Abstract
Functional magnetic resonance imaging (fMRI) studies have observed hyperactivity in the hippocampal region in individuals with Mild Cognitive Impairment (MCI). However, the actual source of such hyperactivity is not well understood. Studies of aged rats observed similar hyperactive signals in the CA3 region of the hippocampus that correlated with spatial memory deficits and, in particular, with their ability to represent novel environments as being distinct from familiar ones (pattern separation). In this study, we tested the hypothesis that patients with amnestic MCI (aMCI) have deficits in pattern separation, along with hyperactive fMRI BOLD activity in the CA3 region of the hippocampus. We used high-resolution fMRI during a continuous recognition task designed to emphasize pattern separation. We conducted hippocampal subfield-level region of interest analyses to test for dysfunctional activity in aMCI patients. We found that patients showed impaired performance on trials that taxed their pattern separation abilities. We also observed hyperactive BOLD signals in the CA3/dentate and hypoactive signals in the entorhinal cortex during the separation condition. In a high-resolution morphometric analysis of hippocampal subfields, aMCI patients also had smaller CA3/dentate and CA1 volumes (no difference in the subiculum). The CA3/dentate region bilaterally also exhibited the largest shape deformations in aMCI patients, suggesting that this locus is affected early in the course of the disease. These findings suggest that structural and functional changes in the CA3/dentate region of the hippocampus contribute to the deficits in episodic memory that are observed in patients with aMCI. The functional hyperactivity may be evidence for a dysfunctional encoding mechanism, consistent with the predictions of computational models of hippocampal learning.
INTRODUCTION
Episodic memory deficits are considered the cognitive hallmark of Alzheimer’s disease (AD) (DeCarli et al., 2004; Kawas et al., 2003; Welsh et al., 1992), and are characteristic of Mild Cognitive Impairment (MCI), a diagnostic entity that is often a preclinical phase of AD (Petersen et al., 1999). The medial temporal lobe, which includes the hippocampus and surrounding cortices, is known to play an important role in the processing of episodic memories (Milner, Squire, Kandel 1998) and is also the site of the earliest pathological changes in AD (Gomez-Isla et al., 1996; Price et al., 2001). In addition to structural magnetic resonance imaging (MRI) changes, a number of studies have also reported functional MRI (fMRI) changes in the medial temporal lobe (MTL) of patients with MCI or AD. While numerous studies report reduced hippocampal activity during memory tasks in mild AD cases (e.g., Dickerson et al., 2005; Small et al., 1999; Sperling 2007), several studies have shown that MCI patients exhibit “hyperactivity” in the hippocampus/parahippocampal region during memory encoding tasks (Dickerson et al., 2004; 2005; Celone et al., 2006; Hämäläinen et al., 2007; Miller et al., 2008a). These changes in functional activity have been linked to both the current level of impairment and the development of future impairments (c.f. Sperling 2007 for review). Similar patterns of “hyperactivity” have also been noted in cognitively intact ApoE4 carriers (Bookheimer et al., 2000), in asymptomatic offspring of autopsy-confirmed AD patients (Bassett et al., 2006), and in healthy aged adults who perform poorly on a subsequent memory task (Miller et al., 2008b). However, the actual source of such hyperactivity is still not well understood.
Recently, models of memory impairment in aged rodents have offered some insight into the neural mechanisms potentially underlying this hyperactivity. Studies have shown that although some aged rats perform as well as young rats on spatial learning tasks, some demonstrate impaired performance (Gallagher and Rapp 1997; Gallagher et al. 1993; 2003). Although these “aged-impaired” rats do not develop AD pathology, their memory deficits are similar in many ways to those observed in MCI patients. The impairments in the rodent have been linked to a failure to encode novel information while navigating similar environments (i.e., there is little to no spatial remapping when environmental cues are shifted) and suggest reliance instead on using familiar information (Tanila et al., 1997; Wilson et al., 2003; 2004; 2005). This “rigidity” in spatial representations is thought to be due to impairment in pattern separation, or the ability to separate similar representations into distinct, non-overlapping representations. This hypothesis about the computational role of the hippocampus was first posited by Marr (1971) as a necessary function of learning in order to facilitate storage of large amounts of information. If pattern separation did not exist, encoding new information would overwrite previously stored information leading to catastrophic interference (McClelland, McNaughton, O'Reilly 1995; Norman and O'Reilly 2003). It is important to note that episodic memory, recollection, and source memory all place strong demands on pattern separation (Norman and O'Reilly 2003). This function appears to be a key function of the dentate gyrus (DG), which then projects the pattern-separated representation onto CA3 neurons via the mossy fibers (Treves and Rolls 1994).
Convergent evidence from computational models (O’Reilly and Norman, 2002; Treves and Rolls, 1994), electrophysiological recordings (Leutgeb et al., 2004; Leutgeb and Leutgeb 2007), immediate-early-gene imaging (Vazdarjanova and Guzowski 2004), and BOLD fMRI analysis (Bakker et al., 2008) have demonstrated that pattern separation signals can be observed in the CA3 subfields of the hippocampus (some studies have also noted this phenomenon in the DG, however due to the DG’s sparse neuronal code, both electrophysiology and imaging of this region has proved very difficult). Recent work by Wilson et al. (2005; 2006) suggests that spatial learning deficits in aged memory-impaired rats are due to a shift in the computational bias in the CA3 region of the hippocampus from pattern separation to pattern completion (the process of reestablishing a pre-existing representation in response to a partial or degraded cue). While the CA3 of young and memory-unimpaired aged rats exhibited normal spatial remapping in similar but, not identical environments (pattern separation), the CA3 of memory impaired aged rats demonstrated place cell firing patterns more consistent with pattern completion. Furthermore, Wilson and colleagues observed that CA3 place cell firing was abnormally elevated in memory impaired aged rats. These findings suggest that the hyperactivity in CA3 contributes to the shift from pattern separation to pattern completion during conditions that tax separation.
The ability of the DG to properly encode and separate overlapping representations relies on the quality of input from layer II of the entorhinal cortex via the perforant path, a vital gateway that is degraded in the course of aging (Burke and Barnes 2006; Geinisman et al., 1992; Wilson et al., 2006) and that is further compromised in AD (Hyman et al., 1986; Killiany et al., 2002; Scheff et al., 2006). In addition, cell loss in the entorhinal cortex early in the course of AD (Gomez-Isla et al., 1996) leads to a partial hippocampal disconnection that deprives the DG from sensory input (Hyman et al., 1984) and thus could shift the computational balance within the hippocampus towards pattern completion and away from encoding novel information (i.e., pattern separation). Consistent with these anatomical changes in the hippocampus, patients with amnestic MCI (aMCI), the MCI subtype that has the highest likelihood of progression to AD, is strongly associated with impairment in the encoding of novel episodic memories (Petersen et al., 1999; Petersen et al. 2004). For example, aMCI patients exhibit disruption in recollection-based recognition (Anderson et al., 2008; Westerberg et al., 2006). Additionally, they have a higher vulnerability to interference (Della Sala et al., 2005; Ebert and Anderson 2009; Loewenstein et al., 2004) and false recognition (Brueckner and Moritz 2009; Hildebrandt, Haldenwanger, Eling 2009).
We hypothesized that a critical feature of the episodic memory impairment in aMCI would be an impairment in pattern separation abilities in the hippocampus. By that view, it is possible that aMCI patients symptomatically represent a more severe form of age-related memory impairment, sharing some important features with aged-impaired rodents. Moreover, a general impairment in pattern separation could contribute to many if not all of the aforementioned deficits in aMCI patients. To address this directly, we hypothesized that aMCI patients would have difficulties with a memory encoding and retrieval task that placed an emphasis on correctly identifying similar (i.e., “lure”) stimuli vs. those that were identical to stimuli that were previously shown (i.e., “old” stimuli). Previous studies of MCI patients have noted elevated BOLD fMRI signals in the hippocampus and parahippocampal gyrus during a variety of memory tasks (Celone et al., 2006; Dickerson et al., 2005; Hamalainen et al., 2007; Johnson et al., 2006), as noted above, although none of the studies localized this hyperactivity to a specific hippocampal subfield. Studies in aged rodents have found hyperactivity at a neural level circumscribed to the CA3 region. It is possible that a similar mechanism is also implicated in aMCI patients. If this is indeed the case, we should be able to localize hyperactive BOLD signals in aMCI patients in the CA3/DG region and not in other hippocampal subfields. To test this prediction, we used high-resolution fMRI capable of differentiating BOLD signals from the hippocampal subfields (CA3/DG, CA1, subiculum). We used both a behavioral task and fMRI contrasts that we have previously used (Kirwan and Stark, 2007) designed to tax pattern separation rather than completion (i.e., during encoding and successful retrieval of similar ‘lure’ items). Our findings showed an increase in fMRI BOLD signal in the CA3/dentate gyrus (CA3/DG) region of the hippocampus in the aMCI group compared to healthy controls. We also found a reduction in activity in the entorhinal cortex (EC) in aMCI patients, and not in controls, during the same conditions. In addition, we found group differences in the shape and volume of the CA3/DG and CA1 subfields.
