Abstract
The worldwide consumption of sucrose, and thus fructose, has risen logarithmically since 1800. Many concerns about the health hazards of calorie-sweetened beverages, including soft drinks and fruit drinks and the fructose they provide, have been voiced over the past 10 years. These concerns are related to higher energy intake, risk of obesity, risk of diabetes, risk of cardiovascular disease, risk of gout in men, and risk of metabolic syndrome. Fructose appears to be responsible for most of the metabolic risks, including high production of lipids, increased thermogenesis, and higher blood pressure associated with sugar or high fructose corn syrup. Some claim that sugar is natural, but natural does not assure safety.
Keywords: cardiometabolic disease, diabetes, obesity, sugar-sweetened beverages
Introduction
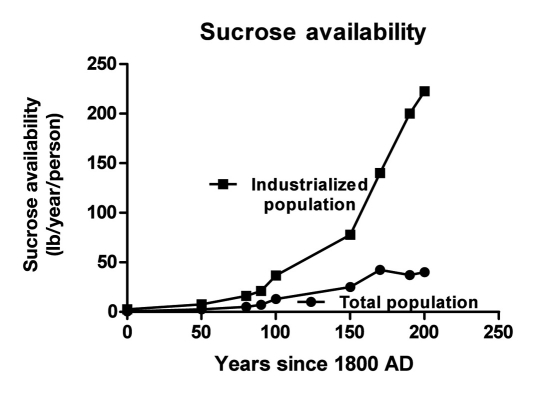
Pure, White and Deadly is the title of a book by Professor Yudkin that describes his view of the dangers of sugar consumption, which has risen exponentially in industrialized countries for the past 200 years (Figure 1).1 This figure shows the per capita production of sugar (lb/year) using population figures for world population growth since 1800 and for industrialized countries for this same time interval. In Germany, the consumption of sugar rose from 2 kg/person in 1825 to 36 kg/person in 1980.1 The exposure to fructose, which is half of the sucrose (sugar) molecule, has also risen exponentially in industrialized countries and is now more than 50-fold higher than in 1800! Exposure to fructose was accelerated by the introduction of high fructose corn syrup (HFCS).2 High fructose corn syrup is made by converting glucose into fructose using enzymes grown in bacteria and then diluting the fructose to provide the commercially available HFCS solutions that have 55% or 42% fructose. Some would argue that sucrose (sugar), as opposed to HFCS, is natural. But natural is no assurance of safety. Morphine, strychnine, and arsenic are all “natural” but not safe.
Figure 1.
Growth in per capita sugar production since 1800.
In this article, I explore the proposition that the large amounts of fructose now consumed from sugar or HFCS are hazardous to our health. Sugar production is big business.3 Prior to the cultivation of the sugar plant more than 2000 years ago or so, fructose in the diet came largely from whole fruits and vegetables. Now, our major sources of dietary fructose are from sugar (sucrose) or HFCS. Fructose is estimated to provide, on average, 54.7 g/day or 10.2% of our daily energy intake.3 For many adolescents, fructose intake is 15% or more of their daily energy intake. Beverages provide 29–45% of the average dietary intake of fructose.4 Fruits and vegetables provide 20–26%, with the remainder coming from sugar or HFCS used in baked goods or other refined products, such as ready-to-eat cereals. To make a distinction between the fructose that comes from fruits and vegetables and the fructose that comes from most other sources, I use the terms “good fructose” and “bad fructose.” The fructose that comes from whole fruits and vegetables, dairy products, and 100% fruit juices is referred to as “good fructose” because it is associated with the vitamins, minerals, and fiber that come with these “natural” products. Biologically, the sweetness of fructose and sucrose found in fruits was a clue that these foods contained other healthful nutrients. Numerous epidemiological studies have provided convincing evidence that higher intakes of fruits and vegetables reduce blood pressure5,6 and reduce risk of cancer7 and heart disease.8“Bad fructose” refers to the fructose that comes in refined foods and beverages that usually have few nutrients.9
Beverages are a major source of our dietary fructose. The amount of calorically sweetened beverages we drink is related to our energy intake, risk of obesity, and risk of cardiometabolic disease. The higher the consumption, the greater the risk. Thus, if fructose poses a health problem, then beverages are a target for change.9
Many of the following studies focus on consumption of soft drinks that are sweetened with either sucrose or HFCS, because these are the major commercial caloric sweeteners and also the major sources of fructose in our diet; these studies do not allow us to separate the effects of fructose from glucose. Studies examining sucrose, fructose, and glucose independently and together, however, argue strongly that it is fructose, NOT glucose, that accounts for the major detrimental effects of soft drinks. Cohen and Schall10 compared the effect on plasma triglycerides of adding 100 g of sucrose, 50 g of fructose, or 50 g of glucose to a standard meal. They found that the lipemic response to the glucose was not significantly different from the ingestion of fat alone. In contrast, the lipemic response increased after adding either fructose or sucrose, suggesting that it was fructose and not glucose that increased triglyceride levels. In the remaining sections, I will review three groups of studies:
The impact of calorically sweetened beverage consumption on energy intake and the risk of developing obesity;
The detrimental health consequences of soft drinks;
Mechanisms for the effects of fructose.
