Abstract
BRCA1 and the DNA helicase FANCJ (also known as BACH1 or BRIP1) have common functions in breast cancer suppression and DNA repair. However, the functional significance of the direct interaction between BRCA1 and FANCJ remains unclear. Here, we have discovered that BRCA1 binding to FANCJ regulates DNA damage repair choice. Thus, when FANCJ binding to BRCA1 is ablated, the molecular mechanism chosen for the repair of damaged DNA is dramatically altered. Specifically, a FANCJ protein that cannot be phosphorylated at serine 990 or bind BRCA1 inhibits DNA repair via homologous recombination and promotes polη-dependent bypass. Furthermore, the polη-dependent bypass promoted by FANCJ requires the direct binding to the mismatch repair (MMR) protein, MLH1. Together, our findings implicate that in human cells BRCA1 binding to FANCJ is critical to regulate DNA repair choice and promote genomic stability. Moreover, unregulated FANCJ function could be associated with cancer and/or chemoresistance.
Keywords: BRCA1, FANCJ, DNA repair
Introduction
BRCA1 function and tumor suppression depend on the BRCA1 C-terminal (BRCT) region, which contains two discrete domains called BRCT repeats. Mutations in the BRCT repeats result in defective DNA damage repair, such as failure to induce cell-cycle checkpoints and sensitivity to DNA double-stranded breaks (DSBs) and interstrand cross-links (ICLs) (Kim and Chen, 2008). These BRCTs also mediate the direct binding of BRCA1 to the FANCJ DNA helicase (also known as FANCJ/BRIP1) (Cantor et al., 2001). FANCJ phosphorylation at serine 990 is required to mediate the BRCT-FANCJ interaction such that a serine to alanine mutation (S990A) ablates this interaction (Yu et al., 2003). Similar to BRCA1, mutations in FANCJ have been associated with hereditary breast cancer (Cantor et al., 2001; Seal et al., 2006). In addition, FANCJ is mutated in the rare childhood disease, Fanconi anemia (FA), within the FANCJ (FA-J) patient complementation group (Levitus et al., 2005; Levran et al., 2005; Litman et al., 2005).
It has been proposed that the FA pathway maintains genomic integrity by coordinating at least two DNA repair mechanisms. One of these mechanisms is homologous recombination (HR), a typically error-free DNA-repair mechanism that uses the homologous sequence in a sister chromatid for repair. HR is critical to repair DSBs, which can form when ICLs fail to be processed and replication forks collapse. Defects in HR characterize BRCA1-, FANCJ-, and BRCA2-deficient cells (Moynahan et al., 1999, 2001; Litman et al., 2005). Evidence also suggests that the FA pathway promotes ICL resolution by a mechanism engaging the error-prone translesion synthesis (TLS) polymerases. Supporting this possibility, fewer TLS-like point mutations are present in the genome of FA cells (Patel and Joenje, 2007). Moreover, the TLS polymerases REV1 and REV3 function with the FA protein FANCC to promote ICL resistance (Niedzwiedz et al., 2004). Consequently, the FA pathway has been proposed to coordinate both TLS and HR to resolve DNA ICLs as well as other DNA lesions thereby limiting the severity of mutagenesis (Niedernhofer et al., 2005; Hinz et al., 2006; Patel and Joenje, 2007).
Current models predict that TLS can function independent of HR. TLS is not expected to repair lesions, but facilitate lesion tolerance or bypass (Dronkert and Kanaar, 2001). In particular, polη has been implicated in recombination-independent repair of ICLs generated by mitomycin C (MMC) (Zheng et al., 2003) as well as in the cellular tolerance to cisplatin (Albertella et al., 2005). Because of the unique structure of its active site, polη replicates through cross-linked DNA (Alt et al., 2007). Depending on the lesion bypassed, TLS can be mutagenic or error-free. For example, polη bypasses ultraviolet (UV) light-induced thymidine–thymidine dimers in an error-free manner and bypasses intrastrand cross-links and ICLs, once unhooked, in an error-prone manner (Zheng et al., 2003; Prakash et al., 2005). Depending on the type and severity of DNA damage, TLS is activated by a Rad6–Rad18-dependent PCNA monoubiquitination (Kannouche and Lehmann, 2004) that loads different TLS polymerases in a lesion-specific manner (Barbour and Xiao, 2003; Papouli et al., 2005).
