Abstract
In mitosis, the duplicated chromosomes are separated and equally distributed to progeny cells under the guidance of the spindle, a dynamic microtubule network. Previous studies revealed a mitotic checkpoint that prevents segregation of the chromosomes until all of the chromosomes are properly attached to microtubules through the kinetochores. A variety of kinetochore-localized proteins, including Mad2 and Cdc20, have been implicated in controlling the mitotic checkpoint. Here we report that both Mad2 and Cdc20 can physically associate with Nek2, a serine/threonine kinase implicated in centrosome functions. We show that, similar to Nek2, the endogenous Cdc20 protein can be detected in the centrosome and the spindle poles. Both Cdc20 and Mad2 can be phosphorylated by Nek2. Moreover, our studies demonstrate that overexpression of Nek2 enhances the ability of Mad2 to induce a delay in mitosis. These observations indicate that Nek2 may act upon the Mad2-Cdc20 protein complex and play a critical role in regulating the mitotic checkpoint protein complex. We propose that overexpression of Nek2 may promote aneuploidy by disrupting the control of the mitotic checkpoint.
Keywords: Cdc20, Centrosome, Kinetochore, Mad2, Mitosis, Nek2
Introduction
In each cell division cycle, the chromosomes are reproduced and equally distributed to the daughter cells through a series of highly coordinated processes. Compromising the fidelity of genome duplication or distribution contributes to malignant transformation (Draviam et al., 2004; Laiho and Latonen, 2003). The mitotic spindle is a key apparatus that guides accurate segregation of the chromosomes. Following the attachment of microtubules to the chromosomes at the site of kinetochore, the chromosomes first align at the equatorial metaphase plate and then, during anaphase, migrate towards the poles of the spindle. A spindle checkpoint mechanism exists to monitor the integrity of the kinetochore–microtubule attachment, and this device also ensures that cells do not enter anaphase until all the chromosomes form solid attachments with microtubules (Musacchio and Hardwick, 2002; Musacchio and Salmon, 2007; Shah and Cleveland, 2000). Disruption of this checkpoint is associated with loss of chromosomes or premature exit from mitosis and, consequently, aneuploidy (Kops et al., 2005).
The spindle checkpoint is controlled through a cascade of signaling molecules. To initiate chromosome segregation, the anaphase-promoting complex/cyclosome (APC/C) is first activated by Cdc20 and, in turn, modulates ubiquitination and proteolytic degradation of securin, an inhibitor of the protease separase (Peters, 2002; Yu, 2007). The subsequent activation of separase causes cleavage of the molecules that mediate the linkage of the sister chromatids (Peters, 2002; Yu, 2007). Both Mad2 and BubR1 can bind to Cdc20 independently and inhibit the activation of APC/C (Fang, 2002; Fang et al., 1998; Tang et al., 2001). It is postulated that, when the kinetochore is not attached to the microtubule and mechanical tension cannot be established, Mad2 is maintained in a unique conformation permissive for binding and sequestrating Cdc20, which thereby constrains APC/C activities (Hoyt, 2001; Luo et al., 2002, 2004). It is a plausible hypothesis that the regulators of APC/C may be regulated by posttranslational modification, such as phosphorylation. Indeed, several earlier studies reported that Cdc20 can be phosphorylated. Bub1, Cdk1, and MAPK are the kinases involved in some of the phosphorylation events (Chung and Chen, 2003; D’Angiolella et al., 2003; Kraft et al., 2003; Tang et al., 2004). In contrast, although a previous study provided evidence that Mad2 can also be phosphorylated, the kinase responsible for this modification has not been identified (Wassmann et al., 2003).
During our studies to identify novel Mad2-binding proteins, we discovered that Mad2 can associate with Nek2, a member of the NIMA-related kinase family of serine/threonine protein kinases (O’Connell et al., 2003; Schultz et al., 1994). Nek2 is localized to the centrosome and modulates centrosome cohesion and separation (Faragher and Fry, 2003; Fry et al., 1998a,b). In addition, Nek2 may contribute to certain aspects of mitotic progression such as chromatin condensation and spindle checkpoint signaling (Chen et al., 2002; Di Agostino et al., 2004; Di Agostino et al., 2002; Draviam et al., 2007; Lou et al., 2004). However, the mechanism by which Nek2 regulates the mitotic checkpoint remains elusive. Here, we demonstrate that Nek2 can form a complex with Mad2 and Cdc20. We have identified the structures involved in the association between Nek2 and Mad2 or Cdc20. We examined the role of Nek2 in phosphorylation of Mad2 and Cdc20. Furthermore, our studies evaluated whether Nek2 affects Mad2-mediated checkpoint control. Our results indicate that Nek2 plays a defining role in spindle assembly checkpoint control by acting upon Mad2 and Cdc20.
Results
Identification of Nek2 as a Mad2-binding protein
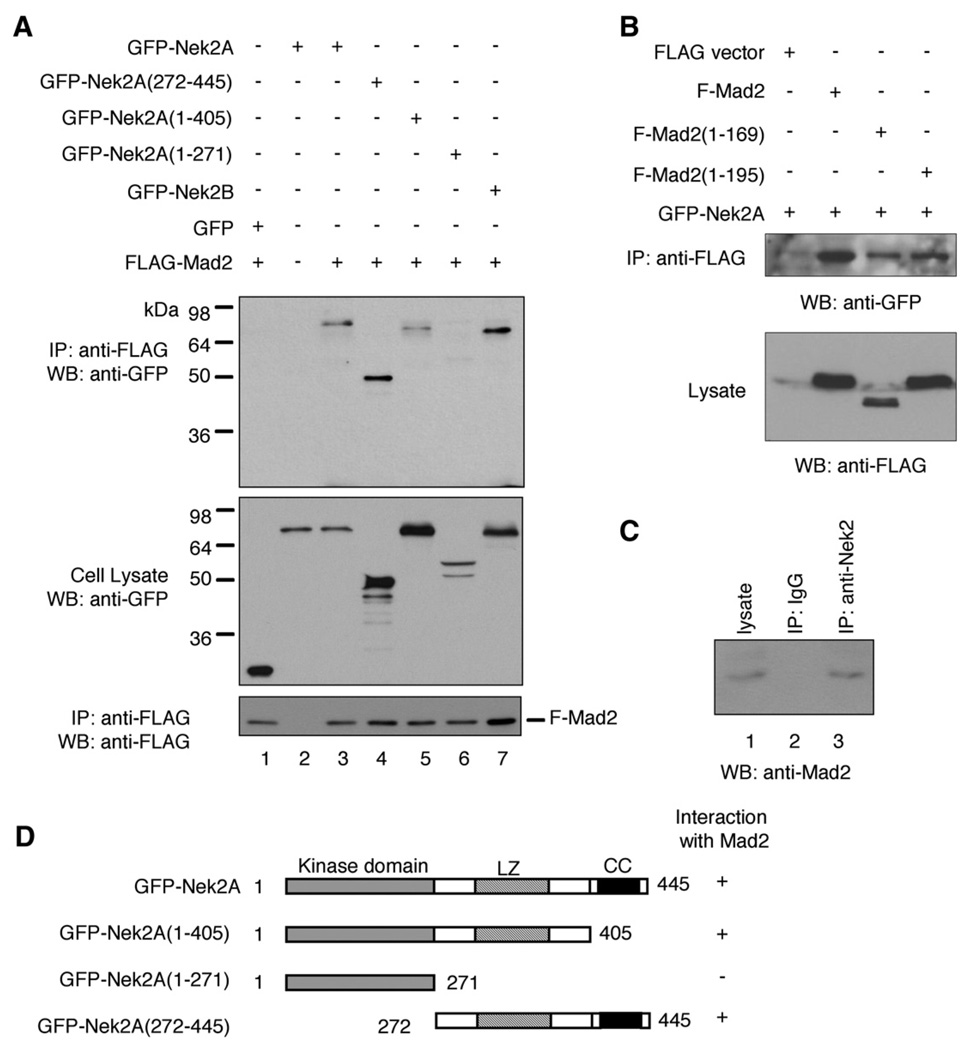
We identified Nek2A as a Mad2-interacting molecules in a yeast two-hybrid screen (SI Supplementary Fig. 1). To confirm that Nek2A can form a complex with Mad2 in mammalian cells, we performed co-immunoprecipitation (co-IP) analyses following cotransfection of GFP-tagged Nek2 and FLAG-tagged Mad2 in 293T cells. We found that the GFP-Nek2 protein can be coprecipitated with FLAG-Mad2 (Fig. 1A, lane 3), which indicates that Nek2A and Mad2 indeed coexist in a protein ensemble in 293T cells. In addition, Mad2 can also associate with Nek2B (Fig. 1A, lane 7), a splicing variant that has a distinct C-terminal region but shares the kinase domain, the coiled-coil domain and a leucine zipper motif with Nek2A (Hames and Fry, 2002). Moreover, the endogenous Mad2 proteins can be detected in the protein complex immunoprecipitated by an anti-Nek2 antibody (Fig. 1C).
