Abstract
As colorectal cancer and colorectal liver metastases become a serious public health problem, new treatment modalities are needed in order to achieve better results. In the last decade there has been very important progress in oncology, with new and more effective chemotherapeutic agents administered alone or in combination improving the resectability rate in up to 40% of patients with colorectal liver metastases. Advances in interventional radiology, in particular, with the use of portal vein embolization and radiofrequency thermal ablation are new strategies allowing major liver resections and treatment of small liver metastases or early recurrences. Surgery, however, remains the gold standard strategy with intention to treat. In this review article we will describe the advanced role of surgery in the multidisciplinary approach to colorectal liver metastases, and the clinical problems the liver surgeon has to deal with, such as the resectability of the metastases, the presence of bilobar liver lesions and extrahepatic disease, the impact of chemotherapy in already resectable liver metastases, the problem of vanishing metastases after chemotherapy and the dilemma of staged or combined liver and colon operations and which organ first in the clinical scenario of synchronous colorectal liver metastases.
Keywords: Metastases, Liver metastases, Colorectal cancer, Surgery
INTRODUCTION
Colorectal cancer (CRC) is an important public health problem: there are nearly one million new cases diagnosed worldwide each year and half a million deaths. Recent reports show that CRC is the third most frequent cancer in the Western world while in the USA it is the most frequent form of cancer among persons aged 75 years and older[1]. Approximately 25% of these patients have liver metastases at the time of diagnosis [colorectal liver metastases (CRLM)] and 25% will develop, CRLM during the course of the disease. Eventually two-thirds of patients with CRLM will die because of liver metastases[2]. Given that the majority of malignancies occur in elderly people and with ageing of the population in mind the above epidemiological observations make the need for prevention and improved treatment strategies very urgent.
In the last decade there has been very important progress in 3 different fields regarding the treatment of CRLM: new and more effective chemotherapeutic agents in oncology administered alone or in combination; an advanced role of interventional radiology with portal vein embolization and radiofrequency ablation; and last, but not least, new strategies and manipulation techniques for safer hepatic resections. The combination of these improvements makes the role of a multidisciplinary approach in the patient with CRLM the only therapeutic modality, which has gradually but effectively improved the resectability rate of metastases to 20%-30% of cases and has resulted in 5-year survival of 35%-50% for selected cases[3,4].
In this review we will discuss the improvement in chemotherapy and interventional radiology as well as the new and advanced role of surgery in this multidisciplinary approach in order to establish treatment strategies and to gain better results in disease-free and overall survival in patients with CRLM.
CHEMOTHERAPY-ONCOLOGY
Chemotherapeutic agents have substantially changed over the last decade. Two new agents, irinotecan and oxaliplatin are used in combination with the traditional regimen of 5-fluorouracil (5-FU) and leucovorin (LV). Irinotecan is an inhibitor of topoisomerase I and its combination with 5-FU and LV is called Folfiri while oxaliplatin is a non-nephrotoxic platinum complex which in combination with 5-FU and LV is called Folfox. While traditional chemotherapy was of limited efficacy, with response rates not exceeding 25%, the new combinations allowed a tumor response in approximately 40%-50% of patients[5,6]. Another very important issue is that the new chemotherapeutic combinations have been reported to facilitate the resection of 9%-40% of initially unresectable metastases, with data emerging from randomized trials suggesting that the addition of targeted agents and a third cytotoxic drug might improve these results even more[7,8]. The use of a triple combination of irinotecan, oxaliplatin and 5-FU or LV seems to further increase the efficacy of systemic chemotherapy, as 3 different studies described a response rate of up to 70% and improvement in the overall survival of up to 26 mo[9-11]. All these studies showed that patients who can undergo surgical resection for their CRLM have better survival rates compared to patients treated only with chemotherapy.
Besides oxaliplatin and irinotecan, the introduction of two new targeted agents opens a new era in the oncological treatment of colorectal liver metastases. Cetuximab is a monoclonal antibody against the epithelial growth factor receptor and bevacizumab is a humanized antibody against the vascular endothelial growth factor (VEGF). The use of the above-mentioned targeted agents in combination with Folfox or Folfiri has increased the resectability rate in patients whose tumors were previously considered unresectable and has also improved the overall survival rate in patients with CRLM as mentioned above. Hurwitz et al[12] published a randomized controlled trial where 813 patients with CRLM were allocated to either Folfiri or Folfiri plus bevacizumab. The addition of bevacizumab was associated with a statistically significant increase in median survival, progression-free survival and overall tumor response rate. There were also other studies where the addition of bevacizumab to Folfox was associated with an increased tumor response rate and median overall survival[13]. However, these studies were not randomized.
