Abstract
This paper provides a conceptual analysis of the endophenotype (EP) construct that is having an increasing role in genetic strategies for unraveling the etiology of psychiatric disorders (PDs). We make six major points illustrated through the method of path analysis. First, it is important to distinguish between mediational and liability-index (or ‘risk indicator’) models for EP, as only the former requires genetic risk for PD to pass through EP. Second, the relative reliability of EP and PD can have a critical role in the interpretation of results. Ignoring them can lead to substantial errors of inference. Third, we need to consider bidirectional relationships between an EP and a PD, and the possibility that genetic effects on PD are only partially mediated by EP. Fourth, EP models typically assume that all genetic effects that have an impact on EP also alter risk for PD. However, among the genetic influences on EP and PD, it is also plausible that some will influence only EP, some only PD and some both. Fifth, we should also consider models incorporating multiple EPs and PDs, which can be well captured by multivariate genetic methods. Sixth, EPs may also reflect the impact of the environment on risk for PDs. The EP concept has important potential lessons for etiological research in PDs that can be optimized by considering it as a special case of a broader set of multivariate genetic models, which can be fitted using currently available methodology.
Keywords: EP, intermediate phenotype, genetics, models of disease, psychiatric illness
Introduction
The term endophenotype (EP) (and its approximate synonym intermediate phenotype) has been used extensively in recent discussions about genetic strategies for unraveling the etiology of psychiatric and substance use disorders.1,1–3 In this report, we attempt to provide a conceptual analysis of this construct, to identify areas of ambiguity in the previous literature and to show how it can be more clearly articulated using methods long familiar to statistical genetics. Our conceptual vehicle is structural equation modeling. Although the EP concept was originally postulated at a time when the dominant model of genetic etiology of psychiatric disorders (PDs) were single genes of moderate to large effect, the underlying logic could be equally applied to multifactorial twin and adoption studies, or linkage or association paradigms seeking to detect genes of moderate to small effect size.
Two concerns have fueled the recent interest in EPs in psychiatric genetics. First, our ‘exophenotypes’— PDs—are seen to be very complex from an etiological and genetic perspective. In seeking to follow Henry David Thoreau’s maxim of ‘simplify, simplify,’ the hope is that by studying EPs we will simplify our problems. Second, PDs seem to be a very long way from DNA variation. The number of physiological steps from a variant in a base pair sequence to a PD, such as schizophrenia, depression or alcohol dependence, is likely very large indeed. In studying EPs, we hope to ‘move closer’ to the ‘DNA level.’ For interest, Table 1 lists three representative definitions of the EP construct by Gottesman and Gould,2 Preston and Weinberger,3 and Cannon and Keller.1
Table 1.
Three recent definitions of endophenotype
Gottesman and Gould2
|
|
Preston and Weinberger3 An intermediate phenotype (often referred to as an endophenotype) is a quantitative biological trait that is reliable and reasonably heritable, i.e., shows greater prevalence in unaffected relatives of patients than in the general population. … If a candidate intermediate phenotype is to provide meaningful information about a disorder, it should be associated with variant alleles that distinguish patients and their unaffected siblings from healthy controls on quantitative measures… The intensive search for such candidates is based in part on (the) … assumption that intermediate phenotypes in schizophrenia (reflect) … a less complex genetic architecture than the disorder as a whole. |
Cannon and Keller1
|
Risk indicator or mediating variable
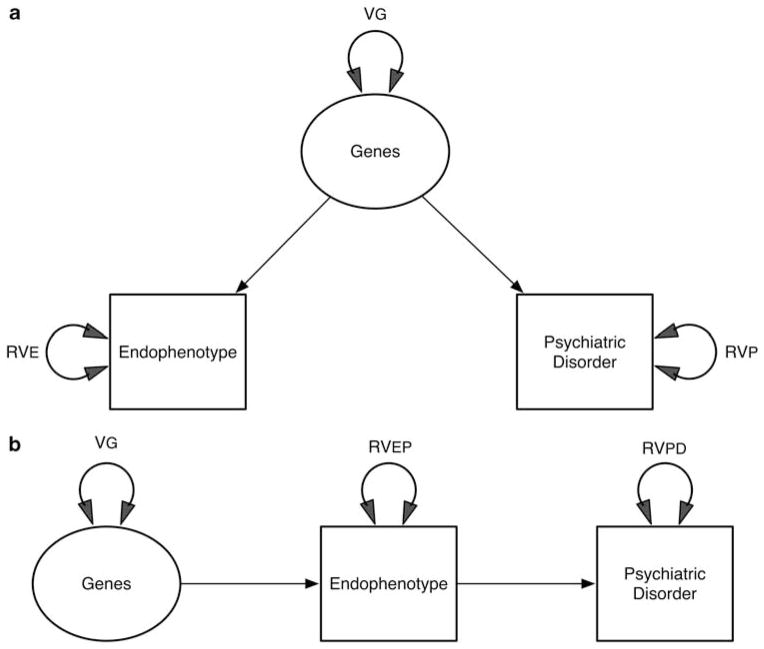
