Abstract
OBJECTIVE
Inflammatory markers such as C-reactive protein (CRP) are related to obesity in adults, but the association is less clear in children. Our objective was to examine relationships between multiple markers of inflammation and children’s weight status; we hypothesized that the prevalence of inflammatory markers would increase as weight status increased.
METHODS
We conducted a cross-sectional analysis of children in the United States aged 1 to 17 years in the National Health and Nutrition Examination Survey, 1999–2006. Children were categorized using weight-for-length when age < 2 years and BMI for ≥2 years, as very obese (≥99th percentile), obese (< 99th and ≥95th percentile), overweight (< 95th and ≥85th percentile), and healthy weight (> 5th to ≤85 thpercentile)according to expert consensus. Our main outcome measures were high-sensitivity CRP and absolute neutrophil count, in addition to a novel third measure: ferritin controlled for iron status using a ferritin/transferrin ratio. We used Cox proportional hazards models to examine risk of abnormal values of inflammatory markers according to weight.
RESULTS
Increased risk of a CRP level of > 1.0 mg/L was evident among very obese children from ages 3 to 5 years (hazard ratio [HR]: 2.29; P < .01) through 15 to 17 years (HR: 4.73; P < .01). Increased risk of abnormal neutrophil count among very obese children began at 6 to 8 years (HR: 2.00; P = .049), and increased prevalence of abnormal ferritin/transferrin ratio began at 9 to 11 years (HR: 7.06; P < .001).
CONCLUSIONS
Multiple inflammatory markers are strongly and positively associated with increasing weight status in children, and this relationship starts as young as age 3. Elevated inflammatory markers in very young obese children are particularly concerning, because inflammation may cause long-term, cumulative vascular damage. This deserves additional research via longitudinal design.
Keywords: inflammation, C-reactive protein, ferritin, obesity, overweight, BMI
WHAT’S KNOWN ON THIS SUBJECT
Inflammation, a risk factor for cardiovascular morbidity, is associated with obesity in adults. Small clinical samples and older nationally representative data have revealed correlations between CRP and BMI percentiles in school-aged children and adolescents.
WHAT THIS STUDY ADDS
Using recent nationally representative data from children aged 1 to 17 and multiple markers, the relationship between inflammation and obesity begins at age 3. This study also provides prevalence of abnormal inflammatory markers in current, clinically relevant BMI subcategories.
Obesity in childhood increases the risk of obesity in adulthood, and obesity in adulthood is associated with cardiovascular disease.1 Although the mechanisms are likely complex, a possible link between these 2 conditions is chronic, low-grade inflammation. Population-based studies have shown a clear relationship between obesity and high-sensitivity C-reactive protein (CRP) levels,2 and others have demonstrated a cause-and-effect relationship between adiposity and inflammation, with changes in weight resulting in directional changes in CRP.3,4 Inflammation may change the risk for cardiovascular disease by its association with traditional cardiovascular risk factors such as high-density lipoprotein, or inflammation may have a direct effect on the endothelium, atherogenesis, or the stability of atherosclerotic plaques.5,6 Multiple prospective studies in adults have shown CRP to be predictive of future cardiovascular disease, independent of obesity.7–9
It is biologically plausible that increased length of exposure to an inflammatory state could increase the risk of vascular damage and morbidity. If overweight or obese children have low-grade inflammation, this inflammation could increase the risk of long-term vascular damage and also indicate risk for future cardiovascular events. A few studies have demonstrated a relationship between CRP and obesity in childhood but were limited by use of small, clinic-based samples,10,11 consideration of only older children or adolescents,12 or limited measures of inflammation.13 Using currently outdated definitions of obesity, Visser et al14 examined inflammation in a nationally representative sample of older children, and Ford et al15,16 examined CRP and BMI in children older than 3 years. It is important to note that an increase in the prevalence of obesity, particularly among very young children,17 since the year 2000 when the most recent of these studies was conducted underscores the need to examine updated, more comprehensive data about the presence of inflammation in children.
A more in-depth and current understanding of the relationship between obesity and inflammation throughout childhood requires the use of multiple waves of recent nationally representative data to increase sample size and validity and analysis by narrow age groupings to identify the age at which any association between obesity and inflammation appears. Also, clinically relevant relationships require using current definitions of obesity that are used in practice, including the increasingly common “severely obese” category (BMI ≥ 99th percentile) with heightened risk of long-term health sequelae. Finally, the use of multiple different inflammatory markers may elucidate whether obesity is largely associated with elevations in CRP level or whether obesity is associated with a widespread inflammatory process that involves multiple different branches of the inflammatory cascade.
To these ends, and to expand previous work, we analyzed the most recent multiple years of data from the National Health and Nutrition Examination Survey (NHANES) to determine the associations between 3 distinct markers of inflammation and increasing weight status in children in the United States with a specific goal of isolating effects according to age. We hypothesized that inflammation and obesity would be highly associated throughout childhood and that the association would begin earlier than previously described.
METHODS
We analyzed data from the NHANES, years 1999–2006. The NHANES is a stratified, multistage probability sample of the civilian, noninstitutionalized population of the United States. It includes computer-based interviews, an in-home questionnaire on a variety of demographic and health topics, an examination that includes a thorough physical examination, and laboratory measures.18
Outcome Variables
We examined 3 markers of inflammation: CRP, absolute neutrophil count (ANC), and ferritin level controlled for iron status. The last measure has not been used previously but is described and tested here as a biologically plausible measure of inflammation that could have a relationship to obesity.
