Abstract
Introduction
Currently, diagnosis of patients with postural instability relies on a rudimentary clinical examination. This article suggests an innovative, portable, and cost-effective prototype to evaluate balance control objectively.
Methods
The proposed system uses low-cost, microelectromechanical sensor, body-worn sensors (BalanSens™) to measure the motion of ankle and hip joints in three dimensions. We also integrated resulting data into a two-link biomechanical model of the human body for estimating the two-dimensional sway of the center of mass (COM) in anterior–posterior (AP) and medial–lateral (ML) directions. A new reciprocal compensatory index (RCI) was defined to quantify postural compensatory strategy (PCS) performance. To validate the accuracy of our algorithms in assessing balance, we investigated the two-dimensional sway of COM and RCI in 21 healthy subjects and 17 patients with diabetic peripheral neuropathic (DPN) complications using the system just explained. Two different conditions were examined: eyes open (EO) and eyes closed (EC) for duration of at least 30 seconds. Results were compared with center of pressure sway (COP) as measured by a pressure platform (Emed-x system, Novel Inc., Germany). To further investigate the contribution of the somatosensory (SOM) feedback to balance control, healthy subjects performed EO and EC trials while standing on both a rigid and a foam surface.
Results
A relatively high correlation was observed between COM measured using BalanSens and COP measured using the pressure platform (r = 0.92). Results demonstrated that DPN patients exhibit significantly greater COM sway than healthy subjects for both EO and EC conditions (p < 0.005). The difference becomes highly pronounced while eyes are closed (197 ± 44 cm2 vs 68 ± 56 cm2). Furthermore, results showed that PCS assessed using RCI is significantly better in healthy subjects compared to DPN subjects for both EO and EC conditions, as well as in both ML and AP directions (p < 0.05). Alteration in SOM feedback in healthy subjects resulted in diminished RCI values that were similar to those seen in DPN subjects (p > 0.05).
Discussion/Conclusion
This study suggested an innovative system that enables the investigation of COM as well as postural control compensatory strategy in humans. Results suggest that neuropathy significantly impacts PCS.
Keywords: balance, body-worn sensor, diabetic peripheral neuropathy, postural compensatory strategy, sensory feedback
Introduction
Balance is a fundamental ability for humans, and its impairment dramatically reduces a person’s ability to perform activities essential to daily living (e.g., walking, turning, and changing posture).1,2 Neurodegenerative diseases such as diabetic neuropathy, Parkinson’s, and stroke may lead to balance-associated disorders. The degradation in balance increases the risk of falling, ultimately leading to increased morbidity, mortality, and health care costs. Falls are also associated with decreased confidence in movement and balance.3–8 Loss ofconfidence or fear of falling often leads to decreased physical activity, which may cause further declines in postural stability and quality of life.9 It comes as no surprise, therefore, that balance instability in persons is strongly linked to an increased prevalence of depression.10,11
Loss of balance control is a key concern for people with diabetes, the elderly, and patients suffering from neurodegenerative diseases. According to the National Diabetes Information Clearinghouse,12 at least 20.8 million people in the United States—7% of the population—live with diabetes. According to the World Health Organization,13 by 2025, the number of diabetes patients will increase by approximately 122% to reach 300 million individuals. Approximately 50% of diabetes patients over 60 years of age exhibit symptoms of diabetic neuropathy, making this the most common symptomatic complication. Individuals with diabetic peripheral neuropathy (DPN) often suffer from postural instability, leading in turn to an increased likelihood of falling, depression, anxiety, and decreased quality of life.14–16 Although previous studies have shown that diabetic neuropathy presents a significant independent risk for falls in elderly people,15,17–24 it is remarkable that only a few studies have considered the postural instability disorder, which is an important consequence of this devastating malady.1,9,15,21,22
There is an unmet need for a convenient, cost-effective tool to identify individuals with postural instability and who are at risk for falls. Falling is among the most serious health problems associated with aging. A third of persons over 65 fall at least once a year, with a quarter of these cases leading to serious injuries. In clinical practice, current physical examination techniques used to diagnose postural instability (e.g., Romberg test) are inexact. The subjective nature of these techniques lacks the optimized sensitivity for diagnosing the presence of the condition early enough while simultaneously assessing its severity. Many strategies are also unsuitable for the busy clinical setting, as they require substantial space and infrastructure (e.g., force platform). Furthermore, no simple, cost-effective, and easy-to-use systems exist that can examine both biomechanical (e.g., body sway) and neurological (e.g., postural control strategy and compensatory mechanisms of upper body for maintaining balance) components of balance control.
In recent years, body-wearable sensor technology based on electromechanical sensors has provided a new avenue for accurately detecting and monitoring body motion and physical activity of an individual under free conditions.8,25–28 For example, combinations of multiple accelerometers and angular-rate sensors (gyroscopes) hold great promise for hybrid kinematic sensor modules for measuring the three-dimensional kinematics of different body segments.5,8,29 Key advantages of using body-wearable sensors are that such technology (1) is inexpensive and (2) does not require a specific environment or installation of any particular infra-structure. These advantages are crucial in developing a suitable tool for clinical applications that enables physicians to better evaluate the postural control of their patients under real conditions and help patients care for themselves.
This article proposes an innovative, wearable, and low-cost system for evaluating posture and balance control that can be used outside of a gait laboratory environment. This system is based on widely available kinematic sensors (i.e., accelerometer, gyroscope, and magnetometer). Using a biomechanical model of the human body, the suggested device assesses both postural stability and postural control strategy objectively.