An immediate concern that arises in reviewing these definitions of EP, as pointed out previously by Walters and Owen,4 is that they do not discriminate between a ‘liability-index’ and a ‘mediational’ model for EPs.5 For the ‘liability-index’ model, Walters and Owen use the somewhat derogatory term ‘epiphenomenon.’ Those who prefer the term intermediate phenotype3 implicitly assume a mediational model but rarely state this explicitly. As seen in Figure 1a, an EP liability-index (or ‘risk indicator’) model specifies that a common set of genes increase risk both for a dichotomous PD and for a continuous EP. It is in essence a model of pleiotropy in which one set of genetic variants causes variation in both EP and disease risk. Figure 1b shows the mediational model for EP, which makes the stronger assumption that the causal pathway from genetic variations to PD passes exclusively through EP.
Figure 1.
(a) A liability-index model for endophenotypes (EPs). Genetic variance VG influences both an EP and a psychiatric disorder (PD). These observed variables also have residual variation, RVEP and RVPD, due to other sources. (b) A mediational model for EPs. Genetic variance causes variation in the EP, which in turn causes variation in PD. EP and PD have residual variance components RVEP and RVPD, respectively.
Figure 1b is equivalent to a standard mediational model as commonly articulated in the psychological literature.6 Both these models generate the pattern of findings required by most of the criteria listed in Table 1. For example, both the liability-index and the mediational model would generate data that satisfy both criteria 4 (cosegregate with illness in families) and 5 (found at increased rates in unaffected family members) of Gottesman and Gould.2 Furthermore, both the liability-index and the mediational models would be useful in a developmental context. In both models, unaffected individuals with high scores on EP would be predicted to be at elevated risk for the development of PD.
The mediational model predicts that the causal processes captured by the ‘genes’ to ‘EP’ path are shared between EP and PD. The liability-index model does not make this assumption. Most importantly, the mediational model predicts that if it were possible to change EP with an exogenous variable (for example, a cognitive or pharmacological treatment), doing so would result in a decline in risk for PD. The risk-indicator model makes no such prediction.
This critical difference between these two models can perhaps be best illustrated by the different predictions they would make about the target of treatment of unaffected individuals at high risk of PD because of elevated EP scores. The mediational model would suggest that treatments could be targeted toward reducing levels of EP with the conviction that a lowering of disease risk would necessarily follow. The risk-indicator model, by contrast, would indicate that treatments should be directed toward correcting the underlying genetic liability, as there would be no guarantee that interventions that reduced EP would prevent illness onset.
Although the distinction between the liability-index and mediational models for EP is conceptually important, it is devilishly difficult in humans to design experiments to discriminate powerfully between them. Rarely can we directly intervene on EP and determine whether risk for PD is altered. Longitudinal and twin models can potentially discriminate between causal and noncausal relationships between an EP and a PD,7,8 but sample sizes sufficient to allow for powerful discrimination may be hard to achieve, especially with relatively rare PDs and/or expensive EPs.9 Developmental studies in which interventions directly on EP in unaffected individuals can be tested to see whether they reduce disease risk would be another way to test the mediational model.
In one study using longitudinal twin data, we have explicitly tested the mediational versus liability-index model for the relationship between the personality trait of neuroticism as EP and major depression as PD.10 Surprisingly, we could reject the mediational model in favor of the liability-index model. Although neuroticism substantially reflected the genetic risk to major depression, we could reject the model in which the pathway from genes to major depression passed exclusively through neuroticism.
