Abstract
Elevated blood alcohol content (BAC) on admission is associated with poorer outcomes, larger burns and more inhalation injury. This study’s purpose was to examine the effects of alcohol through a matched case-controlled study, measuring early and extended markers of clinical outcomes. The hypothesis was that patients with an elevated admission BAC would require more resuscitation and have a longer hospital stay. Admissions 16 to 75 years of age with 15 to 75% TBSA and admission BACs were identified. Patients with BAC >30 mg/dl (BAC+, cases) were matched with patients with undetectable BAC (BAC−, controls), according to age, sex, TBSA, inhalation injury and mechanism. Screening identified 258 patients, 146 with admission BACs. Twenty-seven had a BAC ≥ 30 mg/dl. There were 24 matched pairs. At 24 hours, BAC+ group had larger acute physiology and chronic health evaluation II scores (23.33 vs 18.75, P < .05), fluid requirements (5.25 vs 3.82 L (cc/kg/ TBSA), P < .05), and base deficit (11.15 vs 7.15, P < .05). The duration of mechanical ventilation (14.85 vs 4.23 days, P < .05), intensive care unit length of stay (22.85 vs 9.38, P < .05), hospital length of stay (28.95 vs 15.68, P < .05), and mean hospital charges ($239,507 vs $144,598, P < .05) were increased in the BAC+ patients. Despite matched baseline injury characteristics, elevated BAC was associated with poorer short term and extended clinical outcomes, illustrating the impact of alcohol intoxication on physiologic derangement after burn injury.
It is well recognized that alcohol intoxication is a significant etiologic risk factor for traumatic and burn injury. Of the 100,000 patients hospitalized each year for burn injury, it is estimated that upwards of 50% of adult patients have significant levels of alcohol in their blood at the time of injury.1,2 In addition to alcohol ingestion being a risk factor for burn injury, having an elevated blood alcohol content (BAC) at the time of injury is also a risk factor for increased morbidity and mortality during hospitalization.3–5 Retrospective studies have shown that patients who were intoxicated at the time of burn injury are more difficult to resuscitate with more hypotension as well as increases in the number of operative interventions and infectious complications.6,7 However, some studies have also shown that intoxicated patients are more likely to have more severe injuries: larger, deeper burns, and more inhalation injury,7,8 so one could consider the more difficult resuscitation and increased complication rate to be attributable to the higher injury severity.
Hence, the purpose of this study was to elucidate whether these differences were due to alcohol exposure or to injury severity through a retrospective matched case– control study. We hypothesized that patients with an elevated BAC at the time of injury would require more resuscitation, measured by fluid requirement, base deficit and Acute Physiology and Chronic Health Evaluation score (Apache II), critical care and longer hospital stays.
METHODS
Burn intensive care unit (ICU) admission records from September 2001 to September 2006 were reviewed. To limit bias, reviewers conducting the screening were blinded to BAC and outcomes.
Inclusion Criteria
The burn registry and electronic medical records were studied to identify patients 16 to 75 years of age with burn injuries ranging between 15 and 75% TBSA. Upper and lower limits were placed on the size of the burn to highlight a population with an injury large enough to lead to sequelae from that injury but would also be a survivable injury. BAC was performed at the time of admission to the Burn ICU or by the referring hospital; the earliest documented BAC was recorded. It is burn ICU policy to document BAC on all nonpediatric admissions. All patients in the BAC– group had no alcohol detected. In order to ensure that the BAC would remain positive for the first few hours of hospitalization, a minimum level of 30 mg/dl was established for the cases prior to matching. Cases with alcohol levels greater than 30 mg/dl (BAC+, cases) were matched with patients with undetectable alcohol levels (BAC−, controls) according to age, sex, burn size, presence of inhalation injury and mechanism of burn injury (flame, scald, chemical, or electric). To limit bias, matching was also conducted in a blinded fashion without knowledge of clinical outcomes (Table 1).
Table 1.