MATERIALS AND METHODS
Participants and Clinical Characterization
Ten right-handed aMCI patients (5 female, mean age = 76±7) were recruited from the Alzheimer’s Disease Research Center (ADRC) at the Johns Hopkins University School of Medicine. An additional ten right-handed healthy volunteers (8 female, mean age = 75±7) were recruited from the pool of control participants in the ADRC. All participants underwent medical, neurological, psychiatric, and neuropsychological examination using standardized instruments and methods. A semi-structured interview, the Clinical Dementia Rating (CDR) scale (Hughes et al., 1982), was used to assess function in daily life and to assign a CDR rating reflecting overall functional capacity. All aMCI patients had CDR scores of 0.5. Diagnosis of aMCI was based on the criteria proposed by Petersen (2004), which include a memory complaint (corroborated by an informant), impaired memory function on testing (e.g., 1.5 standard deviations below norm), otherwise preserved cognitive functioning (e.g., within 1 standard deviation of norm), no decline in basic activities of daily living, and no dementia. Final aMCI diagnoses were reached by clinical consensus conferences within the ADRC. At the time of brain imaging all participants were administered the Telephone Interview of Cognitive Status (TICS: Brandt, Spencer, Folstein 1988) to assure consistency with the established clinical diagnoses. The 11-item TICS has 94% sensitivity and 100% specificity in discriminating patients with mild dementia from controls. In addition, scores on the TICS are highly correlated with MMSE scores (r=0.94) (Brandt, Spencer, Folstein 1988). Although all participants were within the unimpaired range (26–41) on the TICS, there was a significant group difference between aMCI patients (mean = 31.3±2.2) and controls (mean TICS = 36.3±1.4). The length of time between clinical assessment and scanning was between 1 and 6 months. See Table 1 for additional details. Our exclusion criteria included major neurological or psychiatric disorders, head trauma with loss of consciousness, history of drug abuse or dependency, and general contraindications to an MRI examination (e.g. cardiac pacemaker, aneurysm coils, claustrophobia).
Table 1.
| Characteristic | Controls | MCI | ||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Subjects | 10 | 10 | ||
| Sex (M/F) | 8/2 | 5/5 | ||
| Age (yrs) | 75 | 7 | 76 | 7 |
| Education (yrs) | 16.2 | 2.0 | 15.7 | 2.3 |
| TICS score | 36.3 | 1.4 | 31.3 | 2.2 |
| Separation Bias | 0.31 | 0.05 | 0.17 | 0.05 |
| CDR (0/0.5/1) | 0/10/0 | |||
TICS: Telephone Interview for Cognitive Status.
CDR: Clinical Dementia Rating.
fMRI Behavioral Paradigm
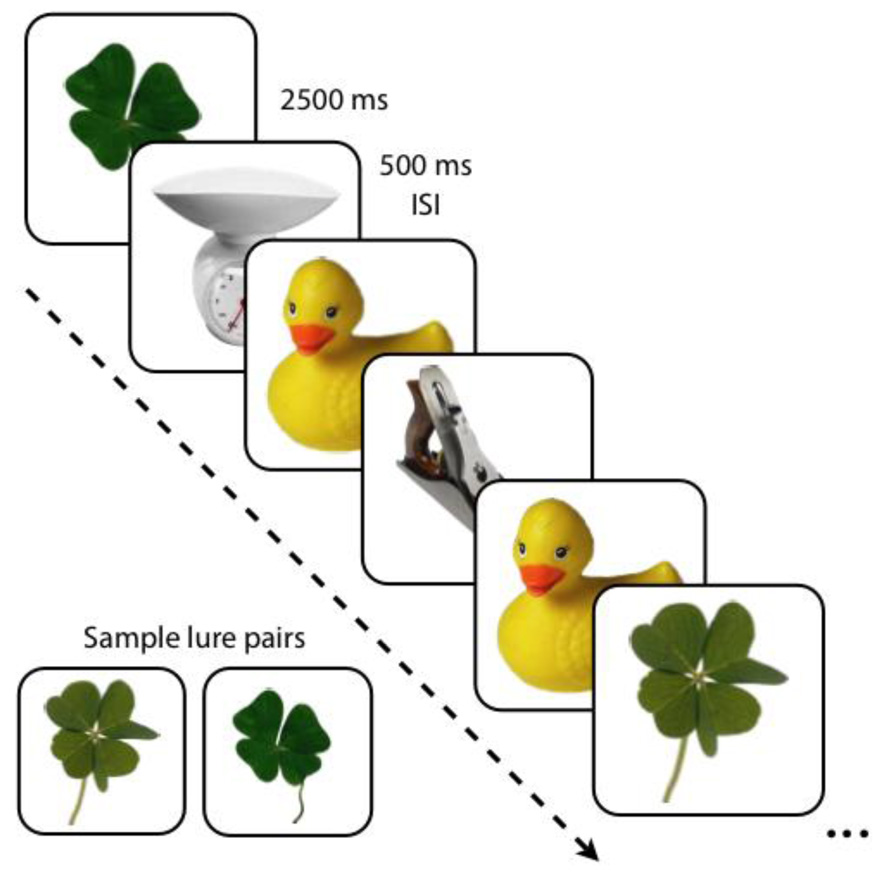
The fMRI behavioral paradigm used in this study has been described earlier in detail by Kirwan and Stark (2007). The paradigm was an explicit 3-alternative forced choice task, in which participants viewed novel (new), repeated (old) and lure (similar) stimuli. Stimuli were color photographs of common objects. Each participant completed six functional runs, each consisting of 16 similar pairs, 16 identical pairs and 44 unrelated novel items (foils), fully randomized throughout the run (Fig. 1). Each stimulus was presented for 2000 milliseconds with a 500-millisecond inter-stimulus-interval. The number of trials separating similar and identical pairs was randomly varied between 10 and 40 trials. Participants were instructed to make a judgment as to whether the object seen was new (i.e., novel items), old (i.e., repeated items) or similar but not identical (i.e., lure items). Of critical interest were the participants’ responses on the lure items. A response of “old” to a lure (i.e., similar) item would suggest that the participant was more biased towards pattern completion, whereas an accurate response of “similar” to a lure would suggest a bias towards pattern separation instead. To avoid confusion, throughout the paper we will refer to items subsequently tested with repetitions of old items as subsequent targets and novel items subsequently tested with similar lures as subsequent lures. The “subsequent” items all refer to 1st presentation trials. During the 2nd presentation, trials will be referred to as targets and lures respectively. We used the Cogent 2000 Toolbox (http://www.fil.ion.ucl.ac.uk) in Matlab 7.0 (The MathWorks, Natick, MA) for stimulus presentation and behavioral data collection.
Figure 1.
Behavioral task. Pictures of single items were presented for 2000 ms followed by a 500 ms ISI. Novel, repeated, and similar lure items were randomly shuffled in the task. A sample pair of similar stimuli used as lures is shown.
MRI Data Acquisition
Imaging data were based on high-resolution methods developed in our laboratory (Miller et al., 2005; Kirwan and Stark 2007; Kirwan et al. 2007). Data were collected on a Phillips 3 Tesla scanner (Eindhoven, The Netherlands) equipped with an 8-channel SENSE (Sensitivity Encoding) head coil, located at the F.M. Kirby Research Center for Functional Brain Imaging at the Kennedy Krieger Institute (Baltimore, MD). High-resolution echo-planar images were collected using a field of view of 96, an acquisition matrix of 64 × 64, a repetition time of 1500 milliseconds, an echo time of 30 milliseconds, a flip angle of 70 degrees, a SENSE factor of 2, and an isotropic resolution of 1.5 mm × 1.5 mm × 1.5 mm with no gap. Nineteen oblique slices were acquired parallel to the principal longitudinal axis of the hippocampus and covered the entire medial temporal lobe region bilaterally. In addition to the functional runs, a whole-brain MPRAGE structural scan was acquired (parameters: 150 oblique slices, 1 mm isotropic resolution, field of view = 192). We used several procedures to correct for fMRI signal distortions in our high-resolution protocol. First, we employed higher-order shims, which can directly compensate for local field distortions. Second, we used SENSE parallel imaging which utilizes multiple surface coils to undersample k-space with fewer phase encoding steps. This resulted in significantly reduced acquisition time, which also limited distortion resulting from magnetic susceptibility. Furthermore, the adoption of high-resolution scanning protocols reduced remaining artifacts substantially as these artifacts are a function of number of slices from the inhomogeneity rather than absolute distance (Buxton 2001). As a result, our high-resolution EPI data suffers from minimal distortions.
fMRI Data Analysis
Data analysis was carried out using the Analysis for Functional NeuroImages (AFNI, release 2007_03_06_0841) software (Cox 1996). Images were first co-registered to correct for within- and across-scan head motion. Acquisitions in which a significant motion event occurred (more than 3 degrees of rotation or 2 mm of translation in any direction relative to prior acquisition), plus and minus one TR were excluded from the analyses. Only a single subject (aMCI patient) had two consecutive time points that were censored due to motion, resulting in a total of 4 TRs removed for this subject. Structural anatomical data were initially registered to standard stereotaxic space (Talairach and Tournoux 1988) and the resulting transformations were subsequently applied to the statistically mapped functional data.
Behavioral vectors were produced to model different trial types. Six vectors of interest were specified: (1) target subsequent hits (sTH), (2) lures subsequently called “similar” (sLS), (3) lures subsequently called “old” (sLO), (4) target hits (TH), (5) lures called “similar” (LS), and (6) lures called “old” (LO). All other response types (misses, false alarms to novel items, etc…) were modeled but were not part of subsequent analyses. The novel foils that were not subsequently tested served as an arbitrary baseline, against which other conditions were compared. All of these vectors were used to individually model each participant’s functional data using a deconvolution approach based on multiple linear regression (Ward 2001). The resultant fit coefficients represent activity versus baseline for a given time point and trial type in a voxel. The sum of the fit coefficients over the expected hemodynamic response (~3–12 sec after trial onset) was taken as the model’s estimate of the response to each trial type (relative to baseline).