Methods
Measuring Joint Angles
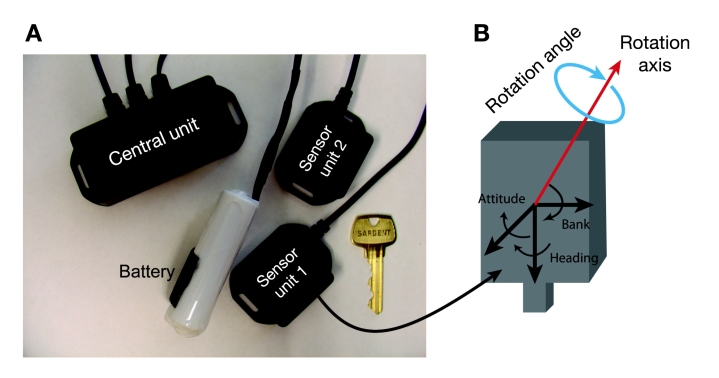
Two sensors (Figure 1A), each including a triaxial accelerometer, triaxial gyroscope, and a triaxial magno-meter, were used to estimate three-dimensional angles of the hip and ankle joints (BalanSens™, BioSensics LLC, Brookline, MA). Each sensor provided real-time (sample frequency 50 to 100 Hz) quaternions (qw, qx, qy, qz) that are subsequently converted to Euler angles denoted as θ, φ, and ψ. The resulting three-dimensional angles are used to estimate the trajectory of the subject’s ankle and hip. Euler angles, used to describe a sequence of three rotations determining the orientation of a rigid body in three dimensions, are defined as (in their order of application): (i) heading (θ), (ii) attitude (φ), and (iii) bank (ψ)(see Figure 1B).
Figure 1.
BalanSens™ system: (A) two sensor units allow providing three-dimensional angles of ankle and hip joints. A central unit allows transferring the estimated angles in real time to a computer via a WiFi communication protocol. A researchable battery allows recording and transferring data unto a 2-hour continuous measurement. If necessary, the battery can be replaced easily. (B) Representation of the axes angles.
The quaternion30,31 output of the calibrated sensor during the balance test, qFINAL, is converted to Euler angles as follows:
| (1) |
| (2) |
| (3) |
| (4) |
In these equations, qx, qy, qw, and qzrepresent components of the quaternion output qFINAL.
Estimation of Center of Mass (COM) Position
Several ways exist for estimating the COM of a subject. For example, a well-known method for monitoring COM is by analyzing signals from an accelerometer placed on the sacrum of the subject, often the best position to monitor the COM.32–34 Although this approach may produce accurate results during quiet standing or walking in a straight line, it may be inappropriate for assessing the COM when the subject sways significantly or during reaching task movements. This approach assumes a single inverted pendulum model in which the body mass rotates around the ankle joint (assuming a negligible motion of the hip joint), which is not always true, especially when the subject sways significantly. To overcome this shortcoming, a two-segment model of the body was used to calculate anterior–posterior (AP) and medial–lateral (ML) angles during movement.
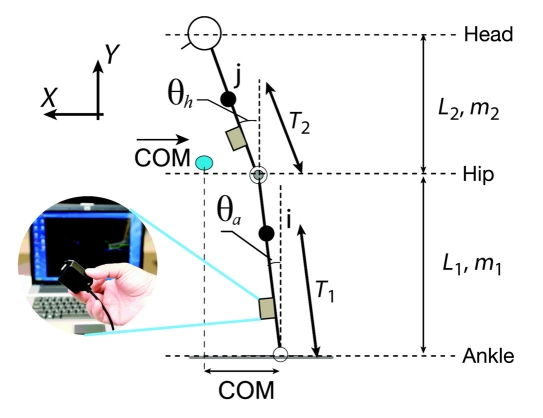
Figure 2 illustrates a two-link model of the human body that can be used to estimate the COM once the joint angles were estimated as described earlier. Figure 3 illustrates an example of sensor attachment. Sensors attached to the subject’s shin and back provide, respectively, the angle of ankle (θa) and hip (θh) joints in the AP plane, as illustrated in Figure 2. Having anthropometric data of each subject (e.g., body mass, m, and height, H), the position of the COM in each link in the saggital plane can be described as:
Figure 2.
Two-link biomechanical model of human body for estimating COM trajectory.
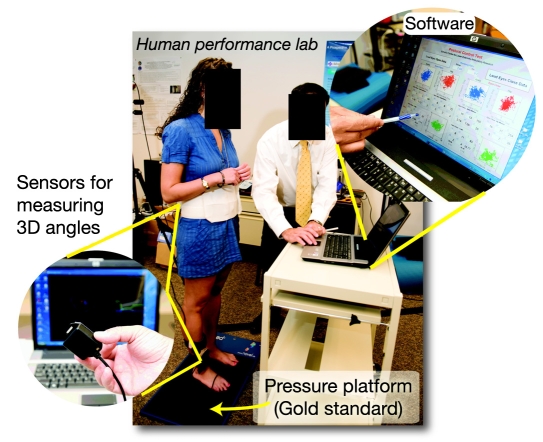
Figure 3.
Experimental setup: two sensors were attached, one to subject’s lower back and one to the shin. These sensors allow measuring three-dimensional angles of ankle and hip joints. Software was developed to estimate the trajectory of COM, ankle, and hip in real time using a two-link biomechanical model of a human body. Balance control and postural compensatory strategy of subjects were assessed during eyes-open and eyes-closed conditions while standing on a hard surface or a soft surface (using a firm and thick foam). A pressure platform was used as the gold standard to examine accuracy of the system to screen balance deterioration due to sensory alteration.
| (5) |
| (6) |
| (7) |
Here, θa′ and θh represent, respectively, the angular displacement of the ankle and hip (see Figure 2). The first component of the COM corresponds to the frontal direction or movement in the AP direction, which can be expressed as:
| (8) |
The equations can be rewritten with three constants, as follows:
| (9) |
The equation of the COM in the ML direction can be derived in an analogous fashion, with angles expressed in the ML direction. The values of mi and Ti(i = 1–3) and Lj(j = 1, 2) can be estimated from the subject’s body mass and height.35 To moderate the noise from artifacts such as skin movement, angles data were filtered using a wavelet transform band-pass filter with a mother wavelet of “Coiflet 5” and a cutoff frequency of 0.06–30 Hz.
Estimation of Reciprocal Compensatory Index (RCI)
To evaluate the best postural strategy for maintaining balance, we explored how the chosen postural strategy compensated the alteration and helped reduce COM variation (i.e., variance; denoted as “var”).The best postural strategy was quantified by estimating the variance of COM from Equation (9) and defined as the reciprocal compensatory index:
| (10) |
Keep in mind that the first two components of the right side of Equation (10) are always positive. However, the third component could be positive, negative, or zero. In case of a negative value for the third component, the variation of COM could be reduced, despite high motion of the ankle and hip joints. This would happen when movement of the ankle joint is in the opposite direction to that of hip joint movement, yielding a negative covariance value. To examine how the third component of Equation (10) could reduce the variance of COM, we normalized the variation of COM by the first two components of the equation according to the following formula:
| (11) |
Equation (11) can be also presented as a function of correlation between movement of the hip and ankle joints, assuming a small range of motion of ankle and hip angles:
| (4) |
where “r” represents the coefficient of correlation between ankle movement and hip movement.