Pairwise comparison of matched patients on baseline characteristics
| BAC− (n = 24) |
BAC+ (n = 24) |
P | |
|---|---|---|---|
| Mean BAC (mg/dl) | 0 | 150 | <.05 |
| Mean patient age (yr) | 37.1 | 38.3 | NS |
| Sex % | |||
| F % (n) | 8 (2) | 17 (4) | NS |
| M % (n) | 92 (22) | 83 (20) | NS |
| Mean TBSA % | 29.3 | 29.17 | NS |
| Inhalation injury % (n) | 42 (10) | 42 (10) | NS |
| Mechanism % | |||
| Scald % (n) | 4 (1) | 4 (1) | NS |
| Flame % (n) | 96 (23) | 96 (23) | NS |
| Mean baux score (Age + TBSA) |
66.4 | 67.4 | NS |
Exclusion Criteria
Patients without a recorded BAC were excluded from the study. Patients with documented comorbidities secondary to prolonged alcohol abuse (eg, portal hypertension, liver failure, esophageal varices) were also disqualified.
Baseline Characteristics
Determination of TBSA was made by the evaluating physicians using an adult Lund and Browder diagram. 9,10 The diagnosis of inhalation injury was made by bronchoscopic visualization, performed on all intubated patients by the attending staff within the first 24 hours after admission. The data were tabulated as either presence or absence of inhalation injury.
Clinical Outcomes
To identify a difficult resuscitation we used fluid requirement, base deficit and the Apache II; scores were retrospectively calculated for the first 24 hours after injury.6 The acute physiology element of the Apache II score is comprised of weighted elements representing the major organ systems: age, Glasgow Coma Scale score, mean arterial pressure, partial pressure of oxygen, white blood count, hematocrit, core temperature, heart rate, respiratory rate, pH, creatinine, sodium, and potassium. Fluid resuscitation was started according to the Parkland formula11,12 and then bolused as required guided by the clinical indicators of shock: blood pressure and urine output.12 None of the patients carried a diagnosis of alcoholism as one of their chronic diseases therefore none of the Apache II scores included alcoholism in the chronic disease assessment. The 24-hour fluid requirements were determined by including all prehospital, referring hospital, and transport fluids. The 24-hour fluid total was normalized to the patients’ weight and burn size (cc/kg/TBSA) for analysis. The clinical outcomes analyzed are shown in Table 2.
Table 2.
Table of measured outcomes
| Short Term Outcomes* | Extended Outcomes |
|---|---|
| Mechanical ventilation | Ventilator days |
| Apache II | Total blood transfusions |
| Highest A-a gradient | Burn ICU days |
| Lowest PaO2:FiO2 ratio | Hospital length of stay |
| Worst base deficit | Hospital charges |
| Fluid requirements | Mortality |
Outcomes measured from the first 24 hours after injury.
Statistical Analysis
