Abstract
Background and Aims
Increased foot skin temperature has been described as a feature of diabetic neuropathy. The aim of this present study was to investigate the association between foot temperature and sudomotor dysfunction in type 2 diabetes mellitus.
Patients and Methods
This study included 51 patients (group A: 25 men, mean age 61.14 ± 6.11 years) without sudomotor dysfunction and 52 patients (group B: 25 men, mean age 59.54 ± 6.18 years) with sudomotor dysfunction. Sudomotor dysfunction was defined as time until complete Neuropad® color change from blue to pink exceeding 600 s in at least one foot. Time until complete color change of the test was also recorded. Foot skin temperature was measured with a handheld infrared thermometer on the plantar aspect of the foot at the level of the first metatarsal head.
Results
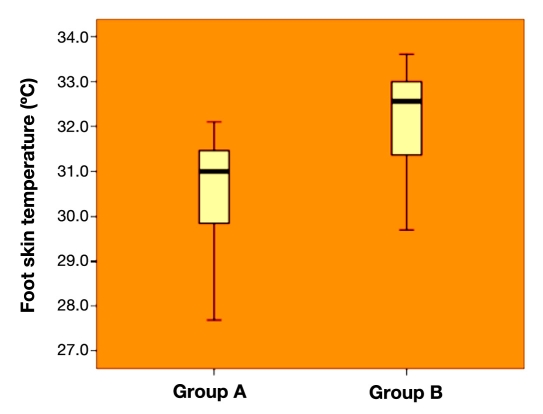
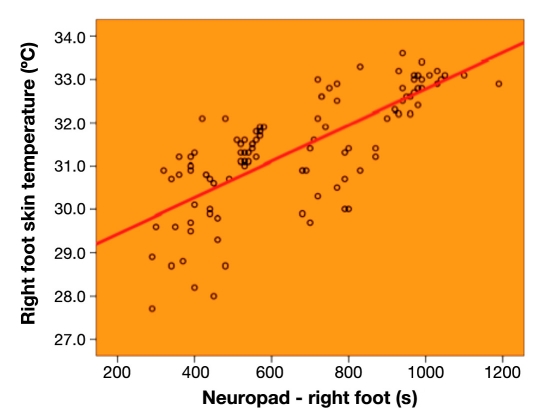
On both feet, temperature was significantly higher in group B than in group A (right foot, group A versus group B, 30.62 ± 1.13 °C versus 32.12 ± 1.06 °C, p < .001; left foot, group A versus group B, 30.65 ± 1.06 °C versus 32.19 ± 1.10 °C, p < .001). There was a significant positive correlation between time to complete Neuropad color change and foot skin temperature (right foot, r = 0.742, p < .001; left foot, r = 0.758, p < .001), which was confirmed in both groups.
Conclusions
Patients with sudomotor dysfunction have significantly higher foot temperature than those without sudomotor dysfunction. Foot temperature is positively correlated with severity of sudomotor dysfunction, as evaluated by the time to complete Neuropad color change.
Keywords: diabetes mellitus, diabetic foot, diabetic neuropathy, Neuropad, temperature measurement
Introduction
Temperature measurement represents a useful, still-growing area of new perspectives for the clinical assessment of the diabetic foot.1,2 Increased foot skin temperature has been described as a classical feature of diabetic neuropathy.3–5 The phenomenon is ascribable to increased blood flow in the foot due to impaired vasoconstriction.4,6,7 The advent of portable infrared thermometers has facilitated temperature measurement and enabled home monitoring of foot skin temperature, thereby preventing re-ulceration among high-risk patients.8,9 Moreover, a significant positive correlation between foot skin temperature and clinical severity of neuropathy has been reported.10
Neuropad® is a new indicator test that evaluates sudomotor dysfunction of the feet in diabetes patients.11 This is based on the evaluation of a color change from blue to pink.11 It has been shown that the time to complete color change is useful in the assessment of the severity of neuropathy, using the Michigan class as the gold standard.12 Of note, the test has been demonstrated to be both highly reproducible13 and very reliable for patient self-examination.14
However, to the best of our knowledge, the association between foot skin temperature and sudomotor dysfunction has not been examined. Thus the aim of the present study was to investigate the potential association between foot temperature and sudomotor dysfunction in type 2 diabetes mellitus.