Given that both FANCJ and BRCA1 are critical for HR, genomic stability, breast cancer suppression, and ICL resistance (Cantor and Andreassen, 2006), we hypothesized that these two proteins likely function together in ICL repair. However, recent studies support independent functions. In particular, the ICL sensitivity of FANCJ-null chicken and patient cells was rescued with mutant versions of FANCJ that cannot interact with BRCA1 (Bridge et al., 2005; Peng et al., 2007). This finding leads one to wonder what is the functional role for the BRCA1–FANCJ interaction.
Here, we identify that when uncoupled from BRCA1, FANCJ functions to inhibit HR and promote polη-dependent bypass. As such, FA-J patient cells expressing the BRCA1-interaction defective mutant, FANCJS990A resist DNA damage in a polη-dependent manner. Furthermore, FANCJS990A requires the MLH1 interaction to promote resistance to agents that induce ICLs. Together, our data implicate that in human cells, BRCA1 binding to FANCJ is critical to regulate DNA repair and bypass mechanisms to promote genomic stability.
Results
The DNA damage response is altered when FANCJ is uncoupled from BRCA1
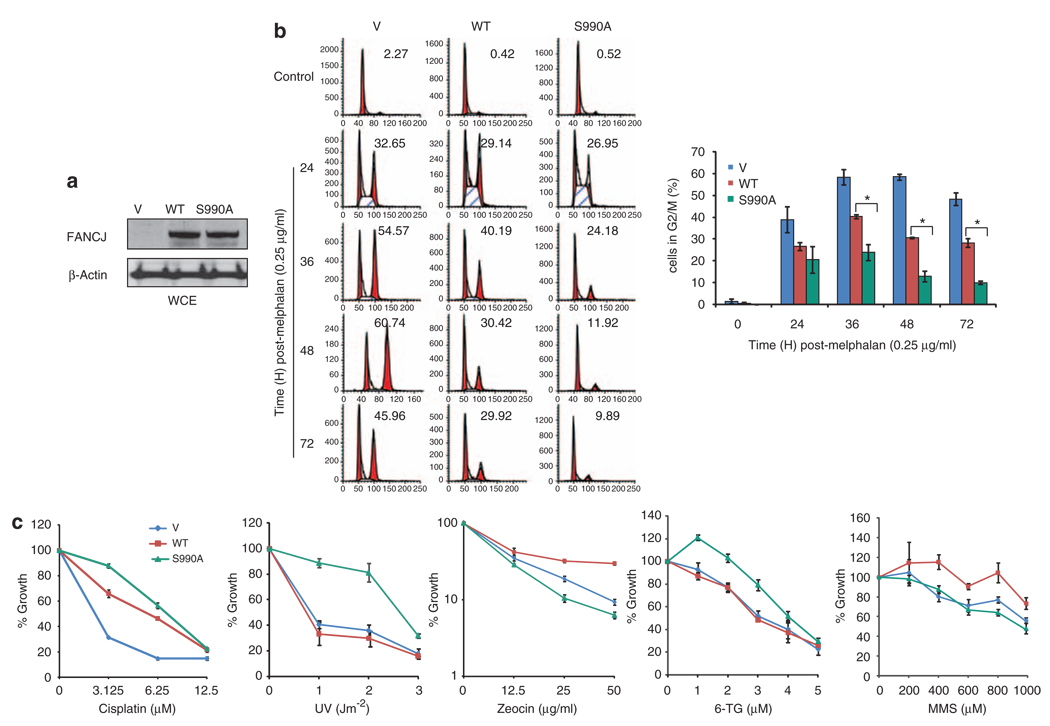
In response to ICLs, FA cells undergo a prolonged G2/M accumulation that correlates with ICL sensitivity (Wang, 2007). Restoration of the missing FA gene, such as FANCJ in FA-J patient cells, restores ICL resistance and reduces the G2/M accumulation, most likely because cells process ICLs and re-enter the cell cycle (Litman et al., 2005; Peng et al., 2007). The finding that correction is also achieved on introduction of the BRCA1-interaction defective mutant FANCJS990A (Peng et al., 2007) could suggest that BRCA1 binding is dispensable for FANCJ to process ICLs. Alternatively, FANCJS990A could promote ICL resistance by a distinct mechanism from FANCJWT. For example, the restored ICL resistance and reduced G2/M accumulation in FA-J cells expressing FANCJS990A could have resulted from a disruption of the ICL-induced checkpoint. To test this idea, we examined the timing of entry and exit from the ICL-induced G2/M accumulation in FA-J cells complemented with vector, FANCJWT, or FANCJS990A. Expression was confirmed by western blot and as before both FANCJWT and FANCJS990A restored ICL resistance (Peng et al., 2007) (Figures 1a and c; Supplementary Figure 1A). Interestingly, the maximum G2/M accumulation in the FANCJS990A complemented FA-J cells was ~25%, as compared with FANCJWT at ~40% (Figure 1b). Fewer FA-J cells accumulated at G2/M at all times when they were complemented with FANCJS990A than when complemented with FANCJWT or vector (Figure 1b). However, the growth of untreated cells was not measurably different (Supplementary Figure 1b). Thus, FANCJS990A reduced, but did not eliminate, the melphalan-induced G2/M accumulation. More importantly, this finding implicated that FA-J cells complemented with FANCJS990A or FANCJWT were distinct in the melphalan-induced response.
