applied to capture the beads for further reactions. After an initial washing step, a premixed sample solution containing TCP and HRP-labeled TCP was introduced into the ‘reactor tube’ containing the antibody-immobilized beads and incubated for ∼20 min. Once the reaction was complete, the beads were washed again and the HRP substrate, TMB, was injected into the ‘reactor tube’ to generate H2O2. Finally, the production of H2O2 product was sent to a thin-layer flow cell for electrochemical measurement (Figure 7). The reduction current required to reduce H2O2 at the electrode surface is proportional to the amount of TCP present in the sample.
Figure 7.
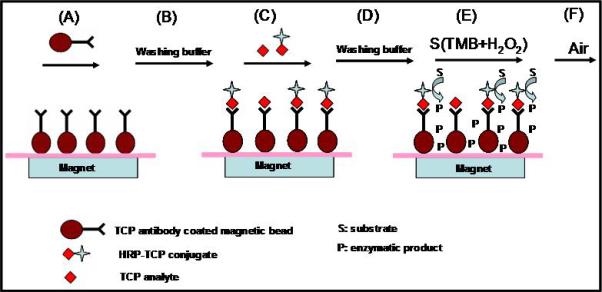
Diagram illustrating competitive immunoassay for 3,5,6-trichloro-2-pyridinol (TCP) determination. (A) immobilization of TCP antibody-coated magnetic beads to the internal wall of reactor tube by magnet; (B) washing beads with buffer; (C) injection of sample solution containing TCP analyte and TCP-horseradish peroxidase (TCP-HRP) for competitive immunoreaction; (D) washing beads with buffer; (E) injection of the substrate solution (TMB + H2O2) to initiate enzymatic reaction; (F) analysis of enzymatic product by electrochemical measurement. Figure adapted from Liu et al. (2005) with permission.
The selectivity of the TCP/HRP-TCP EC immunoassay was determined by using compounds structurally similar to TCP, including 2,4,5-trichlorophenol, 2,4-dichlorophenol, chlorpyrifos, chlorpyrifos-methyl and trichlopyr. The results of these comparisons are presented in Table 1. For each insecticide, metabolite or structurally related chemical, a 50% inhibition concentration (IC50) was determined and compared with the TCP IC50 and the extent of cross reactivity (CR) was calculated (Liu et al., 2006). The structurally related compounds such as 2,4,5-trichlorophenol and 2,4-dichlorophenol demonstrated low cross reactivity (≤2%); whereas, the parent pesticides chlorpyrifos, chlorpyrifos-methyl and trichlopyr showed little to no cross reactivity (<0.01%) (Liu et al., 2006). These results demonstrated that the TCP antibody coated magnetic beads were highly selective for TCP.
Table 1.
Cross reactivity of the bioelectrochemcal magnetic immunoassay for various insecticide, their metabolites or structurally similar compounds
| Compound | I501 (ng/mL) | CR2 (%) |
|---|---|---|
| 3,5,6-trichloro-2-pyridinol (TCP) | 0.45 | 100 |
| 2,4,5-trichlorophenol | 22 | 2.01 |
| 2,4-dichlorophenol | 430 | 0.11 |
| O,O-dimethyl O-3,5,6-trichloro-2-pyridylphosphorothioate (chlorpyrifos-methyl) | >1E4 | <0.01 |
| O,O-diethyl O-3,5,6-trichloro-2-pyridylphosphorothioate (chlorpyrifos) | >1E4 | <0.01 |
| 3,5,6-trichloro-2-pyridyloxyacetic acid (triclopyr) | >1E4 | <0.01 |
Inhibition concentration estimated at 50% I/I0.
Percentage cross reactivity
Performance of the sequential-injection analysis competitive EC immunoassay was optimized by adjusting reaction volumes and incubation times (Liu et al., 2005). Figure 8A shows the typical square-wave voltammagram responses with increasing TCP analyte concentrations. Well-defined peak shapes were used to calculate the concentration of the corresponding TCP in the sample matrix. The normalized signals, expressed as 100(I/I0) (where I and I0 are the reduction peak heights obtained with TCP standards and blank sample respectively) were plotted versus TCP concentration. A sigmoidal-shaped calibration curve for TCP was used to calculate TCP in the sample (as is characteristic of a competitive immunoassay) Figure 8B. The linear measuring range was 0.01−2.0 μg L−1, and the relative standard deviation was below 3.9% (n=6). A detection limit was calculated from competitive curves as the analyte concentration for which the normalized signal was 90%. The detection limit was estimated to be ∼6 ng L−1.
Figure 8.
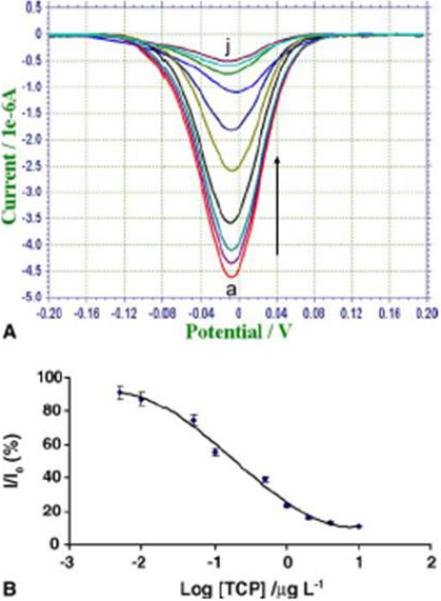
(A, top) Typical square-wave voltammetry of increasing TCP concentration in incubation solution. From bottom to top, the concentrations of TCP are 0, 0.005, 0.01, 0.05, 0.1, 0.5, 1, 2, 8, and 10 ug L−1. (B, btm) Sigmoidal calibration curve of TCP using 20 uL of TCP-Ab-MBs, 50 uL of TCP-HRP in 100 uL of sample solution, 100 uL of TMB-H2O2 substrate solution, reaction time of 20 min. Figure from Liu et al. (2005) with permission.
To build in signal amplification and eliminate the need for enzyme-driven electroactive species, quantum dots can be used as tags along with the appropriate electrode sensor. For the competitive EC immunoassay, a QD can either be conjugated directly onto a metabolite (if the chemistry is appropriate) or conjugated to a chemically compatible structural analog, then used as the competitive analog. EC signal can then be generated by incubating the QD tag with acid (e.g., 1M HCl) and detecting the hundreds to thousands of metal ions that dissolve from each QD.