Impact of measurement error
An inherent appeal of EP concept is that a ‘good’ EP should be closer to the ‘level of gene action’ than the relevant PD. This concept should translate into the empirical observation that the genetic effects on EP should be stronger than on PD. In their recent review, Flint and Munafo11 state this explicitly
Much effort has been devoted to finding such endophenotypes, partly because it is believed that the genetic basis of endophenotypes will be easier to analyze than that of psychiatric disease. This belief depends in part on the assumption that the effect sizes of genetic loci contributing to endophenotypes are larger than those contributing to disease susceptibility, hence increasing the chance that genetic linkage and association tests will detect them.
We can best illustrate this point using path models. Assume, for example, the mediational model for EP as depicted in Figure 1b. As pictured, as long as EP is not perfectly correlated with PD (that is, the standardized path from EP to PD is < |1.00|), it is logically necessary that the genetic effect will be stronger on EP than on PD. However, this model ignores measurement error.
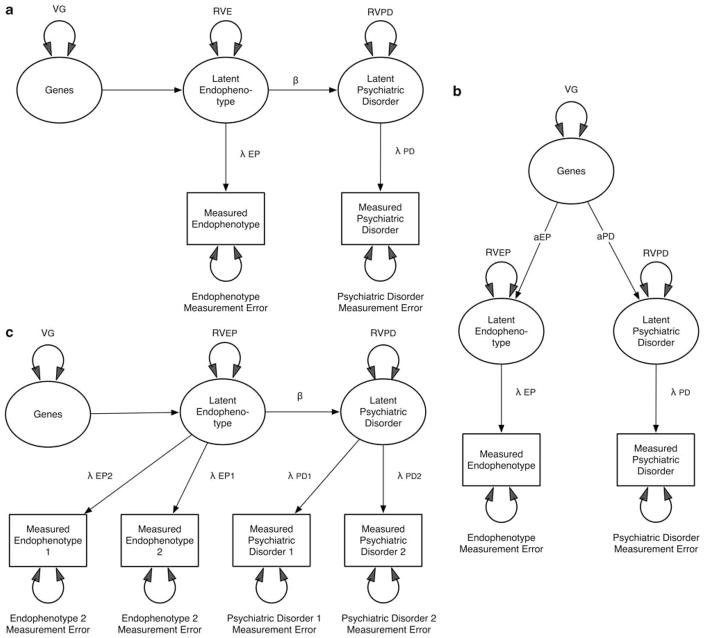
Figure 2a represents a more realistic model incorporating errors of measurement. This figure depicts a mediational model for EP that includes a path from the latent EP to the latent PD (β) and paths from these two latent constructs to the observed constructs, which reflect the accuracy with which they are assessed (λEP and λPD, respectively). (Latent here refers to an unmeasured ‘true’ construct and is traditionally depicted in path diagrams by circles or ovals, whereas measured variables are depicted in squares or rectangles.) Simple algebra allows us to conclude that the genetic effect will be stronger for EP than PD whenever λEP < βλPD. Figure 2b depicts the risk-indicator model for EP, which has a direct path from genes to the latent EP (βEP) and from genes to PD (βPD). For this model, the genetic effects on EP will exceed those on PD whenever aEPλEP > aPDλPD.
Figure 2.
(a) A mediational model for endophenotypes (EPs) including errors of measurement. The residual variance components of EP and psychiatric disorder (PD) are partitioned into reliable and measurement error variance. (b) A liability-index model for EPs including errors of measurement. The residual variance components of EP and PD are partitioned into reliable and measurement error variance. (c) A liability-index model for EPs including two assessments at different times and errors of measurement.