Cross-Participant Alignment
We used a technique for refining cross-participant alignment that our laboratory has developed in recent years (Kirwan and Stark 2007; Miller et al., 2005; Yassa and Stark 2009). The ROI-LDDMM method increases the power of multi-subject regional fMRI studies by focusing the alignment power specifically on the regions of interest using segmentations of each subject’s anatomical image. First, all subjects’ anatomical and functional scans were normalized to the Talairach atlas (Talairach and Tournoux 1988) using AFNI. Subregions of the medial temporal lobe and the hippocampus (bilateral entorhinal cortex, perirhinal cortex, parahippocampal cortex, temporopolar cortex, CA3/DG region, CA1 region, and subiculum) were segmented in three dimensions using the MPRAGE scans according to the methods described in detail in our recent work (Kirwan et al., 2007; Kirwan and Stark 2007; Bakker et al., 2008; Yassa and Stark 2009). Briefly, the temporopolar, entorhinal, perirhinal, and parahippocampal cortices were defined bilaterally in the coronal plane according to the methods described by Insausti et al., (1998). The subfields of the hippocampus were also defined bilaterally as the CA3/DG (CA2/3/4 field + the dentate gyrus), CA1, and SUB (subiculum), following the atlas of Duvernoy (2005) and our previous work. The CA3 region and dentate gyrus (DG) were combined into one label, as it is very difficult to tease apart these subfields on MRI scans. The anatomically defined ROIs were then used to calculate the ROI-LDDMM 3D vector field transformation for each subject that best aligned the individual’s ROIs to a customized model of the ROIs (based on the mean of the entire sample tested). The ROI-LDDMM transformations for each individual subject’s ROIs were then applied to the fit coefficient maps from the functional analyses. Both the realigned structural scans and functional maps were resampled to 1mm3. We have recently shown that this method yields over 70% overlap in hippocampal subfields compared to 30–40% overlap using traditional methods such as SPM whole brain alignment (Yassa and Stark 2009).
Group fMRI Analyses
Group data were analyzed using a two-way Analysis of Variance (ANOVA) with condition (sLS, sLO, sTH, LS LO, TH) and group (aMCI, control) as fixed factors, and with subject as a random factor nested within group. The ANOVA was used, along with the anatomical ROIs, in a hybrid functional/anatomical ROI analysis. A liberal peak threshold of p<0.05 on the overall F statistic, along with a spatial extent threshold of 10 voxels, were used to identify voxels within anatomical ROIs that responded to the task and filter out other, task-irrelevant voxels. This approach, rather than using a direct pair-wise contrast, reduces voxel selection biases (Baker, Hutchison, Kanwisher 2007). This threshold was then combined with the anatomical segmentations to only include voxels inside our regions of interest. This served to exclude voxels that did not change with any of the model’s factors, effectively limiting the analysis to voxels showing any changes with task condition or group. It also maximized our signal-to-noise ratio especially given the anatomical differences across groups in some of the regions of interest. It is worthy of note that although this approach increases our detection power in the sample, the cluster locations are not easily generalized to new samples. See Table 2 for EPI volumes across subfields based on segmentations. Voxels within each anatomical/functional ROI were collapsed for further analysis. This yielded four ROIs: (1) one in the left CA3/DG (54 voxels) (2) one in the left CA1 (122 voxels), (3) one in the left SUB (28 voxels) and (4) one in the left entorhinal cortex (86 voxels). Post hoc t-tests were used in a follow-up analysis to test the difference between aMCI and controls in each of the four ROIs.
Table 2.
| ROI | Volumes (mm3) | Deformation (LogJD) | ||||||
|---|---|---|---|---|---|---|---|---|
| Controls | aMCI | Controls | aMCI | |||||
| Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | |
| LSUB | 1051.0 | 49.4 | 1041.5 | 65.1 | 0.048 | 0.035 | 0.023 | 0.033 |
| RSUB | 943.8 | 32.3 | 917.7 | 47.6 | 0.097 | 0.035 | 0.087 | 0.048 |
| LCA3DG | 1093.7 | 48.3 | 907.7 | 47.0 | 0.033 | 0.030 | −0.148 | 0.038 |
| RCA3DG | 1011.0 | 62.2 | 857.2 | 37.4 | 0.056 | 0.028 | −0.003 | 0.017 |
| LCA1 | 2149.4 | 196.4 | 1804.7 | 93.1 | −0.003 | 0.030 | −0.155 | 0.041 |
| RCA1 | 2086.1 | 122.2 | 1696.7 | 100.2 | 0.021 | 0.032 | −0.055 | 0.041 |
| LPRC | 4036.5 | 174.7 | 3818.5 | 322.1 | −0.031 | 0.045 | −0.073 | 0.037 |
| RPRC | 3911.6 | 247.1 | 3781.3 | 226.2 | 0.055 | 0.035 | 0.035 | 0.049 |
| LERC | 1723.1 | 107.9 | 1554.2 | 111.2 | 0.024 | 0.030 | −0.114 | 0.024 |
| RERC | 1547.0 | 100.1 | 1545.4 | 120.3 | 0.057 | 0.029 | 0.037 | 0.028 |
| LPHC | 2985.5 | 164.9 | 3193.0 | 210.9 | −0.013 | 0.030 | 0.037 | 0.031 |
| RPHC | 3296.6 | 237.8 | 3154.9 | 226.5 | −0.123 | 0.054 | −0.181 | 0.043 |
| LTPC | 2066.3 | 259.0 | 1727.2 | 194.4 | −0.112 | 0.036 | −0.147 | 0.041 |
| RTPC | 2017.7 | 225.5 | 2109.4 | 266.8 | 0.043 | 0.031 | −0.030 | 0.034 |
Abbreviations: LSUB/RSUB: left/right subiculum; LCA3DG/RCA3DG: left/right CA3/dentate gyrus; LCA1/RCA1: left/right CA1; LPRC/RPRC: left/right perirhinal cortex; LERC/RERC: left/right entorhinal cortex; LPHC/RPHC: left/right parahippocampal cortex; LTPC/RTPC: left/right temporopolar cortex; SEM: standard error of the mean; LogJD: Log-Jacobian.
Since neurovascular factors could easily affect cerebral blood flow and the BOLD response (Ances et al., 2009; Fleisher et al., 2008), we hypothesized that there might be a global baseline shift between aMCI patients and controls either due to global metabolic changes or due to cerebrovascular differences. In order to correct for this, we established our critical contrasts by calculating a difference score between two within-subject conditions. First, we compared the 1st presentation conditions by contrasting sLS (lures subsequently called “similar”) with sLO (lures subsequently called “old”). Then we compared the 2nd presentation conditions by contrasting LS (lures called “similar”) with LO (lures called “old”). These contrasts reflect conditions that most heavily emphasize pattern separation, and since they are relative contrasts within-subjects, they tend to be insensitive to any potential baseline shifts that could contaminate the initial analysis.
Structural MRI Analysis
A central tendency anatomical template was created based on all of the MPRAGE scans (normalized to standard Talairach space to get rid of global brain shape and size differences) from all participants using Advanced Normalization Tools (ANTs), which implements SyN (symmetric normalization), a powerful diffeomorphic registration algorithm (Klein et al., 2009). Each participant’s grayscale scan and anatomical ROI segmentation map were used simultaneously to warp the structural scan into the custom template space. This hybrid registration approach uses a pure cross correlation (PR) metric that registers grayscale images using cross-correlation optical flow as well as a point set expectation (PSE) metric that registers label outline information (Yushkevich et al., 2009). The deformations were then quantified using the log-Jacobian (logJD) as a metric because it is symmetric about zero and can capture expansions and contractions. Mean logJDs were then plotted for each MTL and hippocampal subfield ROI per group.
RESULTS
fMRI Task Performance
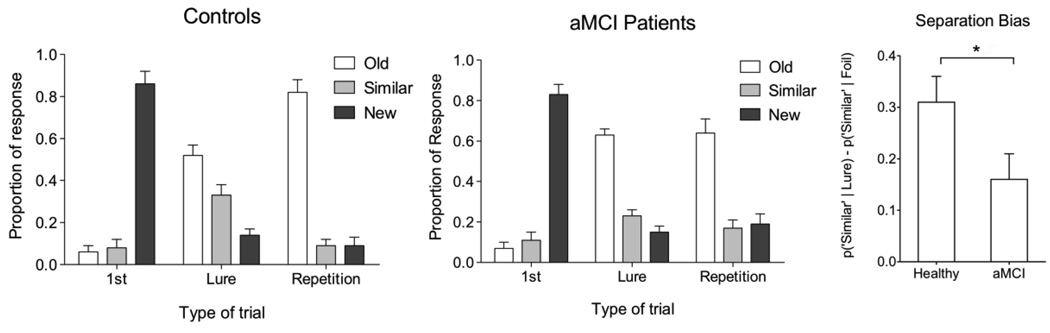
Although there were no significant differences in performance between controls and aMCI patients on the new and repeated items (independent samples t-test, p>0.05), there was a difference in performance on the critical lures (Fig. 2). The aMCI patients had more false alarms to these lure items, mischaracterizing them as old items (63% vs. 52% for controls; t(18)=1.886, P<0.05). Since the “similar” responses are essentially the inverse of the “old” responses to lures, a statistical comparison here would be circular and thus was avoided. When a separation bias score was calculated as the difference between the probability of calling a lure item “similar” and the probability of calling a novel foil item “similar” [p(“Similar”|Lure) – p(“Similar”|Foil)], there was a significant reduction in separation bias for the aMCI patients [t(18) = 1.88, P<0.05]. To determine if there was poorer target recognition in the aMCI group, we conducted a d’ procedure on responses to repetitions and novel foils. We defined the hit rate as the combination of “old” and “similar” responses to repetitions and the false alarm rate as the combination of “old” and similar” responses to foils. We found a statistically significant d’ difference between groups (4.08 in healthy aged adults vs. 2.55 in MCI patients; t(18) = 2.21, P<.05). A closer look at the hit and false alarm rates making up the d’ metric show that this difference is driven by the reduction in hit rate (0.92 in controls vs. 0.81 in patients) and not the false alarm rate (0.14 in controls vs. 0.17 in patients).