Data were tabulated and analyzed using SPSS statistical software (version 13.0 for Windows, SPSS Inc.). Normally distributed outcomes were analyzed using paired t-tests. The Wilcoxon’s rank-sum test was used for paired outcomes that were not normally distributed. The Chi-square test was used for nominal data such as ventilator requirement on admission and mortality.
RESULTS
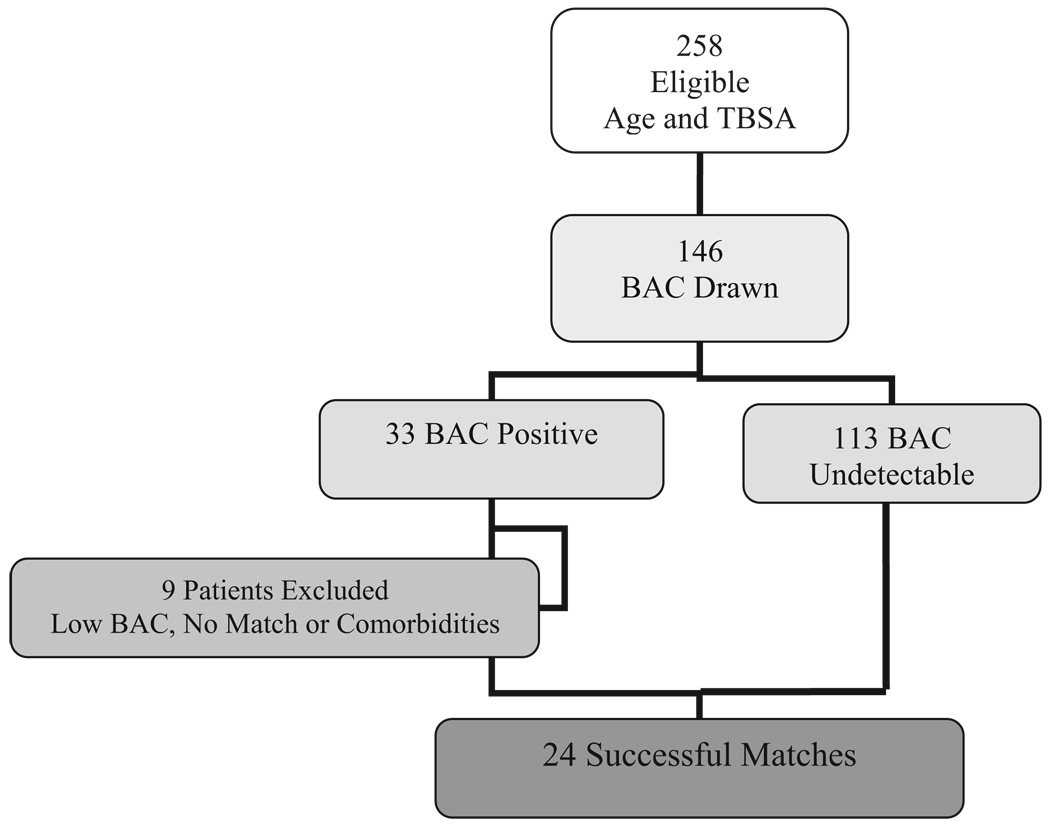
By screening the Burn ICU admission logs for patients age 16 to 75 and with TBSA ranging from 15 to 75%, 258 patients were identified. There were 146 patients with documentation of a BAC drawn at the time of admission or prior to transfer. There were 33 patients with positive BAC and 113 patients with an undetectable BAC. Six patients were below 30 mg/dl and two BAC+ patients did not have a successful BAC− match due to mechanism and inhalation injury. One patient was excluded due to severe alcoholic liver disease. There were 24 successful matches (Figure 1). The positive BACs ranged from 30 to 358 with a mean of 150.6 ± 82.2. The patients were closely matched without any statistical differences in the factors used as matching criteria (Table 1). The average Baux score (age + TBSA), for the BAC+ group was 67.4 ± 20.2 vs 66.4 ± 18.7 in the BAC− group. There was no statistical difference in the Baux score for the two groups, indicating that both groups had the same predicted mortality.13,14
Figure 1.
Consort diagram showing the final number of case– control pairs after screening and application of inclusion and exclusion criteria.
Table 3 shows the results from early clinical outcomes. The Apache II score, the 24-hour worst base deficit, and the 24-hour fluid requirement were found to be significantly different. Extended clinical outcomes are in Table 4. The BAC+ patients had on average more ventilator days, longer length of stay and higher hospital charges. Although the crude mortality rate of BAC+ patients appeared double that of BAC− patients, the difference was not statistically significant. The average increase in hospital stay was 13 days with an increased average charge of $94,909, approximately 66% more than BAC− patients.
Table 3.
Comparison of early clinical outcomes
| BAC− | BAC+ | P | Statistic | |
|---|---|---|---|---|
| Mechanical ventilation* % (n) | 50 (12) | 75 (18) | NS | Chi-square |
| Apache II score* | 18.75 | 23.33 | <.05 | Paired t |
| Highest A-a gradient† | 347.67 | 388.5 | NS | Wilcoxon |
| Lowest PaO2:FiO2 ratio† | 215.27 | 203.72 | NS | Wilcoxon |
| Worst base deficit† | 7.15 | 11.15 | <.05 | Paired t |
| 24 hr fluids [cc/kg/TBSA] (L)* | 3.82 | 5.25 | <.05 | Paired t |
Statistical analysis of outcomes derived from the first 24 hours after injury.
Comparison for all 24 pairs.
Data are for intubated patients only 13 pairs, as these were the only patients on whom arterial blood gas analysis was obtained.
Table 4.