According to this equation, RCI values near zero represent a good postural control strategy (i.e., negative correlation between hip and ankle movements), RCI values more than 1 represent inappropriate postural control strategy (i.e., positive correlation between hip and ankle movements, leading to increase the variation of COM and consequently fall accidents), and RCI values near 1 indicate that no correlation exists between the movement of ankle and hip joints.
Experimental Setup
Subject Recruitment
Two studies were performed to examine performance of the designed biosensor (BalanSens) to assess postural control. The first study evaluated the agreement between results reported by our system and balance evaluation performed using a well-established method based on assessing the area of sway for center of pressure (COP). After initial evaluation, the second study examined the sensitivity of the designed system in screening balance deterioration during eyes-closed condition compared to eyes-open condition in a group of DPN subjects. Both studies were approved by the local ethics committee (institutional review board).
In the first study, 21 healthy subjects (17 males and 4 females) with a mean age of 24.4 ± 1.63 years, a mean body height of 175.5 ± 10.4 cm, a mean body mass of 78.3 ± 14.1 kg, and a mean body mass index (BMI) of 25.3 ± 3.0 were recruited. Participants were a sample of volunteers from the Rosalind Franklin University campus. Participants were all young and healthy individuals that attended the university. Inclusion criterion was any healthy adult student (age >18) with no self-reported gait or balance instability. We excluded those with diabetes or neuropathic complications and those with any history of clinically significant orthopedic, muscular, or neurological disability that would affect their ability to perform the protocol.
In the second study, 17 DPN subjects (age: 59.2 ± 8.5 yrs old; weight: 110.1 ± 13.3 kg; height: 178.4 ± 5.8 cm; BMI: 34.6 ± 4.2 kg/m2) were recruited from the Rosalind Franklin University health system. We included any volunteers with a diagnosis of diabetes mellitus (type 1 or 2), according to American Diabetes Association (ADA) criteria,36 and with evidence of peripheral neuropathy. The diagnosis of peripheral neuropathy (distal symmetric polyneuropathy) was based on criteria explained in the ADA‘s consensus statement.37 More specifically, in this study the diagnosis of DPN consisted of vibratory perception threshold testing using the technique described by Young and colleagues38 and 10-gram Semmes–Weinstein monofilament using criteria described by Armstrong and associates.39 We excluded those subjects with any clinically significant neurological (excluding peripheral neuropathy), orthopedic, or muscular disorder that would affect their ability to perform the protocol.
Subject Preparation and Protocol of Measurement
Subjects were instructed to stand erect with feet together and hands by their sides. Two different conditions were examined as called for by the Romberg’s test: eyes open (EO) and eyes closed (EC) for a duration of at least 30 seconds. During each condition, the area of sway for COM, as well as hip and ankle motions, was estimated using BalanSens as described earlier. Each test was repeated twice, and the average of both measurements was considered as the final outcome. In the first study, we also used a pressure platform (Emed-x system, Novel Inc., Germany) for measuring the area of sway for COP during each condition (Figure 4). Additionally, to alter somatosensory (SOM) feedback (sensation under feet), we repeated both EO and EC tests while subjects were standing on a thick soft and firm foam (Figure 5). This altered SOM feedback in healthy subjects may have challenged postural control similarly to the way a loss of SOM feedback may challenge DPN patients.
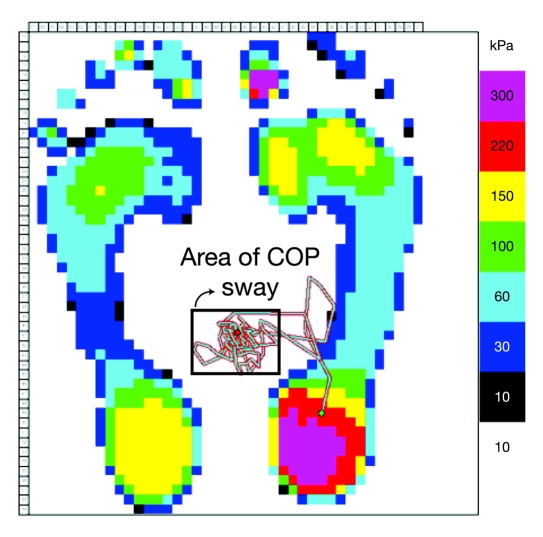
Figure 4.
The area of sway for COP was measured using a standard pressure platform. Only the area of sway during a double stance condition was calculated, and COP values related to a single stance condition were excluded in the analysis.
Figure 5.
To examine the impact of SOM feedback alteration on balance control, subjects were asked to stand on a firm and thick foam.
Statistical Analysis
The area of sway for both COM and COP was calculated by multiplying the range of motion in ML and AP directions after excluding outliers. Outliers were estimated by calculating 5 and 95 percentiles of data. The degree of agreement between COM measured by BalanSens and COP measured by the Emed pressure platform was examined using Pearson’s correlation coefficient (r value). The comparison between EO and EC conditions for both young healthy subjects (group 1) and DPN subjects (group 2) was performed using a paired t test (two-tailed). The comparison between healthy and DPN subjects was performed using a two-sample t test (two-tailed). Intraclass correlation coefficient (ICC)40 was calculated to examine test–retest reliability for both COM and COP values during EO and EC conditions. To interpret ICC values we used benchmarks suggested by Menz and colleagues41 and Najafi and colleagues42 (>0.75 excellent reliability, 0.40–0.75 fair-to-good reliability, and <0.40 poor reliability). Paired sample t tests were performed to demonstrate if any systematic change between test and retest was significant. For all tests, an α level of 0.05 was considered statistically significant. A 95% confidence interval (CI) for t-test results was calculated. A 95% CI for r values and ICC values represents the 95% statistical distribution of r values or ICC values around their mean values. All calculations were made using MATLAB® version 7.4 (R2007z) (The MathWorks, Inc., Natick, MA). To estimate ICC values, we used an open source statistical toolbox developed by the “R” development team, version R2.4.0.43
Results
Comparison between COM and COP
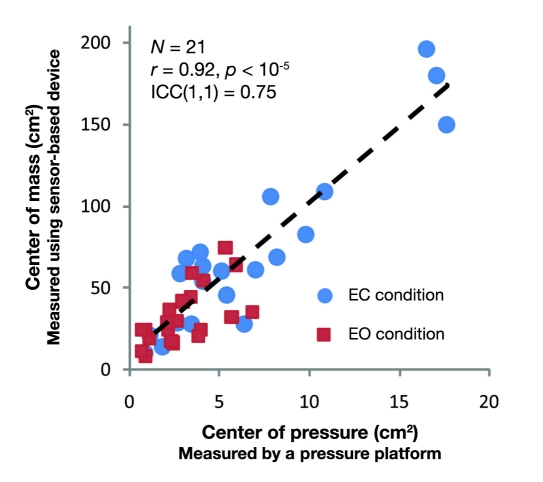
An excellent correlation was observed between the area of sway for COM measured by our biosensor device and the area of sway for COP measured by a pressure platform [r = 0.92; 95% CI = (0.82–0.94), p < 10−6, see Figure 6]. Additionally, test–retest reliability for COM measurements, including both EO and EC trials, was excellent [ICC(1,1) = 0.76, F(41,40.5) = 0.71, 95% CI = (0.57,0.86), p < 10−6]. On the same note, test–retest reliability for COP measurements, including both EC and EO trials, was relatively good [ICC(1,1) = 0.74, F(41,40.1) = 0.66, 95% CI = (0.56–0.85), p < 10−6].
