Abstract
PURPOSE
Heat shock protein 90 inhibition affects the Raf-kinase signaling pathway and could enhance anti-tumor effects of sorafenib, a Raf-kinase inhibitor. The combination of sorafenib and tanespimycin (17-AAG/17-allyl-amino-geldanamycin; NSC # 330507/KOS-953) was evaluated in a phase I trial with the primary objective of defining a phase II dose
PATIENTS AND METHODS
The dose cohorts consisted of fixed continuous oral dosing of sorafenib 400 mg twice daily, starting 14 days prior to tanespimycin which was administered intravenously, at escalating doses, (starting at 300 mg/m,2 with 50 mg/m2 increments), on days 1, 8 and 15, in a 28-day cycle. Toxicity was assessed weekly; response was evaluated every 2 cycles.
RESULTS
Twenty-seven toxicity-evaluable patients were enrolled and treated at four dose levels. Predominant primary malignancies were: renal cancer (12), melanoma (6) and colorectal cancer (4). Dose-limiting toxicities of grade 4 transaminitis and grade 3 hand-foot syndrome in 1 patient each were observed at 450 mg/m2 of tanespimycin. 114 cycles were administered with a median of four cycles (range 1–17 cycles). Plasma concentrations of sorafenib and metabolites reached steady-state after 7 days. Tanespimycin did not alter sorafenib concentrations. Pharmacodynamics showed a decrease in Hsp90 levels and induction of Hsp70. Clinical efficacy was observed in 9 of 12 renal cancer patients and 4 of 6 melanoma patients
CONCLUSIONS
Recommended phase II doses of this combination are sorafenib 400 mg twice daily and tanespimycin 400 mg/m2 on days 1, 8 and 15 every 28 days. Clinical and pharmacodynamic activity was observed in kidney cancer and melanoma.
Keywords: Heat shock protein inhibitors, sorafenib, 17-AAG, tanespimycin, KOS-953, renal cancer, melanoma, phase I clinical trial
INTRODUCTION
Sorafenib is a Raf kinase inhibitor that has proven clinical efficacy in advanced renal and hepatocellular cancer. It is currently approved by the Food and Drug Administration for use in those malignancies1,2. B-Raf and C-Raf (wild-type and mutant) are involved in angiogenic development and are both inhibited by sorafenib3. Activating mutations of B-Raf are found in about 70% of melanoma cell lines and in other common solid tumor cell lines, such as those of breast cancer and lung cancer4.
Heat shock protein 90 (Hsp90) is a chaperone protein integral to maintaining the proper configuration of important cellular signaling proteins5. Raf-1 kinase is one of the client proteins of Hsp 906. Other important client proteins include Akt kinase, Bcr-Abl kinase, CDK4, HER2 and HIF-1alpha.5 Inhibition of Hsp90 causes abnormal folding of its client proteins resulting in their degradation by the ubiquitin proteasome pathway.5–7 17-demethoxy-allylaminogeldanamycin/17-AAG (tanespimycin) is a benzoquinone ansamycin antibiotic with antiproliferative activity related to Hsp90 inhibition8. Tanespimycin acts by binding to the hydrophobic ATP/ADP-binding site of Hsp90. 9 Tumor Hsp90 is present in multi-chaperone complexes with high ATPase activity, and possesses 100-fold higher binding affinity for tanespimycin, which provides a potential tumor-selective effect.9 In a stressful cellular environment, as may exist in tumors due to the continued production of proteins necessary to sustain proliferation, Hsp90 accumulates and has an essential role in maintaining oncogene function and activation.10 The role of Hsp90 inhibition could also be important in augmenting the effects of other anti-cancer therapies that induce tumor cell stress11. Previous in vitro studies exploiting Hsp90 inhibition in combination with Raf-kinase inhibition12 reported cytotoxic synergy with regards to mitochondrial injury and apoptosis induction between tanespimycin and UCN-01, a protein kinase C, and a Raf-kinase inhibitor in leukemia cells. Decreased Akt-activation and marked downregulation of C-Raf, Mek-1 and 2, and MAP-kinases were noted with the combination therapy, compared to either agent alone. Raf-1 kinase inhibition antagonizes the activation of the nuclear factor kappa B (NF-kB) transcription factor signaling pathway13 and also abrogates the stimulation of NF-kB by tumor necrosis factor alpha and interleukin-1 beta, thereby affecting the progression and proliferation of tumor cells13.
The proposed rationale for evaluating tanespimycin and sorafenib in combination is that the raf-kinase inhibition resulting from sorafenib is likely to be increased with the modulation of Hsp90 and could result in enhanced degradation of certain Raf isoforms14.Tanespimycin can also affect a number of other Hsp90 client proteins that are critical to cellular function. Independent of Raf pathway modulation, sorafenib is known to inhibit VEGF-R kinase action directly, and 17-AAG has also demonstrated VEGF-related signaling blockade15. The combination of sorafenib and 17AAG could potentially act at both distinct and related pathways to affect cancer cell proliferation and angiogenesis.
Patients and Methods
This clinical trial was conducted at Wayne State University, and the University of Maryland. The protocol and consent were reviewed and approved by the Human Investigation Committee of each participating institution. Patients had to have a metastatic or unresectable histologically confirmed solid tumor malignancy with radiologic and/or clinical progression. Patients signed a written, informed consent prior to enrollment. Patients allergic to eggs were excluded because 17- AAG is formulated in egg phospholipids. Performance status per ECOG score was required to be ≤ 2 with minimum life expectancy of 12 weeks. Patients had to have acceptable hepatic, renal, and bone marrow function.
Patients with known brain metastases or those taking oral anticoagulation with warfarin were excluded. Patients with prolonged QT interval (> 450 milliseconds in males and > 470 milliseconds in females) were ineligible. 2D ECHO or MUGA scan and pulmonary function tests had to demonstrate minimum ejection fraction of 40% and DLCO ≥ 60% respectively.
Treatment Plan
Therapy was started with a fixed oral sorafenib dose of 400 mg twice daily for the first 14 days (cycle 1). Sorafenib was administered with at least 250 mL of water without regard to meals but preferably with a low to moderate fat meal. Subsequently tanespimycin was started as an intravenous infusion, at escalating dose levels. starting at 300 mg/m,2 with 50 mg/m2 increments in each dose level cohort. Tanespimycin was administered on days 1, 8, and 15 of each 28-day cycle. Sorafenib was continued at the same dose daily continuously without interruption unless dose modification was required.
Evaluation
Primary Objective
The primary objective was to determine the recommended phase II dose of the combination. The maximally administered dose (MAD) was the dose at which at least 33% of patients experienced dose-limiting toxicity (DLT), and was one dose level higher than the recommended phase II dose. DLT was defined as: ANC ≤ 500/µL; platelets ≤ 25,000/µL; drug-related non-hematologic grade 3 or 4 toxicity (despite appropriate prophylactic or therapeutic measures being administered); inability to tolerate one 4-week dosing course due to toxicity; or any drug-related adverse event resulting in a treatment interruption of ≥ 14 days. Toxicity was assessed weekly during the first 6 weeks of therapy and then on the days of tanespimycin administration. The National Cancer Institute (NCI) Common Terminology Criteria for Adverse Event reporting, version 3.0, were used for toxicity grading. Response was evaluated initially after 6 weeks of therapy, and at subsequent 8-week intervals. Response, progression and stable disease were defined per the RECIST criteria. All patients who demonstrated either response or stable disease were defined as having clinical benefit (CB).
Pharmacokinetic (PK) Sampling
Sorafenib trough concentrations (at least 10 hours from the previous sorafenib dose) were obtained pre-therapy and after 7 days of sorafenib administration. On day 14 of sorafenib alone, blood samples were collected in heparinized tubes at the following time points: cycle 1; pre-dose, and at 0.5, 1, 2, 3, 4, 6, 9, and 12 hours after the administration of the first dose of sorafenib. Samples were collected pre-dose, and 0.5, 1, 2, 3, 4, 6, 9, and 12 hours after the start of the tanespimycin infusion.