Figure 2.
Behavioral data in the two groups. There are no significant differences across groups on the repeated items or new items (P>0.05). There is a significant difference across groups on the critical lure items, where aMCI patients are more likely to call these items “old” (63% vs. 52% for controls; P<0.05). The separation bias metric is another way to clearly show this difference. It is calculated as p(“Similar”|Lure)-p(“Similar”|Foil). There is a significant difference on this bias metric between groups (P<0.05). All bars are mean ± s.e.m.
BOLD fMRI Activity
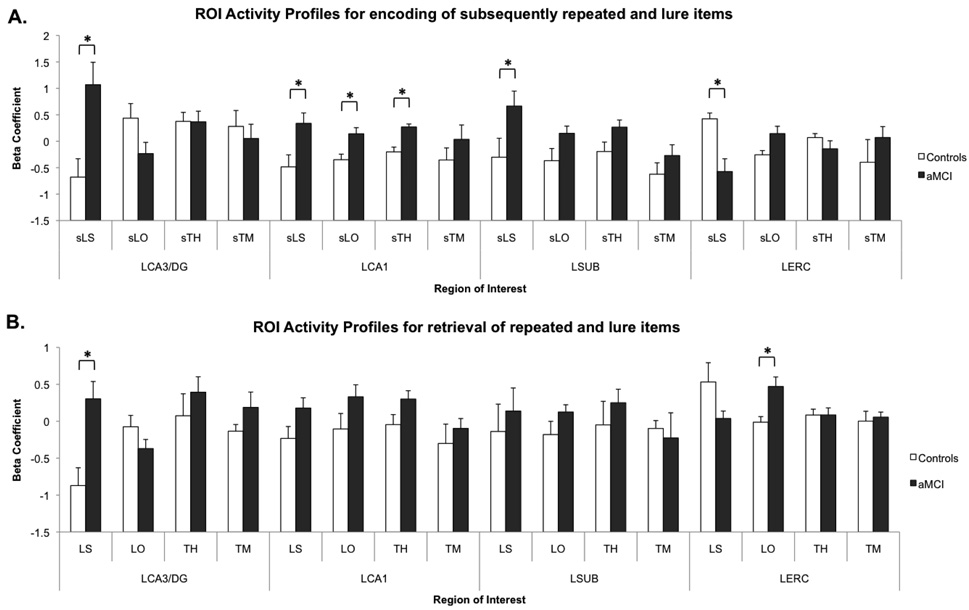
Initial voxel selection yielded four regions in the medial temporal lobe with activity that varied systematically across our trial types of interest. Post hoc t-tests were used to further investigate these effects and determine which regions and conditions showed activity that varied by group (Fig. 3). In the left CA3/DG, we observed a significant increase in BOLD signal in the aMCI patients compared to controls during sLS [t(18) = 3.16, P(two tailed)<0.01] and LS [t(18) = 3.49, P(two tailed)<0.01] relative to our baseline. These contrasts emphasize pattern separation during 1st presentation and 2nd presentation, respectively. In the left CA1 region, we observed significant increases in activity for aMCI over controls during the conditions sTH [t(18) = 4.42, p(two tailed)<0.001], sLS [t(18) = 2.73, P(two tailed)<0.05] and sLO [t(18) = 2.96, P(two tailed)<0.01]. There were no significant differences across groups in this ROI during the 2nd presentation. In the left subiculum, we observed increased activity in aMCI patients during sLS [t(18) = 2.12, P(two tailed)<0.05]. In the left entorhinal cortex, we observed decreased activity in the aMCI patients compared to the controls on sLS [t(18) = 3.75, P(two tailed)<0.01]. During sLO and LO, there was increased BOLD activity in the aMCI patients [sLO: t(18) = 2.54, P(two tailed)<0.05, LO: t(18) = 3.27, P(two-tailed)<0.01]. In addition to these significant pairwise results, there was a global baseline shift taking the form of a statistically significant main effect of group (aMCI >Controls) during 1st presentations [F(1) = 7.14, P<0.05] and during 2nd presentations [F(1) = 4.64, P<0.05]. Consistent with an overall “hyperactivity” in these ROIs, one parsimonious account of this is a shift in the processing of and activity for the novel foil items used as the baseline in the task. It is also possible that there were cerebrovascular or metabolic differences across groups that could contribute to this effect.
Figure 3.
Overall main effect across conditions showing generally increased activity throughout hippocampal subregions during encoding and retrieval. Panel A shows the four ROIs activity profiles during encoding of subsequently repeated and lure items. Of critical interest are the higher activity in MCI patients in multiple hippocampal subregions and the lower activity in the entorhinal cortex. Panel B shows the same ROIs activity profiles during retrieval of repeated and lure items. Here aMCI patients show significantly higher activity in the left CA3/DG during lure correct rejections and higher activity in the left entorhinal cortex during lure false alarms. In general, both these panels illustrate that there is a tendency towards higher activity in aMCI patients across conditions and ROIs, although most are not significant. All bars are mean ± s.e.m. Condition abbreviations: sLS = lures subsequently called “similar”, sLO = lures subsequently called “old”, sTH = target subsequent hits, sTM = target subsequent misses, LS = lures called “similar”, LO = lures called “old”, TH = target hits, TM = target misses.
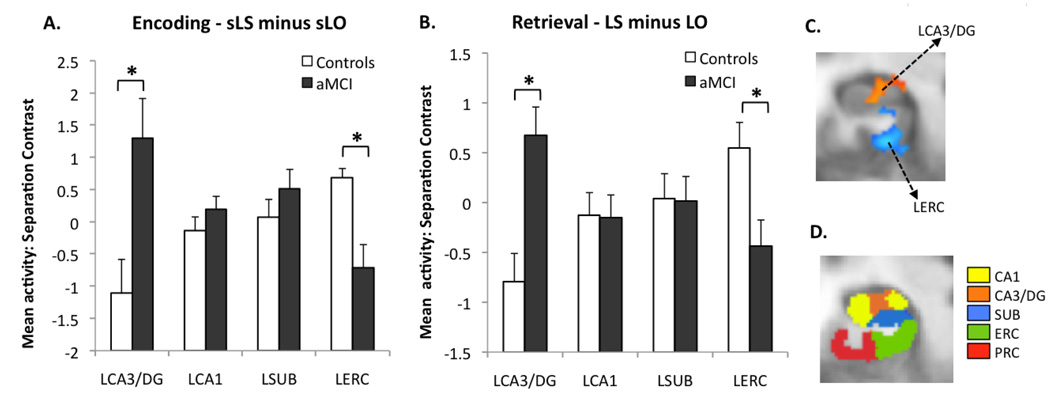
Next, we examined our critical contrasts, which tend to be insensitive to the effects of any such shifts. During 1st presentation (Fig. 4A), the mean difference in beta between sLS and sLO (similar minus old) was significantly higher in aMCI patients than in controls in the left CA3/DG region [t(18) = −2.99, P(two-tailed) <0.01] and significantly lower in the left entorhinal cortex [t(18) = 3.56, P(two-tailed) <0.01]. The results during 2nd presentation (LS minus LO) were almost identical [LCA3/DG: t(18) = −3.59, P(two-tailed) <0.01; LERC: t(18) = 3.42, P(two-tailed)<0.01] (see Figure 4B). Overall, these results illustrate that during encoding and retrieval of lure items, the left CA3/DG region of the hippocampus in aMCI patients exhibited significant increases in activity, while the left entorhinal cortex showed significant decreases in activity.
Figure 4.
Results of the critical contrasts. (A) During encoding (lures subsequently called “similar” minus lures subsequently called “old”) aMCI patients had significantly higher activity in the left CA3/DG and lower activity in the left entorhinal cortex. (B) During retrieval (lures called “similar” minus lures called “old”), there was an identical pattern of group difference. All bars are mean ± s.e.m. (C) The two ROIs of interest in this contrast during encoding shown on an average customized structural template based on the entire sample (functional ROIs based on the t-contrast between groups). Group difference in activity during retrieval was very similar. (D) A sample slice from the anatomical segmentation template in the left medial temporal lobe.
Correlations
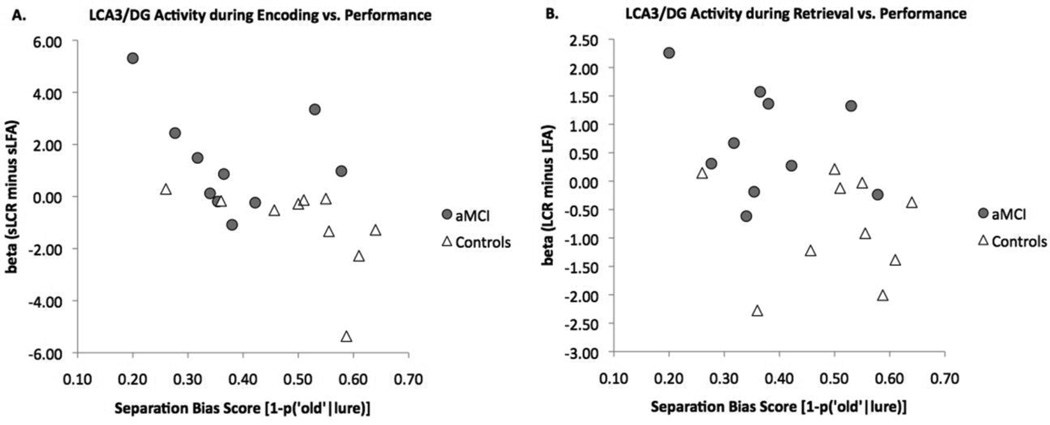
To investigate the potential link between the behavioral ability to pattern separate and CA3/DG hyperactivity, we performed a correlational analysis between each participant’s behavioral separation bias score and his/her activity in the critical lure contrast. Separation bias scores across groups were negatively correlated with magnitude of difference in left CA3/DG BOLD activity during the encoding contrast sLS minus sLO [r = −0.58, t(18) = −3.04, P(two-tailed)<0.01] and during the retrieval contrast LS minus LO [r = −0.45, t(18) = −2.76, P(two-tailed)<0.01] (Fig. 5). Within-group correlations were not significant, likely due to the small sample size, although they all trended in the same direction (negative correlations between performance and magnitude of activity). Correlations between separation bias scores with left entorhinal cortex mean difference in beta were not significant, although they trended in a positive direction [r = 0.3, t(18) = 1.3, P(two-tailed)=0.2] during both encoding and retrieval. There was also a significant negative correlation between BOLD mean difference in activity during encoding in the left CA3/DG and in the left entorhinal cortex [r = −0.53, t(18) = −2.65, P(two-tailed)<0.05].