Figure 6.
An excellent agreement was observed between COM estimated using BalanSens and COP measured using the gold standard.
Impact of Sensory Feedback Alteration in Body Sway and Postural Control
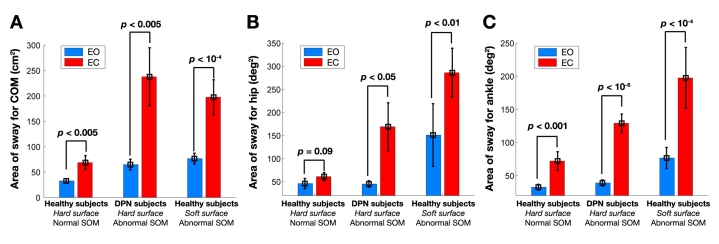
Table 1 summarizes body sway results for healthy and DPN subjects for COM, COP, and ankle and hip sway. Figure 7 illustrates the comparison between healthy and DPN subjects during EO and EC conditions. Results demonstrated that BalanSens is sensitive to screen balance deterioration due to either alteration in visual feedback (EC condition) or alteration in SOM feedback (i.e., standing on a soft surface or DPN complication). In healthy subjects, the area of sway for COM was increased significantly under the EC condition on average by 110% [paired t test, p < 0.005, standard deviation (SD) = 49.9 cm2, degree of freedom (df) = 20, 95% CI = (13.3,58.6) cm2] without altering SOM (standing on hard surface) and approximately by 197% [paired t test, p < 10−6, SD = 120 cm2, df = 20, 95% CI = (76,186) cm2] after altering SOM feedback. Alteration in SOM feedback in healthy subjects increased the area of sway of COM by 103 and 187%, respectively, during EO and EC conditions [paired tests; for EO condition: p < 10−4, SD = 34 cm2, df = 20, 95% CI = (18,50) cm2 ; for EC condition: p < 10−5, SD = 104 cm2, df = 20, 95% CI = (82,174) cm2].
Figure 7.
The area of sway for (A) COM, (B) hip joint, and (C) ankle joint for healthy subjects while standing on a hard surface (healthy SOM feedback), DPN subjects (distorted SOM feedback), and healthy subjects while standing on a soft surface (distorted SOM feedback).
Table 1.
Body Sway and Reciprocal Compensatory Index for Healthy and DPN Subjects
| Healthy subjects | DPN subjects | |||||
|---|---|---|---|---|---|---|
| Hard surface Normal SOM feedback | Soft surface Altered SOM feedback | Hard surface | ||||
| EO | EC | EO | EC | EO | EC | |
| COM sway (cm2 ) | 32.6 ± 17.8a | 68.8 ± 56.4 | 66.4 ± 44.0 | 197.5 ± 142.8 | 64.9 ± 43.7 | 237.7 ± 235.7 |
| COP swayb (cm2 ) | 2.97 ± 1.4 | 5.94 ± 4.0 | 16.3 ± 8.9 | 34.4 ± 28.2 | — | — |
| Ankle sway (deg2 ) | 32 ± 18 | 72 ± 56 | 76 ± 65 | 232 ± 189 | 39 ± 19 | 129 ± 58 |
| Hip sway (deg2 ) | 46 ± 44 | 61.5 | 151 ± 280 | 285 ± 217 | 45 ± 30 | 169 ± 213 |
| RCI—ML | 0.80 ± 0.05 | 0.89 ± 0.05 | 0.94 ± 0.10 | 0.94 ± 0.12 | 0.89 ± 0.14 | 0.96 ± 0.20 |
| RCI—AP | 0.70 ± 0.01 | 0.86 ± 0.10 | 0.79 ± 0.08 | 0.83 ± 0.12 | 0.90 ± 0.11 | 0.95 ± 0.21 |
Pressure platform is unable to measure COP sway on a soft surface.
Mean ± SD.
BalanSens also successfully identified the impact of visual disturbance in DPN subjects as well as balance deterioration due to neuropathic condition (see Figure 7A). Results demonstrated that DPN patients exhibit significantly greater COM sway than healthy subjects during both EO and EC conditions. In DPN subjects, the area of sway for COM was significantly higher than healthy subjects on average by 98% [two-sample t test, p < 0.005, df = 36, SD = 32 cm2, 95% CI = (11,54) cm2]. The difference was highly pronounced during the EC condition and reached greater than 245% [two-sample t test, p < 0.005, df = 36,SD = 163 cm2, 95% CI = (61,274) cm2]. Interestingly, during the EO condition, despite a significant decrease in COM sway in healthy subjects compared to DPN, no significant difference was observed for both ankle and hip sways (p > 0.05), suggesting a more appropriate postural compensatory strategy (PCS) in healthy subjects. During the EC condition, both ankle and hip sways were significantly higher in DPN subjects (p < 0.05; see Table 1 and Figures 7B and 7C).