Blood samples were centrifuged at ambient temperature at 2000×g for 10 minutes, and plasma was collected and stored at −80°C until analysis. Sorafenib plasma concentrations were determined by Bayer Pharmaceuticals (Hamden CT), using a validated liquid chromatography tandem mass spectrometry (LC-MS/MS) method with ([2H3, 15N] sorafenib) as the internal standard.17 The linear calibration curve was constructed over the sorafenib concentration range of 5 to 2,000 ng/mL. The intra- and inter-day precision and accuracy were within 15% of deviations. tanespimycin plasma concentrations were determined using a validated high-performance liquid chromatography method, as reported previously.18
PK parameters for sorafenib and tanespimycin in individual patients were estimated using noncompartmental analysis with the computer software program WinNonlin version 5 (Pharsight Corporation, Mountain View, CA). The maximum plasma concentration (Cmax), the time(s) of occurrence for maximum concentration (Tmax), and pre-dosing trough plasma concentration (Cmin) were obtained by visual inspection of the plasma concentration versus time curves. The total area under the plasma concentration versus time curve from time zero to the last sampling time point (AUC0-t) was calculated using the linear and logarithmic trapezoidal method for ascending and descending plasma concentrations, respectively. The total area under the plasma concentration versus time curve from time zero to infinity (AUC0-∝) was calculated as the sum of AUC0-t and the extrapolated area, which was calculated by dividing the last observed plasma concentration by the terminal rate constant (λz), where λz was estimated from the terminal log-linear phase of the plasma concentration versus time curve. Terminal plasma half-life (t1/2) was calculated as 0.693/λz. Clearance (CL) was calculated as Dose/AUC0-∞.
Pharmacodynamic (PD) Sample Collection and Processing
Blood samples were collected in two BD Vacutainer CPT™ tubes with sodium heparin before therapy, 7 days after beginning sorafenib and 72 hours after the first and third tanespimycin administrations (days 1 and 15 of cycle 2). Stability studies have shown that such samples are stable for 24 hours at room temperature. The blood was centrifuged within 2 hours at room temperature and at 1800×g for 15 minutes following the manufacturer’s instructions. The plasma layer was carefully removed without disturbing the peripheral blood mononuclear cells (PBMC). The PBMC of each patient were pooled and washed twice, first with ice-cold phosphate-buffered saline (PBS) followed by Bio-Plex™ (BioRad, Hercules, CA) cell wash buffer before being subjected to lysis using 75µl Bio-Plex™Cell Lysis buffer. Lysates were processed following the Bio-Plex™Cell Lysis Kit protocol and stored at −80°C until they were used for Western blotting. The Bio-Plex™ lysis buffer is formulated to stabilize phosphoproteins. Just prior to cell lysis, a mixture of protease inhibitors and phosphatase inhibitors were added.
Western Blotting
PBMC were analyzed for Hsp90, Hsp70 (monocloncal antibodies from Assay Designs, Ann Arbor, MI); CDK4, pAKT, pERK, c-Raf (antibodies from Cell Signaling Technologies, Boston, MA)) and beta-actin (antibodies from Sigma, St. Louis, MO) expression. We loaded either 25 or 50 µg of total cellular protein as determined by Bradford assay (Sigma) dependent on the protein yields from the PBMC. Each Western blot membrane contained all available time points for 2 patients and was probed with 6 antibodies. Beta-actin was used as a loading control. A chemiluminescence-based developing system from Millipore was used with Kodak Biomax films that were scanned and analyzed. To account for differences in amounts of protein loaded and variability of assay conditions, the quantification of signal intensities was performed by relating the expression of a particular protein before and after treatment to the intensity of the actin band in the same specimen. Signals were quantified using NIH Image J software to measure the mean signal intensity (grey scale) value and its integrated density. Resulting absolute intensities were determined, and a relative intensity value was obtained by dividing the absolute intensity of protein X by that of the beta-actin loading control. Whole cell lysates from the Hs578T breast cancer cell line, and the PC-3 prostate cancer cell line were used as positive controls.
Statistical methods
The data from this Phase I trial were summarized using descriptive statistics, including point estimates and confidence interval (CI) estimates. The PD variables were presented using multiple box plots. Baseline (Day −14) levels of the PD variables were compared by clinical benefit (CB) status (yes/no) using the exact version of the Wilcoxon rank sum test (2-sided). CB was defined as either partial response (PR) or stable disease (SD). Among CB patients, the levels of PD variables at Day −1, Day +4, and Day +18 were compared vs baseline (Day −14) using the exact version of the Wilxocon signed-rank test. Since these analyses of the PD variables were hypothesis generating only, no adjustment for multiple comparisons was applied.
Sorafenib Cmax and AUCτ values (on day −1) and in combination with tanespimycin (on day 1) were compared using the paired t-test.
Time to progression (TTP) was measured from date of treatment start, until date of documented disease progression..If no progression occurred, patients were censored for TTP as of the date of their last tumor assessment. Overall survival (OS) was measured from date of treatment start, until date of death from any cause. Patients still alive were censored for OS as of the date of last follow-up for vital status determination. The censored TTP and OS distributions were estimated with standard Kaplan-Meier (K–M) methods. Due to the modest sample size (or number of events), time-to-event (TTE) statistics (e.g., median, 6-month rate, ctc.) were estimated more conservatively using linear interpolation among successive event times on the K–M curves.16
Results
Patient Characteristics
Twenty-eight patients were consented, however one was deemed ineligible due to presence of brain metastases in pre-study screening. One patient died during the first cycle of therapy, considered to be possibly related to study medication. Three patients had to discontinue therapy after the first cycle due to severe toxicity from sorafenib alone. All twenty-seven patients were evaluable for the primary endpoint of toxicity assessment, but twenty three patients were response evaluable. Twelve patients with renal cancer were enrolled, of which 2 were untreated, 7 had received prior VEGF inhibitor therapy, 2 had received prior chemotherapy and 1 had received immunotherapy. None of the patients had received prior sorafenib therapy. Patient characteristics are summarized in Table 1.
Table 1.
Patient Characteristics (N = 27)
| Characteristic | N (%) |
|---|---|
| Median age | 56 years (range 31–76 years) |
| Gender | |
| Male | 18(70) |
| Female | 9 (30) |
| Zubrod Performance status | |
| 0 | 6 (22) |
| 1 | 21 (78) |
| Primary site | |
| Kidney | 12 (44) |
| Melanoma | 6 (22) |
| Adrenal | 1 (4) |
| Colorectal | 4 (15) |
| Thyroid | 1 (4) |
| Cervix | 1 (4) |
| Pancreas | 1 (4) |
| Tongue | 1 (4) |
| Histology# | |
| Clear cell | 7 (26) |
| Papillary | 3 (11) |
| Sarcomatoid | 2 (7) |
| Adenocarcinoma | 5 (19) |
| Squamous | 2 (7) |
| Melanoma | 7 (26) |
| Thyroid papillary | 1 (4) |
| Adrenocortical | 1 (4) |
| Visceral Metastases- Liver or Lung | |
| Present | 21 (78) |
| Absent | 6 (22) |
| Number of organ sites involved | |
| One | 4 (15) |
| Two | 9 (33) |
| Three or more | 14 (52) |
| Prior Systemic Therapy | |
| None | 4 (15) |
| Chemotherapy ≥2 regimens | 16 (60) |
| Biotherapy ≥ 1 regimens | 7 (25) |
| Prior resections/surgeries* | |
| Kidney cancer; nephrectomy | 12 (100) |
| Melanoma resections | 6 (100) |
Percentages may not add up to 100 due to rounding or overlapping categories.
Among only the 12 patients with kidney cancer, and 6 patients with melanoma.