Figure 1.
FANCJS990A, as compared with FANCJWT, promotes a distinct DNA damage response in FA-J cells. (a) FANCJ-null FA-J cells were complemented with vector, FANCJWT, or FANCJS990A and lysates were analyzed by immunoblot with the indicated antibodies (Abs). (b) The FA-J cell lines were treated constitutively with 0.25 µg/ml melphalan, collected at the indicated times, and analyzed by FACS to determine the percentage of cells in G2/M. A representative experiment is shown. The bar graph represents mean ± s.d. from three independent experiments. The asterisk indicates a significant difference (P<0.05, unpaired t-test). (c) The FA-J cells expressing vector, FANCJWT, or FANCJS990A were plated at low density, treated with the indicated doses of cisplatin, UV, zeocin, 6-thioguanine, or methyl methanesulfonate and allowed to grow for 5–8 days. The cells were then collected and counted to analyze percent growth. Data represent mean percent ± s.d. of growth from three independent experiments.
To further assess whether FANCJS990A, as compared with FANCJWT, promoted a distinct DNA damage repair response, the complemented FA-J cell lines were treated with different forms of DNA damage. Interestingly, FANCJS990A-, as compared with FANCJWT-, complemented FA-J cells were slightly more resistant to cisplatin, but dramatically more resistant to UV and 6-thioguanine (6-TG). In contrast, FANCJS990A-, as compared with FANCJWT-, complemented FA-J cells were more sensitive to zeocin or methyl methanesulfonate (MMS) (Figure 1c). Together with the G2/M accumulation data, it appears that the DNA damage response is distinct in FA-J cells complemented with FANCJS990A as compared to FANCJWT.
FANCJS990A reduces RAD51 foci and HR
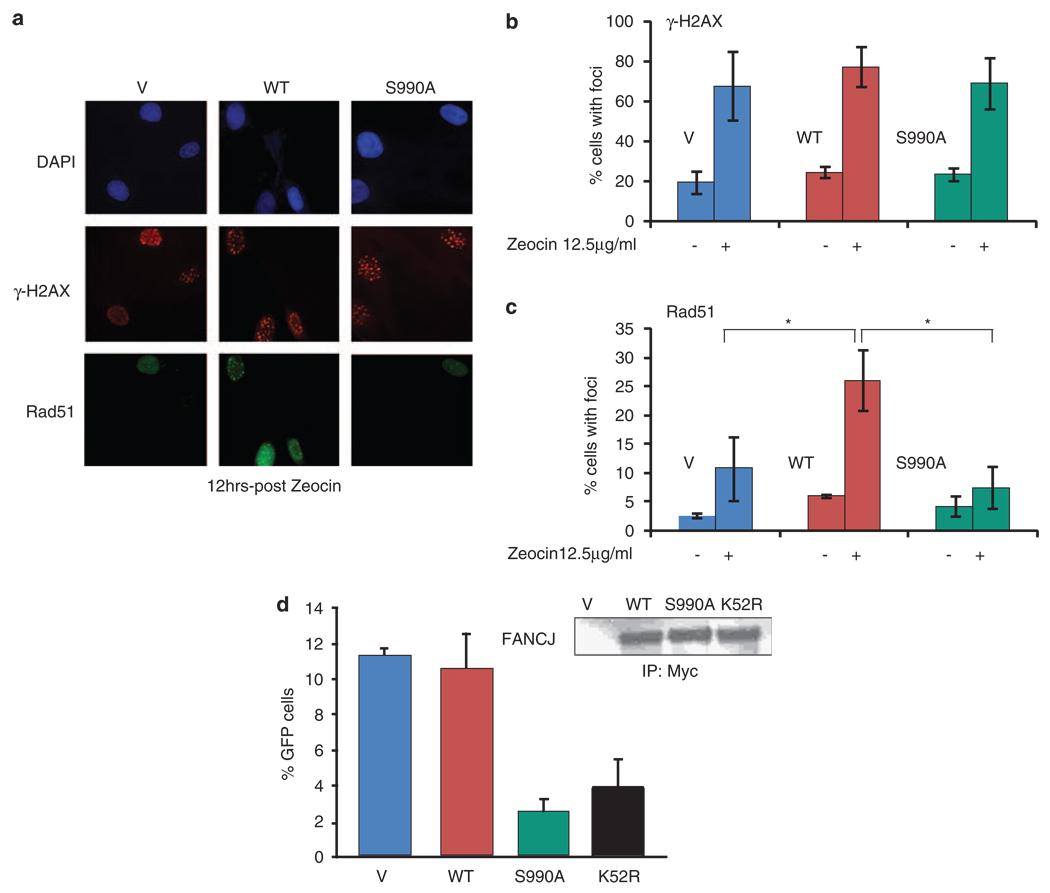
Zeocin induces DSBs and sensitizes HR-defective cells (Delacote et al., 2007). Thus, we reasoned the zeocin sensitivity of FA-J cells complemented with FANCJS990A could result from defects in double-strand break repair and/or HR. Formation of DSBs corresponds with nuclear γ-H2AX foci formation. Treatment with zeocin induced the formation of γ-H2AX foci to the same extent in FANCJWT- and FANCJS990A- complemented FA-J cells as detected by immunofluorescence (Figures 2a and b). In contrast, RAD51 foci were not similarly detected (Figures 2a and c; Supplementary Figure 2). As compared with