Abstract
Background
Insulin pump therapy is a complex technology prone to errors when employed in the hospital setting. When patients on insulin pump therapy require hospitalization, practitioners caring for them must decide whether to allow continued pump use. We provide the largest review regarding transitioning insulin pump therapy from the outpatient to inpatient setting.
Method
Records of inpatient insulin pump users were retrospectively analyzed at a metropolitan Phoenix hospital between January 2006 and December 2009. Adherence to institutional procedures on insulin pump use was assessed, glycemic control was determined, and adverse events were examined.
Results
We examined records on 65 patients with insulin pumps, totaling 125 hospitalizations. Mean (standard deviation) patient age was 55 (17) years, diabetes duration was 27 (14) years, pump duration was 6 (5) years, length of hospital stay was 4.7 (6.3) days, hemoglobin A1c was 7.3 (1.3)%, 85% had type 1 diabetes mellitus, 57% were women, and 97% were white. Admissions involving insulin pumps increased (23 in 2006, 17 in 2007, 40 in 2008, and 45 in 2009). Insulin pump therapy was continued in 83 (66%) hospitalizations. Among these hospitalizations, endocrinology consultations were obtained in 89%, consent agreements were found in 83%, insulin pump order sets were completed in 89%, admission glucose was checked in 100%, and nursing assessments of pump insertion sites were documented in 89%, but bedside insulin pump flow sheets were found in only 55%. Mean glucose of 175 (57) mg/dl was not significantly different than that in hospitalizations where insulin pumps were discontinued [175 (42) mg/dl] or used intermittently [177 (7) mg/dl]. There was one instance of a pump catheter kinking; however, no other adverse events (pump site infections, mechanical pump failure, diabetic ketoacidosis) were observed, and there were no use-related fatalities.
Conclusions
Most patients using insulin pumps can safely have their therapy transitioned when hospitalized. A policy on inpatient continuous subcutaneous insulin infusion use can be successfully implemented. Compliance with required procedures can be achieved, although there was room to improve adherence with some process measures. Further study is needed to determine how to optimize glycemic control when pumps are allowed during hospitalization.
Keywords: continuous subcutaneous insulin infusion, diabetes mellitus, hospitalization, inpatient, insulin pumps
Introduction
Estimates indicate that more than 100,000 patients with diabetes mellitus in the United States are being managed with continuous subcutaneous insulin infusion (CSII; insulin pump) therapy.1 Continuous subcutaneous insulin infusion is complex, requiring specialized training by the patient and the diabetes care specialists who assist with management. Moreover, insulin is a high-alert medication prone to errors in the hospital, which further increases the risks associated with the already complex nature of CSII treatment.2 Finally, surveys of inpatient practitioners confirm a lack of familiarity with the use of insulin pumps.3–6 Thus inpatient physicians may justifiably struggle with whether to allow CSII users to continue their therapy during a hospital stay.
This combination of complex technology, high-risk medication, and inpatient unfamiliarity with CSII may create an unsafe situation for patients who wish to continue using their insulin pumps when hospitalized. Errors are possible unless a standardized inpatient policy and set of procedures are in place that provide guidance about how to manage the patient on an insulin pump.7 Despite the possible risks, many clinicians believe that patients can continue to use their CSII treatment in the acute care setting.8–10 Limited data suggest that this is the patient’s preference as well, and patient satisfaction is high when insulin pump therapy is allowed in the hospital.11,12
No national data exist on current hospital practices regarding insulin pump use. Likewise, consensus is lacking on how to employ CSII therapy in the hospital setting. We previously published a strategy that assists practitioners with the management of CSII in the hospital.13 Our recommendations were developed to supplement preexisting but limited guidelines.14 We sought to protect the patient’s desire for autonomy by allowing insulin pump self-management while simultaneously maximizing safety.13 The formation of a collaborative relationship between the patient and the hospital staff was implied and reinforced by maintaining lines of communication through a daily review of pump operation, surveillance of point-of-care glucose data, and discussion with the patient and staff about any related issues. Since our first publication on this area, others have also presented their approach to inpatient insulin pump management.12,15
Our earlier reports indicated that our policy and procedures permitted a safe outpatient-to-inpatient transition of CSII therapy.16,17 Prior reviews involved smaller numbers of individual patients and hospitalizations.16,17 The purpose of this analysis was to provide updated information relating to the use of inpatient CSII that includes an examination of adherence to our institutional policies over time, assessment of glycemic control, and evaluation of adverse events related to inpatient pump use.
Methods
Description of Facility
Our academic medical center is located in metropolitan Phoenix, Arizona. All adult general medical and surgical specialties are represented; pediatric care and obstetrical care are not available. An electronic medical record links inpatient and outpatient notes and laboratory data. Patient care orders are input via a computerized order entry system.
