Abstract
Diabetes around the globe results in one major limb amputation every 30 seconds, over 2500 limbs lost per day. The underlying pathophysiology sometimes leads to a chronic inflammatory stage, which may prevent appropriate healing, and therefore, the need for a clear strategy for assessing and classifying wounds and wound healing cannot be overstated. Temperature is a surrogate marker for inflammation. Quantitative thermography using a numerical index provides a useful way to assess wound healing. Advances in technology have afforded the availability of low-cost, high-resolution thermal imaging systems, which can be used to quantify sensitive changes on the skin surface and may be particularly useful to develop monitoring strategies for wounds. This article provides a standardized technique for calculating a thermal index (TI) supported with a case report from assessment of a diabetic foot ulcer. In this single case study, the TI/wound inflammatory index indicates a shift from negative to positive (p < .05) before it reaches zero.
Keywords: diabetic foot ulcers, thermal index, thermography, thermometry, wound healing
Introduction
The development of lower extremity complications of diabetes, commonly called diabetic foot disease, is associated with increased morbidity and mortality.1,2 The relationship between diabetes and lower extremity amputations is well recognized. Diabetes patients are prone to develop lower extremity ulcerations and infections, both of which serve as major risk factors for lower extremity amputations.3,4 Studies have demonstrated that the five-year mortality rate in patients with diabetes following major amputation is significant—even greater than many major forms of cancer.5 Diabetic foot amputations have been compared to landmine-related amputations. This intriguing comparison emphasizes the silent nature of the “warfare” and the sinister consequences on the life of patients/victims. Diabetes around the globe results in one major limb amputation every 30 seconds, over 2500 limbs lost per day.6
The incidence of diabetic foot disease is growing worldwide, leading to the increased socioeconomic burden on health care systems of both developed and developing nations.7–10 Several studies have suggested prevention of foot ulcers by identifying individuals at high risk and treating for lower extremity complications.11–13
Peripheral neuropathy is the most important causal component in persons with diabetes, leading to foot ulcers and traumatic amputations.14 Due to loss of sensation under the sole of the foot, patients do not recognize an injury until full thickness ulcerations occur. The assessment of diabetic foot ulcers is therefore a complex and challenging process. A consensus statement release recommends clear methodologies for assessing and classifying a wound ulcer.3 The variables associated with classifying wounds include compliance issues with orthotic or pharmacological interventions, quality of wound granulation tissue, host immunity, clinical signs of infection, peripheral vascular disease, nutritional status, and any existing comorbidities.4 Three well-documented risk factors associated with wound assessment as well as wound healing include the depth of the wound, presence of infection, and presence of concomitant ischemia.4,15,16 From a clinical as well as research standpoint, other supplementary details such as location of the wound, wound size trajectory, base of the wound, wound temperature, thermal patterns and wound margins may also be important.
Temperature Measurement in Diabetic Foot Ulcers
Research in this area has focused on developing simple and robust tools to quantify the risk factors such as inflammation, presence of infection, pain, and wound characteristics. Inflammation is a potential marker of diabetic foot ulcers and can be easily identified by temperature assessments of the affected limbs. Bharara and colleagues12 and Roback and associates17 have reviewed various thermological techniques relevant to the diabetic foot disease and emphasize the importance of using thermo-metry for lower extremities as a tool for supplementary evidence of neuropathy. Armstrong et al.18 have, on the other hand, shown skin temperature monitoring to reduce risk of diabetic foot ulceration. This study is further supported by multiple independent randomized controlled trials reporting similar findings and approximately 4- to 10-fold reductions in reulceration for patients using home-based thermometry devices.16,19
There has been a growing interest in home monitoring and ambulatory measurements of foot temperatures in the diabetic neuropathic foot to prevent foot ulceration. Typically, simple digital thermometers,19 liquid crystal thermography technology,20 Spectrasole Pro 1000 indicator sheets for quick assessment of the diabetic feet (SpectraSole Foot Indicators, Sweden; ), and smart insoles (Zephyr Technology, Ltd., Auckland, New Zealand) are available. It must be emphasized that only handheld dermal thermometers based on infrared technology have been validated by randomized controlled trials for preventing recurring ulcers in the home-based environment.16,18,19
It is evident that most supplementary techniques based on thermometry have targeted prevention and home monitoring. Two independent proof-of-concept studies from the authors’ group have reported results for the effectiveness of cold stress testing and warm immersion testing for the neuropathic patient population.21,22 Evidence from clinical thermometry systems is therefore required to bridge the existing gaps in adoption of this technology. This article provides a proof of concept for a novel methodology using infrared thermography to assess wound healing in patients with diabetic foot ulcers. The authors describe an objective assessment of thermographic images to calculate a unitless index termed “wound inflammatory index” (WII)” for lower extremity ulcers. The terms thermal index (TI) and WII are used interchangeably throughout this article.