untreated cells, at 12 h post-zeocin treatment RAD51 foci were induced ~5-fold in FANCJWT, as compared with ~2-fold in FANCJS990A or ~3.5-fold in vector complemented FA-J cells (Figure 2c). To rule out the possibility that zeocin differentially affected the FA-J cell lines and the number of cells in S-phase, in which RAD51 foci are most prominent, we measured cell-cycle distributions and found no significant differences (Supplementary Figure 3A).
Figure 2.
FANCJS990A reduces DSB-induced RAD51 foci and HR. (a) The FA-J cell lines were treated with 12.5 µg/ml of zeocin and immunofluorescence was performed with γ-H2AX and RAD51 Abs. A representative image is shown to depict how staining was observed. (b) The γ-H2AX and (c) RAD51 foci were quantitated based on a cell being positive (> 10) foci per 300 DAPI positive cells from three independent experiments. Asterisk indicates significant difference (P<0.05, unpaired t-test). (d) DR-U2OS cells were cotransfected with the I-Sce-1 endonuclease and vector, FANCJWT, FANCJS990A, or FANCJK52R, collected and either lysed and immunoprecipitated followed by immunoblot with the indicated Abs or analyzed by FACS. The bar graph shows the percentage of GFP-positive cells.
Next, we examined the consequence of expressing FANCJ mutants on DSB-induced HR. DSBs were induced by transient expression of I-Sce1 in an established U2OS cell line containing an integrated copy of the pDR-GP reporter. With this reporter, if HR occurs, GFP is expressed, which is quantifiable by flow cytometric analysis (Pierce et al., 1999). Consistent with the possibility that HR was reduced as a result of more unbound FANCJ, expression of FANCJS990A reduced the number of cells with restored GFP. Compared with that in U2OS cells with FANCJWT or vector, U2OS cells with FANCJS990A and FANCJK52R reduced HR, 4.5- and 3-fold, respectively (Figure 2d). FANCJ species were expressed similarly (Figure 2d). FANCJWT precipitated similar ratio of BRCA1 as endogenous FANCJ, and as expected, the FANCJS990A did not precipitate BRCA1 (Supplementary Figure 4A). Despite reduced HR due to expression of FANCJS990A or FANCJK52R, only U20S cells expressing FANCJK52R were sensitive to MMC (Supplementary Figure 4B). Together, these data suggested that expression of FANCJS990A in U20S or FA-J cells reduced RAD51-based HR, but not crosslink resistance.