Patients and Methods
This study included 51 patients (group A: 25 men, mean age 61.14 ± 6.11 years, mean diabetes duration 8.11 ± 4.09 years) without sudomotor dysfunction and 52 patients (group B: 25 men, mean age 59.54 ± 6.18 years, mean diabetes duration 8.02 ± 4.25 years) with sudomotor dysfunction. The two groups were matched for age, sex, and diabetes duration. Patients were recruited from the Outpatient Clinic of the Diabetic Foot at the Second Department at Democritus University of Thrace, Greece. The study was conducted in accordance with the Helsinki Declaration of Human Rights, and all patients gave their informed consent. Recruitment was consecutive and performed in a tertiary care setting.
Exclusion criteria were age <17 or >75 years; peripheral arterial disease; other potential causes of peripheral neuropathy; peripheral nerve lesions; current foot ulceration; trauma or infection; active or past Charcot osteoarthropathy; pedal edema; chronic renal, liver, or heart failure; thyroid disease; other systemic disease; skin diseases (neurodermatitis, psoriasis, scleroderma, allergy to metals, Raynaud syndrome, hyperhidrosis, acrocyanosis); as well as medication that might interfere with sweating or body temperature.10,11 Peripheral arterial disease was defined as ankle–brachial index <0.9 in at least one limb, as measured by the authors using a handheld 8 MHz Doppler device (MiniDop handheld Doppler, Hadeco, Inc., Japan) and a sphygmomanometer.15 Other potential causes of peripheral neuropathy and peripheral nerve lesions were excluded on the basis of patients’ medical history.
Sudomotor dysfunction was diagnosed by means of the indicator test Neuropad.11 Patients were allowed to rest in constant room temperature (25 °C) for 10 min after they had taken off their socks and shoes. Indicator tests were applied to both soles at the level of the 1st–2nd metatarsal heads.11 Time until complete color change of the test from blue to pink was recorded in seconds with an exactitude of 10 s. Sudomotor dysfunction was defined as time until complete color change of the test exceeding 600 s in at least one foot.11
Foot skin temperature was measured with a handheld infrared thermometer (KM 814, Kane-May, United Kingdom) on the plantar aspect of the foot at the level of the first metatarsal head (plantar temperature).10 Foot skin temperature was measured by a physician who was blinded as to the presence of sudomotor dysfunction. Measurement was performed in constant room temperature (25 °C), and a 10-min interval was allocated for patient acclimatization after removal of socks and shoes.10
Statistical analysis was performed using the Statistical Package for Social Sciences 13.0. Quantitative variables had normal distribution (as documented by Kolmogorov Smirnov test). Data were expressed as mean ± standard deviation and were compared by t-test. The correlation between temperature measurements and time until complete Neuropad color change was evaluated by Pearson’s correlation coefficient. Significance was defined at the 5% level (p < .05).
Results
Time to complete Neuropad color change differed significantly between the two groups, as expected (right foot, group A versus group B, 455.49 ± 85.26 s versus 870.58 ± 130.54 s, p < .001; left foot, group A versus group B, 456.08 ± 84.67 s versus 870.77 ± 131.01 s, p < .001). On both feet, temperature was significantly higher in group B than in group A (right foot, group A versus group B, 30.62 ± 1.13 °C versus 32.12 ± 1.06 °C, p < .001, as shown in Figure 1; left foot, group A versus group B, 30.65 ± 1.06 °C versus 32.19 ± 1.10 °C, p < .001).
Figure 1.
Right foot skin temperature in the two groups (group A versus group B, 30.62 ± 1.13 °C versus 32.12 ± 1.06 °C, p <.001).