Analysis of late clinical outcomes in matched pairs
| BAC− | BAC+ | P | Statistic | |
|---|---|---|---|---|
| Ventilator days* | 4.23 | 14.85 | <.05 | Paired t |
| Total blood transfusions† | 7.33 | 5.79 | NS | ANOVA |
| Burn ICU days‡ | 9.38 | 22.85 | <.05 | Paired t |
| Length of stay‡ | 15.68 | 28.95 | <.05 | Paired t |
| Hospital chargesठ| $144,598 | $239,507 | <.05 | Paired t |
| Mortality%† (n) | 8 (2) | 16 (4) | NS | Chi-square |
Thirteen pairs were compared.
Comparison of 24 pairs
Analysis includes patients alive until discharge. Nineteen pairs were compared.
Data from the burn registry
DISCUSSION
The presence of elevated BAC was associated with notable changes in both early and extended clinical outcomes in patients closely matched for age, sex, burn size, burn mechanism, and inhalation injury. From our review of the literature, there have been no studies in burn patients matching for injury severity to examine the physiologic differences after the combined insult of alcohol intoxication and burn injury. We chose not to look at the presence of other drugs. Based on findings from our institution15 and others, 16 there is evidence that other drugs do not have an appreciable effect on clinical outcomes and are not a significant predictor of death in burn patients.
One of the major limitations of the study is the lack of information regarding the acute or chronic nature of the patients’ alcohol use. From the medical records, there was no way to elicit if the patients are more likely to be acute binge drinkers or chronic dependent drinkers. In future studies, using our ongoing screening with the Alcohol Use Disorders Identification Test (AUDIT), we will be able to better assess the presence and severity of alcohol use disorders, in burn patients with positive BAC.8The AUDIT could also be used to differentiate between the impact of heavy sustained alcohol use and episodic drinking; as the AUDIT is quantitative, the scores could be statistically compared with clinical outcomes. Another potential limitation of this study is that we did not collect information on time from injury to burn center resuscitation. However, while the issue of long-distance transfer has been an concern in the past, emergency medical systems have improved and removed some of the impact of transport on outcomes and mortality in burn patients.17,18 Unfortunately, data on time from injury to BAC measurement was not readily available. It is true that some patients who had an undetectable BAC may have had alcohol in their system at the time of injury, leading to misclassification as controls or omission as cases. To prevent some of this, we chose a BAC threshold of 30 mg/dl to ensure that the patients would continue to have alcohol present during the first few hours of hospitalization and to test the effect of alcohol on resuscitation. In our small study population, it was necessary to analyze alcohol exposure as an all or none phenomenon, not in a dose-response manner. The number of outcomes chosen also limits the study. Additional clinical outcomes would be interesting to review, for example, number of operations and types of interventions as well as time to healing, infectious complications (eg, ventilator associated pneumonia, cellulites, etc.) and discharge status (eg, home, acute rehabilitation facility, nursing home). In this study population, we considered correlating alcohol exposure with biomarkers of nutritional status, as this can influence outcomes, but too many of these data were missing for successful analysis. However, nutritional aspects of our burn service are guided by strict protocols. The same dietitian follows all patients with uniform goals driving their care.
More information is required to examine the demographics of alcohol consumption in the burn population in order to tailor therapies as well as to study clinical outcomes more thoroughly. We can, however, extrapolate from the research in the trauma population, showing that most patients who drink are binge, or heavy episodic drinkers and a smaller percentage are dependent drinkers.19,20 Though these data are highly variable by institution, this gives some indication that our patients are more likely to be acutely intoxicated and not dependent drinkers. However, we can definitively conclude that routine BAC testing is an essential part of the initial assessment of burn patients as they are a group at high risk for complications associated with alcohol intoxication.
Correlating our findings with prior clinical studies in burn patients, others have shown a 2- to 6-fold increase in mortality in burn patients with who consumed alcohol prior to sustaining their injuries.15,21 Although in our study the clinical effects of elevated BAC were readily apparent in multiple clinical outcomes, the crude mortality was not statistically different. Though there was an apparent 100% increased in the number of deaths in the BAC+ group, the study population is too small and underpowered to show statistical significance with respect to crude mortality. However, as our clinical findings are consistent with others we can predict that there would likely be differences in mortality in larger study populations.