MMC-induced polη foci are enhanced in U2OS cells expressing FANCJS990A
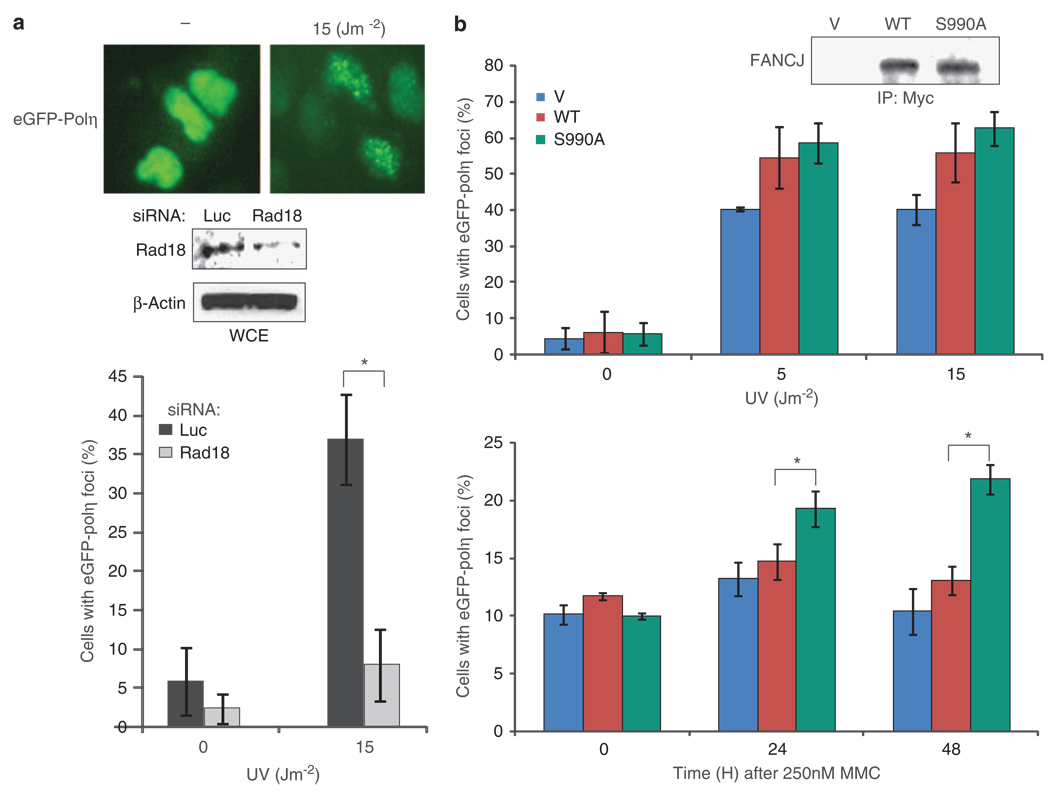
Given these findings, we hypothesized that resistance to agents that induce ICLs was mediated by a recombination-independent mechanism such as TLS. In particular, the TLS polymerase polη can facilitate bypass of lesions that escape excision repair such as unhooked ICLs and UV lesions (Kannouche and Lehmann, 2004; Nojima et al., 2005). Thus, we sought to examine whether the reduced HR in U2OS cells expressing FANCJS990A corresponded with elevated eGFP-polη foci that have been shown to form in response to UV (Kannouche et al., 2001). First, we sought to confirm this UV-dependent eGFP-polη foci formation and its dependence on Rad18 (Watanabe et al., 2004). U2OS cells were cotransfected with eGFP-polη and -siRNA targeting luciferase (luc) or Rad18; UV irradiated and cells positive for eGFP-polη foci were counted. In response to UV, polη foci clearly formed in B35% of the cells (Figure 3a). Moreover, the number of cells with eGFP-polη foci was reduced ~4-fold when Rad18 was depleted. Next, the U2OS cells stably expressing vector, FANCJWT, or FANCJS990A (Figure 3b) were transfected with the eGFP-polη fusion protein, UV irradiated, and analyzed for eGFP-polη foci. Expression of FANCJS990A did not affect the number of polη foci in untreated cells, but in UV-irradiated cells expressing FANCJS990A or FANCJWT, ~30% more cells with eGFP-polη foci were detected as compared with the vector controls (Figure 3b). In contrast to UV, MMC-treated cells showed few cells with polη foci, suggesting that repair of ICLs does not readily activate polη. However, when cells expressed FANCJS990A, polη foci were readily observed (Figure 3b) consistent with the finding that FANCJS990A enhances TLS in response to MMC. To rule out the possibility that DNA damage differentially affects the number of cells in S-phase, in which polη foci are most prominent (Kannouche et al., 2001), we measured cell-cycle distributions before and after UV or MMC in control or FANCJ over-expressed cells and found no significant changes (Supplementary Figure 3b).