Treatment Administered and Dose Modifications
A total of 114 cycles of treatment were administered, with a median of 4 cycles (Range 1 – 17). Fifteen patients did not require any dose adjustments. Dose modifications of sorafenib to 400 mg once daily and of tanespimycin to one dose level lower were required in 3 and 4 patients, respectively. Treatment interruptions of either sorafenib or tanespimycin were noted in 5 and 2 patients, respectively. If patients had toxicities requiring a dose reduction in the first 2 cycles this was considered a dose limiting toxicity. If patients required a dose reduction of sorafenib during the first 14 days of therapy, then they were not started on tanespimycin. Hence all the dose reductions were in the patients who stayed on therapy beyond 2 cycles, as they were deriving clinical benefit.
Toxicity
Twenty-seven patients were evaluable for toxicity (Table 2). At the 450 mg/m2 dose of tanespimycin, dose-limiting toxicities of grade 4 transaminitis and grade 3 hand-foot syndrome were observed in 1 patient, and grade 3 hand-foot syndrome was seen in a second patient. Four treatment-related hospitalizations occurred and these patients demonstrated multiple grade 3 and 4 toxicities. One was due to diarrhea, dehydration, abdominal pain and transaminitis, and another was due to severe hand-foot syndrome and fatigue. The other reasons for admission were vaginal hemorrhage and anemia in a patient with primary cervix cancer, and chest pain and dyspnea in another. The predominant toxicities attributed to sorafenib were hand-foot syndrome and diarrhea. The most prevalent toxicities related to the combination were nausea and emesis. One death, possibly attributed to study medication occurred.
Table 2.
Toxicity
| Toxicity by Tanespimycin dose level |
Grade1 | Grade2 | Grade3 | Grade4 |
|---|---|---|---|---|
| 300 mg/m2 (n = 3) | ||||
| Liver function abnormality | 0 | 1 | 0 | 0 |
| Hand-Foot syndrome | 1 | 0 | 0 | 0 |
| Nausea | 1 | 0 | 0 | 0 |
| Diarrhea | 1 | 1 | 0 | 0 |
| Fatigue | 3 | 0 | 0 | 0 |
| Headache | 1 | 0 | 0 | 0 |
| Other toxicity | 0 | 3 | 0 | 0 |
| 350 mg/m2 ( n = 7 ) | ||||
| Hemoglobin | 1 | 0 | 0 | 0 |
| Platelets | 1 | 0 | 0 | 0 |
| Liver function abnormality | 2 | 0 | 0 | 0 |
| Hand-Foot syndrome | 0 | 2 | 0 | 0 |
| Nausea | 3 | 2 | 0 | 0 |
| Emesis | 2 | 3 | 1 | 0 |
| Diarrhea | 3 | 1 | 1 | 0 |
| Fatigue | 2 | 0 | 0 | 0 |
| Creatinine | 1 | 1 | 0 | 0 |