However, apart from these positive effects of chemotherapy on resectability and survival rates, there were also some severe adverse effects on the liver, as histological lesions are known to occur in the liver parenchyma following chemotherapy, with the type of lesion being specific for the agent used. Sinusoidal obstruction syndrome is characterized by erythrocytic congestion and can be accompanied by perisinusoidal fibrosis and fibrotic venous occlusion. This syndrome has been described in association with oxaliplatin, and the incidence of these histological changes is between 20% and 29%[14,15]. The administration of oxaliplatin-based chemotherapy can also be associated with vascular lesions in the liver such as hemorrhagic centrilobular necrosis, and carry a higher risk of operative bleeding and transfusion requirement, as well as impaired liver regeneration and increased post-hepatectomy mortality[16,17]. The second adverse effect is a form of steatohepatitis that has been related to irinotecan administration. Chemotherapy-associated steatohepatitis is characterized by the simultaneous presence of severe steatosis, lobular inflammation and ballooning of the hepatocytes[18]. Analysis of the impact of steatosis on surgical outcome suggests that morbidity is increased and that there is also an increased rate of infectious complications[19,20]. Steatohepatitis linked to irinotecan treatment is associated with increased 90-d mortality because of liver failure after surgery[18,21].
At this point there is an important question raised whether the above-mentioned chemotherapy-linked liver damage is related to the duration of treatment or not. There are 2 pertinent studies in the literature which clearly showed that the morbidity rate was related to the number of cycles of chemotherapy administered[17,21]. More recent data on postoperative complications in the EORTC 40983/EPOC study show that 3 mo of preoperative chemotherapy with Folfox-4 had a relatively mild impact on surgical outcome[22]. Another issue regarding the safety of preoperative chemotherapy is the potent effect of the monoclonal antibody bevacizumab, as targeting VEGF could augment hepatic damage and diminish regeneration after resection. One study showed that there was no major effect of bevacizumab on the incidence of postoperative complications if stopped at least 5 wk prior to surgery[23]. Data from another study described that the use of this agent may even reduce the incidence of liver failure after hepatic resection[24]. One can conclude that neoadjuvant chemotherapy can induce liver injury but with little clinical impact if patients are not overtreated and if a proper time interval is maintained between chemotherapy and surgery[25].
INTERVENTIONAL RADIOLOGY
Advances in interventional radiology have contributed to the treatment of patients with CRLM, in particular the use of portal vein embolization (PVE) and radiofrequency thermal ablation (RFA). Since 1982 when Makuuchi et al[26] first used PVE in order to provoke compensatory hypertrophy of the future remnant liver in patients planned for major hepatic resections, much experience has been gained and this technique is today available in every specialized hepatobiliary center[27,28]. In patients planned for major hepatectomy and with an otherwise normal liver, preoperative PVE is recommended when the ratio of the remnant liver to total liver volume is estimated to be less than 30%, whereas in patients with neoadjuvant chemotherapy this ratio is considered to be 40%[29,30]. PVE is a safe procedure, but movement of the embolic material to the main portal vein or into branches that supply the future remnant liver remains a risk.
RFA was initially and widely used for local treatment of hepatocellular carcinoma and recently has gained popularity for the management of CRLM, where its indications are still under debate. Critical review of the results of RFA shows that RFA must be restricted in cases with a maximum of 3 lesions with the size of the biggest lesion less than 3 cm[31]. Another limitation for the use of RFA in the management of CRLM is the anatomic location of the lesion. When the metastases are near big vessels the risk of incomplete ablation is increased as the heat effect is minimized[32]. Because of the above-mentioned limitations there is still no place for RFA in patients with resectable CRLM. The use of RFA is actually limited in cases with early recurrence after resection, detected as small lesions, because it is not mandatory to stop chemotherapy[33].
THE ROLE OF SURGERY
The liver surgeon of the multidisciplinary team for the treatment of CRLM will face some clinical problems and scenarios such as the resectability of the metastases, the presence of bilobar liver lesions or extrahepatic disease, the impact of neoadjuvant chemotherapy in the case of resectable metastases, the problem of vanishing metastases after chemotherapy, and the dilemma of staged or combined liver and colon resections in the clinical scenario of synchronous CRLM. For historical reasons we mention that liver metastases were classified as unresectable if they were large in size, bilobar or there were more than 4[34,35]. Today there has been substantial progress in liver surgery owing to improved preoperative diagnosis and intraoperative and postoperative care, and new strategies are being developed in order to achieve larger and safer resections. Many tumors that were previously considered unresectable are now amenable to complete resection. In 2007, Figueras et al[36] published a study where no predefined criteria of resectability were mentioned with regard to number, size, location of the tumor or presence of extrahepatic disease. The only prerequisite is that the resection was possible and that the potential liver remnant was able to sustain metabolic, synthetic and detoxifying functions[37]. It is in general accepted that where possible it is better to remove a metastasis than to leave it and that even R1 resections might become an acceptable clinical strategy provided that they confer meaningful patient benefit[25].
The presence of bilobar CRLM is a challenging issue for the liver surgeon as this problem requires extended resections. As mentioned above, interventional radiology with PVE is an important preoperative tool in order to increase the volume of the remnant liver after an extended resection. However, the legitimate concern that metastases in the non-embolized hemiliver might grow more rapidly after right PVE[38] has led to the proposal of a 2-stage procedure. In the first stage all visible metastases in the left hemiliver are removed in association with right portal vein ligation. In the second stage, which takes place between 2 and 4 wk after the first stage, an extended right hemihepatectomy is performed[37] (Figure 1).
