Abstract
Background
Optimal continuous subcutaneous insulin infusion (CSII) therapy emphasizes the relationship between insulin dose and carbohydrate consumption. One widely used tool (bolus calculator) requires the user to enter discrete carbohydrate values; however, many patients might not estimate carbohydrates accurately. This study assessed carbohydrate estimation accuracy in type 1 diabetes CSII users and compared simulated blood glucose (BG) outcomes using the bolus calculator and the “bolus guide,” an alternative system based on ranges of carbohydrate load.
Methods
Patients (n = 60) estimated the carbohydrate load of a representative sample of meals of known carbohydrate value. The estimated error distribution [coefficient of variation (CV)] was the basis for a computer simulation (n = 1.6 million observations) of insulin recommendations for the bolus guide and bolus calculator, translated into outcome blood glucose (OBG) ranges (≤60, 61–200, >201 mg/dl). Patients (n = 30) completed questionnaires assessing satisfaction with the bolus guide.
Results
The CV of typical meals ranged from 27.9% to 44.5%. The percentage of simulated OBG for the calculator and the bolus guide in the <60 mg/dl range were 20.8% and 17.2%, respectively, and 13.8% and 15.8%, respectively, in the >200 mg/dl range. The mean and median scores of all bolus guide satisfaction items and ease of learning and use were 4.17 and 4.2, respectively (of 5.0).
Conclusion
The bolus guide recommendation based on carbohydrate range selection is substantially similar to the calculator based on carbohydrate point estimation and appears to be highly accepted by type 1 diabetes insulin pump users.
Keywords: bolus, bolus calculator, bolus guide, carbohydrates, glycemic control, range
Introduction
The Diabetes Control and Complications Trial (DCCT) confirmed the benefits of intensive treatment via multiple daily injections (MDI) or continuous subcutaneous insulin infusion (CSII) therapy.1 A cornerstone of intensive therapyin the DCCT was meal-planning strategies that emphasized the relationship between prandial insulin dose selection and the anticipated amount of carbohydrate to be consumed.2,3 Since the results of the DCCT were published, others have reported on glycemic and patient-reported outcome benefits of carbohydrate counting for individuals with type 1 diabetes. These benefits include lower hemoglobin A1c (HbA1c) levels,4–6 improved coping ability5,6 and diabetes-specific quality of life,4,6 and greater treatment satisfaction.4
Precision with carbohydrate counting was associated with lower HbA1c in one study of children with diabetes,7 and inaccuracy of carbohydrate counting has been noted as one of the two major sources of error in predicting blood glucose (BG) levels, along with BG monitor inaccuracy.8 Others have reported high rates of inaccurate carbohydrate counting and consequent inappropriate prandial insulin dosing. In the FinnDiane study,9 adults with type 1 diabetes estimated their prandial need inappropriately in 62% of meals; in the Carbohydrate Counting for Adolescents with Type 1 Diabetes study,10 only 23% of study participants estimated daily carbohydrate within 10 g of the true amount.
There is evidence that using a bolus calculator may help patients more accurately meet prandial insulin dose requirements, reduce postprandial glycemic excursions, and achieve closer to normal overall glycemic control.11 In one randomized clinical trial,12 patients who used a diabetes interactive diary (DID), a tool incorporating a bolus calculator, and patients who were taught standard carbohydrate counting had similar reductions in HbA1c levels, but DID arm patients experienced greater improvements in diabetes treatment satisfaction and in some aspects of health-related quality of life.
A study addressed the question of how precise carbohydrate quantification has to be to maintain post-prandial glycemic control.13 The study found that, in children and adolescents using either CSII or MDI therapy, an individually calculated insulin dose for 60 g of carbohydrate maintained post-prandial BG levels for meals containing between 50 and 70 g of carbohydrate. This suggests that a single mealtime insulin dose will cover a range of carbohydrate amounts without deterioration in postprandial control.
Use of a bolus guide, based on carbohydrate range selection, might have advantages over a bolus calculator that requires carbohydrate point estimation if both systems yield substantially the same bolus recommendations and BG outcomes. Patients might find it easier to use estimate ranges, and this could lead to high treatment satisfaction with a bolus guide. Treatment satisfaction is important because it may be associated with adherence to treatment,14 treatment preference,15,16 and glycemic control.17–19
The current study of current CSII therapy users was designed to answer three questions: (1) how accurately do patients in the study estimate carbohydrate loads?; (2) how do simulated BG outcomes using the bolus guide compare with those using the bolus calculator?; and (3) what are patient perceptions of the bolus guide? We hypothesize that rates of inaccurate carbohydrate assessment will be high, that simulated BG outcomes for the bolus guide and the bolus calculator will be similar, and that patients will have favorable perceptions of the carbohydrate range selection used in the bolus guide as compared with entry of specific carbohydrate values as used in the bolus calculator.