Synopsis of Inpatient Insulin Pump Policy
We formalized our inpatient CSII guidelines into a written policy consisting of three basic elements: (1) contraindications for insulin pump use in the hospital; (2) procedures that guide the medical staff in insulin pump management after patient admission; and (3) a requirement for signed patient informed consent that details the conditions for use of CSII in the hospital.13 Our contraindications to continued insulin pump use are a patient with altered state of consciousness, a critically ill patient (i.e., one in the intensive care unit), suicide risk, the patient refuses or is otherwise unable to participate in his care, a family member is unavailable to assist with pump management if the patient cannot self-manage, or another reason deemed appropriate by physician. This policy and associated requirements have been previously published.17
Case Definitions
A patient registry was developed to track insulin pump patients and was updated as hospitalizations were identified. We reviewed electronic medical records of patients on insulin pump therapy who were admitted between January 1, 2006, and December 31, 2009. This analysis was approved by our Institutional Review Board. Some patients had multiple admissions. Each hospitalization was considered an independent opportunity to implement our inpatient insulin pump policy, so the unit of analysis was the hospital stay rather than the patient. Per our previous definitions, if CSII therapy was only temporarily suspended or discontinued (typically for 1–2 hours) for a procedure, then restarted and otherwise maintained for the duration of the hospitalization, we designated these patients as having continued CSII therapy.16,17
Chart Review
We have proposed a set of inpatient insulin pump metrics consisting of patient demographics and a group of process measures.13,16,17 Thus we retrieved data on age, sex, race or ethnicity, length of hospital stay, type of diabetes, duration of diabetes, and length of time that the patient reported being on CSII therapy. Compliance with process measures was assessed by reviewing the electronic medical record and determining if documentation of the following occurred: nursing staff notation of pump presence at admission, admission glucose measurement, signed patient consent form present in the medical record, standard insulin pump orders completed, endocrinology consultation placed, and, finally, documentation of a completed bedside flow sheet.13,16,17
Glucose Data
Bedside glucose monitoring was conducted with an instrument that scanned and stored patient identification from a barcode. These bedside glucose values were then retrieved by linking patient identifiers to the electronic laboratory database. Commercial software (Medical Automation Systems, Inc, Charlottesville, VA) facilitates the glucometer interface with the electronic laboratory file.18 Joint Commission requires a hemoglobin A1c (HbA1c) test be done at admission if not performed within 60 days prior to the hospitalization.19 Obtaining a measurement too far into the hospital stay might result in alterations in HbA1c (e.g., due to phlebotomies). To gauge the level of outpatient glycemic control, we elected to extract HbA1c values obtained 90 days before admission through day 7 of the hospitalization.
Outcome Measures
We determined compliance with CSII process measures for hospitalizations overall and across the four-year review period. The bedside glucose measurements for the entire length of stay were averaged for each patient, and the composite bedside glucose average (BedGlucavg) was calculated.16–18,20 The proportion of hospitalizations with hypoglycemia (at least one measurement <70 mg/dl) or hyperglycemia (at least one measurement >200 mg/dl) was calculated and compared based on inpatient insulin pump status. The percentage of bedside glucose measure-ments with hypoglycemic (bedside glucose <40, <50, <60, or <70 mg/dl) or hyperglycemic values (bedside glucose >200, >250, >300, >350, or >400 mg/dl) was calculated by counting the number of these events for each patient, dividing by the total number of bedside measurements per patient, and then multiplying by 100.16,17,20 This method allowed adjustment for different numbers of measurements per patient, and it captured information on multiple episodes of hypo- or hyper-glycemia in individual patients.16,17,20,21 The frequency of hypo- and hyperglycemic measurements was compared according to inpatient insulin pump status.
In addition to these measures, we examined records for any evidence of adverse events that might be specific for CSII. These were any evidence of mechanical failure, problems with infusion sites (e.g., infection, kinking of infusion catheter), and diabetic ketoacidosis while on therapy. We also examined for inpatient mortality.
Data Analysis
Data are reported as mean (standard deviation) where applicable. Statistical differences between continuous variables (e.g., glucose levels and hypo- and hyperglycemic frequencies) were tested using nonparametric methods, and differences between categorical variables (hospital hypo- and hyperglycemia prevalence) were tested using the χ2 test.