The differences in the Apache II score, 24-hour fluid requirements, and base deficit suggest an early acting mechanism of physiologic impairment. This is most likely secondary to further exacerbation of the already substantial inflammatory response to burn injury. 22,23 Moreover, the fact that these early clinical outcomes were significantly different in this limited number of paired samples demonstrates a more profound influence of ethanol exposure than previously demonstrated. The finding that early physiologic changes occurred in association with alterations in the extended outcomes, such as ventilator time and hospital length of stay, suggests a prolonged systemic response to the alcohol insult and the involvement of multiple systems. The current experimental research paradigm using rodent models of alcohol exposure and burn injury demonstrate aberrant inflammatory and immune responses that results in an increased susceptibility to infections, sepsis, and wound healing complications.7,24 Due to the complexity of the host response to burn injury and the lack of clinical correlation with existing laboratory studies, the underlying mechanism of action of alcohol’s effects on inflammatory and immune function in burn patients remains unclear. That alcohol exposure contributes to immune suppression is well supported by animal models in which burn size and depth, alcohol exposure, subject age, sex and experimental conditions are well controlled. Studies utilizing rodent models of burn injury demonstrate that alcohol exposure magnifies the burn-induced impairment of immune function resulting in impaired gut barrier function,25 macrophage function,26,27 T-cell function,28 and angiogenesis, 29 in addition to aberrant production of pro-inflammatory cytokines,30 and increased susceptibility to infection.26,31,32
Examining the considerable differences in increased ventilator days and length of stay, the data strongly support a clinical picture of a more severe pulmonary insult in the intoxicated cases. Other clinical studies have also documented increased pulmonary complications, like pneumonia.16 The experimental work on the pulmonary complications of alcohol and burn injury relates mostly to pulmonary edema and increased vascular permeability.31,33,34 Looking at these factors in combination, the intoxicated burn patient presents a complicated clinical balance. Similar to trauma patients with pulmonary contusions, it would seem that the goal would be to maintain a conservative fluid resuscitation strategy in intoxicated burn patients. However, the early increased fluid requirement of intoxicated burn patients makes this a difficult equilibrium. A larger study utilizing prospective data collection and translational studies for correlation with data from animal models is clearly necessary to clarify the pathways and mechanisms responsible for the observed pulmonary morbidity. In conclusion, this study adds to the body of literature showing that alcohol intoxication is not only a risk factor for injury and increased injury severity, but also plays a role in the physiologic response surrounding burn injury.
CONCLUSION
In this retrospective case– control study, the presence of a positive BAC on admission was associated with early physiologic impairment leading to later morbidity. The increased ventilator time and length of stay were substantial and led to increased hospital charges in the surviving patients. The mechanisms responsible for the early and late effects of ethanol require further study utilizing a translational approach with a prospective sampling and data collection protocol. This study demonstrates the need for additional prospective study and the potential value of new information that would lead to new and improved patient management strategies.
ACKNOWLEDGMENTS
We thank Melanie D. Bird, PhD for her discussion on this project.
This study was supported by a grant NIH R01 AA12034 (to E.J.K.), T32 AA013527 (to E.J.K.), R01 GM042577 (to R.L.G.), R01 AA015067 (to C.R.S.). The Illinois Excellence in Academic Medicine Grant (to E.J.K.), and The Ralph and Marian C. Falk Medical Research Trust (to R.L.G.).