Methods
The overall study incorporated three components: (1) a study to assess patient accuracy in estimating the carbohydrate content of meals, (2) a simulation study comparing insulin bolus recommendations for the bolus guide and bolus calculator and consequent BG levels, and (3) a usability study to assess patient perceptions of the bolus guide.
Carbohydrate Content Accuracy Study
Type 1 diabetes pump users (n = 60, pump usage time 6.4 ± 3.9 years) familiar with carbohydrate quantification participated in this study. The study was approved by an institutional research review board provided by Chesapeake Research Review. Participants signed an informed consent form. Table 1 shows patient demographics.
Table 1.
Baseline Data
| Demographics | N | % |
|---|---|---|
| Age | ||
| 18–40 years | 20 | 33.3 |
| 41–59 years | 31 | 51.7 |
| 60+ years | 9 | 15.0 |
| All | 60 | 100.0 |
| Gender | ||
| Male | 16 | 26.7 |
| Female | 44 | 73.3 |
| All | 60 | 100.0 |
Eight packaged meals (Table 2) of known carbohydrate values, containing typical amounts of carbohydrate in meals consumed by the intended use population, were shown to participants, and they were asked to record their estimates of the carbohydrate content of each meal on a form provided. A total of 48 participants estimated the carbohydrate load of meals A–D, and 42 participants estimated the carbohydrate load of meals E–H (30 participants estimated all meals, 18 participants estimated only meals A–D, and 12 participants estimated only meals E–H).
Table 2.
Meals Displayed
| Meal | Content | True Carbohydrates (g) |
|---|---|---|
| D | Classic Cobb salad with dressing and a breadstick | 37 |
| H | Chicken Caesar salad with dressing and a breadstick | 39 |
| A | Southwest steak panini sandwich | 66 |
| E | Cheese and tomato flatbread pizza | 68 |
| C | Chicago cheesecake with strawberries and whipped cream | 76 |
| G | Burger and French fries | 83 |
| B | Chicken and broccoli fettuccine and a breadstick | 131 |
| F | Plate of cookies | 154 |
The results of this study (i.e., human error in carbohydrate estimation and error distribution) served as the basis for subsequent computer simulations.
Simulation Study
Bolus calculators typically ask the user to identify a specific amount of carbohydrate to be consumed in order to make a recommendation for the amount of insulin to be administered in a bolus. While mathematically appropriate, this does not take into account the difficulty of precisely estimating carbohydrate load in a meal. The bolus guide tool was designed to recommend a bolus insulin dose based on user input of the estimated carbohydrate load within a range rather than a single point.
Tables of carbohydrate and glucose ranges are stored in the bolus guide software memory, with each table related to a specific combination of target blood glucose (TBG), insulin sensitivity factor (ISF), and insulin-to-carbohydrate ratio (ITC). The number of tables stored in the database is limited to combinations of the TBG, ISF, and ITC values [minimum, maximum, and increments (in brackets)] as shown in Table 3. Should data entered exceed these limits, the bolus guide will present an error message. Future work will be done to increase these ranges to cover patients with even greater insulin sensitivity.
Table 3.
Combinations of the Target Blood Glucose, Insulin Sensitivity Factor, and Insulin-to-Carbohydrate Ratio Values Supported by the Bolus Guide
| Parameter | Minimum-Maximum (Increments) |
|---|---|
| TBG (mg/dl) | 70–180 (10) |
| ISF (1 U: X mg/dl) | 16–24 (2) 25–45 (5) 50–150 (10) |
| ITC (1 U: X g) | 1–20 (1) 22–60 (2) |
| ISF/ITC | 3.5–5 |
The columns and rows of the tables stored in the bolus guide include ranges of carbohydrate and current blood glucose (CBG), respectively [range boundaries (low carbohydrate, high carbohydrate; low BG, high BG) are shown in Table 4]. Each cell in Table 4 represents a pair of discrete reference values, one for carbohydrate load range and one for CBG range. The bolus (in units) recommended in each cell (in Table 4, for example, 3.2 U for the cell represented by carbohydrate range 61–75 g and BG range 71–100 mg/dl) is precalculated according to the formula using the reference values (in the given example, 64 g and 98 mg/dl, respectively). The reference values were selected to minimize hypoglycemia related to the bolus dose recommendation of each cell. The reference values are not the mid values of carbohydrate and CBG range boundaries and are different for each bolus guide grid (defined by various combinations of TBG, ISF, and ITC). The reference values were chosen in each cell to produce the smallest error for the extreme cases of carbohydrates and BG in the cell (lowest and highest corner values in the carbohydrates × BG cell). However, the error was made artificially large if it resulted in an outcome blood glucose (OBG; as explained further later) under 60 or over 200. These values were originally given as constraints on the accuracy requirements of the bolus guide tool.