Closing of the eyes in DPN subjects caused a significant deterioration in COM sway by an average of 266% [paired t test, p < 0.005, SD = 205 cm2, df = 16, 95% CI = (61,250) cm2], which is more than twice than healthy subjects without altering SOM feedback [two-sample t test, p < 0.005, SD = 141 cm2, df = 36, 95% CI = (57,305) cm2]. Interestingly, deterioration of balance in DPN subjects due to eye closing was almost the same as healthy subjects by alteration of their SOM feedback [two-sample t test, p = 0.44, SD = 163 cm2, df = 36, 95% CI = (–67,152) cm2].
On the same note, except for healthy subjects without alteration of SOM feedback, after closing the eyes, the area of sway for both hip and ankle joints was increased significantly (paired t test, p < 0.05, see Figures 7B and 7C). The amount of sway for both ankle and hip joints and during both EO and EC conditions was significantly higher in healthy subjects when their SOM feedback was altered compared to DPN subjects (two-sample t test, p < 0.05). During the EO condition, alteration of SOM feedback in healthy subjects caused increased body sway compared to DPN subjects on average by 49 and 70%, respectively, for ankle and hip joints. This increase in the ankle joint was significant [two-sample t test: p < 0.05, df = 36, SD = 50.2 deg2, 95% CI = (4,71) deg2], but was insignificant for the hip joint [two-sample t test: p = 0.12; SD = 209 deg2, df = 36, 95% CI = (–30, 243) deg2].
Impact of Sensory Feedback Alteration in Postural Compensatory Strategy
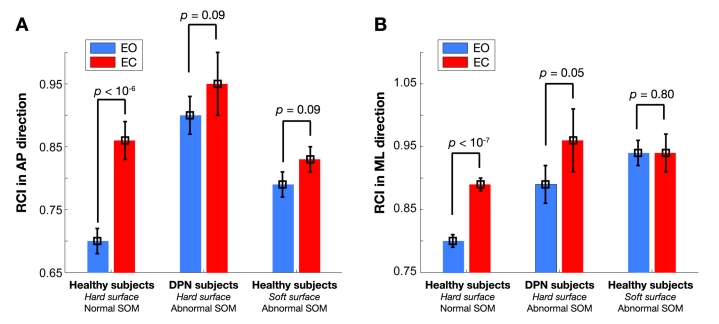
Postural control strategy in both ML and AP directions was reported as RCI—ML and RCI—AP, respectively (see Table 1). Results of RCI are also illustrated in Figure 8. Interestingly, results demonstrate that the PCS is significantly better in healthy subjects compared to DPN subjects during the EO condition in both AP and ML directions [two-sample t test in AP: p < 10−7, SD = 0.09, df = 36, 95% CI = (–0.24,–0.12); in ML: p < 0.05, SD = 0.10, df = 36, 95% CI =(–0.18,–0.02)]. After alteration of SOM feedback in healthy subjects, the PCS was still better in the AP direction compared to DPN subjects [p < 0.001, SD = 0.09, df = 36, 95% CI = (–0.18,–0.06)]. No significant difference was observed for postural control strategy in the ML direction between DPN subjects and healthy subjects with altered SOM feedback (p = 0.3).
Figure 8.
Postural compensatory strategy quantified by RCI value in (A) anterior–posterior (AP)direction and (B) medial–lateral (ML)directions.
As illustrated in Figure 8, in healthy subjects, closing the eyes significantly deteriorates postural control strategy by an average of 22 and 10%, respectively, in AP and ML directions. Furthermore, alteration of SOM feedback in healthy subjects during the EO condition significantly deteriorates RCI on average by 16 and 13%, respectively, for ML and AP directions (p < 10−4 ). However, alteration of SOM feedback during the EC condition did not impact RCI (p > 0.05).
In DPN subjects, alteration in visual feedback (EC) significantly deteriorated RCI approximately by 7 and 6%, respectively, for ML and AP directions. However, the observed deterioration was only significant in the ML direction [paired t test, p < 0.05, SD = 0.11, df = 16,95% CI = (–0.12,–0.008)]. More interestingly, results suggest that the PCS during the EO condition is significantly better in healthy subjects compared to DPN subjects on average by 10 and 28%, respectively, for ML and AP directions (p < 10−4 ). During the EC condition, although postural control strategy was better in healthy subjects on average of more than 7% for both AP and ML directions, the observed difference was not significant (p > 0.05).
Discussion
Traditionally, assessments of COM sway and postural anticipatory strategy were performed using standard optic, magnetic, or sonic technologies.5,7,25 However, body-wearable sensor technology based on electromechanical sensors has provided a new avenue for accurately detecting and monitoring body motion and physical activity of an individual under free conditions.8,25–28 This study has suggested an innovative, portable, and cost-effective prototype based on body-worn sensor technology and used BalanSens to evaluate balance control objectively. This system uses low-cost, microelectromechanical sensor, body-worn sensors (i.e., accelerometer, gyroscope, and magnetometer) to measure the motion of ankle and hip joints in three dimensions. We also integrated resulting data into a two-link biomechanical model of the human body for estimating the two-dimensional sway of the COM. A new RCI was defined to quantify PCS performance. Reciprocal compensatory index values may range from 0 to 2 with lower RCI values associated with better PCS.
The validity and reliability of the suggested system were examined by several measurements. First, the COM estimated using BalanSens was compared with COP measured using a standard pressure platform in 21 healthy subjects. Results suggested a relatively high correlation (r = 0.92) between two measurements during all conditions. Interestingly, measuring COM seems to be more than 12 times more sensitive than measuring COP (i.e., 12 times higher range of sway for COM compared to range of sway for COP). Second, we examined test–retest reliability (repeatability) of the measurement by repeating each condition during the same session. Results demonstrated an excellent test–retest reliability for measuring the area of sway for COM (ICC >0.75). Third, to examine the sensitivity of the device for screening balance deterioration, we compared the output of the system between several conditions in which one or both visual and SOM feedback were altered. Results demonstrated that our system is highly sensitive to detect balance alterations due to challenges in visual (EC condition) and SOM (standing on a soft surface) feedback. Finally, the clinical validity of the system was assessed by comparing balance control of healthy subjects with a group of diabetes patients suffering from lack of SOM feedback (i.e., DPN). The proposed technology allowed screening balance impairment due to DPN complication during both EO and EC conditions.
