Abstract
Background
Diabetic foot complications represent significant morbidity and precede most of the lower extremity amputations performed. Peripheral neuropathy is a frequent complication of diabetes shown to affect gait. Glycosylation of soft tissues can also affect gait. The purpose of this review article is to highlight the changes in gait for persons with diabetes and highlight the effects of glycosylation on soft tissues at the foot–ground interface.
Methods
PubMed, the Cochrane Library, and EBSCOhost® on-line databases were searched for articles pertaining to diabetes and gait. Bibliographies from relevant manuscripts were also searched.
Findings
Patients with diabetes frequently exhibit a conservative gait strategy where there is slower walking speed, wider base of gait, and prolonged double support time. Glycosylation affects are observed in the lower extremities. Initially, skin thickness decreases and skin hardness increases; tendons thicken; muscles atrophy and exhibit activation delays; bones become less dense; joints have limited mobility; and fat pads are less thick, demonstrate fibrotic atrophy, migrate distally, and may be stiffer.
Interpretation
In conclusion, there do appear to be gait changes in patients with diabetes. These changes, coupled with local soft tissue changes from advanced glycosylated end products, also alter a patient’s gait, putting them at risk of foot ulceration. Better elucidation of these changes throughout the entire spectrum of diabetes disease can help design better treatments and potentially reduce the unnecessarily high prevalence of foot ulcers and amputation.
Keywords: biomechanics, diabetes, foot
Background
Proper gait function (i.e., quality of gait) requires the ability to maintain a safe gait while navigating in complex and changing environments and to conform one’s gait to different task demands. Furthermore, a person’s quality of gait is closely linked to his or her overall state of health. For example, walking speed inversely correlates with the individual’s ability to live independently, perform various activities of daily life (such as crossing a traffic intersection safely), and risk of falling.1,2
Normal walking requires sensory input to adapt and modify motor patterns and muscle output to carry out the desired task.3 Fully functioning joints and bones, combined with adequate muscle strength, are also needed.4 The result of this activity is also coupled with local soft tissue mechanics affecting the foot–ground interface. These can be affected by the frictional properties of the sole, gait velocity, and internal muscle activity.5 The purpose of this review article is to highlight the changes in gait for persons with diabetes. The effects of glycosylation on soft tissues at the foot–ground interface are also described.
Methods
A systematic literature search was conducted using PubMed, U.S. National Library of Medicine’s database of biomedical citations, and abstracts searchable on the Web. It includes over 16 million citations from over 4800 journals published in the United States and more than 70 other countries primarily from 1966 to the present. Additional database searches included the Cochrane Library and EBSCOhost®. The search phrases “diabetes” and “gait” were used to have the largest sensitivity for retrieving papers. In PubMed, 264 papers were identified. In the Cochrane Library, 13 trials were identified. In EBSCOhost, one paper was identified. Additional papers were identified from the bibliographies of select papers. Criteria for inclusion included a well-described methods section for describing the patient population and sampling technique. Additional inclusion criteria included valid statistical techniques and presentation of data.
Are Patients with Diabetes Less Active?
Patients with worsening diabetes appear to be less active than people without diabetes. They are less likely to get the recommended amount of exercise per week and may tend to walk less.6,7 Morrato and colleagues7 examined the Medical Expenditure Panel Survey of approximately 23,000 U.S. adults for 2003. They found that only 39% of patients with diabetes report engaging in at least 30 minutes of moderate exercise three days per week. This is compared to 58% of the healthy population reporting this level of exercise.7 In terms of average activity level, this may be assessed with walking in steps per day. Healthy U.S. adults average ~6000–7000 steps/day.8 The same research group studied walking in patients with type 2 diabetes. On average, this group walked 6662 steps/day.9 Maluf and Mueller6 also studied the activity level in patients with diabetes. Patients with neuropathy and diabetes took ~7816 steps on average, whereas patients with a history of foot ulcer took just 5454 steps/day on average.6 This finding was corroborated by Armstrong and colleagues,10 who reported that those at high risk for diabetic foot ulceration took only 4548 steps/day, on average. Approximately 52% of these steps were taken inside the home.10 It appears that worsening complications from diabetes may affect the average steps per day for patients with diabetes. However, healthy patients with diabetes do not demonstrate less daily walking per se. As exercise and activity levels decrease in patients with diabetes, are there changes in the activity itself?
Is the Quality of Activity Different in Patients with Diabetes?
Many of the aforementioned studies utilized pedometers in approximating the number of steps taken per day in a patient’s natural environment. To date, few studies have attempted to investigate the quality of this activity in the patient’s natural environment. With the emergence of body-worn or fixed-body sensors, this technology is now available. The reliability of gait parameters can change at varying distances and gait speeds.11 Najafi and colleagues11 studied 24 elderly patients over shorter (<10 meters) and longer (>20 meters) walking distances. They found that the reliability of spatiotemporal parameters of gait improved with longer walking distances, although gait variability over both distances was still poor.11 Patients with diabetes will also change their gait strategy based on differences in terrain.12 Outside of gait perturbation studies, this is difficult to assess in a laboratory environment. Allet and colleagues12 studied 16 patients with diabetes with and without neuropathy. Patients wore fixed-body sensors, including four uniaxial gyroscopes attached to each shank and thigh segments using elastic bands. They were asked to walk with their habitual speed over three different surfaces, including tarred, grass, and cobbled stone. The order of walking surface was randomized by subject to remove any potential bias due to learning or fatigue. After 8 days, they were tested again. They reported excellent reliability across the three different conditions. Their results suggested that surfaces have an effect on spatiotemporal parameters of gait in diabetic subjects (p < 0.05). Specifically, the enrolled subjects tended to walk slower on stones on average by 8% compared to walking on grass surface (1.12 ± 0.23 m/s on stones vs 1.21 ± 0.21 m/s on grass). On the same note, they walked slower on grass than on the tarred surface (1.25 ± 0.20 m/s on tar vs 1.21 ± 0.21 m/s on grass).12
Are There Gait Differences in Patients with Diabetes?
Patients with diabetes tend to take shorter steps with a wider base of support.13,14 They also walk slower and demonstrate a longer double support time.13,14 Psycho-logical factors may influence one’s gait pattern beyond aging alone.15,16 Patients with diabetes mellitus and peri-pheral neuropathy (DMPN) have been described to have gait instability.17,18 An unsteadiness in gait demonstrated the strongest association with depressive symptoms in a study by Vileikyte and colleagues.16 Using the Quality of Life Outcomes in Neurological Disorders, the team studied 522 patients with peripheral neuropathy defined using both the neuropathy disability score and vibratory perception threshold testing. Unsteadiness was one of three domains that included pain and loss of feeling.16 These findings were corroborated by Brach and colleagues15 when they studied explanatory variables for gait speed and base support in 558 patients with diabetes. Potential explanatory variables included demographics, health status, mood, cognition, peripheral circulation, sensation, visual impairment, strength, physical activity, and body mass index (BMI). They found that mood and cognition attenuated the relationship between diabetes and gait speed by 50%. Strength, as assessed by repeated chair stand time, explained the greatest proportion of changes in gait speed. None of the variables could explain the increased step width. They attributed this lack of association to be because of potential changes in the motor circuit of the basal ganglia or vestibular system.15
Petrofsky and colleagues14 studied this potential area in 15 patients with diabetes and no strength deficits via manual muscle testing or loss of protective sensation using 10-gram monofilaments. Gait was assessed in a linear path as well during two turning tasks (0.66 and 0.33 meter). Reaction times were assessed as the time taken to stop walking in reaction to a strobe flash. The reaction time was twofold higher in diabetic patients versus age-matched controls. They also demonstrated slower speed and wider step length. Coupled with greater motor error at the joints, the authors suggested that results were due to damage in the vestibular, autonomic, and somatic nervous systems.14 Other authors have observed gait impairment preceding sensory loss.19,20
Courtemanche and colleagues21 observed similar findings in a study of 12 patients with DMPN compared with 7 age-matched controls. Neuropathy was defined using a clinical scoring system. They found a prolonged reaction time in DMPN patients. This was measured using an upper extremity reaction time test to auditory stimulus. These results led the authors to conclude that increased attentional demands with more conservative gait patterns suggest lack of proprioception affecting control of gait.21 Yavuzer and colleagues4 conducted a cross-sectional study of patients with DMPM (n = 20), diabetes (n = 26), and age–gender–BMI-matched control patients (n = 20). They described patients with diabetes as having slower gait, shorter steps, limited knee and ankle mobility, and lower plantar flexion moment and power than the control group. These differences were not significant for the DMPN group. Neuropathic patients were defined by electrophysiological testing, and the duration of diabetes was similar between the groups at 19 and 15 years. They also found that increased glycated hemoglobin (HbA1c) and F-wave distal latency were significantly associated with decreased ankle mobility and peak plantar flexion moment and power.4 Using electromyography (EMG) studies, Sacco and Amadio22 described delayed EMG responses in the thigh and leg compared to normal recruitment patterns for patients with DMPN (n = 16; mean age of 52 years) and a healthy control group (n = 20; mean age of 40 years). There were larger significant activation delays in tibialis anterior and vastas lateralis. This may have an effect on the roles of both muscles in shock attenuation. Based on these observations, they concluded that in addition to somatosensory and motor changes, there are also changes in intrinsic mechanisms of motor control to decrease ankle efficiency in DMPN patients.22 The muscles in patients with diabetes have also been investigated using magnetic resonance imaging (MRI) and isokinetic muscle testing.13,23 Mueller and colleagues13 described gait characteristics in 10 DMPN patients (defined by previous foot ulcer) and 10 healthy age-, gender-, height-, and weight-matched controls. The DMPN patients had significantly less walking speed and stride length with subsequent decreased ankle mobility, moment, and power. Plantar flexor peak torque was measured in a supine position using an isokinetic dynamo-meter. The mean plantar flexor peak torque for the DMPN group was 55% of the age-, gender-, height-, and weight-matched control group.13 Andersen and colleagues23 studied ankle and knee maximal isokinetic muscle strength using an isokinetic dynamometer in 8 DMPN patients, 8 insulin-dependent diabetes mellitus patients without neuropathy, and 16 age-, gender-, height-, and weight-matched controls. They found that peak isokinetic muscle strength in the DMPN group was 59% of the ankle strength of controls and 73% of the knee strength for controls. For the DMPN group, there was a 32% reduction in muscle volume. This group also demonstrated atrophy in the midleg (43%) and distal leg (65%) compared to controls.23 Based on these investigations, muscles in DMPN patients exhibit decreased isokinetic muscle strength, atrophy, and delayed EMG responses.