Overall, there was a significant positive correlation between time to complete Neuropad color change and foot skin temperature (right foot, r = 0.742, p < .001, as shown in Figure 2; left foot, r = 0.758, p < .001). This correlation was observed separately both in group A (right foot, r = 0.618, p < .001; left foot, r = 0.640, p < .001) and in group B (right foot, r = 0.656, p < .001; left foot, r = 0.663, p < .001).
Figure 2.
Correlation between time to complete Neuropad color change and foot skin temperature on the right foot in the entire patient series (r = 0.742, p < .001).
Discussion
The present work examined the association between Neuropad and foot skin temperature measured with a portable infrared thermometer. Both tests have already been reported to be reproducible10,13 and to have value for patient self-examination.8,9,14 It was shown that patients with sudomotor dysfunction exhibited significantly higher foot temperature than those without sudomotor dysfunction. This difference held true for the right and left foot. The difference in temperature may be explained by the fact that the neuropathic foot is often warmer.3,6,7,16 We have previously shown that patients with neuropathy exhibit significantly higher skin temperature on the plantar aspect of the foot.10 This study extends our previous findings to the specific setting of sudomotor dysfunction. The latter is a manifestation of neuropathy due to involvement of sympathetic postganglionic cholinergic fibers that innervate sweat glands, leading to dry skin in the foot.5,17,18 Essentially, it is due to this sudomotor neuropathy that warmer feet do not, as expected, produce more sweat but are instead, paradoxically, associated with dry skin. Interestingly, Neuropad has a very high sensitivity and a modest specificity for peripheral neuropathy.11,12,19 Hence the novel finding that sudomotor dysfunction is associated with increased foot temperature is in harmony with the notion that neuropathic feet are warmer.3,6,7,16
Moreover, a significant positive correlation was observed between time to complete Neuropad color change and foot temperature. Again, this correlation was demonstrable in both legs. Of note, time to complete Neuropad color change has been shown to be a sensitive measure of the severity of neuropathy.12,19 Thus our findings confirm previous evidence that the elevation in foot temperature is associated with the clinical severity of neuropathy.10 Furthermore, given that Neuropad color change is based on a chemical reaction, i.e., the absorption of six water molecules by each molecule of the blue complex salt anhydrous cobalt-II-chloride that is contained in the test, the time required for complete color change is inversely related to skin humidity.20 Therefore, time to complete color change may be regarded as a marker of the severity of sudomotor dysfunction. Accordingly, we have shown that temperature elevation is correlated with the severity of sudomotor dysfunction.
The limitations of this study may be outlined as follows. First, we included patients from a tertiary care setting. Thus its findings are not directly applicable to the general diabetes population. Indeed, it would be interesting to replicate the results in a primary care setting. One must be careful, however, to respect the exclusion criteria suggested in the present work. Secondly, we did not assess diabetes control (mainly hemoglobin A1c), which may have exerted an unrecognized role.
The practical implications of the present work may be described as follows. In type 2 diabetes patients, sudomotor dysfunction is associated with warmer feet. The former has been recently identified as an important risk factor for foot ulceration,21 while the latter is a risk factor for re-ulceration in high-risk patients.8,9 The association herein reported gives rise to the speculation that increased foot temperature might prove of value for the identification of patients at risk for foot ulceration. This argument is enhanced by reported findings, in which multivariate logistic regression analysis revealed that an abnormal Neuropad test was very strongly correlated with presence of foot ulceration.22 Nevertheless, due to the aforementioned limitations, some caution is needed before suggesting increased foot temperature as a risk factor for ulceration in the general diabetes population. Indeed, reexamination in a large prospective survey would be useful.