The Impact of Calorically Sweetened Beverage Consumption on Energy Intake and the Risk of Developing Obesity
Concerns about the detrimental effects of soft drinks sweetened with sugar or high fructose corn syrup have been on the rise for more than a decade.2,1112 In one review of this problem, Malik and colleagues13 found 30 publications (15 cross-sectional, 10 prospective, and 5 experimental) that met their selection criteria. Findings from large cross-sectional studies, in conjunction with those from well-powered prospective cohort studies with long periods of follow-up, showed a positive association between greater intake of sugar-sweetened soft drinks and weight gain and obesity in both children and adults. Findings from short-term feeding trials in adults also support induction of positive energy balance and weight gain from consuming sugar-sweetened sodas, but these trials are few.
Another meta-analysis by Olsen and Heitmann14 concluded that consumption of soft drinks was a determinant of obesity. Their analysis included 14 prospective and 5 experimental studies. The majority of the 14 prospective studies found positive associations between intake of calorically sweetened beverages and obesity, and none found that these beverages were beneficial. Three experimental studies found positive effects of calorically sweetened beverages and subsequent changes in body fat, but two experimental studies did not. Eight prospective studies adjusted statistically for energy intake. Seven of these eight studies reported associations that were essentially similar before and after energy adjustment, suggesting that the caloric sweeteners might offer “additional” effects. The authors concluded that a high intake of calorically sweetened beverages was a determinant of obesity.
Detrimental Health Consequences of Soft Drinks
Consumption of soft drinks predicts the risk of developing cardiometabolic disease.15 Malik and associates15 identified 8 prospective cohort studies evaluating sweetened soft drink consumption and weight gain, and 10 prospective cohort studies evaluating soft drink consumption and the risk of cardiometabolic disease. Six of them related soft drink consumption to the risk of diabetes mellitus,16–21 3 to the risk of metabolic syndrome,21–23 and 1 to the risk of coronary heart disease.23 The meta-analysis included 294,617 participants, with 10,010 cases of type 2 diabetes, 6,236 cases of metabolic syndrome, and 3,105 cases of coronary heart disease. There was a clear and consistent positive association between consumption of sweetened soft drinks and weight gain, particularly in larger studies with longer durations of follow-up. Individuals in the highest quantile of soft drink intake had a 24% greater risk of cardiometabolic disease than those in the lowest quantile [RR:1.24 (95% CI: 1.12, 1.34)]. This increased to 30% when studies that adjusted for mediating effects of energy intake and BMI were excluded from analysis [RR: 1.31 (95% CI: 1.16, 1.48)]. The authors concluded that higher consumption of calorie-sweetened soft drinks was associated with weight gain and increased risk of cardiometabolic diseases.15 Fructose intake is also related to the risk of developing gout in men.24 Fructose intake has also been related to the increased level of small dense LDL-cholesterol (bad cholesterol) in children.25 Clearly, fructose can be hazardous to the health of some people.
