Abstract
The overall survival for patients with advanced hepatocellular carcinoma (HCC) is still limited. Although the multi-kinase inhibitor sorafenib has recently been approved for this disease, response rates are still low and patients often face dose-limiting toxicities which lead to a reduction in prognosis and treatment success. We here report a patient with metastasized HCC who shows a sustained response for more than 30 mo to sorafenib therapy after failure of a first line therapy with gemcitabine, oxaliplatin and bevacizumab.
Keywords: Hepatocellular carcinoma, Sorafenib, Bevacizumab, Angiogenesis
INTRODUCTION
Hepatocellular carcinoma (HCC) belongs to the most common malignancies worldwide and shows a rising incidence in Western countries due to high prevalence of chronic viral hepatitis, alcoholic liver damage and metabolic disorders associated with fatty liver degeneration[1-3]. Surgical and loco-regional approaches provide satisfying results for patient with early stage HCC, while advanced diseases with underlying cirrhosis have an unfavourable prognosis[4].
Angiogenesis and neo-vascularization are considered “hallmarks” of malignant tumors and several strategies have been established to interfere with the underlying signalling cascade[5], e.g. the anti-vascular endothelial growth factor (VEGF) antibody bevacizumab or the small molecule tyrosine kinase inhibitor sorafenib. HCC represents a highly vascularised tumor[6] and various anti-angiogenic treatment strategies have therefore been applied to this disease[7]. So far, only the multi-kinase inhibitor sorafenib provided a significant patient benefit in this setting and has therefore been approved as the first-line therapy for advanced HCC in patients with good metabolic liver capacity (Child-Pugh A)[8,9]. Although sorafenib showed a good tolerability in the study populations, most patients in clinical practice suffer from underlying liver cirrhosis with impaired metabolic function and experience dose-limiting toxicities with the need to reduce the overall dose of sorafenib subsequently[10].
We herein report a case of advanced metastatic HCC which was treated with a reduced dose of sorafenib for more than 30 mo after failure of a first-line therapy with gemcitabine, oxaliplatine and bevacizumab and intermittent radiation therapy.
CASE REPORT
In January 2007, a 56-year-old man presented in our hospital for clinical diagnosis of elevated liver enzymes with suspicion of ethyltoxic liver cirrhosis due to regular alcohol consumption of 60-80 g/d. Blood tests and ultrasonic investigation confirmed liver cirrhosis of Child-Pugh B (score 7) with elevated bilirubin (2.3 mg/dL) and acquired coagulation deficiency (INT 1.39). Ascites or hepatic encephalopathy were not present. Other aetiologies, e.g. chronic viral or autoimmune hepatitis, haemochromatosis or α1-antitrypsin deficiency were excluded by corresponding blood tests. Moreover, multilocular (n = 6), extended lesions (diameter > 1 cm) were detected by contrast enhanced ultrasound and magnetic resonance imaging scan (largest lesions in segments II: 1.2 cm × 1.5 cm; IVa: 4.9 cm × 3.7 cm × 5.6 cm; VIII: 1.9 cm × 2.9 cm). Portal vein thrombosis or invasion were not identified. High levels of α-fetoprotein (1900 ng/mL) substantiated the diagnosis of HCC according to AASLD guidelines[4,11]. Biopsy proved a well differentiated HCC (Figure 1).
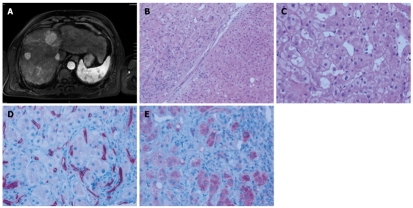
Figure 1.
Baseline radiologic and pathologic assessment of the patient in late 2006 before initiation of therapy. A: Initial magnetic resonance imaging (MRI) scan revealing multiple hepatocellular carcinoma (HCC) nodules; B: Regenerative nodule (lower right) juxtaposed to well circumscribed HCC (upper left) separated by a fibrous septum with a sparse lymphocytic infiltrate (magnification, × 100); C: Representative high power magnification of the HCC showed large tumor cells with occasional vacuoles and microvesicular fatty degeneration of hepatocytes (magnification, × 200); D: Abnormally increased microvessel density within the HCC as demonstrated by CD34 immunohistochemistry (magnification, × 200); E: Tumor cells express VEGFR1 at the tumor front (right) contrasting with weak expression towards to the tumor centre (left; magnification, × 200).