The NHANES measured CRP in all children aged 3 and older in 1999–2002 and all children aged 1 and older in 2003–2006. CRP was quantified by using latex-enhanced nephelometry, and a high-sensitivity assay was performed on a Behring (Deerfield, IL) nephelometer.19 The lower limit of detection for this test was 0.2 mg/L. For continuous analyses, all values of < 0.2 mg/L were coded as equal to 0.1. Research in adults has indicated increased risk of cardiovascular disease beginning at values of ≥1.0 mg/L,20 so we used this value as a cut point for our analyses. However, because of a lack of evidence defining important cut points for CRP in children, we also used a definition of elevated CRP of 4.0 mg/L, representing the survey-weighted 95th percentile. There is insufficient data to suggest a cutoff related to health outcomes in children; therefore, our use of the 95th percentile is intended only to analyze weight-related trends. We excluded children with CRP levels of > 10.0 mg/L from all analyses, because values that high likely indicate a separate, active inflammatory clinical process.
Complete blood count was performed on all children aged 1 year and older, and we used the component ANC as an inflammatory marker because of previous research that demonstrated that it may be predictive of cardiovascular disease in adults.21 We considered neutrophils elevated when the ANC was > 6.6 × 103 μL or when the percentage of white blood cells that were neutrophils was > 67.2%, representing the survey-weighted 95th percentile of the final analytic sample.
Iron studies were completed for all children 1 year and older in 1999–2002. In 2003–2006, these studies were available only for children aged 3 to 5 years and girls aged 12 to 18 years. Ferritin is increasingly recognized as not only a test for serum iron status but also an acute phase reactant that is elevated in inflammatory conditions.22 However, there are no established cutoff values for ferritin in children that represent low-grade inflammation. In addition, interpretation of ferritin values depends on whole-body iron status including transferrin saturation. Therefore, we introduce a ratio of ferritin to transferrin (F/T) saturation as a ferritin-based inflammatory outcome with control for iron status. Because no clinical cutoffs exist for this measure, we defined F/T saturation as high when the value was greater then the weighted 95th percentile in our final analytic sample. Therefore, for categorical analyses, F/T was considered elevated if it was > 4.81.
These 3 markers of inflammation were chosen because they are incited in part by exposure to shared inflammatory factors, but also each has unique branches of the inflammatory cascade: CRP increases directly with interleukin 6 levels, the neutrophil count largely depends on granulocyte colony-stimulating factor, and the ferritin level increases with macrophage activation.23 Although F/T has not previously been validated as an inflammatory marker that is related to future health outcomes, we included this measure of inflammation controlled for iron status to establish the relationship of obesity and inflammation across multiple inflammatory cascades and measures.
Independent Variables
We used height and weight as measured during the examination component to determine age- and gender-specific weight-for-length for children younger than 2 and those aged 2 or older to calculate BMI. SAS (SAS Institute, Inc, Cary, NC) code developed for the purpose determined BMI percentiles.24 Children were then categorized using weight-for-length or BMI percentiles and current Centers for Disease Control and Prevention and expert committee recommendations as very obese (≥99th percentile), obese (< 99th and ≥95th percentile), overweight (< 95th and ≥85th percentile), or healthy weight(< 85th percentile).25 It should be noted that these definitions are based on BMI percentile and do not represent the distribution of the sample or of the current population. Rather, they derive from BMI distribution in the population from 1963 to 1994,26 and on the basis of current distributions, > 5% of our study population will be above the 95th percentile. We excluded underweight children (BMI < 5th percentile) because we were primarily interested in the relationship between excess weight and inflammation, and because these children may be more likely to have inflammation associated with other chronic illnesses. European growth charts, which do include BMI for children from birth, demonstrate generally good agreement between BMI and weight-for-length.27
To avoid confounding between inflammation related to chronic illness (particularly ferritin) and inflammation associated with obesity, we excluded all children with anemia, which we de-fined by using hemoglobin level (< 11.0g/dL for children aged 0–4 years, < 11.5g/dL for children aged 5–12 years, and < 12.0g/dL for children aged 13 years and older)28 or parental report of taking medication for iron-deficiency anemia. In addition, all girls who reported using oral contraceptives were also excluded because of known relationships between estrogen use and inflammation.29
We controlled for gender, race/ethnicity, current illness, age, and smoking exposure (children with serum cotinine concentrations of ≥12 ng/mL were classified as exposed to cigarette smoke either actively or passively30), the latter because of its known relationship to inflammation.31 Race/ethnicity was categorized as white, black, Hispanic, or other. Because of minimal differences noted in descriptive statistics, we chose to control for, rather than stratify according to, race and gender. To control for current illness, we adjusted for 3 binary variables: having a “head cold” or “influenza or an ear infection” in the last 30 days or having a diagnosis of asthma.
Statistical Methods
We began by examining the bivariate relationships between each inflammatory marker and obesity. We examined mean differences in the outcome measures according to weight category, testing with an adjusted Wald test. We also examined differences in prevalence of abnormal values according to weight category, testing differences by using a Pearson χ2 test. Consistent with recommendations, we did not adjust for multiple comparisons, because each comparison has an independent null hypothesis determined before analysis.32
Multivariate analyses used Cox proportional hazards models to develop hazard ratios (HRs) of the effect of weight on abnormal outcomes. This model performs well and provides HRs that are more clearly interpretable than odds ratios from logistic regression.33
All analyses were adjusted for the complex survey design of the NHANES and performed by using the survey estimation routines in Stata 10.0 (Stata Corp, College Station, TX). The study was deemed exempt from human subjects review by the University of North Carolina public health/nursing institutional review board (07–1967) because it used only deidentified secondary data.