Recently, we demonstrated the ultrasensitive detection and selection capability of QD-tagged sandwich immunoassays. Using commercially available monoclonal antibodies and QD conjugation kits (Invitrogen, Eugene, OR), EC immunosensors were developed for ng mL−1 or pg mL−1 detection of interleukin-1α (Figure 9) or prostate specific antigen (Figure 10), respectively, in human plasma (Wu et al. 2007; Liu et al., 2007). For detection of interleukin-1α, an immune and inflammatory response cytokine, primary interleukin-1α antibody was bound to streptavidin-coated magnetic beads and QDs consisting of a CdSe core/ZnS coat were conjugated to secondary antibodies to sandwich the antigen, followed by acid hydrolysis (1M HCl) of the QDs and detection of Cd+2 ions with a bismuth/mercury coated screen-printed electrode using square-wave voltammetry (Figure 9). A detection limit of 18 pM (or 1.8 fmoles) of cytokine could be detected in 200 uL of sample with a linear range of 0.5−50 ng mL−1. For detection of prostate specific antigen in human serum, a disposable EC immunosensors was manufactured containing separate zones for primary and secondary antigen capture (Figure 10). Primary monoclonal antibodies were conjugated with QDs and immobilized onto a glassy fiber pad and incubated with sample to capture the antigen, then the QD-antibody-antigen complex was migrated to a second zone containing immobilized secondary antibody for capture of tagged complex, followed by the addition of HCl at the second zone, solubilization of the QD and EC detection of Cd+2 ions as the ions traversed laterally to the electrode surface hidden beneath a nitrocellulose membrane (Figure 10 and Figure 11A). Prostate specific antigen could be detected down to 20 pg mL−1 with 20 min of total incubation time. The EC assay was compared with results obtained from a commercially available ELISA prostate specific antigen kit (Alpha Diagnostics International) and similar concentration values were obtained for each assay but the reproducibility of the EC assay did vary more at lower concentrations; at 0.5 ng mL−1 the EC assay had a %CV of 24.5% while ELISA produced 7.1%, but at 2 ng mL−1 the EC assay had a 2.5% CV while ELISA produced 8.2%. These first set of experiments are quite promising and further optimizations and improvements are underway to enhance sample migration, analyte capture, and signal detection. In addition, we have integrated the disposable EC immunoassay into a self-contained platform that can be interfaced with a computer containing the appropriate software (Figure 11B). Our focus is to develop low cost disposable immunochromatographic strips that can be rapidly evaluated in a fashion similar to glucose monitors used by diabetic patients.
Figure 9.

Solubilization and detection of quantum dot (QD) tags. Scheme of an EC sandwich immunoassay (A) using primary monoclonal anti-interleukin-1α antibody immobilized on magnetic bead and QD tagged secondary anti-interleukin-1α antibody, followed by acid solubilization of CdSe core/ZnS coated QDs and detection of cadmium ions (B) by SWV (C). Adapted from Wu et al. (2007) with permission.
Figure 10.
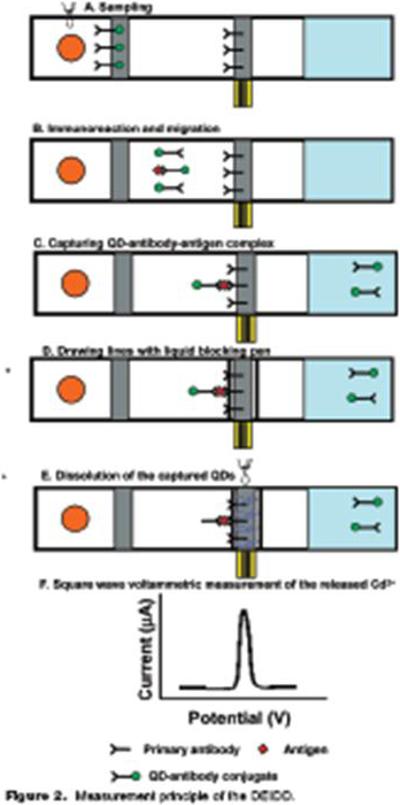
Development of a disposable EC sandwich immunoassay for the detection of prostate specific antigen, protein biomarkers of prostate cancer. Primary monoclonal antibodies were conjugated with QD and immobilized onto a glassy fiber pad and incubated with sample to capture the antigen (A), then the QD-antibody-antigen complex was migrated (B) to a second zone containing immobilized secondary antibody for capture of tagged antigen (C), followed by drawing two insulator lines with liquid blocker (super PAP pen, D) and the addition of HCl at the second zone, solubilization of the QD and EC detection of Cd+2 ions as the ions traversed laterally to the electrode surface hidden beneath a nitrocellulose membrane (E) to produce a square-wave voltammetry signal (F). Adapted from Liu et al. 2007, with permission.
Figure 11.

(A) Schematic of a disposable EC immunosensors containing a primary capture region using an immobilized QD-tagged anti-prostate antigen antibody, a secondary capture regions containing secondary antibodies. Upon treatment with 1M HCl at the secondary region, cadmium ions are released and electrophorectically migrate to a bismuth/mercury-coated screen-printed electrode beneath a nitrocellulose membrane. (B) Sample is added to the strip and the electrical pins of the strip are inserted into an electronic control box of the portable EC platform. Voltage is applied to the strip and the signal output is displayed on a PC. Figure is adapted from Liu et al. 2007 with permission.
Interpretation of Sensor Biomonitoring Results
An important consideration is whether the sensor platforms have adequate sensitivity to detect and quantify at environmentally relevant exposure concentrations. In this regard, computational dosimetry modeling has been used to establish the sensitivity to detect TCP in saliva and blood of humans following repeated oral exposures to chlorpyrifos (Timchalk et al., 2007b). To accomplish this, a physiologically based pharmacokinetic and pharmacodynamic model was used to simulate dosimetry and dynamic response in humans (Timchalk et al., 2002). The model simulated repeated dietary exposure to chlorpyrifos over a 72 hour period at the established EPA Reference Dose (RfD) of 0.003 mg/kg/day (EPA, 1994). The results from these simulations and the reported detection limits for a commercial TCP ELISA and the electrochemical immunoassay are presented in Figure 12. These simulations suggest that the electrochemical immunoassay theoretically has the needed sensitivity to detect TCP in both blood and saliva based upon the anticipated range of concentrations at the RfD level. Future in vivo studies are needed to substantiate the model simulations and validate the detection limits for the sensor platforms.
Figure 12.
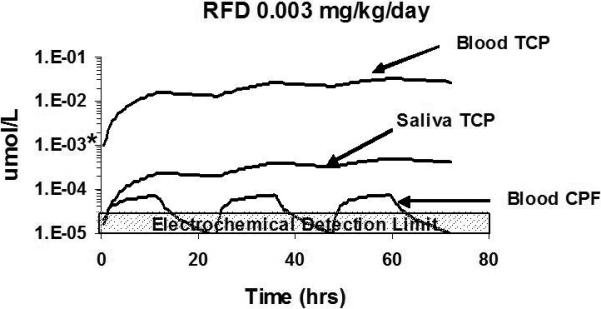
Computational pharmacokinetic model simulation of 3,5,6-trichloro-2-pyridinol (TCP) in blood and saliva and chlorpyrifos in blood following a repeated (3-day) dietary exposure (12 hr/day) to 0.003 mg/kg/day (RfD). The detection limit (*) for the ELISA assay is based on the value reported by the manufacturer of the TCP rapid Assay® kit (0.25 μg/L or 1E−3 μmol/L). Figure adapted from Timchalk et al. (2007) with permission.