REFERENCES
- 1.Thal ER, Bost RO, Anderson RJ. Effects of alcohol and other drugs on traumatized patients. Arch Surg. 1985;120:708–712. doi: 10.1001/archsurg.1985.01390300058010. [DOI] [PubMed] [Google Scholar]
- 2.Nelson S, Kolls JK. Alcohol, host defence and society. Nat Rev Immunol. 2002;2:205–209. doi: 10.1038/nri744. [DOI] [PubMed] [Google Scholar]
- 3.Howland J, Hingson R. Alcohol as a risk factor for injuries or death due to fires and burns: review of the literature. Public Health Rep. 1987;102:475–483. [PMC free article] [PubMed] [Google Scholar]
- 4.Rutledge R, Messick WJ. The association of trauma death and alcohol use in a rural state. J Trauma. 1992;33:737–742. doi: 10.1097/00005373-199211000-00024. [DOI] [PubMed] [Google Scholar]
- 5.Barillo DJ, Goode R. Substance abuse in victims of fire. J Burn Care Rehabil. 1996;17:71–76. doi: 10.1097/00004630-199601000-00014. [DOI] [PubMed] [Google Scholar]
- 6.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829. [PubMed] [Google Scholar]
- 7.Choudhry MA, Chaudry IH. Alcohol intoxication and post-burn complications. Front Biosci. 2006;11:998–1005. doi: 10.2741/1857. [DOI] [PubMed] [Google Scholar]
- 8.Soderstrom CA, Dischinger PC, Kerns TJ, et al. Screening trauma patients for alcoholism according to NIAAA guidelines with alcohol use disorders identification test questions. Alcohol Clin Exp Res. 1998;22:1470–1475. [PubMed] [Google Scholar]
- 9.Wachtel TL, Berry CC, Wachtel EE, Frank HA. The inter-rater reliability of estimating the size of burns from various burn area chart drawings. Burns. 2000;26:156–170. doi: 10.1016/s0305-4179(99)00047-9. [DOI] [PubMed] [Google Scholar]
- 10.Scott-Conner CE, Clarke KM, Conner HF. Burn area measurement by computerized planimetry. J Trauma. 1988;28:638–641. doi: 10.1097/00005373-198805000-00013. [DOI] [PubMed] [Google Scholar]
- 11.Baxter C. Fluid resuscitation, burn percentage, and physiologic age. J Trauma. 1979;19 Suppl 11:864–865. [PubMed] [Google Scholar]
- 12.Cartotto RC, Innes M, Musgrave MA, Gomez M, Cooper AB. How well does the Parkland formula estimate actual fluid resuscitation volumes? J Burn Care Rehabil. 2002;23:258–265. doi: 10.1097/00004630-200207000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Jeng JC. Patrimonie de Docteur Baux-Baux scores ≫ 100 gleaned from 170,791 admissions: a glimmer from the National Burn Repository. J Burn Care Res. 2007;28:380–381. doi: 10.1097/BCR.0B013E318053D3F4. [DOI] [PubMed] [Google Scholar]
- 14.Wibbenmeyer LA, Amelon MJ, Morgan LJ, et al. Predicting survival in an elderly burn patient population. Burns. 2001;27:583–590. doi: 10.1016/s0305-4179(01)00009-2. [DOI] [PubMed] [Google Scholar]
- 15.McGill V, Kowal-Vern A, Fisher SG, Kahn S, Gamelli RL. The impact of substance use on mortality and morbidity from thermal injury. J Trauma. 1995;38:931–934. doi: 10.1097/00005373-199506000-00019. [DOI] [PubMed] [Google Scholar]
- 16.Grobmyer SR, Maniscalco SP, Purdue GF, Hunt JL. Alcohol, drug intoxication, or both at the time of burn injury as a predictor of complications and mortality in hospitalized patients with burns. J Burn Care Rehabil. 1996;17(6 Pt 1):532–539. doi: 10.1097/00004630-199611000-00010. [DOI] [PubMed] [Google Scholar]
- 17.Klein MB, Nathens AB, Heimbach DM, Gibran NS. An outcome analysis of patients transferred to a regional burn center: transfer status does not impact survival. Burns. 2006;32:940–945. doi: 10.1016/j.burns.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 18.Klein MB, Nathens AB, Emerson D, Heimbach DM, Gibran NS. An analysis of the long-distance transport of burn patients to a regional burn center. J Burn Care Res. 2007;28:49–55. doi: 10.1097/BCR.0B013E31802C894B. [DOI] [PubMed] [Google Scholar]