Results
Patient Characteristics
We identified 125 hospital admissions between January 1, 2006, and December 31, 2009, that involved 65 individual patients on CSII. Just 21 patients accounted for 83 of these hospitalizations. Reasons for admission were diverse but were mostly acute problems (e.g., nausea, vomiting, abdominal pain, fever, chest pain, seizures), with many patients having multiple complaints. The mean age of the 65 patients was 55 (17) years, the mean diabetes duration was 27 (14) years, and the self-reported duration of CSII therapy was 6 (5) years (Table 1). Of these 65 patients, 85% had type 1 diabetes, 57% were women, and 97% were white. Patients who experienced multiple admissions were comparable in age, diabetes duration, sex, and HbA1c to individuals who had single admissions (all p > .15), but patients with more than one admission had slightly longer duration of reported insulin pump therapy than persons with only a single hospitalization [8 (5) versus 5 (5) years, p = .013,by Mann–Whitney test]. The number of hospitalizations involving CSII patients increased over time (23 hospital-izations in 2006, 17 in 2007, 40 in 2008, and 45 in 2009).
Table 1.
Characteristics of 65 Patients with 125 Hospitalizations between January 1, 2006, and December 31, 2009, Involving Insulin Pump Therapy
| Characteristic | Valuea |
|---|---|
| Age, years | 55 (17) |
| Diabetes duration, years | 27 (14) |
| Duration of insulin pump therapy, years | 6 (5) |
| Type 1 diabetes mellitus, % | 85 |
| Female sex, % | 57 |
| White race, % | 97 |
| Length of hospital stay, days | 4.7 (6.3) |
| Number of bedside glucose measurements per person per day | 5.8 (2.9) |
| BedGlucavg, mg/dl | 176 (53) |
| HbA1c, % | 7.3 (1.3) |
Values are mean (standard deviation) unless indicated otherwise.
The average length of stay for the 125 hospitalizations was 4.7 (6.3) days. More than 40% of these hospitalizations were extremely short stays, with 17 hospitalizations (14%) having a length of stay <24 hours and 37 (30%) having a stay of 1–2 days. The average glucose measurement per patient per day was 5.8 (2.9), and the BedGlucavgwas 176 (53) mg/dl (Table 1). Mean HbA1c was 7.3% (1.3%) (Table 1). Mean interval between admission and the HbA1c measurement was 7.8 (18) days (range, 81 days before admission to 5 days after admission).
Characterization of Inpatient Pump Status
We identified three subsets of hospitalizations. We had previously designated the first group as “pump on” hospitalizations—hospitalizations where patients were deemed to be candidates for CSII use per our policy and who remained on treatment from admission to discharge.16,17 Of the 125 hospitalizations, there were 83 (66%) “pump on” hospitalizations. We had also designated a second group as “pump off” hospitalizations16,17 when CSII was discontinued from admission to discharge. There were 18 (14%) “pump off” hospitalizations. The reasons (number of patients) for pump discontinuation at admission were diabetic ketoacidosis (2), altered mental status (2), one-day admissions for procedures (3), severe hypoglycemia (2), pump malfunction (1), patient request (1), surgeon preference (1), suicide attempt (1), hyperglycemia requiring insulin drip (1), and unable to ascertain reason (4). If CSII was discontinued, patients received alternate therapy (e.g., basal and short-acting insulin).
Further review identified a third group not described before, which we have now designated as an “intermittent pump” group. These were instances where patients may have had CSII therapy continued at admission but then stopped during the course of their hospitalization or patients who had CSII stopped at admission but then restarted it as their clinical status improved. Twenty-four (19%) of the 125 hospitalizations involved intermittent pump use.
Adherence to Process Measures
All CSII patients at admission should have had an initial nursing assessment and an admission glucose value recorded. Of all 125 admissions, 104 (83%) had nursing documentation that the pump was present, 78 (62%) had the pump brand recorded, and 99 (79%) had the type of insulin entered into the electronic medical record. All hospitalizations had a glucose level obtained at admission.
Next, we determined adherence with insulin pump process measures, evaluating just “pump on” and “intermittent pump” hospitalizations (N = 107). “Intermittent pump” hospitalizations were included because, at some point during their stay, these individuals should have had the required procedures and documentation completed similar to those for “pump on” patients (Table 2). Although 100% compliance was not achieved, procedures were typically being accomplished in ≥80% of the hospitalizations. The two biggest areas requiring improvement were the recording of the pump brand and documentation that the bedside flow sheet had been completed (Table 2).
Table 2.
Compliance with Hospital Procedures in “Pump On” and “Intermittent Pump” Usersa
| Process measure | Pump onb no. (%) | Intermittent pumpc no. (%) | On or intermittentd no. (%) |
|---|---|---|---|
| Admission nursing assessment | |||
| Presence of pump | 75 (90) | 19 (79) | 94 (88) |
| Brand of pump | 58 (70) | 13 (54) | 71 (66) |
| Type of insulin in pump | 71 (86) | 19 (79) | 90 (84) |
| Admission glucose | 83 (100) | 24 (100) | 107 (100) |
| Patient consent in record | 69 (83) | 20 (83) | 89 (83) |
| Insulin pump order set | 74 (89) | 18 (75) | 92 (86) |
| Endocrinology consultation | 74 (89) | 21 (88) | 95 (89) |
| Skin site assessment | 74 (89) | 21 (88) | 95 (89) |
| Bedside flow sheet | 46 (55) | 11 (46) | 57 (53) |
Values are percentage of hospitalizations in each category.
n = 83
n = 24
n = 107
Lastly, we examined adherence with process measures over time (Table 3). High compliance was generally achieved each year. In some cases—such as use of the insulin pump orders, endocrine consultations, and skin site assessments—90% compliance was achieved in 2008 and 2009. Documentation of the pump brand and bedside flow sheet had the lowest percentage of completed requirements over time (Table 3).