Although biomonitoring offers one of the best approaches for accurately assessing human dosimetry and for determining risk from both occupational and environmental exposure to xenobiotics (Friberg and Elinder, 1993; Christensen, 1995) the ability to better interpret the results of biomonitoring is also needed (Hays et al., 2007). One potentially useful approach is to use “reverse dosimetry” to back-extrapolate a population-based distribution of biomonitoring data to a distribution of exposure doses (Hays et al., 2007). In this regard, Tan et al. (2007) recently used a “reverse dosimetry” approach to integrate outputs from physiologically based pharmacokinetic modeling, exposure characterizations, and Monte Carlo and statistical analyses to estimate the distribution of exposures to trihalomethanes which could then be compared with animal-based health standards (i.e. RfD). With regard to the organophosphate insecticide chlorpyrifos a similar approach was used based on a simple pharmacokinetic model of TCP urinary excretion kinetics to estimate dosimetry in children (Rigas et al., 2001). In this particular study, however, the reverse pharmacokinetic modeling of urinary TCP generally over predicted dose. As suggested by Rigas et al. (2001) and others, exposure to organophosphate breakdown products such as TCP as residues on food or in the environment could contribute to the greater than expected amounts of TCP in urine (Timchalk et al., 2007b; Lu et al., 2005; Morgan et al., 2005; Wilson et al. 2003). One way to strengthen predictive assessments would be to develop computational dosimetry models based on metabolic endpoints and multiple biomarkers (e.g. for pesticide exposures: cholinesterase inhibition, metabolites, and protein biomarkers).
Discussion
Summary Considerations for EC Sensors and Future Opportunities
Disposable electrochemical sensors offer a wide-range of applications for analysis of chemical compounds within biological systems, given they are minimally susceptible to matrix effects and are selective for the compound of interest. An important consideration for sensor development is the optimization of the sensor performance in a broad range of biological matrices (i.e. blood, urine, saliva) that are routinely used to biological monitoring (Yantasee et al., 2007a). Specifically, the EC sensor must be validated for analytical performance and cross-validated with well-established analytical methods, such as mass spectrometry and ELISA. Characteristics such as accuracy, precision, sensitivity, selectivity, repeatability and stability should be addressed. Secondly, a robust evaluation is needed to assess the potential for detection interference using biological matrices. Thirdly, in vivo animal and in vitro human validation studies are needed to assess the selectivity, sensitivity, and robustness of the sensor platforms. And finally, appropriate field evaluation studies are needed to ascertain the performance of the sensor platforms under a broad range of robust conditions that exist in the “real-world”.
Future opportunities exist to investigate the use of alternative affinity or sequestration stationary phases, such as titanium oxide, zirconium oxide, aptamers (small nucleic acids strands), chelators, self-assembled monolayers, polymer-modified nanoparticles, and nanostructured hydrogels (Singh et al., 1999; Flounders et al., 1999; Liu and Lin, 2005; Mattigod et. al., 2005; Nayak and Lyon, 2005; Castellana et al., 2006; Yantasee, et. al., 2006; 2007b; Li and Ho, 2008; Xiao and Li, 2008; Li et al., 2008). In addition, throughput could be extended by developing multichannel, parallel, and multiplexed detection of multiple analytes (Tang et al., 2002; Dong et al., 2008; Polsky et al. 2008).
Conclusion
We are all routinely exposed to a broad range of chemicals that are present within the environment; including pollutants within the air we breathe, the food we eat, and the water we drink. Exposure to these chemicals may be associated with our home or work environments, as part of our jobs, or as lifestyle choices. Epidemiology studies have been utilized to ascertain an association between environmental chemical exposures and human disease. As recently noted, current epidemiology study designs are seriously hampered due to the inability to make definitive associations between chemical exposures and disease (Weis et al., 2005). Biomonitoring studies that can routinely measure chemical and biological molecular markers of exposure and the development of dosimetry models that can factor in these markers offers the potential to better understand the risk factors associated with adverse health. Inexpensive, sensitive, flexible and portable biomonitoring tools need to be developed that can handle the logistics of monitoring the myriad of markers associated with chemical exposures among different populations. The development of portable nanotechnology-based electrochemical sensors has the potential to meet the needs for low cost, rapid, high-throughput and ultrasensitive bioassays for biomonitoring an array of chemical markers. Highly selective EC sensors capable of pM sensitivity, high-throughput (3600 samples/h) with low sample requirements (<50uL) have already been demonstrated; and improved EC sensors are on the horizon. To advance the utility and improve the quality of these sensors for risk assessment and epidemiological studies, it would be ideal to form close collaborations between chemists, toxicologists, and epidemiologists.
Acknowledgements
This work was performed at Pacific Northwest National Laboratory (PNNL) supported partially by grant number NS058161-01 from the National Institutes of Health CounterACT Program through the National Institute of Neurological Disorders and Stroke, partially by CDC/NIOSH Grant R01 OH008173-01, and partially by grant number U54 ES16015 from the National Institute of Environmental Health Sciences (NIEHS), NIH. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the Federal Government. The research described in this paper was partly performed at the Environmental Molecular Sciences Laboratory, a national scientific user facility sponsored by the DOE's Office of Biological and Environmental Research and located at PNNL. PNNL is operated by Battelle for DOE under Contract DE-AC05-76RL01830.