Figure 1.
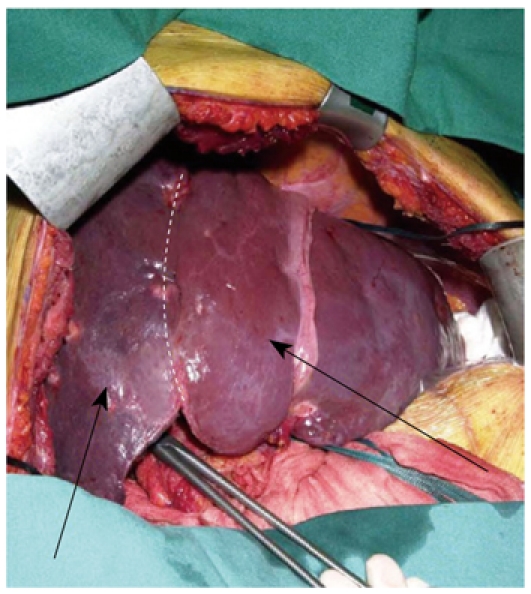
Atrophy of the right hemiliver (right arrow) and hypertrophy of the left hemiliver (left arrow) after right portal vein ligation.
Despite the great effort that has been made both in preoperative liver volume manipulation and in intraoperative and postoperative care there are still many patients who present to the liver surgeon with initially unresectable metastases. Current treatment practice for these patients is a combination of classical chemotherapy with the targeted agents cetuximab and bevacizumab, which facilitates resection in 9%-40% of lesions initially considered unresectable[7]. As a consequence, 5-year survival rates of 50% after combined treatment are becoming increasingly common[36]. The next step is to define the most effective chemotherapeutic regimen for this treatment strategy. Currently, data from randomized trials are beginning to show the added benefit conferred by the targeted agent cetuximab on response and resection rates achieved with standard first-line therapies in patients with advanced CRC[39,40]. The rate of surgery with curative intent was higher in patients who received Folfiri plus cetuximab in comparison with Folfiri alone and the R0 resection was also increased[40]. In this setting the requirement for a delay between the end of chemotherapy treatment and the planning of surgery, as well as the presence of K-ras mutation in the primary tumor are 2 very important considerations for the management of these complex cases[41-43]. Mutations in this gene have been shown to be predictive of reduced disease-free and overall survival. Furthermore, it has been demonstrated that those with K-ras mutations do not benefit in the same manner from the traditional chemotherapeutic regimens as those with the wild type. Analysis of recent data show that patients with wild type K-ras had a significant advantage in terms of tumor response with the addition of bevacizumab compared to standard Folfiri treatment. Further studies are needed in the era of biomarkers in order to achieve better tumor response rates and increased survival[44,45]. At this point it is important to mention that the presence of extrahepatic disease is no longer considered in the criteria for unresectability, provided that it is also resectable[46].
The conventional way of thinking in patients with resectable synchronous CRLM is to offer an upfront operation, and the reason for this attitude is the fear that the CRLM will not respond to chemotherapy and that during the time of chemotherapy the tumors will grow beyond the possibility of surgical cure. However, chemotherapy before surgery, even in patients with resectable CRLM, can increase the complete resection rate, facilitate limited hepatectomies, improve postoperative recovery, treat micrometastases, provide a test for chemoresponsiveness, identify aggressive disease and spare ineffective chemotherapy. All the above parameters are supported by the results of the EORTC 40983 study where the progression-free survival rate at 3 years was increased by 8.1% in those patients who received perioperative chemotherapy when compared with surgery alone. Furthermore, single-center non-randomized studies support the use of neoadjuvant protocols for resectable CRLM[47]. A specific problem that has emerged with the use of effective neoadjuvant chemotherapy regimens is known as the “missing” or “vanishing” metastases. This terminology reflects lesions that were present on initial radiological examinations and can no longer be identified by imaging performed after chemotherapy. Because these lesions are very difficult to localize and resect during surgery the situation is well characterized by the sentence “when the dream of the oncologist becomes the nightmare of the surgeon”. While a publication suggested that missing metastases are cured in 70% of cases[48], another study showed persistent microscopic or macroscopic residual disease or very early recurrence in 83% of cases with a complete radiological response[49]. Thus a complete radiological response does not mean a complete histological response and some authors suggest that when the detailed intraoperative ultrasound examination fails to detect missing metastases, the corresponding parenchymal region should be resected in the basis of vascular landmarks[33].