Role of Advanced Glycosylation End Products (AGEs) Affecting the Foot
As described earlier, limited joint mobility (LJM) changes have been observed in DMPN patients. Coupled with the aforementioned described gait changes in patients with diabetes, advanced glycosylation end products (AGEs) might also affect the soft tissues of the foot. These could include skin,24–26 tendons,27,28 joints,28–34bones,35collagen, and fat pads.24–32,35–49
Skin Changes in Response to AGEs in Diabetes
In a medical hypothesis piece, Wang and Sanders26 described skin adaptation in response to mechanical stresses and how skin may eventually become load tolerant.26 The skin will tend to break down first when subjected to high dynamic shear and compression forces. In response to these stresses, individual collagen fibrils will increase in size even though the total cross section may not change. Proteoglycans and glycosaminoglycans are also believed to be important in this response. The leading hypothesis for this loading response is both fibril degradation (low-load areas) and formation of new fibrils.26 The skin in patients with diabetes has been studied using skin autofluorescence (AF) and a durometer.24,25,49 Thomas and colleagues25 studied 36 patients with diabetes (mean age 45 years) and 18 controls (mean age 57 years). They measured foot sole thickness using ultrasound, skin hardness with a durometer, and plantar pressures. They found plantar pressures, thickness, and hardness increased at ulcer sites compared to controls and nonulcerated areas. During initial DMPN, there was a loss of skin thickness and increased sole hardness that may increase local pressure. With progression of DMPN, increased pressure may increase both thickness and hardness.25Tajaddini and colleagues24 used laser-induced autofluorescence in a cross-sectional (age- and gender-matched) study of 16 patients having a history of diabetes-related foot ulcer (DFU). The plantar skin excited with weak laser light (337 nm) at three sites with measurement of the spectral area under the curve (AUC). The AUC was significantly higher (29%) in DFU patients and decreased prior to reulceration. This was thought to represent intermolecular cross-linking and thinning of skin.24 Using a similar technology, Gerrits and colleagues49 studied 973 patients with diabetes. After a mean follow-up time of 3.1 years, 881 patients were available for follow-up. In a multivariate model, AF was a better predictor of developing subsequent neuropathy than all other clinical predictors, including gender, HbA1c, diabetes duration, and smoking.49
Tendon Changes in Response to Diabetes
Several investigations have looked into changes in tendon from diabetes.27,28 Bolton and colleagues27 used computerized tomography scans to evaluate the thickness of the plantar aponeurosis and flexor hallucis longus (FHL) tendon in patients with DMPN (n = 16 with BMI mean of 32) and healthy controls (n = 10 with BMI mean of 37) that were matched on age, gender, and shoe size. The DMPN patients had significantly thicker plantar aponeurosis (4.2 mm vs 3.6 mm) and thicker FHL that approached significance [4.8 mm vs 4.3 mm (p = 0.051)].27 Giacomozzi and colleagues28 also studied the plantar fascia and the Achilles tendon using ultrasound. They studied DMPN patients (n = 19), patients with diabetes (n = 27), patients with diabetes and previous foot ulcer [(n = 15) DFU], and healthy controls (n = 21). Patients were matched on age, BMI, metabolic control, and diabetes duration. There was a trend of increased thickness of both structures as diabetes severity worsened. Differences were significant when diabetes patients were pooled and compared with controls. This also led to changes in ground reactive forces (GRFs), force × time integrals, and equivalent maximum loading times. For foot ulcer patients, vertical and mediolateral GRF were larger than controls. The equivalent maximum foot loading time was also higher than controls in vertical, anterior–posterior, and mediolateral directions. For DPMN patients, vertical GRFs were larger than controls. The equivalent maximum foot loading time was also higher than controls in vertical, anterior–posterior, and mediolateral directions.28
Joint Mobility Changes in Response to Diabetes
Several authors have also described LJM in patients with diabetes.28–34 Zimny and colleagues29 studied LJM at the ankle and first metatarsophalangeal (MTP) joint in patients with diabetes (n = 35), DMPN (n = 35), and healthy controls (n = 30) matched on age and BMI. In a supine nonweight-bearing position, the DMPN group had significantly less total ankle (17.9° vs 31 or 28.4°) and first MTP joint mobility (35.3° vs 59.4 or 62°) than either healthy or diabetes controls.29 In the previous described study by Giacomozzi and colleagues,28 first MTP joint mobility was also reduced in the DMPN group (55° vs 100°). Wrobel and colleagues32,33 studied end range of motion dorsiflexion in the ankle and first MTP joint in patients with diabetes. They found a significant reduction of about 2–3° for each of these measures in the DMPN group vs the remaining patients with diabetes.32,33 Delbridge and colleagues30 investigated subtalar joint (STJ) mobility in a control group (n = 20), a group with diabetes and no known foot disorders (n = 24), and a group with DFU (n = 18). Subtalar joint mobility was measured in the supine and STJ neutral position measuring total frontal plane motion using a goniometer. Subtalar joint mobility was reduced significantly in the DFU group (18°) vs control (35°) and diabetes control (31°).30 Using similar methods, Fernando and colleagues31 reported similar results to the Delbridge and colleagues30 study. They studied a LJM and neuropathy group (n = 12), a nonneuropathy and LJM group (n = 11), a DMPN group (n = 15), and a diabetes control (n = 11) and a nondiabetes control (n = 15). The LJM and neuropathy group had similar STJ mobility (18°) vs the diabetes control group (29°).31 All of these studies assessed passive range of motion. More recently, Turner and colleagues34 assessed passive and active range of motion at the ankle and first MTP joint in a cross section of patients with diabetes (n = 25), neuropathy (n = 28), ulcer (n = 25), and a nondiabetes reference group (n = 25). They found significant reductions in both measures for first MTP joint dorsiflexion for the ulcer group vs the reference group. Reductions in active inversion/eversion and dorsiflexion/plantar flexion did not reach significance at the ankle. The method did not control for differences in self-selected walking speed, BMI, and age across patient strata.34 By assessing both plantar flexion and dorsiflexion at the ankle, changes in passive and active torque could be related to muscle stiffness.