| Headache | 1 | 1 | 0 | 0 |
| Myalgias | 1 | 0 | 0 | 0 |
| Bleeding | 1 | 1 | 0 | 0 |
| Other toxicity | 5 | 1 | 1 | 0 |
| 400 mg/m2 ( n = 11 ) | ||||
| Neutropenia | 0 | 0 | 1 | 0 |
| Hemoglobin | 2 | 1 | 0 | 0 |
| Platelets | 2 | 0 | 0 | 0 |
| Liver function abnormality | 1 | 0 | 0 | 0 |
| Hand-Foot syndrome | 0 | 3 | 2 | 0 |
| Nausea | 6 | 1 | 1 | 0 |
| Emesis | 5 | 0 | 0 | 0 |
| Diarrhea | 1 | 1 | 0 | 0 |
| Fatigue | 5 | 2 | 1 | 0 |
| Creatinine | 0 | 1 | 0 | 0 |
| Infection | 0 | 1 | 0 | 0 |
| Headache | 1 | 0 | 0 | 0 |
| Myalgias | 1 | 0 | 0 | 0 |
| Dyspnea | 0 | 0 | 1 | 1 |
| Other toxicity | 3 | 3 | 2 | 0 |
| 450 mg/m2 ( n = 6 ) | ||||
| Hemoglobin | 1 | 0 | 0 | 0 |
| Platelets | 0 | 0 | 1 | 0 |
| Liver function abnormality | 0 | 0 | 0 | 1 |
| Hand-Foot syndrome | 1 | 1 | 2 | 0 |
| Nausea | 4 | 0 | 0 | 0 |
| Emesis | 1 | 0 | 0 | 0 |
| Diarrhea | 2 | 0 | 1 | 0 |
| Fatigue | 4 | 1 | 0 | 0 |
| Hypertension | 0 | 1 | 0 | 0 |
| Infection | 0 | 0 | 1 | 0 |
| Headache | 2 | 0 | 0 | 0 |
| Abdominal pain | 2 | 0 | 1 | 0 |
| Myalgias | 1 | 0 | 0 | 0 |
| Other toxicity | 3 | 0 | 1 | 0 |
Response and Survival
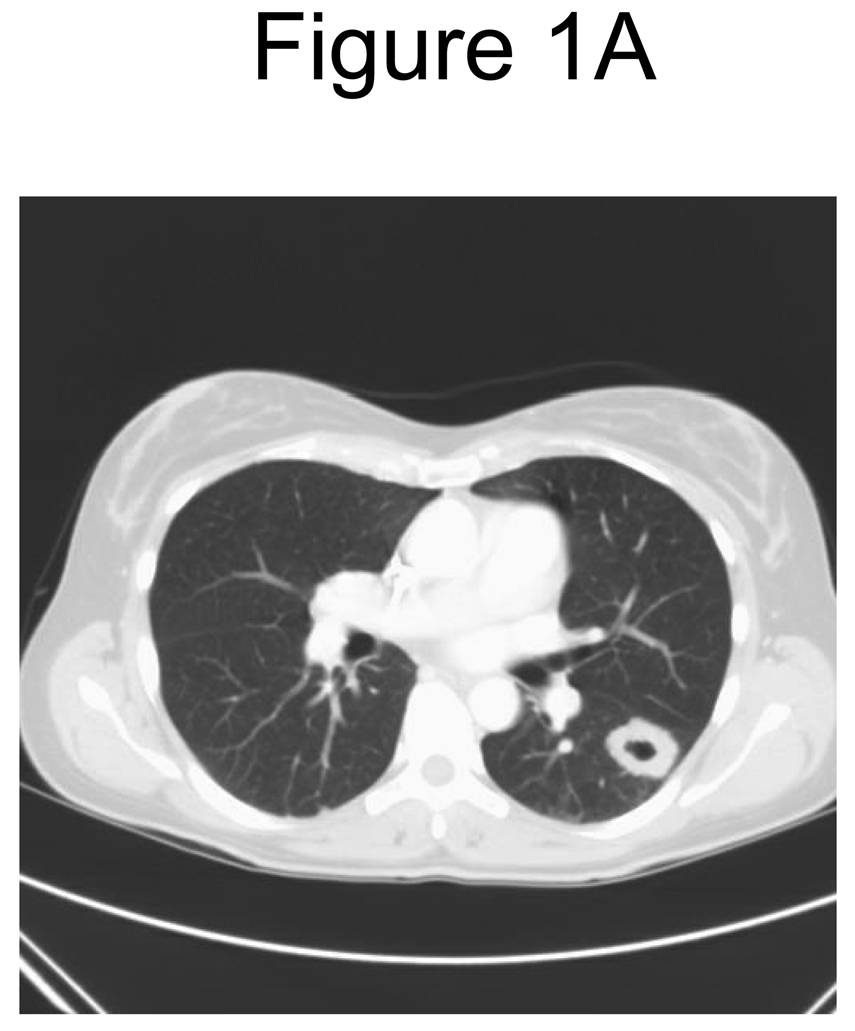
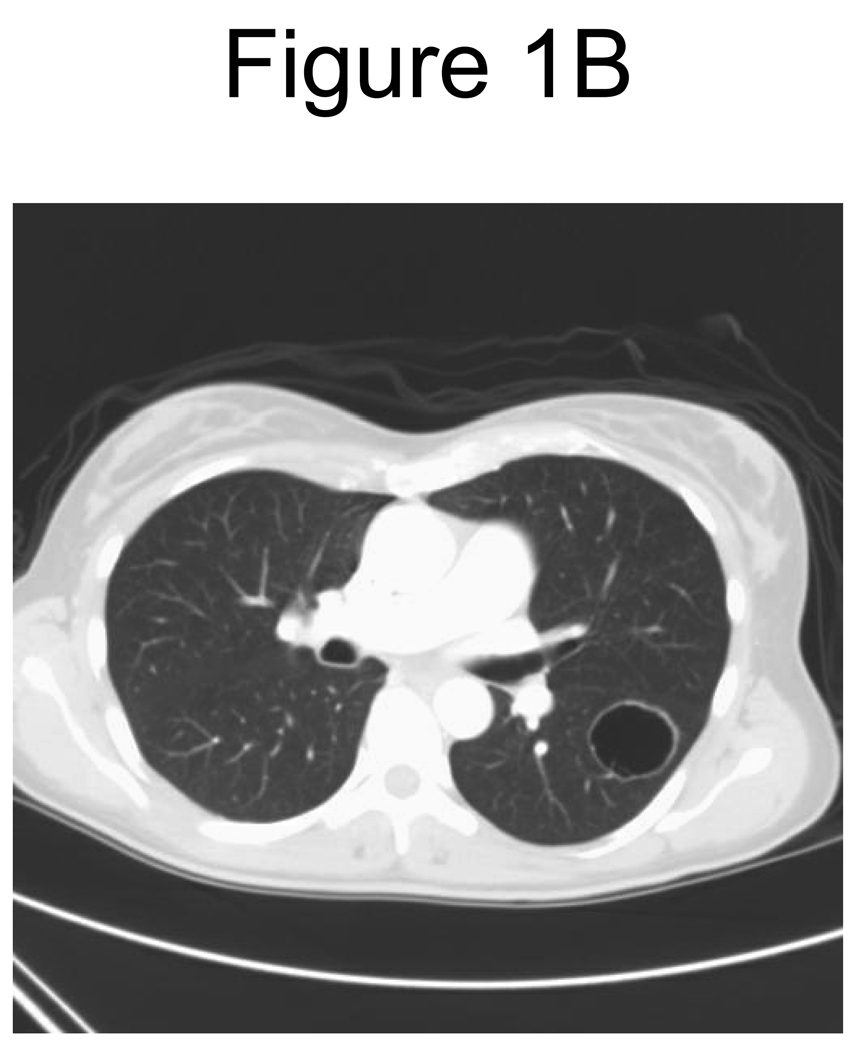
Twenty-three patients received at least 2 cycles and were evaluable for response. Of these, two (9%) demonstrated a partial remission (PR); one with thyroid cancer and one with renal cancer, and fourteen (61%) had stable disease (SD). Disease stabilization was noted in eight patients with renal cancer and four with melanoma and one patient each with colorectal cancer and cancer of cervix. Clinical benefit (PR+ SD) was noted in a total of sixteen (70%) patients. Of the twelve patients with renal cancer, nine (75%) had clinical benefit; one PR, and eight SD. One patient with renal cancer had SD, and continued on therapy for 17 cycles. Among the 10 SD patients the median duration was 3.4 months (range 2.6 – 15.9 months). Of the six patients with melanoma, four demonstrated SD. Figure 1 reveals an example of remarkable change in tumor consistency with no change in tumor dimensions. Median TTP for all patients (N=27) was 2.9 months (90% CI 2.3 – 4.1 months), and median OS was 12.1 months (90% CI 7.9 – 18.6 months).
Figure 1.
A. Pre-therapy CT scan of chest
B. Post-therapy CT scan of chest
Pharmacokinetics (Figures 2A and 2B)
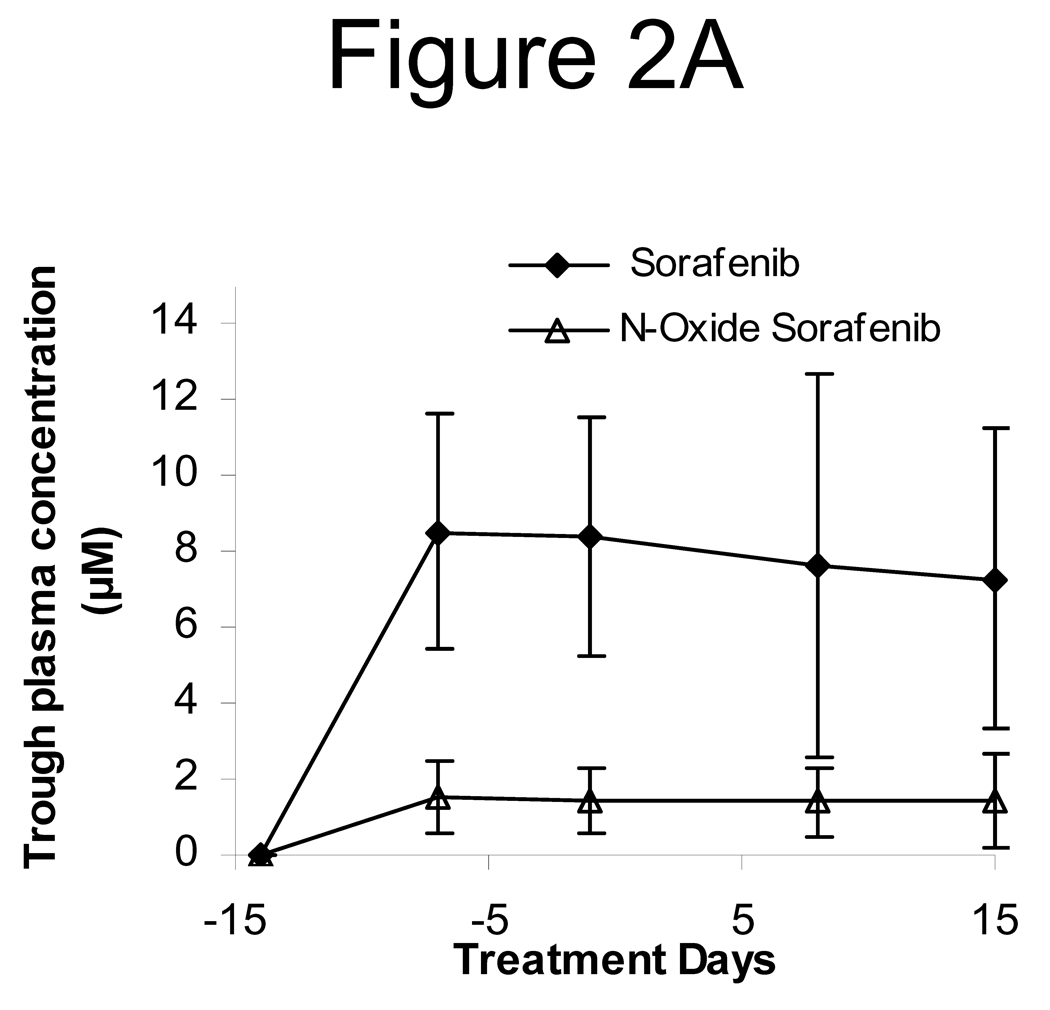
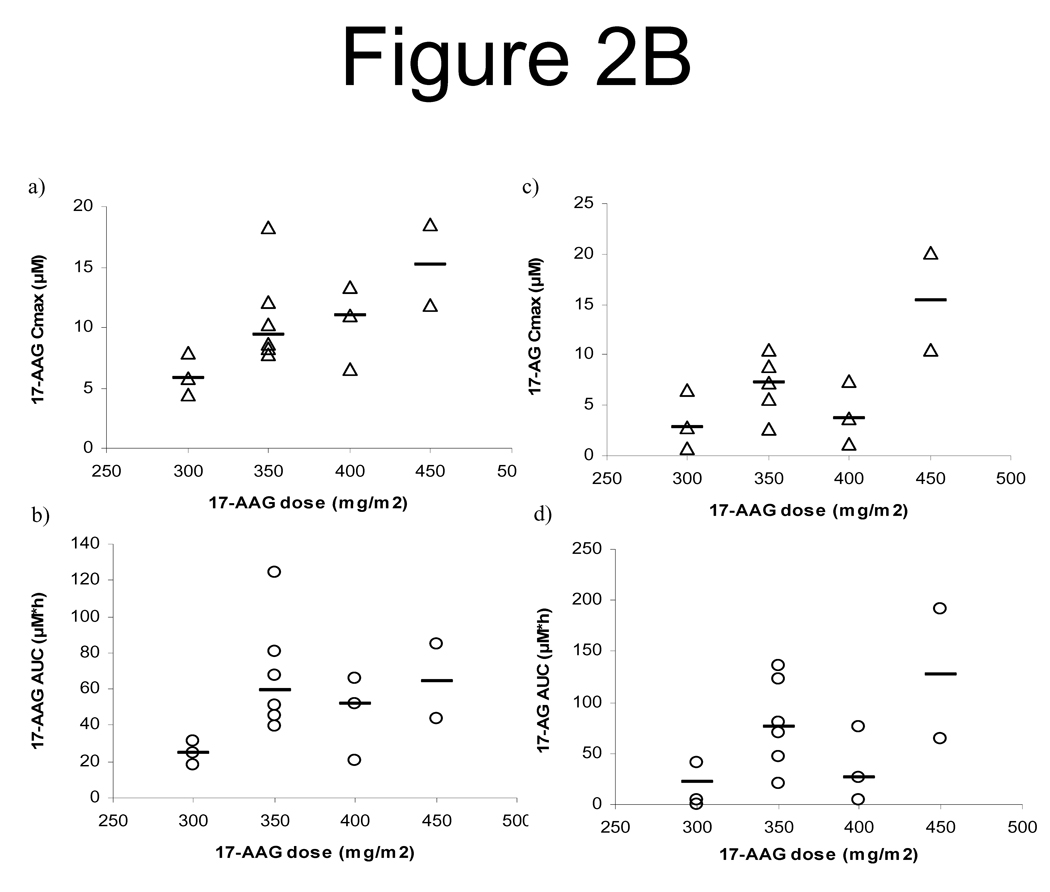
Figure 2.
