Abstract
From the historic and simple assessment of temperature by the clinical thermometer, modern infrared technology has opened up new perspectives, especially in the use of thermal imaging to map body surface temperature with a remote sensing camera. Since the 1960s, there is now a greater understanding of thermal physiology and the relationship between skin temperature and blood perfusion. Furthermore, the examination technique, and the advantages of computer-aided digital imaging has greatly improved the reliability of this technology in medicine. Studies in diabetology have shown the value of this new facility and its relevance to clinical assessment of peripheral perfusion and tissue viability.
Keywords: amputation, diabetes mellitus, infrared, skin temperature, thermal imaging, ulceration
Introduction
The association between disease and temperature is as old as medicine itself. However, the early physicians had only primitive means of assessing the presence of fever in their patients, mostly by using the natural sense of touch. From the 17th century onward, thermometers were developed, but it was not until the 18th century that Dr. Carl Wunderlich in Leipzig, Germany, systematically studied the course of temperature in his patients and could monitor the progress of fever. He developed the clinical thermometer that has been used universally in medicine for some 200 years.1
The last half century has brought dramatic changes in the way in which the human body can be investigated. In particular, we now have many different imaging techniques. Initially, the microscope, followed by radiology with the use of X rays, were the most useful tools in medicine. Today, a wider range of different physical energies from ultrasound to magnetic resonance is available that opens up greater opportunities for diagnostic imaging.
Thermal imaging is a technique that uses infrared radiation but is not widely used in routine medical diagnostics. First uses were made in the 1960s, with large and noisy scanners. These were employed in some research studies and applied to the noninvasive study of human skin temperature.2
The human body is an efficient thermal system. We are entirely dependent on the ability of the body to self-regulate human body temperature. Vital organs are dependent on regular perfusion of blood carrying oxygen, and our ability to survive in arctic or tropical conditions is dependent on the adjustments in human circulation to the organs of the body, of which the heart is the central pumping mechanism.
One of the key factors in thermoregulation of the human body is the skin, which is the dynamic interface between the body and its environment. In excessive heat, the need to lose heat is accelerated by sweating, which evaporates and cools the skin. In very cold conditions, the peripheral blood vessels constrict, reducing the opportunity for blood cooling at the body surface.3
Infrared thermal imaging, unlike many other imaging techniques used in medicine, is not an internal imaging system for anatomical information. The human skin surface is a highly efficient radiator, with an emissivity of 0.98. A perfect black body radiator is considered to be at unity, meaning the ability to absorb and radiate are maximal.4
Infrared imaging therefore provides information on skin temperature distribution. Since the environment in which the human body is situated will affect this, it is obviously vital that conditions for any examination using this method must be as neutral as possible, and standardized.5
Great progress has been made both in the understanding of the physiological conditions required to harness this technique as a medical investigation and in the technology itself.
The early scanning cameras were not as reliable or stable as those available today. Images of heat distribution, where white represents hot and black represents cold, were obtained. Two important factors in the creation of such images are thermal and spatial resolution. The former is the ability of the imaging system to discriminate between small areas of differing temperatures, and the latter relates to image quality. To overcome the slow scan speed often required with the earlier camera systems, multi-element detectors were introduced. However, there was still a compromise needed between high speed and low resolution or low speed to obtain high resolution.6
Modern thermal imaging cameras now provide high speed and high resolution. Furthermore, the stability of the earlier cameras has improved dramatically, and calibration of the imager against a stable temperature reference can be achieved to ensure reliability. This is of particular importance when repeated investigations are made with this technique.7
One great advantage of thermal imaging is that it is both noncontact and noninvasive. By remote temperature sensing, the camera is merely receiving the natural thermal energy emitted by the body. Since no harmful energy is used in the imaging process, it is very suitable for repeated investigations over time.
Thermal Imaging Technique
All medical imaging procedures have benefited from the use of modern digital technology. A reliable thermal imaging system will be used online with a computer that will be operating specialized software. This will be able to indicate to the operator when the camera is stable and ideally will provide confirmation from a temperature reference source that the calibration is at the correct level. The examination should be performed in a temperature-controlled environment with a humidity of <50%. The disrobing cubicle should also be at the constant temperature, which is typically 22 °C (70 °F). A period of thermal equilibration is required before any imaging takes place, and this can be variable depending on whether peripheral or trunk areas are to be examined. Fifteen minutes is quite often found to be adequate to at least achieve a reasonable level of stability in blood pressure and skin temperature.