Muscle Stiffness Changes in Response to Diabetes
Muscle stiffness is a concept related to the previously described changes in delayed muscle activation, weakness, atrophy, tendon thickening, and LJM. Passive muscle stiffness relates to the resistance of a muscle to elongation. This resistance affects passive and active tension develop-ment. Several investigators have tried to better elucidate the contributions of strength, stiffness, and range of motion in subsequent gait impairment.45–48 Farley and Morganroth45 studied leg stiffness during human hopping. The methods may give some better understanding to how patients with diabetes might alter their leg stiffness during gait. The investigators evaluated hip, knee, and ankle stiffness during hopping trials. They reported that modulation of ankle stiffness was the preferred mechanism for controlling overall leg stiffness. Even though knee stiffness increased 1.7-fold, this had no effect on overall leg stiffness.45 Salsich and colleagues46 investigated ankle stiffness in DMPN patients. They studied active46 and passive peak torque48 in 17 patients with DMPN and 17 age-matched controls. In DMPN patients, they found a positive association between all passive plantar flexor torque variables and concentric peak torque, suggesting that intramuscular structures contribute to both strength and stiffness. The DMPN patients also use passive torque for a larger proportion of total torque output. The 36% decrease in concentric plantar flexor peak torque may lead to instability when the center of mass passes anterior to the ankle joint.48 The authors failed to find a significant correlation of passive stiffness and range of motion. They surmised that muscle strength and sensation may be more related to dorsiflexion at the ankle.46 Furthermore, passive stiffness was not different compared to controls. One potential explanation is that changes in muscle atrophy and collagen cross-linking may have negated each other. However, passive stiffness described a significant amount of variance in walking speed. This may have clinical bearing in brace use in this population to increase passive stiffness.47
Fat Pad Changes in Response to Diabetes
Several authors have described atrophy, relocation, and changes in absorption and shear properties in fat pads for patients with diabetes.36–40 In 1986, Gooding and colleagues39 studied the plantar heel and forefoot fat pad using ultrasound. They studied 24 controls, 38 patients with diabetes, and 11 DFU patients. They found that controls had statistically significant thicker fat pads at the heel and first and second metatarsal heads over patients with diabetes. For the heel fat pad, these differences were statistically significant across all three patient groups.39 Using MRI, Bus and colleagues37 studied a finer distinction of patient groupings. They studied age- and gender-matched DMPN patients (n = 13) and DMPN patients with foot deformity (n = 13). They found that the foot deformity group demonstrated significantly less at the metatarsal head level over the phalangeal level, suggesting thinning and distal displacement (dislocation) of the fat pad due to contracture of the digit.37 The activity level of fat pads has been studied using MRI and pseudo-elastic mathematical modeling.36,40 Brash and colleagues36 studied the magnetization transfer (MT) of fat pads in DM controls (n = 11) and DMPN patients (n = 19). The observed differences in MT were attributed to muscle atrophy and fibrotic fat pad atrophy.36 Using cadaver specimens, Hsu and colleagues40 investigated the heel pad stress–strain relationship in loaded and unloaded states. They used electron microscopy to examine six cadaver heels from age-matched diabetic and nondiabetic patients. Using pseudoelastic modeling they concluded that curvature results could explain poor rebound resulting from high-impact energy.40 Stiffness of the heel fat pad in patients with diabetes was investigated by Cheung and colleagues.38 In a cross-sectional study of 12 healthy (mean age 44) and 4 DM patients (mean age 54), they used a prototype MR elastographic apparatus to measure stiffness. Mean elastic moduli results of the pilot study suggested that heel pads trended toward being stiffer in DM patients.38
Bone Changes in Response to Diabetes
Bone has been studied in patients with diabetes.35,50 While Bonds and colleagues50 reported higher bone mineral density (BMD) in women patients with diabetes, Sinacore and colleagues35 found a decrease of BMD in the calcaneus. Bonds and colleagues50 used multivariate analysis to look at the independent risk of falls in women patients with diabetes, whereas Sinacore and colleagues35 studied DMPN (n = 22) and age-, gender-, and race-matched healthy controls (n = 29). They found that control subjects had 13% higher calcaneal BMD than DMPN (p = 0.02). Calcaneal BMD was 16% lower in the foot with deformity compared to the foot without deformity (p = 0.04).35
In summary, for patients with diabetes, local changes occur in the foot. Initially, skin thickness decreases and skin hardness increases; tendons thicken; muscles atrophy; bones become less dense; joints have limited mobility; and fat pads are less thick, demonstrate fibrotic atrophy, dislocate distally, and may be stiffer. A summary of these effects is described in the phases of the gait cycle in Figure 1.
Figure 1.
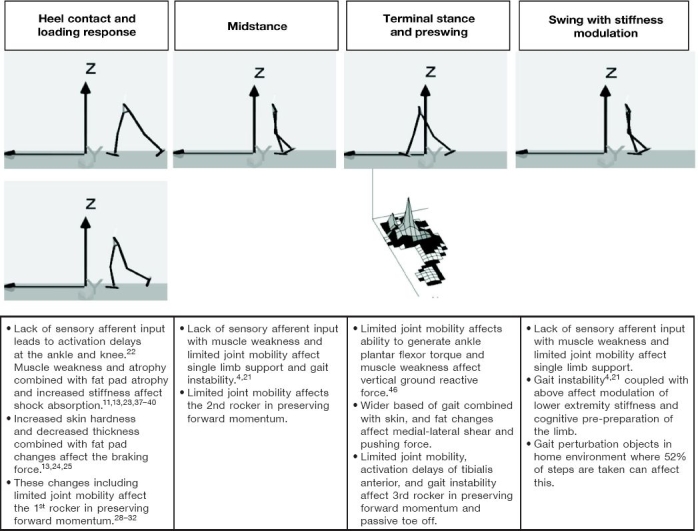
Gait characteristic changes in persons with diabetes.
How Are These Changes Reflected in the Foot–Ground Interface?
How do the aforementioned described changes affect the foot–ground interface? While some authors have described high pressure areas in the plantar aspect of the diabetic foot as being predictive of foot ulcer,51–55 the relationship between activity and ulceration is less clear. Diabetic patients developing foot ulcers seem to have less cumulative plantar stress than those that do not develop foot ulcers.6 Patients with a greater variability of activity have a higher likelihood of developing a foot ulcer.58 Clinical evidence suggests that pressure reduction strategies alone do not have the greatest effect sizes at preventing reulceration. For example, bench studies described a modest effect of reducing pressures using total contact insoles and rocker sole shoes.41,44,57–66 However, clinical footwear trials are equivocal and 26–42% of these patients still reulcerated within 12–18 months.67–70 Part of the results in these trials may be explained by lack of patient adherence to foot wear.71,72 Namely, patients view their homes as “safe zones” and may not wear their prescribed foot wear there, despite taking over 50% of their steps at home.10 Thermometry demonstrates larger effect sizes for preventing reulceration. There are approximately 4- to 10-fold reductions in reulceration for patients using home-based thermometry devices due, ostensibly, to the ability of elevated skin temperatures to act as a surrogate marker for otherwise imperceptible inflammation in the extremity devoid of nociceptive feedback.10,73–77
Is There a Peak Pressure Threshold for Ulceration?
Identifying a peak pressure may be no better than flipping a coin in determining who will develop a subsequent foot ulcer.51,54 In a case-control study of 219 patients with diabetes, Armstrong and colleagues51 measured peak pressure and found that there was no optimal cutoff for peak pressures in patients that ulcerated. Lavery and colleagues54 conducted a large 2-year cohort study of 1666 patients with diabetes; 16% (n = 263) of patients subsequently developed a foot ulcer. The sensitivity and specificity for peak pressures (using an optimal cutoff value of 87.5 N/cm2) were 64 and 46%, respectively.54 The isolated measure of peak pressure does not incorporate a time dimension.78 Two studies have investigated the role of pressure time integral (PTI) and cumulative plantar stress in the development of DFU.52,79 In a cross-sectional study of controls with diabetes (n = 34), DMPN (n = 14), and previous DFU patients (n = 49), Stess and colleagues52 investigated PTI. They found that DFU patients had significantly higher forefoot peak pressure and PTI than controls. The DFU patients also had significantly higher PTI in three out of the four forefoot masks versus one out of the four fore-foot masks for peak pressure over controls. One potential confounding variable was that DFU patients weighed more than controls.52 Maluf and Mueller6 went on to study cumulative plantar stress. In their prospective cohort, they matched patients on age, gender, and BMI. Each study group had 10 patients with controls, DMPN, and DFU patients. They studied peak pressures, PTI, and steps per day. Patients with a history of DFU were significantly less active (46%) than controls and compiled 41% less cumulative daily stress.6 Surprisingly, there are little published data looking at correlations between the actual location of peak pressure and the location of subsequent ulcer development.5,55 Veves and colleagues55 reported that only 38% of ulcer locations matched the peak pressure location. They also found that the peak pressure location actually changed in 59% of patients over the mean follow-up time of 30 months.55
Does Pressure Gradient Offer Advances over Peak Pressure?