The final but very important issue that the liver surgeon has to deal with is whether to proceed to combined (liver and colon) or staged surgery and if he chooses a staged procedure which organ first. The ideal solution for this complicated problem would be a simultaneous colon and liver operation. The advantage of the combined procedure is that we have one operation with less psychological considerations for the patient, less financial costs and shorter hospitalization time. On the other hand the advantages of the staged procedure are that there is no accumulation of the risks of liver and bowel resections at the same time, a neoadjuvant chemotherapy may be given before liver resection, and an extended hepatectomy or difficult bowel resection can be performed with the full attention of the surgical team focused on the liver or bowel disease. However, the critical issue for decision-making is the patient’s safety. Considering the initial experience with simultaneous versus staged resections, a French multicenter study showed an operative mortality of 7% for simultaneous vs 2% for staged surgery[50], while in a single center US study the mortality was 12% for simultaneous vs 4% for staged resections[51]. It was also shown in several studies that simultaneous operations can be performed without death[52-55]. These studies, however, were retrospective and patients for simultaneous resection were selected by experienced hepatobiliary surgeons. In conclusion, simultaneous liver and bowel operations can be performed on selected patients but should be avoided in cases of major hepatectomies, in elderly patients, and in difficult rectal surgery.
In the case of a staged operative procedure, the standard treatment recommendations in the pertinent literature until now were resection of the primary tumor followed by chemotherapy for 3-6 mo and then liver surgery. Given that liver metastases rather than the primary tumor determine survival the above-mentioned standard approach has some disadvantages. Firstly, chemotherapy which is effective against liver metastases cannot be given during the treatment of the primary tumor, especially if complications of colorectal surgery are encountered as the risk for an anastomotic leak in rectal surgery varies from 6% to 12%[56,57]. Even if the colon surgery runs uneventfully the recommended treatment for locally advanced rectal cancer is a long course of radio-chemotherapy (5 wk).
Surgery is usually planned 6-10 wk after finishing neoadjuvant therapy. During these 3 mo no treatment is given to hepatic metastases and these may progress beyond cure[58]. The second disadvantage is that there rapid growth of metastases described after removal of the primary tumor in several mouse models[59,60]. The underlying mechanism for these experimental data seems to be the loss of primary tumor-induced inhibition of angiogenesis in the metastases. It was also demonstrated in human CRLM that vascular density increased after resection of the primary tumor[61]. However, the clinical significance of these experimental demonstrations is still unknown.
These two major disadvantages gave impetus to a new approach for the treatment of CRLM. This new approach is the “liver first” approach, where, after initial treatment with chemotherapy, the liver metastases were operated first followed by removal of the primary tumor. The new reverse approach includes the risk that during the period between chemotherapy and liver resection the primary tumor might become obstructive. This rare possibility can easily be solved by performing Hartmann’s procedure. There have already been pertinent studies published in the literature showing that the “liver first” approach is a safe procedure and brings excellent results[58,62]. The results of these studies suggest that patients with advanced synchronous CRLM and non-obstructive primary tumors can be safely and effectively treated with highly effective neoadjuvant chemotherapy towards the metastases, followed by liver surgery and, finally, colorectal resection.
CONCLUSION
Many changes have occurred in the treatment of CRLM in the last decade. Progress in neoadjuvant chemotherapy, new targeted agents and improvement of interventional radiology with PVE and RFA as well as diagnostic imaging provide useful tools. However, since surgery is still the only treatment modality that has curative potential on its own, this may appear the most attractive approach in some situations, even if resistance to medical treatment generally means an unfavorable prognosis. The 2-stage hepatectomy as well as the “liver first” approach seem to create new treatment strategies and improve the rate of survival of patients in whom an R0 resection can be achieved with curative intention. The multidisciplinary treatment modality in these complex cases of patients with CRLM helps to make the best treatment selection for patients in order to offer each of them the best strategy.