In a clinical setting, the ideal camera will be mounted on a parallax-free stand (not a tripod) to ensure that positioning is reproducible and free from unwanted angles between the patient and the camera.
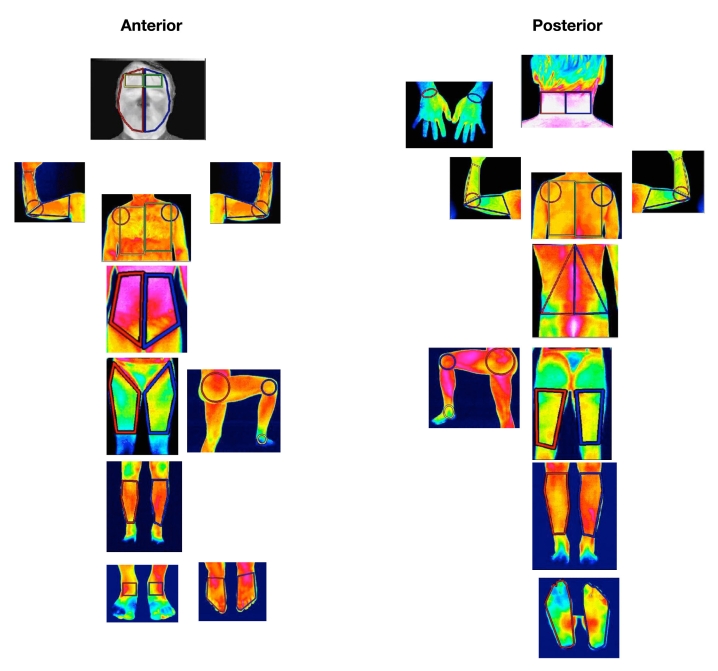
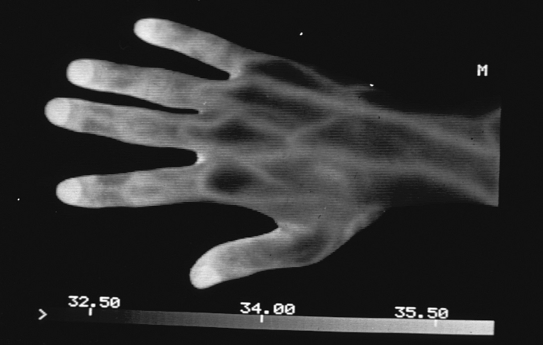
A series of standard images of the body regions required for the investigation are obtained within minutes, since almost all modern cameras are real time. The images are stored on the computer to be analyzed by the image processing software. Standardization of image capture can be improved by the use of software masks providing an outline of the region, e.g., both dorsal hands (Figure 1). By this means, the camera is moved closer or farther from the patient until the image fits the outline mask. Subsequent images from the same patient will therefore be at a constant distance and position. A series of such masks were developed at the University of Glamorgan to assist practitioners (Figure 2).
Figure 1.
Thermographic examination of hands.
Figure 2.
Standard views for infrared thermography.
After the images have been captured to the computer, the analysis of temperature distribution is made by selecting regions of interest, followed by a statistical measure of the thermal data.8 These may also be preformed in the software, or the operator draws the region according to protocol, using anatomical points to delineate the area required. Drawing regions of interest from memory can be another source of error, and when serial images are required, the variance can be up to 10%, even in so-called expert thermographers. Most software systems for thermal imaging provide basic image analysis based on mean maximum and minimum temperature. Real-time temperature changes, if required, such as recovery from a cold challenge, will require the patient to remain as still as possible, and most procedures used limit the length of time to a practical level, such as 10 minutes.9,10
Thermal Imaging Applied to Diabetic Diseases
Skin temperature, the information that can be investigated by this technique, is dependent on blood circulation. In ischemic conditions, where blood perfusion may be reduced, especially at the periphery of the human body and limbs (hands and feet), reduced temperature may be found. This requires a period of stabilization, where the patient is resting in the stable room temperature (and low humidity) for some 15 minutes prior to examination. During this time, areas of the body to be examined must be unclothed.