Mueller and associates79 and Zou and colleagues80 went on to study pressure gradient in the foot as a possible predictor of foot ulcer. Pressure gradient was defined as a spatial change in plantar pressure around the peak pressure location. In 20 DMPN patients, the peak pressure forefoot-to-rearfoot ratio was 1.48. This was contrasted with a pressure gradient forefoot-to-rearfoot ratio of 2.84. Furthermore, peak pressure accounted for 57% of variance in pressure gradient of the rearfoot and only 35% of the variance in the forefoot. The authors concluded that pressure gradient provided unique information beyond peak pressures alone.79 This research group later studied 20 patients with DMPN and previous DFU. Three-dimensional stresses were calculated from measured pressure at the peak plantar pressure time frame during the gait cycle. The peak maximum shear stress at each depth level was compared to the calculated measure at each point at the same depth level. They found that peak maximal shear stress was significantly higher (1.29) and closer to the surface (2.61 rearfoot to forefoot depth) in the forefoot compared to the rearfoot. Significant correlations were found between peak maximum shear stress and peak pressure gradient (r = 0.61) and peak pressure (r = 0.91). Significant negative correlations were found between depth of peak maximum shear stress and peak pressure gradient (r = –0.61) and peak pressure (r = –0.77).80 Armstrong and colleagues56 studied neuropathic patients with diabetes (n = 100) for a mean of 37 weeks with 8% of patients developing a foot ulcer. Consistent with Maluf and Mueller,6 foot ulcer patients were less active (809 activity cycles vs 1395 activity cycles; p = 0.03). The group found that activity levels in foot ulcer patients had a much higher degree of variation prior to ulceration. The coefficient of variation was twice the value of neuropathic patients (p = 0.0001). This variability increased further 2 weeks prior to ulceration.56
Major Shortcomings of Measuring Peak Plantar Pressure
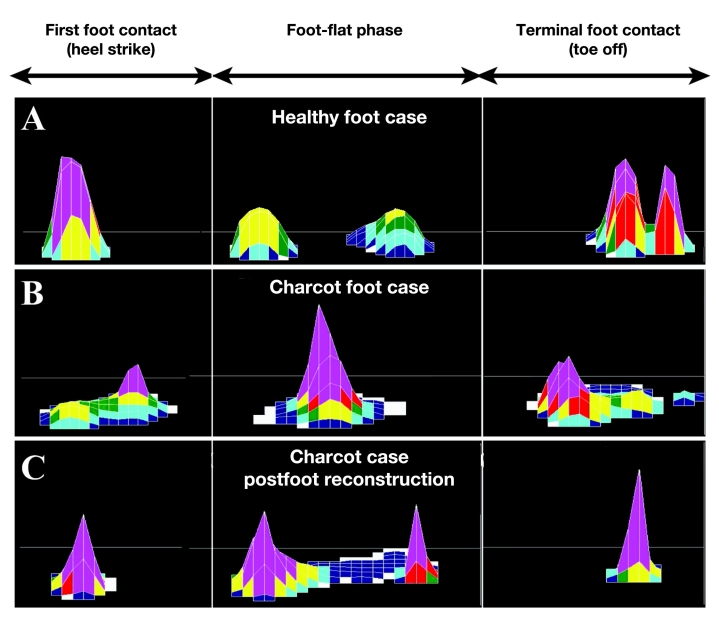
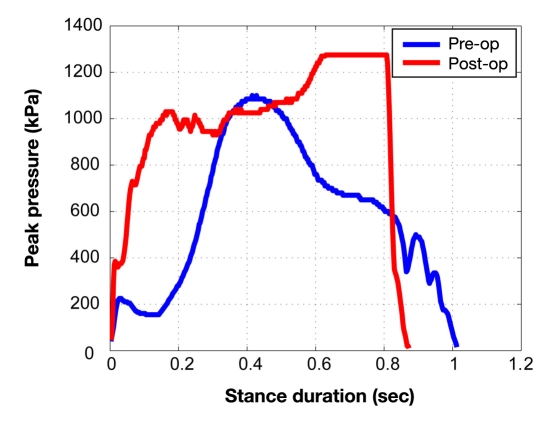
Although many studies have proposed peak plantar pressure (PPP) as a surrogate measure of trauma to the plantar foot, current evaluation methods suffer from various shortcomings. For example, there does not appear to be a specific threshold of PPP that predicts the development of foot ulceration.81 Additionally, PPP can be difficult to interpret due to factors such as gait speed. For example, a postoperative increase in walking speed may be deemed a functional improvement in gait; however, the increased speed could result in increased PPP that would traditionally be viewed as detrimental. For example, Figure 2 illustrates the pattern of plantar pressure spatial distribution during different phases of walking in a typical healthy subject (A), a Charcot neuroarthropathy subject (B), and the same subject postfoot reconstruction (C). Although the shape of plantar distribution postoperation became similar to the healthy subject’s plantar loading pattern, the magnitude of plantar pressure became higher postoperation (see Figure 3), suggesting that measuring PPP is not an accurate measurement of improvement postfoot reconstruction. This is because following reconstructive foot surgery, patients may increase their gait speed as a result of greater confidence and stability, thus demonstrating a more efficient gait pattern. Although this increase may be practically advantageous, it may also result in increased PPP, historically viewed as a negative outcome. To overcome this shortcoming, Najafi and colleagues81 suggested another alternative parameter, which is independent of gait speed and can be used for screening normal plantar loading during walking. This parameter, regression factor (RF), is based on analyzing the pattern of statistical distribution of plantar pressure instead of spatial distribution of plantar loading. In the suggested approach, the duration of stance was normalized using a timescale normalization scheme. To examine whether the statistical distribution of plantar pressure was normal, a customized normal distribution curve was fitted to actual plantar pressure distribution measured at each step. This technique yielded a RF, which represents the similarity of the actual pressure distribution with a normal distribution. Regression factor values may range from negative 1 to positive 1, and as the value increases positively so does the similarity between actual and normalized pressure distributions. The authors tested this novel score on the plantar pressure pattern of healthy subjects (n = 15), Charcot patients preoperation (n = 3), and a Charcot patient postfoot reconstruction (n = 1). In healthy subjects, the RF was 0.46 ± 0.1. When subjects increased their gait speed by 29%, PPP was increased by 8% (p < 10-5), whereas RF was unchanged (p = 0.55), suggesting that the RF value is independent of gait speed. In preoperative Charcot patients, the RF <0; however, the RF increased postsurgery (RF = 0.42), indicating a transition to normal plantar distribution after Charcot reconstruction.81
Figure 2.
Spatial distribution of plantar loading for (A) a typical healthy foot case, (B) a Charcot foot case, and (C) the same Charcot case postfoot reconstruction, respectively, during first foot contact, foot flat, and terminal foot contact phases.
Figure 3.
Magnitude of plantar pressure during walking for a typical Charcot foot case (blue color) and the same case postfoot reconstruction (red case). Postfoot reconstruction, subject walked faster (stance duration was reduced approximately 15%). However, the peak of plantar pressure was increased by 14% postfoot reconstruction, which is viewed historically as a negative outcome.