Table 4.
Bolus Guide Grid and Range Boundaries
| ISF = 60 ITC = 20 TBG = 100 |
Low carbohydrate | 0 | 5 | 16 | 31 | 46 | 61 | 76 | 91 | 106 | 121 | 136 | 151 | 166 | 181 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| High carbohydrate | 0 | 15 | 30 | 45 | 60 | 75 | 90 | 105 | 120 | 135 | 150 | 165 | 180 | 195 | ||
| Low BG | High BG | Reference | 64 | |||||||||||||
| 50 | 70 | |||||||||||||||
| 71 | 100 | 98 | 3.2U | |||||||||||||
| 101 | 130 | |||||||||||||||
| 131 | 160 | |||||||||||||||
| 161 | 190 | |||||||||||||||
| 191 | 220 | |||||||||||||||
| 221 | 250 | |||||||||||||||
| 251 | 280 | |||||||||||||||
| 281 | 310 | |||||||||||||||
| 311 | 340 | |||||||||||||||
The bolus guide process for recommending an insulin bolus dose is based on the user’s input of TBG, ISF, and ITC, entered once at the initial setting. The CBG and a particular carbohydrate range selected from a set of ranges that the system prespecifies are entered every time the bolus guide is used. Carbohydrate range is the range comprising the total amount of carbohydrates to be consumed. The software also enables entry of a discrete carbohydrate value that is attributed to the relevant carbohydrate range. For example, if a user enters a discrete carbohydrate value of 68 g, the reference value (i.e., 64 g) of the relevant carbohydrate range (i.e., 61–75 g) is used to calculate the bolus dose (Table 4).
The remaining insulin or “bolus on board” (BOB) is based on delivery times and doses of previous boluses and is calculated in the same manner as in available bolus calculators.
A computer simulation was performed wherein hundreds of carbohydrate estimation error values were generated within the coefficient of variation (CV) range (30–50%) resulting from the carbohydrate estimation study outcome for each of the meals displayed. In addition to CVs yielded by the study, simulation results for lower CV values of 0–30% were also evaluated. The carbohydrate estimation error values were also simulated according to the error distribution patterns resulting from the carbohydrate estimation study outcome. Normal (Gaussian) and gamma (an asymmetrical distribution with certain skewness) distributions around the estimated and true carbohydrate (TC) values (four different error distribution patterns) were thus simulated. Shape and scale parameters for gamma distribution were extracted by mean and standard deviation (scale = CV2× mean and shape = 1/CV2; as mean, the TC was taken for each meal, and CV was fixed at 0% to 50% by step of 10%).
An additional error that was considered and simulated is the error of the BG measurement (CBG). The simulated CVs of the CBG error were 0% (i.e., accurate BG measurements) and 10%. The BG measurement errors were assumed to be normally distributed around the true BG level with a standard deviation equal to the true BG times the given error CV for BG.
Recommended insulin boluses were simulated to yield three bolus recommendations as follows:
-
Bolus guide recommendation based on
carbohydrate—reference value representing the user-estimated bolus guide range,
CBG—reference value representing bolus guide range that includes the user’s CBG value, and
ITC and ISF—rounded to the nearest available bolus guide value (Table 3).
- Calculator recommendation based on CBG, ITC, ISF, and user-estimated carbohydrate (discrete value) according to the following formula:
Correct recommendation based on CBG, ITC, ISF, and TC value (Table 2). The correct recommendation is based on no error of the intake of carbohydrate. The carbohydrate intake is expressed as a discrete value rather than as a range of values.
The ISF and ITC are assumed to be accurate and therefore do not contribute to the error of the calculator recommendation or the correct recommendation.
The process of generating the three bolus recommendation comparisons (bolus guide, calculator, and correct) was repeated for 140 random combinations of ISF, ITC, and TBG for each of eight meal types (A–H). These 1120 combinations of parameters were simulated with 12 different values of CBG (total 13,140). Each combination was simulated with 10 different estimated carbohydrate values based on a given error CV for carbohydrate (yielding 131,400 observations). These comparisons were repeated for six carbohydrate estimation CVs of 0%, 10%, 20%, 30%, 40%, and 50% (yielding 806,400 observations). These comparisons were repeated for two BG measure-ment errors of 0% and 10% for each of the 12 simulated CBG values (yielding 1,612,800 observations).
An additional simulation (875,712 observations) with uniform distribution running over a narrower range of the most commonly used user parameters (ISF 40–110, TBG 80–120, ITC 8–31) was also completed. Such a simulation resembles a more realistic distribution of the combinations of ISF, ITC, and TBG in the population.