Table 3.
| Process measure | 2006c no. (%) | 2007d no. (%) | 2008e no. (%) | 2009f no. (%) |
|---|---|---|---|---|
| Admission nursing assessment | ||||
| Presence of pump | 14 (78) | 13 (93) | 32 (91) | 35 (88) |
| Brand of pump | 10 (56) | 9 (64) | 27 (77) | 25 (63) |
| Type of insulin in pump | 14 (78) | 12 (86) | 32 (91) | 32 (80) |
| Admission glucose | 18 (100) | 14 (100) | 35 (100) | 40 (100) |
| Patient consent form on file | 14 (78) | 11 (79) | 30 (86) | 34 (85) |
| Insulin pump order set | 13 (72) | 11 (79) | 32 (91) | 36 (90) |
| Endocrinology consultation | 16 (89) | 11 (79) | 32 (91) | 36 (90) |
| Skin site assessment | 14 (78) | 12 (86) | 32 (91) | 37 (93) |
| Bedside flow sheet | 8 (44) | 5 (36) | 18 (51) | 26 (65) |
Values are percentage of hospitalizations in each category.
n = 107
n = 18
n = 14
n = 35
n = 40
Adverse Events
Among patients using their insulin pumps in the hospital, there was one instance of an infusion catheter kinking, resulting in nonfatal hyperglycemia until corrected. Otherwise, there were no unexpected complications related to inpatient insulin pump use (e.g., insertion site infections or mechanical failure of the pump or diabetic ketoacidosis), and there was no inpatient mortality among CSII users.
Glycemic Control
The BedGlucavgvalues were comparable for all hospital-izations (p > .5 between groups by the Mann–Whitney test): “pump on” BedGlucavg= 175 (57) mg/dl, “pump off” BedGlucavg= 175 (42) mg/dl, and “intermittent pump” BedGlucavg= 177 (7) mg/dl. There was no difference in length of stay among the three categories or in the number of daily bedside glucose measurements (p > .3 for all by the Mann–Whitney test).
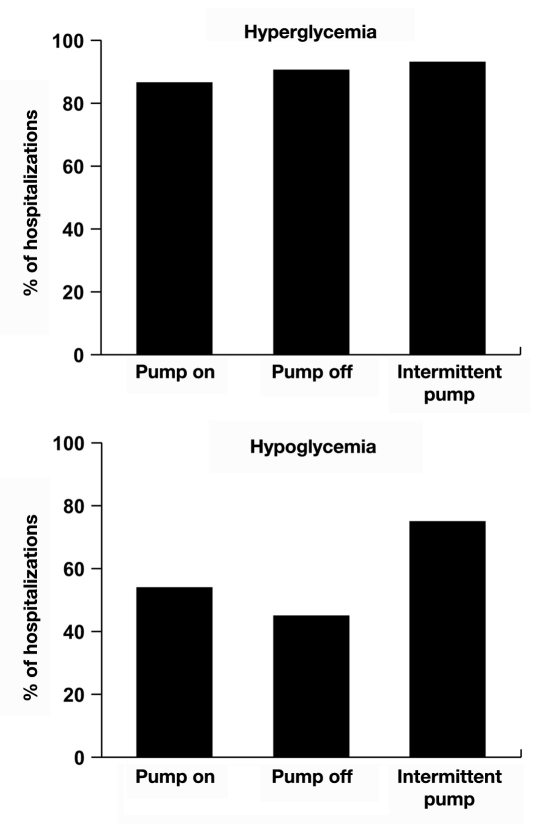
The prevalence of hyperglycemia was common among all three classes of insulin pump hospitalizations (Figure 1). Eighty-five percent of the “pump on,” 89% of the “pump off,” and 92% of the “intermittent pump” hospitalizations were characterized by at least one bedside glucose value >200 mg/dl; there were no differences among groups. The prevalence of hypoglycemia (at least one bedside glucose <70 mg/dl) was 54% in “pump on,” 44% in “pump off,” and 75% among the “intermittent pump” hospitalizations (Figure 1). The prevalence of hypo-glycemia tended to be higher among hospitalizations identified as “intermittent pump” compared with that of the “pump on” hospitalizations (p = .07 by the χ2 test), and it was significantly greater (p = .04 by the χ2 test) than that of the “pump off” hospitalizations (Figure 1). There were no differences in hypoglycemia between “pump on” and “pump off” hospitalizations.