For treatment of multifocal HCC, based on AASLD practice guidelines, neither systemic chemotherapy nor therapy options for a better overall survival were established in spring 2007. Locoregional methods like high frequency thermotherapy (HFTT) or radiofrequency ablation as well as transarterial chemoembolization could not be applied in this patient due to the multifocal disease[12]. It was not possible to perform selective internal radiotherapy (SIRT) representing an experimental therapy option in multilocular hepatic cancer[13]. Extrahepatic tumor manifestation was detected by positron emission tomography/computed tomography (CT) presenting a single thoracic metastasis of the 2nd rib at the right side of 8.8 cm × 7.7 cm. Consequently, SIRT was not performed. No histological analysis of this lesion was obtained as osseous metastases are typical extrahepatic manifestations of advanced HCC[14-16]. According to the Barcelona Clinic Liver Cancer staging system, our patient was classified as stage C (advanced stage) with good ECOG performance (status 1-2)[17]. We therefore considered the patient for new agents or randomized controlled trials[6].
Off-label use of systemic drug therapy seemed to offer the best option to sustain this patient at this time. A review of the literature available in January 2007 disclosed a phase II trial with moderate response rates in palliative HCC treatment by a combination of gemcitabine, oxaliplatin (GemOx) and the angiogenesis inhibitor bevacizumab[18]. After informed consent of the patient, an individualized chemotherapy with this regimen was started in May 2007. The patient received 10 mg/kg bevacizumab on day 1 together with 1000 mg/m2 gemcitabine followed by oxaliplatin at 85 mg/m2 on day 2. First staging was performed after four cycles (14 d/cycle) resulting in a moderate tumor progression on thoracic CT scan and ultrasound but showed a good overall tolerability and stable primary tumors in the liver. Therapy was continued based on a good overall tolerability, the strong wish of the patient for continuing and the fact that no other therapy option was available at this time. The ongoing systemic chemotherapy induced a moderate pancytopenia, slight renal dysfunction and weight loss (8 kg in 8 mo). Yet, discontinuation of the therapy regimen was not necessary until 2nd staging in September 2007. Here, further progression of the thoracic metastasis with stable disease of hepatic lesions was demonstrated by CT and ultrasound scans (Figure 2).
Figure 2.
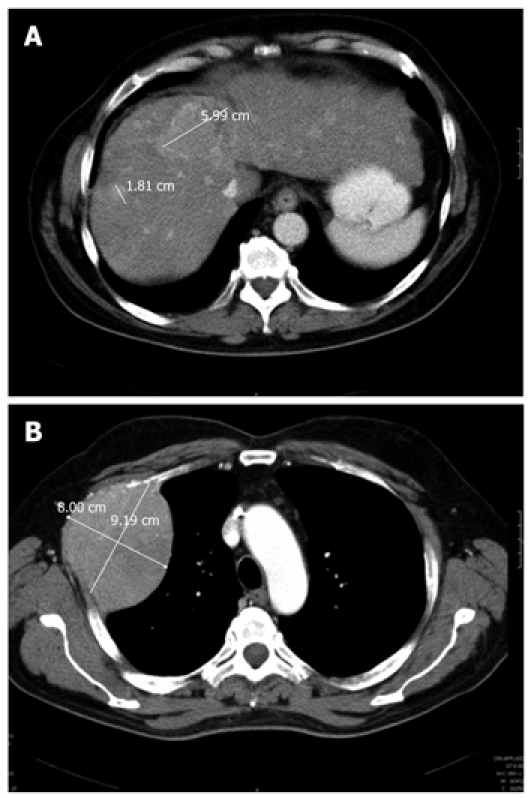
Radiologic assessment of the primary liver tumors and the thoracic metastasis before initiation of sorafenib therapy in September 2007. A: Multiple hepatocellular carcinoma lesions (maximum diameter 6 cm) in computed tomography scan of the liver; B: Thoracic metastasis of the 2nd rib (maximum diameter 8.00 cm × 9.19 cm).