An analysis was conducted to evaluate the clinical significance of the bolus guide and the calculator recommendation errors in comparison to the correct recommendation. The BG levels that result from administration of a certain amount of insulin (either that recommended by the bolus guide or the calculator) can be predicted using the following equation:
wherein U is insulin units (provided by either calculator or bolus guide). Note that BOB is considered 0 in the simulations since it would affect both tools equally. When generating a bolus dose using the discrete values of perfectly accurate BG and carbohydrate estimations (i.e., the “correct” recommendation), the OBG always equals exactly the TBG value.
Outcome BG levels were assigned to one of the following OBG categories ≤60, 61–90, 91–120, 121–150, 151–200, 201–250, and >250 mg/dl, and the percentage of OBG for the bolus guide and the bolus calculator in each category was compared. In addition, we compared the proportion of inaccurate OBG with each tool. Outcome BG was considered inaccurate if it fell outside the TBG range (70–180 mg/dl) or if the OBG was not in the same category as the TBG.
Usability Test
Type 1 diabetes pump users (n = 30) participated in a usability study (n = 30) to estimate patient satisfaction using the bolus guide. The study was approved by Chesapeake Research Review. Participants signed an informed consent form. The bolus guide application was implemented as a software program on a personal computer [(PC) yet it can be implemented using any device that includes memory and computing capabilities, e.g., an insulin pump, a PC, a cell phone, or a personal digital assistant]. Its use and application were demonstrated with the PC software. After training by a facilitator, the participants practiced until they felt confident and then responded to four written scenarios (demonstrated on cue cards) to submit bolus recommendation requests (Table 5). In order to receive a bolus recommendation, the participants had to enter BG value, carbohydrate range, ISF, ITC, TBG, and the previous boluses delivered (for BOB calculation). Distractions and errors (Table 5) were intentionally introduced, and the participants needed to arrive at the correct recommendation by responding to error messages and making the needed corrections. Participants completed a 15-item questionnaire (Table 6) about their experience using the bolus guide, with responses on a five-point scale, with 1 being “strongly disagree” and 5 being “strongly agree.” Questions included an item “choosing carbohydrate values from a ranges list is easier than estimating exact amounts.”
Table 5.
Four Written User Scenarios with Distractions and Errors
| Scenario | Distractions | Tasks to complete (pathway) | |
|---|---|---|---|
| 1 | Normal entry | Someone will open the door and talk on the phone | Enter data as written on cue card, press the “Submit” button, receive bolus guide recommendation |
| 2 | Out of range value | None | Enter data as written on cue card, respond to messages by pressing “OK,” take corrective action, enter corrected values (to be provided on cue card), press the “Submit” button, receive bolus guide recommendation |
| 3 | BG measurement time error (>10min) | Radio | Enter data as written on cue card, respond to messages, take corrective action, enter new time (to be provided on cue card), press the “Submit” button, receive bolus guide recommendation |
| 4 | Previous bolus list entry error | Telephone call | Enter data as written on cue card, respond to messages, take corrective action, enter missing time (to be provided on cue card), press the “Submit” button, receive bolus guide recommendation |
Table 6.
Descriptive Statistics for the User’s Agreement by Item
| Question | Mean | Standard deviation | Median | |
|---|---|---|---|---|
| 1 | The introduction was enough to get me started using the bolus guide. | 4.17 | 0.66 | 4.00 |
| 2 | It is easy to enter data correctly. | 4.34 | 0.61 | 4.00 |
| 3 | The screen layout is simple to follow. | 4.21 | 0.86 | 4.00 |
| 4 | The messages and alarms help prevent errors. | 4.34 | 0.72 | 4.00 |
| 5 | Messages are easy to understand. | 4.45 | 0.57 | 4.00 |
| 6 | I think that pump users can easily learn to use this tool. | 4.31 | 0.71 | 4.00 |
| 7 | I understood easily how to correct input errors. | 4.48 | 0.51 | 4.00 |
| 8 | Choosing carbohydrate values from a ranges list is easier than estimating exact amounts. | 4.17 | 0.85 | 4.00 |
| 9 | I felt confident with the bolus recommendations I received. | 3.97 | 0.73 | 4.00 |
| 10 | I would be able to easily complete all the test tasks a second time. | 4.41 | 0.82 | 5.00 |
| 11 | It is hard to make a mistake using this system. | 3.86 | 1.03 | 4.00 |
| 12 | It is easy to find information in the user guide. | 3.25 | 1.71 | 3.50 |
| 13 | The bolus guide meets my expectations for a bolus recommendation tool. | 3.83 | 1.04 | 4.00 |
| 14 | By the end of the tasks, I felt confident using the tool. | 4.45 | 0.63 | 5.00 |
| 15 | It is practical to have a bolus recommendations tool separate from my insulin pump. | 2.55 | 1.38 | 2.00 |
Results
Carbohydrate Content Accuracy Study
Table 7 shows human error and CV values of carbohydrate estimates of the displayed meals.
Table 7.