Footnotes
Peer reviewers: Patricia Sylla, MD, General and Colorectal Surgery, Massachusetts General Hospital, WACC 460, 15 Parkman Street, Boston, MA 02114, United States; Kjetil Soreide, MD, PhD, Associate Professor, Department of Surgery, Stavanger University Hospital, Armauer Hansensvei 20, PO Box 8100, N-4068, Stavanger, Norway
S- Editor Wang YR L- Editor Cant MR E- Editor Lin YP
References
- 1.Boyle P, Leon ME. Epidemiology of colorectal cancer. Br Med Bull. 2002;64:1–25. doi: 10.1093/bmb/64.1.1. [DOI] [PubMed] [Google Scholar]
- 2.Khatri VP, Petrelli NJ, Belghiti J. Extending the frontiers of surgical therapy for hepatic colorectal metastases: is there a limit? J Clin Oncol. 2005;23:8490–8499. doi: 10.1200/JCO.2004.00.6155. [DOI] [PubMed] [Google Scholar]
- 3.Rees M, Tekkis PP, Welsh FK, O'Rourke T, John TG. Evaluation of long-term survival after hepatic resection for metastatic colorectal cancer: a multifactorial model of 929 patients. Ann Surg. 2008;247:125–135. doi: 10.1097/SLA.0b013e31815aa2c2. [DOI] [PubMed] [Google Scholar]
- 4.Choti MA, Sitzmann JV, Tiburi MF, Sumetchotimetha W, Rangsin R, Schulick RD, Lillemoe KD, Yeo CJ, Cameron JL. Trends in long-term survival following liver resection for hepatic colorectal metastases. Ann Surg. 2002;235:759–766. doi: 10.1097/00000658-200206000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, Boni C, Cortes-Funes H, Cervantes A, Freyer G, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18:2938–2947. doi: 10.1200/JCO.2000.18.16.2938. [DOI] [PubMed] [Google Scholar]
- 6.Douillard JY, Cunningham D, Roth AD, Navarro M, James RD, Karasek P, Jandik P, Iveson T, Carmichael J, Alakl M, et al. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet. 2000;355:1041–1047. doi: 10.1016/s0140-6736(00)02034-1. [DOI] [PubMed] [Google Scholar]
- 7.Nordlinger B, Van Cutsem E, Rougier P, Köhne CH, Ychou M, Sobrero A, Adam R, Arvidsson D, Carrato A, Georgoulias V, et al. Does chemotherapy prior to liver resection increase the potential for cure in patients with metastatic colorectal cancer? A report from the European Colorectal Metastases Treatment Group. Eur J Cancer. 2007;43:2037–2045. doi: 10.1016/j.ejca.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 8.Van Cutsem E, Nordlinger B, Adam R, Köhne CH, Pozzo C, Poston G, Ychou M, Rougier P. Towards a pan-European consensus on the treatment of patients with colorectal liver metastases. Eur J Cancer. 2006;42:2212–2221. doi: 10.1016/j.ejca.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 9.Falcone A, Masi G, Allegrini G, Danesi R, Pfanner E, Brunetti IM, Di Paolo A, Cupini S, Del Tacca M, Conte P. Biweekly chemotherapy with oxaliplatin, irinotecan, infusional Fluorouracil, and leucovorin: a pilot study in patients with metastatic colorectal cancer. J Clin Oncol. 2002;20:4006–4014. doi: 10.1200/JCO.2002.12.075. [DOI] [PubMed] [Google Scholar]
- 10.Seium Y, Stupp R, Ruhstaller T, Gervaz P, Mentha G, Philippe M, Allal A, Trembleau C, Bauer J, Morant R, et al. Oxaliplatin combined with irinotecan and 5-fluorouracil/leucovorin (OCFL) in metastatic colorectal cancer: a phase I-II study. Ann Oncol. 2005;16:762–766. doi: 10.1093/annonc/mdi154. [DOI] [PubMed] [Google Scholar]
- 11.Sheikh HY, Valle JW, Palmer K, Sjursen A, Craven O, Wilson G, Swindell R, Saunders MP. Concurrent irinotecan, oxaliplatin and UFT in first-line treatment of metastatic colorectal cancer: a phase I study. Br J Cancer. 2007;96:38–43. doi: 10.1038/sj.bjc.6603521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 13.Hochster HS, Hart LL, Ramanathan RK, Childs BH, Hainsworth JD, Cohn AL, Wong L, Fehrenbacher L, Abubakr Y, Saif MW, et al. Safety and efficacy of oxaliplatin and fluoropyrimidine regimens with or without bevacizumab as first-line treatment of metastatic colorectal cancer: results of the TREE Study. J Clin Oncol. 2008;26:3523–3529. doi: 10.1200/JCO.2007.15.4138. [DOI] [PubMed] [Google Scholar]
- 14.Rubbia-Brandt L, Audard V, Sartoretti P, Roth AD, Brezault C, Le Charpentier M, Dousset B, Morel P, Soubrane O, Chaussade S, et al. Severe hepatic sinusoidal obstruction associated with oxaliplatin-based chemotherapy in patients with metastatic colorectal cancer. Ann Oncol. 2004;15:460–466. doi: 10.1093/annonc/mdh095. [DOI] [PubMed] [Google Scholar]