Potential Mechanisms for the Detrimental Effects of Fructose
At least two main mechanisms2,12,15 can account for most of the detrimental effects of calorically sweetened beverages and the fructose they provide: (1) failure to suppress intake of other foods to compensate for the calories in soft drinks;26–28 and (2) differences in the metabolic pathways for fructose and glucose that facilitate conversion of carbon from fructose into the backbone of triglycerides2,12 and the formation of uric acid during metabolism of fructose.29,30
Failure to suppress caloric intake to offset the calories from calorie-sweetened beverages is part of the mechanism for increased energy intake and the risk for obesity.26-28 Drinking sugar-sweetened beverages at lunch did not suppress intake of solid food; rather, the calories from beverages added on to total calorie intake.28 For example, drinking apple juice reduced energy intake less than when the same amount of apple was eaten as applesauce or as pieces of apple.27
Short-term Effects of Fructose on Human Metabolism
Fructose alone is poorly absorbed from the gastro-intestinal tract, but when ingested with glucose, its absorption is enhanced.11,12 Both fructose and glucose enter the portal blood supply and are then transported to the liver. Since the transport system for fructose is absent in most cells, the liver and kidney are the main site for fructose metabolism. Upon entering the liver, glucose is phosphorylated at the 6-position to form glucose-6-phosphate, whereas fructose is phosphorylated at the 1-position by ketohexokinase to form fructose-1- phosphate.30 The fructose-1-phosphate is readily converted to the backbone of triglyceride, whereas glucose-6-phosphate is not so readily converted to triglyceride because its metabolism is regulated by phosphofructokinase. This probably explains why fructose—but not glucose—stimulates the formation of lipids in the liver and increases circulating levels of triglycerides, particularly at night.31
To compare the acute effects of fructose and glucose on plasma lipids, Teff and colleagues.32 gave 17 individuals with obesity meals with beverages containing either fructose or glucose that provided 30% of the total calories. Following fructose, the rise in plasma glucose was significantly smaller than after glucose. Following fructose, triglycerides rose almost 200% higher than after glucose, and lactate production was five times greater. Insulin and leptin both rose less in response to fructose than to glucose. These responses in lipids are seen primarily in men, with smaller or limited responses seen in women.33,34
Fructose increases thermogenesis and blood pressure more than glucose,35,36 but both have this effect. When a 75 g oral load of glucose or fructose was given to 17 volunteers, fructose stimulated oxygen consumption more than glucose but produced a much smaller stimulation of insulin.35 Fructose increased the respiratory quotient more than glucose, which is consistent with de novo lipogenesis. Both patients with obesity and patients with diabetes had a similar stimulation of oxygen uptake after infusion of glucose that was smaller than the response to fructose.35
Effects of Ingesting Fructose Over Several Weeks in Human Subjects
Two carefully conducted 10-week studies compared beverages. In one study, 21 adults received a minimum amount of sugar-containing beverages or sugar-sweetened foods each day for 10 weeks that contributed about 23% to their energy intake (about 2 g sucrose per kg body weight per day).37 The other 20 received comparable amounts of beverages (80%) or foods (20%) sweetened with aspartame. For their other foods, subjects could select freely from items available at a kiosk run by the study group. After 10 weeks, energy intake had increased by 380 kcal/day (1.6 MJ/day) and sucrose to 28% of calorie intake in the group receiving the sugar-containing beverages. Protein and fat intakes declined.37 Body weight and fat mass increased by 1.6 and 1.3 kg respectively in the sugared-beverage group and decreased by 1.0 and 0.3 kg in the aspartame-sweetened group. Blood pressure increased by 3.8/4.1 mm Hg in the sugared-beverage consuming group but did not change in the other group, mimicking the acute response of blood pressure to fructose.36 Concentrations of several inflammatory markers were also changed. In the group consuming sucrose, haptoglobin increased by 13%, transferrin by 5%, and C-reactive protein by 6%, whereas in the group receiving the aspartame-sweetened beverages, haptoglobin decreased by 16%, C-reactive protein decreased by 26% and transferrin was basically unchanged with a small 2% fall.38
In a second 10-week study, fructose or glucose were provided as 25% solutions as part of the total diet. Stanhope and co-workers31 found that visceral fat was significantly increased in the group drinking fructose-containing beverages. When 32 men and women ate a diet with 15% protein, 30% fat, and 55% carbohydrate, with either 25% of calories as glucose-sweetened beverages or as fructose-sweetened beverages, visceral fat increased by 14% in the fructose-consuming group compared to about 5% in the control group, with no significant change in body weight or subcutaneous fat. De novo lipogenesis increased and post-prandial triglycerides increased, particularly at night.31
Conclusion
The rising consumption of calorie-sweetened beverages, both soft drinks and fruit drinks, provides a readily identifiable pathway for an increasing amount of energy intake and dietary fructose. Based on this review of the literature, I conclude that in the amounts currently ingested, fructose is hazardous to the health of some people. The term “good fructose” is used to identify the fructose that comes from fruits and vegetables, and “bad fructose” the fructose that comes from calorie-sweetened beverages and refined products. Is fructose the “pure, white and deadly” that Yudkin had in mind?