RESULTS
After removal of children who were anemic (2.7%), taking oral contraceptives (< 1%), and with CRP levels of > 10.0 mg/L, the final sample size was 16 335. Those excluded were more likely to be black, possibly because of greater prevalence of anemia in black children, and more likely to be female, because of the use of oral contraceptive use as an exclusion criteria. The prevalence of potential confounders (Table 1) included cotinine levels indicative of tobacco use or significant secondary exposure (3%), a “head cold” in the previous 30 days (27%), having “flu or ear infection” in previous 30 days (7.2%), or an asthma diagnosis (15%).
TABLE 1.
Descriptive Statistics and Percent With Abnormal Values According to Demographic and Confounding Factors
| Total | CRP > 1.0, % | CRP > 4.0, % | Neutrophils > 6600 or 67.2%, % | F/T Saturation > 4.81, % | |
|---|---|---|---|---|---|
| Total, N = 16 335 | 24.5 | 5.2 | 7.3 | 5.0 | |
| Gender | |||||
| Female | 48.2 | 25.2 | 5.1 | 8.3a | 5.6 |
| Male | 51.8 | 23.9 | 5.2 | 6.4 | 4.5 |
| Race/ethnicity | |||||
| White | 60.0 | 22.3a | 4.4a | 7.4a | 4.5 |
| Black | 13.9 | 24.4 | 5.8 | 3.9 | 6.5 |
| Hispanic | 19.5 | 32.1 | 7.2 | 9.6 | 5.1 |
| Other | 6.6 | 22.9 | 4.3 | 6.8 | 5.7 |
| Age, y | |||||
| 1–2 | 11.1 | 20.7a | 5.9b | 3.5a | 8.5a |
| 3–5 | 15.9 | 19.6 | 4.1 | 5.2 | 5.2 |
| 6–8 | 17.7 | 22.3 | 4.1 | 7.7 | 5.0 |
| 9–11 | 16.8 | 27.4 | 6.7 | 4.8 | 7.0 |
| 12–14 | 17.1 | 23.7 | 4.7 | 8.0 | 3.0 |
| 15–17 | 16.2 | 29.0 | 5.8 | 12.2 | 4.6 |
| Head cold in previous 30 d | |||||
| Yes | 26.5 | 30.7a | 8.6a | 9.9a | 9.0a |
| No | 73.5 | 22.4 | 4.1 | 6.4 | 3.6 |
| Flu or ear infection in previous 30 d | |||||
| Yes | 7.2 | 33.0a | 7.8b | 6.9 | 7.4 |
| No | 92.8 | 23.9 | 5.1 | 7.3 | 4.8 |
| Ever told have asthma | |||||
| Yes | 14.9 | 27.8a | 6.6a | 7.4 | 5.2 |
| No | 85.1 | 23.9 | 4.9 | 7.3 | 5.0 |
| Cotinine > 12 ng/mL | |||||
| Yes | 2.9 | 31.3b | 6.8 | 10.8b | 4.6 |
| No | 97.1 | 24.2 | 5.1 | 7.2 | 5.0 |
| Weight category | |||||
| Very obese | 3.5 | 71.0a | 23.7a | 13.0a | 14.0a |
| Obese | 11.4 | 53.8 | 12.1 | 11.5 | 9.8 |
| Overweight | 15.3 | 31.4 | 5.4 | 8.4 | 2.8 |
| Healthy weight | 69.8 | 15.2 | 2.9 | 6.0 | 4.2 |
P < .01 and
P < .05,
adjusted Wald test for differences in inflammation prevalence within demographic category.
Correlations between CRP, ANC, neutrophil percent, and F/T were significant but weak, ranging from r = 0.10 to 0.36 and confirming that each is, at least in part, acting independently in its association with obesity. Table 2 presents percentile distributions of each inflammatory marker. Median CRP was 0.3 mg/L (mean: 0.89 mg/L), median ANC was 3.4 × 103 μL (mean: 3.67 × 103 μL), and median F/T was 1.38 (mean: 1.86). Only the prevalence of elevated ANC (not CRP or F/T) differed according to gender. Some ethnicity- and race-related differences in inflammatory markers were noted (Table 1).
TABLE 2.
Distribution of Inflammatory Markers
| Percentile | CRP, mg/L | ANC, × 103 μL | Ferritin |
|---|---|---|---|
| 1st | 0.10 | 1.10 | 0.26 |
| 5th | 0.10 | 1.60 | 0.44 |
| 10th | 0.10 | 2.00 | 0.58 |
| 25th | 0.10 | 2.60 | 0.87 |
| 50th | 0.30 | 3.40 | 1.38 |
| 75th | 0.90 | 4.50 | 2.20 |
| 95th | 4.00 | 6.60 | 4.81 |
| 99th | 7.60 | 8.90 | 8.00 |
| Mean | 0.89 | 3.67 | 1.86 |
The prevalence of a CRP level > 1.0 mg/L was significantly greater in very obese children beginning at age 3 and continuing through adolescence (Table 3). In children aged 15 to 17 years, 83.3% of those who were very obese had a CRP level of > 1.0 mg/L, compared with only 18.1% of healthy-weight children (P < .001). Similarly, a weight-related increase in prevalence of elevated F/T and ANC began very early in life. However, the prevalence of elevated F/T in the very obese was not significantly different from healthy-weight children until the age of 6. Increased prevalence of abnormal ANC began at age 9.