- 15.Rubbia-Brandt L, Mentha G, Terris B. Sinusoidal obstruction syndrome is a major feature of hepatic lesions associated with oxaliplatin neoadjuvant chemotherapy for liver colorectal metastases. J Am Coll Surg. 2006;202:199–200. doi: 10.1016/j.jamcollsurg.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 16.Parikh AA, Gentner B, Wu TT, Curley SA, Ellis LM, Vauthey JN. Perioperative complications in patients undergoing major liver resection with or without neoadjuvant chemotherapy. J Gastrointest Surg. 2003;7:1082–1088. doi: 10.1016/j.gassur.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 17.Aloia T, Sebagh M, Plasse M, Karam V, Lévi F, Giacchetti S, Azoulay D, Bismuth H, Castaing D, Adam R. Liver histology and surgical outcomes after preoperative chemotherapy with fluorouracil plus oxaliplatin in colorectal cancer liver metastases. J Clin Oncol. 2006;24:4983–4990. doi: 10.1200/JCO.2006.05.8156. [DOI] [PubMed] [Google Scholar]
- 18.Vauthey JN, Pawlik TM, Ribero D, Wu TT, Zorzi D, Hoff PM, Xiong HQ, Eng C, Lauwers GY, Mino-Kenudson M, et al. Chemotherapy regimen predicts steatohepatitis and an increase in 90-day mortality after surgery for hepatic colorectal metastases. J Clin Oncol. 2006;24:2065–2072. doi: 10.1200/JCO.2005.05.3074. [DOI] [PubMed] [Google Scholar]
- 19.Kooby DA, Fong Y, Suriawinata A, Gonen M, Allen PJ, Klimstra DS, DeMatteo RP, D'Angelica M, Blumgart LH, Jarnagin WR. Impact of steatosis on perioperative outcome following hepatic resection. J Gastrointest Surg. 2003;7:1034–1044. doi: 10.1016/j.gassur.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 20.Veteläinen R, van Vliet A, Gouma DJ, van Gulik TM. Steatosis as a risk factor in liver surgery. Ann Surg. 2007;245:20–30. doi: 10.1097/01.sla.0000225113.88433.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karoui M, Penna C, Amin-Hashem M, Mitry E, Benoist S, Franc B, Rougier P, Nordlinger B. Influence of preoperative chemotherapy on the risk of major hepatectomy for colorectal liver metastases. Ann Surg. 2006;243:1–7. doi: 10.1097/01.sla.0000193603.26265.c3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, Bechstein WO, Primrose JN, Walpole ET, Finch-Jones M, et al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet. 2008;371:1007–1016. doi: 10.1016/S0140-6736(08)60455-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gruenberger B, Tamandl D, Schueller J, Scheithauer W, Zielinski C, Herbst F, Gruenberger T. Bevacizumab, capecitabine, and oxaliplatin as neoadjuvant therapy for patients with potentially curable metastatic colorectal cancer. J Clin Oncol. 2008;26:1830–1835. doi: 10.1200/JCO.2007.13.7679. [DOI] [PubMed] [Google Scholar]
- 24.Mahfud M, Breitenstein S, El-Badry AM, Puhan M, Rickenbacher A, Samaras P, Pessaux P, Lopez-Ben S, Jaeck D, Figueras J, et al. Impact of preoperative bevacizumab on complications after resection of colorectal liver metastases: case-matched control study. World J Surg. 2010;34:92–100. doi: 10.1007/s00268-009-0251-8. [DOI] [PubMed] [Google Scholar]
- 25.Nordlinger B, Van Cutsem E, Gruenberger T, Glimelius B, Poston G, Rougier P, Sobrero A, Ychou M. Combination of surgery and chemotherapy and the role of targeted agents in the treatment of patients with colorectal liver metastases: recommendations from an expert panel. Ann Oncol. 2009;20:985–992. doi: 10.1093/annonc/mdn735. [DOI] [PubMed] [Google Scholar]
- 26.Makuuchi M, Thai BL, Takayasu K, Takayama T, Kosuge T, Gunvén P, Yamazaki S, Hasegawa H, Ozaki H. Preoperative portal embolization to increase safety of major hepatectomy for hilar bile duct carcinoma: a preliminary report. Surgery. 1990;107:521–527. [PubMed] [Google Scholar]
- 27.de Baere T, Roche A, Elias D, Lasser P, Lagrange C, Bousson V. Preoperative portal vein embolization for extension of hepatectomy indications. Hepatology. 1996;24:1386–1391. doi: 10.1053/jhep.1996.v24.pm0008938166. [DOI] [PubMed] [Google Scholar]
- 28.Imamura H, Shimada R, Kubota M, Matsuyama Y, Nakayama A, Miyagawa S, Makuuchi M, Kawasaki S. Preoperative portal vein embolization: an audit of 84 patients. Hepatology. 1999;29:1099–1105. doi: 10.1002/hep.510290415. [DOI] [PubMed] [Google Scholar]
- 29.Abdalla EK, Barnett CC, Doherty D, Curley SA, Vauthey JN. Extended hepatectomy in patients with hepatobiliary malignancies with and without preoperative portal vein embolization. Arch Surg. 2002;137:675–680; discussion 680-681. doi: 10.1001/archsurg.137.6.675. [DOI] [PubMed] [Google Scholar]