Methods
Use of thermal imaging may facilitate the assessment of wound and wound healing, especially with the intent to address the risk factors discussed earlier. It is important that any proposed methodology for analyzing the thermal image must be standardized.
Typically, when using infrared thermography, the anatomical surfaces of the foot are examined to identify potential hot or cold spots where inflammation or circulatory loss is occurring, respectively. The question of how large or extensive the wound site is is addressed by examining the infrared and visible imagery to determine the shape, area, curvature, and eccentricity characteristics. Identifying the shape will focus on the pattern of the infrared signature to determine if it is round, elliptical, or oval or has a mottled appearance. Describing the base of the wound ulcer in terms of being granular, fibrotic, or necrotic is also helpful. The existence of undermining of the leading edge may indicate an interruption in the skin matrix due to excessive vertical and shear stress forces on the edges.
While this approach provides a general qualitative process for analyzing the thermal images for wounds, the need for an objective parameter (an index based on thermal profile) cannot be overstated. This is especially important when tracking the wound healing over time. The progression of tissue injury or healing can be determined by calculating a TI of the wound based on the thermal profile or thermal signature, as discussed earlier. The processed image can be used to calculate a unitless index, described as follows.
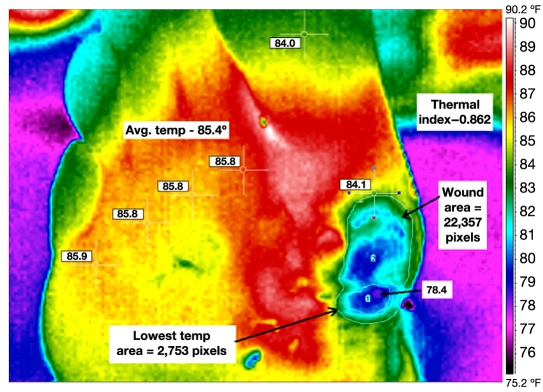
The authors propose a new tool for quantifying wound conditions, which includes the thermal features and wound size, both of which may play a key role in determining the duration of would healing. Because temperature is a surrogate marker for inflammation, the authors term this as WII for feet. The concept of a TI was first proposed by Collins and colleagues23 and was later applied to objective assessment of arthritis in clinical studies.24–26 The proposed TI for wound healing is, however, independent of these prior clinical studies. To the authors’ knowledge, this is a first case report evaluating a TI for wound healing. where DT is the temperature difference between the ulcer and mean foot temperature, a is the area of the isotherm (highest or lowest temperature) in the ulcer area, and A is the area of the wound bed. Area is calculated in terms of the pixels for this analysis. The choice of highest or lowest isotherm must be made at the beginning of the analysis and followed consistently. Currently, these features are manually extracted from the thermal image. Figure 1 illustrates a typical example for calculation of TI/WII for a wound.
Figure 1.
Calculation of TI/WII at baseline for the test subject (63 years, white male).