A. The trough plasma concentrations of sorafenib and its N-oxide metabolites following first cycle administration of sorafenib 400 mg twice daily in cancer patients. Values shown are mean ± standard deviation of the trough levels from 13, 12, 17, 12, and 17 patients, respectively, on treatment days −14, −7, −1, 8, and 15.
B. Relationship of tanespimycin dose with the Cmax and AUC of tanespimycin (a and b) as well as with the Cmax and AUC of its metabolite 17-AG (c and d).
Sorafenib exhibited an irregular or erratic absorption profile, with the peak time (Tmax) ranging from 1 to 10 h. The plasma concentration of sorafenib and its N-oxide metabolite reached the steady-state after 7 days of chronic treatment, with mean steady-state trough levels of 8.0 and 1.5 µM, respectively [Figure 2A]. The coadministration of 17-AAG did not significantly alter the systemic exposure of sorafenib and its O-oxide metabolite. When administered orally alone and in combination with 17-AAG, sorafenib achieved the Cmax, expressed as the mean (with % CV in parenthesis), of 12.5 µM (39%) and 13.1 µM (46%), respectively, P = 0.437; it had the mean AUCτ of 90.7 µM*h (44%) and 85.0 µM*h (39%), respectively, P = 0.435 [Table 3].
Table 3.
PK Parameters of Sorafenib and Metabolite at the Steady-State when Given Alone (on Day −1) and in Combination with Tanespimycin (on Day 1) a
| Tmax (h) | Cmax (µM) | AUClast (µM*h) | ||||
|---|---|---|---|---|---|---|
| Day −1 | Day 1 | Day-1 | Day1 | Day-1 | Day1 | |
| Sorafenib | ||||||
| Mean | 5.1 | 8.5 | 12.5 | 13.1 | 90.7 | 85.0 |
| Standard Deviation | 3.0 | 2.7 | 4.8 | 6.0 | 39.8 | 33.1 |
| Coefficient of Variation (%) | 58.0 | 31.5 | 38.8 | 45.5 | 43.9 | 39.0 |
| Maximum | 10.0 | 10.0 | 22.5 | 26.6 | 181.2 | 146.8 |
| Minimum | 1.0 | 1.0 | 5.8 | 6.6 | 44.3 | 46.7 |
| N-oxide sorafenib | ||||||
| Mean | 5.9 | 8.5 | 2.0 | 2.6 | 15.5 | 16.1 |
| Standard Deviation | 2.9 | 1.8 | 1.4 | 2.0 | 11.4 | 11.4 |
| Coefficient of Variation (%) | 49.4 | 21.2 | 71.5 | 79.8 | 73.7 | 70.9 |
| Maximum | 10.0 | 10.0 | 6.4 | 7.3 | 45.7 | 40.5 |
| Minimum | 1.0 | 4.0 | 0.5 | 0.7 | 3.2 | 5.5 |
Eleven patients had these PK parameters available at both time points (Day −1 and Day 1).
Following intravenous infusion, Cmax (end of infusion) and AUC of tanespimycin and its major metabolite, 17-AG, increased with increasing tanespimycin dose (Figure 2B). On day 1, following the 3-hour intravenous infusions of 300, 350, 400, and 450 mg/m2, the mean tanespimycin Cmax were 6.0 µM (range 4.4 to 7.9 µM, n = 3), 10.9 µM (range 7.8 to 18.3 µM, n = 6), 10.3 µM (range 6.6 to 13.3 µM, n = 3), and 15.2 µM (range 11.9 to 18.6 µM, n = 2), respectively (Figure 2B). Tanespimycin Cmax and 17-AG C max were similar on days 1 and 15, suggesting that tanespimycin PK were not changed with time or by concurrent administration of sorafenib. Tanespimycin exhibited a short elimination half-life, with a mean value of 3.6 h (range 1.1 to 6.6 h). The clearance (Mean ± SD) was 15.0 ± 7.9 L/h/m2 (range 4.8 to 32.9 L/h/m2). PK variability of tanespimycin, as determined with interindividual variability in tanespimycin clearance and assessed by CV%, was 53.0%.
Pharmacodynamics
The markers below were evaluated, and their individual associations with clinical benefit were explored (Figure 3). Clinical benefit was defined as either PR, MR or SD. Biomarker levels are reported cumulatively for all dose levels because PK revealed that the minimum tanespimycin concentrations achieved in our patient samples were considerably higher (see Figure 2 and PK Results above) than the minimum concentrations reported to produce pharmacodynamic effects in prior studies conducted in xenograft models (123 nM to 20 mM)19, 20.