TABLE 3.
Prevalence of Abnormal Values of Inflammatory Markers
| Age, y | Very Obese (N = 691), % | Obese (N = 1996), % | Overweight (N = 2529), % | Healthy Weight (N = 10 609), % | P | n |
|---|---|---|---|---|---|---|
| CRP > 1.0 mg/L | ||||||
| 1–2 | 25.9 | 20.2 | 27.7 | 18.8 | .285 | 600 |
| 3–5 | 42.5 | 28.4 | 21.1 | 16.8 | < .001 | 1460 |
| 6–8 | 62.6 | 42.2 | 29.7 | 15.6 | < .001 | 1588 |
| 9–11 | 84.3 | 67.3 | 34.6 | 12.9 | < .001 | 1739 |
| 12–14 | 86.9 | 55.3 | 29.2 | 12.1 | < .001 | 2830 |
| 15–17 | 83.3 | 60.4 | 38.1 | 18.1 | < .001 | 2636 |
| CRP > 4.0 mg/L | ||||||
| 1–2 | 3.1 | 3.9 | 11.1 | 5.0 | .134 | 600 |
| 3–5 | 5.8 | 6.4 | 3.2 | 3.9 | .536 | 1460 |
| 6–8 | 16.7 | 5.2 | 7.1 | 2.7 | < .001 | 1588 |
| 9–11 | 41.1 | 17.3 | 6.2 | 2.6 | < .001 | 1739 |
| 12–14 | 34.0 | 13.1 | 2.8 | 1.8 | < .001 | 2830 |
| 15–17 | 24.6 | 13.9 | 5.9 | 3.2 | < .001 | 2636 |
| Neutrophils > 6600 or 67.2% | ||||||
| 1–2 | 6.4 | 2.8 | 4.4 | 3.2 | .676 | 1334 |
| 3–5 | 6.7 | 4.4 | 7.5 | 5.0 | .765 | 1582 |
| 6–8 | 16.7 | 10.3 | 7.1 | 6.7 | .258 | 1635 |
| 9–11 | 15.5 | 9.6 | 3.9 | 3.3 | < .001 | 1766 |
| 12–14 | 14.0 | 14.1 | 9.2 | 6.1 | < .001 | 2855 |
| 15–17 | 13.9 | 17.1 | 15.9 | 10.3 | .047 | 2660 |
| F/T saturation > 4.81 | ||||||
| 1–2 | 21.2 | 4.2 | 2.0 | 10.2 | .075 | 525 |
| 3–5 | 9.5 | 5.5 | 2.2 | 5.3 | .296 | 1440 |
| 6–8 | 2.1 | 14.8 | 0.8 | 4.4 | .020 | 863 |
| 9–11 | 30.0 | 14.0 | 5.8 | 4.5 | < .001 | 867 |
| 12–14 | 10.2 | 7.1 | 2.6 | 1.9 | < .001 | 2149 |
| 15–17 | 24.4 | 9.8 | 2.7 | 3.3 | < .001 | 1907 |
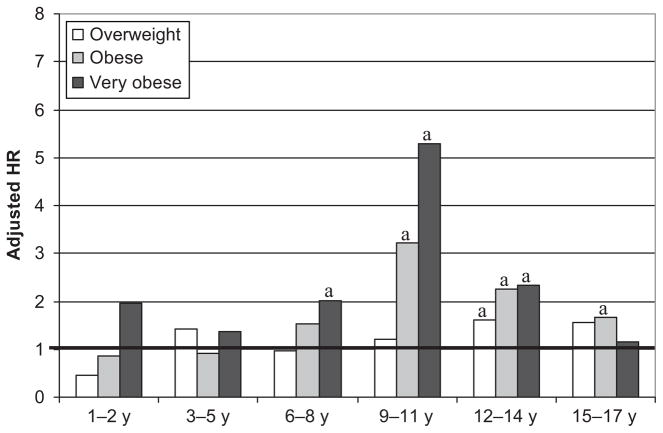
The increased prevalence of abnormal inflammatory markers among obese children remained when controlling for demographic and other potentially proinflammatory factors. Increased risk of a CRP level of > 1.0 mg/L (Fig 1) was evident at 3 years of age among very obese children(HR: 2.29 [95% confidence interval (CI): 1.52–3.44]), peaked among children aged 9 to 14 years and continued through adolescence (HR: 4.73 [95% CI: 3.72–6.02]; ages 15–17). HRs were generally larger with similar distribution for risk of CRP levels of > 4.0 mg/L. Similar but somewhat smaller increases in risk were also seen for abnormal ANC (Fig 2) beginning at age 6 and F/T beginning at age 9 (Fig 3).
FIGURE 1.
Relative risk of a CRP of > 1.0 mg/L, compared with healthy-weight children. a P < .01. (Tabular data are available in Table 4.)
FIGURE 2.
Relative risk of an ANC of > 6600, compared with healthy-weight children. a P < .05. (Tabular data are available in Table 4.)
FIGURE 3.
Relative risk of an F/T of > 4.81, compared with healthy-weight children. a P < .05. (Tabular data are available in Table 4.)
DISCUSSION
To our knowledge, this is the first study to demonstrate a strong and consistent association among inflammatory markers and obesity in a large, recent nationally representative sample of US children across the full childhood age range. Although associations between inflammatory markers and obesity were greatest for older children, this relationship was observed among children as young as age 3 and was consistently demonstrated for 3 different measures of inflammation. Our analysis also provides national prevalence estimates of elevated inflammatory markers in children at varying weight statuses and age groups.