The maintenance of postural stability depends on the ability of the sensory system to extract SOM, vestibular, and visual inputs relating to body orientation. This information is then integrated within the central nervous system (CNS) and an appropriate motor response is activated.44,45 Somatosensory function is thought to be the most important sensory information for control of postural stability, contributing at least 60–75% of the control of stance posture when a subject stands on a firm surface.46,49 Somatosensory loss is a characteristic of patients with diabetic neuropathy.14,46–48 Although more than 50% of diabetes patients older than 60 years of age show evidence of peripheral neuropathy, only a few studies have focused on the postural instability disorder, which is an important consequence of this devastating malady.15,17–24 This study, for the first time, explored balance determination in DPN subjects by measuring the area of sway in COM rather than the area of sway in COP. Results were consistent with other studies that indicated DPN may significantly impact the ability of the subject to maintain balance, particularly during the EC condition. For example, Lafond et al.50 reported that DPN patients show larger root mean square values of COP displacement in both AP and ML directions compared to control subjects. According to their study, the root mean square value for COP during the EO condition was 2.77 ± 0.97 cm2 in DPN patients versus 1.97 ± 0.53 cm2 in control subjects, which represents approximately 41% deterioration of balance due to neuropathy. In our study, during the EO condition, the area of sway for COM was 33 ± 18 and 66 ± 44 cm2, respectively, for healthy and DPN subjects, which shows an approximately 100% increase in COM sway due to DPN complication. On the same note, Lafond and associates50 reported that COP swayduring the EC condition is 2.39 ± 0.81 and 4.13 ± 3.69 cm2, respectively, for healthy and DPN subjects, which indicates a 73% deterioration of balance due to diabetic neuropathy. Our study found the area of COM sway during the EC condition was 69 ± 56 cm2 in healthy subjects versus 198 ± 143 cm2 in DPN subjects, which is equivalent to 187% deterioration in balance due to DPN complication. These results may suggest that measuring COM is more sensitive than COP for screening balance performance.
The authors of this article provided primary evidence for potential impairment in PCS in diabetes patients. Balance disorder in DPN has been found to be associated with abnormal SOM feedback (proprioceptive and tactile), which is used for formation of an internal representation of body position and motion (internal model) in the CNS.14,46–48 It has been well established that, in healthy subjects, this internal model is formed and tuned with practice, based on error-dependent learning rules between prior motor action and desired action.44,45 Despite long sensory delays, noise from multiple sources, and many interdependent muscles to control, this internal model enables individuals to produce motor commands (feedforward prediction) appropriate for arbitrary actions. However, there unfortunately is still a paucity of evidence on whether and how alteration in sensory feedback such as a DPN complication affect the feedforward compensatory mechanism. In this study, to examine PCS, we proposed evaluation of postural reciprocal coordination between motion around hip (proximal segment) and ankle (distal segment). This reciprocal coordination allows healthy subjects to compensate movement of the proximal segment via anticipation of the distal segment movement. To quantify this anticipatory strategy, we proposed a simple index called the reciprocal compensatory index. This index may range from 0 to 2 with lower RCI values associated with better compensation of distal segment movement through anticipation of proximal segment movement. Interestingly, results revealed that closing of the eyes or altering SOM feedback will reduce PCS in healthy subjects. Additionally, results suggest that DPN significantly impacts subject’s PCS. On the same note, results suggest that removing visual feedback from DPN patients completely diminishes their reciprocal compensatory strategy for reducing the sway of COM. This consequently may trigger a fall accident in a DPN patient, as contrary to healthy subjects, their motion of distal segments cannot be compensated by motion of their proximal segments.
Interestingly, altering SOM feedback in healthy subjects caused a significant increase in both ankle and hip joint sway compared to DPN subjects. This is an important finding and suggests that neuropathic patients may compensate their distorted SOM information via a prior experience/adaptation process. Further investigation to understand the mechanism of this compensation may open new avenues to design a smart balance training program to improve the balance in DPN.
Results of this study have some limitations. First, we used a small number of subjects; these results will need to be confirmed with a larger sample. Second, these subjects were a convenience sample and may not be representative. Third, we did not match age, gender, and BMI of our healthy group with the DPN group. Another study should be addressed to explore the impact of age, gender, and BMI on the observed results.
Despite these limitations, we believe this system has the potential for extended clinical as well as research applications. It is envisaged that this system could help assess a subject’s PCS, an important component underlying postural control. Additionally, the system allows direct measurement of COM, which may be more sensitive than COP for examining balance control. Finally, the suggested technology is highly portable and does not require installation of any infrastructure, gait laboratory, or highly trained personnel for data acquisition/analysis.
Conclusion
This study suggested an innovative technology based on low-cost, wearable kinematic sensors. Using a two-link biomechanical model of the human body, our device assesses both postural stability and PCS objectively (i.e., anticipatory strategy to compensate movement of the proximal segment by movement of the distal segment). Clinical results of this investigation suggest that alteration in SOM feedback due to DPN will significantly impact postural reciprocal coordination between ankle and hip joints movements. Additionally, in DPN subjects, completely closing the eyes diminished this coordination, which is used by healthy subjects to anticipate motion of the proximal segment by motion of the distal segment. This compensatory strategy in healthy subjects allows them to reduce the variation of COM during both voluntary and involuntary movements. Deterioration in PCS in a DPN subject may make him or her vulnerable in maintaining balance while closing the eyes or in face of high amplitude of sway for either proximal or distal segments.
Acknowledgment
Mr. Samuel Marclay was a master student visiting fellow from Ecole Polytechnique Federale de Lausanne (Lausanne, Switzerland), who partially contributed to this study as a part of his master project.