Conclusions
Patients with sudomotor dysfunction have significantly higher foot temperature than those without sudomotor dysfunction. Foot temperature is positively correlated with severity of sudomotor dysfunction, as evaluated by time to complete Neuropad color change. These findings should be interpreted in the context that both reduced sweating and increased temperature are associated with the risk for foot ulceration and appear to be useful in identifying at-risk patients. Of particular importance, both Neuropad and the handheld infrared thermometer are very easy to use and have been evaluated for patient self-examination.8,9,14 The present work adds to the body of knowledge that the neuropathic foot is warmer and encourages the future application of temperature measurement in the study of the diabetic foot.
References
- 1.Bharara M, Cobb JE, Claremont DJ. Thermography and thermometry in the assessment of diabetic neuropathic foot: a case for furthering the role of thermal techniques. Int J Low Extrem Wounds. 2006;5(4):250–260. doi: 10.1177/1534734606293481. [DOI] [PubMed] [Google Scholar]
- 2.Naicker AS, Roohi SA, Lee CS, Chan WH, Tay LS, Din XJ, Eow LH. Alteration of foot temperature in diabetic neuropathy: is it another piece of puzzle? Med J Malaysia. 2006;61(Suppl A):10–13. [PubMed] [Google Scholar]
- 3.Boyko EJ, Ahroni JH, Stensel VL. Skin temperature in the neuropathic diabetic foot. J Diabetes Complications. 2001;15(5):260–264. doi: 10.1016/s1056-8727(01)00156-8. [DOI] [PubMed] [Google Scholar]
- 4.Donnelly R, Emslie-Smith AM, Gardner ID, Morris AD. ABC of arterial and venous disease: vascular complications of diabetes. BMJ. 2000;320(7241):1062–1066. doi: 10.1136/bmj.320.7241.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boulton AJ. The diabetic foot: from art to science. The 18th Camillo Golgi lecture. Diabetologia. 2004;47(8):1343–1353. doi: 10.1007/s00125-004-1463-y. [DOI] [PubMed] [Google Scholar]
- 6.Ward JD, Boulton AJ, Simms JM, Sandler DA, Knight G. Venous distension in the diabetic neuropathic foot (physical sign of arteriovenous shunting) J R Soc Med. 1983;76(12):1011–1014. doi: 10.1177/014107688307601206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Archer AG, Roberts VC, Watkins PJ. Blood flow patterns in painful diabetic neuropathy. Diabetologia. 1984;27(6):563–567. doi: 10.1007/BF00276968. [DOI] [PubMed] [Google Scholar]
- 8.Lavery LA, Higgins KR, Lanctot DR, Constantinides GP, Zamorano RG, Armstrong DG, Athanasiou KA, Agrawal CM. Home monitoring of foot skin temperatures to prevent ulceration. Diabetes Care. 2004;27(11):2642–2647. doi: 10.2337/diacare.27.11.2642. [DOI] [PubMed] [Google Scholar]
- 9.Lavery LA, Higgins KR, Lanctot DR, Constantinides GP, Zamorano RG, Athanasiou KA, Armstrong DG, Agrawal CM. Preventing diabetic foot ulcer recurrence in high-risk patients: use of temperature monitoring as a self-assessment tool. Diabetes Care. 2007;30(1):14–20. doi: 10.2337/dc06-1600. [DOI] [PubMed] [Google Scholar]
- 10.Papanas N, Papatheodorou K, Papazoglou D, Monastiriotis C, Maltezos E. Foot temperature in type 2 diabetic patients with or without peripheral neuropathy. Exp Clin Endocrinol Diabetes. 2009;117(1):44–47. doi: 10.1055/s-2008-1081498. [DOI] [PubMed] [Google Scholar]