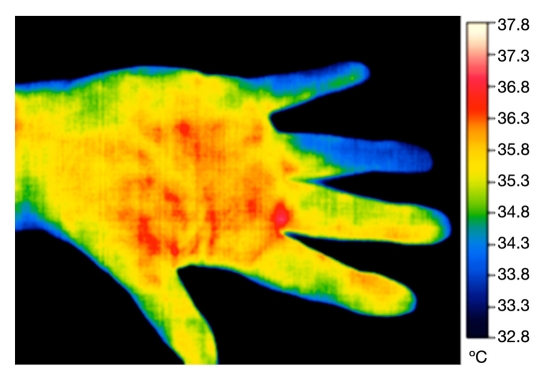
In many situations, it can be more useful to apply a challenge to the peripheral circulation and skin region. This can be chemical, mechanical, or thermal. In the latter case, a widely used test is the cold challenge test. Here the dorsal surface of the hands is imaged after the period of stabilization, and more than one image may be recorded to establish this. Hands are then covered with thin plastic gloves and given timed immersion in water (often 20 °C) for 1 minute or longer. After removal of the gloves, the hands are then followed for thermal recovery by a series of thermal images at fixed time intervals (Figure 3). Rapid recovery in a time such as 10 minutes postcooling by immersion usually indicates a normal response. Some may produce overspill of recovery, reactive hyperemia, where fingers become hotter for a time than the rest of the hand. A delayed and protracted recovery, leaving fingers colder than before immersion, may be typical of Raynaud’s phenomenon and may be a feature of another condition such as rheumatoid arthritis or diabetes mellitus (Figure 4). An advantage of thermal imaging is that this test can be graded for severity by quantification, and the effects of any prescribed medication to improve the symptoms can be measured.
Figure 3.
Dorsal view of hand during reactive hyperemia.
Figure 4.
Palmar view of hand showing cold fourth and fifth fingers.
Brånemark and colleagues11 noted characteristic abnor-malities in the thermal patterns over the hands and feet of 16 diabetes patients with and without vascular complications. They concluded that thermography was a useful technique for the study of circulation and metabolism in diabetes. Cold stimulation was used by Jiang and associates12 in 2003 to assess metabolic status in diabetes, which they considered to be highly specific. In another study of 60 patients, Marcinkowska-Gapińska and Kowal13 analyzed the rheological parameters of blood in 18 diabetes patients and compared them with a group of 20 postmyocardial infarction patients, the remainder being healthy controls. They compared the results with the skin temperature data obtained by thermography. Using a cold stress at 20 °C, a Japanese study found a high correlation between the thermal recovery measured by thermography and laser Doppler flowmetry at the feet.14
From these studies, the different authors all found that infrared thermography was a valid measure of skin temperature that directly related to peripheral circulation, especially after the application of a mild cold stress to the extremities.
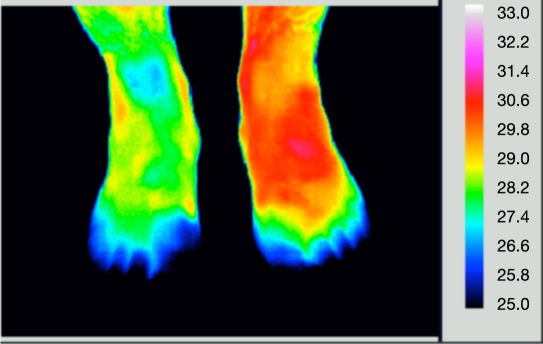
In diabetes mellitus, circulatory dysfunction can occur in the feet as well as hands, which can lead to skin ulceration (Figure 5). Thermal imaging has been used as one method to monitor skin temperature in the area of ulceration, which again can be a means of assessing the efficacy of treatment used to improved the perfusion to the affected limb. The allocations of thermography and thermometry in lower extremity wounds and the associated vascular complications have been reviewed by Bharara and coworkers,15 who also studied the low-cost technology of contact liquid crystal thermography.
Figure 5.
Dorsal view of feet. The left forefoot is inflamed (red) and toes are cold.