References
- Al-Saleh IA. Pesticides: a review article. J Environ Path Toxicol Oncol. 1994;13:151–161. [PubMed] [Google Scholar]
- Andreescu S, Marty J-L. Twenty years research in cholinesterase biosensors: from basic research to practical applications. Biomol Engineer. 2006;23:1–15. doi: 10.1016/j.bioeng.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Angerer J, Bird MG, Burke TA, Doerrer NG, Needham L, Robison SH, et al. Strategic biomonitoring initiatives: Moving the science forward. Toxicol Sci. 2006;93(1):3–10. doi: 10.1093/toxsci/kfl042. [DOI] [PubMed] [Google Scholar]
- Arnold EK, Beasley VR. The pharmacokinetics of chlorinated phenoxy acid herbicidnes: a literature review. Vet Hum Toxicol. 1989;31(2):121–125. [PubMed] [Google Scholar]
- Ashley K. Developments in electrochemical sensors for occupational and environmental health applications. J Haz Mat. 2003;102:1–12. doi: 10.1016/s0304-3894(03)00198-5. [DOI] [PubMed] [Google Scholar]
- Aspelin AL. Pesticide Industry Sales and Usage: 1990 and 1991 Market Estimates. Office of Pesticide Programs, US Environmental Protection Agency, EPA; Washington, DC: 1992. 733-K-92−001. [Google Scholar]
- Aspelin AL. Pesticide Industry Sales and Usage: 1992 and 1993 Market Estimates. Office of Pesticide Programs, US Environmental Protection Agency, EPA; Washington, DC: 1994. 733-K-94−001. [Google Scholar]
- Authier L, Grossiord C, Brossier P, Limoges B. Gold nanoparticle-based quantitative electrochemical detection of amplified human cytomegalovirus DNA using disposable microband electrodes. Anal Chem. 2001;73:4450–4456. doi: 10.1021/ac0103221. [DOI] [PubMed] [Google Scholar]
- Badihi-Mossberg M, Buchner V, Rishpon J. Electrochemical Biosensors for pollutants in the environment. Electroanalysis. 2007;19:2015–2028. [Google Scholar]
- Bard AJ, Faulkner LR. Electrochemical methods: fundamentals and applications. John Wiley and Sons Inc; New York: 1980. pp. 413–414. [Google Scholar]
- Barr DB, Bravo R, Weerasekera G, Caltabiano LM, Whitehead RD, Jr, et al. Concentrations of dialkyl phosphate metabolites of organophosphorus pesticides in the U. S. population. Environ Health Perspect. 2004;112:186–200. doi: 10.1289/ehp.6503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmont C, Tercier ML, Buffle J, Fiaccabrino GC, Koudelka-Hep Mercury-plated iridium-based microelectrode array for trace metals detection by voltammetry: optimum conditions and reliability. Anal Chim Acta. 1996;329:203–214. [Google Scholar]
- Berkowitz GS, Wetmur JG, Birman-Deych E, Obel J, Lapinski RH, et al. In utero pesticide exposure, maternal paraoxonase activity, and head circumference. Environ Health Perspect. 2004;112(3):388–391. doi: 10.1289/ehp.6414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black RM, Harrison JM, Read RW. The interaction of sarin and soman with plasma proteins: the identification of a novel phosphonylation site. Arch Toxicol. 1999;73:123–126. doi: 10.1007/s002040050596. [DOI] [PubMed] [Google Scholar]
- Borzelleca JF, Skalsky HL. The excretion of pesticides in saliva and its value in assessing exposure. J Environ Sci Health. 1980;B15(6):843–866. doi: 10.1080/03601238009372220. [DOI] [PubMed] [Google Scholar]
- Boter HL, Ooms AJJ. Stereospecificity of hydrolytic enzymes in their reaction with optically active organophosphorus compounds. II. The inhibition of aliesterase, acetylesterase, chymotrypsin and trypsin by S-alkyl para-nitrophenyl methylphosphonothiolates. Biochem Pharmacol. 1967;16:1563–1569. doi: 10.1016/0006-2952(67)90134-7. [DOI] [PubMed] [Google Scholar]
- Bradman A, Eskenazi B, Barr D, Bravo R, Castorina R, et al. Organophosphate urinary metabolite levels during pregnancy and after delivery in women living in an agricultural community. Environ Health Persp. 2005;12(113):1802–1807. doi: 10.1289/ehp.7894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter-Pokras O, Zabrana RE, Peppell CF, Logie LA, Guerrero-Preston R. The environmental health of latino children. J Pediat Health Care. 2007;21:307–314. doi: 10.1016/j.pedhc.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellana ET, Kataoka S, Albertorio F, Cremer PS. Direct writing of metal nanoparticle films inside sealed microfluidic channels. Anal Chem. 2006;78:107–112. doi: 10.1021/ac051288j. [DOI] [PubMed] [Google Scholar]
- CDC . Centers for Disease Control and Prevention. Third national report on human exposure to environmental chemicals. Department of Health and Human Services; 2005. NCEH Pub. # 05−0570. [Google Scholar]
- Chambers JE, Chambers HW. Oxidative desulfation of chlorpyrifos, chlorpyrifosmethyl, and leptophos by rat brain and liver. J Biochem Tox. 1989;4(1):201–203. doi: 10.1002/jbt.2570040310. [DOI] [PubMed] [Google Scholar]
- Chen G, Lin YH, Wang J. Monitoring environmental pollutants by microchip capillary electrophoresis with electrochemical detection. Talanta. 2006a;68:497–503. doi: 10.1016/j.talanta.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Chen JC, Shih JL, Liu CH, Kuo MY, Zen JM. Disposable electrochemical sensor for determination of nitroaromatic compounds by a single-run approach. Anal Chem. 2006b;78:3752–3757. doi: 10.1021/ac060002n. [DOI] [PubMed] [Google Scholar]
- Chen SH, Yuan R, Chai YQ, Zhang LY, Wang N, Li XL. Amperometric third-generation hydrogen peroxide biosensor based on the immobilization of hemoglobin on multiwall carbon nanotubes and gold colloidal nanoparticles. Biosens Bioelectron. 2007;22:1268–1274. doi: 10.1016/j.bios.2006.05.022. [DOI] [PubMed] [Google Scholar]
- Chester G. Evaluation of agricultural worker exposure to and absorption of pesticides. Occup Hyg. 1993;37:509–523. doi: 10.1093/annhyg/37.5.509. [DOI] [PubMed] [Google Scholar]
- Christensen JM. Human exposure to toxic metals: factors influencing interpretation of biomonitoring results. Sci Total Environ. 1995;166:89–135. doi: 10.1016/0048-9697(95)04478-j. [DOI] [PubMed] [Google Scholar]
- Collins GE, Lu Q. Microfabricated capillary electrophoresis sensor for uranium (VI). Anal Chim Acta. 2001;436:181–189. [Google Scholar]
- Cui R, Pan HC, Zhu JJ, Chen HY. Versatile immunosensor using CdTe quantum dots as electrochemical and fluorescent labels. Anal Chem. 2007;79:8494–8501. doi: 10.1021/ac070923d. [DOI] [PubMed] [Google Scholar]