Abbreviations
- ADA
American Diabetes Association
- AP
anterior–posterior
- BMI
body mass index
- CI
confidence interval
- CNS
central nervous system
- COM
center of mass
- COP
center of pressure
- df
degree of freedom
- DPN
diabetic peripheral neuropathy
- EC
eyes closed
- EO
eyes open
- ICC
intraclass correlation
- ML
medial–lateral
- PCS
postural compensatory strategy
- RCI
reciprocal compensatory index
- SD
standard deviation
- SOM
somatosensory
References
- 1.Tinetti ME, Speechley M, Ginter SF. Risk factors for falls among elderly persons living in the community. N Engl J Med. 1988;319(26):1701–1707. doi: 10.1056/NEJM198812293192604. [DOI] [PubMed] [Google Scholar]
- 2.Doughty K, Lewis R, McIntosh A. The design of a practical and reliable fall detector for community and institutional telecare. J Telemed Telecare. 2000;6(Suppl 1):S150–S154. doi: 10.1258/1357633001934483. [DOI] [PubMed] [Google Scholar]
- 3.De Bruin ED, Najafi B, Murer K, Uebelhart D, Aminian K. Quantification of everyday motor function in a geriatric population. J Rehabil Res Dev. 2007;44(3):417–428. doi: 10.1682/jrrd.2006.01.0003. [DOI] [PubMed] [Google Scholar]
- 4.Maki BE. Gait changes in older adults: predictors of falls or indicators of fear. J Am Geriatr Soc. 1997;45(3):313–320. doi: 10.1111/j.1532-5415.1997.tb00946.x. [DOI] [PubMed] [Google Scholar]
- 5.Najafi B. Physical activity monitoring and risk of falling evaluation in elderly people [dissertation] Lausanne: Ecole Polytechnique Federale de Lausanne (EPFL); 2003. pp. 1–178. [Google Scholar]
- 6.Najafi B, Aminian K, Loew F, Blanc Y, Robert PA. Measurement of stand-sit and sit-stand transitions using a miniature gyroscope and its application in fall risk evaluation in the elderly. IEEE Trans Biomed Eng. 2002;49(8):843–851. doi: 10.1109/TBME.2002.800763. [DOI] [PubMed] [Google Scholar]
- 7.Najafi B, Aminian K, Paraschiv-Ionescu A, Loew F, Büla CJ, Robert P. Ambulatory system for human motion analysis using a kinematic sensor: monitoring of daily physical activity in the elderly. IEEE Trans Biomed Eng. 2003;50(6):711–723. doi: 10.1109/TBME.2003.812189. [DOI] [PubMed] [Google Scholar]
- 8.Zijlstra W, Aminian K. Mobility assessment in older people: new possibilities and challenges. Eur J Ageing. 2007;4:3–12. doi: 10.1007/s10433-007-0041-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vileikyte L, Leventhal H, Gonzalez JS, Peyrot M, Rubin RR, Ulbrecht JS, Garrow A, Waterman C, Cavanagh PR, Boulton AJ. Diabetic peripheral neuropathy and depressive symptoms: the association revisited. Diabetes Care. 2005;28(10):2378–2383. doi: 10.2337/diacare.28.10.2378. [DOI] [PubMed] [Google Scholar]
- 10.Morasso PG, Baratto L, Capra R, Spada G. Internal models in the control of posture. Neural Netw. 1999;12(7-8):1173–1180. doi: 10.1016/s0893-6080(99)00058-1. [DOI] [PubMed] [Google Scholar]
- 11.Massion J. Postural control system. Curr Opin Neurobiol. 1994;4(6):877–887. doi: 10.1016/0959-4388(94)90137-6. [DOI] [PubMed] [Google Scholar]
- 12.National Diabetes Information Clearing house. National diabetes statistics. [cited 2008 Apr]. Available from: http://diabetes.niddk.nih.gov/dm/pubs/statistics.
- 13.Diabetes Mellitus. Fact sheet no. 138.” World Health Organization revised 2002.
- 14.Lord SR, Caplan GA, Colagiuri R, Colagiuri S, Ward JA. Sensori-motor function in older persons with diabetes. Diabet Med. 1993;10(7):614–618. doi: 10.1111/j.1464-5491.1993.tb00133.x. [DOI] [PubMed] [Google Scholar]
- 15.Van Schie CH. Neuropathy: mobility and quality of life. Diabetes Metab Res Rev. 2008;24(Suppl 1):S45–S51. doi: 10.1002/dmrr.856. [DOI] [PubMed] [Google Scholar]
- 16.Tilling LM, Darawil K, Britton M. Falls as a complication of diabetes mellitus in older people. J Diabetes Complications. 2006;20(3):158–162. doi: 10.1016/j.jdiacomp.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 17.Boucher P, Teasdale N, Courtemanche R, Bard C, Fleury M. Postural stability in diabetic polyneuropathy. Diabetes Care. 1995;18(5):638–645. doi: 10.2337/diacare.18.5.638. [DOI] [PubMed] [Google Scholar]
- 18.Peterka RJ. Sensorimotor integration in human postural control. J Neurophysiol. 2002;88(3):1097–1118. doi: 10.1152/jn.2002.88.3.1097. [DOI] [PubMed] [Google Scholar]
- 19.Peterka RJ, Loughlin PJ. Dynamic regulation of sensorimotor integration in human postural control. J Neurophysiol. 2004;91(1):410–423. doi: 10.1152/jn.00516.2003. [DOI] [PubMed] [Google Scholar]
- 20.Nardone A, Galante M, Pareyson D, Schieppati M. Balance control in sensory neuron disease. Clin Neurophysiol. 2007;118(3):538–550. doi: 10.1016/j.clinph.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 21.Nardone A, Grasso M, Schieppati M. Balance control in peripheral neuropathy: are patients equally unstable under static and dynamic conditions? Gait Posture. 2006;23(3):364–373. doi: 10.1016/j.gaitpost.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 22.Nardone A, Schieppati M. Balance control under static and dynamic conditions in patients with peripheral neuropathy. G Ital Med Lav Ergon. 2007;29(1):101–104. [PubMed] [Google Scholar]
- 23.Richardson JK, Ching C, Hurvitz EA. The relationship between electromyographically documented peripheral neuropathy and falls. J Am Geriatr Soc. 1992;40(10):1008–1012. doi: 10.1111/j.1532-5415.1992.tb04477.x. [DOI] [PubMed] [Google Scholar]
- 24.van Deursen RW, Simoneau GG. Foot and ankle sensory neuropathy, proprioception, and postural stability. J Orthop Sports Phys Ther. 1999;29(12):718–726. doi: 10.2519/jospt.1999.29.12.718. [DOI] [PubMed] [Google Scholar]
- 25.Aminian K, Najafi B. Capturing human motion using body-fixed sensors: outdoor measurement and clinical applications. Comput Animation Virtual Worlds. 2004;15(2):79–94. [Google Scholar]