Shear
Shear has also been implicated in the development of foot ulcers and has been the subject of recent investi-gations.5,82–85 It would stand to reason that shear stress is likely higher around bony contours.85 The importance of shear around bony contours may also be deduced as the local prevalence of foot ulcer and subsequent osteomyelitis from the radiology literature. Ledermann and colleagues86 studied 161 diabetic patients with suspected osteomyelitis. All patients had bone biopsy and MRI imaging. The highest prevalence was at the fifth metatarsal phalangeal joint (MPJ), first MPJ, and Hallux.86 In studying 20 patients with DMPN, Perry and associates83 found that only half of DMPN patients exhibited peak shear at the same location as peak pressure. The importance of actual measurement of shear versus mathematical modeling was described by Yavuz and colleagues.84 In analyzing two models, location errors ranged from 2.2 to 3.1 cm. The most accurate magnitude estimation was 76% root mean squared error to actual ratio. For temporal parameters, the range of root mean squared error was 15–19%.84 In an examination of peak pressure and peak shear locations, Yavuz and colleagues5 studied 10 DMPN patients and 20 healthy controls. In DMPN patients, 20% exhibited peak pressure and peak shear at the same location with 60% having this distance being greater than 2.5 cm. In the control group, none exhibited peak pressure and peak shear at the same location with 35% having this distance being greater than 2.5 cm.5 This research group also reported on the temporal characteristics of these changes using PTI and shear-time integrals (STI). The cross-sectional study consisted of 15 DMPN patients (mean BMI of 29) and 20 healthy controls (mean BMI of 25; p = 0.064). The DMPN group had significant increases in PTI by 54%, peak shear 32%, and STI 61–132% despite walking at slower speeds.85
Thermometry: Elevated Temperatures Predict Foot Complications
Relative temperature changes using thermometry may represent antecedent inflammatory changes occurring prior to frank ulceration. Initial clinical trial effect sizes of a 4- to 10-fold reduction in reulceration are on top of patients already having pressure reduction addressed through total contact insoles, rocker sole shoes, and callus debridement.73–76 Lavery and colleagues77 observed a 4-fold reduction in foot reulceration patients with a history of foot ulcer. In a single-blinded, randomized clinical trial, 85 patients were assigned to either standard therapy or enhanced therapy using twice-daily temperature measures at six sites on each foot. Both groups received therapeutic footwear, diabetic foot education, and regular podiatric evaluation. If temperature differences were ≥4 °F between corresponding sites, patients were advised to reduce activity levels until ≤4 °F. Results were collected over 1987 weeks of data. No significant differences were observed in age, duration of diabetes, or foot risk category. There were significantly more complications (20%) in the standard therapy group compared to the enhanced therapy group. Patients in the thermometry group were 10 times less likely to develop a foot complication (95% confidence interval = 1.2–85.3, p < 0.05).77 In an analysis of subjects participating in a randomized controlled trial of personal thermometry devices, Armstrong and colleagues73 reported that people who ulcerated had a temperature difference 4.8 times greater at the site of ulceration in the week prior to ulceration than a random 7 consecutive-day sample of 50 other subjects that did not ulcerate (3.50 ± 1.0 vs 0.74 ± 0.05, p = 0.001).
Clinical Relevance and Technology Opportunities
The highlighted differences given earlier were observed primarily in patients with clinically detectable DMPN. There are potential implications for clinical practice. Major considerations are ulcer prevention and possible prevention of Charcot arthropathy of the foot. Routine questioning of gait instability and testing for sensory neuropathy and subsequent monitoring for inflammatory changes via thermometry can offer opportunities for prevention. While counterintuitive, exercise training may modify the history of DMPN and/or improve balance,87,88 other rehabilitative medicine techniques such as stretching (or surgical lengthening) may be used with caution due to the previously described passive stiffness changes and reliability on passive torque at the ankle.46 As these patients likely function at the end range of motion, a stretching protocol may alleviate some stiffness symptoms at the potential cost of total ankle torque generation with walking. For the same reasons, lower extremity bracing or foot orthoses might be considered.89 Similar considerations would be given to the third rocker of walking at the MTP level. Improved mobility can be provided with rocker soles or surgical considerations. Footwear with total contact innersoles and rocker soles combined with continued foot care and education may prevent reulceration. Foot care behaviors, such as routine skin hydration, have been described as preventing DFU.90 These results may be from the aforementioned skin changes or may also describe some thermometry findings of increased foot surveillance. Refractory reulceration patients might benefit from newer developmental materials being tested to mitigate shear. These patients may also benefit from referral for surgical reconstruction.
In conclusion, there do appear to be gait changes in patients with diabetes.91,92 These changes, coupled with local soft tissue changes from AGEs, also alter a patient’s gait and puts him or her at risk of foot ulceration. Better elucidation of these changes throughout the entire spectrum of diabetes disease can help design better treatments and potentially reduce the unnecessarily high prevalence of foot ulcers and amputation.87,88
Acknowledgment
We are grateful to David Armstrong, D.P.M., Ph.D.; Ryan Crews, M.S.; and Manish Bharara, Ph.D., for their thoughtful review of this manuscript and assistance with creating the figures.
Abbreviations
- AF
skin autofluorescence
- AGEs
advanced glycosylation end products
- AUC
area under the curve
- BMD
bone mineral density
- BMI
body mass index
- DFU
diabetes-related foot ulcer
- DM
diabetes mellitus
- DMPN
diabetes mellitus and peripheral neuropathy
- EMG
electromyography
- FHL
flexor hallucis longus
- GRFs
ground reactive forces
- HbA1c
glycated hemoglobin
- LJM
limited joint mobility
- MPJ
metatarsal phalangeal joint
- MRI
magnetic resonance imaging
- MT
magnetization transfer
- MTP
metatarsophalangeal
- PPP
peak plantar pressure
- PTI
pressure time integral
- RF
regression factor
- STI
shear-time integral
- STJ
subtalar joint
References
- 1.Bohannon RW, Andrews AW, Thomas MW. Walking speed: reference values and correlates for older adults. J Orthop Sports Phys Ther. 1996;24(2):86–90. doi: 10.2519/jospt.1996.24.2.86. [DOI] [PubMed] [Google Scholar]
- 2.Aminian K, Najafi B, Bula C, Leyvraz P, Robert P. Spatiotemporal parameters of gait measured by an ambulatory system using miniature gyroscopes. J Biomech. 2002;35:689–699. doi: 10.1016/s0021-9290(02)00008-8. [DOI] [PubMed] [Google Scholar]
- 3.Shaw JE, van Schie CH, Carrington AL, Abbott CA, Boulton AJ. An analysis of dynamic forces transmitted through the foot in diabetic neuropathy. Diabetes Care. 1998;21(11):1955–1959. doi: 10.2337/diacare.21.11.1955. [DOI] [PubMed] [Google Scholar]