Fig. 1.
Co-immunoprecipitation analysis of Mad2-Nek2 association. (A) 293T cells were transfected with the plasmids, as indicated. Top panel: Immunoprecipitation (IP) using anti-FLAG antibody followed by Western blot (WB) using anti-GFP antibody. Middle panel: Western blot of cell lysate using anti-GFP antibody. Bottom panel: Reprobing of the top panel blot using anti-FLAG antibody. (B) 293T cells were transfected with the plasmids, as indicated. Upper panel: IP using anti-FLAG antibody and Western blot using anti-GFP antibody. Lower panel: Western Blot of cell lysate using anti-FLAG antibody. (C) 293T cell lysate was subjected to IP using anti-Nek2 antibody (lane 3) or control mouse IgG (lane 2) followed by Western blot using anti-Mad2 antibody. The endogenous Mad2 level in the cell lysate was also examined (lane 1). (D) Diagram of Nek2A and its deletion mutants. LZ: leucine zipper; CC: coiled-coil domain.
To map the Nek2A region responsible for association with Mad2, we generated a series of deletion mutants of Nek2A and tested their ability to form complexes with Mad2 by co-IP. The C-terminal portion of the Nek2 protein, which contains the leucine zipper domain and the coiled-coil motif, was sufficient to bind to Mad2 (Fig. 1A, lane 4). Moreover, the Nek2A deletion mutant that lacks the coiled-coil structure retained its ability to bind Mad2 (Fig. 1A, lane 5). In contrast, further deletion of leucine zipper domain abolished the association with Mad2 (Fig. 1A, lane 6). Collectively, these observations indicate that the leucine zipper of Nek2A is essential for forming a complex with Mad2 (Fig. 1D). The C-terminal coiled-coil motif, however, is not required for formation of the Nek2–Mad2 ensemble.
A similar approach was used to map the Mad2 region involved in association with Nek2. In previous studies, it was found that the 10 amino acid residues in the C-terminus of Mad2 is required for interaction with Cdc20 and Mad1 (Luo et al., 2000; Sironi et al., 2001). We therefore created two deletion mutants of Mad2, namely, Mad2 (1–169) and Mad2(1–195), both lacking the region essential for binding to Cdc20 and Mad1. We found that Nek2 could still be coprecipitated with these deletion mutants (Fig. 1B). Thus, the Mad2 region involved in binding to Nek2 is distinct from the region of the molecule involved in modulating the interaction with Mad1 or Cdc20.
Nek2 phosphorylates Mad2
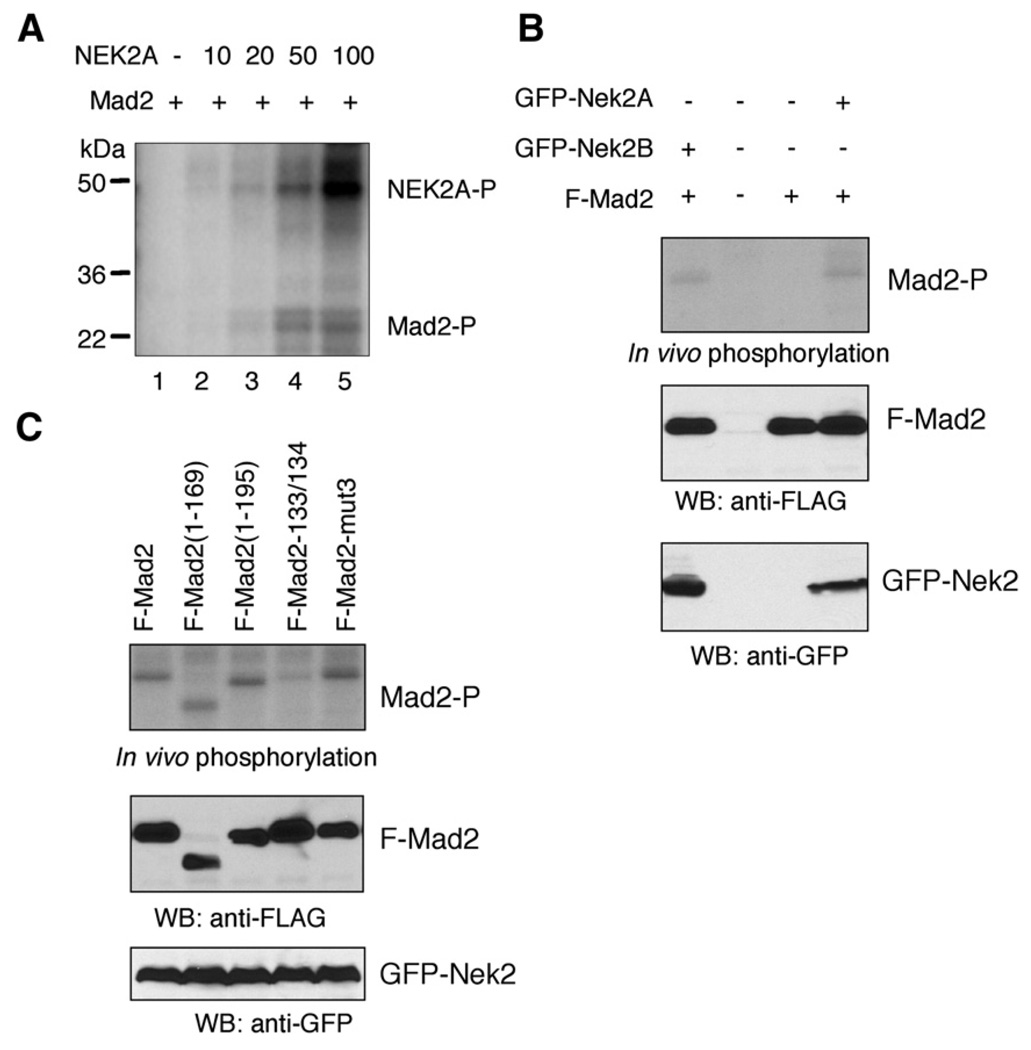
Since Nek2 and Mad2 reside in a protein complex it was reasonable to examine if Mad2 could be a substrate for Nek2. We first performed in vitro kinase assays using purified recombinant Nek2A and Mad2 proteins. We found that Nek2A can indeed phosphorylate Mad2 in vitro in a dose-dependent manner (Fig. 2A). We noted that the level of Mad2 phosphorylation by Nek2 is low in this in vitro setting, compared with that of autophosphorylation of Nek2 (Fig. 2A).
Fig. 2.
Phosphorylation of Mad2 by Nek2. (A) Purified recombinant CBP-Mad2 protein (10 µg) was incubated with the indicated amount of Nek2 (ng) in the presence of 32P-γ-ATP. Protein phosphorylation was evaluated by SDS–PAGE and phosphorimaging. (B) In vivo phosphorylation of Mad2 by Nek2. 293T cells were transfected with the plasmids as indicated followed by metabolic labeling. Immunoprecipitation (IP) was performed using anti-FLAG antibody. Top panel: Phosphorylation was evaluated by phosphorimaging. Middle and bottom panels: Western blot analysis using anti-FLAG and anti-GFP antibody, respectively. (C) FLAG-Mad2 or its mutants were coexpressed with GFP-Nek2A in 293T cells followed by metabolic labeling. IP and phosphorimaging were performed in the same way as in (B). The Mad2 mutants are Mad2-133/134 (R133E, Q134A) and Mad2-mut3 (S170G, S178A, S195A).