- Dabek-Zlotorzynska E, Chen H, Ding LY. Recent advances in capillary electrophoresis and capillary electrochromatography of pollutants. Electrophoresis. 2003;24:4128–4149. doi: 10.1002/elps.200305658. [DOI] [PubMed] [Google Scholar]
- Delsingh AK. MEMS technology in analytical chemistry. The Analyst. 2003;128:9–11. doi: 10.1039/b211229a. [DOI] [PubMed] [Google Scholar]
- Drummond TG, Hill MG, Barton JK. Electrochemical DNA sensors. Nature Biotechnology. 2003;21(10):1192–1199. doi: 10.1038/nbt873. [DOI] [PubMed] [Google Scholar]
- Dong H, Li CM, Zhang YF, Cao XD, Gan Y. Screen-printed microfluidic device for electrochemical immunoassay. Lab Chip. 2007;7(12):1752–1758. doi: 10.1039/b712394a. [DOI] [PubMed] [Google Scholar]
- Du D, Ding JW, Cai J, Zhang AD. One-step electrochemically deposited interface of chitosan-gold nanoparticles for acetylcholinesterase biosensor design. J Electroanal Chem. 2007;605:53–60. [Google Scholar]
- Ecobichon DJ, Comeau AM. Pseudocholinesterases of mammalian plasma: physicochemical properties and organophosphate inhibition in eleven species. Toxicol Appl Pharmacol. 1973;24(1):92–100. doi: 10.1016/0041-008x(73)90184-1. [DOI] [PubMed] [Google Scholar]
- Ecobichon DJ. Toxic effects of pesticides. In: Klassen CD, editor. Casarett & Doull's Toxicology The basic science of poisons. 6th edn. McGraw-Hill; New York: 2001. pp. 763–810. [Google Scholar]
- Elhanany E, Ordentlich A, Dgany O, Kaplan D, Segall Y, et al. Resolving pathways of interaction of covalent inhibitors with the active site of acetylcholinesterases: MALDITOS/MS analysis of various nerve agent phosphyl adducts. Chem Res Toxicol. 2001;14:912–918. doi: 10.1021/tx0100542. [DOI] [PubMed] [Google Scholar]
- EPA, U.S. Environmental Protection Agency . Integrated Risk Information System Database. Washington, DC: 1994. pp. 5–54. [Google Scholar]
- Eskenazi B, Harley K, Bradman A, Weltzien E, Jewell NP, et al. Association of in utero organophosphate pesticide exposure and fetal growth and length of gestation in an agricultural population. Environ Health Perspect. 2004;112(10):1116–1124. doi: 10.1289/ehp.6789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidder A, Noort D, Hulst AG, De Ruiter R, Van der Schans MJ, et al. Retrospective detection of exposure to organophosphorus anti-cholinesterases: Mass spectrometric analysis of phosphylated human buturylcholinesterase. Chem Res Toxicol. 2002;15:582–590. doi: 10.1021/tx0101806. [DOI] [PubMed] [Google Scholar]
- Flounders AW, Singh AK, Volponi JV, Carichner SC, Wally K, Simonian AS, et al. Development of sensors for direct detection of organophosphates. Part II: sol-gel modified field effect transistor with immobilized organophosphate hydrolase. Biosens Bioelectron. 1999;14:715–722. doi: 10.1016/s0956-5663(99)00044-5. [DOI] [PubMed] [Google Scholar]
- Fonnum F, Sterri SH, Aas P, Johnsen H. Carboxylesterase, importance for detoxification of organophosphorus anticholinesterases and trichothecenes. Fundam Appl Toxicol. 1985;5:S29–S38. doi: 10.1016/0272-0590(85)90112-5. [DOI] [PubMed] [Google Scholar]
- Friberg L, Elinder CG. Biological monitoring of toxic metals. Scand J Work Environ Health. 1993;19(Suppl 1):7–13. [PubMed] [Google Scholar]
- Galve R, Nichkova M, Camps F, Sanchez-Baeza F, Marco MP. Development and evaluation of an immunoassay for biological monitoring chlorophenols in urine as potential indicators of occupational exposure. Anal Chem. 2002;74(2):468–478. doi: 10.1021/ac010419n. [DOI] [PubMed] [Google Scholar]
- Gil F, Pla A. Biomarkers as biological indicators of xenobiotic exposure. J Appl Toxicol. 2001;21:245–255. doi: 10.1002/jat.769. [DOI] [PubMed] [Google Scholar]
- Gilbert DM, Sale TC. Sequential electrolytic oxidation and reduction of aqueous phase energetic compounds. Environ Sci Technol. 2005;39:9270–9277. doi: 10.1021/es051452k. [DOI] [PubMed] [Google Scholar]
- Guerrieri A, Palmisano F. An Acetylcholinesterase/choline oxidase-based amperometric biosensor as a liquid chromatography detector for acetylcholine and choline determination in brain tissue. Anal Chem. 2001;73:2875–2882. doi: 10.1021/ac000852h. [DOI] [PubMed] [Google Scholar]
- Guo SJ, Wang EK. Synthesis and electrochemical applications of gold nanoparticles. Anal Chim Acta. 2007;598:181–192. doi: 10.1016/j.aca.2007.07.054. [DOI] [PubMed] [Google Scholar]
- Hainfeld JF, Powell RD. New frontiers in gold labeling. J Histochem Cytochem. 2000;48:471–480. doi: 10.1177/002215540004800404. [DOI] [PubMed] [Google Scholar]
- Halamek J, Hepel M, Skladal P. Investigation of highly sensitive piezoelectric immunosensors for 2,4-dichlorophenoxyacetic acid. Biosens Bioelectron. 2001;16(4−5):253–260. doi: 10.1016/s0956-5663(01)00132-4. [DOI] [PubMed] [Google Scholar]
- Hanrahan G, Patil DG, Wang J. Electrochemical sensors for environmental monitoring: design, development and applications. J Environ Monitor. 2004;6:657–664. doi: 10.1039/b403975k. [DOI] [PubMed] [Google Scholar]
- Hardt J, Angerer J. Determination of dialkyl phosphates in human urine using gas chromatography–mass spectrometry. J Anal Toxicol. 2000;8(24):678–684. doi: 10.1093/jat/24.8.678. [DOI] [PubMed] [Google Scholar]
- Hays SM, Becker RA, Leung HW, Aylward LL, Pyatt DW. Biomonitoring equivalents: A screening approach for interpreting biomonitoring results from a public health risk perspective. Reg Toxicol Pharmacol. 2007;47:96–109. doi: 10.1016/j.yrtph.2006.08.004. [DOI] [PubMed] [Google Scholar]
- He L, Toh CS. Recent advances in Anal Chem - A material approach. Anal Chim Acta. 2006;556:1–15. doi: 10.1016/j.aca.2005.08.042. [DOI] [PubMed] [Google Scholar]
- Henn BC, McMaster S, Padilla S. Measuring cholinesterase in human saliva. J Toxicol Environ Health A. 2006;69:1805–1818. doi: 10.1080/15287390600631458. [DOI] [PubMed] [Google Scholar]
- Hrapovic S, Majid E, Liu Y, Male K, Luong JHT. Metallic nanoparticle-carbon nanotube composites for electrochemical determination of explosive nitroaromatic compounds. Anal Chem. 2006;78:5504–5512. doi: 10.1021/ac060435q. [DOI] [PubMed] [Google Scholar]
- Jain KK. Applications of nanobiotechnology in clinical diagnostics. Clin Chem. 2007;53:2002–2009. doi: 10.1373/clinchem.2007.090795. [DOI] [PubMed] [Google Scholar]