This assessment removes the subjective comparison of temperature. In Figure 1, the average foot temperature of 85.4°F/29.67°C was obtained by recording the temperature at six anatomical sites (metatarsal heads 1–5 and hallux). These sites are manually selected based on the evidence from clinical literature.12, 21 The active wound bed was measured to be 22,387 pixels, while the area of the lowest temperature had a pixel count of 2753. The lowest temperature was 78.4°F/25.78°C. The resultant TI was calculated to be 0.862. The image processing is typically carried out using the thermal imager’s software. Image J Software was used for postprocessing of data and feature extraction from the thermal images.
The following section describes a case report where a single patient with a diabetic foot ulcer on the plantar foot was followed up weekly until healing, and TI was measured as well as correlated with the conventional wound size measurement.
Case Report: Wound Inflammatory Index for a 63-Year-Old White Male
In order to provide a proof of concept for WII, a 63-year-old white male diabetes patient (history of 13 years) with a plantar neuropathic ulcer was recruited from the wound clinic at the Southern Arizona Limb Salvage Alliance (SALSA), College of Medicine, University of Arizona, for a detailed analysis. This patient was a high-risk candidate with a previous history of toe amputation. The ulcer under consideration had existed for three years, and the patient did not have any peripheral vascular disease. The patient was provided standard wound care with offloading using total contact cast. Thermal image data were collected at baseline and 7, 14, 21, 35, and 48 days. The ulcer on the plantar foot was healed at day 48. The change in TI was correlated with wound healing trajectory using Pearson’s correlation. Image processing was carried out using the Irisys IRI 4010 Imager Software and Image J Software.
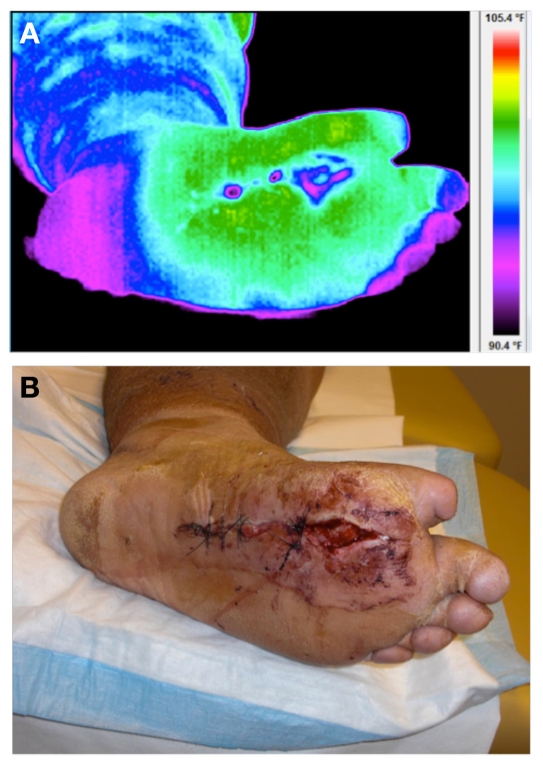
Figure 2 illustrates the thermal and visual image of the ulcer under consideration at baseline. Thermal images were acquired with the patient in supine position after a 20-minute acclimatization period. All images were acquired before the surgical debridement.
Figure 2.
(A) Thermal and (B) visual image of the diabetic foot ulcer on plantar aspect at day 0 (baseline).