In the present era of an obesity epidemic, when 14% of preschool-aged children (2–5 years) are overweight,17 our study is an important addition to the literature. Previous research used ultrasound techniques to show that increased CRP seems to be related to vascular intima media thickness in children and early atherosclerotic changes related to inflammation.11,34 Taken together with these pathophysiologic findings in small clinic samples, the results of our work raise concerns about the entire population of overweight children’s risk for long-term cardiovascular disease. Ford et al analyzed NHANES 1999–2000 and demonstrated associations between BMI percentile and increases in CRP.35 Since that study, the definitions of overweight/obesity have changed, and the epidemic of obesity has progressed within all age groups of the population. Our study, in which we used larger, more recent nationally representative data with a broader age range and multiple inflammatory markers, therefore extends earlier work by Ford et al and other authors.10–15,36
Our study results also demonstrated the persistence of the relationship between weight status and inflammation despite using inflammatory markers based on different terminal inflammatory pathways. The weak correlations between the measures suggest that each is measuring a somewhat different process yet is independently and similarly associated with weight status. Each inflammatory pathway may have different effects on health outcomes. Future research will need to examine their usefulness, individually or combined, in predicting future cardiovascular and other health outcomes.
Whereas our study has important strengths, there are also limitations. First, we were unable to fully control for pubertal age, which may affect cardiovascular risk factors including inflammation. NHANES no longer includes Tanner staging for measurement of puberty, and there are also few other sexual development proxies available. However, our results are similar for age groups in which there may be significant variation in puberty and for age groups in which the great majority of children have likely begun puberty, strongly suggesting that the lack of control for puberty had minimal impact on our analyses. Second, given the cross-sectional design, we are unable to determine the direction of association between inflammation and obesity. Although there is evidence that adipose tissue acts as an active endocrine organ that stimulates inflammatory cascades,5,6,37 it is possible that inflammation could also incite obesity. More importantly, although the relationship between inflammation and adiposity is evident in very young children, the cross-sectional design does not permit us to comment on whether the relationship is maintained for individual children across time or certainly into adulthood. Third, because inflammatory markers do vary somewhat according to age, our use of the same cutoff for all ages may obscure subtle relationships. However, using age-specific 95th percentile as cutoffs for abnormal values did not significantly change the results. Finally, we used 2 less-established markers of chronic inflammation, in addition to the more accepted and conventional marker, CRP. Whereas the F/T marker (described here for the first time) may ultimately be a novel way to examine ferritin while controlling for iron status, there are currently no descriptions of its distribution in the population, and its use here is based on face validity alone.
Why we did not identify a relationship between elevated inflammatory markers and obesity in infants and children younger than 3 years of age is unclear, although very obese 1- to 2-year-olds did have a trend toward elevated inflammatory markers. It is possible that inflammatory markers are not elevated immediately with obese status, and therefore obese children in the youngest age range would not yet have elevated markers. Longitudinal study would better test this hypothesis. Possibly, the weight-for-length percentile used for the youngest children altered the relationship between weight status (otherwise assessed via BMI) and inflammatory markers. Because the number of 1- to 2-year-old children in our sample was smaller, it is possible that a more balanced sample distribution would have conferred statistically significant differences at all ages.
It is also unclear why the association between obesity and inflammatory markers seems to peak at ages 9 to 11. One possibility is that the lack of pubertal controls disguises a more complex relationship between adiposity changes in the early pubertal period and inflammation perhaps related to insulin resistance.38 In addition to specific puberty-based differences, there may be biological or physiologic differences in this developmental period that we are unable to elucidate using our data.
Our research brings to the forefront many important questions. First, with so many children potentially at risk for health-related sequelae from obesity, does the presence of increased inflammatory markers predict those who will fare poorest? Future research needs to focus on the ability to use inflammatory markers to predict cardiovascular risk in children, as has been done in adults. In this way, inflammatory markers could be used as a second layer of screening to determine who would most benefit from obesity interventions. Although cardiovascular screening of adults by using CRP is debated,39 it is clear that there is a relationship between CRP level and future cardiovascular morbidity.40,41 In addition, there is evidence that treating older adults with an elevated CRP but normal lipid levels using 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors (statins) reduces mortality.42 To be enrolled in the 3-hydroxy-3-methylglutaryl coenzyme A reductase treatment trial (JUPITER trial), subjects had to have a CRP level of > 2 mg/L,42 which makes it concerning that a significant proportion of obese children have CRP levels of > 4 mg/L. Although current recommendations and debate about statin use in children focus on the reduction of hyperlipidemia, the JUPITER trial may stimulate researchers to investigate the benefits of statin usage in children beyond cholesterol reduction alone.
Another question is whether biomarkers could change quickly with lifestyle change and thereby be useful to motivate and support patients early in the course of improved lifestyles. Because there is evidence that physical activity, specific dietary behaviors, and other environmental factors play a role in the inflammatory process,37,43 could counseling that focuses on improved diet and activity improve inflammation even before weight decreases?
CONCLUSIONS
Obese children in this nationally representative sample already had measurably elevated markers of inflammation. Additional research should determine whether inflammation incites a cascade that over many years leads to cardiovascular damage and subsequent cardiovascular events and whether earlier exposure to inflammation causes cumulative damage. If such processes were confirmed, inflammation may transform the current epidemic of childhood obesity into a future epidemic of cardiovascular morbidity and mortality in adults, further justifying early obesity prevention efforts.