- Jamieson T, Bakhshi R, Petrova D, Pocock R, Imani M, Seifalian AM. Bioapplications of quantum dots. Biomaterials. 2007;28:4717–4732. doi: 10.1016/j.biomaterials.2007.07.014. [DOI] [PubMed] [Google Scholar]
- Johnson MK, Glynn P. Neuropathy target esterase (NTE) and organophosphorus-induced delayed polyneuropathy (OPIDP): recent advances. Toxicol Lett. 1995;82−83:459–463. doi: 10.1016/0378-4274(95)03495-1. [DOI] [PubMed] [Google Scholar]
- Johnson MK. Organophosphorus esters causing delayed neurotoxic effects—mechanism of action and structure/activity studies. Arch Toxicol. 1975;34:259–288. doi: 10.1007/BF00353848. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Gobi KV, Iwasaka H, Tanaka H, Miura N. Novel minature SPR immunosensor equipped with all-in-one multi-microchannel sensor chip for detecting low-molecular-weight analytes. Biosens Bioelectron. 2007;23(5):701–707. doi: 10.1016/j.bios.2007.08.010. [DOI] [PubMed] [Google Scholar]
- Kim SN, Rusling JF, Papadimitrakopoulos F. Carbon nanotubes for electronic and electrochemical detection of biomolecules. Adv Materials. 2007;19:3214–3228. doi: 10.1002/adma.200700665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knecht E, Martinez-Ramon A, Grisolia S. Electron microscopic localization of glutamate dehydrogenase in rat liver mitochondria by an immunogold procedure and monoclonal and polyclonal antibodies. J Histochem Cytochem. 1986;34:912–922. doi: 10.1177/34.7.3519755. [DOI] [PubMed] [Google Scholar]
- Kousba AA, Poet TS, Timchalk C. Characterization of the in vitro kinetic interaction of chlorpyrifos-oxon with rat saliva cholinesterase: A potential biomonitoring matrix. Toxicol. 2003;188:219–232. doi: 10.1016/s0300-483x(03)00090-8. [DOI] [PubMed] [Google Scholar]
- Kroger S, Setford SJ, Turner AP. Immunosensor for 2,4-dichlorophenoxyacetic acid in aqueous/organic solvent soil extracts. Anal Chem. 1998;70(23):5047–5053. doi: 10.1021/ac9805100. [DOI] [PubMed] [Google Scholar]
- Li N, Ho C-M. Aptamer-based optical probes with separated molecular recognition and signal transduction modules. J Am Chem Soc. 2008 doi: 10.1021/ja076787b. epub Feb 5. [DOI] [PubMed] [Google Scholar]
- Li B, Schopfer LM, Hinrichs SH, Masson P, Lockridge O. Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry assay for organophosphorus toxicants bound to human albumin at Tyr411. Anal Biochem. 2007;361:263–272. doi: 10.1016/j.ab.2006.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Wang Y, Dong S. Amplified electrochemical apatasensor taking AuNPs based sandwich sensing platform as a model. Biosens Bioelectron. 2008;23(7):965–970. doi: 10.1016/j.bios.2007.09.019. [DOI] [PubMed] [Google Scholar]
- Lin YH, Lu F, Wang J. Disposable carbon nanotube modified screen-printed biosensor for amperometric detection of organophosphorus pesticides and nerve agents. Electroanalysis. 2004;16:145–149. [Google Scholar]
- Lin YH, Yantasee W, Wang J. Carbon nanotubes CNTs for the development of electrochemical biosensors. Frontiers in Bioscience. 2005;10:492–505. doi: 10.2741/1545. [DOI] [PubMed] [Google Scholar]
- Lin YY, Liu GD, Wai CM, Lin YH. Magnetic beads-based bioelectrochemical immunoassay of polycyclic aromatic hydrocarbons. Electrochem Comm. 2007;9:1547–1552. [Google Scholar]
- Liu GD, Riechers SL, Timchalk C, Lin Y. Sequential injection/electrochemical immunoassay for quantifying the pesticide metabolite 3,5,6-trichloro-2-pyridinol. Electrochem Comm. 2005;7:1463–1470. [Google Scholar]
- Liu GD, Lin YH. Electrochemical sensor for organophosphate pesticides and nerve agents using zirconia nanoparticles as selective sorbents. Anal Chem. 2005;77:5894–5901. doi: 10.1021/ac050791t. [DOI] [PubMed] [Google Scholar]
- Liu G, Timchalk C, Lin Y. Bioelectrochemical magnetic immunosensing of trichloropyridinol: A potential insecticide biomarker. Electroanalysis. 2006;18(16):1605–1613. [Google Scholar]
- Liu G, Lin Y. Biosensor based on self-assembling acetylcholinesterase on carbon nanotubes for flow injection/amperometric detection of organophosphate pesticides and nerve agents. Anal Chem. 2006;78:835–843. doi: 10.1021/ac051559q. [DOI] [PubMed] [Google Scholar]
- Liu G, Wang J, Wu H, Lin Y-Y, Lin Y. Nanovehicles based bioassay labels. Electroanalysis. 2007a;19:777–785. [Google Scholar]
- Liu G, Lin Y-Y, Wang J, Wu H, Chien MW, Lin Y. Disposable electrochemical immunosensor diagnosis device based nanoparticle probe and immunochromatographic strip. Anal Chem. 2007b;79:7644–7653. doi: 10.1021/ac070691i. [DOI] [PubMed] [Google Scholar]
- Liu J, Lu Y. A colorimetric lead biosensor using DNAzyme-directed assembly of gold nanoparticles. J Am Chem Soc. 2003;125:6642–6643. doi: 10.1021/ja034775u. [DOI] [PubMed] [Google Scholar]
- Lu C, Bravo R, Caltabiano LM, Irish RM, Weerasekera G, et al. The presence of dialkylphosphate in fresh fruit juices: implication for organophosphorus pesticide exposure and risk assessments. J Toxicol Environ Health A. 2005;68:209–227. doi: 10.1080/15287390590890554. [DOI] [PubMed] [Google Scholar]
- Ma T, Chambers JE. Kinetic parameters of desulfuration and dearylation of parathion and chlorpyrifos by rat liver microsomes. Fd Chem Tox. 1994;32(8):763–767. doi: 10.1016/s0278-6915(09)80009-4. [DOI] [PubMed] [Google Scholar]
- Manz A, Fettinger JC, Verpoorte E, Ludi H, Widmer HM, Harrison DJ. Micromachining of monocrystalline silicon and glass for chemical analysis systems. Trends Anal Chem. 1991;10:144–149. [Google Scholar]
- Mattigod SV, Fryxell GE, Alford K, Gilmore T, Parker K, et al. Functionalized TiO2 nanoparticles for use in situ anion immobilization. Environ Sci Technol. 2005;39(18):7306–7310. doi: 10.1021/es048982l. [DOI] [PubMed] [Google Scholar]
- Medintz I, Uyeda HT, Goldman ER, Mattoussi H. Quantum dot bioconjugates for imaging, labelling and sensing. Nature Materials. 2005;4:435–436. doi: 10.1038/nmat1390. [DOI] [PubMed] [Google Scholar]
- Merkoci A, Aldavert M, Tarrason G, Eritja R, Alegret S. Toward and ICPMS-linked DNA assay based on gold nanoparticles immunoconnected through peptide sequences. Anal Chem. 2005;77:6500–6503. doi: 10.1021/ac050539l. [DOI] [PubMed] [Google Scholar]
- Morgan MK, Sheldon LS, Croghan CW, Jones PA, Robertson GL, et al. Exposures of preschool children to chlorpyrifos and its degradation product 3,5,6-trichloro-2-pyridinol in their everyday environment. J Exp Anal Environ Epidemiol. 2005;15:297–309. doi: 10.1038/sj.jea.7500406. [DOI] [PubMed] [Google Scholar]