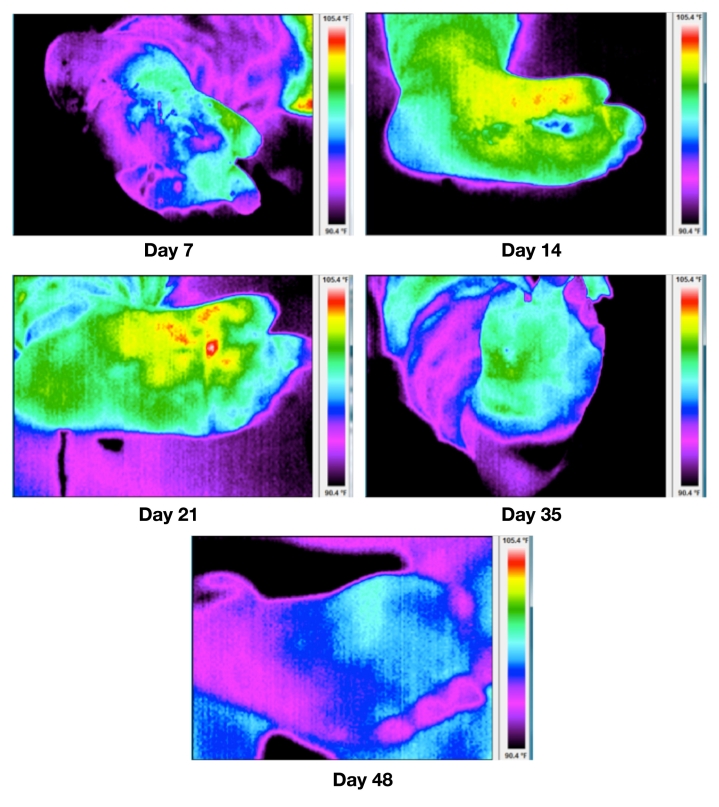
Figure 3 illustrates the processed thermal images from first follow-up visit until healing. Table 1 describes the various parameters for the TI calculation at each visit.
Figure 3.
Thermal imagery at days 7, 14, 21, 35 and 48. The ulcer was healed at day 48. All images are at the same temperature scale, i.e., 90.4 to 105.4 °F.
Table 1.
Calculation of Thermal Index/Wound Inflammatory Index at Each Patient Visit
| Day | Average foot temp (°C) | Wound area (pixels)–A | Isotherm area (pixels)–a | Wound temp (°C) | WII | Wound area (L × B, cm2) |
|---|---|---|---|---|---|---|
| 0 | 37.28 | 20907.00 | 8216.00 | 36.39 | −0.63 | 5.44 |
| 7 | 36.56 | 13949.00 | 3158.00 | 35.17 | −0.57 | 5.67 |
| 14 | 38.24 | 4615.00 | 2701.00 | 38.00 | −0.26 | 4.8 |
| 21 | 37.87 | 1821.00 | 279.00 | 40.39 | 0.70 | 1.4 |
| 35 | 36.78 | 1715.00 | 174.00 | 36.96 | 0.03 | 0.84 |
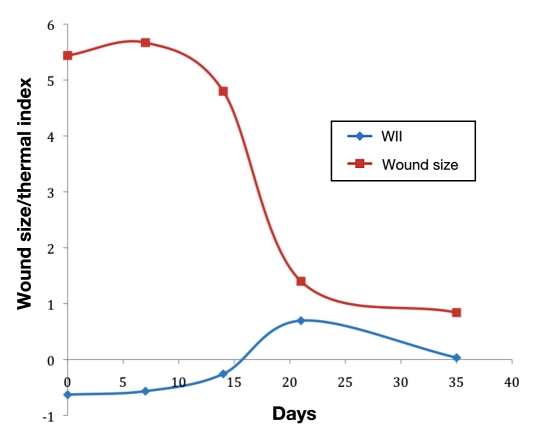
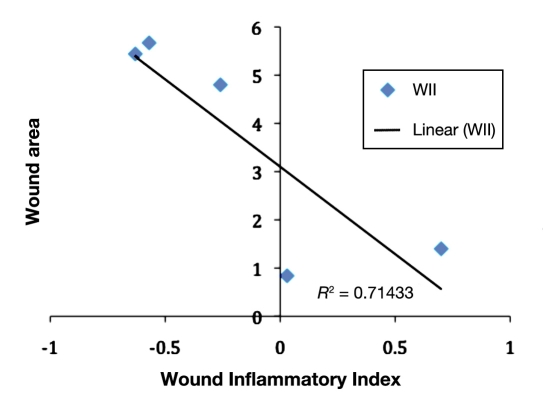
From Figure 3, the changes in thermal patterns or thermal morphology indicate a flare response at the wound periphery, which triggers at around day 14, and this acute inflammation around the wound begins to subside, leading to healing. The WII shows a strong negative correlation (-85%) with the conventional wound area calculation (multiplying the longest height by longest width). Figure 4 represents a plot of WII and wound size trajectory versus the number of days to healing. Figure 5 illustrates a scatter plot between the WII and wound area. The TI/WII indicates a shift from negative to positive (p < .05) before it reaches zero. From a wound-healing perspective, TI/WII at zero may indicate complete healing of the wound. A comparison between WII and wound size indicates that WII may have a quicker response time to predict healing versus wound size, and therefore, it may be a robust indicator of tissue health.