- 30.Vauthey JN, Chaoui A, Do KA, Bilimoria MM, Fenstermacher MJ, Charnsangavej C, Hicks M, Alsfasser G, Lauwers G, Hawkins IF, et al. Standardized measurement of the future liver remnant prior to extended liver resection: methodology and clinical associations. Surgery. 2000;127:512–519. doi: 10.1067/msy.2000.105294. [DOI] [PubMed] [Google Scholar]
- 31.Berber E, Pelley R, Siperstein AE. Predictors of survival after radiofrequency thermal ablation of colorectal cancer metastases to the liver: a prospective study. J Clin Oncol. 2005;23:1358–1364. doi: 10.1200/JCO.2005.12.039. [DOI] [PubMed] [Google Scholar]
- 32.Terraz S, Constantin C, Majno PE, Spahr L, Mentha G, Becker CD. Image-guided multipolar radiofrequency ablation of liver tumours: initial clinical results. Eur Radiol. 2007;17:2253–2261. doi: 10.1007/s00330-007-0626-x. [DOI] [PubMed] [Google Scholar]
- 33.Mentha G, Majno P, Terraz S, Rubbia-Brandt L, Gervaz P, Andres A, Allal AS, Morel P, Roth AD. Treatment strategies for the management of advanced colorectal liver metastases detected synchronously with the primary tumour. Eur J Surg Oncol. 2007;33 Suppl 2:S76–S83. doi: 10.1016/j.ejso.2007.09.016. [DOI] [PubMed] [Google Scholar]
- 34.Adam R, Avisar E, Ariche A, Giachetti S, Azoulay D, Castaing D, Kunstlinger F, Levi F, Bismuth F. Five-year survival following hepatic resection after neoadjuvant therapy for nonresectable colorectal. Ann Surg Oncol. 2001;8:347–353. doi: 10.1007/s10434-001-0347-3. [DOI] [PubMed] [Google Scholar]
- 35.Bismuth H, Adam R, Lévi F, Farabos C, Waechter F, Castaing D, Majno P, Engerran L. Resection of nonresectable liver metastases from colorectal cancer after neoadjuvant chemotherapy. Ann Surg. 1996;224:509–520; discussion 520-522. doi: 10.1097/00000658-199610000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Figueras J, Torras J, Valls C, Llado L, Ramos E, Marti-Ragué J, Serrano T, Fabregat J. Surgical resection of colorectal liver metastases in patients with expanded indications: a single-center experience with 501 patients. Dis Colon Rectum. 2007;50:478–488. doi: 10.1007/s10350-006-0817-6. [DOI] [PubMed] [Google Scholar]
- 37.Clavien PA, Petrowsky H, DeOliveira ML, Graf R. Strategies for safer liver surgery and partial liver transplantation. N Engl J Med. 2007;356:1545–1559. doi: 10.1056/NEJMra065156. [DOI] [PubMed] [Google Scholar]
- 38.Kokudo N, Tada K, Seki M, Ohta H, Azekura K, Ueno M, Ohta K, Yamaguchi T, Matsubara T, Takahashi T, et al. Proliferative activity of intrahepatic colorectal metastases after preoperative hemihepatic portal vein embolization. Hepatology. 2001;34:267–272. doi: 10.1053/jhep.2001.26513. [DOI] [PubMed] [Google Scholar]
- 39.Bokemeyer C, Bondarenko I, Makhson A, Hartmann JT, Aparicio J, de Braud F, Donea S, Ludwig H, Schuch G, Stroh C, et al. Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2009;27:663–671. doi: 10.1200/JCO.2008.20.8397. [DOI] [PubMed] [Google Scholar]
- 40.Van Cutsem E, Köhne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A, D'Haens G, Pintér T, Lim R, Bodoky G, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408–1417. doi: 10.1056/NEJMoa0805019. [DOI] [PubMed] [Google Scholar]
- 41.Amado RG, Wolf M, Peeters M, Van Cutsem E, Siena S, Freeman DJ, Juan T, Sikorski R, Suggs S, Radinsky R, et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:1626–1634. doi: 10.1200/JCO.2007.14.7116. [DOI] [PubMed] [Google Scholar]
- 42.De Roock W, Piessevaux H, De Schutter J, Janssens M, De Hertogh G, Personeni N, Biesmans B, Van Laethem JL, Peeters M, Humblet Y, et al. KRAS wild-type state predicts survival and is associated to early radiological response in metastatic colorectal cancer treated with cetuximab. Ann Oncol. 2008;19:508–515. doi: 10.1093/annonc/mdm496. [DOI] [PubMed] [Google Scholar]
- 43.Tabernero J, Cervantes A, Ciardiello F, Vega-Villegas E, Vega-Villegas E, Macar-ulla T, Rodriguez-Braun E, Martinelli E, Stroh C, Nippgen J, et al. Correlation of efficacy to KRAS status (wt vs. mut) in patients (pts) with metastatic colorectal cancer (mCRC), treated with weekly (q1w) and q2w schedules of cetuximab combined with FOLFIRI. Proc ASCO GI Cancer Symposium. 2008:A435. [Google Scholar]
- 44.Esteller M, González S, Risques RA, Marcuello E, Mangues R, Germà JR, Herman JG, Capellà G, Peinado MA. K-ras and p16 aberrations confer poor prognosis in human colorectal cancer. J Clin Oncol. 2001;19:299–304. doi: 10.1200/JCO.2001.19.2.299. [DOI] [PubMed] [Google Scholar]
- 45.Hurwitz HI, Yi J, Ince W, Novotny WF, Rosen O. The clinical benefit of bevacizumab in metastatic colorectal cancer is independent of K-ras mutation status: analysis of a phase III study of bevacizumab with chemotherapy in previously untreated metastatic colorectal cancer. Oncologist. 2009;14:22–28. doi: 10.1634/theoncologist.2008-0213. [DOI] [PubMed] [Google Scholar]