Abbreviations
- CI
confidence interval
- HFCS
high fructose corn syrup
- LDL
low density lipoprotein
- RR
relative risk
References
- 1.Yudkin J. Pure, White and Deadly. London: Penguin Books; 1989. [Google Scholar]
- 2.Bray GA, Nielsen SJ, Popkin BM. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am J Clin Nutr. 2004;79(4):537–543. doi: 10.1093/ajcn/79.4.537. [DOI] [PubMed] [Google Scholar]
- 3.Mintz S. Sweetness and power: the place of sugar in history. London: Penguin Books; 1986. [Google Scholar]
- 4.Vos MB, Kimmons JE, Gillespie C, Welsh J, Blanck HM. Dietary fructose consumption among US children and adults: the third national health and nutrition examination survey. Medscape J Med. 2008;10(7):160. [PMC free article] [PubMed] [Google Scholar]
- 5.Appel LJ, Moore TJ, Obarzanek E, Vollmer W, Svetkey LP, Sacks FM, Bray GA, Vogt TM, Cutler JA, Windhauser MM, Lin PH, Karanja N. A clinical trial of the effects of dietary patterns on blood pressure. New Engl J Med. 1997;336(16):1117–1124. doi: 10.1056/NEJM199704173361601. [DOI] [PubMed] [Google Scholar]
- 6.Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha DW, Obarzanek E, Conlin PR, Miller ER, Simons-Morton DG, Karanja N, Lin PH, DASH-Sodium Collaborative Research Group Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. N Engl J Med. 2001;344(1):3–10. doi: 10.1056/NEJM200101043440101. [DOI] [PubMed] [Google Scholar]
- 7.Vainio H, Weiderpass E. Fruit and vegetables in cancer prevention. Nutr Cancer. 2006;54(1):111–142. doi: 10.1207/s15327914nc5401_13. [DOI] [PubMed] [Google Scholar]
- 8.He FJ, Nowson CA, Lucas M, MacGregor GA. Increased consumption of fruit and vegetables is related to a reduced risk of coronary heart disease: meta-analysis of cohort studies. J Hum Hypertens. 2007;21(9):717–728. doi: 10.1038/sj.jhh.1002212. [DOI] [PubMed] [Google Scholar]
- 9.Bray GA. The low-fructose approach to weight control. Pittsburgh: Dorrance Publishing; 2009. [Google Scholar]
- 10.Cohen JC, Schall R. Reassessing the effects of simple carbohydrates on the serum triglyceride responses to fat meals. Am J Clin Nutr. 1988;48(4):1031–1034. doi: 10.1093/ajcn/48.4.1031. [DOI] [PubMed] [Google Scholar]
- 11.Elliott SS, Keim NL, Stern JS, Teff K, Havel PJ. Fructose, weight gain, and the insulin resistance syndrome. Am J Clin Nutr. 2002;76(5):911–922. doi: 10.1093/ajcn/76.5.911. [DOI] [PubMed] [Google Scholar]
- 12.Havel PJ. Dietary fructose: implications for dysregulation of energy homeostasis and lipid/carbohydrate metabolism. Nutr Rev. 2005;63(5):133–157. doi: 10.1301/nr.2005.may.133-157. [DOI] [PubMed] [Google Scholar]
- 13.Malik VS, Schulze MB, Hu FB. Intake of sugar-sweetened beverages and weight gain: a systematic review. Am J Clin Nutr. 2006;84(2):274–288. doi: 10.1093/ajcn/84.1.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olsen NJ, Heitmann BL. Intake of calorically sweetened beverages and obesity. Obes Rev. 2009;10(1):68–75. doi: 10.1111/j.1467-789X.2008.00523.x. [DOI] [PubMed] [Google Scholar]