Figure 4.
Plot of WII and wound size versus number of days to healing.
Figure 5.
Scatter plot between WII and wound area.
Discussion
Pathways leading to diabetic foot ulceration and under-lying pathophysiology for subsequent complications are well understood. Clinicians typically assess circulatory function, medical history, neuropathic complications, and pressure distribution in the lower extremities to identify risk of foot ulceration. Several technological advances have provided innovative strategies to treat and manage the patients with diabetic foot disease, especially the ones that facilitate early diagnosis, surgical planning, and prevention. Thermometry is a prime example of one such modality, which has been shown to be clinically effective. Plantar temperatures are not just useful in preventing recurring ulcers, rather they may also be indicative of subclinical neuropathy and assess the wound-healing trajectory. This gap in the technological implementation has triggered the use of alternative, thermal modalities such as infrared thermography. This has empowered clinician scientists to develop novel assessment protocols relevant to diabetic foot disease.
The important advantages of this technique are safety of examination, noninvasive nature, simplicity, and speed. Such a system promotes a coupling between prevalent sensory testing modalities and itself, aimed to objectively characterize the wound. This article has presented a novel methodology to characterize wounds and objectively measure the wound-healing trajectory. There are several limitations of the proposed technique in its current form. Some of these include patient positioning, camera positioning, image registration (overlay of visual image on the thermal image), and automation of the analysis. These can, however, be corrected in an elaborate feasibility study, with extended efforts in developing the analytical tools/processes to minimize computational errors for the wound area (in terms of pixels). Furthermore, these analytical tools can help to develop tissue health characterization tools and quantify the flare response in the figures.
On the other hand, developing and testing mutimodal techniques such as ultrasonography, hyperspectral imaging, and thermography can be useful for identifying preulcerous symptoms, microvascular abnormalities, and monitoring (as well as quantifying) wound healing.27,28 In addition to providing a whole-field image of the anatomic site, the technique also provides subclinical metabolic information and tissue health status invisible to the naked eye. Further evidence from translational research studies is necessary to further the role of diagnosis, prevention, and therapy monitoring in diabetes patients using multimodal techniques. In the future, we may witness a combination of these modalities coupled with information technology workflow to develop a pervasive health care regime that provides economical and standardized treatment using expert decision support systems. From a clinical standpoint, this will result in avoiding outpatient visits, unnecessary hospitalization, and traumatic complications such as amputations. The most important benefit of the imaging modalities is real-time education and empowerment of patients, as visual feedback on health status may motivate patients to monitor their pathological state and maximize adherence to therapy.
Conclusion
Wound inflammatory index or thermal index may provide an adequate scoring range for assessing wounds by considering physiological as well as morphological changes on the skin surface. To the authors’ knowledge, this is the first study using the proposed specific definition of WII to assess the wound-healing trajectory. This technique could be applied to other kinds of wounds with different pathologies as well. However, detailed follow-up studies are required to ascertain the reliability and feasibility of this proposed methodology.