- 4.Yavuzer G, Yetkin I, Toruner FB, Koca N, Bolukbasi N. Gait deviations of patients with diabetes mellitus: looking beyond peripheral neuropathy. Eura Medicophys. 2006;42(2):127–133. [PubMed] [Google Scholar]
- 5.Yavuz M, Erdemir A, Botek G, Hirschman GB, Bardsley L, Davis BL. Peak plantar pressure and shear locations: relevance to diabetic patients. Diabetes Care. 2007;30(10):2643–2645. doi: 10.2337/dc07-0862. [DOI] [PubMed] [Google Scholar]
- 6.Maluf KS, Mueller MJ. Novel Award 2002. Comparison of physical activity and cumulative plantar tissue stress among subjects with and without diabetes mellitus and a history of recurrent plantar ulcers. Clin Biomech (Bristol, Avon) 2003;18(7):567–575. doi: 10.1016/s0268-0033(03)00118-9. [DOI] [PubMed] [Google Scholar]
- 7.Morrato EH, Hill JO, Wyatt HR, Ghushchyan V, Sullivan PW. Physical activity in U.S. adults with diabetes and at risk for developing diabetes, 2003. Diabetes Care. 2007;30(2):203–209. doi: 10.2337/dc06-1128. [DOI] [PubMed] [Google Scholar]
- 8.Tudor-Locke C, Bassett DR., Jr How many steps/day are enough? Preliminary pedometer indices for public health. Sports Med. 2004;34(1):1–8. doi: 10.2165/00007256-200434010-00001. [DOI] [PubMed] [Google Scholar]
- 9.Tudor-Locke CE, Bell RC, Myers AM, Harris SB, Lauzon N, Rodger NW. Pedometer-determined ambulatory activity in individuals with type 2 diabetes. Diabetes Res Clin Pract. 2002;55(3):191–199. doi: 10.1016/s0168-8227(01)00317-5. [DOI] [PubMed] [Google Scholar]
- 10.Armstrong DG, Abu-Rumman PL, Nixon BP, Boulton AJ. Continuous activity monitoring in persons at high risk for diabetes-related lower-extremity amputation. J Am Podiatr Med Assoc. 2001;91(9):451–455. doi: 10.7547/87507315-91-9-451. [DOI] [PubMed] [Google Scholar]
- 11.Najafi B, Helbostad JL, Moe-Nilssen R, Zijlstra W, Aminian K. Does walking strategy in older people change as a function of walking distance? Gait Posture. 2009;29(2):261–266. doi: 10.1016/j.gaitpost.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 12.Allet L, Armand S, de Bie RA, Golay A, Monnin D, Aminian K, de Bruin ED. Reliability of diabetic patients’ gait parameters in a challenging environment. Gait Posture. 2008;28(4):680–686. doi: 10.1016/j.gaitpost.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 13.Mueller MJ, Minor SD, Sahrmann SA, Schaaf JA, Strube MJ. Differences in the gait characteristics of patients with diabetes and peripheral neuropathy compared with age-matched controls. Phys Ther. 1994;74(4):299–308. doi: 10.1093/ptj/74.4.299. discussion 309-13. [DOI] [PubMed] [Google Scholar]
- 14.Petrofsky J, Lee S, Bweir S. Gait characteristics in people with type 2 diabetes mellitus. Eur J Appl Physiol. 2005;93(5-6):640–647. doi: 10.1007/s00421-004-1246-7. [DOI] [PubMed] [Google Scholar]
- 15.Brach JS, Talkowski JB, Strotmeyer ES, Newman AB. Diabetes mellitus and gait dysfunction: possible explanatory factors. Phys Ther. 2008;88(11):1365–1374. doi: 10.2522/ptj.20080016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vileikyte L, Leventhal H, Gonzalez JS, Peyrot M, Rubin RR, Ulbrecht JS, Garrow A, Waterman C, Cavanagh PR, Boulton AJ. Diabetic peripheral neuropathy and depressive symptoms: the association revisited. Diabetes Care. 2005;28(10):2378–2383. doi: 10.2337/diacare.28.10.2378. [DOI] [PubMed] [Google Scholar]
- 17.Cavanagh PR, Simoneau GG, Ulbrecht JS. Ulceration, unsteadiness, and uncertainty: the biomechanical consequences of diabetes mellitus. J Biomech. 1993;26(Suppl 1):23–40. doi: 10.1016/0021-9290(93)90077-r. [DOI] [PubMed] [Google Scholar]
- 18.Resnick HE, Vinik AI, Schwartz AV, Leveille SG, Brancati FL, Balfour J, Guralnik JM. Independent effects of peripheral nerve dysfunction on lower-extremity physical function in old age: the Women’s Health and Aging Study. Diabetes Care. 2000;23(11):1642–1647. doi: 10.2337/diacare.23.11.1642. [DOI] [PubMed] [Google Scholar]
- 19.Turgut N, Karasalihoglu S, Kücükugurluoglu Y, Balci K, Ekuklu G, Tütüncüler F. Clinical utility of dorsal sural nerve conduction studies in healthy and diabetic children. Clin Neurophysiol. 2004;115(6):1452–1456. doi: 10.1016/j.clinph.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 20.Vinik AI, Freeman R, Erbas T. Diabetic autonomic neuropathy. Semin Neurol. 2003;23(4):365–372. doi: 10.1055/s-2004-817720. [DOI] [PubMed] [Google Scholar]
- 21.Courtemanche R, Teasdale N, Boucher P, Fleury M, Lajoie Y, Bard C. Gait problems in diabetic neuropathic patients. Arch Phys Med Rehabil. 1996;77(9):849–855. doi: 10.1016/s0003-9993(96)90269-5. [DOI] [PubMed] [Google Scholar]
- 22.Sacco IC, Amadio AC. Influence of the diabetic neuropathy on the behavior of electromyographic and sensorial responses in treadmill gait. Clin Biomech (Bristol, Avon) 2003;18(5):426–434. doi: 10.1016/s0268-0033(03)00043-3. [DOI] [PubMed] [Google Scholar]
- 23.Andersen H, Gadeberg PC, Brock B, Jakobsen J. Muscular atrophy in diabetic neuropathy: a stereological magnetic resonance imaging study. Diabetologia. 1997;40(9):1062–1069. doi: 10.1007/s001250050788. [DOI] [PubMed] [Google Scholar]
- 24.Tajaddini A, Scoffone HM, Botek G, Davis BL. Laser-induced auto-fluorescence (LIAF) as a method for assessing skin stiffness preceding diabetic ulcer formation. J Biomech. 2007;40(4):736–741. doi: 10.1016/j.jbiomech.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 25.Thomas VJ, Patil KM, Radhakrishnan S, Narayanamurthy VB, Parivalavan R. The role of skin hardness, thickness, and sensory loss on standing foot power in the development of plantar ulcers in patients with diabetes mellitus--a preliminary study. Int J Low Extrem Wounds. 2003;2(3):132–139. doi: 10.1177/1534734603258601. [DOI] [PubMed] [Google Scholar]
- 26.Wang YN, Sanders JE. How does skin adapt to repetitive mechanical stress to become load tolerant? Med Hypotheses. 2003;61(1):29–35. doi: 10.1016/s0306-9877(03)00100-2. [DOI] [PubMed] [Google Scholar]
- 27.Bolton NR, Smith KE, Pilgram TK, Mueller MJ, Bae KT. Computed tomography to visualize and quantify the plantar aponeurosis and flexor hallucis longus tendon in the diabetic foot. Clin Biomech (Bristol, Avon) 2005;20(5):540–546. doi: 10.1016/j.clinbiomech.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 28.Giacomozzi C, D’Ambrogi E, Uccioli L, Macellari V. Does the thickening of Achilles tendon and plantar fascia contribute to the alteration of diabetic foot loading? Clin Biomech (Bristol, Avon) 2005;20(5):532–539. doi: 10.1016/j.clinbiomech.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 29.Zimny S, Schatz H, Pfohl M. The role of limited joint mobility in diabetic patients with an at-risk foot. Diabetes Care. 2004;27(4):942–946. doi: 10.2337/diacare.27.4.942. [DOI] [PubMed] [Google Scholar]
- 30.Delbridge L, Perry P, Marr S, Arnold N, Yue DK, Turtle JR, Reeve TS. Limited joint mobility in the diabetic foot: relationship to neuropathic ulceration. Diabet Med. 1988;5(4):333–337. doi: 10.1111/j.1464-5491.1988.tb01000.x. [DOI] [PubMed] [Google Scholar]
- 31.Fernando DJ, Masson EA, Veves A, Boulton AJ. Relationship of limited joint mobility to abnormal foot pressures and diabetic foot ulceration. Diabetes Care. 1991;14(1):8–11. doi: 10.2337/diacare.14.1.8. [DOI] [PubMed] [Google Scholar]
- 32.Wrobel JS, Birkmeyer NJ, Dercoli JL, Connolly JE. Do clinical examination variables predict high plantar pressures in the diabetic foot? J Am Podiatr Med Assoc. 2003;93(5):367–372. doi: 10.7547/87507315-93-5-367. [DOI] [PubMed] [Google Scholar]