As EPs are often measured using sophisticated imaging, neurophysiological or neuropsychological measures, there is a tendency to assume that such ‘harder’ measures are, of necessity, more reliable than the ‘softer’ psychiatric diagnoses. That is, we commonly assume that λEP exceeds λPD. However, this assumption may be incorrect. Many putative EPs are measured over short time intervals and can be influenced by transient state effects such as ambient noise, temperature, time of day, or variations in machine functioning—as well as temporary changes in mental state of the participant due to stressors, or consumption of or withdrawal from nicotine, caffeine or alcohol. By contrast, some PDs are assessed using years of medical records and the recording of symptoms occurring over similar periods, or with carefully constructed psychological instruments. For example, Gur et al.12 report that the 1-year stability for a commonly used measure of the Continuous Performance Test was ‘found to be 0.65 for schizophrenia patients and 0.72 for healthy subjects,’ whereas another such measure had stability over 2 years, which ranged from 0.56 to 0.73. The reliability of brain functional magnetic resonance imaging (fMRI) is quite variable and for some paradigms is under +0.30.13–15 However, other EPs, such as structural MRI, might be highly reliable as indicated by a recent report showing test–retest correlations > +0.95 for measures of cortical thickness.16 By contrast, in the Irish Study of High-Density Schizophrenia Families, which used both in-depth personal interviews and extensive reviews of hospital records, the diagnostic reliability, assessed using a weighted κ, was +0.94.17 Conversely, in the Virginia Adult Twin Study of Psychiatric and Substance Use Disorders, the long-term stability of an interview-based assessment of lifetime major depression was κ = +0.43,18 considerably lower than the reliability of the short form of the Eysenck’s neuroticism scale (r = +0.69) which has been proposed as an EP for MD—measured over a comparable time period. The major point here is that the relative ‘performance’ of EPs versus PD in assessing a ‘genetic signal’ cannot be divorced from the problems of measurement. It is perfectly possible for us to study an EP that is ‘truly’ closer to gene action than our PD. But if our measures of EP are less reliable than those of our PD, unless we account for this unreliability in our models, we would get the wrong answer—that EP cannot be sitting in the causal path to our PD.
There are several ways by which this problem can be approached. One is to obtain good measures of λEP and λPD that could be incorporated into the analytic models. This might be carried out by obtaining test–retest reliability in a subset of the studied sample. An even more powerful approach would be to obtain longitudinal measures of EP and PD in the entire sample. Then, making the reasonable assumption that the genetic risks for them were temporally stable, the model depicted in Figure 2c could be applied. In this model, the estimates of β are now unconfounded from those of λEP and λPD. Such data would also now be much more likely to be able to discriminate the risk indicator from the mediating variable model for EP. A third possibility is to use data collected from pairs of relatives, which can also potentially discriminate risk indicator from mediational models.
Direction of causation between EP and PD
Our treatment of the mediating variable EP model above assumed that the causal relationship between an EP and a PD all flowed in one direction, EP → PD. Is this a realistic assumption? Take the well-studied association between attentional difficulties and risk for schizophrenia. Although having attentional difficulties might contribute to risk for schizophrenia, the state of schizophrenic psychosis might itself also impair attention. Thus, in this instance, the association between EP and PD may be sometimes bidirectional (EP↔PD) rather than unidirectional (EP → PD). Furthermore, the ‘return’ path from EP to PD might vary as a function of clinical state, being stronger during times of acute symptoms and weaker in times of remission.
Complete versus partial mediation
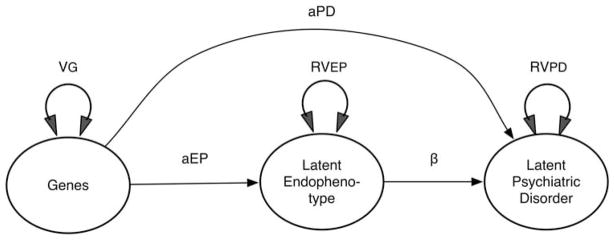
The mediational model for EPs depicted in Figure 1b assumes that all the genetic risk factor for PD operates through EP. This model may be less realistic than the partial mediational one depicted in Figure 3. In this partial mediation model, some of the genetic effect on PD is mediated through EP but some bypasses EP to have a direct impact on PD. This scenario would provide another possible explanation for the not uncommon observation that the genetic influence on EP (be it an odds ratio in an association design or heritability from a twin study) is less than that on PD. For the present, ignoring problems of reliability of measurement, we can see from Figure 3 that the genetic signal for EP will exceed that for PD only when aEP > (aPD+aEPβ+2aPDaEPβ).
Figure 3.
A nonexclusive mediational model for endophenotypes (EPs) including a direct causal path from genes to the psychiatric disorder (aPD) that does not pass through EP.