Descriptive Statistics of Carbohydrate Estimate and Error by Meal in Increasing True Carbohydrate Order
| Meal | TC (g) | n | Estimated carbohydrate | Error | |||
|---|---|---|---|---|---|---|---|
| Mean (g) | Standard deviation | CV (%) | Mean (g) deviation from TC | Mean error (%) | |||
| D | 37 | 48 | 37.2 | 16.9 | 45.6 | 0.2 | 0.5 |
| H | 39 | 42 | 38.9 | 16.7 | 43.0 | -0.1 | 0.3 |
| A | 66 | 48 | 58.2 | 19.2 | 32.9 | -7.8 | 11.8 |
| E | 68 | 42 | 73.2 | 32.1 | 43.9 | 5.2 | 7.6 |
| C | 76 | 48 | 63.4 | 27.3 | 43.1 | -12.6 | 16.6 |
| G | 83 | 42 | 74.3 | 20.7 | 27.9 | -8.7 | 10.5 |
| B | 131 | 48 | 86.8 | 30.8 | 35.5 | -44.2 | 33.7 |
| F | 154 | 42 | 80.6 | 36.1 | 44.8 | -73.4 | 47.7 |
Error is defined as mean estimated carbohydrate minus TC or as mean percent error [(absolute value of the mean error expressed in grams/TC) × 100].
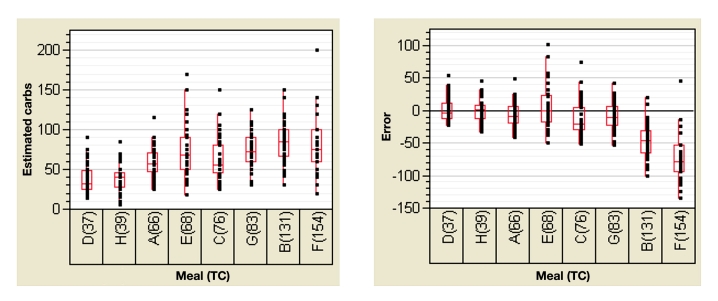
Key points from the clinical study analysis (Figure 1 and Table 7) are as follows:
Figure 1.
Estimated carbohydrate and error by meals (TC). The dots represent the population distribution, the bottom and top of the box represent the 25th and 75th percentile, the band inside the box represents the 50th percentile, and the ends of the whiskers represent the 9th and 91st percentile. Carbs, carbohydrates.
Mean percent error tended to increase with increasing carbohydrate load.
The standard deviations of the estimated carbo-hydrate were large for both low carbohydrate and high carbohydrate meals and increase with increasing carbohydrate loads.
The CV of the estimated carbohydrate was high for all meals, ranging from 28% to 46%; values of 0%, 10%, 20%, 30% 40%, and 50% were used in the simulation.
The error distribution of some of the meals followed a normal pattern, while other meals followed a gamma pattern. Since no consistency was observed in error distribution, the simulation was done with both normal and gamma error distributions. The best estimated meal was the hamburger and fries. This might point to the effect of familiarity on estimation accuracy.
Simulation Study
The percentage of accurate carbohydrate estimation data points was very similar for the bolus guide and bolus calculator (Table 8), with accuracy defined as an OBG in the same category (i.e., ≤60, 61–90, 91–120, 121–150, 151–200, 201–250, and >250 mg/dl) as the TBG. Nearly identical results were obtained for a simulation with uniform distribution running over a narrower range of the most commonly used user parameters (ISF 40–110, TBG 80–120, ITC 8–31, ISF/ITC 3.5–5).
Table 8.
Accuracies of Calculator Versus Bolus Guide for Meals A-H Averaged for Carbohydrate Estimation Error Coefficients of Variation of 0%, 10%, 20%, 30%, 40%, and 50% and Blood Glucose Measurement Error Coefficients of Variation of 0% and 10% over the Entire Range of Target Blood Glucose, Insulin-to-Carbohydrate Ratio, and Insulin Sensitivity Factor According to Normal and Gamma Carbohydrate Estimation Error Distribution around the Estimated Mean and True Carbohydrate Value
| Distribution/Carbohydrates | Calculator | Bolus guide | Difference | |
|---|---|---|---|---|
| Normal | TCs | 71.0% | 72.2% | 1.2% |
| Estimated Carbohydrates | 61.7% | 61.8% | 0.1% | |
| Gamma | TCs | 72.5% | 73.4% | 0.9% |
| Estimated Carbohydrates | 62.0% | 61.9% | 0.0% | |
The bolus recommendations provided by the bolus guide led to slightly less hypoglycemia (<60 mg/dl) and slightly more hyperglycemia (>200 mg/dl) than the bolus recommendations provided by the bolus calculator (Table 9). Bolus recommendations by the bolus guide also led to slightly more normoglycemia (90–150 mg/dl) than the bolus calculator recommendations (Table 10).