TABLE 4.
HRs of the Effect of Weight on the Likelihood of Abnormal Values of Inflammatory Markers
| Age, y | CRP > 1.0 mg/L |
CRP > 4.0 mg/L |
Neutrophils > 6600 or 67.2% |
F/T Saturation > 4.81 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | |
| 1–2 | ||||||||||||
| Very obese | 1.16 | 0.44–3.05 | .763 | 0.61 | 0.07–5.03 | .638 | 1.96 | 0.48–8.03 | .344 | 3.19 | 0.53–19.28 | .203 |
| Obese | 1.19 | 0.57–2.48 | .635 | 0.89 | 0.29–2.75 | .843 | 0.87 | 0.22–3.46 | .839 | 0.31 | 0.08–1.31 | .110 |
| Overweight | 1.58 | 0.85–2.92 | .145 | 1.89 | 0.68–5.25 | .219 | 0.45 | 0.16–1.24 | .120 | 0.18a | 0.06–0.57 | .004 |
| 3–5 | ||||||||||||
| Very obese | 2.29a | 1.52–3.44 | < .001 | 1.14 | 0.46–2.83 | .770 | 1.38 | 0.37–5.17 | .627 | 1.82 | 0.56–5.89 | .318 |
| Obese | 1.49 | 0.94–2.37 | .088 | 1.34 | 0.51–3.55 | .545 | 0.90 | 0.30–2.71 | .853 | 1.11 | 0.42–2.93 | .829 |
| Overweight | 1.14 | 0.70–1.85 | .590 | 0.71 | 0.24–2.07 | .526 | 1.42 | 0.62–3.23 | .402 | 0.37 | 0.13–1.05 | .061 |
| 6–8 | ||||||||||||
| Very obese | 3.03a | 2.12–4.33 | < .001 | 4.05a | 1.52–10.79 | .006 | 2.00b | 1.00–3.99 | .049 | 0.41 | 0.08–2.06 | .277 |
| Obese | 2.39a | 1.63–3.51 | < .001 | 1.78 | 0.75–4.20 | .187 | 1.53 | 0.59–3.99 | .378 | 3.78 | 0.94–15.18 | .060 |
| Overweight | 1.68b | 1.14–2.47 | .010 | 2.21 | 0.93–5.26 | .073 | 0.97 | 0.44–2.12 | .929 | 0.19b | 0.05–0.78 | .021 |
| 9–11 | ||||||||||||
| Very obese | 6.36a | 4.86–8.31 | < .001 | 19.79a | 11.76–33.31 | < .001 | 5.29a | 2.02–13.87 | < .001 | 7.06a | 2.31–21.62 | < .001 |
| Obese | 5.12a | 4.08–6.42 | < .001 | 7.00a | 3.89–12.59 | < .001 | 3.21a | 1.54–6.70 | .002 | 2.93b | 1.26–6.80 | .013 |
| Overweight | 2.63a | 1.85–3.73 | < .001 | 2.43b | 1.17–5.05 | .018 | 1.21 | 0.47–3.12 | .689 | 1.31 | 0.41–4.15 | .642 |
| 12–14 | ||||||||||||
| Very obese | 7.19a | 5.59–9.24 | < .001 | 18.88a | 11.04–32.28 | < .001 | 2.32b | 1.13–4.79 | .023 | 4.20a | 2.06–8.57 | < .001 |
| Obese | 4.50a | 3.63–5.59 | < .001 | 6.61a | 3.85–11.34 | < .001 | 2.24a | 1.47–3.42 | < .001 | 4.03a | 1.83–8.83 | < .001 |
| Overweight | 2.41a | 1.84–3.16 | < .001 | 1.50 | 0.70–3.22 | .291 | 1.60b | 1.05–2.44 | .030 | 1.49 | 0.56–3.95 | .420 |
| 15–17 | ||||||||||||
| Very obese | 4.73a | 3.72–6.02 | < .001 | 8.14a | 4.81–13.77 | < .001 | 1.16 | 0.53–2.56 | .706 | 5.50a | 2.43–12.48 | < .001 |
| Obese | 3.64a | 3.03–4.37 | < .001 | 5.00a | 3.04–8.21 | < .001 | 1.67b | 1.03–2.71 | .039 | 2.72a | 1.58–4.67 | < .001 |
| Overweight | 2.09a | 1.71–2.57 | < .001 | 1.86 | 0.96–3.61 | .065 | 1.56 | 0.97–2.49 | .063 | 0.77 | 0.27–2.20 | .620 |
Adjusted for age, race, presence of recent cold or influenza, asthma, and smoking.
P < .01.
P < .05.
Acknowledgments
Dr Skinner was supported by National Institutes of Health (NIH) training grant 5 T32 NR008856, and is currently supported by a BIRCWH career development award (5K12HD001441-10). Dr Perrin is supported by NIH career development award 5 K23 HD051817.
We thank Drs Kyrie Shomaker, Morris Weinberger, and Thomas Keyserling for helpful comments during preparation of the manuscript.
Funded by the National Institutes of Health (NIH).