EPs and PDs as a bivariate problem
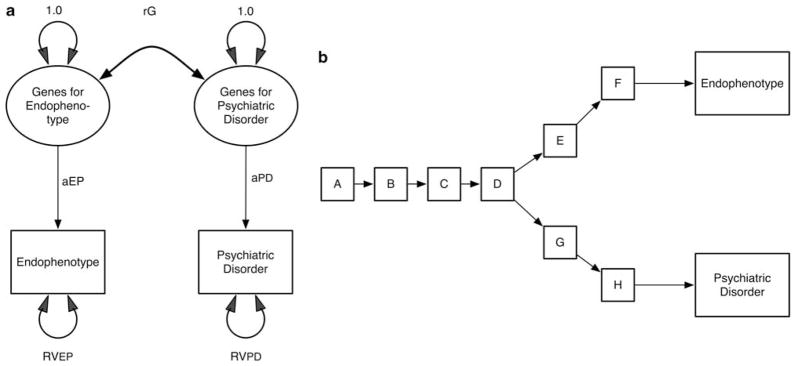
The model in Figure 3 assumes that all genetic effects that have an impact on EP either directly or indirectly affect PD. This is probably an unrealistic assumption as well. Rather, it is more likely that, among all the possible genetic effects on EP and PD, some will influence only EP, others only PD and yet others will influence both. This kind of relationship is well captured in quantitative genetics in a standard bivariate correlated liability model,19 which can be understood as an expanded version of the ‘liability-index’ model for EP (Figure 4a). Such a model assumes two sets of genetic risk factors: one for EP and one for PD. The magnitude of the association between these two sets of risk factors is captured by the genetic correlation (rg). The correlation will be lowered both by genes that affect EP but not PD, and genes that affect PD but not EP.
Figure 4.
(a) A bivariate model for genetic risk factors for an endophenotype (EP) and a psychiatric disorder (PD). The correlation between them is represented by rg. (b) A conceptual bivariate model for genetic risk factors for an EP and a PD. One biological pathway splits into two, with one path leading to EP and the other to PD. Genetic factors may influence each of the steps of the pathway.
For readers of a more biological bent, it might help to illustrate one possible scenario that would be described by Figure 4a. As seen in Figure 4b, imagine we had a shared pathophysiological pathway from A to D, with each step having its own genetic influences. Then one etiological pathway goes in one direction through steps E and F and influences EP. The other direction, which includes sets G and H, leads to PD. In this case, EP and PD would share genetic influences on A, B, C and D, but have unique genetic influences that affect stages E, F, G and H.
As rg approaches 1.0, Figure 4a becomes equivalent to Figure 1a (and this becomes equivalent to the paths for EP and PD coming both from step D in Figure 4b). Obviously, the interest and utility of an EP for a given PD would relate to the value of rg. If rg were quite low, measuring EP would tell you relatively little about the genetic risk for PD.
It is also possible to expand the mediational model for EP in a similar manner but we will not do that here.
EPs as a multivariate problem
Many EPs have been proposed for individual PDs, and Preston and Weinberger3 (Figure 1 in their work) specifically assume that each of the multiple EPs for schizophrenia will reflect somewhat different aspects of the genetic risk for the disorder. Thus, the models that we have presented hitherto, focusing on a single EP and a single PD, are likely too simplistic.
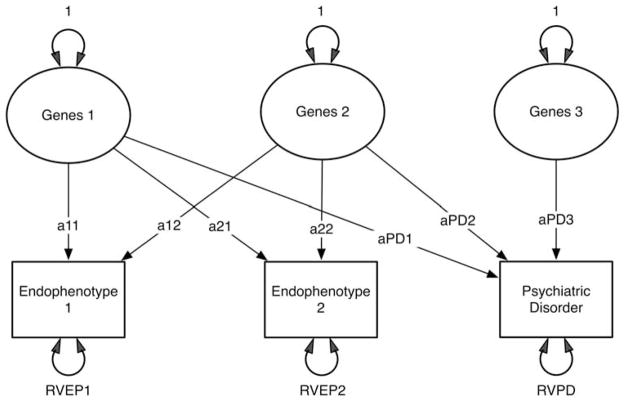
Assuming EPs as risk indicators, we illustrate in Figure 5 one such possible model. This model includes three sets of genetic risk factors (which we assume for simplicity are uncorrelated), two EPs (EP1 and EP2) and one PD. We further assume that the third genetic risk factor affects only PD. The paths from these sets of genes to EPs and PD are identified by path names so that a11 reflects the path from the first risk factor to the first EP, and path aPD2 reflects the path from the second genetic risk factor to PD. Finally, imagine a situation in which paths a12 and a21 are very low. In such a situation, you would expect (at least from a genetic perspective) that the correlation between the scores on EP1 and EP2 would be quite low. However, both could be valuable and complementary from a research perspective. That is, if paths a11 and aPD1 are high, then EP1 will function as a good risk indicator for the first genetic risk factor. If paths a22 and aPD2 are high, then EP2 will function as a good risk indicator for the second genetic risk factor. Thus, in this situation, each EP indexes a different set of risk genes for PD.