Table 9.
Percentage of Results Blood Glucose ≤60, 61-200, and >200 mg/dl of Calculator Versus Bolus Guide for Meals A-H Averaged for Carbohydrate Estimation Error Coefficients of Variation of 0%, 10%, 20%, 30%, 40%, and 50% and Blood Glucose Measurements Error Coefficients of Variation of 0% and 10% over the Entire Range of Target Blood Glucose, Insulin-to-Carbohydrate Ratio, and Insulin Sensitivity Factor According to Normal and Gamma Carbohydrate Estimation Error Distribution around the Estimated Mean and True Carbohydrate Value
| Normal | Gamma | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Around TC | Around estimated carbohydrate | Around TC | Around estimated carbohydrate | |||||||||
| ≤60 mg/dl | 61–200 mg/dl | >200 mg/dl | ≤60 mg/dl | 61–200 mg/dl | >200 mg/dl | ≤60 mg/dl | 61–200 mg/dl | >200 mg/dl | ≤60 mg/dl | 61–200 mg/dl | >200 mg/dl | |
| Calculator | 20.3% | 66.2% | 13.5% | 12.5% | 55.3% | 32.3% | 18.5% | 67.1% | 14.4% | 11.8% | 55.2% | 33.0% |
| Bolus guide | 17.0% | 67.6% | 15.4% | 10.0% | 54.9% | 35.0% | 15.5% | 67.9% | 16.6% | 9.6% | 54.6% | 35.8% |
| Difference | -3.3% | 1.4% | 1.9% | -2.5% | -0.4% | 2.7% | -3.0% | 0.8% | 2.2% | -2.2% | -0.6% | 2.8% |
Table 10.
Percentage of Results Blood Glucose ≤90, 91-150, and >150 mg/dl of Calculator Versus Bolus Guide for Meals A-H Averaged for Carbohydrate Estimation Error Coefficients of Variation of 0%, 10%, 20%, 30%, 40%, and 50% and Blood Glucose Measurements Error Coefficients of Variation of 0% and 10% over the Entire Range of Insulin-to-Carbohydrate Ratio, Insulin Sensitivity Factor, and Target Blood Glucose Range of 90-150 mg/dl According to Normal and Gamma Carbohydrate Estimation Error Distribution around the Estimated Mean and True Carbohydrate Value
| Normal | Gamma | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Around TC | Around estimated carbohydrate | Around TC | Around estimated carbohydrate | |||||||||
| ≤90 mg/dl | 91-150 mg/dl | >150 mg/dl | ≤90 mg/dl | 91-150 mg/dl | >150 mg/dl | ≤90 mg/dl | 91-150 mg/dl | >150 mg/dl | ≤90 mg/dl | 91-150 mg/dl | >150 mg/dl | |
| Calculator | 35. 9% | 34.0% | 30.1% | 22.4% | 27.7% | 49.9% | 33.4% | 34.3% | 32.3% | 21.2% | 27.6% | 51.2% |
| Bolus guide | 27.7% | 39.5% | 32.8% | 16.1% | 29.3% | 54.7% | 25.5% | 39.3% | 35.2% | 15.1% | 28.8% | 56.1% |
| Difference | -8.2% | 5.5% | 2.7% | -6.3% | 1.6% | 4.8% | -7.9% | 5.0% | 2.9% | -6.1% | 1.2% | 4.9% |
Carbohydrate estimations were assumed to be (1) normally distributed around the mean estimated carbohydrate load of the meal, (2) gamma distribution pattern around the estimated mean, (3) normally distributed around the TC value, and (4) gamma distribution pattern around the TC value. The results are a simple average of equally distributed combinations of carbohydrate estimation error CVs of 0%, 10%, 20%, 30%, 40%, and 50% and BG measurement error CVs of 0% and 10% over the entire range of TBG, ITC, and ISF for meals A–H.
Usability Study
Table 11 presents descriptive statistics for task completion as recorded by the study’s facilitator. Task completion was scored using a scale of 0 to 3, 0 being “completed alone” and 3 being “not completed.”
Table 11.
Descriptive Statistics for Task Completion
| Scenario | Mean | Standard deviation | Median | N |
|---|---|---|---|---|
| Scenario 1: normal entry | 0.63 | 0.67 | 1.00 | 30 |
| Scenario 2: out-of-range value | 0.57 | 0.68 | 0.50 | 30 |
| Scenario 3: BG measurement time error | 0.45 | 0.63 | 0.00 | 29 |
| Scenario 4: previous bolus list time error | 0.31 | 0.60 | 0.00 | 29 |
From Table 11, we see that the mean scores of task completion for all scenarios were below 0.70 points, ranging between 0.31 and 0.63.
There seems to be a trend for improving scores across the scenarios. The highest mean was in the first scenario, and lowest was in the last scenario. This suggests a short learning curve to reaching a criterion of independent completion.