Figure 5.
Correlations between hyperactivity in the left CA3/DG and behavioral performance during encoding (A) and during retrieval (B). There is a significant negative correlation between performance and hyperactivity during both encoding and retrieval.
Structural MRI Morphometry
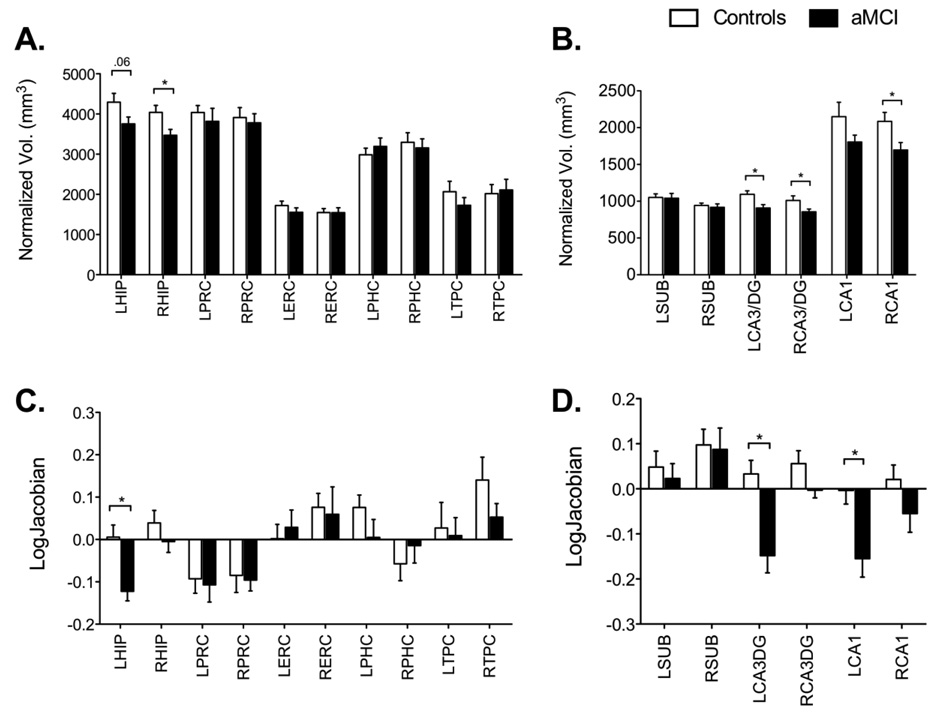
Volumetric analyses across MTL regions showed significant group differences only in the hippocampus (Right t(18) = 2.52, P(two-tailed)<0.05; Left t(18) = 1.94, P(two tailed) =0.06). No other region of the MTL showed a significant group difference in volume (Figure 6A). An analysis of hippocampal subregions (Figure 6B) showed that this difference was driven primarily by the CA3/DG and CA1 subfields and not by the subiculum (left CA3/DG t(18) = 2.76, P(two-tailed)<0.05, right CA3/DG t(18) = 2.12, P(two-tailed)<0.05; left CA1 t(18) = 1.59, P(two-tailed)=0.1; right CA1 t(18) = 2.46, P(two-tailed)<0.05; left subiculum t(18)=0.12, n.s., right subiculum t(18)=0.45, n.s.). Morphometric shape analyses across MTL regions were largely consistent with the volumetric analysis. The hippocampus was significantly contracted in the aMCI participants compared to the controls (left t(18) = 3.52, P(two-tailed)<0.05; right t(18) = 1.11, P(two tailed)=0.2 (Figure 6C). A detailed subregion analysis (Figure 6D) once again showed that this difference was driven primarily by the CA3/DG and CA1 regions and not the subiculum (left CA3/DG t(18) = 3.73, P(two-tailed)<0.05, right CA3/DG t(18) = 1.76, P(two-tailed)=0.1; left CA1 t(18) = 2.97, P(two-tailed)<0.05; right CA1 t(18) = 1.45, P(two-tailed)=0.1; left subiculum t(18)=0.5, n.s., right subiculum t(18)=0.17, n.s.). Actual volumes and deformations are also shown in Table 2.
Figure 6.
Volumetric and morphometric analyses. Panel A shows that only the hippocampus underwent volumetric changes in the aMCI patients. Panel B shows a more detailed analysis of hippocampal subfields and shows that volumetric decreases were limited to the CA3/DG and CA1 subfields only and not the subiculum. Panel C shows again that the left hippocampus is the only region that underwent morphometric (i.e., shape) changes in the aMCI patients. Panel D shows a detailed morphometric analysis of hippocampal subfields and shows that once again this change can be ascribed to the CA3/DG and CA1 subfields and not the subiculum. All bars are mean ± s.e.m.
DISCUSSION
In this study, we hypothesized that aMCI patients would be selectively impaired on an fMRI task that emphasized pattern separation and that fMRI hyperactivity would be found specifically in the CA3/DG region on this task. Consistent with previous fMRI recognition memory tasks in MCI patients (Celone et al., 2006; Dickerson et al., 2005; Johnson et al., 2006; Kircher et al., 2007), the aMCI patients in our study performed as well as controls in discriminating the new items from the repeated items. However, the aMCI patients struggled with lure items that were similar but not identical to ones they had seen before. These items placed heavier demands on pattern separation than completely novel or identically repeated items. In aMCI patients, the response distribution is strikingly similar for lures and repetitions (Figure 2). While controls show a markedly different distribution of responses for these two types, patients do not. This is a further indication of the aMCI patients’ failure to discriminate between these two trials types (i.e., a failure in pattern separation). Our first prediction, that aMCI patients would show pattern separation deficits, was clearly supported by our data. Recently, a study by Toner et al. (2009) reported similar deficits on the same task in nondemented older adults compared to young individuals, a finding that we have also recently replicated in our laboratory (Yassa et al., submitted). Thus, while aging itself can impair memory performance on this task, a further loss is noted in aMCI.
In addition to the reported deficits in lure discrimination, aMCI patients also demonstrated an impairment in target recognition, as evidenced by the significantly different d’ scores across groups. This deficit is not surprising, given the nature of the amnestic deficit. It is important to note, however, that the recognition memory deficit is qualitatively different from the patients’ deficit in lure discrimination, which constitutes a higher false alarm rate to lures. If there were an overall degraded memory representation driving both effects, one would expect an increase in “new” responses to both lures and repetitions, which is not the case. Diminished target recognition likely involves a combination of mnemonic deficits as well as failures in other capacities such as working memory, attention and executive function. Compared to young adults, both aged adults and aMCI patients exhibit a lure discrimination deficit. However, it is clear that aMCI patients also suffer from additional behavioral impairments that likely interact with and may share common or overlapping neural substrates with the lure discrimination deficit.
Although previous studies of aMCI patients have shown hyperactive BOLD signals in the hippocampal/parahippocampal region (Celone et al., 2006; Dickerson et al., 2004; 2005; Hämäläinen et al., 2007), none have localized this hyperactivity to a specific subfield or subregion of the hippocampus. Both the computational models and findings from the animal studies described earlier predict this hyperactivity to occur in the CA3 region of the hippocampus. We found general increases in activity across task conditions and across hippocampal subregions in the aMCI patients compared to controls, replicating findings from other studies. One possible explanation for this finding is that the aMCI patients treated the novel foil baseline in the task differently. It is possible, for example, that aMCI patients have an attenuated novelty response, and thus the difference between activity on task conditions and the novel foil baseline is magnified. When we removed baseline effects by comparing participants on a contrast of lure correct rejections vs. lure false alarms (heavily biased towards separation), hyperactivity only persisted in the CA3/DG region, suggesting that there is a specific impairment in this region. This hyperactivity was negatively correlated to participants’ separation bias scores, suggesting that it is a marker for network impairment. Taken together, evidence from the current study as well as from electrophysiological recordings in memory impaired aged rats, are providing convergent evidence that CA3/DG hyperactivity is likely part of a dysfunctional MTL network, or at a minimum is the result of inefficient processing (Grady 2008). In this study, we also found hypoactivity in the entorhinal cortex that would have been obscured if we did not separate hippocampal from parahippocampal activity. It is quite possible that the entorhinal hypoactivity contributes to the impairment perhaps by depriving the CA3/DG of its input via the perforant path. However, since direct correlations between ERC activity and behavior were not significant in our study, we hesitate to make strong claims regarding the extent to which this pattern is dysfunctional.