Figure 5.
A model incorporating three genetic risk factors, two endophenotypes and one psychiatric disorder. Residual variation, specific to each of these three phenotypes, is shown as RVEP1, RVEP2 and RVPD.
As should be clear by now, the degree to which the individual phenotypes reflect different sets of risk factors is a function of the magnitude of the different paths. For example, unlike the above situation, if paths a11 and a12, and paths a21 and a22 are similar in magnitude, then EP1 and EP2 will be substantially correlated and they will be nonspecific, indexing in a similar manner the first and second genetic factor. A model analogous to Figure 5 could be developed that assumed a mediational model for EPs. It would be considerably more complex and will not be presented here.
EPs and environmental risk factors
Hitherto, nearly all treatments in the literature as well as our models have assumed that EPs ought to reflect genetic risk factors for PD. In fact, it has been seen as a problem that EPs might reflect environmental etiological factors. However, just as EPs might help researchers index genetic risk factors, the same logic can be applied to environmental risk factors. That is, a researcher might be interested in finding EPs that provide more useful indexes of environmental factors. An example would be finding a neuroanatomic consequence of the teratogenic effects of maternal substance use, which in turn affects adolescent antisocial behavior. This EP could prove useful in identifying specific substances that have more distal effects on the antisocial behavior outcome. All the points made above to ‘genetic EPs’ would apply equally to such ‘environmental EPs.’ For example, EP might index only the liability or it might mediate the pathway from the environmental trauma to the disorder.
To make matters more complex, it is perfectly possible that certain EPs reflect both the genetic and the environmental risk factors for a disorder. For example, the cognitive abnormalities considered to be an EP for schizophrenia are likely influenced both by genetic risk factors for schizophrenia, as well as environmental risk factors such as famine,20 in utero viral infections21 or birth complications.22
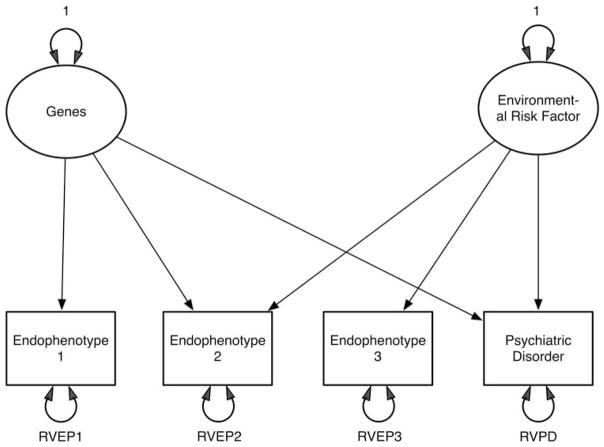
We present in Figure 6a a highly simplified version of such a model, which contains one set of genetic risk factors, one set of environmental risk factors, three EPs and a PD. In the model, EP1 reflects only the genetic risk factors and EP3 only the environmental risk factors. However, both sets of risk factors affect EP2. More complex models could easily be conceptualized. In particular, it seems likely that there exist joint models in which there are both shared risk factors and direct causal pathways between EPs and PDs. Cross-sectional data from relatives are generally not sufficient to identify all the parameters of such models, but there is the potential to do so with longitudinal studies of related individuals.
Figure 6.
A model incorporating one set each of genetic and environmental risk factors, three endophenotypes and one psychiatric disorder. Residual variation (RV) components are shown for all four phenotypes.
Discussion
This paper sought to elucidate the construct of EP and to place it in the context of other efforts in genetic epidemiology and statistical genetics to understand the causes of PDs. In so doing, we hope to have clarified certain issues about the nature of EPs and how their action can be best understood. We wish to emphasize five major points in this discussion.