Table 6 presents descriptive statistics for the user’s (n = 29) overall assessment of usability, scored after all tasks had been completed. Questionnaire items were completed on a five-point scale from 1 to 5, with 1 being “strongly disagree” and 5 being “strongly agree.”
The mean, median, and standard deviation of questions related to ease of learning and use for the four scenarios were 4.17, 4.2, and 0.72, respectively, with a score of 5 being “strongly agree” and a score of 4 being “agree.” The mean, median, and standard deviation for the question “choosing carbohydrate values from a ranges list is easier than estimating exact amounts” were 4.17, 4.0, and 0.85, respectively.
Discussion
In persons with type 1 diabetes, the total amount of carbohydrate consumed strongly predicts glycemic response, so monitoring carbohydrate consumption is essential for achieving glycemic control. Our results are consistent with those of earlier studies4,5 in finding that substantial estimation inaccuracies were common. In addition, we found that the mean percent error was significantly larger in meals containing high carbohydrate loads (>100 g). This may relate to patient reluctance to take what seems like an unusually large bolus.
The simulation results demonstrated that the bolus guide and bolus calculator provided similar bolus recommendations and OBG levels overall, with the bolus calculator providing recommendations leading to BG levels in the <60 mg/dl (hypoglycemia) category more often than the bolus guide, and the bolus guide providing bolus recommendations leading to BG levels in the >200 mg/dl and >150 mg/dl categories more often than the calculator.
As described, our comparison of the bolus guide and the calculator was done via a simulation. Since simulation procedures can increase “sample size” indefinitely, there is no issue of sampling error and hence of formal statistical testing. The problem is well suited to modeling because the consequences (normal, hyper, or hypoglycemia) follow logically and inevitably from the starting conditions (exact or inaccurate carbohydrate load estimation that leads to accurate or inaccurate insulin bolus doses, respectively).
Given the similar simulated bolus recommendations and OBG levels for the bolus guide and bolus calculator, patient reports regarding use of the bolus guide are of special interest. In the usability study, patients were highly satisfied with the performance and ease of use of the bolus guide, and most patients (average score 4.2 out of 5, with a score of 5 indicating strong agreement) reported that choosing carbohydrate values from a range list is easier than estimating exact amounts. This suggests that using a bolus guide might be associated with greater treatment satisfaction than using a bolus calculator. Others have found that treatment satisfaction may be associated with adherence to treatment,14 treatment preference,15,16 and glycemic control.17–19
Strengths of the current study include the fact that the comparison of bolus guide and bolus calculator outcomes was based on a simulation that permitted assessment of a very large number of possible events. The inclusion of a user acceptance study in which patients were asked to compare the ease of using the bolus guide and bolus calculator is also a study strength. Future studies should allow patients to use the bolus guide on their own over extended periods and should compare real clinical outcomes rather than simulated ones for the bolus guide and bolus calculator.
Conclusions
In the study simulation, the bolus recommendations provided by the bolus guide led to slightly less hypoglycemia (<60 mg/dl) than the bolus calculator. The bolus guide recommendations, however, did result in slightly more hyperglycemia (>200 mg/dl) than the bolus recommendations provided by the bolus calculator.
Responses in the usability study suggest that patients might find the bolus guide’s carbohydrate range estimation system easier to use than the bolus calculator’s carbohydrate values estimation system.