- 33.Wrobel JS, Connolly JE, Beach ML. Associations between static and functional measures of joint function in the foot and ankle. J Am Podiatr Med Assoc. 2004;94(6):535–541. doi: 10.7547/0940535. [DOI] [PubMed] [Google Scholar]
- 34.Turner DE, Helliwell PS, Burton AK, Woodburn J. The relationship between passive range of motion and range of motion during gait and plantar pressure measurements. Diabet Med. 2007;24(11):1240–1246. doi: 10.1111/j.1464-5491.2007.02233.x. [DOI] [PubMed] [Google Scholar]
- 35.Sinacore DR, Bohnert KL, Hastings MK, Johnson JE. Mid foot kinetics characterize structural polymorphism in diabetic foot disease. Clin Biomech (Bristol, Avon) 2008;23(5):653–661. doi: 10.1016/j.clinbiomech.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brash PD, Foster J, Vennart W, Anthony P, Tooke JE. Magnetic resonance imaging techniques demonstrate soft tissue damage in the diabetic foot. Diabet Med. 1999;16(1):55–61. doi: 10.1046/j.1464-5491.1999.00005.x. [DOI] [PubMed] [Google Scholar]
- 37.Bus SA, Maas M, Cavanagh PR, Michels RP, Levi M. Plantar fat-pad displacement in neuropathic diabetic patients with toe deformity: a magnetic resonance imaging study. Diabetes Care. 2004;27(10):2376–2381. doi: 10.2337/diacare.27.10.2376. [DOI] [PubMed] [Google Scholar]
- 38.Cheung YY, Doyley M, Miller TB, Kennedy F, Lynch F, Jr, Wrobel JS, Paulson K, Weaver J. Magnetic resonance elastography of the plantar fat pads: preliminary study in diabetic patients and asymptomatic volunteers. J Comput Assist Tomogr. 2006;30(2):321–326. doi: 10.1097/00004728-200603000-00031. [DOI] [PubMed] [Google Scholar]
- 39.Gooding GA, Stess RM, Graf PM, Moss KM, Louie KS, Grunfeld C. Sonography of the sole of the foot. Evidence for loss of foot pad thickness in diabetes and its relationship to ulceration of the foot. Invest Radiol. 1986;21(1):45–48. [PubMed] [Google Scholar]
- 40.Hsu TC, Lee YS, Shau YW. Biomechanics of the heel pad for type 2 diabetic patients. Clin Biomech (Bristol, Avon) 2002;17(4):291–296. doi: 10.1016/s0268-0033(02)00018-9. [DOI] [PubMed] [Google Scholar]
- 41.Lott DJ, Hastings MK, Commean PK, Smith KE, Mueller MJ. Effect of footwear and orthotic devices on stress reduction and soft tissue strain of the neuropathic foot. Clin Biomech (Bristol, Avon) 2007;22(3):352–359. doi: 10.1016/j.clinbiomech.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maluf KS, Mueller MJ, Strube MJ, Engsberg JR, Johnson JE. Tendon Achilles lengthening for the treatment of neuropathic ulcers causes a temporary reduction in forefoot pressure associated with changes in plantar flexor power rather than ankle motion during gait. J Biomech. 2004;37(6):897–906. doi: 10.1016/j.jbiomech.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 43.Salsich GB, Mueller MJ, Hastings MK, Sinacore DR, Strube MJ, Johnson JE. Effect of Achilles tendon lengthening on ankle muscle performance in people with diabetes mellitus and a neuropathic plantar ulcer. Phys Ther. 2005;85(1):34–43. [PubMed] [Google Scholar]
- 44.Bus SA, Ulbrecht JS, Cavanagh PR. Pressure relief and load redistribution by custom-made insoles in diabetic patients with neuropathy and foot deformity. Clin Biomech (Bristol, Avon) 2004;19(6):629–638. doi: 10.1016/j.clinbiomech.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 45.Farley CT, Morgenroth DC. Leg stiffness primarily depends on ankle stiffness during human hopping. J Biomech. 1999;32(3):267–273. doi: 10.1016/s0021-9290(98)00170-5. [DOI] [PubMed] [Google Scholar]
- 46.Salsich GB, Brown M, Mueller MJ. Relationships between plantar flexor muscle stiffness, strength, and range of motion in subjects with diabetes-peripheral neuropathy compared to age-matched controls. J Orthop Sports Phys Ther. 2000;30(8):473–483. doi: 10.2519/jospt.2000.30.8.473. [DOI] [PubMed] [Google Scholar]
- 47.Salsich GB, Mueller MJ. Effect of plantar flexor muscle stiffness on selected gait characteristics. Gait Posture. 2000;11(3):207–216. doi: 10.1016/s0966-6362(00)00047-3. [DOI] [PubMed] [Google Scholar]
- 48.Salsich GB, Mueller MJ, Sahrmann SA. Passive ankle stiffness in subjects with diabetes and peripheral neuropathy versus an age-matched comparison group. Phys Ther. 2000;80(4):352–362. doi: 10.1093/ptj/80.4.352. [DOI] [PubMed] [Google Scholar]
- 49.Gerrits EG, Lutgers HL, Kleefstra N, Graaff R, Groenier KH, Smit AJ, Gans RO, Bilo HJ. Skin autofluorescence: a tool to identify type 2 diabetic patients at risk for developing microvascular complications. Diabetes Care. 2008;31(3):517–521. doi: 10.2337/dc07-1755. [DOI] [PubMed] [Google Scholar]
- 50.Bonds DE, Larson JC, Schwartz AV, Strotmeyer ES, Robbins J, Rodriguez BL, Johnson KC, Margolis KL. Risk of fracture in women with type 2 diabetes: the Women’s Health Initiative Observational Study. J Clin Endocrinol Metab. 2006;91(9):3404–3410. doi: 10.1210/jc.2006-0614. [DOI] [PubMed] [Google Scholar]
- 51.Armstrong DG, Peters EJ, Athanasiou KA, Lavery LA. Is there a critical level of plantar foot pressure to identify patients at risk for neuropathic foot ulceration? J Foot Ankle Surg. 1998;37(4):303–307. doi: 10.1016/s1067-2516(98)80066-5. [DOI] [PubMed] [Google Scholar]
- 52.Stess RM, Jensen SR, Mirmiran R. The role of dynamic plantar pressures in diabetic foot ulcers. Diabetes Care. 1997;20(5):855–858. doi: 10.2337/diacare.20.5.855. [DOI] [PubMed] [Google Scholar]
- 53.Frykberg RG, Lavery LA, Pham H, Harvey C, Harkless L, Veves A. Role of neuropathy and high foot pressures in diabetic foot ulceration. Diabetes Care. 1998;21(10):1714–1719. doi: 10.2337/diacare.21.10.1714. [DOI] [PubMed] [Google Scholar]
- 54.Lavery LA, Armstrong DG, Wunderlich RP, Tredwell J, Boulton AJ. Predictive value of foot pressure assessment as part of a population-based diabetes disease management program. Diabetes Care. 2003;26(4):1069–1073. doi: 10.2337/diacare.26.4.1069. [DOI] [PubMed] [Google Scholar]
- 55.Veves A, Murray HJ, Young MJ, Boulton AJ. The risk of foot ulceration in diabetic patients with high foot pressure: a prospective study. Diabetologia. 1992;35(7):660–663. doi: 10.1007/BF00400259. [DOI] [PubMed] [Google Scholar]
- 56.Armstrong DG, Lavery LA, Holtz-Neiderer K, Mohler MJ, Wendel CS, Nixon BP, Boulton AJ. Variability in activity may precede diabetic foot ulceration. Diabetes Care. 2004;27(8):1980–1984. doi: 10.2337/diacare.27.8.1980. [DOI] [PubMed] [Google Scholar]
- 57.Albert SF, Christensen LC. Diabetic foot pressure studies: comparison study of patient-selected shoes versus clinician selected shoes. Lower Extremity. 1994;1:21–27. [Google Scholar]
- 58.Ashry HR, Lavery LA, Murdoch DP, Frolich M, Lavery DC. Effectiveness of diabetic insoles to reduce foot pressures. J Foot Ankle Surg. 1997;36(4):268–271. doi: 10.1016/s1067-2516(97)80071-3. discussion 328-9. [DOI] [PubMed] [Google Scholar]
- 59.Brown M, Rudicel S, Esquenazi A. Measurement of dynamic pressures at the shoe-foot interface during normal walking with various foot orthoses using the FSCAN system. Foot Ankle Int. 1996;17(3):152–156. doi: 10.1177/107110079601700306. [DOI] [PubMed] [Google Scholar]
- 60.Long JT, Klein JP, Sirota NM, Wertsch JJ, Janisse D, Harris GF. Biomechanics of the double rocker sole shoe: gait kinematics and kinetics. J Biomech. 2007;40(13):2882–2890. doi: 10.1016/j.jbiomech.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 61.Lord M, Hosein R. Pressure redistribution by molded inserts in diabetic footwear: a pilot study. J Rehabil Res Dev. 1994;31(3):214–221. [PubMed] [Google Scholar]
- 62.Mueller MJ, Lott DJ, Hastings MK, Commean PK, Smith KE, Pilgram TK. Efficacy and mechanism of orthotic devices to unload metatarsal heads in people with diabetes and a history of plantar ulcers. Phys Ther. 2006;86(6):833–842. [PubMed] [Google Scholar]