To determine whether Mad2 can be phosphorylated by Nek2 in vivo, we transfected 293T cells with FLAG-tagged Mad2 combined with GFP-Nek2A or GFP-Nek2B and performed metabolic labeling using 32P-orthophosphate. Our results confirmed that the Mad2 protein was hyperphosphorylated in the presence of ectopic Nek2A protein (Fig. 2B). Similarly, overexpression of GFP-Nek2B also increased the phosphorylation levels of Mad2 (Fig. 2B).
We also sought to determine the Mad2 structure features involved in the phosphorylation event. An earlier study demonstrated that Mad2 can be phosphorylated in vivo on three amino acid residues, including serine 170, serine 179, and serine 195 (Wassmann et al., 2003). However, our results indicate that the Nek2-induced phosphorylation level of Mad2 was not affected by simultaneous alteration of these three sites (Fig. 2C). The C-terminal deletion mutants Mad2 (1–195) and Mad2(1–169) can also be phosphorylated following Nek2 overexpression (Fig. 2C). In contrast, the phosphorylation levels of Mad2 were diminished as a result of the double mutations of arginine 133 to glutamate and glutamine 134 to alanine (Fig. 2C).
Nek2 associates with and phosphorylates Cdc20
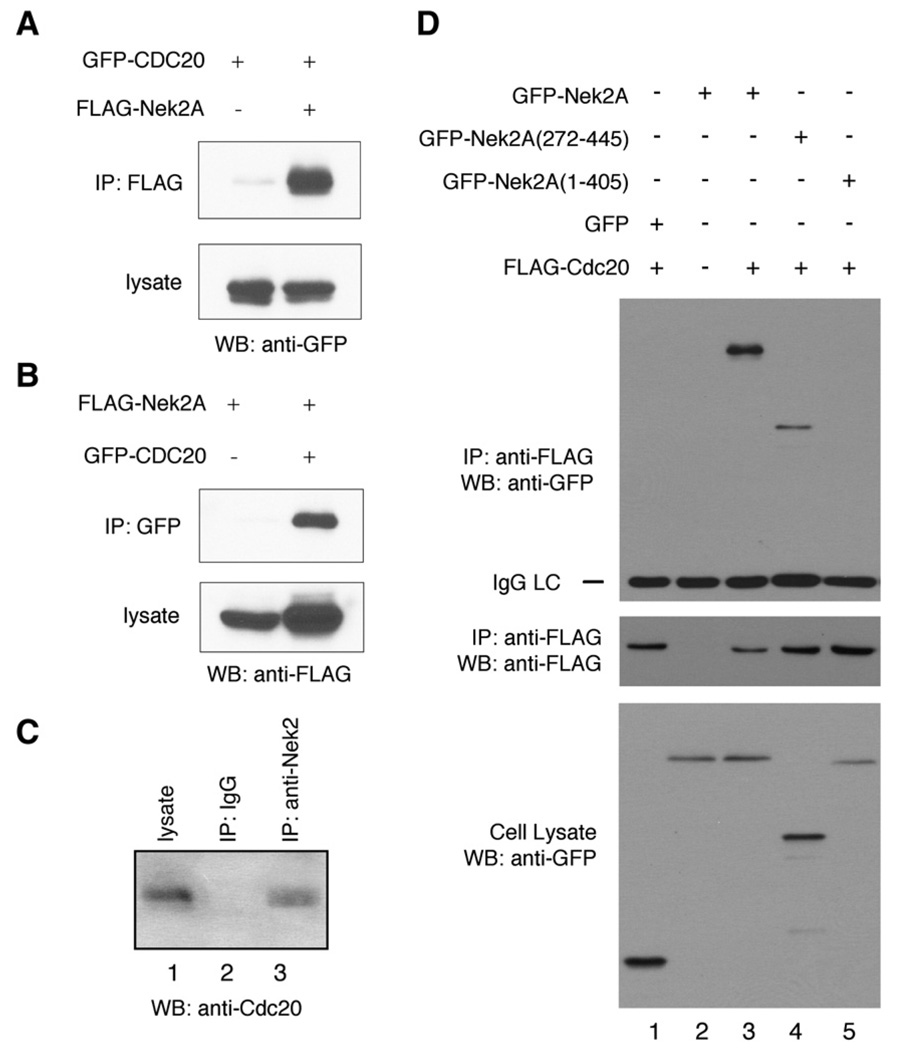
The finding of Nek2–Mad2 association further prompted us to examine whether Nek2 can form a complex with other Mad2-interacting proteins, such as Cdc20. By co-IP, we demonstrated that Cdc20 can be copurified with Nek2A (Fig. 3A). The interaction was confirmed in a reciprocal assay (Fig. 3B). Furthermore, the association between endogenous Nek2 and Cdc20 proteins was also detected by co-IP (Fig. 3C).
Fig. 3.
Nek2-Cdc20 interaction. (A) Co-immunoprecipitation analysis of Mad2-Cdc20 interaction. 293T cells were transfected with GFP-Cdc20 in combination with FLAG-Nek2A or the empty control plasmid. Upper panel: IP with anti-FLAG antibody; immunoblotting with anti-GFP antibody. Lower panel: Western Blot using anti-GFP antibody. (B) Reciprocal co-IP. 293T cells were transfected with the plasmids, as indicated. Upper panel: IP with anti-GFP antibody; Western blot using anti-FLAG antibody. Lower panel: Western blot using anti-FLAG antibody. (C) 293T cell lysate was subjected to IP using anti-Nek2 antibody (lane 3) or control mouse IgG (lane 2) followed by Western blot analysis using anti-Cdc20 antibody. The endogenous Cdc20 level in the cell lysate was also examined (lane 1). (D) The Nek2 region involved in association with Cdc20. 293T cells were transfected with the plasmids, as indicated. Top panel: IP with anti-FLAG antibody; Western blot (WB) using anti-GFP antibody. Middle panel: IP and WB using anti-FLAG antibody. Bottom panel: Western blot analysis of cell lysate using anti-GFP antibody.
We then carried out co-IP to identify the Nek2A region responsible for association with Cdc20. Deletion of the C-terminal fragment of Nek2, which includes the leucine zipper and the coiled-coil, did not affect formation of a complex with Cdc20 (Fig. 3D, lane 4). In contrast, removal of the coiled-coil structure abolished the interaction (Fig. 3D, lane 5). Therefore, the Nek2 region involved in interacting with Cdc20 is distinct from that is needed for Mad2 interactions (Fig. 1B).