ABBREVIATIONS
- CRP
C-reactive protein
- NHANES
National Health and Nutrition Examination Survey
- ANC
absolute neutrophil count
- F/T
ferritin/transferrin ratio
- HR
hazard ratio
- CI
confidence interval
Footnotes
Dr Skinner had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
References
- 1.Poirier P, Giles TD, Bray GA, et al. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss. Arterioscler Thromb Vasc Biol. 2006;26(5):968–976. doi: 10.1161/01.ATV.0000216787.85457.f3. [DOI] [PubMed] [Google Scholar]
- 2.Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB. Elevated C-reactive protein levels in overweight and obese adults. JAMA. 1999;282(22):2131–2135. doi: 10.1001/jama.282.22.2131. [DOI] [PubMed] [Google Scholar]
- 3.Fogarty AW, Glancy C, Jones S, Lewis SA, McKeever TM, Britton JR. A prospective study of weight change and systemic inflammation over 9 y. Am J Clin Nutr. 2008;87(1):30–35. doi: 10.1093/ajcn/87.1.30. [DOI] [PubMed] [Google Scholar]
- 4.Selvin E, Paynter NP, Erlinger TP. The effect of weight loss on C-reactive protein: a systematic review. Arch Intern Med. 2007;167(1):31–39. doi: 10.1001/archinte.167.1.31. [DOI] [PubMed] [Google Scholar]
- 5.Das UN. Is obesity an inflammatory condition? Nutrition. 2001;17(11–12):953–966. doi: 10.1016/s0899-9007(01)00672-4. [DOI] [PubMed] [Google Scholar]
- 6.Calabro P, Limongelli G, Pacileo G, Di Salvo G, Golino P, Calabro R. The role of adiposity as a determinant of an inflammatory milieu. J Cardiovasc Med (Hagerstown) 2008;9(5):450–460. doi: 10.2459/JCM.0b013e3282eee9a8. [DOI] [PubMed] [Google Scholar]
- 7.Greenfield JR, Samaras K, Jenkins AB, et al. Obesity is an important determinant of baseline serum C-reactive protein concentration in monozygotic twins, independent of genetic influences. Circulation. 2004;109(24):3022–3028. doi: 10.1161/01.CIR.0000130640.77501.79. [DOI] [PubMed] [Google Scholar]
- 8.Danesh J, Collins R, Appleby P, Peto R. Association of fibrinogen, C-reactive protein, albumin, or leukocyte count with coronary heart disease: meta-analyses of prospective studies. JAMA. 1998;279(18):1477–1482. doi: 10.1001/jama.279.18.1477. [DOI] [PubMed] [Google Scholar]
- 9.Danesh J, Wheeler JG, Hirschfield GM, et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med. 2004;350(14):1387–1397. doi: 10.1056/NEJMoa032804. [DOI] [PubMed] [Google Scholar]
- 10.Nagel G, Rapp K, Wabitsch M, et al. Prevalence and cluster of cardiometabolic biomarkers in overweight and obese schoolchildren: results from a large survey in southwest Germany. Clin Chem. 2008;54(2):317–325. doi: 10.1373/clinchem.2007.094821. [DOI] [PubMed] [Google Scholar]
- 11.Kapiotis S, Holzer G, Schaller G, et al. A proinflammatory state is detectable in obese children and is accompanied by functional and morphological vascular changes. Arterioscler Thromb Vasc Biol. 2006;26(11):2541–2546. doi: 10.1161/01.ATV.0000245795.08139.70. [DOI] [PubMed] [Google Scholar]
- 12.de Ferranti SD, Gauvreau K, Ludwig DS, Newburger JW, Rifai N. Inflammation and changes in metabolic syndrome abnormalities in US adolescents: findings from the 1988–1994 and 1999–2000 National Health and Nutrition Examination Surveys. Clin Chem. 2006;52(7):1325–1330. doi: 10.1373/clinchem.2006.067181. [DOI] [PubMed] [Google Scholar]
- 13.Lande MB, Pearson TA, Vermilion RP, Auinger P, Fernandez ID. Elevated blood pressure, race/ethnicity, and C-reactive protein levels in children and adolescents. Pediatrics. 2008;122(6):1252–1257. doi: 10.1542/peds.2007-3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB. Low-grade systemic inflammation in overweight children. Pediatrics. 2001;107(1) doi: 10.1542/peds.107.1.e13. Available at: www.pediatrics.org/cgi/content/full/107/1/e13. [DOI] [PubMed]
- 15.Ford ES, Galuska DA, Gillespie C, Will JC, Giles WH, Dietz WH. C-reactive protein and body mass index in children: findings from the Third National Health and Nutrition Examination Survey, 1988–1994. J Pediatr. 2001;138(4):486–492. doi: 10.1067/mpd.2001.112898. [DOI] [PubMed] [Google Scholar]
- 16.Ford ES. C-reactive protein concentration and cardiovascular disease risk factors in children: findings from the National Health and Nutrition Examination Survey 1999–2000. Circulation. 2003;108(9):1053–1058. doi: 10.1161/01.CIR.0000080913.81393.B8. [DOI] [PubMed] [Google Scholar]
- 17.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295(13):1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 18.National Center for Health Statistics. NHANES General Data Release Documentation. Hyattsville, MD: National Center for Health Statistics; 2008. [Google Scholar]
- 19.National Center for Health Statistics. Laboratory Procedures Manual. Hyattsville, MD: National Center for Health Statistics; 2000. [Google Scholar]
- 20.Ridker PM. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation. 2003;107(3):363–369. doi: 10.1161/01.cir.0000053730.47739.3c. [DOI] [PubMed] [Google Scholar]