- 26.Favre J, Aissaoui R, Jolles B, Siegrist O, de Guise J, Aminian K. 3D joint rotation measurement using MEMs inertial sensors: application to the knee joint. Valenciennes, France: ISB-3D; 2006. [Google Scholar]
- 27.Favre J, Jolles BM, Siegrist O, Aminian K. Quaternion-based fusion of gyroscopes and accelerometers to improve 3D angle measurement. Electron Lett. 2006;42(11):612–614. [Google Scholar]
- 28.Favre J, Luthi F, Jolles BM, Siegrist O, Najafi B, Aminian K. A new ambulatory system for comparative evaluation of the three-dimensional knee kinematics, applied to anterior cruciate ligament injuries. Knee Surg Sports Traumatol Arthrosc. 2006;14(7):592–604. doi: 10.1007/s00167-005-0023-4. [DOI] [PubMed] [Google Scholar]
- 29.Aminian K, Favre J. Body fixed sensors and their application in sport science. Lausanne, Switzerland: ECSS; 2006. p. 261. [Google Scholar]
- 30.Hart JC, Francis GK, Kauffman LH. Visualizing quaternion rotation. ACM Trans. Graphics. 1994;13(3):256–276. [Google Scholar]
- 31.Kingston DB, Beard RW. Real-time attitude and position estimation for small UAVs using low-cost sensors. Brigham Young University; 2004. [Google Scholar]
- 32.Adlerton AK, Moritz U, Moe-Nilssen R. Forceplate and accelerometer measures for evaluating the effect of muscle fatigue on postural control during one-legged stance. Physiother Res Int. 2003;8(4):187–199. doi: 10.1002/pri.289. [DOI] [PubMed] [Google Scholar]
- 33.Moe-Nilssen R, Helbostad JL. Estimation of gait cycle characteristics by trunk accelerometry. J Biomech. 2004;37(1):121–126. doi: 10.1016/s0021-9290(03)00233-1. [DOI] [PubMed] [Google Scholar]
- 34.Smedal T, Lygren H, Myhr KM, Moe-Nilssen R, Gjelsvik B, Gjelsvik O, Strand LI. Balance and gait improved in patients with MS after physiotherapy based on the Bobath concept. Physiother Res Int. 2006;11(2):104–116. doi: 10.1002/pri.327. [DOI] [PubMed] [Google Scholar]
- 35.Winter DA. Biomechanics and motor control of human movement. Hoboken: John Wiley & Sons; 2009. [Google Scholar]
- 36.Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20(7):1183–1197. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 37.Boulton AJ, Vinik AI, Arezzo JC, Bril V, Feldman EL, Freeman R, Malik RA, Maser RE, Sosenko JM, Ziegler D. American Diabetes Association. Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care. 2005;28(4):956–962. doi: 10.2337/diacare.28.4.956. [DOI] [PubMed] [Google Scholar]
- 38.Young MJ, Breddy JL, Veves A, Boulton AJ. The prediction of diabetic neuropathic foot ulceration using vibration perception thresholds. A prospective study. Diabetes Care. 1994;17(6):557–560. doi: 10.2337/diacare.17.6.557. [DOI] [PubMed] [Google Scholar]
- 39.Armstrong DG, Lavery LA, Vela SA, Quebedeaux TL, Fleischli JG. Choosing a practical screening instrument to identify patients at risk for diabetic foot ulceration. Arch Intern Med. 1998;158(3):289–292. doi: 10.1001/archinte.158.3.289. [DOI] [PubMed] [Google Scholar]
- 40.Alexander E, Young ME. Analyzing rater agreement: manifest variable methods. London: Lawrence Erlbaum Associates Publishers; 2005. [Google Scholar]
- 41.Menz HB, Latt MD, Tiedemann A, Mun San Kwan M, Lord SR. Reliability of the GAITRite walkway system for the qualification of temporo-spatial parameters of gait in young and older people. Gait Posture. 2004;20(1):20–25. doi: 10.1016/S0966-6362(03)00068-7. [DOI] [PubMed] [Google Scholar]
- 42.Najafi B, Helbostad JL, Moe-Nilssen R, Zijlstra W, Aminian K. Does walking strategy in older people change as a function of walking distance? Gait Posture. 2009;29(2):261–266. doi: 10.1016/j.gaitpost.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 43.R-Development-Core-Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing. 2006. Available from: http://www.R-project.org. Accessed on June 1, 2009.
- 44.Smith MA, Ghazizadeh A, Shadmehr R. Interacting adaptive processes with different timescales underlie short-term motor learning. PLoS Biol. 2006;4(6):e179. doi: 10.1371/journal.pbio.0040179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shadmehr R, Mussa-Ivaldi FA. Adaptive representation of dynamics during learning of a motor task. J Neurosci. 1994;14(5 Pt 2):3208–3224. doi: 10.1523/JNEUROSCI.14-05-03208.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Horak FB, Dickstein R, Peterka RJ. Diabetic neuropathy and surface sway-referencing disrupt somatosensory information for postural stability in stance. Somatosens Mot Res. 2002;19(4):316–326. doi: 10.1080/0899022021000037782. [DOI] [PubMed] [Google Scholar]
- 47.DeMott TK, Richardson JK, Thies SB, Ashton-Miller JA. Falls and gait characteristics among older persons with peripheral neuropathy. Am J Phys Med Rehabil. 2007;86(2):125–132. doi: 10.1097/PHM.0b013e31802ee1d1. [DOI] [PubMed] [Google Scholar]
- 48.Richardson JK, Ashton-Miller JA, Lee SG, Jacobs K. Moderate peripheral neuropathy impairs weight transfer and unipedal balance in the elderly. Arch Phys Med Rehabil. 1996;77(11):1152–1156. doi: 10.1016/s0003-9993(96)90139-2. [DOI] [PubMed] [Google Scholar]
- 49.Simoneau GG, Ulbrecht JS, Derr JA, Cavanagh PR. Role of somatosensory input in the control of human posture. Gait Posture. 1995;3:115. [Google Scholar]
- 50.Lafond D, Corriveau H, Prince F. Postural control mechanisms during quiet standing in patients with diabetic sensory neuropathy. Diabetes Care. 2004;27(1):173–178. doi: 10.2337/diacare.27.1.173. [DOI] [PubMed] [Google Scholar]
- 51.Horak FB, Nashner LM, Diener HC. Postural strategies associated with somatosensory and vestibular loss. Exp Brain Res. 1990;82(1):167–177. doi: 10.1007/BF00230848. [DOI] [PubMed] [Google Scholar]