- 46.Elias D, Sideris L, Pocard M, Ouellet JF, Boige V, Lasser P, Pignon JP, Ducreux M. Results of R0 resection for colorectal liver metastases associated with extrahepatic disease. Ann Surg Oncol. 2004;11:274–280. doi: 10.1245/aso.2004.03.085. [DOI] [PubMed] [Google Scholar]
- 47.Gruenberger B, Scheithauer W, Punzengruber R, Zielinski C, Tamandl D, Gruenberger T. Importance of response to neoadjuvant chemotherapy in potentially curable colorectal cancer liver metastases. BMC Cancer. 2008;8:120. doi: 10.1186/1471-2407-8-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Elias D, Youssef O, Sideris L, Dromain C, Baton O, Boige V, Ducreux M. Evolution of missing colorectal liver metastases following inductive chemotherapy and hepatectomy. J Surg Oncol. 2004;86:4–9. doi: 10.1002/jso.20039. [DOI] [PubMed] [Google Scholar]
- 49.Benoist S, Brouquet A, Penna C, Julié C, El Hajjam M, Chagnon S, Mitry E, Rougier P, Nordlinger B. Complete response of colorectal liver metastases after chemotherapy: does it mean cure? J Clin Oncol. 2006;24:3939–3945. doi: 10.1200/JCO.2006.05.8727. [DOI] [PubMed] [Google Scholar]
- 50.Nordlinger B, Guiguet M, Vaillant JC, Balladur P, Boudjema K, Bachellier P, Jaeck D. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Association Française de Chirurgie. Cancer. 1996;77:1254–1262. [PubMed] [Google Scholar]
- 51.Bolton JS, Fuhrman GM. Survival after resection of multiple bilobar hepatic metastases from colorectal carcinoma. Ann Surg. 2000;231:743–751. doi: 10.1097/00000658-200005000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weber JC, Bachellier P, Oussoultzoglou E, Jaeck D. Simultaneous resection of colorectal primary tumour and synchronous liver metastases. Br J Surg. 2003;90:956–962. doi: 10.1002/bjs.4132. [DOI] [PubMed] [Google Scholar]
- 53.Chua HK, Sondenaa K, Tsiotos GG, Larson DR, Wolff BG, Nagorney DM. Concurrent vs. staged colectomy and hepatectomy for primary colorectal cancer with synchronous hepatic metastases. Dis Colon Rectum. 2004;47:1310–1316. doi: 10.1007/s10350-004-0586-z. [DOI] [PubMed] [Google Scholar]
- 54.Tanaka K, Shimada H, Matsuo K, Nagano Y, Endo I, Sekido H, Togo S. Outcome after simultaneous colorectal and hepatic resection for colorectal cancer with synchronous metastases. Surgery. 2004;136:650–659. doi: 10.1016/j.surg.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 55.Minagawa M, Yamamoto J, Miwa S, Sakamoto Y, Kokudo N, Kosuge T, Miyagawa S, Makuuchi M. Selection criteria for simultaneous resection in patients with synchronous liver metastasis. Arch Surg. 2006;141:1006–1012; discussion 1013. doi: 10.1001/archsurg.141.10.1006. [DOI] [PubMed] [Google Scholar]
- 56.Chiappa A, Biffi R, Bertani E, Zbar AP, Pace U, Crotti C, Biella F, Viale G, Orecchia R, Pruneri G, et al. Surgical outcomes after total mesorectal excision for rectal cancer. J Surg Oncol. 2006;94:182–193; discussion 181. doi: 10.1002/jso.20518. [DOI] [PubMed] [Google Scholar]
- 57.Tran CL, Udani S, Holt A, Arnell T, Kumar R, Stamos MJ. Evaluation of safety of increased time interval between chemoradiation and resection for rectal cancer. Am J Surg. 2006;192:873–877. doi: 10.1016/j.amjsurg.2006.08.061. [DOI] [PubMed] [Google Scholar]
- 58.Verhoef C, van der Pool AE, Nuyttens JJ, Planting AS, Eggermont AM, de Wilt JH. The "liver-first approach" for patients with locally advanced rectal cancer and synchronous liver metastases. Dis Colon Rectum. 2009;52:23–30. doi: 10.1007/DCR.0b013e318197939a. [DOI] [PubMed] [Google Scholar]
- 59.O'Reilly MS, Holmgren L, Shing Y, Chen C, Rosenthal RA, Moses M, Lane WS, Cao Y, Sage EH, Folkman J. Angiostatin: a novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. Cell. 1994;79:315–328. doi: 10.1016/0092-8674(94)90200-3. [DOI] [PubMed] [Google Scholar]
- 60.O'Reilly MS, Holmgren L, Chen C, Folkman J. Angiostatin induces and sustains dormancy of human primary tumors in mice. Nat Med. 1996;2:689–692. doi: 10.1038/nm0696-689. [DOI] [PubMed] [Google Scholar]
- 61.Peeters CF, de Waal RM, Wobbes T, Westphal JR, Ruers TJ. Outgrowth of human liver metastases after resection of the primary colorectal tumor: a shift in the balance between apoptosis and proliferation. Int J Cancer. 2006;119:1249–1253. doi: 10.1002/ijc.21928. [DOI] [PubMed] [Google Scholar]
- 62.Mentha G, Roth AD, Terraz S, Giostra E, Gervaz P, Andres A, Morel P, Rubbia-Brandt L, Majno PE. 'Liver first' approach in the treatment of colorectal cancer with synchronous liver metastases. Dig Surg. 2008;25:430–435. doi: 10.1159/000184734. [DOI] [PubMed] [Google Scholar]