First, the field has paid insufficient attention to the potential causal claims surrounding EPs. A mediational model for EPs is a stronger scientific claim than a liability-index model. It is more falsifiable and hence, from a Popperian perspective, a ‘stronger’ hypothesis. However, because it contains causal claims, it is more difficult to test, especially in a nonexperimental setting. It is not an accident that models in this paper have largely used the simpler but less informative liability-index model. Walters and Owen4 would save the term EP solely for variables fitting our mediational model and would use a new term like ‘biomarker’ for risk-indicator variables. Yet it is difficult in humans to actually discriminate between liability-index and mediational models, especially when joint models (genetic factors operate directly on both EP and PD but there is also a causal path from EP to PD) are plausible alternatives. Whether an alternative term such as biomarker is warranted for genetic correlates of a PD remains a matter of debate.
Second, measurement error is generally conceived of as a ‘nuisance’ variable, which is rarely visible on the radar screen of researchers. However, the models presented above illustrate that researchers using an EP strategy ignore measurement error at their peril. The wrong answer can be obtained about the nature of EP–PD relationship if such error is not properly modeled.
Third, EP models have, to date, been relatively restricted and have incorporated what are likely to be unrealistic assumptions such as all the genetic influences on EP will affect risk for PD, and vice versa, or that environmental risk factors for PD will have no impact on EP. As this article suggests, EPs are probably best considered as part of a family of bi- and multivariate models that can be well captured by a range of developments in statistical genetics.
Fourth, our models illustrate some of the complexity inherent in comparing the predictive power of an EP and PD. EP have one important starting advantage in which they are typically quantitative, thereby providing more information than the dichotomous diagnostic assessment used for PD. This is especially true for relatively rare PDs like schizophrenia, bipolar illness or anorexia nervosa in which being unaffected is rather uninformative, only indicating that the individual is somewhere in the lower 99% of risk in the population. But the predictive power of EP will be attenuated when it is measured with low reliability, if the genetic risk factors of EP are only partially correlated with those of PD, or if EP is reflecting only a subset of the risk genes.
Finally, our results have potential implications for the optimal ways in which studies of EPs and PDs can be conducted. For example, given awareness of the problems of error, researchers might modify their protocols to improve the reliability of their EPs by including either multiple ratings or multiple measurements over time. Both strategies have been shown to substantially increase the heritability for PDs that are assessed with significant error.18,23 Furthermore, multiple measurements, longitudinal designs and/or the incorporation of specific risk genes in statistical models, have the capacity to clarify the causal interrelationships for EPs and PDs.24
Acknowledgments
This work was supported in part by Grants AA-011408, MH-068643, DA-011287 and DA-18673.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Cannon TD, Keller MC. Endophenotypes in the genetic analyses of mental disorders. Annu Rev Clin Psychol. 2006;2:267–290. doi: 10.1146/annurev.clinpsy.2.022305.095232. [DOI] [PubMed] [Google Scholar]
- 2.Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- 3.Preston GA, Weinberger DR. Intermediate phenotypes in schizophrenia: a selective review. Dialogues Clin Neurosci. 2005;7:165–179. doi: 10.31887/DCNS.2005.7.2/gpreston. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walters JT, Owen MJ. Endophenotypes in psychiatric genetics. Mol Psychiatry. 2007;12:886–890. doi: 10.1038/sj.mp.4002068. [DOI] [PubMed] [Google Scholar]
- 5.Kendler KS, Prescott CA. Genes, Environment, and Psychopathology: Understanding the Causes of Psychiatric and Substance Use Disorders. 1. Guilford Press; New York: 2006. [Google Scholar]
- 6.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 7.Neale MC, Kendler KS. Models of comorbidity for multifactorial disorders. Am J Hum Genet. 1995;57:935–953. [PMC free article] [PubMed] [Google Scholar]