- 15.Malik VS, Popkin BM, Bray GA, Després JP, Hu FB. Sugar-sweetened beverages, obesity, type 2 diabetes mellitus, and cardiovascular disease risk. Circulation. 2010;121(11):1356–1364. doi: 10.1161/CIRCULATIONAHA.109.876185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palmer JR, Boggs DA, Krishnan S, Hu FB, Singer M, Rosenberg L. Sugar-sweetened beverages and incidence of type 2 diabetes mellitus in African American women. Arch Intern Med. 2008;168(14):1487–1492. doi: 10.1001/archinte.168.14.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paynter NP, Yeh HC, Voutilainen S, Schmidt MI, Heiss G, Folsom AR, Brancati FL, Kao WH. Coffee and sweetened beverage consumption and the risk of type 2 diabetes mellitus: the atherosclerosis risk in communities study. Am J Epidemiol. 2006;164(11):1075–1084. doi: 10.1093/aje/kwj323. [DOI] [PubMed] [Google Scholar]
- 18.Montonen J, Järvinen R, Knekt P, Heliövaara M, Reunanen A. Consumption of sweetened beverages and intakes of fructose and glucose predict type 2 diabetes occurrence. J Nutr. 2007;137(6):1447–1454. doi: 10.1093/jn/137.6.1447. [DOI] [PubMed] [Google Scholar]
- 19.Bazzano LA, Li TY, Joshipura KJ, Hu FB. Intake of fruit, vegetables, and fruit juices and risk of diabetes in women. Diabetes Care. 2008;31(7):1311–1317. doi: 10.2337/dc08-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nettleton JA, Lutsey PL, Wang Y, Lima JA, Michos ED, Jacobs DR. Diet soda intake and risk of incident metabolic syndrome and type 2 diabetes in the Multi-Ethnic Study of Atherosclerosis (MESA) Diabetes Care. 2009;32(4):688–694. doi: 10.2337/dc08-1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dhingra R, Sullivan L, Jacques PF, Wang TJ, Fox CS, Meigs JB, D’Agostino RB, Gaziano JM, Vasan RS. Soft drink consumption and risk of developing cardiometabolic risk factors and the metabolic syndrome in middle-aged adults in the community. Circulation. 2007;116(5):480–488. doi: 10.1161/CIRCULATIONAHA.107.689935. [DOI] [PubMed] [Google Scholar]
- 22.Lutsey PL, Steffen LM, Stevens J. Dietary intake and the development of the metabolic syndrome: the Atherosclerosis Risk in Communities study. Circulation. 2008;117(6):754–761. doi: 10.1161/CIRCULATIONAHA.107.716159. [DOI] [PubMed] [Google Scholar]
- 23.Fung TT, Malik V, Rexrode KM, Manson JE, Willett WC, Hu FB. Sweetened beverage consumption and risk of coronary heart disease in women. Am J Clin Nutr. 2009;89(4):1037–1042. doi: 10.3945/ajcn.2008.27140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choi HK, Curhan G. Soft drinks, fructose consumption, and the risk of gout in men: prospective cohort study. BMJ. 2008;336(7639):309–312. doi: 10.1136/bmj.39449.819271.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aeberli I, Zimmermann MB, Molinari L, Lehmann R, l’Allemand D, Spinas GA, Berneis K. Fructose intake is a predictor of LDL particle size in overweight schoolchildren. Am J Clin Nutr. 2007;86(4):1174–1178. doi: 10.1093/ajcn/86.4.1174. [DOI] [PubMed] [Google Scholar]
- 26.Mattes RD. Fluid energy—Where’s the problem? J Am Diet Assoc. 2006;106(12):1956–1961. doi: 10.1016/j.jada.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 27.Mattes RD, Campbell WW. Effects of food form and timing of ingestion on appetite and energy intake in lean young adults and in young adults with obesity. J Am Diet Assoc. 2009;109(3):430–437. doi: 10.1016/j.jada.2008.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rolls BJ, Kim S, Fedoroff IC. Effects of drinks sweetened with sucrose or aspartame on hunger, third and food intake in men. Physiol Behav. 1990;48(1):19–26. doi: 10.1016/0031-9384(90)90254-2. [DOI] [PubMed] [Google Scholar]