- Nayak S, Lyon LA. Soft nanotechnology and soft nanoparticles. Angew Chem Int Ed. 2005;44:7686–7708. doi: 10.1002/anie.200501321. [DOI] [PubMed] [Google Scholar]
- Neufeld T, Eshkenazi I, Cohen E, Rishpon J. A micro flow injection electrochemical biosensor for organophosphorus pesticides. Biosens Bioelectron. 2000;15:323–329. doi: 10.1016/s0956-5663(00)00073-7. [DOI] [PubMed] [Google Scholar]
- Nolan RJ, Rick DL, Freshour NL, Saunders JH. Chlorpyrifos: pharmacokinetics in human volunteers. Toxicol Appl Pharmacol. 1984;73:8–15. doi: 10.1016/0041-008x(84)90046-2. [DOI] [PubMed] [Google Scholar]
- Ooms AJJ, van Dijk C. The reaction of organophosphorus compounds with hydrolytic enzymes—III: The inhibition of chymotrypsin and trypsin. Biochem Pharmacol. 1966;15:1361–1377. doi: 10.1016/0006-2952(66)90106-7. [DOI] [PubMed] [Google Scholar]
- Park S, McGrath MJ, Smyth MR, Diamond D, Lunte CE. Voltammetric detection for capillary electrophoresis. Anal Chem. 1997;69:2994–3001. doi: 10.1021/ac970156q. [DOI] [PubMed] [Google Scholar]
- Patolsky F, Zheng GF, Lieber CM. Fabrication of silicon nanowire devices for ultrasensitive, label-free, real-time detection of biological and chemical species. Nature Protocols. 2006;1:1711–1724. doi: 10.1038/nprot.2006.227. [DOI] [PubMed] [Google Scholar]
- Peeples ES, Schopfer LM, Duysen EG, Spaulding R, Voelker T, et al. Albumin, a new biomarker for organophosphorus toxicant exposure, identified by mass spectrometry. Toxicol Sci. 2005;83:303–312. doi: 10.1093/toxsci/kfi023. [DOI] [PubMed] [Google Scholar]
- Peoples SA, Knaak J. Monitoring pesticide blood cholinesterase and analyzing blood and urine for pesticides and their metabolites. In: Plimmer JR, editor. Pesticide Residues and Exposure, Am Chem Soc Symp Series. 182. American Chemical Society; Washington, D.C.: 1982. pp. 41–57. [Google Scholar]
- Piras L, Reho S. Colloidal gold based electrochemical immunoassays for the diagnosis of actute myocardial infarction. Sensors and Actuators B. 2005;111−112:450–454. [Google Scholar]
- Polhuijs M, Langenberg JP, Benschop HP. New method for retrospective detection of exposure to organophosphorus anticholinesterases: Application to alleged sarin victims of Japanese terrorists. Toxicol Appl Pharmacol. 1997;146:156–161. doi: 10.1006/taap.1997.8243. [DOI] [PubMed] [Google Scholar]
- Polsky R, Harper JC, Wheeler DR, Brozik SM. Multifunctional electrode arrays: toward a universial detection platform. Electroanalysis. 2008 epub Feb 13. [Google Scholar]
- Pumera M, Sanchez S, Ichinose I, Tang J. Electrochemical nanobiosensors. Sensors and Actuators B-Chem. 2007;123:1195–1205. [Google Scholar]
- Rigas ML, Okino MS, Quackenboss JJ. Use of a pharmacokinetic model to assess chlorpyrifos exposure and dose in children, based on urinary biomarker measurements. Toxicol Sci. 2001;61:374–381. doi: 10.1093/toxsci/61.2.374. [DOI] [PubMed] [Google Scholar]
- Renedo OD, Alonso-Lomillo MA, Martinez MJA. Recent developments in the field of screen-printed electrodes and their related applications. Talanta. 2007;73:202–219. doi: 10.1016/j.talanta.2007.03.050. [DOI] [PubMed] [Google Scholar]
- Ryhanen RJ. Pseudocholinesterase activity in some human body fluids. Gen Pharmacol. 1983;14(4):459–460. doi: 10.1016/0306-3623(83)90030-7. [DOI] [PubMed] [Google Scholar]
- Sadik OA, Van Emon JM. Applications of electrochemical immunosensors to environmental monitoring. Biosens Bioelectron. 1996;11(8):1–9. doi: 10.1016/0956-5663(96)85936-7. [DOI] [PubMed] [Google Scholar]
- Saravanan NP, Venugopalan S, Senthilkumar N, Santhosh P, Kavita B, Prabu HG. Voltammetric determination of nitroaromatic and nitramine explosives contamination in soil. Talanta. 2006;69:656–662. doi: 10.1016/j.talanta.2005.10.041. [DOI] [PubMed] [Google Scholar]
- Savolainen K. Understanding the toxic actions of organophosphates. In: Krieger RI, editor. Handbook of Pesticide Toxicology. Vol. 2. Academic Press; San Diego: 2001. pp. 1013–1041. [Google Scholar]
- Simonian AL, Good TA, Wang S-S, Wild JR. Nanoparticle-based optical biosensors for the direct detection of organophosphate chemical warfare agents and pesticides. Anal Chim Acta. 2005;534:69–77. [Google Scholar]
- Singh AK, Flounders AW, Volponi JV, Ashley CS, Wally K, Schoeninger JS. Development of sensors for direct detection of organophosphates. Part I: immobilization, characterization and stabilization of acetylcholinesterase and organophosphate hydrolase on silica supports. Biosens Bioelectron. 1999;14:703–713. doi: 10.1016/s0956-5663(99)00044-5. [DOI] [PubMed] [Google Scholar]
- Skoog DA, Leary JJ. Principles of instrumental analysis. 4th Ed. Harcourt Brace College Publishers; New York: 1992. pp. 535–564. [Google Scholar]
- Sole S, Alegret S. Environmental toxicity monitoring using electrochemical biosensing systems. Environ Sci Pollut Res Int. 2001;8:256–264. doi: 10.1007/BF02987403. [DOI] [PubMed] [Google Scholar]
- Tan YM, Liao KH, Clewell HJ., 3rd Reverse dosimetry: interpreting trihalomethanes biomonitoring data using physiologically based pharmacokinetic modeling. J Expo Sci Environ Epidemiol. 2007;17(7):591–603. doi: 10.1038/sj.jes.7500540. [DOI] [PubMed] [Google Scholar]
- Tang T-C, Deng A, Huang H-J. Immunoassay with microtiter plate incorporated multichannel electrochemical detection system. Anal Chem. 2002;74:2617–2621. doi: 10.1021/ac0112925. [DOI] [PubMed] [Google Scholar]
- Tansil NC, Gao ZQ. Nanoparticles in biomolecular detection. Nano Today. 2006;1:28–37. [Google Scholar]
- Tasis D, Tagmatarchis N, Bianco A, Prato M. Chemistry of carbon nanotubes. Chem Rev. 2006;106:1105–1136. doi: 10.1021/cr050569o. [DOI] [PubMed] [Google Scholar]
- Timchalk C, Poet TS, Lin Y, Weitz KK, Zhao R, et al. Development of an integrated microanalytical system for analysis of lead in saliva and linkage to a physiologically based pharmacokinetic model describing lead saliva secretion. AIHAJ. 2001;62:295–302. doi: 10.1080/15298660108984631. [DOI] [PubMed] [Google Scholar]
- Timchalk C, Nolan RJ, Mendrala AL, Dittenber DA, Brzak KA, Mattsson JL. A physiologically based pharmacokinetic and pharmacodynamic (PBPK/PD) model for the organophosphate insecticida chlorpyrifos in rats and humans. Tox Sci. 2002;66:34–53. doi: 10.1093/toxsci/66.1.34. [DOI] [PubMed] [Google Scholar]