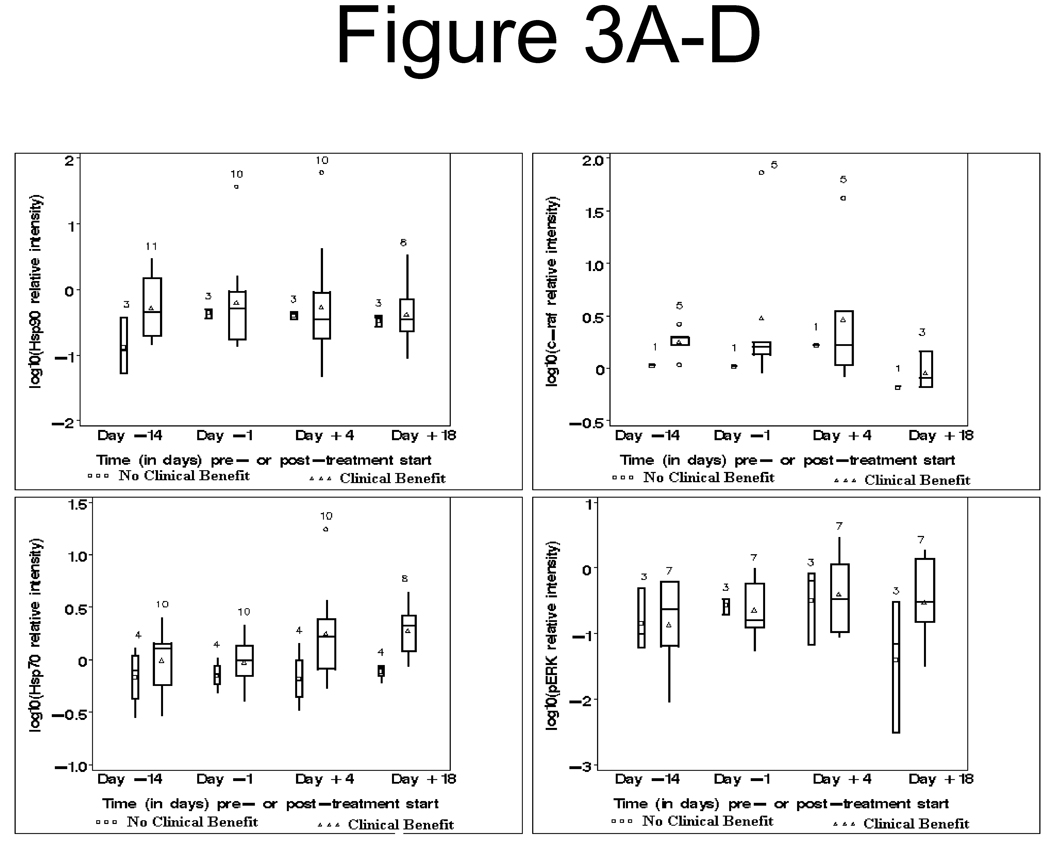
Figure 3. Box plot graphs of log10Hsp 70, log10pErk, log10Hsp90 and log10c-raf in patients with and without clinical benefit.
A. Upper left: Log10Hsp90 relative intensity, pre- and post-therapy, clinical benefit (Δ) vs no clinical benefit (□).
B. Lower left: Log10Hsp70 relative intensity, pre- and post-therapy, clinical benefit (Δ) vs no clinical benefit (□)
C. Upper right: Log10c-raf relative intensity, pre- and post-therapy, clinical benefit (Δ) vs no clinical benefit (□)
D. Lower right: Log10pERK relative intensity, pre- and post-therapy, clinical benefit (Δ) vs no clinical benefit (□)
Hsp90 and Hsp70 levels
Baseline (Day −14) Hsp90 levels were weakly significantly different (p = 0.0604) between patients with vs without clinical benefit (CB). There was a decrease in Hsp90 levels after tanespimycin therapy in 6 of the 14 patient samples tested. All 6 patients with decline in Hsp90 levels demonstrated clinical benefit, and all 4 patients with progression had an increase in Hsp90 levels. (Figure 3A). Hsp90 levels were not affected by sorafenib therapy. Among the CB patients, Hsp90 levels were not significantly different at any time point vs baseline (p ≥ 0.3750 for each of the 3 time points: Day −1, Day +4, and Day +18.
Baseline (Day −14) Hsp70 levels did not differ significantly (p = 0.3736) between patients with vs those without CB. Hsp70 induction was noted in 11 of 14 samples after treatment with tanespimycin. An increase in Hsp70 level was noted in 8 of the 10 patients showing clinical benefit after tanespimycin therapy, whereas the levels in the 4 patients without clinical benefit were unchanged (Figure 3B). Among the CB patients, Hsp70 levels were weakly significantly different from baseline only at Day +4 (p = 0.0547) and at Day +18 (p = 0.0313).
Other Correlates
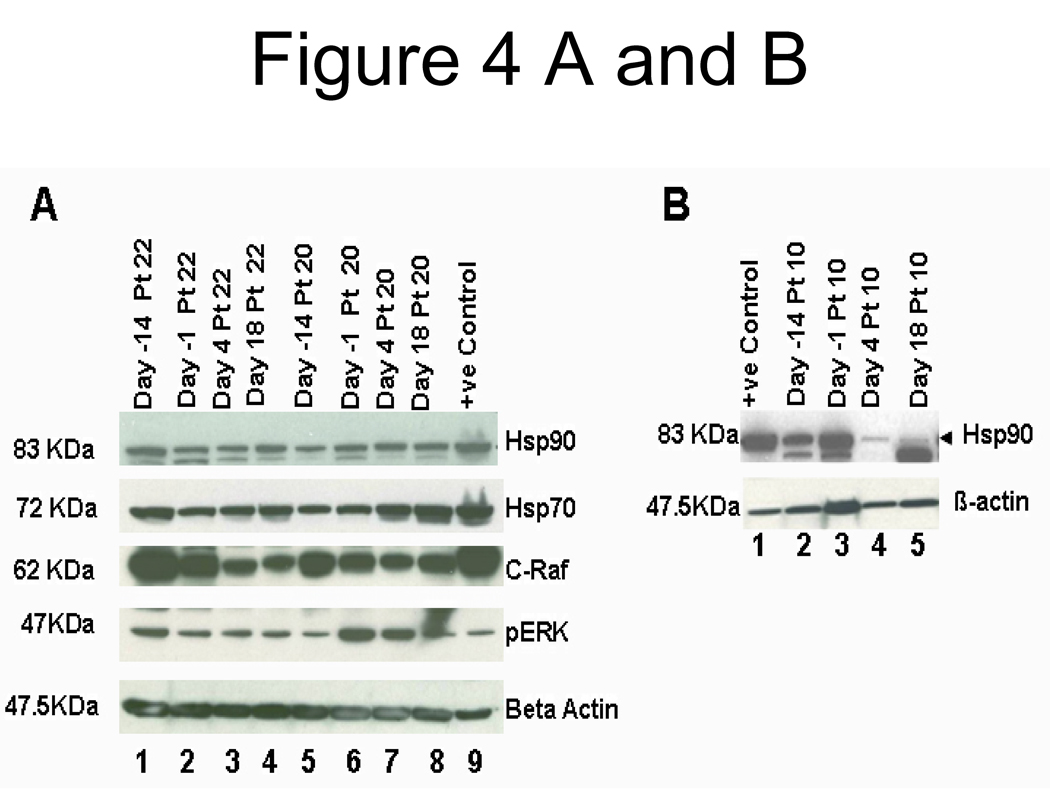
Four of six patients had a decrease in c-Raf. Three of the four patients demonstrated clinical benefit (Figure 3C). Three patient samples showed a decline in pErk levels (Figure 3D]. An example of a remarkable post-therapy decline in c-Raf is shown in patient # 22 (Figure 4A). Figure 4B demonstrates a western blot for samples from a patient with prolonged SD (17 cycles), with a high baseline level of Hsp90 and reduction after therapy.
Figure 4.
A. Western blot analysis of Hsp90, Hsp70, c-Raf, and phosphor-ERK in PBMC whole cell lysates from patients 22 and 20. We used a cell lysate from the prostate cancer cell line PC-3 as a positive control (+ve). Time points are indicated on top of the lanes. In both patients, a reduction of c-Raf and pERK is seen under therapy with more marked effects in the combination. Noticeable induction of Hsp70 is seen in patient # 20 PBMC after 17-AAG treatment.
B. A patient with prolonged clinical benefit (17 cycles) with high baseline levels of Hsp90, and a marked decrease on days 4 and 18 upon treatment with tanespimycin on days 1, 8 and 15.
Beta-actin was used to probe for equal loading in A and B.
Discussion
This phase I trial established the recommended phase II doses of sorafenib at 400 mg twice daily and tanespimycin at 400 mg/m2. The study demonstrated feasibility of administering clinically effective doses of sorafenib in combination with tanespimycin. Hand-foot syndrome and hepatotoxicity were the dose-limiting toxicities.