Fushimi and colleagues16 proposed a new index of autonomic neuropathy in diabetes mellitus by using a warm water bath immersion for one leg while studying the other using infrared thermography. They noted that thermography was one of the most reliable, reproducible, and noninvasive methods for detecting and monitoring sympathetic abnormalities in this disease. Early diagnosis of neuropathic joint disease is important and provides the opportunity to introduce prompt protective treatment. A group of orthopedic surgeons successfully used thermo-graphy to detect early presence of warmth associated with early neuropathic disease and concluded that infrared thermography was a reliable diagnostic tool in this application.17
Thermal symmetry in the feet is a normal finding in healthy subjects. It is interesting to note that some studies have been performed with low-cost radiation thermometers. Lavery and associates18 carried out a clinical trial involving 173 diabetes patients with a history of ulceration. The patients were instructed to self-monitor by measuring temperatures on the great toe; first, third, and fifth metatarsal heads; midfoot; and heel on a regular basis. They were instructed to call the study nurse if a temperature difference of 4 °F was found. Over 50% of patients did call the nurse, and 8% of them went on to develop ulcers. In another study, Armstrong and coworkers19 evaluated the effectiveness of home temperature monitoring over an 18-month period with a randomized controlled trial involving 225 patients. They also found that 8.4% ulcerated over the study period. Those patients who ulcerated had a temperature difference that was 4.8 times greater at the site of ulceration in the week before the ulcers appeared than did a random seven-consecutive-day sample of 50 other subjects who did not ulcerate. These studies clearly demonstrate that high temperature gradients between feet may predict the onset of neuropathic ulceration and that simple temperature measurements recorded in home monitoring is of benefit in the management of these patients.
Some early work was carried out in Scotland to assess the use of infrared thermography in the determination of amputation level in those patients whose limbs could not be saved. Spence and colleagues20 studied 104 patients with ischemic limbs prior to amputation in 1981 and concluded that the thermographic method as they described was a reliable indicator of the tissue viability and could be used to indicate the level of a major limb amputation. In a later paper in 1984, they discussed three methods in use for amputation level assessment: partial oxygen pressure, skin blood flow, and infrared thermography.21 One group, that compared thermography with Doppler flowmetry and clinical judgment of an experienced surgeon, did not support their findings. Their conclusion was that the experienced surgeon was the most reliable method and that other methods are only an adjunct to clinical assessment.22
However, these earlier studies were not based on the modern and improved camera technology, which, combined with good technique and computer-aided image processing, is now known to provide improved data.
Conclusions
Infrared thermal imaging, also referred to as thermo-graphy, is a reliable noninvasive technique for imaging skin temperature distribution. When used in a stable environment, it has been shown to be useful in the assessment of tissue viability and peripheral circulation applied to diabetes mellitus. It is especially suitable for serial measurements used in the follow-up of response to treatment.
References
- 1.Wunderlich CA. In: On the temperature in diseases, a manual of medical thermometry. Woodman WB, translator. London: The New Sydenham Society; 1871. [Google Scholar]
- 2.Wolfe WL. Infrared imaging devices in infrared medical radiography. Ann NY Acad Sci. 1964;121:57–70. doi: 10.1111/j.1749-6632.1964.tb13685.x. [DOI] [PubMed] [Google Scholar]
- 3.Houdas Y, Ring EF. Human body temperature, its measurement and regulation. New York: Plenum Press; 1982. [Google Scholar]
- 4.Hardy JD. The radiation of heat from the human body: III. The human skin as a black-body radiator. J Clin Invest. 1934;13(4):615–620. doi: 10.1172/JCI100609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ring EF, Ammer K. The technique of infrared imaging in medicine. Thermol Int. 2000;10(1):7–14. [Google Scholar]