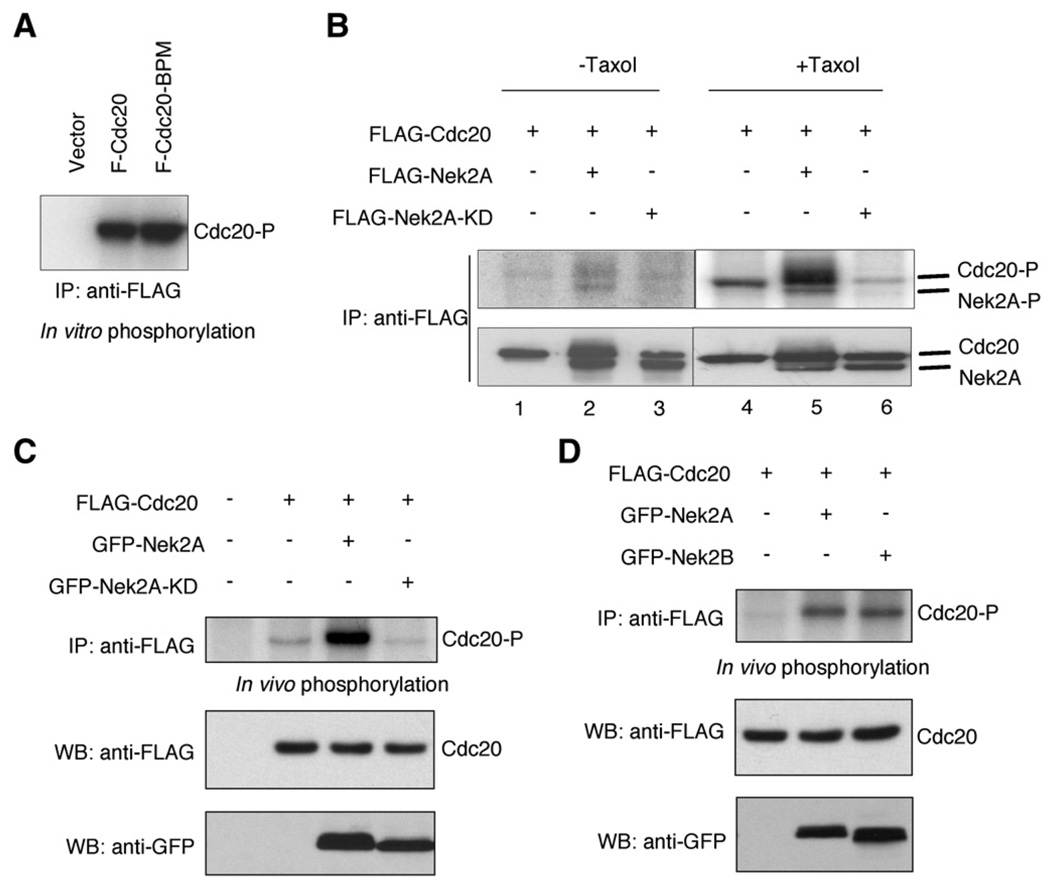
We performed in vitro kinase assays to explore the possibility that Cdc20 phosphorylation is mediated by Nek2. FLAG-Cdc20 was expressed in 293T cells and was isolated by IP. The FLAG-Cdc20 protein, which by itself showed little phosphorylation, was then incubated with purified recombinant Nek2 protein in the presence of 32P-γ-ATP. We found that Cdc20 was phosphorylated by Nek2A (Fig. 4A). An earlier study reported that Cdc20 can be phosphorylated by Bub1 and the phosphorylation is abolished upon mutagenesis of six serine/threonine residues of Cdc20 (Tang et al., 2004). In our study, we found that this mutated form of Cdc20 (i.e., Cdc20BP) can be phosphorylated by Nek2 (Fig. 4A). Therefore, the sites subjected to phosphorylation by Nek2 are different from those modified by Bub1.
Fig. 4.
Phosphorylation of Cdc20. (A) In vitro phosphorylation. FLAG-Cdc20 or FLAG-Cdc20-BPM was expressed in 293T cell and immunoprecipitated using anti-FLAG antibody. The proteins were incubated with purified recombinant Nek2A protein in the presence of 32P-γ-ATP. Protein phosphorylation was visualized by SDS–PAGE and phosphorimaging. (B) Effects of Taxol treatment on phosphorylation of Cdc20 by Nek2. 293T cells were transfected with the plasmids as indicated, treated with Taxol (lanes 1–3) or DMSO (lanes 4–6), and then metabolically labeled. Upper panels: IP with anti-FLAG antibody followed by phosphorimaging. Lower panels: IP and Western blot using anti-FLAG antibody. (C) In vivo phosphorylation of Cdc20. 293T cells were metabolically labeled following transfection of the plasmids as indicated. Top panel: IP with anti-FLAG antibody followed by phosphorimaging. Middle panel: Western blotting of cell lysate using anti-FLAG antibody. Bottom panel: Western blot analysis of cell lysate using anti-GFP antibody. (D) FLAG-Cdc20 was coexpressed with GFP-Nek2A or GFP-NEK2B in 293T cells, followed by metabolic labeling. Top panel: IP with anti-FLAG antibody followed by phosphorimaging. Middle panel: Western blotting of cell lysate (anti-FLAG antibody). Bottom panel: Western blot of cell lysate (anti-GFP antibody).
We next investigated in vivo phosphorylation of Cdc20 by Nek2. In asynchronized 293T cells transfected with Cdc20, the levels of Cdc20 phosphorylation was modestly elevated upon ectopic expression of the wild type Nek2 but not the kinase-deficient mutant Nek2-KD (Fig. 4B, lanes 1–3). Remarkably, phosphorylation of Cdc20 was more profound in cells synchronized in mitosis by Taxol treatment (Fig. 4B). Moreover, coexpression of Nek2 drastically raised the levels of Cdc20 phosphorylation (Fig. 4B). Under the same condition, ectopic expression of Nek2-KD did not enhance but reduced Cdc20 phosphorylation levels (Fig. 4B). Since Nek2-KD can associate Cdc20, it is likely that this form of the Nek2 mutant competes with endogenous Nek2 to associate with Cdc20 and, thus, results in attenuation of Cdc20 phosphorylation. Similarly, ectopic expression of GFP-Nek2A, but not the kinase-deficient form, also led to hyperphosphorylation of Cdc20 (Fig. 4C). In addition, both GFP-Nek2A and GFP-Nek2B can elevate Cdc20 phosphorylation levels in vivo (Fig. 4D).
Subcellular localization of Nek2, Cdc20, and Mad2
To corroborate the physiological relevance of the interaction between Nek2 and Mad2/Cdc20, we compared the subcellular localization patterns of these molecules in the cell at different stages of the cell cycle. In an earlier study, Nek2 was found in the kinetochore during mitosis in a pattern reminiscent of that of Mad1 and Mad2 (Lou et al., 2004). In addition to its localization on the kinetochore, GFP-Cdc20 was also found on the centrosome during interphase and on the spindle poles during mitosis (Kallio et al., 2002). However, it was unclear if the endogenous Cdc20 species is targeted to the centrosome or the spindle poles during various stages of the cell cycle.