Abbreviations
- BG
blood glucose
- BOB
bolus on board
- CBG
current blood glucose
- CSII
continuous subcutaneous insulin infusion
- CV
coefficient of variation
- DCCT
Diabetes Control and Complications Trial
- DID
diabetes interactive diary
- HbA1c
hemoglobin A1c
- ISF
insulin sensitivity factor
- ITC
insulin-to-carbohydrate ratio
- MDI
multiple daily injection
- OBG
outcome blood glucose
- PC
personal computer
- TBG
target blood glucose
- TC
true carbohydrate
References
- 1.The Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–85. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 2.The DCCT Research Group. Anderson EJ, Richardson M, Castle G, Cercone S, Delahanty L, Lyon R, Mueller D, Snetselaar L. Nutrition interventions for intensive therapy in the Diabetes Control and Complications Trial. J Am Diet Assoc. 1993;93(7):768–772. doi: 10.1016/0002-8223(93)91750-k. [DOI] [PubMed] [Google Scholar]
- 3.Delahanty LM, Halford BN. The role of diet behaviors in achieving improved glycemic control in intensively treated patients in the Diabetes Control and Complications Trial. Diabetes Care. 1993;16(11):1453–1458. doi: 10.2337/diacare.16.11.1453. [DOI] [PubMed] [Google Scholar]
- 4.DAFNE Study Group Training in flexible, intensive insulin management to enable dietary freedom in people with type 1 diabetes: dose adjustment for normal eating (DAFNE) randomised controlled trial. BMJ. 2002;325(7367):746. doi: 10.1136/bmj.325.7367.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trento M, Borgo E, Kucich C, Passera P, Trinetta A, Charrier L, Cavallo F, Porta M. Quality of life coping ability, and metabolic control in patients with type 1 diabetes managed by group care and a carbohydrate counting program. Diabetes Care. 2009;32(11):e134. doi: 10.2337/dc09-0903. [DOI] [PubMed] [Google Scholar]
- 6.Lowe J, Linjawi, Mensch M, James K, Attia J. Flexible eating and flexible insulin dosing in patients with diabetes: results of an intensive self-management course. Diabetes Res Clin Pract. 2008;80(3):439–443. doi: 10.1016/j.diabres.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 7.Mehta SN, Quinn N, Volkening LK, Laffel LM. Impact of carbohydrate counting on glycemic control in children with type 1 diabetes. Diabetes Care. 2009;32(6):1014–1016. doi: 10.2337/dc08-2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kildegaard J, Randløv J, Poulsen JU, Hejlesen OK. The impact of non-model-related variability on blood glucose prediction. Diabetes Technol Ther. 2007;9(4):363–371. doi: 10.1089/dia.2006.0039. [DOI] [PubMed] [Google Scholar]
- 9.Ahola AJ, Mäkimattila S, Saraheimo M, Mikkilä V, Freese R, Groop PH. FinnDiane Study. Poster 1680. New Orleans: 2009. Patients with type 1 diabetes frequently estimate their prandial insulin need inappropriately. ADA meeting. [DOI] [PubMed] [Google Scholar]
- 10.Bishop FK, Maahs DM, Spiegel G, Owen D, Klingensmith GJ, Bortsov A, Thomas J, Mayer-Davis EJ. The carbohydrate counting in adolescents with type 1 diabetes (CCAT) study. Diabetes Spectr. 2009;22(1):56–62. [Google Scholar]
- 11.Gross TM, Kayne D, King A, Rother C, Juth S. A bolus calculator is an effective means of controlling postprandial glycemia in patients on insulin pump therapy. Diabetes Technol Ther. 2003;5(3):365–369. doi: 10.1089/152091503765691848. [DOI] [PubMed] [Google Scholar]
- 12.Rossi MC, Nicolucci A, Di Bartolo P, Bruttomesso D, Girelli A, Ampudia FJ, Kerr D, Ceriello A, De la Mayor C, Pellegrini F, Horwitz D, Vespasiani G. Diabetes Interactive Diary: a new telemedicine system enabling flexible diet and insulin therapy while improving quality of life: an open-label, international, multicenter, randomized study. Diabetes Care. 2010;33(1):109–115. doi: 10.2337/dc09-1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smart CE, Ross K, Edge JA, Collins CE, Colyvas K, King BR. Children and adolescents on intensive insulin therapy maintain postprandial glycaemic control without precise carbohydrate counting. Diabet Med. 2009;26(3):279–285. doi: 10.1111/j.1464-5491.2009.02669.x. [DOI] [PubMed] [Google Scholar]
- 14.Charpentier G, Fleury F, Dubroca I, Vaur L, Clerson P. Electronic pill-boxes in the evaluation of oral hypoglycemic agent compliance. Diabetes Metab. 2005;31(2):189–195. doi: 10.1016/s1262-3636(07)70185-4. [DOI] [PubMed] [Google Scholar]
- 15.Peyrot M, Rubin RR. Validity and reliability of an instrument for assessing health-related quality of life and treatment preference: the Insulin Delivery System Rating Questionnaire. Diabetes Care. 2005;28(1):53–58. doi: 10.2337/diacare.28.1.53. [DOI] [PubMed] [Google Scholar]
- 16.Peyrot M, Rubin RR. How does treatment satisfaction work? Modeling determinants of treatment satisfaction and preference. Diabetes Care. 2009;32(8):1411–1417. doi: 10.2337/dc08-2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marra G, DIAB. & TE.S Project Study Group The DIAB. & TE.S Project: how patients perceive diabetes and diabetes therapy. Acta Biomed. 2004;75(3):164–170. [PubMed] [Google Scholar]
- 18.Kelley K, Dempsey C. An evaluation of an insulin transfer programme delivered in a group setting. J Clin Nurs. 2007;16(7B):152–158. doi: 10.1111/j.1365-2702.2007.01772.x. [DOI] [PubMed] [Google Scholar]
- 19.Tahrani AA, Digwood S, Lee C, Moulik P. Evaluation of glargine group-start sessions in patients with type 2 diabetes as a strategy to deliver the service. Int J Clin Pract. 2007;61(2):329–335. doi: 10.1111/j.1742-1241.2006.01190.x. [DOI] [PubMed] [Google Scholar]