- 6.Norton PR, Horn SB, Pellegrino JG, Perconti P. Infrared detectors and detector arrays. In: Diakides NA, Bronzino JD, editors. Medical infrared imaging. Boca Raton: CRC Press; 2006. [Google Scholar]
- 7.Mekhontsev SN, Prokhorov AV, Hanssen LM. Experimental characterization of blackbody radiation sources. In: Zhang ZM, Tsai BK, Machin G, editors. Radiometric temperature measurements. II. Applications. Elsevier: 2010. pp. 57–136. [Google Scholar]
- 8.Ammer K, Ring EF. Standard procedures for infrared imaging in medicine. In: Diakides NA, Bronzino JD, editors. Medical infrared imaging. Boca Raton: CRC Press; 2006. [Google Scholar]
- 9.Ring EF. Cold stress test for the hands. In: Ammer K, Ring EF, editors. The thermal image in medicine and biology. Vienna: Uhlen-Verlag; 1995. pp. 237–240. [Google Scholar]
- 10.Merla A, Romani GL. Biomedical applications of functional infrared imaging. In: Diakides N, Bronzino JD, editors. Medical infrared imaging. Boca Raton: CRC Press; 2006. [Google Scholar]
- 11.Brånemark PI, Fagerberg SE, Langer L, Säve-Söderbergh J. Infrared thermography in diabetes mellitus. A preliminary study. Diabetologia. 1967;3(6):529–532. doi: 10.1007/BF01213572. [DOI] [PubMed] [Google Scholar]
- 12.Jiang G, Shang Z, Zhang M. Metabolism parameter analysis of diabetics based on thermography. Engineering in medicine and biology. 24th Annual Conference and Meeting of the Biomedical Engineering Society; 2002. pp. 2226–2227. [Google Scholar]
- 13.Marcinkowska-Gapińska A, Kowal P. Blood fluidity and thermo-graphy in patients with diabetes mellitus and coronary heart disease in comparison to healthy subjects. Clin Hemorheol Microcir. 2006;35(4):473–479. [PubMed] [Google Scholar]
- 14.Hosaki Y, Takata S, Mitsunobu F, Mifune T, Ashida K, Tsugeno H, Okamoto M, Harada S, Tsuji T. Evaluation of body surface temperature by thermography. 3. Correlation between the peripheral circulation estimated by laser-Doppler blood flowmetry and thermography. Annual Reports of Misasa Medical Branch; Okayama University Medical School, Tottori Japan. 1999. pp. 36–42. [Google Scholar]
- 15.Bharara M, Cobb JE, Claremont DJ. Thermography and thermo-metry in the assessment of diabetic neuropathic foot: a case for furthering the role of thermal techniques. Int J Low Extrem Wounds. 2006;5(4):250–260. doi: 10.1177/1534734606293481. [DOI] [PubMed] [Google Scholar]
- 16.Fushimi H, Inoue T, Nishikawa M, Matsuyama Y, Kitagawa J. A new index of autonomic neuropathy in diabetes mellitus: heat stimulated thermographic patterns. Diabetes Res Clin Pract. 1985;1(2):103–107. doi: 10.1016/s0168-8227(85)80035-8. [DOI] [PubMed] [Google Scholar]
- 17.Sandrow RE, Torg JS, Lapayowker MS, Resnick EJ. The use of thermography in the early diagnosis of neuropathic arthropathy in the feet of diabetics. Clin Orthop Relat Res. 1972;88:31–33. doi: 10.1097/00003086-197210000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Lavery LA, Higgins KR, Lanctot DR, Constantinides GP, Zamorano RG, Athanasiou KA, Armstrong DG, Agrawal CM. Preventing diabetic foot ulcer recurrence in high-risk patients: use of temperature monitoring as a self-assessment tool. Diabetes Care. 2007;30(1):14–20. doi: 10.2337/dc06-1600. [DOI] [PubMed] [Google Scholar]
- 19.Armstrong DG, Holtz-Neiderer K, Wendel C, Mohler MJ, Kimbriel HR, Lavery LA. Skin temperature monitoring reduces the risk for diabetic foot ulceration in high-risk patients. Am J Med. 2007;120(12):1042–1046. doi: 10.1016/j.amjmed.2007.06.028. [DOI] [PubMed] [Google Scholar]
- 20.Spence VA, Walker WF, Troup IM, Murdoch G. Amputation of the ischemic limb: selection of the optimum site by thermography. Angiology. 1981;32(3):155–169. doi: 10.1177/000331978103200302. [DOI] [PubMed] [Google Scholar]
- 21.Spence VA, McCollum PT, Walker WF, Murdoch G. Assessment of tissue viability in relation to the selection of amputation level. Prosthet Orthot Int. 1984;8(2):67–75. doi: 10.3109/03093648409145351. [DOI] [PubMed] [Google Scholar]
- 22.Luk KD, Yeung PS, Leong JC. Thermography in the determination of amputation levels in ischaemic limbs. Int Orthop. 1986;10(2):79–81. [PubMed] [Google Scholar]