- 8.Heath AC, Kessler RC, Neale MC, Hewitt JK, Eaves LJ, Kendler KS. Testing hypotheses about direction of causation using crosssectional family data. Behav Genet. 1993;23:29–50. doi: 10.1007/BF01067552. [DOI] [PubMed] [Google Scholar]
- 9.Rhee SH, Hewitt JK, Lessem JM, Stallings MC, Corley RP, Neale MC. The validity of the Neale and Kendler model-fitting approach in examining the etiology of comorbidity. Behav Genet. 2004;34:251–265. doi: 10.1023/B:BEGE.0000017871.87431.2a. [DOI] [PubMed] [Google Scholar]
- 10.Kendler KS, Neale MC, Kessler RC, Heath AC, Eaves LJ. A longitudinal twin study of personality and major depression in women. Arch Gen Psychiatry. 1993;50:853–862. doi: 10.1001/archpsyc.1993.01820230023002. [DOI] [PubMed] [Google Scholar]
- 11.Flint J, Munafo MR. The endophenotype concept in psychiatric genetics. Psychol Med. 2007;37:163–180. doi: 10.1017/S0033291706008750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gur RE, Calkins ME, Gur RC, Horan WP, Neuchterlein KK, Seidman LJ, et al. The Consortium on the Genetics of Schizophrenia: neurocognitive endophenotypes. Schizophr Bull. 2007;33:49–68. doi: 10.1093/schbul/sbl055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clement F, Belleville S. Test-retest reliability of fMRI verbal episodic memory paradigms in healthy older adults and in persons with mild cognitive impairment. Hum Brain Mapp. 2009;30:4033–4047. doi: 10.1002/hbm.20827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kong J, Gollub RL, Webb JM, Kong JT, Vangel MG, Kwong K. Test-retest study of fMRI signal change evoked by electroacupuncture stimulation. Neuroimage. 2007;34:1171–1181. doi: 10.1016/j.neuroimage.2006.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aron AR, Gluck MA, Poldrack RA. Long-term test-retest reliability of functional MRI in a classification learning task. Neuroimage. 2006;29:1000–1006. doi: 10.1016/j.neuroimage.2005.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wonderlick JS, Ziegler DA, Hosseini-Varnamkhasti P, Locascio JJ, Bakkour A, van der Kouwe A, et al. Reliability of MRI-derived cortical and subcortical morphometric measures: effects of pulse sequence, voxel geometry, and parallel imaging. Neuroimage. 2009;44:1324–1333. doi: 10.1016/j.neuroimage.2008.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kendler KS, O’Neill FA, Burke J, Murphy B, Duke F, Straub RE, et al. Irish study on high-density schizophrenia families: field methods and power to detect linkage. Am J Med Genet. 1996;67:179–190. doi: 10.1002/(SICI)1096-8628(19960409)67:2<179::AID-AJMG8>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 18.Foley DL, Neale MC, Kendler KS. Reliability of a lifetime history of major depression: implications for heritability and co-morbidity. Psychol Med. 1998;28:857–870. doi: 10.1017/s0033291798006977. [DOI] [PubMed] [Google Scholar]
- 19.Neale MC, Kendler KS. Models of comorbidity for multifactorial disorders. Am J Hum Genet. 1995;57:935–953. [PMC free article] [PubMed] [Google Scholar]
- 20.Susser E, Neugebauer R, Hoek HW, Brown AS, Lin S, Labovitz D, et al. Schizophrenia after prenatal famine. Further evidence. Arch Gen Psychiatry. 1996;53:25–31. doi: 10.1001/archpsyc.1996.01830010027005. [DOI] [PubMed] [Google Scholar]
- 21.Mednick SA, Machon RA, Huttunen MO, Bonett D. Adult schizophrenia following prenatal exposure to an influenza epidemic. Arch Gen Psychiatry. 1988;45:189–192. doi: 10.1001/archpsyc.1988.01800260109013. [DOI] [PubMed] [Google Scholar]
- 22.Jones PB, Rantakallio P, Hartikainen AL, Isohanni M, Sipila P. Schizophrenia as a long-term outcome of pregnancy, delivery, and perinatal complications: a 28-year follow-up of the 1966 north Finland general population birth cohort. Am J Psychiatry. 1998;155:355–364. doi: 10.1176/ajp.155.3.355. [DOI] [PubMed] [Google Scholar]
- 23.Kendler KS, Prescott CA, Jacobson K, Myers J, Neale MC. The joint analysis of personal interview and family history diagnoses: evidence for validity of diagnosis and increased heritability estimates. Psychol Med. 2002;32:829–842. doi: 10.1017/s0033291702005858. [DOI] [PubMed] [Google Scholar]
- 24.van den Oord EJ, Snieder H. Including measured genotypes in statistical models to study the interplay of multiple factors affecting complex traits. Behav Genet. 2002;32:1–22. doi: 10.1023/a:1014474711118. [DOI] [PubMed] [Google Scholar]