- Timchalk C, Poet T, Kousba A, Campbell J, Lin Y. Noninvasive biomonitoring approaches to determine dosimetry and risk following acute chemical exposure: analysis of lead or organophosphate insecticide in saliva. J Toxicol Environ Health A. 2004a;67:635–650. doi: 10.1080/15287390490428035. [DOI] [PubMed] [Google Scholar]
- Timchalk C. Comparative inter-species pharmacokinetics of phenoxyacetic acid herbicides and related organic acids. Evidence that the dog is not a relevant species for evaluation of human health risk. Toxicol. 2004b;200:1–19. doi: 10.1016/j.tox.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Timchalk C, Campbell JA, Liu G, Lin Y, Kousba AA. Development of a non-invasive biomonitoring approach to determine exposure to the organophosphorus insecticide chlorpyrifos in rat saliva. Toxicol Appl Pharmacol. 2007a;219:217–225. doi: 10.1016/j.taap.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Timchalk C, Busby A, Campbell JA, Needham LL, Barr DB. Comparative pharmacokinetics of the organophosphorus insecticide chlorpyrifos and its major metabolites diethylphosphate, diethylthiophosphate and 3,5,6-trichloro-2-pyridinol in the rat. Toxicol. 2007b;237:145–157. doi: 10.1016/j.tox.2007.05.007. [DOI] [PubMed] [Google Scholar]
- Trojanowicz M. Analytical applications of carbon nanotubes: a review. Trac-Trends Anal Chem. 2006;25:480–489. [Google Scholar]
- Vamakaki V, Chaniotakis NA. Carbon nanotubes as transducers in biosensors. Sensors and Actuators B. 2007;126:193–197. [Google Scholar]
- Velasco-Arjona A, Manclús JJ, Montoya A, Luque de Castro MD. Robotic sample pretreatment-immunoassay determination of chlorpyrifos metabolite (TCP) in soil and fruit. Talanta. 1997;45:371–377. doi: 10.1016/s0039-9140(97)00140-9. [DOI] [PubMed] [Google Scholar]
- Vertelov GK, Olenin AY, Lisichkin GV. Use of nanoparticles in the electrochemical analysis of biological samples. J Anal Chem. 2007;62:813–824. [Google Scholar]
- Wang J, Li R. On-valve electrochemical detector for high-speed flow injection analysis. Anal Chem. 1990;62:2414–2416. doi: 10.1021/ac00220a032. [DOI] [PubMed] [Google Scholar]
- Wang J, Chatrathi MP, Mulchandani A, Chen W. Capillary electrophoresis microchips for separation and detection of organophosphate nerve agents. Anal Chem. 2001;73:1804–1808. doi: 10.1021/ac001424e. [DOI] [PubMed] [Google Scholar]
- Wang J, Pumera M, Chatrathi MP, Escarpa A, Musameh M, Collins G, Mulchandani A, Lin Y, Olsen K. Single-channel microchip for fast screening and detailed identification of nitroaromatic explosives or organophosphate nerve agents. Anal Chem. 2002;74:1187–1191. [PubMed] [Google Scholar]
- Wang J, Li JH, Baca AJ, Hu JB, Zhou FM, Yan W, Pang DW. Amplified voltammetric detection of DNA hybridization via oxidation of ferrocene caps on gold nanoparticle/streptavidin conjugates. Anal Chem. 2003;75:3941–3945. doi: 10.1021/ac0344079. [DOI] [PubMed] [Google Scholar]
- Wang J, Liu GD, Engelhard MH, Lin YH. Sensitive immunoassay of a biomarker tumor necrosis factor-alpha based on polyguanine-functionalized silica nanoparticle label. Anal Chem. 2006a;78:6974–6979. doi: 10.1021/ac060809f. [DOI] [PubMed] [Google Scholar]
- Wang J, Liu GD, Lin YH. Amperometric choline biosensor fabricated through electrostatic assembly of bienzyme/polyelectrolyte hybrid layers on carbon nanotubes. Analyst. 2006b;131:477–483. doi: 10.1039/b516038c. [DOI] [PubMed] [Google Scholar]
- Wang J. Nanoparticle-based electrochemical bioassays of proteins. Electroanalysis. 2007;19:769–776. [Google Scholar]
- Wang J, Liu GD, Timchalk C, Lin Y. Carbon Nanotube-Based Electrochemical Sensor for Assay of Salivary Cholinesterase Enzyme Activity: An Exposure Biomarker of Organophosphate Pesticides and Nerve Agents. Environ Sci Technol. 2008a doi: 10.1021/es702335y. In press. [DOI] [PubMed] [Google Scholar]
- Wang J, Liu GD, Wu H, Lin YH. Quantum dot-based electrochemical immunosensor for high throughput screening of prostate-specific antigen. Small. 2008b;4:82–86. doi: 10.1002/smll.200700459. [DOI] [PubMed] [Google Scholar]
- Wang J, Liu GD, Wu H, Lin YH. Sensitive electrochemical immunoassay for 2, 4, 6-trinitrotoluene based on functionalized silica nanoparticle labels. Anal Chim Acta. 2008c doi: 10.1016/j.aca.2008.01.024. In press. [DOI] [PubMed] [Google Scholar]
- Weis BK, Balshaw D, Barr JR, Brown D, Ellisman M, Lioy P, et al. Personalized exposure assessment: promising approaches for human environmental health research. Environ Health Perspect. 2005;131:840–848. doi: 10.1289/ehp.7651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson NK, Chuang JC, Lyu C, Menton R, Morgan MK. Aggregate exposures of nine preschool children to persistent organic pollutants at day care and at home. J Exp Anal Environ Epidemiol. 2003;13:187–202. doi: 10.1038/sj.jea.7500270. [DOI] [PubMed] [Google Scholar]
- Wu J, Fu ZF, Yan F, Ju HX. Biomedical and clinical applications of immunoassays and immunosensors for tumor markers. Trac-Trends in Anal Chem. 2007;26:679–688. [Google Scholar]
- Wu H, Liu G, Wang J, Lin Y. Quantum-dots based electrochemical immunoassay of interleukin-1α. Electrochem Comm. 2007;9:1573–1577. [Google Scholar]
- Xiao Y, Li CM. Nanocomposites: from fabrications to electrochemical bioapplications. Electroanalysis. 2008 epub Feb 8. [Google Scholar]
- Xu JJ, Wang AJ, Chen HY. Electrochemical detection modes for microchip capillary electrophoresis. Trac-Trends in Anal Chem. 2007;26:125–132. [Google Scholar]
- Yantasee W, Lin Y, Hongsirikarn K, Fryxell GE, Addleman R, Timchalk C. Electrochemcal sensors for the detection of lead and other toxic heavy metals: The next generation of personal exposure biomonitors. Environ Health Perspect. 2007a;115(12):1683–1690. doi: 10.1289/ehp.10190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yantasee W, Timchalk C, Lin Y. Microanalyzer for biomonitoring lead (Pb) in blood and urine. Anal Bioanal Chem. 2007b;387(1):335–341. doi: 10.1007/s00216-006-0940-1. [DOI] [PubMed] [Google Scholar]
- Zacco E, Galve R, Marco MP, Alegret S, Pividori MI. Electrochemical biosensing of pesticide residues based on affinity biocomposite platforms. Biosens Bioelectron. 2007;22:1707–1715. doi: 10.1016/j.bios.2006.07.037. [DOI] [PubMed] [Google Scholar]