Abbreviations
- TI
thermal index
- WII
wound inflammatory index
References
- 1.Izumi Y, Satterfield K, Lee S, Harkless LB, Lavery LA. Mortality of first-time amputees in diabetics: a 10-year observation. Diabetes Res Clin Pract. 2009;83(1):126–131. doi: 10.1016/j.diabres.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 2.Moulik PK, Mtonga R, Gill GV. Amputation and mortality in new-onset diabetic foot ulcers stratified by etiology. Diabetes Care. 2003;26(2):491–494. doi: 10.2337/diacare.26.2.491. [DOI] [PubMed] [Google Scholar]
- 3.Boulton AJ, Armstrong DG, Albert SF, Frykberg RG, Hellman R, Kirkman MS, Lavery LA, LeMaster JW, Mills JL, Sr, Mueller MJ, Sheehan P, Wukich DK. Task Force of the Foot Care Interest Group of the American Diabetes Association. Comprehensive foot examination and risk assessment. Endocr Pract. 2008;14(5):576–583. doi: 10.4158/EP.14.5.576. [DOI] [PubMed] [Google Scholar]
- 4.Lavery LA, Armstrong DG, Wunderlich RP, Mohler MJ, Wendel CS, Lipsky BA. Risk factors for foot infections in individuals with diabetes. Diabetes Care. 2006;29(6):1288–1293. doi: 10.2337/dc05-2425. [DOI] [PubMed] [Google Scholar]
- 5.Armstrong DG, Wrobel J, Robbins J. Guest editorial: are diabetes-related wounds and amputations worse than cancer? Int Wound J. 2007;4(4):286–287. doi: 10.1111/j.1742-481X.2007.00392.x. [DOI] [PubMed] [Google Scholar]
- 6.Bharara M, Mills JL, Suresh K, Rilo HL, Armstrong DG. Diabetes and landmine-related amputations: a call to arms to save limbs. Int Wound J. 2009;6(1):2–3. doi: 10.1111/j.1742-481X.2009.00587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gordois A, Scuffham P, Shearer A, Oglesby A, Tobian JA. The health care costs of diabetic peripheral neuropathy in the US. Diabetes Care. 2003;26(6):1790–1795. doi: 10.2337/diacare.26.6.1790. [DOI] [PubMed] [Google Scholar]
- 8.Abbott CA, Garrow AP, Carrington AL, Morris J, Van Ross ER, Boulton AJ. North-West Diabetes Foot Care Study. Foot ulcer risk is lower in South-Asian and African-Caribbean compared with European diabetic patients in the U.K.: the North-West diabetes foot care study. Diabetes Care. 2005;28(8):1869–1875. doi: 10.2337/diacare.28.8.1869. [DOI] [PubMed] [Google Scholar]
- 9.Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA. 2005;293(2):217–228. doi: 10.1001/jama.293.2.217. [DOI] [PubMed] [Google Scholar]
- 10.Reiber GE, Lipsky BA, Gibbons GW. The burden of diabetic foot ulcers. Am J Surg. 1998;176(2A Suppl):5S–10S. doi: 10.1016/s0002-9610(98)00181-0. [DOI] [PubMed] [Google Scholar]
- 11.Boulton AJ, Connor H, Cavanagh PR, editors. The foot in diabetes. Chichester: John Wiley & Sons; 1998. [Google Scholar]
- 12.Bharara M, Cobb JE, Claremont DJ. Thermography and thermo-metry in the assessment of diabetic neuropathic foot: a case for furthering the role of thermal techniques. Int J Low Extrem Wounds. 2006;5(4):250–260. doi: 10.1177/1534734606293481. [DOI] [PubMed] [Google Scholar]
- 13.Reiber GE. Diabetic foot care. Finanacial implications and practical guidelines. Diabetes Care. 1992;15(Suppl 1):29–31. doi: 10.2337/diacare.15.1.s29. [DOI] [PubMed] [Google Scholar]
- 14.Pecoraro RE, Reiber GE, Burgess EM. Pathways to diabetic limb amputation. Basis for prevention. Diabetes Care. 1990;13(5):513–521. doi: 10.2337/diacare.13.5.513. [DOI] [PubMed] [Google Scholar]