At this time, a significant benefit for advanced HCC was proven in a randomized controlled phase III trial using the novel raf-kinase inhibitor sorafenib as a single agent[8] which lead to the approval of sorafenib for the first-line therapy of advanced HCC in December 2008[9]. Treatment was started in October 2007 with the recommended dose of 800 mg/d. Known side effects (e.g. diarrhea, hand-foot-reaction, oral ulceration) developed during the first 2 wk of treatment and were handled symptomatically (e.g. loperamide, oily creams, mucositis solutions) and with a concomitant dose-reduction of sorafenib to 400 mg/d. Staging after 12 mo revealed an extraordinary regression of both hepatic and thoracic tumor masses (Figure 3). To avoid potential tumor progression by drug resistance, the patient underwent local radiation of the osseous metastases with a total of 66 Gy in November 2008. In March 2009 and 18 mo after initiation of sorafenib therapy, a single remaining calcifying lesion of hepatic segment IVa was ablated by ultrasound-controlled HFTT (Figure 4). Facial and cleavage erythema, dry cough and hoarseness remained under continued sorafenib therapy, but were at a tolerable level. Liver function was stabilized at Child-Pugh A after treatment of a temporarily acquired coagulation deficiency with vitamin K. Now, 36 mo after first diagnosis of advanced HCC, the patient still consults our outpatient ward for regular surveillance in excellent general condition.
Figure 3.
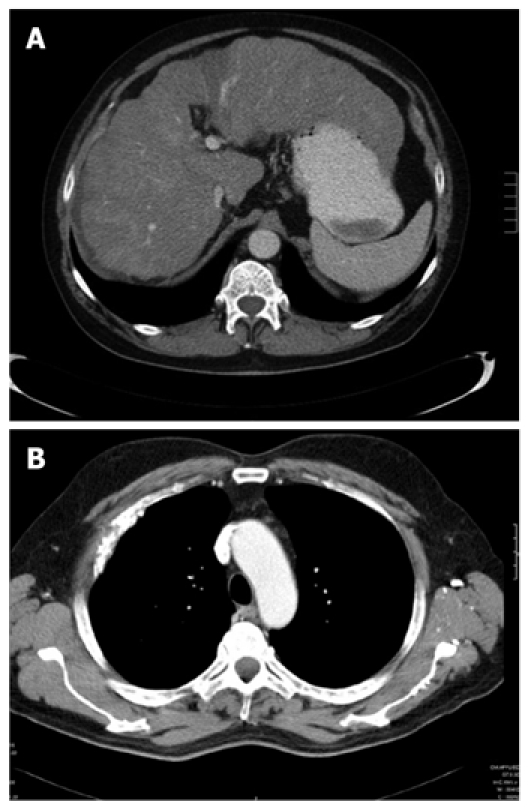
Computed tomography scans of liver (A) and thorax (B) taken in September 2008, 12 mo after initiation of sorafenib therapy showing a dramatic regression of previously described tumor lesions.
Figure 4.
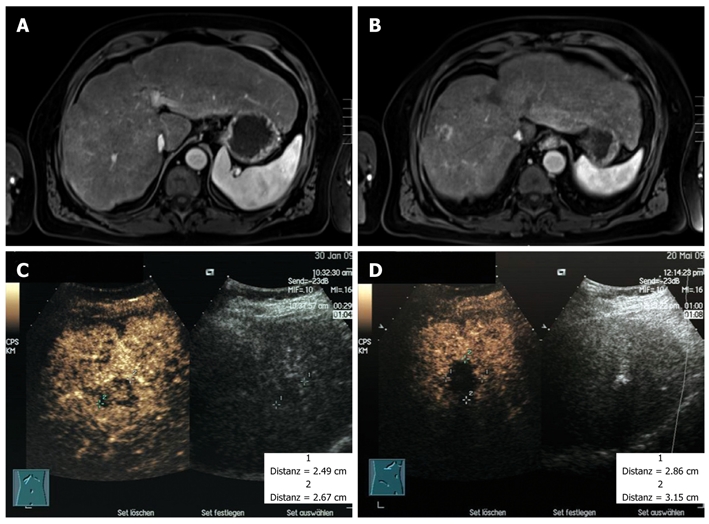
Magnetic resonance imaging scans of the liver in spring 2009, 18 mo after initiation of sorafenib therapy (A, B) and the contrast-enhanced ultrasound investigation of a single calcifying lesion of segment IVa before (C) and after (D) ablation by ultrasound controlled high frequency thermotherapy in spring 2009.