The sorafenib concentrations noted were consistent with previously published data 21. The PK revealed a dose-dependent increase in tanespimycin and metabolite concentrations. The tanespimycin concentrations were similar to those reported in prior trials22–24.and tanespimycin did not significantly alter the systemic exposure of sorafenib and its N-oxide metabolite.
Tanespimycin modifies Hsp90 and Hsp70 levels in tumor cells in vitro and in vivo19,20,25. This led us to include Hsp90 and Hsp70 assessment as correlates in our trial design, to establish proof of principle of target inhibition. The PK data showed that tanespimycin was detected in the serum of all patients, independent of the administered dose and above the concentrations that we have previously defined necessary to modulate the target, and Hsp90 client proteins, in cell lines, and human xenograft tumor tissues19,20. Banerji et al. have previously confirmed the PK-PD relationship of tanespimycin in animal models, and compared PD in tumor to PBMC samples. Their results showed, that changes in molecular biomarkers that occur in tumors, are well represented in PBMCs at drug concentrations required for therapeutic efficacy 23. The latter suggests that the use of PBMCs to assess PD effects of tanespimycin is informative, even at the lowest concentrations achieved in our study. However, the association between Hsp90 decrease in PBMCs, and observed clinical efficacy is hypothesis-generating only and is limited by the small sample size and variations in dose and tumor type that are inherent in a phase I study.
Response determination as a surrogate for clinical outcome presents a challenge in the era of targeted therapy, especially when the most frequently induced response is stable disease. The RECIST criteria27 are based on single-dimension measurements of tumor masses. A remarkable radiologic change in tumor density in the absence of any appreciable change in tumor dimensions was noted in our study, as shown in Figure 1. The Choi26 criteria (≥10% decrease in dimensions on RECIST, or ≥15% decrease in tumor density as measured by Hounsfield units on CT scan) could supplement RECIST in assessing response to targeted therapies. The Choi criteria have been validated in gastrointestinal stromal tumors28 and were recently evaluated in targeted therapy of renal cancers, where they were found to be better predictors of survival than RECIST. The change in tumor lesion density/consistency should thus be considered when reviewing responses to targeted therapies and their combinations.
Sorafenib has demonstrated efficacy specifically in renal cancer and hepatocellular cancers1,2. The geldanamycin-based Hsp90 inhibitors were tested in numerous phase I trials, but demonstrated single agent efficacy in patients with refractory multiple myeloma, acute myeloid leukemia and breast cancer30–32. The response rate with sorafenib in renal cancer, with no prior VEGF inhibitor therapy, was 10%, with SD rate of 74%. Phase II trial of tanespimycin in melanoma33 revealed no objective responses or disease stabilization, and none of the patients received therapy beyond the initial 6 weeks. In kidney cancer, a phase II trial of tanespimycin34 revealed no objective responses, even though all the patients enrolled had not received prior VEGF inhibitor therapy. The 70% PR+ SD rate noted in our trial, despite the pretreated population of renal cancer and melanoma patients enrolled, compares favorably with that seen in the phase II trials of tanespimycin mentioned above. Sorafenib appeared to enhance the efficacy of tanespimycin in renal cancer and melanoma even in a pretreated population. This should prompt the therapeutic development of combinations of Hsp90 and raf-kinase inhibition in kidney cancer and melanoma. In addition, the overall tolerability of the combination and the possible association between Hsp90 and Hsp70 levels and clinical benefit, make a strong rationale for testing the combination in metastatic renal cancer and melanoma.
In conclusion, the recommended doses for phase II trials are 400 mg twice daily orally of sorafenib and 400 mg/m2 of intravenous tanespimycin on days 1, 8, and 15 every 28 days. The combination demonstrated clinical efficacy in metastatic renal cancer and melanoma. and pharmacodynamic effects of Hsp90 inhibition and Hsp 70 induction.
Translational Relevance.
Hsp90 inhibitors are a new class of emerging anticancer agents. Hsp90 is a critical molecular chaperone for the activation of many oncogenic proteins, including C-Raf. Raf signaling plays an important role in many cancers, but particularly, renal carcinoma and malignant melanoma.
Tanespimycin has shown minimal single agent activity in phase I/II clinical trials. We tested the hypothesis that simultaneous inhibition of Raf activation, by inhibition of Hsp90 with tanespimycin, and blockage of the Raf/MEK/ERK signaling pathway by sorafenib, could demonstrate synergy. Proof of concept studies of Hsp90 and Raf-associated signaling transduction inhibition were done using peripheral blood mononuclear cells. PK of each of the agents were not altered by the combination, and drug plasma concentrations at every dose level were above those required to achieve in vitro PD effects. PD analyses demonstrated down-regulation of Hsp90, increase in Hsp70 levels, and inhibition of C-Raf in responders. Clinical responses and disease stabilization were mainly seen in renal carcinoma and melanoma patients. The results of the trial suggest that the combination of sorafenib and tanespimycin is well tolerated and phase II studies should be performed in metastatic renal cancer and melanoma.
Acknowledgements
We are indebted to Johann deBono M.D. at the Royal Marsden Hospital, U.K. for his intellectual contribution to the initial protocol preparation. We extend our sincere thanks to Alex Lorusso and Jie Zhang for help with data collection, Colette Burgess for technical support, and Daryn Smith for statistical support. We thank CTEP for supplying both medications for the study.
Research Support: Supported by NIH# 5UO1CA062487-14, NIH# UO1CA099168, 2MO1RR016500-006 and Cancer Center Support Grant CA-22543. Sorafenib/BAY43-9006 and tanespimycin (17-AAG) were supplied by NCI-CTEP and correlatives were supported by NCI Translational Research Initiative grant 26XS162, (P6972). The initial version of the protocol was designed in the 2004 ECCO-AACR-ASCO Workshop, “Methods in Clinical Cancer Research”, Flims, Switzerland. Dr Egorin is the recipient of an ASCO Cancer Foundation Translational Research Professorship.
Footnotes
Presented, in part, at the American Society of Clinical Oncology Meeting in June 2007 and the ASCO Genitourinary Cancers Symposium in February 2008.