It is important to note that CA3/DG hyperactivity in our study was observed in a contrast that weighs heavily pattern separation. In other words, aMCI patients seem to engage the CA3/DG network to a greater extent on trials where they successfully discriminated between similar and old stimuli. Although we have designed our contrasts such that lures called “similar” would require more pattern separation than lures called “old”, it is unclear how much pattern completion is involved in each trial type. For example, in lures called “similar”, recall of the previous item's presentation may be used to correctly reject the item by saying “similar” and not “old” (a “recall to reject” strategy). Thus, both “old” and “similar” responses to lures likely involve some amount of pattern completion. The exact link between the relative load on separation and completion per trial and the hyperactivity at this point remains unclear, although overall separation behavior has an inverse relationship with the hyperactivity.
This study opens up an interesting question: if hyperactivity in the CA3 region is the neural basis of the observed episodic memory deficits in aMCI patients, would any attempt to bring down the level of activity in this region be an effective therapeutic tool to improve memory performance and perhaps slow down progression of dementia? Recent work in the rat suggests that this may indeed be the case. Targeted expression of an inhibitory neuropeptide to the CA3 of aged rats with cognitive impairment improves performance on a spatial memory task (Koh et al., 2009). Koh and colleagues (2009) also showed that using antiepileptic agents such as sodium valproate and levetiracetam produced similar improvements. It therefore seems possible that therapeutic approaches that target this hyperactivity in aMCI patients may be effective in reversing memory deficits and improving their performance on memory tasks. It may also be possible to investigate other groups that are at increased risk for AD and have also been reported to show hippocampal hyperactivity, such as ApoE4 allele carriers (Bookheimer et al., 2000) and offspring of autopsy-confirmed AD cases (Bassett et al., 2006), to see if we can isolate the fMRI hyperactivity specifically to the CA3/DG region of the hippocampus and see links to episodic memory impairment. These groups would also be ideal to target with therapeutic interventions.
In addition to functional findings in the CA3 region, we also found a significant decrease in activity in the left entorhinal cortex in aMCI patients in the critical separation contrast. This is consistent with the model, as input from layer II of entorhinal cortex to the dentate gyrus and to the CA3 region is reduced with aging (Geinisman et al., 1992; Smith et al., 2000; Scheff et al., 2006). Morevoer, the entorhinal cortex is also one of the first regions to undergo significant neuronal cell loss in MCI and AD dementia (Gomez-Isla et al., 1996; Kordower et al., 2001; Price et al., 2001). A natural question here would be why we find a functional difference in this region but not a structural difference. One possibility is that impending structural changes have not yet manifested and that functional changes in the region precede frank neuronal loss. Although speculative, this could be the case, given that our aMCI participants had mild deficits compared to more advanced aMCI and AD cases. It should also be noted that the entorhinal cortex is both the recipient of hippocampal processing as well as the source for hippocampal innervation. Since we do observe atrophy in the CA3/DG region it is possible this precedes entorhinal changes early on in the course of AD. Perhaps retrograde degeneration of the perforant path is a precursor to entorhinal atrophy. This is a question that should be the subject of future investigation, however, our reported evidence suggests that this may be the case.
The issue is further complicated by the imprecise nature of the BOLD signal and the myriad factors that influence its magnitude (e.g. synaptic, cerebrovascular, metabolic, etc…), which makes it all the more difficult to draw strong conclusions regarding whether functional deficits are the result of structural changes (i.e., activity from fewer cells) or purely functional changes (i.e., reduced activity per cell). Additionally, one must exercise extreme caution when interpreting group differences (e.g., hyperactivity vs. hypoactivity), especially when these differences are in specific conditions or contrasts. For example, it may be tempting to simply treat activity increases as “good” and activity decreases as “bad”. We show here that this is not the case, and that there are instances where increases in activity could be evidence for a dysfunctional mechanism.
We found structural differences between groups specifically in the hippocampus, and more specifically in the CA3/DG and CA1 regions, suggesting that these regions may be the first to suffer from early loss. Previous morphometric MRI reports have emphasized that the CA1 region of the hippocampus is structurally compromised early in the course of AD (Chetelat et al., 2008; Csernansky et al., 2000; Csernansky et al., 2005; Qiu et al., 2008; Wang et al., 2006). However, these approaches may suffer from an inherent bias against the deeper subfields. Consider, for example, the shape and location of the CA3/DG. There are areas of the hippocampus in which this region is nearly or even entirely surrounded by the other subfields. If there were a true change in shape in the CA3/DG region, surface-based approaches (Chetelat et al., 2008; e.g. Wang et al., 2006) would detect a change in the shape of the surface and ascribe it to the lateral subfields (CA1 and subiculum). Grayscale-based methods (Chetelat et al., 2008; e.g. Csernansky et al., 2000; Csernansky et al., 2005) would need sufficient detail in the internal structure of the hippocampus in order for the transformation to accurately reflect the actual location of the change. However, if such detail is not present (as is typical in MRI structural images using 1mm voxels), a more general contraction of the hippocampus will result and the localization of the actual change will be incorrect. Our approach estimates the deformations at the level of each subfield individually and thus is less likely to suffer from the above issues.
Recent volumetric MRI reports have also found CA3/DG structural deficits in those at increased risk for Alzheimer’s disease by virtue of carrying the e4 allele variant of the apolipoprotein E (ApoE) gene (Mueller et al., 2008; Mueller and Weiner 2009). Our results in aMCI patients are quite consistent with the results from the Weiner group (whose volumetric approach was based on precise anatomical subfield segmentations). We also show that shape and volumetric analyses can offer different, but complementary, methods to investigate structural changes in the hippocampus.
One limitation of the current work is that the exact relationship between pattern separation performance and CA3/DG structure and function to the severity of clinical impairment in aMCI remains unclear. Several studies have suggested that there is a relationship between hippocampal hyperactivity and the degree of clinical impairment in these patients (Celone et al., 2006; Dickerson et al., 2004; Dickerson et al., 2005; Miller et al., 2008a). Due to the small sample size, we were not been able to make this link. However, if this sample is followed longitudinally, we may be able to determine which aMCI patients progress to AD within a short period of time and which ones do not, and better understand the predictive value of our structural and functional markers. It is also noteworthy that our sample was not matched on gender, and given our small sample size we did not have enough power to assess potential gender differences in this study. This will be an important question for future research.
In summary, we have shown that aMCI patients demonstrate impairments on a recognition memory task that specifically taxes pattern separation abilities. We found evidence for the previously reported pattern of hyperactivity in aMCI patients across task conditions. Critically, we also found evidence for a specific pattern of hyperactivity as well as shape and volume changes in the CA3/DG region of the hippocampus in aMCI patients. These results suggest that structural and functional changes in the CA3/DG region could be an indicator for dysfunction that can be the possible target of future therapeutic interventions.
Acknowledgements
We would like to thank Dr. Barry Gordon and Dr. Jason Brandt for help with participant recruitment and clinical diagnosis, and the staff of the F.M. Kirby Center for Functional Brain Imaging for their assistance in data collection. This work was supported by National Institute on Aging/National Institutes of Health Grants P50AG05146 and 2P01 AG09973 and Glenn Foundation Award. Dr. Michela Gallagher is the founder of AgeneBio Incorporated, a biotechnology company that is dedicated to commercializing therapies to treat cognitive impairment in aging and she has a financial interest in the company.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ances BM, Liang CL, Leontiev O, Perthen JE, Fleisher AS, Lansing AE, Buxton RB. Effects of aging on cerebral blood flow, oxygen metabolism, and blood oxygenation level dependent responses to visual stimulation. Human Brain Mapping. 2009;30(4):1120–1132. doi: 10.1002/hbm.20574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson ND, Ebert PL, Jennings JM, Grady CL, Cabeza R, Graham SJ. Recollection-and familiarity-based memory in healthy aging and amnestic mild cognitive impairment. Neuropsychology. 2008;22(2):177–187. doi: 10.1037/0894-4105.22.2.177. [DOI] [PubMed] [Google Scholar]
- Baker CI, Hutchison TL, Kanwisher N. Does the fusiform face area contain subregions highly selective for nonfaces? Nature Neuroscience. 2007;10(1):3–4. doi: 10.1038/nn0107-3. [DOI] [PubMed] [Google Scholar]