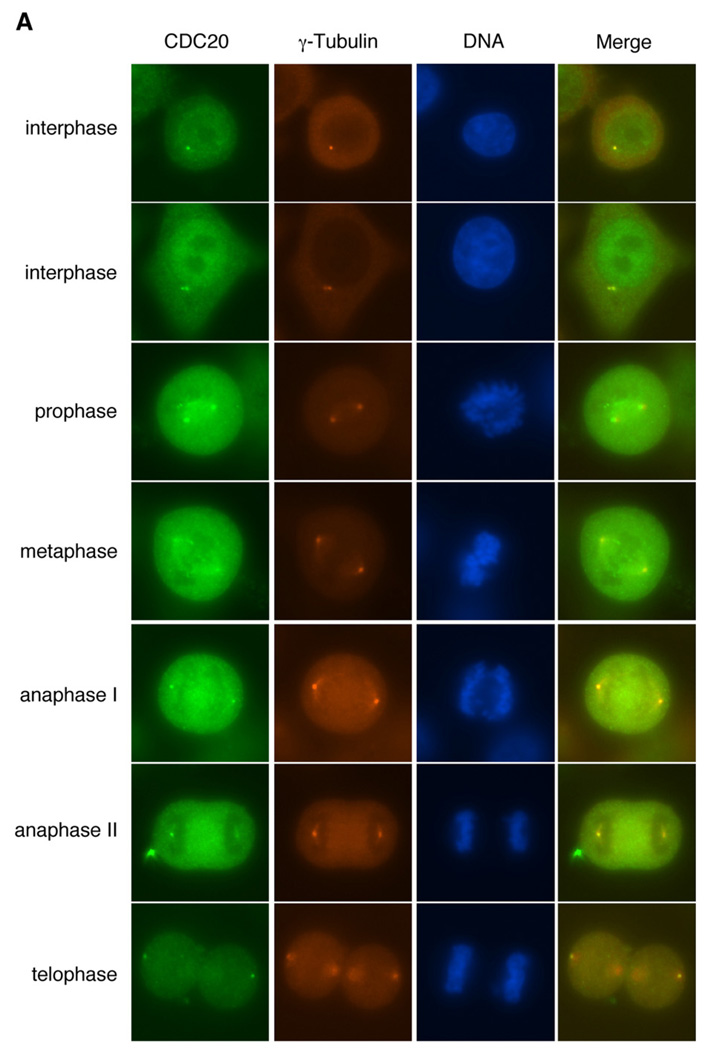
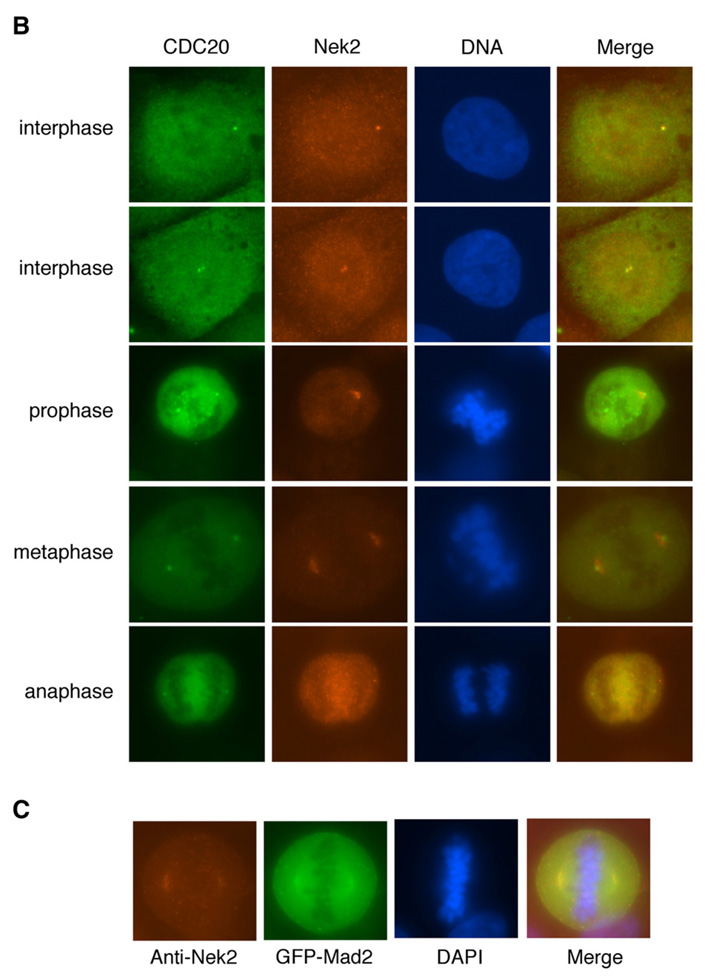
We therefore examined whether the endogenous Cdc20 and Nek2 proteins are colocalized to the centrosome or the spindles poles. Using a specific monoclonal anti-Cdc20 antibody, we detected dot-like structures that are colocalized with γ-tubulin in nonmitotic HeLa cells (Fig. 5A), which suggests that the endogenous Cdc20 protein is targeted to the centrosome. Similarly, Cdc20 also colocalized with Nek2 to the centrosome before the onset of mitosis (Fig. 5B). GFP-Mad2 was found in colocalization with Nek2 in the poles of the mitotic spindle (Fig. 5C).
Fig. 5.
Subcellular localization of Cdc20 and Nek2. (A) Localization of endogenous Cdc20 to the centrosome and the spindle poles in HeLa cells. Green: Cdc20; red: γ-tubulin; blue: DAPI. (B) Colocalization of endogenous Cdc20 and Nek2 to the centrosome and the spindle poles. Green: Cdc20; red: Nek2; blue: DAPI. (C) Colocalization of Mad2 and Nek2 on the spindle poles. Cells were transfected with GFP-Mad2 (green). Red: Nek2; blue: DAPI.
In mitotic cells, Cdc20 can be detected on both the kinetochore and the spindle poles, at different focusing points, by immunofluorescence microscopy (Fig. 5 and Supplementary Fig. 3). Although localization of Nek2 on the kinetochore has been reported previously (Lou et al., 2004), the antibody we obtained was not able to detect Nek2 in the kinetochore. It is possible that Nek2 is only targeted to this site in a very specific but short period of time during mitosis, which makes it difficult to be traced. Nevertheless, our results clearly show that endogenous Nek2 is disposed to the centrosome and the areas around the poles of the spindle throughout mitosis until the stage of cytokinesis (Fig. 5). Thus, the colocalization patterns of Nek2 and Cdc20 are consistent with the identification of the Nek2-Cdc20 ensemble and thus implicate a functional relationship at the centrosome.
Nek2A cooperates with Mad2 to cause mitotic delay
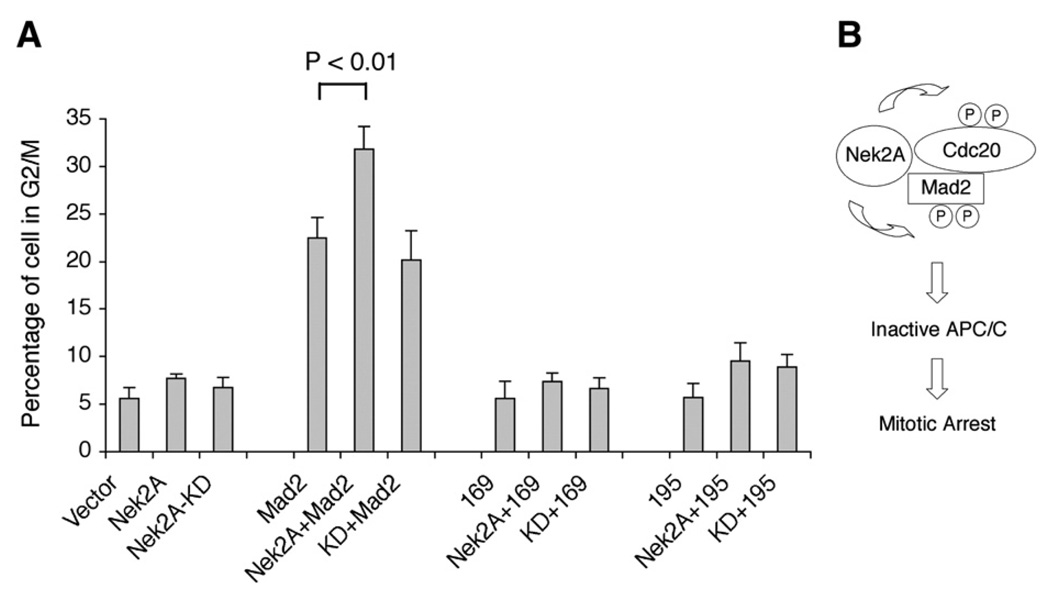
To evaluate the biological significance of the interactions involving Nek2, Mad2, and Cdc20, we took advantage of a previous observation that overexpression of Mad2 leads to prolonged mitosis as a manifestation of its role in mitotic checkpoint (Wassmann et al., 2003). We reasoned that, if Nek2 can affect the functions of Mad2, ectopic expression of Nek2 may either enhance or limit the ability of Mad2 to delay cell cycle progression. Indeed, our results show that, whereas transfection of Mad2 alone led to accumulation of cells in G2/ M phase of the cell cycle, coexpression of Nek2A with Mad2 further increased the percentage of cells in this cell cycle stage (Fig. 6A). In contrast, the kinase-deficient Nek2A mutant was not able to enhance the delay caused by Mad2 (Fig. 6A), which ruled out the possibility that Nek2A stalled cell cycle progression because it saturated APC/C activity as a substrate. In these assays, the Mad2 levels were not affected by coexpression of the Nek2A species (data not shown). Taken together, these results indicate that Nek2 may act cooperatively with Mad2 to cause mitotic delay and that the kinase activity of Nek2 is required for this function.
Fig. 6.
Overexpression of Nek2 enhances the ability of Mad2 to induce cell cycle arrest. (A) 293T cells were transfected with plasmids as indicated, followed by PI staining and FACS. The percentage of cells in G2/M phase is shown. The error bars represent standard deviation (n = 3; p<0.01). KD: kinase-deficient Nek2A; 169: Mad2(1–169); 195: Mad2(1–195). (B) Model depicting the proposed role ofNek2 in mitotic checkpoint control. Nek2 binds and phosphorylates Cdc20 and Mad2. These phosphorylation events may prevent activation of APC/C and arrest cells in mitosis.