REFERENCES
- 1.Escudier B, Eisen T, Stadler WM, et al. TARGET Study Group. Sorafenib in Advanced Clear-Cell renal cell carcinoma. N Engl J Med. 2007;356:125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 2.Llovet JM, Ricci S, Mazzaferro V, et al. SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 3.Adnane L, Trail PA, Taylor I, et al. Sorafenib (BAY 43–9006, Nexavar), a dual-action inhibitor that targets RAF/MEK/ERK pathway in tumor cells and tyrosine kinases VEGFR/PDGFR in tumor vasculature. Methods Enzymol. 2006;407:597–612. doi: 10.1016/S0076-6879(05)07047-3. [DOI] [PubMed] [Google Scholar]
- 4.Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 5.Sharp S, Workman P. Inhibitors of the HSP90 molecular chaperone: current status. Adv Cancer Res. 2006;95:323–348. doi: 10.1016/S0065-230X(06)95009-X. [DOI] [PubMed] [Google Scholar]
- 6.Da Rocha Dias, Friedlos F, Light Y, et al. Activated B-RAF is an Hsp90 client protein that is targeted by the anticancer drug 17-allylamino-17-demethoxygeldanamycin. Cancer Res. 2005;65:10686–10691. doi: 10.1158/0008-5472.CAN-05-2632. [DOI] [PubMed] [Google Scholar]
- 7.Stebbins CE, Russo AA, Schneider C, et al. Crystal structure of an Hsp90-geldanamycin complex: targeting of a protein chaperone by an antitumor agent. Cell. 1997;89:239–250. doi: 10.1016/s0092-8674(00)80203-2. [DOI] [PubMed] [Google Scholar]
- 8.Supko JG, Hickman RL, Grever MR, et al. Preclinical pharmacologic evaluation of geldanamycin as an antitumor agent. Cancer Chemother Pharmacol. 1995;36:305–315. doi: 10.1007/BF00689048. [DOI] [PubMed] [Google Scholar]
- 9.Grenert JP, Sullivan WP, Fadden P, et al. The amino-terminal domain of heat shock protein 90 (hsp90) that binds geldanamycin is an ATP/ADP switch domain that regulates hsp90 conformation. J Biol Chem. 1997;272:23843–23850. doi: 10.1074/jbc.272.38.23843. [DOI] [PubMed] [Google Scholar]
- 10.Neckers L, Neckers K. Heat-shock protein 90 inhibitors as novel cancer chemotherapeutics - an update. Expert Opin Emerg Drugs. 2005;10:137–149. doi: 10.1517/14728214.10.1.137. [DOI] [PubMed] [Google Scholar]
- 11.Banerji U, Judson I, Wokman P. The clinical applications of heat shock protein inhibitors in cancer - present and future. Curr Cancer Drug Targets. 2003;3:385–390. doi: 10.2174/1568009033481813. [DOI] [PubMed] [Google Scholar]
- 12.Jia W, Yu C, Rahmani M, et al. Synergistic antileukemic interactions between 17-AAG and UCN-01 involve interruption of RAF/MEK- and AKT-related pathways. Blood. 2003;102:1824–1832. doi: 10.1182/blood-2002-12-3785. [DOI] [PubMed] [Google Scholar]
- 13.Yeung KC, Rose DW, Dhillon AS, et al. Sedivy Raf Kinase Inhibitor Protein Interacts with NF-κB-Inducing Kinase and TAK1 and Inhibits NF-κB Activation Mol. Cell. Biol. 2001;21:7207–7217. doi: 10.1128/MCB.21.21.7207-7217.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grbovic OM, Basso AD, Sawai A, et al. V600E B-Raf requires the Hsp90 chaperone for stability and is degraded in response to Hsp90 inhibitors. Proc Natl Acad Sci U S A. 2006;103:57–62. doi: 10.1073/pnas.0609973103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gollob JA. Sorafenib: scientific rationales for single-agent and combination therapy in clear-cell renal cell carcinoma. Clin Genitourin Cancer. 2005;4:167–174. doi: 10.3816/CGC.2005.n.028. [DOI] [PubMed] [Google Scholar]
- 16.Lee ET, Wang JW. Statistical Methods for Survival Data Analysis. 3rd Ed. Hoboken, NJ: Wiley and Sons, Inc; 2003. pp. 76–91. [Google Scholar]
- 17.Zhao M, Rudek MA, He P, et al. A rapid and sensitive method for detection of sorafenib in human plasma using a liquid chromatography/tandem mass spectrometry assay. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;846:1–7. doi: 10.1016/j.jchromb.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 18.Ramanathan RK, Trump DL, Eiseman JL, et al. Phase I pharmacokinetic-pharmacodynamic study of 17-(allylamino)-17-demethoxygeldanamycin (17AAG, NSC 330507), a novel inhibitor of heat shock protein 90, in patients with refractory advanced cancers. Clin Cancer Res. 2005;11:3385–3391. doi: 10.1158/1078-0432.CCR-04-2322. [DOI] [PubMed] [Google Scholar]
- 19.Burger AM, Fiebig HH, Stinson SF, et al. 17-(Allylamino)-17-demethoxygeldanamycin activity in human melanoma models. AntiCancer Drugs. 2004;15:377–387. doi: 10.1097/00001813-200404000-00011. [DOI] [PubMed] [Google Scholar]
- 20.Smith V, Sausville EA, Camalier RF, et al. Comparison of 17-dimethylaminoethylamino-17-demethoxy-geldanamycin (17DMAG) and 17-allylamino-17-demethoxygeldanamycin (17AAG) in vitro: effects on Hsp90 and client proteins in melanoma models. Cancer Chemother Pharmacol. 2005;56:126–137. doi: 10.1007/s00280-004-0947-2. [DOI] [PubMed] [Google Scholar]
- 21.Strumberg D, Clark JW, Awada A, et al. Safety, pharmacokinetics, and preliminary antitumor activity of sorafenib: a review of four phase I trials in patients with advanced refractory solid tumors. Oncologist. 2007;12:426–437. doi: 10.1634/theoncologist.12-4-426. [DOI] [PubMed] [Google Scholar]
- 22.Goetz MP, Toft D, Reid J, et al. Phase I trial of 17-allylamino-17-demethoxygeldanamycin in patients with advanced cancer. J Clin Oncol. 2005;23:1078–1087. doi: 10.1200/JCO.2005.09.119. [DOI] [PubMed] [Google Scholar]
- 23.Banerji U, Walton M, Raynaud F, et al. Pharmacokinetic-pharmacodynamic relationships for the heat shock protein 90 molecular chaperone inhibitor 17-allylamino, 17-demethoxygeldanamycin in human ovarian cancer xenograft models. Clin Cancer Res. 2005;11:7023–7032. doi: 10.1158/1078-0432.CCR-05-0518. [DOI] [PubMed] [Google Scholar]
- 24.Tse AN, Klimstra DS, Gonen M, et al. A phase I dose escalation study of irinotecan in combination with 17- allylamino-17-demethoxygeldanamycin in patient with solid tumors. Clin Cancer Res. 2008;14:6704–6711. doi: 10.1158/1078-0432.CCR-08-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eiseman JL, Lan J, Lagattuta TF, et al. Pharmacokinetics and pharmacodynamics of 17-demethoxy 17-[[(2-dimethylamino) ethyl]amino]geldanamycin (17DMAG, NSC 707545) in C.B-17 SCID mice bearing MDA-MB-231 human breast cancer xenografts. Cancer Chemother Pharmacol. 2005;55:21–32. doi: 10.1007/s00280-004-0865-3. [DOI] [PubMed] [Google Scholar]
- 26.Benjamin RS, Choi H, Macapinlac HA, et al. We should desist using RECIST, at least in GIST. J Clin Oncol. 2007;25:1760–1764. doi: 10.1200/JCO.2006.07.3411. [DOI] [PubMed] [Google Scholar]
- 27.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 28.Choi H, Charnsangavej C, Faria SC, et al. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol. 2007;25:1753–1759. doi: 10.1200/JCO.2006.07.3049. [DOI] [PubMed] [Google Scholar]
- 29.Van der Veldt A, Meijerink MR, Van den Eertwegh AJ, et al. Choi response criteria for prediction of clinical outcome in patients with metastatic renal cell cancer treated with sunitinib. Proc ASCO. 2009 doi: 10.1038/sj.bjc.6605567. abstract # 5044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Banerji U. Heat shock protein as a drug target- some like it hot. Clin Cancer Res. 2009;15:9–14. doi: 10.1158/1078-0432.CCR-08-0132. [DOI] [PubMed] [Google Scholar]
- 31.Banerji U, O’Donell A, Scurr M, et al. Phase I pharmacokinetic and pharmacodynamic study of 17-allylamino, 17-demethoxygeldanamycin in patients with advanced malignancies. J Clin Oncol. 2005;23:4152–4161. doi: 10.1200/JCO.2005.00.612. [DOI] [PubMed] [Google Scholar]
- 32.Taldone T, Gozman A, Maharaj R, et al. Targeting Hsp90: small-molecule inhibitors and their clinical development. Curr Opinion Pharmacol. 2008;8:370–374. doi: 10.1016/j.coph.2008.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Solit DB, Osman I, Polsky D, et al. Phase II Trial of 17-Allylamino-17-Demethoxygeldanamycin in Patients with metastatic melanoma. Clin Cancer Res. 2008;14:8302–8307. doi: 10.1158/1078-0432.CCR-08-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ronnen EA, Kondagunta GV, Ishill N, et al. A phase II trial of 17-(Allylamino)-17-demethoxygeldanamycin in patients with papillary and clear cell renal cell carcinoma. Invest New Drugs. 2006;24:543–546. doi: 10.1007/s10637-006-9208-z. [DOI] [PubMed] [Google Scholar]