- Bakker A, Kirwan CB, Miller NI, Stark CEL. Pattern separation in the human hippocampal CA3 and dentate gyrus. Science. 2008;319:1640–1642. doi: 10.1126/science.1152882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett SS, Yousem DM, Cristinzio C, Kusevic I, Yassa MA, Caffo BS, Zeger SL. Familial risk for Alzheimer's disease alters fMRI activation patterns. Brain : A Journal of Neurology. 2006;129(Pt 5):1229–1239. doi: 10.1093/brain/awl089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookheimer SY, Strojwas MH, Cohen MS, Saunders AM, Pericak-Vance MA, Mazziotta JC, Small GW. Patterns of brain activation in people at risk for Alzheimer's disease. The New England Journal of Medicine. 2000;343(7):450–456. doi: 10.1056/NEJM200008173430701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt J, Spencer M, Folstein M. The Telephone Interview for Cognitive Status. Neuropsychiatry, Neuropsychol Behav Neurol. 1988;1:111–117. [Google Scholar]
- Brueckner K, Moritz S. Emotional valence and semantic relatedness differentially influence false recognition in mild cognitive impairment, Alzheimer's disease, and healthy elderly. Journal of the International Neuropsychological Society. 2009;15(2):268–276. doi: 10.1017/S135561770909047X. [DOI] [PubMed] [Google Scholar]
- Burke SN, Barnes CA. Neural plasticity in the ageing brain. Nature Reviews. Neuroscience. 2006;7(1):30–40. doi: 10.1038/nrn1809. [DOI] [PubMed] [Google Scholar]
- Buxton R. Introduction to functional magnetic resonance imaging: principles and techniques. New York: Cambridge University Press; 2001. [Google Scholar]
- Celone KA, Calhoun VD, Dickerson BC, Atri A, Chua EF, Miller SL, DePeau K, Rentz DM, Selkoe DJ, Blacker D. Alterations in memory networks in mild cognitive impairment and Alzheimer's disease: An independent component analysis. Journal of Neuroscience. 2006;26(40):10222–10231. doi: 10.1523/JNEUROSCI.2250-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chetelat G, Fouquet M, Kalpouzos G, Denghien I, De la Sayette V, Viader F, Mezenge F, Landeau B, Baron JC, Eustache F. Three-dimensional surface mapping of hippocampal atrophy progression from MCI to AD and over normal aging as assessed using voxel-based morphometry. Neuropsychologia. 2008;46(6):1721–1731. doi: 10.1016/j.neuropsychologia.2007.11.037. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research, an International Journal. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Csernansky JG, Wang L, Swank J, Miller JP, Gado M, McKeel D, Miller MI, Morris JC. Preclinical detection of Alzheimer's disease: Hippocampal shape and volume predict dementia onset in the elderly. NeuroImage. 2005;25:783–792. doi: 10.1016/j.neuroimage.2004.12.036. [DOI] [PubMed] [Google Scholar]
- Csernansky JG, Wang L, Joshi S, Miller JP, Gado M, Kido D, McKeel D, Morris JC, Miller MI. Early DAT is distinguished from aging by high-dimensional mapping of the hippocampus. Neurology. 2000;55:1636–1643. doi: 10.1212/wnl.55.11.1636. [DOI] [PubMed] [Google Scholar]
- DeCarli C, Mungas D, Harvey D, Reed B, Weiner M, Chui H, Jagust W. Memory impairment, but not cerebrovascular disease, predicts progression of MCI to dementia. Neurology. 2004;63(2):220–227. doi: 10.1212/01.wnl.0000130531.90205.ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della Sala S, Cowan N, Beschin N, Perini M. Memory. 3–4. Vol. 13. England: Hove; 2005. Just lying there, remembering: Improving recall of prose in amnesic patients with mild cognitive impairment by minimising interference; pp. 435–440. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Salat DH, Bates JF, Atiya M, Killiany RJ, Greve DN, Dale AM, Stern CE, Blacker D, Albert MS. Medial temporal lobe function and structure in mild cognitive impairment. Annuals of Neurology. 2004;56:27–35. doi: 10.1002/ana.20163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson BC, Salat DH, Greve DN, Chua EF, Rand-Giovannetti E, Rentz DM, Bertram L, Mullin K, Tanzi RE, Blacker D. Increased hippocampal activation in mild cognitive impairment compared to normal aging and AD. Neurology. 2005;65(3):404–411. doi: 10.1212/01.wnl.0000171450.97464.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvernoy H, Cattin F, Naidich TP, Raybaud CRPY, Salvolini U, Scarabino U, Vannson JL. The human hippocampus: Functional anatomy, vascularization and serial sections with MRI. Third ed. New York: Springer; 2005. [Google Scholar]
- Ebert PL, Anderson ND. Proactive and retroactive interference in young adults, healthy older adults, and older adults with amnestic mild cognitive impairment. Journal of the International Neuropsychological Society. 2009;15(1):83–93. doi: 10.1017/S1355617708090115. [DOI] [PubMed] [Google Scholar]
- Fleisher AS, Podraza KM, Bangen KJ, Taylor C, Sherzai A, Sidhar K, Liu TT, Dale AM, Buxton RB. Cerebral perfusion and oxygenation differences in Alzheimer's disease risk. Neurobiology of Aging. 2008;30(11):1737–1748. doi: 10.1016/j.neurobiolaging.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher M, Rapp PR. The use of animal models to study the effects of aging on cognition. Annual Reviews of Psychology. 1997;48:339–370. doi: 10.1146/annurev.psych.48.1.339. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Burwell R, Burchinal M. Severity of spatial learning impairment in aging: development of a learning index for performance in the Morris water maze. Behav Neurosci. 1993;107:618–626. doi: 10.1037//0735-7044.107.4.618. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Bizon JL, Hoyt EC, Helm KA, Lund PK. Effects of aging on the hippocampal formation in a naturally occurring animal model of mild cognitive impairment. Experimental Gerontology. 2003;38(1–2):71–77. doi: 10.1016/s0531-5565(02)00159-6. [DOI] [PubMed] [Google Scholar]
- Geinisman Y, de Toledo-Morrell L, Morrell F, Persina IS, Rossi M. Age-related loss of axospinous synapses formed by two afferent systems in the rat dentate gyrus as revealed by the unbiased stereological dissector technique. Hippocampus. 1992;2(4):437–444. doi: 10.1002/hipo.450020411. [DOI] [PubMed] [Google Scholar]
- Gomez-Isla T, Price JL, McKeel DW, Jr, Morris JC, Growdon JH, Hyman BT. Profound loss of layer II entorhinal cortex neurons occurs in very mild Alzheimer's disease. Journal of Neuroscience. 1996;16(14):4491–4500. doi: 10.1523/JNEUROSCI.16-14-04491.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL. Cognitive neuroscience of aging. Annals of the New York Academy of Sciences. 2008;1124:127–144. doi: 10.1196/annals.1440.009. [DOI] [PubMed] [Google Scholar]
- Hämäläinen A, Pihlajamäki M, Tanila H, Hänninen T, Niskanen E, Tervo S, Karjalainen PA, Vanninen RL, Soininen H. Increased fMRI responses during encoding in mild cognitive impairment. Neurobiology of Aging. 2007;28(12):1889–1903. doi: 10.1016/j.neurobiolaging.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Hildebrandt H, Haldenwanger A, Eling P. False recognition correlates with amyloid-beta (1–42) but not with total tau in cerebrospinal fluid of patients with dementia and mild cognitive impairment. Journal of Alzheimer's Disease : JAD. 2009;16(1):157–165. doi: 10.3233/JAD-2009-0931. [DOI] [PubMed] [Google Scholar]
- Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- Hyman BT, Van Hoesen GW, Kromer LJ, Damasio AR. Perforant pathway changes and the memory impairment of Alzheimer's disease. Annals of Neurology. 1986;20(4):472–481. doi: 10.1002/ana.410200406. [DOI] [PubMed] [Google Scholar]
- Hyman BT, Van Hoesen GW, Damasio AR, Barnes CL. Alzheimer's disease: Cell-specific pathology isolates the hippocampal formation. Science. 1984;225(4667):1168–1170. doi: 10.1126/science.6474172. [DOI] [PubMed] [Google Scholar]
- Insausti R, Juottonen K, Soininen H, Insausti AM, Partanen K, Vainio P, Laakso MP, Pitkanen A. MR volumetric analysis of the human entorhinal, perirhinal, and temporopolar cortices. American Journal of Neuroradiology. 1998;19(4):659–671. [PMC free article] [PubMed] [Google Scholar]
- Johnson SC, Schmitz TW, Moritz CH, Meyerand ME, Rowley HA, Alexander AL, Hansen KW, Gleason CE, Carlsson CM, Ries ML. Activation of brain regions vulnerable to Alzheimer's disease: The effect of mild cognitive impairment. Neurobiology of Aging. 2006;27(11):1604–1612. doi: 10.1016/j.neurobiolaging.2005.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawas CH, Corrada MM, Brookmeyer R, Morrison A, Resnick SM, Zonderman AB, Arenberg D. Visual memory predicts Alzheimer's disease more than a decade before diagnosis. Neurology. 2003;60(7):1089–1093. doi: 10.1212/01.wnl.0000055813.36504.bf. [DOI] [PubMed] [Google Scholar]
- Killiany RJ, Hyman BT, Gomez-Isla T, Moss MB, Kikinis R, Jolesz F, Tanzi R, Jones K, Albert MS. MRI measures of entorhinal cortex vs hippocampus in preclinical AD. Neurology. 2002;58(8):1188–1196. doi: 10.1212/wnl.58.8.1188. [DOI] [PubMed] [Google Scholar]
- Kircher TT, Weis S, Freymann K, Erb M, Jessen F, Grodd W, Heun R, Leube DT. Hippocampal activation in patients with mild cognitive impairment is necessary for successful memory encoding. Journal of Neurology, Neurosurgery, and Psychiatry. 2007;78(8):812–818. doi: 10.1136/jnnp.2006.104877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirwan CB, Stark CEL. Overcoming interference: An fMRI investigation of pattern separation in the medial temporal lobe. Learning and Memory. 2007;14 doi: 10.1101/lm.663507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirwan CB, Jones C, Miller MI, Stark CEL. High-resolution fMRI investigation of the medial temporal lobe. Human Brain Mapping. 2007;28(1):959–966. doi: 10.1002/hbm.20331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein A, Andersson J, Ardekani BA, Ashburner J, Avants B, Chiang MC, Christensen GE, Collins DL, Gee J, Hellier P. Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. NeuroImage. 2009;46(3):786–802. doi: 10.1016/j.neuroimage.2008.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh MT, Haberman RP, Foti S, McCown TJ, Gallagher M. Treatment strategies targeting excess hippocampal activity benefit aged rats with cognitive impairment. Neuropsychopharmacology. 2009 doi: 10.1038/npp.2009.207. doi:10.1038/npp.2009.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordower JH, Chu Y, Stebbins GT, DeKosky ST, Cochran EJ, Bennett D, Mufson EJ. Loss and atrophy of layer II entorhinal cortex neurons in elderly people with mild cognitive impairment. Annals of Neurology. 2001;49(2):202–213. [PubMed] [Google Scholar]