Figure 1.
Prevalence of hyperglycemia and hypoglycemia in hospitalizations involving insulin pump therapy. Insulin pump therapy was continued (“pump on,” n = 83), discontinued (“pump off,” n = 18), or used intermittently (“intermittent pump,” n = 24).
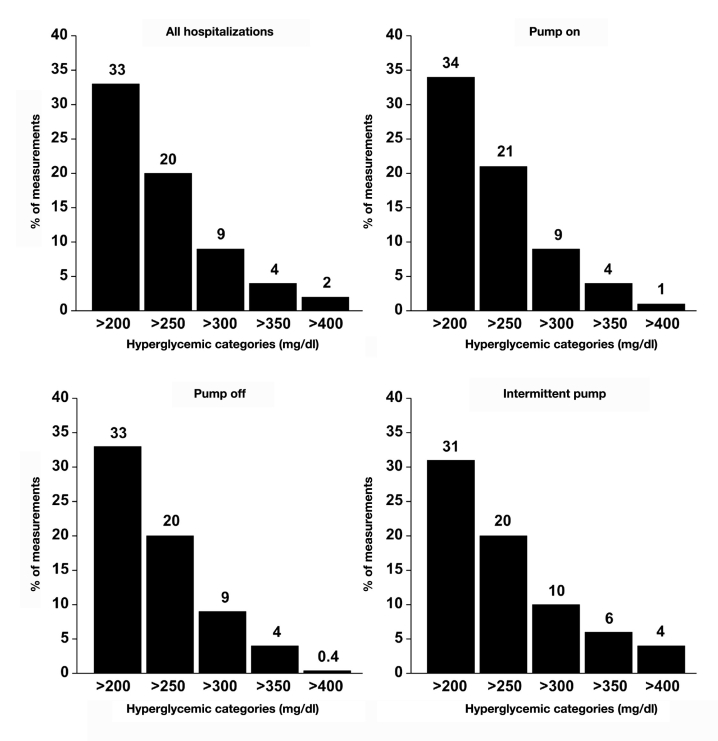
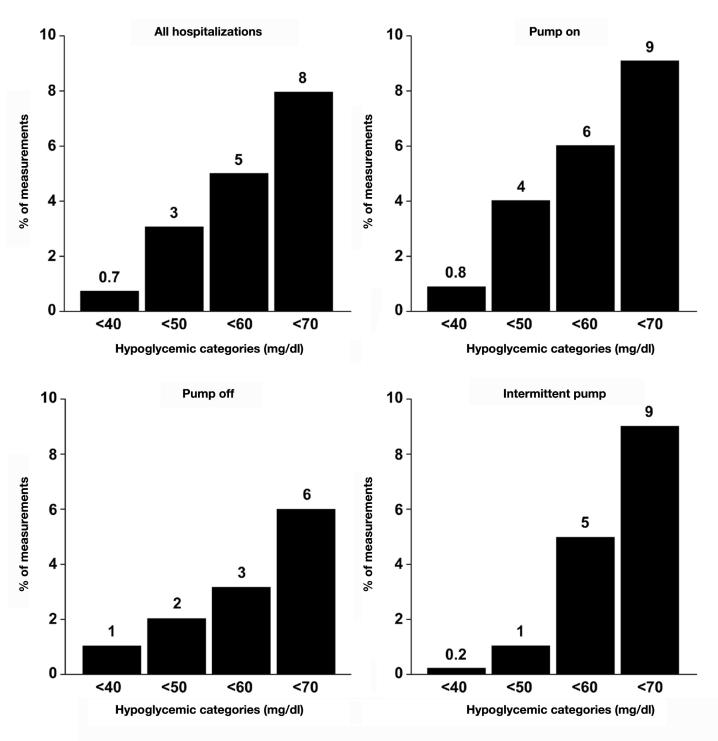
An examination of the percentage of bedside glucose values that were hyperglycemic showed that nearly one-third of the measurements were >200 mg/dl for all insulin pump groups combined as well as for individual categories (Figure 2); severe hyperglycemia (e.g., >300 mg/dl) was uncommon. Hypoglycemic values were rare (Figure 3), particularly severe hypoglycemia (e.g., <50 mg/dl). There were no differences in the frequency of hyperglycemic or hypoglycemic measure-ments among any of the categories (p > .15 for all).
Figure 2.
Percentage of per person bedside glucose measurements with hyperglycemia. Data shown are for all insulin pump hospitalizations combined and separately for “pump on,” “pump off,” and “intermittent pump” hospitalizations.