- 11.Papanas N, Papatheodorou K, Christakidis D, Papazoglou D, Giassakis G, Piperidou H, Monastiriotis C, Maltezos E. Evaluation of a new indicator test for sudomotor function (Neuropad) in the diagnosis of peripheral neuropathy in type 2 diabetic patients. Exp Clin Endocrinol Diabetes. 2005;113(4):195–198. doi: 10.1055/s-2005-837735. [DOI] [PubMed] [Google Scholar]
- 12.Papanas N, Giassakis G, Papatheodorou K, Papazoglou D, Monastiriotis C, Christakidis D, Piperidou H, Maltezos E. Use of the new indicator test (Neuropad) for the assessment of the staged severity of neuropathy in type 2 diabetic patients. Exp Clin Endocrinol Diabetes. 2007;115(1):58–61. doi: 10.1055/s-2007-955098. [DOI] [PubMed] [Google Scholar]
- 13.Papanas N, Papatheodorou K, Papazoglou D, Christakidis D, Monastiriotis C, Maltezos E. Reproducibility of the new indicator test for sudomotor function (Neuropad) in patients with type 2 diabetes mellitus: short communication. Exp Clin Endocrinol Diabetes. 2005;113(10):577–581. doi: 10.1055/s-2005-872912. [DOI] [PubMed] [Google Scholar]
- 14.Tentolouris N, Achtsidis V, Marinou K, Katsilambros N. Evaluation of the self-administered indicator plaster Neuropad for the diagnosis of neuropathy in diabetes. Diabetes Care. 2008;31(2):236–237. doi: 10.2337/dc07-1942. [DOI] [PubMed] [Google Scholar]
- 15.Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FGR and the TASC II Working Group. Bell K, Caporusso J, Durand-Zaleski I, Komori K, Lammer J, Liapis C, Novo S, Razavi M, Robbs J, Schaper N, Shigematsu H, Sapoval M, White C, White J, Clement D, Creager M, Jaff M, Mohler E, 3rd, Rutherford RB, Sheehan P, Sillesen H, Rosenfield K. Inter-Society Consensus for the management of vascular disease (TASC II) Eur J Vasc Endovasc Surg. 2007;33(supp 1):S1–S75. doi: 10.1016/j.ejvs.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 16.Rayman G, Hassan A, Tooke JE. Blood flow in the skin of the foot related to posture in diabetes mellitus. Br Med J (Clin Res Ed) 1986;292(6513):87–90. doi: 10.1136/bmj.292.6513.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boulton AJ, Vinik AI, Arezzo JC, Bril V, Feldman EL, Freeman R, Malik RA, Maser RE, Sosenko JM, Ziegler D, American Diabetes Association Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care. 2005;28(4):956–962. doi: 10.2337/diacare.28.4.956. [DOI] [PubMed] [Google Scholar]
- 18.Low PA. Evaluation of sudomotor function. Clin Neurophysiol. 2004;115(7):1506–1513. doi: 10.1016/j.clinph.2004.01.023. [DOI] [PubMed] [Google Scholar]
- 19.Spallone V, Morganti R, Siampli M, Fedele T, D’Amato C, Cacciotti L, Maiello MR. Neuropad as a diagnostic tool for diabetic autonomic and sensorimotor neuropathy. Diabet Med. 2009;26(7):686–692. doi: 10.1111/j.1464-5491.2009.02760.x. [DOI] [PubMed] [Google Scholar]
- 20.Papanas N, Ziegler D. New diagnostic tests for diabetic distal symmetric polyneuropathy. J Diabetes Complications. 2009 doi: 10.1016/j.jdiacomp.2009.09.006. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 21.Tentolouris N, Marinou K, Kokotis P, Karanti A, Diakoumopoulou E, Katsilambros N. Sudomotor dysfunction is associated with foot ulceration in diabetes. Diabet Med. 2009;26(3):302–305. doi: 10.1111/j.1464-5491.2009.02677.x. [DOI] [PubMed] [Google Scholar]
- 22.Tentolouris N, Voulgari C, Liatis S, Kokkinos A, Eleftheriadou I, Makrilakis K, Marinou K, Katsilambros N. Moisture status of the skin of the feet assessed by the visual test neuropad correlates with foot ulceration in diabetes. Diabetes Care. 2010;33(5):1112–1114. doi: 10.2337/dc09-2027. [DOI] [PMC free article] [PubMed] [Google Scholar]