- Leutgeb S, Leutgeb JK. Pattern separation, pattern completion, and new neuronal codes within a continuous CA3 map. Learning & Memory. 2007;14(11):745–757. doi: 10.1101/lm.703907. [DOI] [PubMed] [Google Scholar]
- Leutgeb S, Leutgeb JK, Treves A, Moser MB, Moser EI. Distinct ensemble codes in hippocampal areas CA3 and CA1. Science. 2004;305(5688):1295–1298. doi: 10.1126/science.1100265. [DOI] [PubMed] [Google Scholar]
- Loewenstein DA, Acevedo A, Luis C, Crum T, Barker WW, Duara R. Semantic interference deficits and the detection of mild Alzheimer's disease and mild cognitive impairment without dementia. Journal of the International Neuropsychological Society. 2004;10(1):91–100. doi: 10.1017/S1355617704101112. [DOI] [PubMed] [Google Scholar]
- Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neuropsychological investigation of the basis of the fMRI signal. Nature. 2001;412(6843):128–130. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- Marr D. Simple memory: A theory for Archicortex. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 1971;262(841):23–81. doi: 10.1098/rstb.1971.0078. [DOI] [PubMed] [Google Scholar]
- McClelland JL, McNaughton BL, O'Reilly RC. Why there are complementary learning systems in the hippocampus and neocortex: Insights from the successes and failures of connectionist models of learning and memory. Psychological Review. 1995;102(3):419–457. doi: 10.1037/0033-295X.102.3.419. [DOI] [PubMed] [Google Scholar]
- Miller MI, Beg MF, Ceritoglu C, Stark C. Increasing the power of functional maps of the medial temporal lobe by using large deformation diffeomorphic metric mapping. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(27):9685–9690. doi: 10.1073/pnas.0503892102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SL, Fenstermacher E, Bates J, Blacker D, Sperling RA, Dickerson BC. Hippocampal activation in adults with mild cognitive impairment predicts subsequent cognitive decline. Journal of Neurology, Neurosurgery, and Psychiatry. 2008a;79(6):630–635. doi: 10.1136/jnnp.2007.124149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SL, Celone K, DePeau K, Diamond E, Dickerson BC, Rentz D, Pihlajamaki M, Sperling RA. Age-related memory impairment associated with loss of parietal deactivation but preserved hippocampal activation. Proceedings of the National Academy of Sciences of the United States of America. 2008b;105(6):2181–2186. doi: 10.1073/pnas.0706818105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner B, Squire LR, Kandel ER. Cognitive neuroscience and the study of memory. Neuron. 1998;20(3):445–468. doi: 10.1016/s0896-6273(00)80987-3. [DOI] [PubMed] [Google Scholar]
- Mueller SG, Weiner MW. Selective effect of age, apo e4, and Alzheimer's disease on hippocampal subfields. Hippocampus. 2009;19(6):558–564. doi: 10.1002/hipo.20614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller SG, Schuff N, Raptentsetsang S, Elman J, Weiner MW. Selective effect of Apo e4 on CA3 and dentate in normal aging and Alzheimer's disease using high resolution MRI at 4 T. NeuroImage. 2008;42(1):42–48. doi: 10.1016/j.neuroimage.2008.04.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman KA, O'Reilly RC. Modeling hippocampal and neocortical contributions to recognition memory: A complementary-learning-systems approach. Psychological Review. 2003;110(4):611–646. doi: 10.1037/0033-295X.110.4.611. [DOI] [PubMed] [Google Scholar]
- O'Reilly RC, Norman KA. Hippocampal and neocortical contributions to memory: Advances in the complementary learning systems framework. Trends in Cognitive Sciences. 2002;6(12):505–510. doi: 10.1016/s1364-6613(02)02005-3. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: Clinical characterization and outcome. Archives of Neurology. 1999;56(3):303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- Petesen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256(3):183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- Price JL, Ko AI, Wade MJ, Tsou SK, McKeel DW, Morris JC. Neuron number in the entorhinal cortex and CA1 in preclinical Alzheimer disease. Archives of Neurology. 2001;58(9):1395–1402. doi: 10.1001/archneur.58.9.1395. [DOI] [PubMed] [Google Scholar]
- Qiu A, Younes L, Miller MI, Csernansky JG. Parallel transport in diffeomorphisms distinguishes the time-dependent pattern of hippocampal surface deformation due to healthy aging and the dementia of the Alzheimer's type. NeuroImage. 2008;40(1):68–76. doi: 10.1016/j.neuroimage.2007.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheff SW, Price DA, Schmitt FA, Mufson EJ. Hippocampal synaptic loss in early Alzheimer's disease and mild cognitive impairment. Neurobiology of Aging. 2006;27(10):1372–1384. doi: 10.1016/j.neurobiolaging.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Small SA, Perera GM, DeLaPaz R, Mayeux R, Stern Y. Differential regional dysfunction of the hippocampal formation among elderly with memory decline and Alzheimer's disease. Annals of Neurology. 1999;45(4):466–472. doi: 10.1002/1531-8249(199904)45:4<466::aid-ana8>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Smith TD, Adams MM, Gallagher M, Morrison JH, Rapp PR. Circuit-specific alterations in hippocampal synaptophysin immunoreactivity predict spatial learning impairment in aged rats. Journal of Neuroscience. 2000;20(17):6587–6593. doi: 10.1523/JNEUROSCI.20-17-06587.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling R. Functional MRI studies of associative encoding in normal aging, mild cognitive impairment, and Alzheimer's disease. Annals of the New York Academy of Sciences. 2007;1097:146–155. doi: 10.1196/annals.1379.009. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. A co-planar stereotaxic atlas of the human brain. New York: Thieme Medical; 1988. [Google Scholar]
- Tanila H, Shapiro M, Gallagher M, Eichenbaum H. Brain aging: Changes in the nature of information coding by the hippocampus. Journal of Neuroscience. 1997;17(13):5155–5166. doi: 10.1523/JNEUROSCI.17-13-05155.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toner CK, Pirogovsky E, Kirwan CB, Gilbert PE. Visual object pattern separation deficits in nondemented older adults. Learning and Memory. 2009;16:338–342. doi: 10.1101/lm.1315109. [DOI] [PubMed] [Google Scholar]
- Treves A, Rolls ET. Computational analysis of the role of the hippocampus in memory. Hippocampus. 1994;4(3):374–391. doi: 10.1002/hipo.450040319. [DOI] [PubMed] [Google Scholar]
- Vazdarjanova A, Guzowski JF. Differences in hippocampal neuronal population responses to modifications of an environmental context: Evidence for distinct, yet complementary, functions of CA3 and CA1 ensembles. Journal of Neuroscience. 2004;24(29):6489–6496. doi: 10.1523/JNEUROSCI.0350-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Miller JP, Gado MH, McKeel DW, Rothermich M, Miller MI, Morris JC, Csernansky JG. Abnormalities of hippocampal surface structure in very mild dementia of the Alzheimer type. NeuroImage. 2006;30(1):52–60. doi: 10.1016/j.neuroimage.2005.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward BD. Deconvolution analysis of fMRI time series data. 2001 http://afni.nimh.nih.gov/pub/dist/doc/manual/Deconvolvem.pdf.
- Welsh KA, Butters N, Hughes JP, Mohs RC, Heyman A. Detection and staging of dementia in Alzheimer's disease. Use of the neuropsychological measures developed for the consortium to establish a registry for Alzheimer's disease. Archives of Neurology. 1992;49(5):448–452. doi: 10.1001/archneur.1992.00530290030008. [DOI] [PubMed] [Google Scholar]
- Westerberg CE, Paller KA, Weintraub S, Mesulam MM, Holdstock JS, Mayes AR, Reber PJ. When memory does not fail: Familiarity-based recognition in mild cognitive impairment and Alzheimer's disease. Neuropsychology. 2006;20(2):193–205. doi: 10.1037/0894-4105.20.2.193. [DOI] [PubMed] [Google Scholar]
- Wilson IA, Gallagher M, Eichenbaum H, Tanila H. Neurocognitive aging: Prior memories hinder new hippocampal encoding. Trends in Neurosciences. 2006;29(12):662–670. doi: 10.1016/j.tins.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson IA, Ikonen S, Gallagher M, Eichenbaum H, Tanila H. Age-associated alterations of hippocampal place cells are subregion specific. Journal of Neuroscience. 2005;25(29):6877–6886. doi: 10.1523/JNEUROSCI.1744-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson IA, Ikonen S, McMahan RW, Gallagher M, Eichenbaum H, Tanila H. Place cell rigidity correlates with impaired spatial learning in aged rats. Neurobiology of Aging. 2003;24(2):297–305. doi: 10.1016/s0197-4580(02)00080-5. [DOI] [PubMed] [Google Scholar]
- Wilson IA, Ikonen S, Gurevicius K, McMahan RW, Gallagher M, Eichenbaum H, Tanila H. Place cells of aged rats in two visually identical compartments. Neurobiology of Aging. 2005;26(7):1099–1106. doi: 10.1016/j.neurobiolaging.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Wilson IA, Ikonen S, Gureviciene I, McMahan RW, Gallagher M, Eichenbaum H, Tanila H. Cognitive aging and the hippocampus: How old rats represent new environments. Journal of Neuroscience. 2004;24(15):3870–3878. doi: 10.1523/JNEUROSCI.5205-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Stark CEL. A quantitative evaluation of cross-participant registration techniques for MRI studies of the medial temporal lobe. NeuroImage. 2009;44(2):319–327. doi: 10.1016/j.neuroimage.2008.09.016. [DOI] [PubMed] [Google Scholar]
- Yushkevich PA, Avants BB, Pluta J, Das S, Minkoff D, Mechanic-Hamilton D, Glynn S, Pickup S, Liu W, Gee JC. A high-resolution computational atlas of the human hippocampus from postmortem magnetic resonance imaging at 9.4 T. NeuroImage. 2009;44(2):385–398. doi: 10.1016/j.neuroimage.2008.08.04. [DOI] [PMC free article] [PubMed] [Google Scholar]