Discussion
We have demonstrated that Nek2 can bind and phosphorylate Mad2 and Cdc20. We also provide evidence that Nek2 can co-operate with Mad2 to cause delays in cell division. These findings are consistent with earlier observations of the association between Nek2 and another kinetochore-associated protein, Mad1 (Lou et al., 2004). Notably, Nek2 was found to be required for localization of Mad2 to the kinetochore (Lou et al., 2004). In addition, a separate study indicated that Nek2 can phosphorylate Hec1, another kinetochore-localized protein that is essential for proper chromosome segregation (Chen et al., 2002). NEK2A-mediated Hec1 phosphorylation is essential for faithful kinetochore-microtubule attachments (Du et al., 2008). Furthermore, a recent report indicate that Nek2 is required for mitotic checkpoint control (Draviam et al., 2007). Thus, an accumulating body of evidence suggests that, in addition to its role in centrosome separation, Nek2 may mediate other critical functions, particularly in the mitotic kinetochore.
Our study has identified Nek2 as a kinase that can phosphorylate Cdc20. However, the Nek2 phosphorylation sites on Cdc20 are different from those targeted by Bub1 (Tang et al., 2004). Of note, phosphorylation of Cdc20 in vivo is significantly reduced in the presence of a kinase-deficient form of Nek2. This mutant form of Nek2 presumably exerts a dominant-negative effect and interferes with phosphorylation of Cdc20 by endogenous Nek2. Thus, these results highlight the importance of Nek2 in phosphorylation of Cdc20. We believe that, besides Bub1, Cdk1 and MAPK, Nek2 constitutes an additional layer of regulation of Cdc20.
Although an earlier study found that Mad2 is subjected to phosphorylation, the kinase responsible for this modification was not identified (Wassmann et al., 2003). The finding of phosphorylation of Mad2 by Nek2 represents the first report of a kinase involved in this biological process. Our studies indicated that the N-terminal region of Mad2 is sufficient to bind to Nek2 and undergoes phosphorylation following Nek2 overexpression. Interestingly, the double mutant Mad2R133E/Q134A cannot be readily phosphorylated. Previous studies showed that this mutant form of Mad2 cannot form the dimeric structure that modulates the assembly of the kinetochore protein complex (De Antoni et al., 2005a,b; Mapelli et al., 2007; Yang et al., 2007). Thus, our results support the notion that Mad2 undergoes phosphorylation in the dimeric form. The C-terminus of Mad2, which plays a vital role in binding to other checkpoint regulating molecules such as Mad1 and Cdc20, is not required for Nek2-mediated phosphorylation.
We have provided functional evidence for a role of Nek2 in the Mad2-mediated mitotic checkpoint. We demonstrated that overexpression of Nek2 can enhance the ability of Mad2 to cause delays in cell division. Our data also indicate that the kinase activity of Nek2 is essential for the cooperation between Nek2 and Mad2. One plausible explanation for the inhibitory effect of Mad2 in the mitotic transition is that Mad2 can form a protein complex with Cdc20, which subsequently blocks APC/C activity and prevents mitotic exit (Fang et al., 1998; Wassmann et al., 2003). Coexpression of Nek2 may therefore inhibit APC/C by facilitating the formation of the Mad2-Cdc20 complex, possibly through phosphorylation of Mad2 and Cdc20 (Fig. 6B). A recent study demonstrated that Cdc20 is a target of ubiquitination and that the Mad2-Cdc20 checkpoint complex can be stabilized by the process of deubiquitination (Stegmeier et al., 2007). Thus, it would be of great interest to examine whether Nek2 modulates the ubiquitination levels of Cdc20. Furthermore, it is also conceivable that Nek2 may modulate the APC/C activity and the checkpoint control through effecting the posttranslational modification of other kinetochore proteins that are associated with Mad2 or Cdc20.
Nek2A itself can directly bind to APC/C and is a substrate of APC/C-mediated proteolysis (Hames et al., 2001; Hayes et al., 2006). Curiously, it appears that degradation of Nek2A can occur even in pro-metaphase, although it is not clear how APC/C can be activated at this early stage of mitosis (Hayes et al., 2006). Thus, while Nek2A may act in cooperation with Mad2 as a negative regulator of APC/C, initial activation of APC/C may in return lead to degradation of Nek2A. The outcome of this sequence of events would be amplified APC/C activity. In contrast, Nek2B lacks the structural signatures targeted by APC/C and maintains its expression levels throughout mitosis (Hames and Fry, 2002; Hayes et al., 2006). Our results have also revealed that Nek2B can associate with Mad2 and Cdc20 just as well as Nek2A. Therefore, Nek2B may represent the major isoform that acts upon Mad2 and Cdc20 and sustains the inhibitory effects on the APC/C activity towards the later stages of mitosis.
The functional linkage of Nek2 with the Mad2/Cdc20 kinetochore protein complex provides additional insights into how deregulation of Nek2 activities may promote aneuploidy and oncogenesis. Indeed, elevation of Nek2 expression has been found in a variety of cancer cell lines, as well as in the ductal carcinoma in situ of the breast and in invasive breast carcinomas (Hayward et al., 2004). Conceivably, one mechanism by which Nek2 causes chromosome instability is through inducing centrosome supernumerary (Hayward et al., 2004). Our findings suggest that Nek2 overexpression may also contribute to malignant transformation by disrupting the mitotic checkpoint that is modulated by Mad2 and Cdc20. Importantly, Mad2 overexpression leads to aneuploidy and tumorigenesis in a transgenic mouse model (Sotillo et al., 2007). Thus, excessive Nek2 and Mad2 levels may act in synergy to corrupt the mitotic checkpoint and cause aneuploidy.
Cdc20 and Nek2 have been found colocalized to the centrosome in nonmitotic cells. The biological function of Cdc20 on the centrosome at this stage of the cell cycle is not clear. It has been documented that the ubiquitin-mediated proteolytic activities, such as SCF complexes, are implicated in the events of centrosome duplication and separation (Freed et al., 1999; Murphy, 2003; Wojcik et al., 2000). These proteolytic activities may be involved in the cleavage of proteins that maintains the physical linkage between the old and the nascent centrosomes. Because Cdc20 is an activator of a functionally similar protein complex, namely the APC/C complex, it is possible that this protein may play a role in the centrosome cycle, in addition to its function in mitotic checkpoint control. Indeed, a recent study demonstrated that the APC/C and separase activities are required for disengagement of the nascent centriole from its template, which constitutes a mechanism to prevent excessive centrosome duplication (Tsou and Stearns, 2006). It is conceivable that Cdc20, originally identified as an activator of the APC/C complex and regulator of mitotic checkpoint, may thus play additional roles in controlling a critical step of the centrosome duplication cycle. In this respect, phosphorylation of Cdc20 by Nek2 may represent a mechanism that regulates Cdc20 functions in the centrosome.
In summary, we have demonstrated that Nek2 can associate with and phosphorylate Mad2 and Cdc20. The results presented here support a model in which Nek2 modulates the functions of Mad2 and Cdc20 in the mitotic checkpoint and elevation of Nek2 levels may contribute to chromosome instability by interfering with the control of the checkpoint. Our data, combined with a number of other recent findings, also suggest a potential role of Cdc20 in the centrosome cycle.
Materials and methods
Plasmids and antibodies
The Mad2 mutants were created using the Quickchange® site-directed mutagenesis kit (Sratagene, La Jolla, CA). The following antibodies were used: anti-centromere autoantibody (ACA), kindly provided by Dr. Tim Yen (Fox Chase Cancer Center, Philadelphia, PA); anti-Nek2 and anti-Cdc20 antibodies (BD Biosciences); anti-GFP antibody (Roche Applied Science, Indianapolis, IN); anti-FLAG (M2) and anti-γ-tubulin antibody (GTU88) (Sigma, St. Louis, MO); horseradish peroxidase-conjugated anti-mouse κ light chain antibody (Invitrogen).
Immunoprecipitation and Western blot
293T cells were transfected using FuGene6 (Roche). Forty-eight hours after transfection, cell lysates were prepared in lysis buffer (120 mM NaCl, 25 mM Tris-HCl (pH = 7.4), 0.5% NP-40, and protease inhibitor cocktail (Roche)). IP and Western blot were performed using antibodies as indicated. For IP of endogenous protein, 3 × 107 untransfected cells were used and cell lysate was homogenized by sonication.