- 19.Schermer CR. Alcohol and injury prevention. J Trauma. 2006;60:447–451. doi: 10.1097/01.ta.0000196956.49282.91. [DOI] [PubMed] [Google Scholar]
- 20.Danielsson PE, Rivara FP, Gentilello v, Maier RV. Reasons why trauma surgeons fail to screen for alcohol problems. Arch Surg. 1999;134:564–568. doi: 10.1001/archsurg.134.5.564. [DOI] [PubMed] [Google Scholar]
- 21.Haum A, Perbix W, Hack HJ, Stark GB, Spilker G, Doehn M. Alcohol and drug abuse in burn injuries. Burns. 1995;21:194–199. doi: 10.1016/0305-4179(95)80008-c. [DOI] [PubMed] [Google Scholar]
- 22.Dehne MG, Sablotzki A, Hoffmann A, et al. Alterations of acute phase reaction and cytokine production in patients following severe burn injury. Burns. 2002;28:535–542. doi: 10.1016/s0305-4179(02)00050-5. [DOI] [PubMed] [Google Scholar]
- 23.Barret JP, Herndon DN. Modulation of inflammatory and catabolic responses in severely burned children by early burn wound excision in the first 24 hours. Arch Surg. 2003;138:127–132. doi: 10.1001/archsurg.138.2.127. [DOI] [PubMed] [Google Scholar]
- 24.Messingham KA, Faunce DE, Kovacs EJ. Alcohol, injury, and cellular immunity. Alcohol. 2002;28:137–149. doi: 10.1016/s0741-8329(02)00278-1. [DOI] [PubMed] [Google Scholar]
- 25.Kavanaugh MJ, Clark C, Goto M, et al. Effect of acute alcohol ingestion prior to burn injury on intestinal bacterial growth and barrier function. Burns. 2005;31:290–296. doi: 10.1016/j.burns.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 26.Faunce DE, Gregory MS, Kovacs EJ. Effects of acute ethanol exposure on cellular immune responses in a murine model of thermal injury. J Leukoc Biol. 1997;62:733–740. doi: 10.1002/jlb.62.6.733. [DOI] [PubMed] [Google Scholar]
- 27.Messingham KA, Heinrich SA, Kovacs EJ. Estrogen restores cellular immunity in injured male mice via suppression of interleukin-6 production. J Leukoc Biol. 2001;70:887–895. [PubMed] [Google Scholar]
- 28.Choudhry MA, Messingham KA, Namak S, et al. Ethanol exacerbates T cell dysfunction after thermal injury. Alcohol. 2000;21:239–243. doi: 10.1016/s0741-8329(00)00093-8. [DOI] [PubMed] [Google Scholar]
- 29.Radek KA, Matthies AM, Burns AL, Heinrich SA, Kovacs EJ, Dipietro LA. Acute ethanol exposure impairs angiogenesis and the proliferative phase of wound healing. Am J Physiol Heart Circ Physiol. 2005;289:H1084–H1090. doi: 10.1152/ajpheart.00080.2005. [DOI] [PubMed] [Google Scholar]
- 30.Faunce DE, Gregory MS, Kovacs EJ. Acute ethanol exposure prior to thermal injury results in decreased T-cell responses mediated in part by increased production of IL-6. Shock. 1998;10:135–140. doi: 10.1097/00024382-199808000-00009. [DOI] [PubMed] [Google Scholar]
- 31.Murdoch EL, Brown HG, Gamelli RL, Kovacs EJ. Effects of ethanol on pulmonary inflammation in post-burn intratracheal infection. J Burn Care Res. 2008;29:323–330. doi: 10.1097/BCR.0b013e3181667599. [DOI] [PubMed] [Google Scholar]
- 32.Faunce DE, Garner JL, Llanas JN, et al. Effect of acute ethanol exposure on the dermal inflammatory response after burn injury. Alcohol Clin Exp Res. 2003;27:1199–1206. doi: 10.1097/01.ALC.0000075833.92139.35. [DOI] [PubMed] [Google Scholar]
- 33.Patel PJ, Faunce DE, Gregory MS, Duffner LA, Kovacs EJ. Elevation in pulmonary neutrophils and prolonged production of pulmonary macrophage inflammatory protein-2 after burn injury with prior alcohol exposure. Am J Respir Cell Mol Biol. 1999;20:1229–1237. doi: 10.1165/ajrcmb.20.6.3491. [DOI] [PubMed] [Google Scholar]
- 34.Li X, Kovacs EJ, Schwacha MG, Chaudry IH, Choudhry MA. Acute alcohol intoxication increases interleukin-18-mediated neutrophil infiltration and lung inflammation following burn injury in rats. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1193–L1201. doi: 10.1152/ajplung.00408.2006. [DOI] [PubMed] [Google Scholar]