- 15.Boulton AJ, Armstrong DG, Albert SF, Frykberg RG, Hellman R, Kirkman MS, Lavery LA, Lemaster JW, Mills JL, Sr, Mueller MJ, Sheehan P, Wukich DK. American Diabetes Association, American Association of Clinical Endocrinologists. Comprehensive foot examination and risk assessment: a report of the task force of the foot care interest group of the American Diabetes Association, with endorsement by the American Association of Clinical Endocrinologists. Diabetes Care. 2008;31(8):1679–1685. doi: 10.2337/dc08-9021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lavery LA, Higgins KR, Lanctot DR, Constantinides GP, Zamorano RG, Athanasiou KA, Armstrong DG, Agrawal CM. Preventing diabetic foot ulcer recurrence in high-risk patients: use of temperature monitoring as a self-assessment tool. Diabetes Care. 2007;30(1):14–20. doi: 10.2337/dc06-1600. [DOI] [PubMed] [Google Scholar]
- 17.Roback K, Johansson M, Starkhammar A. Feasibility of a thermo-graphic method for early detection of foot disorders in diabetes. Diabetes Technol Ther. 2009;11(10):663–667. doi: 10.1089/dia.2009.0053. [DOI] [PubMed] [Google Scholar]
- 18.Armstrong DG, Holtz-Neiderer K, Wendel C, Mohler MJ, Kimbriel HR, Lavery LA. Skin temperature monitoring reduces the risk for diabetic foot ulceration in high-risk patients. Am J Med. 2007;120(12):1042–1046. doi: 10.1016/j.amjmed.2007.06.028. [DOI] [PubMed] [Google Scholar]
- 19.Lavery LA, Higgins KR, Lanctot DR, Constantinides GP, Zamorano RG, Armstrong DG, Athanasiou KA, Agrawal CM. Home monitoring of foot skin temperatures to prevent ulceration. Diabetes Care. 2004;27(11):2642–2647. doi: 10.2337/diacare.27.11.2642. [DOI] [PubMed] [Google Scholar]
- 20.Frykberg RG, Tallis A, Tierney E. Diabetic foot self examination with the Tempstat as an integral component of a comprehensive prevention program. J Diabetic Foot Comp. 2009;1(1):13–18. [Google Scholar]
- 21.Bharara M, Viswanathan V, Cobb JE. Cold immersion recovery responses in the diabetic foot with neuropathy. Int Wound J. 2008;5(4):562–569. doi: 10.1111/j.1742-481X.2008.00454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bharara M, Viswanathan V, Cobb JE. Warm immersion recovery test in assessment of diabetic neuropathy—a proof of concept study. Int Wound J. 2008;5(4):570–576. doi: 10.1111/j.1742-481X.2008.00455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Collins AJ, Ring EF, Cosh JA, Bacon PA. Quantitation of thermo-graphy in arthritis using multi-isothermal analysis. I. The thermo-graphic index. Ann Rheum Dis. 1974;33(2):113–115. doi: 10.1136/ard.33.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ring EF. Thermography and rheumatic diseases. Bibl Radiol. 1975;6:97–106. [PubMed] [Google Scholar]
- 25.Ring EF, Collins AJ, Bacon PA, Cosh JA. Quantitation of thermo-graphy in arthritis using multi-isothermal analysis. II. Effect of nonsteroidal anti-inflammatory therapy on the thermographic index. Ann Rheum Dis. 1974;33(4):353–356. doi: 10.1136/ard.33.4.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ring EF, Collins AJ. Quantitative thermography. Rheumatol Phys Med. 1970;10(7):337–341. doi: 10.1093/rheumatology/10.7.337. [DOI] [PubMed] [Google Scholar]
- 27.Chen MH, Kerechanin CW, II, Greenspan DG, Criss TB, Franckowiak SC, Vincent JA, Pattay RS. Development of a thermal and hyperspectral imaging system for wound characterization and metabolic correlation. Johns Hopkins Apl Technical Digest. 2005;26(1):67–74. [Google Scholar]
- 28.Nishide K, Nagase T, Oba M, Oe M, Ohashi Y, Iizaka S, Nakagami G, Kadowaki T, Sanada H. Ultrasonographic and thermo-graphic screening for latent inflammation in diabetic foot callus. Diabetes Res Clin Pract. 2009;85(3):304–309. doi: 10.1016/j.diabres.2009.05.018. [DOI] [PubMed] [Google Scholar]