Immunofluorescence microscopy
Immunostaining was performed using a previously published protocol (Wang et al., 2001). Briefly, cells were subsequently fixed in buffer A (3% paraformaldehyde in phosphate-buffered saline) and in methanol, permeabilized in buffer B (0.5% Triton X-100 in 20 mM HEPES, pH 7.5, and 50 mM NaCl, 3 mM MgCl2), and placed in blocking buffer (buffer B plus 0.1% Triton X-100 and 2% BSA). After subsequent incubation with the primary and the secondary antibodies, the images were obtained on a Leica DM IRBE fluorescence microscope.
Cell cycle analysis
Cells were fixed in methanol, washed with PBS, and stained with propidium iodide (60 µg/ml, Sigma) in the presence of RNase (100 µg/ml, Roche Applied Science) for 60 min at 4 °C. FACS analysis was performed using a Becton Dickinson luorescence-activated cell analyzer (Becton Dickinson Immunocytometry Systems, Mansfield, MA). Cell cycle distribution was quantified using the ModFit LT version 1.01 software (Verity Software House Inc., Topsham, ME).
Kinase assay
In vitro kinase assays
Mad2, Cdc20, or their mutant forms were transfected into 293T cells. After 48 hours, cell lysates were collected and subjected to immuno-precipitation. The immunocomplex was then incubated at 30 °C for 20 minutes in 40 µl of kinase assay buffer containing 10 µCi of 32P-γ-ATP and 300 ng of purified recombinant Nek2. Alternatively, purified CBP-Mad2 protein was used as the substrate. The protein mixture was separated by SDS–PAGE and visualized by phosphorimaging.
In vivo kinase assays
Mad2, Cdc20, or their mutant forms were transfected into 293T cells. Twenty-four hours after transfection, the cells were placed in phosphate-free media containing 10% dialyzed FBS and cultured overnight. The cells were then metabolically labeled with 32P-orthophosphate (1 mCi/ml) for 2 hours followed by immunoprecipitation. The immunocomplex was then separated by SDS–PAGE and visualized by phosphorimaging. A parallel set of unlabeled cells was used to confirm protein expression and IP efficiency.
Supplementary Material
Acknowledgments
This work was funded by the Abramson Family Cancer Research Institute of the University of Pennsylvania and by the National Institute of Health. We thank Dr. Tim Yen for providing the anti-ACA antibody and Dr. Hongtao Yu for the Cdc20 constructs.
Footnotes
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.yexmp.2009.12.004.
References
- Chen Y, Riley DJ, Zheng L, Chen PL, Lee WH. Phosphorylation of the mitotic regulator protein Hec1 by Nek2 kinase is essential for faithful chromosome segregation. J. Biol. Chem. 2002;277:49408–49416. doi: 10.1074/jbc.M207069200. [DOI] [PubMed] [Google Scholar]
- Chung E, Chen RH. Phosphorylation of Cdc20 is required for its inhibition by the spindle checkpoint. Nat. Cell Biol. 2003;5:748–753. doi: 10.1038/ncb1022. [DOI] [PubMed] [Google Scholar]
- D’Angiolella V, Mari C, Nocera D, Rametti L, Grieco D. The spindle checkpoint requires cyclin-dependent kinase activity. Genes Dev. 2003;17:2520–2525. doi: 10.1101/gad.267603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Antoni A, Pearson CG, Cimini D, Canman JC, Sala V, Nezi L, Mapelli M, Sironi L, Faretta M, Salmon ED, Musacchio A. The Mad1/Mad2 complex as a template for Mad2 activation in the spindle assembly checkpoint. Curr. Biol. 2005a;15:214–225. doi: 10.1016/j.cub.2005.01.038. [DOI] [PubMed] [Google Scholar]
- De Antoni A, Sala V, Musacchio A. Explaining the oligomerization properties of the spindle assembly checkpoint protein Mad2. Philos. Trans. R. Soc. Lond., B Biol. Sci. 2005b;360:637–647. doi: 10.1098/rstb.2004.1618. discussion 447–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Agostino S, Rossi P, Geremia R, Sette C. The MAPK pathway triggers activation of Nek2 during chromosome condensation in mouse spermatocytes. Development. 2002;129:1715–1727. doi: 10.1242/dev.129.7.1715. [DOI] [PubMed] [Google Scholar]
- Di Agostino S, Fedele M, Chiefi P, Fusco A, Rossi P, Geremia R, Sette C. Phosphorylation of high-mobility group protein A2 by Nek2 kinase during the first meiotic division in mouse spermatocytes. Mol. Biol. Cell. 2004;15:1224–1232. doi: 10.1091/mbc.E03-09-0638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draviam VM, Xie S, Sorger PK. Chromosome segregation and genomic stability. Curr. Opin. Genet. Dev. 2004;14:120–125. doi: 10.1016/j.gde.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Draviam VM, Stegmeier F, Nalepa G, Sowa ME, Chen J, Liang A, Hannon GJ, Sorger PK, Harper JW, Elledge SJ. A functional genomic screen identifies a role for TAO1 kinase in spindle-checkpoint signalling. Nat. Cell Biol. 2007;9:556–564. doi: 10.1038/ncb1569. [DOI] [PubMed] [Google Scholar]
- Du J, Cai X, Yao J, Ding X, Wu Q, Pei S, Jiang K, Zhang Y, Wang W, Shi Y, et al. The mitotic checkpoint kinase NEK2A regulates kinetochore microtubule attachment stability. Oncogene. 2008;27:4107–4114. doi: 10.1038/onc.2008.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang G. Checkpoint protein BubR1 acts synergistically with Mad2 to inhibit anaphase-promoting complex. Mol. Biol. Cell. 2002;13:755–766. doi: 10.1091/mbc.01-09-0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang G, Yu H, Kirschner MW. The checkpoint protein MAD2 and the mitotic regulator CDC20 form a ternary complex with the anaphase-promoting complex to control anaphase initiation. Genes Dev. 1998;12:1871–1883. doi: 10.1101/gad.12.12.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faragher AJ, Fry AM. Nek2A kinase stimulates centrosome disjunction and is required for formation of bipolar mitotic spindles. Mol. Biol. Cell. 2003;14:2876–2889. doi: 10.1091/mbc.E03-02-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freed E, Lacey KR, Huie P, Lyapina SA, Deshaies RJ, Stearns T, Jackson PK. Components of an SCF ubiquitin ligase localize to the centrosome and regulate the centrosome duplication cycle. Genes Dev. 1999;13:2242–2257. doi: 10.1101/gad.13.17.2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry AM, Mayor T, Meraldi P, Stierhof YD, Tanaka K, Nigg EA. C-Nap1, a novel centrosomal coiled-coil protein and candidate substrate of the cell cycle-regulated protein kinase Nek2. J. Cell Biol. 1998a;141:1563–1574. doi: 10.1083/jcb.141.7.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry AM, Meraldi P, Nigg EA. A centrosomal function for the human Nek2 protein kinase, a member of the NIMA family of cell cycle regulators. EMBO J. 1998b;17:470–481. doi: 10.1093/emboj/17.2.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hames RS, Fry AM. Alternative splice variants of the human centrosome kinase Nek2 exhibit distinct patterns of expression in mitosis. Biochem. J. 2002;361:77–85. doi: 10.1042/0264-6021:3610077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hames RS, Wattam SL, Yamano H, Bacchieri R, Fry AM. APC/C-mediated destruction of the centrosomal kinase Nek2A occurs in early mitosis and depends upon a cyclin A-type D-box. EMBO J. 2001;20:7117–7127. doi: 10.1093/emboj/20.24.7117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes MJ, Kimata Y, Wattam SL, Lindon C, Mao G, Yamano H, Fry AM. Early mitotic degradation of Nek2A depends on Cdc20-independent interaction with the APC/C. Nat. Cell Biol. 2006;8:607–614. doi: 10.1038/ncb1410. [DOI] [PubMed] [Google Scholar]