- 63.Novick A, Stone J, Birke JA, Brasseaux DM, Broussard JB, Hoard AS, Hawkins ES. Reduction of plantar pressure with the rigid relief orthosis. J Am Podiatr Med Assoc. 1993;83(3):115–122. doi: 10.7547/87507315-83-3-115. [DOI] [PubMed] [Google Scholar]
- 64.Postema K, Burm PE, Zande ME, Limbeek J. Primary metatarsalgia: the influence of a custom moulded insole and a rockerbar on plantar pressure. Prosthet Orthot Int. 1998;22(1):35–44. doi: 10.3109/03093649809164455. [DOI] [PubMed] [Google Scholar]
- 65.Uccioli L, Toffolo M, Volpe A, Pasqualitto P, Ferri A, Monticone G, Menzinger G. Efficacy of different shoes and insoles in reducing plantar pressures in diabetic neuropathic patients. Diabetologia. 1997;40(Suppl 1):A-489. [Google Scholar]
- 66.Hastings MK, Mueller MJ, Pilgram TK, Lott DJ, Commean PK, Johnson JE. Effect of metatarsal pad placement on plantar pressure in people with diabetes mellitus and peripheral neuropathy. Foot Ankle Int. 2007;28(1):84–88. doi: 10.3113/FAI.2007.0015. [DOI] [PubMed] [Google Scholar]
- 67.Busch K, Chantelau E. Effectiveness of a new brand of stock “diabetic” shoes to protect against diabetic foot ulcer relapse. A prospective cohort study. Diabet Med. 2003;20(8):665–659. doi: 10.1046/j.1464-5491.2003.01003.x. [DOI] [PubMed] [Google Scholar]
- 68.Colagiuri S, Marsden LL, Naidu V, Taylor L. The use of orthotic devices to correct plantar callus in people with diabetes. Diabetes Res Clin Pract. 1995;28(1):29–34. doi: 10.1016/0168-8227(95)01050-n. [DOI] [PubMed] [Google Scholar]
- 69.Reiber GE, Smith DG, Wallace CM, Vath CA, Sullivan K, Hayes S, Yu O, Martin D, Maciejewski M. Footwear used by individuals with diabetes and a history of foot ulcer. J Rehabil Res Dev. 2002;39(5):615–622. [PubMed] [Google Scholar]
- 70.Uccioli L, Faglia E, Monticone G, Favales F, Durola L, Aldeghi A, Quarantiello A, Calia P, Menzinger G. Manufactured shoes in the prevention of diabetic foot ulcers. Diabetes Care. 1995;18(10):1376–1378. doi: 10.2337/diacare.18.10.1376. [DOI] [PubMed] [Google Scholar]
- 71.Knowles EA, Boulton AJ. Do people with diabetes wear their prescribed footwear? Diabet Med. 1996;13(12):1064–1068. doi: 10.1002/(SICI)1096-9136(199612)13:12<1064::AID-DIA253>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 72.Nixon BP, Armstrong DG, Wendell C, Vazquez JR, Rabinovich Z, Kimbriel HR, Rosales MA, Boulton AJ. Do US veterans wear appropriately sized shoes?: the Veterans Affairs shoe size selection study. J Am Podiatr Med Assoc. 2006;96(4):290–292. doi: 10.7547/0960290. [DOI] [PubMed] [Google Scholar]
- 73.Armstrong DG, Holtz-Neiderer K, Wendel C, Mohler MJ, Kimbriel HR, Lavery LA. Skin temperature monitoring reduces the risk for diabetic foot ulceration in high-risk patients. Am J Med. 2007;120(12):1042–1046. doi: 10.1016/j.amjmed.2007.06.028. [DOI] [PubMed] [Google Scholar]
- 74.Armstrong DG, Lavery LA. Predicting neuropathic ulceration with infrared dermal thermometry. J Am Podiatr Med Assoc. 1997;87(7):336–337. doi: 10.7547/87507315-87-7-336. [DOI] [PubMed] [Google Scholar]
- 75.Armstrong DG, Lavery LA, Liswood PJ, Todd WF, Tredwell JA. Infrared dermal thermometry for the high-risk diabetic foot. Phys Ther. 1997;77(2):169–175. doi: 10.1093/ptj/77.2.169. discussion 176-7. [DOI] [PubMed] [Google Scholar]
- 76.Armstrong DG, Sangalang MB, Jolley D, Maben F, Kimbriel HR, Nixon BP, Cohen IK. Cooling the foot to prevent diabetic foot wounds: a proof-of-concept trial. J Am Podiatr Med Assoc. 2005;95(2):103–107. doi: 10.7547/0950103. [DOI] [PubMed] [Google Scholar]
- 77.Lavery LA, Higgins KR, Lanctot DR, Constantinides GP, Zamorano RG, Armstrong DG, Athanasiou KA, Agrawal CM. Home monitoring of foot skin temperatures to prevent ulceration. Diabetes Care. 2004;27(11):2642–2647. doi: 10.2337/diacare.27.11.2642. [DOI] [PubMed] [Google Scholar]
- 78.Brand PW. Tenderizing the foot. Foot Ankle Int. 2003;24(6):457–461. doi: 10.1177/107110070302400602. [DOI] [PubMed] [Google Scholar]
- 79.Mueller MJ, Zou D, Lott DJ. “Pressure gradient” as an indicator of plantar skin injury. Diabetes Care. 2005;28(12):2908–2912. doi: 10.2337/diacare.28.12.2908. [DOI] [PubMed] [Google Scholar]
- 80.Zou D, Mueller MJ, Lott DJ. Effect of peak pressure and pressure gradient on subsurface shear stresses in the neuropathic foot. J Biomech. 2007;40(4):883–890. doi: 10.1016/j.jbiomech.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 81.Najafi B, Crews RT, Armstrong DG, Rogers LC, Aminian K, Wrobel J. Can we predict outcome of surgical reconstruction of Charcot neuroarthropathy by dynamic plantar pressure assessment?--A proof of concept study. Gait Posture. 2010;31(1):87–92. doi: 10.1016/j.gaitpost.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 82.Mackey JR, Davis BL. Simultaneous shear and pressure sensor array for assessing pressure and shear at foot/ground interface. J Biomech. 2006;39(15):2893–2897. doi: 10.1016/j.jbiomech.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 83.Perry JE, Hall JO, Davis BL. Simultaneous measurement of plantar pressure and shear forces in diabetic individuals. Gait Posture. 2002;15(1):101–107. doi: 10.1016/s0966-6362(01)00176-x. [DOI] [PubMed] [Google Scholar]
- 84.Yavuz M, Botek G, Davis BL. Plantar shear stress distributions: comparing actual and predicted frictional forces at the foot-ground interface. J Biomech. 2007;40(13):3045–3049. doi: 10.1016/j.jbiomech.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yavuz M, Tajaddini A, Botek G, Davis BL. Temporal characteristics of plantar shear distribution: relevance to diabetic patients. J Biomech. 2008;41(3):556–559. doi: 10.1016/j.jbiomech.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ledermann HP, Morrison WB, Schweitzer ME. MR image analysis of pedal osteomyelitis: distribution, patterns of spread, and frequency of associated ulceration and septic arthritis. Radiology. 2002;223(3):747–755. doi: 10.1148/radiol.2233011279. [DOI] [PubMed] [Google Scholar]
- 87.Balducci S, Iacobellis G, Parisi L, Di Biase N, Calandriello E, Leonetti F, Fallucca F. Exercise training can modify the natural history of diabetic peripheral neuropathy. J Diabetes Complications. 2006;20(4):216–223. doi: 10.1016/j.jdiacomp.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 88.Richardson JK, Sandman D, Vela S. A focused exercise regimen improves clinical measures of balance in patients with peripheral neuropathy. Arch Phys Med Rehabil. 2001;82(2):205–209. doi: 10.1053/apmr.2001.19742. [DOI] [PubMed] [Google Scholar]
- 89.MacLean C, Davis IM, Hamill J. Influence of a custom foot orthotic intervention on lower extremity dynamics in healthy runners. Clin Biomech (Bristol, Avon) 2006;21(6):623–630. doi: 10.1016/j.clinbiomech.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 90.Suico JG, Marriott DJ, Vinicor F, Litzelman DK. Behaviors predicting foot lesions in patients with non-insulin-dependent diabetes mellitus. J Gen Intern Med. 1998;13(7):482–484. doi: 10.1046/j.1525-1497.1998.00138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Payne CB. Biomechanics of the foot in diabetes mellitus. Some theoretical considerations. J Am Podiatr Med Assoc. 1998;88(6):285–289. doi: 10.7547/87507315-88-6-285. [DOI] [PubMed] [Google Scholar]
- 92.Van Schie CH. A review of the biomechanics of the diabetic foot. Int J Low Extrem Wounds. 2005;4(3):160–170. doi: 10.1177/1534734605280587. [DOI] [PubMed] [Google Scholar]