Histological aspects
Histological examination of both the primary biopsy and the biopsy material following treatment revealed essentially similar findings (Figure 1). The liver parenchyma showed micronodular cirrhosis with moderate steatosis without significant necroinflammatory activity. The tumor was composed of large polyhedral tumor cells with centrally located vesicular nuclei and prominent nuclei, arranged in trabeculae and pseudo-acinar structures. The cytoplasm was eosinophilic to pale or clear. Immunohistochemistry revealed a prominent vascularisation. In addition, tumor cells at the periphery expressed the VEGFR1, but were negative for VEGFR2/3. The proliferative index (Ki67) was well below 5%. The cytological features were consistent with a well differentiated HCC in a background of cirrhosis.
DISCUSSION
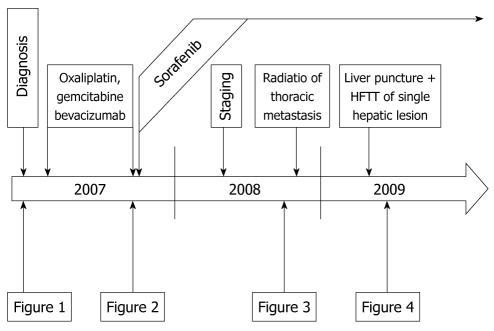
We here report the extraordinary success in treating metastatic HCC by a sequential therapy with gemcitabine, oxaliplatin, bevacizumab, internal radiation and treatment with the novel multi-kinase inhibitor sorafenib in a single patient with a survival time of more than 40 mo since the initial time of diagnosis and more than 30 mo since beginning sorafenib therapy (Figure 5).
Figure 5.
Schematic course of the disease from diagnosis in early 2007 until late 2009 indicating the time points of therapy and staging examinations. Representative examples are shown in Figures 1 to 4 as indicated here. HFTT: High frequency thermotherapy.
Although sorafenib was established as first-line therapy for advanced HCC and approximately 70% of patients showed a stable disease in the initial trials[8,9], the overall response rate is below 3% and it does not therefore provide a significant survival benefit compared to other recently established experimental therapies[7]. Interestingly, the initial report on GemOx and bevacizumab in advanced HCC showed an overall response rate of 20% as well as progression free survival and overall survival rates comparable to the SHARP trial[7,9,18]. The tumor of our patient was well differentiated which indicates a good metabolic and detoxifying capacity of the tumor cells. In addition, the proliferation index was below 5% which indicates that drugs with a strong S-phase effect like pyrimidine-analogues are ineffective in this case.
Several recent studies highlighted the importance of anti-angiogenesis in HCC[19]. As angiogenesis-inhibitory effects represent the main mechanisms of action for both sorafenib and bevacizumab, additive effects are therefore conceivable. This concept has been proven successfully by Azad et al[20] in a phase I trial with advanced solid tumors and in a phase II trial in epithelial ovarian cancer[21], although enhanced toxicities were observed. In contrast to this study where both agents were applied simultaneously, we used a sequential approach of GemOx and bevacizumab before applying sorafenib, which may explain the good tolerability of this regime in our case. We started sorafenib therapy 26 d after the last application of bevacizumab, which is still in the range of bevacizumab’s half-life in humans (approx 20 d, range 11-50 d)[22,23].
Preclinical data suggest that radiation therapy ameliorates the effect of anti-angiogenic therapeutics in rectal cancer, non-small cell lung cancer or malignant glioma[24]. Several mechanisms are discussed for this effect, e.g. increasing vascular permeability with improved delivery of cytotoxic agents to the tumor, and further investigations on determining the optimal biological dose as well as drug sequencing are therefore urgently needed. Yet, several studies showed that radiation therapy can sensitize human tumors, including HCC, for sequential sorafenib therapy[25-27].