- Hayward DG, Clarke RB, Faragher AJ, Pillai MR, Hagan IM, Fry AM. The centrosomal kinase Nek2 displays elevated levels of protein expression in human breast cancer. Cancer Res. 2004;64:7370–7376. doi: 10.1158/0008-5472.CAN-04-0960. [DOI] [PubMed] [Google Scholar]
- Hoyt MA. A new view of the spindle checkpoint. J. Cell Biol. 2001;154:909–911. doi: 10.1083/jcb.200108010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallio MJ, Beardmore VA, Weinstein J, Gorbsky GJ. Rapid microtubuleindependent dynamics of Cdc20 at kinetochores and centrosomes in mammalian cells. J. Cell Biol. 2002;158:841–847. doi: 10.1083/jcb.200201135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kops GJ, Weaver BA, Cleveland DW. On the road to cancer: aneuploidy and the mitotic checkpoint. Nat. Rev. Cancer. 2005;5:773–785. doi: 10.1038/nrc1714. [DOI] [PubMed] [Google Scholar]
- Kraft C, Herzog F, Gieffers C, Mechtler K, Hagting A, Pines J, Peters JM. Mitotic regulation of the human anaphase-promoting complex by phosphorylation. EMBO J. 2003;22:6598–6609. doi: 10.1093/emboj/cdg627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laiho M, Latonen L. Cell cycle control, DNA damage checkpoints and cancer. Ann. Med. 2003;35:391–397. doi: 10.1080/07853890310014605. [DOI] [PubMed] [Google Scholar]
- Lou Y, Yao J, Zereshki A, Dou Z, Ahmed K, Wang H, Hu J, Wang Y, Yao X. NEK2A interacts with MAD1 and possibly functions as a novel integrator of the spindle checkpoint signaling. J. Biol. Chem. 2004;279:20049–20057. doi: 10.1074/jbc.M314205200. [DOI] [PubMed] [Google Scholar]
- Luo X, Fang G, Coldiron M, Lin Y, Yu H, Kirschner MW, Wagner G. Structure of the Mad2 spindle assembly checkpoint protein and its interaction with Cdc20. Nat. Struct. Biol. 2000;7:224–229. doi: 10.1038/73338. [DOI] [PubMed] [Google Scholar]
- Luo X, Tang Z, Rizo J, Yu H. The Mad2 spindle checkpoint protein undergoes similar major conformational changes upon binding to either Mad1 or Cdc20. Mol. Cell. 2002;9:59–71. doi: 10.1016/s1097-2765(01)00435-x. [DOI] [PubMed] [Google Scholar]
- Luo X, Tang Z, Xia G, Wassmann K, Matsumoto T, Rizo J, Yu H. The Mad2 spindle checkpoint protein has two distinct natively folded states. Nat. Struct. Mol. Biol. 2004;11:338–345. doi: 10.1038/nsmb748. [DOI] [PubMed] [Google Scholar]
- Mapelli M, Massimiliano L, Santaguida S, Musacchio A. The Mad2 conformational dimer: structure and implications for the spindle assembly checkpoint. Cell. 2007;131:730–743. doi: 10.1016/j.cell.2007.08.049. [DOI] [PubMed] [Google Scholar]
- Murphy TD. Drosophila skpA, a component of SCF ubiquitin ligases, regulates centrosome duplication independently of cyclin E accumulation. J. Cell Sci. 2003;116:2321–2332. doi: 10.1242/jcs.00463. [DOI] [PubMed] [Google Scholar]
- Musacchio A, Hardwick KG. The spindle checkpoint: structural insights into dynamic signalling. Nat. Rev. Mol. Cell Biol. 2002;3:731–741. doi: 10.1038/nrm929. [DOI] [PubMed] [Google Scholar]
- Musacchio A, Salmon ED. The spindle-assembly checkpoint in space and time. Nat. Rev. Mol. Cell Biol. 2007;8:379–393. doi: 10.1038/nrm2163. [DOI] [PubMed] [Google Scholar]
- O’Connell MJ, Krien MJ, Hunter T. Never say never. The NIMA-related protein kinases in mitotic control. Trends Cell Biol. 2003;13:221–228. doi: 10.1016/s0962-8924(03)00056-4. [DOI] [PubMed] [Google Scholar]
- Peters JM. The anaphase-promoting complex: proteolysis in mitosis and beyond. Mol. Cell. 2002;9:931–943. doi: 10.1016/s1097-2765(02)00540-3. [DOI] [PubMed] [Google Scholar]
- Schultz SJ, Fry AM, Sutterlin C, Ried T, Nigg EA. Cell cycle-dependent expression of Nek2, a novel human protein kinase related to the NIMA mitotic regulator of Aspergillus nidulans. Cell Growth Differ. 1994;5:625–635. [PubMed] [Google Scholar]
- Shah JV, Cleveland DW. Waiting for anaphase: Mad2 and the spindle assembly checkpoint. Cell. 2000;103:997–1000. doi: 10.1016/s0092-8674(00)00202-6. [DOI] [PubMed] [Google Scholar]
- Sironi L, Melixetian M, Faretta M, Prosperini E, Helin K, Musacchio A. Mad2 binding to Mad1 and Cdc20, rather than oligomerization, is required for the spindle checkpoint. EMBO J. 2001;20:6371–6382. doi: 10.1093/emboj/20.22.6371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotillo R, Hernando E, Diaz-Rodriguez E, Teruya-Feldstein J, Cordon-Cardo C, Lowe SW, Benezra R. Mad2 overexpression promotes aneuploidy and tumorigenesis in mice. Cancer Cell. 2007;11:9–23. doi: 10.1016/j.ccr.2006.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegmeier F, Rape M, Draviam VM, Nalepa G, Sowa ME, Ang XL, McDonald ER, III, Li MZ, Hannon GJ, Sorger PK, et al. Anaphase initiation is regulated by antagonistic ubiquitination and deubiquitination activities. Nature. 2007;446:876–881. doi: 10.1038/nature05694. [DOI] [PubMed] [Google Scholar]
- Tang Z, Bharadwaj R, Li B, Yu H. Mad2-independent inhibition of APCCdc20 by the mitotic checkpoint protein BubR1. Dev. Cell. 2001;1:227–237. doi: 10.1016/s1534-5807(01)00019-3. [DOI] [PubMed] [Google Scholar]
- Tang Z, Shu H, Oncel D, Chen S, Yu H. Phosphorylation of Cdc20 by Bub1 provides a catalytic mechanism for APC/C inhibition by the spindle checkpoint. Mol. Cell. 2004;16:387–397. doi: 10.1016/j.molcel.2004.09.031. [DOI] [PubMed] [Google Scholar]
- Tsou MF, Stearns T. Mechanism limiting centrosome duplication to once per cell cycle. Nature. 2006 doi: 10.1038/nature04985. [DOI] [PubMed] [Google Scholar]
- Wang Q, Zhang H, Guerrette S, Chen J, Mazurek A, Wilson T, Slupianek A, Skorski T, Fishel R, Greene MI. Adenosine nucleotide modulates the physical interaction between hMSH2 and BRCA1. Oncogene. 2001;20:4640–4649. doi: 10.1038/sj.onc.1204625. [DOI] [PubMed] [Google Scholar]
- Wassmann K, Liberal V, Benezra R. Mad2 phosphorylation regulates its association with Mad1 and the APC/C. EMBO J. 2003;22:797–806. doi: 10.1093/emboj/cdg071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojcik EJ, Glover DM, Hays TS. The SCF ubiquitin ligase protein slimb regulates centrosome duplication in Drosophila. Curr. Biol. 2000;10:1131–1134. doi: 10.1016/s0960-9822(00)00703-x. [DOI] [PubMed] [Google Scholar]
- Yang M, Li B, Tomchick DR, Machius M, Rizo J, Yu H, Luo X. p31comet blocks Mad2 activation through structural mimicry. Cell. 2007;131:744–755. doi: 10.1016/j.cell.2007.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H. Cdc20: a WD40 activator for a cell cycle degradation machine. Mol. Cell. 2007;27:3–16. doi: 10.1016/j.molcel.2007.06.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.