Figure 3.
Percentage of bedside glucose measurements per person with hypoglycemia. Data shown are for all insulin pump hospitalizations combined and separately for “pump on,” “pump off,” and “intermittent pump” hospitalizations.
Discussion
Our findings in this study confirm those in our earlier reports that CSII therapy can be maintained in most insulin pump patients during a hospitalization.16,17 A smaller group of these patients had to be transitioned off CSII therapy and placed on alternative insulin regimens because they did not meet the criteria for continued CSII use as set forth by our hospital policy guidelines. Aside from the one instance of transient hyperglycemia due to a catheter kinking, no adverse events occurred among those patients who remained on CSII. The only other study examining the topic of inpatient CSII use also noted no significant safety issues.12
In the current study, a third category of inpatient CSII users was identified, one in which insulin pumps were used intermittently. Others have recognized that patients may not be on insulin pumps for the entire length of their hospital stay.12 This intermittent use of insulin pumps in the hospital probably reflected a dynamic interplay between the desire of patients to continue using their technology whenever possible and their changing clinical status, which would require constant reevaluation of the appropriateness of the therapy. The findings of three categories of CSII users underscores the diversity of these patients in the hospital and further supports the need for the type of institutional standards described here.
This article reflects an assessment of the largest number of hospitalizations involving insulin pump users, with the number of unique patients encountered nearly doubling since our last report and the number of hospitalizations being evaluated increasing more than two-fold.16,17 It is not clear why the number of cases with CSII encountered in our hospital has been increasing. This rise likely is not due simply to a general increase in the number of diabetes hospitalizations. The proportion of diabetes-associated hospitalizations in our institution was 18% in 2006, 22% in 2007, and 19% in both 2008 and 2009. Thus, when our greatest number of diabetes hospitalizations occurred, the number of CSII admissions was least (in 2007). Moreover, the number of hospitalizations involving insulin pump patients increased in 2008 and 2009 despite the percentage of overall inpatient diabetes cases remaining flat in these years.
One explanation of why we have witnessed more patients admitted with insulin pumps is that use of CSII itself may be increasing. Testing this hypothesis would require some knowledge of the regional insulin pump outpatient population, but such data do not exist in the form of a disease management registry in the public domain. As we are a regional referral center, another possibility for the rise in insulin pump patient cases may be that the most seriously ill CSII patients are presenting to our institution. It would be of interest to know if other hospitals are observing similar increases in the number of insulin pump patients. Ongoing surveillance will establish whether the increasing trend continues.
The result of this analysis is greater insight into the characteristics of the insulin pump patients we encounter in the hospital. One-third of the patients accounted for nearly two-thirds of the hospitalizations, which may imply a high degree of chronic illness in some insulin pump users. Overall, patients had a long duration of diabetes, and on average, they had been on CSII therapy for several years. Hence they were likely to be familiar both with management of their diabetes and with insulin pump technology.
In general, institutional compliance with insulin pump procedures was high. Adherence remained high over time and even improved for some measures. It cannot be ascertained from a retrospective analysis why some measures improved, but possibilities include improved familiarity with inpatient insulin pump processes as more cases were encountered or the result of ongoing staff educational efforts on the topic. Underperformance with the bedside flow sheet was a consistent shortfall. We were not able to determine whether these were missing because of not being completed or simply because the documents had not been scanned into the electronic medical record. The bedside flow sheet is a key method by which the patient communicates pump data (e.g., basal insulin rates and bolus amounts and glucose data) to the hospital staff that should be documented in the medical record. An examination of the chain of custody of the bedside flow sheet needs to occur, from the patient bedside to the scanning process.
This updated analysis does continue to raise a question as to whether CSII therapy is more advantageous than other insulin regimens for controlling inpatient hyperglycemia. The average glucose during “pump on” hospitalizations and the prevalence of hyperglycemia in these patients was similar to those values in the “pump off” and “intermittent pump” groups. Mean bedside glucose levels, the prevalence of hypoglycemia and hyperglycemia, and the frequencies of hypoglycemic and hyperglycemic measurements in this analysis were consistent with what we have reported previously.17 Moreover, hyperglycemia and hypoglycemia among these CSII patients continues to be more frequent than what we have historically reported for our general diabetes inpatient population.20 We had earlier thought that maintaining CSII in the hospital offered an advantage regarding less hypo-glycemia, but our findings from this larger data set did not confirm this expectation.16 Since CSII patients potentially face the same stressors in the hospital as those affecting any other diabetes patient, insulin pumps may not offer superior glycemic control compared with other insulin treatment strategies. Additionally, the short length of stay in many cases would not allow sufficient time to optimize pump settings and monitor effects.