Our patient experienced a rapid need for dose reduction of sorafenib which was accompanied by a good response of the tumor lesions. As dose reductions for sorafenib are usually not associated with the here observed rapid response, we conclude that the combination therapy and not sorafenib monotherapy is responsible for the observed therapeutic effect.
Based on these findings from the literature and our own experience, we assume that the sequential therapy with bevacizumab and sorafenib inhibits angiogenetic signalling pathways from both upstream at the receptor level and downstream at the level of signalling kinases and that this dual effect is further supported by the influence of radiation therapy. This strategy should therefore be validated in a larger series of patients with advanced HCC and other solid malignancies.
Acknowledgments
We thank Dr. Ernst Fellner, Bad Windsheim, Germany, for providing CT scans and radiologic assessments shown in Figures 2 and 3.
Footnotes
Peer reviewers: Chih-Chi Wang, MD, Department of Surgery, Chang Gung Memorial Hospital-Kaohsiung Medical Center, 123 Ta-Pei Road, Niao-Sung, Kaohsiung 833, Taiwan, China; Hitoshi Yoshiji, MD, PhD, Third Department of Internal Medicine, Nara Medical University, 840 Shijo-cho, Kashihara, Nara 634-8522, Japan
S- Editor Wang JL L- Editor O’Neill M E- Editor Zheng XM
References
- 1.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 2.Bugianesi E. Non-alcoholic steatohepatitis and cancer. Clin Liver Dis. 2007;11:191–207, x-xi. doi: 10.1016/j.cld.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 3.El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340:745–750. doi: 10.1056/NEJM199903113401001. [DOI] [PubMed] [Google Scholar]
- 4.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 5.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 6.Okamoto K, Neureiter D, Ocker M. Biomarkers for novel targeted therapies of hepatocellular carcinoma. Histol Histopathol. 2009;24:493–502. doi: 10.14670/HH-24.493. [DOI] [PubMed] [Google Scholar]
- 7.Greten TF, Korangy F, Manns MP, Malek NP. Molecular therapy for the treatment of hepatocellular carcinoma. Br J Cancer. 2009;100:19–23. doi: 10.1038/sj.bjc.6604784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abou-Alfa GK, Schwartz L, Ricci S, Amadori D, Santoro A, Figer A, De Greve J, Douillard JY, Lathia C, Schwartz B, et al. Phase II study of sorafenib in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2006;24:4293–4300. doi: 10.1200/JCO.2005.01.3441. [DOI] [PubMed] [Google Scholar]
- 9.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 10.Wörns MA, Weinmann A, Pfingst K, Schulte-Sasse C, Messow CM, Schulze-Bergkamen H, Teufel A, Schuchmann M, Kanzler S, Düber C, et al. Safety and efficacy of sorafenib in patients with advanced hepatocellular carcinoma in consideration of concomitant stage of liver cirrhosis. J Clin Gastroenterol. 2009;43:489–495. doi: 10.1097/MCG.0b013e31818ddfc6. [DOI] [PubMed] [Google Scholar]
- 11.Zhou L, Liu J, Luo F. Serum tumor markers for detection of hepatocellular carcinoma. World J Gastroenterol. 2006;12:1175–1181. doi: 10.3748/wjg.v12.i8.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lopez RR Jr, Pan SH, Hoffman AL, Ramirez C, Rojter SE, Ramos H, McMonigle M, Lois J. Comparison of transarterial chemoembolization in patients with unresectable, diffuse vs focal hepatocellular carcinoma. Arch Surg. 2002;137:653–657; discussion 657-658. doi: 10.1001/archsurg.137.6.653. [DOI] [PubMed] [Google Scholar]
- 13.Khodjibekova M, Szyszko T, Khan S, Nijran K, Tait P, Al-Nahhas A. Selective internal radiation therapy with Yttrium-90 for unresectable liver tumours. Rev Recent Clin Trials. 2007;2:212–216. doi: 10.2174/157488707781662742. [DOI] [PubMed] [Google Scholar]