- 29.Johnson RJ, Perez-Pozo SE, Sautin YY, Manitius J, Sanchez-Lozada LG, Feig DI, Shafiu M, Segal M, Glassock RJ, Shimada M, Roncal C, Nakagawa T. Hypothesis: could excessive fructose intake and uric acid cause type 2 diabetes? Endo Rev. 2009;30(1):96–116. doi: 10.1210/er.2008-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cirillo P, Gersch MS, Mu W, Scherer PM, Kim KM, Gesualdo L, Henderson GN, Johnson RJ, Sautin YY. Ketohexokinase-dependent metabolism of fructose induces proinflammatory mediators in proximal tubular cells. J Am Soc Nephrol. 2009;20(3):545–553. doi: 10.1681/ASN.2008060576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stanhope KL, Schwarz JM, Keim NL, Griffen SC, Bremer AA, Graham JL, Hatcher B, Cox CL, Dyachenko A, Zhang W, McGahan JP, Seibert A, Krauss RM, Chiu S, Schaefer EJ, Ai M, Otokozawa S, Nakajima K, Nakano T, Beysen C, Hellerstein MK, Berglund L, Havel PJ. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J Clin Invest. 2009;119(5):1322–1334. doi: 10.1172/JCI37385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Teff KL, Grudziak J, Townsend RR, Dunn TN, Grant RW, Adams SH, Keim NL, Cummings BP, Stanhope KL, Havel PJ. Endocrine and metabolic effects of consuming fructose- and glucose-sweetened beverages with meals in obese men and women: influence of insulin resistance on plasma triglyceride responses. J Clin Endocrinol Metab. 2009;94(5):1562–1569. doi: 10.1210/jc.2008-2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bantle JP, Raatz SK, Thomas W, Georgopoulos A. Effects of dietary fructose on plasma lipids in healthy subjects. Am J Clin Nutr. 2000;72(5):1128–1134. doi: 10.1093/ajcn/72.5.1128. [DOI] [PubMed] [Google Scholar]
- 34.Couchepin C, Lê KA, Bortolotti M, da Encarnaçao JA, Oboni JB, Tran C, Schneiter P, Tappy L. Markedly blunted metabolic effects of fructose in healthy young female subjects compared with male subjects. Diabetes Care. 2008;31(6):1254–1256. doi: 10.2337/dc07-2001. [DOI] [PubMed] [Google Scholar]
- 35.Tappy L, Randin JP, Felber JP, Chiolero R, Simonson DC, Jequier E, DeFronzo RA. Comparison of thermogenic effect of fructose and glucose in normal humans. Am J Physiol. 1986;250(6 Pt 1):E718–E724. doi: 10.1152/ajpendo.1986.250.6.E718. [DOI] [PubMed] [Google Scholar]
- 36.Brown CM, Dulloo AG, Yepuri G, Montani JP. Fructose ingestion acutely elevates blood pressure in healthy young humans. Am J Physiol Regul Integr Comp Physiol. 2008;294(3):R730–R737. doi: 10.1152/ajpregu.00680.2007. [DOI] [PubMed] [Google Scholar]
- 37.Raben A, Vasilaras TH, Møller AC, Astrup A. Sucrose compared with artificial sweeteners: different effects on ad libitum food intake and body weight after 10 wk of supplementation in overweight subjects. Am J Clin Nutr. 2002;76(4):721–729. doi: 10.1093/ajcn/76.4.721. [DOI] [PubMed] [Google Scholar]
- 38.Sørensen LB, Raben A, Stender S, Astrup A. Effect of sucrose on inflammatory markers in overweight humans. Am J Clin Nutr. 2005;82(2):421–427. doi: 10.1093/ajcn.82.2.421. [DOI] [PubMed] [Google Scholar]