There are some limitations to our analysis. The sample sizes, particularly in the “pump off” and “intermittent pump” categories, are very small, and definite conclusions about differences in glucose control among the three inpatient scenarios cannot be made. Although this review examines the largest number of hospitalizations involving insulin pump patients reported to date, it still took four years to accrue just 125 cases for analysis. This low volume of encounters probably will not allow hospital staff to become proficient with insulin pump technology and reinforces the need for a standardized approach that practitioners can implement with such patients when hospital admission is required.
Another limitation is that we cannot be certain that we identified every hospitalized patient who was receiving insulin pump therapy. The data indicate that not every patient received an endocrinology consultation, so the endocrinology consultants may not have been aware of some cases until toward the end of the hospital stay or even after discharge. Patients on insulin pumps are co-mingled with the general diabetes patient population from a coding perspective, i.e., they do not receive a unique diagnostic code that can distinguish them from other diabetes patients in an electronic database. Due to the lack of a specific identifier for insulin pump patients, it will be difficult to conduct large scale analyses of hospitalizations involving pump users unless institutions develop registries like ours. Applying existing ICD-9 codes to CSII users would allow for easier identification in electronic data for future retrospective analysis. Our process of identifying patients on insulin pumps still requires an endocrinology consultation request from the admitting team, which makes it all the more necessary to continue educating inpatient staff about this policy so that all pump patients can be seen and the outcomes tracked.
A third limitation is that we have not yet addressed patient satisfaction with our policy and procedures. A study did report data on the topic of patient satisfaction with regard to inpatient insulin pump therapy, which is something we need to begin evaluating.12 Additionally, we should evaluate staff satisfaction with our approach to these patients.
Beyond the challenges and limitations noted here is how best to provide ongoing education to inpatient staff regarding insulin pump procedures. Staff turnover occurs constantly. We have been providing ongoing briefings to the nursing staff about the insulin pump policy, and resident physicians have access to online modules on inpatient diabetes that include information about our inpatient CSII procedures,22 but we need to develop a more comprehensive approach that includes all inpatient providers.
Despite these limitations and challenges, this analysis provided us the most detail yet about characteristics of the inpatient population using insulin pumps. A policy on inpatient CSII use can be successfully implemented, and patients on outpatient treatment can have therapy safely transitioned to the hospital. High compliance with required process measures can be achieved, although there continues to be room to improve in some areas. Hospital glycemic control among CSII users was no worse than that achieved when alternative insulin regimens were required. Further study is needed to determine how best to optimize glycemic control when CSII therapy is used in the hospital, to improve case identification, and to provide continuing education on the processes needed to care for these patients.
Abbreviations
- BedGlucavg
bedside glucose average
- CSII
continuous subcutaneous insulin infusion
- HbA1c
hemoglobin A1c
References
- 1.Pickup J, Keen H. Continuous subcutaneous insulin infusion at 25 years: evidence base for the expanding use of insulin pump therapy in type 1 diabetes. Diabetes Care. 2002;25(3):593–598. doi: 10.2337/diacare.25.3.593. [DOI] [PubMed] [Google Scholar]
- 2.Institute for Safe Medication Practices. ISMP’s list of high-alert medications. 2010. Jan 18, http://www.ismp.org/Tools/highalertmedications.pdf. Accessed March 19, 2010.
- 3.Cheekati V, Osburne RC, Jameson KA, Cook CB. Insulin therapy for inpatients with diabetes: perceptions of resident physicians from disparate geographic training programs. Insulin. 2009;4:106–113. [Google Scholar]
- 4.Cheekati V, Osburne RC, Jameson KA, Cook CB. Perceptions of resident physicians about management of inpatient hyperglycemia in an urban hospital. J Hosp Med. 2009;4(1):E1–E8. doi: 10.1002/jhm.383. [DOI] [PubMed] [Google Scholar]
- 5.Cook CB, Jameson KA, Hartsell ZC, Boyle ME, Leonhardi BJ, Farquhar-Snow M, Beer KA. Beliefs about hospital diabetes and perceived barriers to glucose management among inpatient midlevel practitioners. Diabetes Educ. 2008;34(1):75–83. doi: 10.1177/0145721707311957. [DOI] [PubMed] [Google Scholar]
- 6.Cook CB, McNaughton DA, Braddy CM, Jamesonn KA, Roust LR, Smith SA, Roberts DL, Thomas SL, Hull BP. Management of inpatient hyperglycemia: assessing perceptions and barriers to care among resident physicians. Endocr Pract. 2007;13(2):117–124. doi: 10.4158/EP.13.2.117. [DOI] [PubMed] [Google Scholar]
- 7.Cook CB. Are two insulin pumps better than one? 2009. Jan, Agency for Healthcare Research and Quality, Morbidity and Mortality Rounds on the Web. http://webmm.ahrq.gov/case.aspx?caseID=192. Accessed January 28, 2010.