- 21.Madjid M, Awan I, Willerson JT, Casscells SW. Leukocyte count and coronary heart disease: implications for risk assessment. J Am Coll Cardiol. 2004;44(10):1945–1956. doi: 10.1016/j.jacc.2004.07.056. [DOI] [PubMed] [Google Scholar]
- 22.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340(6):448–454. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 23.Gallin JI, Snyderman R, editors. Inflammation: Basic Principles and Clinical Correlates. Philadelphia, PA: Lippincott, Williams & Wilkins; 1999. [Google Scholar]
- 24.National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention. A SAS Program for the CDC Growth Charts. Atlanta, GA: Centers for Disease Control and Prevention; 2005. [Google Scholar]
- 25.Barlow SE. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007;120 (suppl 4):S164–S192. doi: 10.1542/peds.2007-2329C. [DOI] [PubMed] [Google Scholar]
- 26.Kuczmarski R, Ogden C, Guo S, et al. 2000 CDC growth charts for the United States: methods and development. Vital Health Stat 11. 2002;246:1–190. [PubMed] [Google Scholar]
- 27.van’t Hof MA, Haschke F. Euro-Growth references for body mass index and weight for length. Euro-Growth Study Group. J Pediatr Gastroenterol Nutr. 2000;31(suppl 1):S48–S59. doi: 10.1097/00005176-200007001-00005. [DOI] [PubMed] [Google Scholar]
- 28.World Health Organization. Worldwide Prevalence of Anaemia 1993–2005. Geneva, Switzerland: World Health Organization; 2008. [Google Scholar]
- 29.Haarala A, Eklund C, Pessi T, et al. Use of combined oral contraceptives alters metabolic determinants and genetic regulation of C-reactive protein. The Cardiovascular Risk in Young Finns Study. Scand J Clin Lab Invest. 2009;69(2):168–174. doi: 10.1080/00365510802449642. [DOI] [PubMed] [Google Scholar]
- 30.Jarvis MJ, Fidler J, Mindell J, Feyerabend C, West R. Assessing smoking status in children, adolescents and adults: cotinine cut-points revisited. Addiction. 2008;103(9):1553–1561. doi: 10.1111/j.1360-0443.2008.02297.x. [DOI] [PubMed] [Google Scholar]
- 31.Yanbaeva DG, Dentener MA, Creutzberg EC, Wesseling G, Wouters EF. Systemic effects of smoking. Chest. 2007;131(5):1557–1566. doi: 10.1378/chest.06-2179. [DOI] [PubMed] [Google Scholar]
- 32.Perneger TV. What’s wrong with Bonferroni adjustments. BMJ. 1998;316(7139):1236–1238. doi: 10.1136/bmj.316.7139.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barros AJ, Hirakata VN. Alternatives for logistic regression in cross-sectional studies: an empirical comparison of models that directly estimate the prevalence ratio. BMC Med Res Methodol. 2003;3:21. doi: 10.1186/1471-2288-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reinehr T, Kiess W, de Sousa G, Stoffel-Wagner B, Wunsch R. Intima media thickness in childhood obesity: relations to inflammatory marker, glucose metabolism, and blood pressure. Metabolism. 2006;55(1):113–118. doi: 10.1016/j.metabol.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 35.Ford ES, Giles WH, Myers GL, Rifai N, Ridker PM, Mannino DM. C-reactive protein concentration distribution among US children and young adults: findings from the National Health and Nutrition Examination Survey, 1999–2000. Clin Chem. 2003;49(8):1353–1357. doi: 10.1373/49.8.1353. [DOI] [PubMed] [Google Scholar]
- 36.Akinci G, Akinci B, Coskun S, Bayindir P, Hekimsoy Z, Ozmen B. Evaluation of markers of inflammation, insulin resistance and endothelial dysfunction in children at risk for overweight. Hormones (Athens) 2008;7(2):156–162. doi: 10.1007/BF03401507. [DOI] [PubMed] [Google Scholar]
- 37.Wärnberg J, Nova E, Romeo J, Moreno LA, Sjostrom M, Marcos A. Lifestyle-related determinants of inflammation in adolescence. Br J Nutr. 2007;98(suppl 1):S116–S120. doi: 10.1017/S0007114507839614. [DOI] [PubMed] [Google Scholar]
- 38.Moran A, Jacobs DR, Jr, Steinberger J, et al. Insulin resistance during puberty: results from clamp studies in 357 children. Diabetes. 1999;48(10):2039–2044. doi: 10.2337/diabetes.48.10.2039. [DOI] [PubMed] [Google Scholar]
- 39.Hackam DG, Anand SS. Emerging risk factors for atherosclerotic vascular disease: a critical review of the evidence. JAMA. 2003;290(7):932–940. doi: 10.1001/jama.290.7.932. [DOI] [PubMed] [Google Scholar]
- 40.Wang TJ, Gona P, Larson MG, et al. Multiple biomarkers for the prediction of first major cardiovascular events and death. N Engl J Med. 2006;355(25):2631–2639. doi: 10.1056/NEJMoa055373. [DOI] [PubMed] [Google Scholar]
- 41.Zethelius B, Berglund L, Sundstrom J, et al. Use of multiple biomarkers to improve the prediction of death from cardiovascular causes. N Engl J Med. 2008;358(20):2107–2116. doi: 10.1056/NEJMoa0707064. [DOI] [PubMed] [Google Scholar]
- 42.Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359(21):2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 43.Egger G, Dixon J. Should obesity be the main game? Or do we need an environmental makeover to combat the inflammatory and chronic disease epidemics? Obes Rev. 2009;10(2):237–249. doi: 10.1111/j.1467-789X.2008.00542.x. [DOI] [PubMed] [Google Scholar]