- 14.Verslype C, Van Cutsem E, Dicato M, Arber N, Berlin JD, Cunningham D, De Gramont A, Diaz-Rubio E, Ducreux M, Gruenberger T, et al. The management of hepatocellular carcinoma. Current expert opinion and recommendations derived from the 10th World Congress on Gastrointestinal Cancer, Barcelona, 2008. Ann Oncol. 2009;20 Suppl 7:vii1–vii6. doi: 10.1093/annonc/mdp281. [DOI] [PubMed] [Google Scholar]
- 15.Katyal S, Oliver JH 3rd, Peterson MS, Ferris JV, Carr BS, Baron RL. Extrahepatic metastases of hepatocellular carcinoma. Radiology. 2000;216:698–703. doi: 10.1148/radiology.216.3.r00se24698. [DOI] [PubMed] [Google Scholar]
- 16.Talwalkar JA, Gores GJ. Diagnosis and staging of hepatocellular carcinoma. Gastroenterology. 2004;127:S126–S132. doi: 10.1053/j.gastro.2004.09.026. [DOI] [PubMed] [Google Scholar]
- 17.Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329–338. doi: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]
- 18.Zhu AX, Blaszkowsky LS, Ryan DP, Clark JW, Muzikansky A, Horgan K, Sheehan S, Hale KE, Enzinger PC, Bhargava P, et al. Phase II study of gemcitabine and oxaliplatin in combination with bevacizumab in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2006;24:1898–1903. doi: 10.1200/JCO.2005.04.9130. [DOI] [PubMed] [Google Scholar]
- 19.Finn RS, Zhu AX. Targeting angiogenesis in hepatocellular carcinoma: focus on VEGF and bevacizumab. Expert Rev Anticancer Ther. 2009;9:503–509. doi: 10.1586/era.09.6. [DOI] [PubMed] [Google Scholar]
- 20.Azad NS, Posadas EM, Kwitkowski VE, Steinberg SM, Jain L, Annunziata CM, Minasian L, Sarosy G, Kotz HL, Premkumar A, et al. Combination targeted therapy with sorafenib and bevacizumab results in enhanced toxicity and antitumor activity. J Clin Oncol. 2008;26:3709–3714. doi: 10.1200/JCO.2007.10.8332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee JM, Sarosy GA, Annunziata CM, Azad N, Minasian L, Kotz H, Squires J, Houston N, Kohn EC. Combination therapy: intermittent sorafenib with bevacizumab yields activity and decreased toxicity. Br J Cancer. 2010;102:495–499. doi: 10.1038/sj.bjc.6605514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu JF, Bruno R, Eppler S, Novotny W, Lum B, Gaudreault J. Clinical pharmacokinetics of bevacizumab in patients with solid tumors. Cancer Chemother Pharmacol. 2008;62:779–786. doi: 10.1007/s00280-007-0664-8. [DOI] [PubMed] [Google Scholar]
- 23.Shih T, Lindley C. Bevacizumab: an angiogenesis inhibitor for the treatment of solid malignancies. Clin Ther. 2006;28:1779–1802. doi: 10.1016/j.clinthera.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 24.Senan S, Smit EF. Design of clinical trials of radiation combined with antiangiogenic therapy. Oncologist. 2007;12:465–477. doi: 10.1634/theoncologist.12-4-465. [DOI] [PubMed] [Google Scholar]
- 25.Hsieh CH, Jeng KS, Lin CC, Chen CK, Liu CY, Lin CP, Tai HC, Wang CH, Shueng PW, Chen YJ. Combination of sorafenib and intensity modulated radiotherapy for unresectable hepatocellular carcinoma. Clin Drug Investig. 2009;29:65–71. doi: 10.2165/0044011-200929010-00007. [DOI] [PubMed] [Google Scholar]
- 26.Kasibhatla M, Steinberg P, Meyer J, Ernstoff MS, George DJ. Radiation therapy and sorafenib: clinical data and rationale for the combination in metastatic renal cell carcinoma. Clin Genitourin Cancer. 2007;5:291–294. doi: 10.3816/CGC.2007.n.007. [DOI] [PubMed] [Google Scholar]
- 27.Gay HA, Cavalieri R, Allison RR, Finley J, Quan WD Jr. Complete response in a cutaneous facial metastatic nodule from renal cell carcinoma after hypofractionated radiotherapy. Dermatol Online J. 2007;13:6. [PubMed] [Google Scholar]