- 8.Fain JA. Pump up your knowledge of insulin pumps. Nursing. 2003;33(6):51–53. doi: 10.1097/00152193-200306000-00045. [DOI] [PubMed] [Google Scholar]
- 9.Hess-Fischl A. Practical management of patients with diabetes in critical care: from a diabetes educator’s perspective. Crit Care Nurs Q. 2004;27:189–200. doi: 10.1097/00002727-200404000-00011. [DOI] [PubMed] [Google Scholar]
- 10.Korytkowski MT. Treatment options for safely achieving glycemic targets in the hospital. In: Moghissi ES, editor. Revisiting inpatient hyperglycemia: new recommendations, evolving data, and practical implications for implementation. Suppl. ACP Hospitalist, Postgraduate Institute of Medicine; 2009. pp. 15–23. [Google Scholar]
- 11.Quinn C. Infusion devices: understanding the patient perspective to avoid errors. Prof Nurse. 2003;19(2):79–83. [PubMed] [Google Scholar]
- 12.Noschese ML, DiNardo MM, Donihi AC, Gibson JM, Koerbel GL, Saul M, Stefanovic-Racic M, Korytkowski MT. Patient outcomes after implementation of a protocol for inpatient insulin pump therapy. Endocr Pract. 2009;15(5):415–424. doi: 10.4158/EP09063.ORR. [DOI] [PubMed] [Google Scholar]
- 13.Cook CB, Boyle ME, Cisar NS, Miller-Cage V, Bourgeois P, Roust LR, Smith SA, Zimmerman RS. Use of continuous subcutaneous insulin infusion (insulin pump) therapy in the hospital setting: proposed guidelines and outcome measures. Diabetes Educ. 2005;31(6):849–857. doi: 10.1177/0145721705281563. [DOI] [PubMed] [Google Scholar]
- 14.Lee SW, Im R, Magbual R. Current perspectives on the use of continuous subcutaneous insulin infusion in the acute care setting and overview of therapy. Crit Care Nurs Q. 2004;27(2):172–184. doi: 10.1097/00002727-200404000-00009. [DOI] [PubMed] [Google Scholar]
- 15.Dalton MF, Klipfel L, Carmichael K. Safety issues: use of continuous subcutaneous insulin infusion (CSII) pumps in hospitalized patients. Hosp Pharm. 2006;41(10):956–969. [Google Scholar]
- 16.Bailon RM, Partlow BJ, Miller-Cage V, Boyle ME, Castro JC, Bourgeois PB, Cook CB. Continuous subcutaneous insulin infusion (insulin pump) therapy can be safely used in the hospital in select patients. Endocr Pract. 2009;15(1):24–29. doi: 10.4158/EP.15.1.24. [DOI] [PubMed] [Google Scholar]
- 17.Leonhardi BJ, Boyle ME, Beer KA, Seifert KM, Bailey M, Miller-Cage V, Castro JC, Bourgeois PB, Cook CB. Use of continuous subcutaneous insulin infusion (insulin pump) therapy in the hospital: a review of one institution’s experience. J Diabetes Sci Technol. 2008;2(6):948–962. doi: 10.1177/193229680800200605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cook CB, Moghissi E, Joshi R, Kongable GL, Abad VJ. Inpatient point-of-care bedside glucose testing: preliminary data on use of connectivity informatics to measure hospital glycemic control. Diabetes Technol Ther. 2007;9(6):493–500. doi: 10.1089/dia.2007.0232. [DOI] [PubMed] [Google Scholar]
- 19.The Joint Commission. Inpatient diabetes certification. http://www.jointcommission.org/CertificationPrograms/Inpatient+Diabetes/. Accessed February 14, 2008.
- 20.Cook CB, Castro JC, Schmidt RE, Gauthier SM, Whitaker MD, Roust LR, Argueta R, Hull BP, Zimmerman RS. Diabetes care in hospitalized non-critically ill patients: more evidence for clinical inertia and negative therapeutic momentum. J Hosp Med. 2007;2(4):203–211. doi: 10.1002/jhm.188. [DOI] [PubMed] [Google Scholar]
- 21.Queale WS, Seidler AJ, Brancati FL. Glycemic control and sliding scale insulin use in medical inpatients with diabetes mellitus. Arch Intern Med. 1997;157(5):545–552. [PubMed] [Google Scholar]
- 22.Cook CB, Wilson RD, Hovan MJ, Hull BP, Gray RJ, Apsey HA. Development of computer-based training to enhance resident physician management of inpatient diabetes. J Diabetes Sci Technol. 2009;3(6):1377–1387. doi: 10.1177/193229680900300618. [DOI] [PMC free article] [PubMed] [Google Scholar]