Figure 3.
FANCJ enhances DNA damage-induced polη foci. (a) U2OS cells were cotransfected with siRNA for luc or Rad18 and eGFP-polη and either collected for immunoblot with the indicated Abs or UV irradiated and assessed for eGFP-polη foci. Cells were assessed for eGFP-polη foci by autofluorescence. (b) U2OS cells stably expressing vector, FANCJS990A, or FANCJWT were transfected with eGFP-polη and either collected for immunoblot with the indicated Abs or UV irradiated with indicated dose with 4 h incubation or treated with 250 nm MMC with incubation at varying times. Data represent the mean percent ± s.d. cells positive (> 10) green foci per 300 DAPI positive cells from three independent experiments. Asterisk indicates significant difference (P<0.05, unpaired t-test).
Cells expressing FANCJS990A rely on polη for MMC resistance
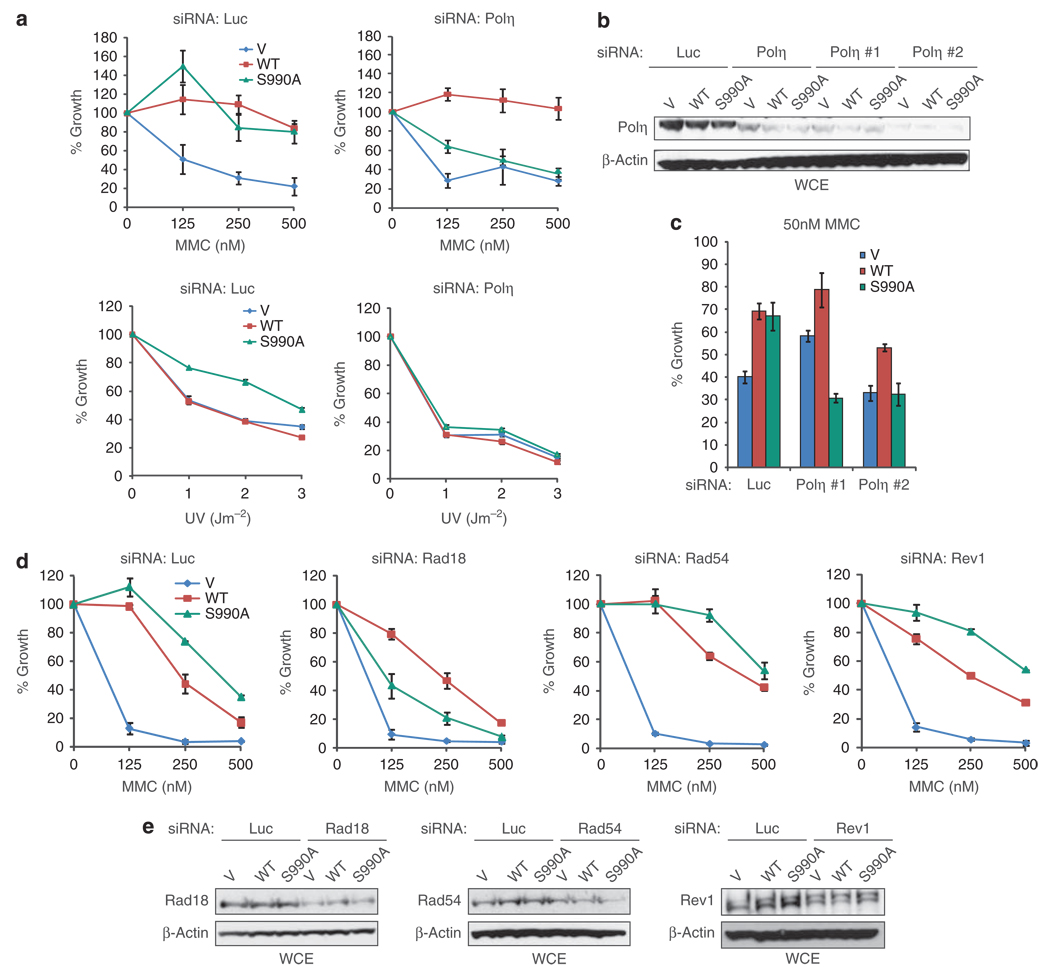
Given that polη readily bypasses UV-induced thymidine–thymidine dimers as well as MMC-induced ICLs (Zheng et al., 2003; Prakash et al., 2005), we considered that the MMC and hyper UV resistance of FA-J cells expressing FANCJS990A (Figure 1c) could rely on polη. To address this possibility, the FA-J cells lines were transfected with siRNA targeting polη or luc control. Consistent with FANCJS990A promoting DNA cross-link resistance in a manner that requires polη, polη-depletion uniquely reversed the MMC and UV resistance in FANCJS990A-, as compared with FANCJWT-, or vector complemented FA-J cells (Figures 4a and b). Consistent with this effect being due to polη-depletion and not off-target affects, depletion of polη with either of two distinct siRNAs was substantial B90% (Figure 4b) and also uniquely reversed the MMC sensitivity of FA-J cells expressing FANCJS990A (Figure 4c). As Rad18 is required for polη foci formation (Figure 3a) (Watanabe et al., 2004), we tested how Rad18 depletion affected the MMC resistance of the FA-J cell lines. Similar to polη-depletion, Rad18-depletion, uniquely sensitized FA-J cells complemented with FANCJS990A, as compared with FANCJWT or vector (Figure 4d). Likewise, depletion of Rad18 with either of two distinct siRNAs reversed the ICL resistance of FA-J cells expressing FANCJS990A (Supplementary Figure 5A). Furthermore, the affect did not appear restricted to FA-J cells as transient over-expression of FANCJS990A, as compared with FANCJWT, in U2OS cells also promoted UV and MMC resistance in a Rad18 and polη-dependent manner (Supplementary Figure 5B and Figure 6). In contrast, Rad54- or Rev1-siRNAs did not affect the MMC resistance of the FA-J cell lines (Figure 4d). Introduction of siRNAs into the FA-J cell lines had minimal affect on cell viability (Supplementary Figure 7). Immunoblot analysis revealed that Rad18, Rad54, and Rev1 were depleted; however, Rad54 and Rev1 expression was lower in vector FA-J cells and only partially reduced by siRNAs (Figure 4e). Thus, the data most clearly show that FA-J cells expressing FANCJS990A, in contrast to FANCJWT, rely on polη-dependent bypass for ICL resistance.
Figure 4.
FANCJS990A promotes ICL and UV resistance in a polη-dependent manner. (a) FA-J cells stably expressing vector, FANCJWT, or FANCJS990A (Figure 1a) were transfected with siRNA to Luc or polη, incubated for 48 h, treated with MMC or UV, and percent growth was assessed as in Figure 1. (b) FA-J cell lines transfected with siRNA to luc, polη, polη #1, or polη #2 were collected for immunoblot with the indicated Abs. (c) The FA-J cell lines with indicated siRNAs were incubated for 48 h, treated with MMC at the IC50 dose, and percent growth was assessed as in Figure 1. Graph shows the percent growth mean±s.d. (d) FA-J cells stably expressing vector, FANCJWT, or FANCJS990A were transfected with siRNA to luc, Rad18, Rad54, or Rev1 and incubated for 48 h, treated with MMC, and percent growth was assessed as in Figure 1. (e) Cells used in (d) were collected and lysed for immunoblot with the indicated Abs.
To confirm the polη dependence of our findings, we next tested whether over-expression of FANCJS990A differentially affected the MMC or UV resistance of polη-null or polη-complemented XPV patient cells (Kannouche et al., 2001). As expected, the polη-complemented XPV cells expressed polη and were more resistant to UV than the non-complemented XPV cells (Kannouche et al., 2001) (Supplementary Figures 8A and B). Next, vector and polη-complemented lines were transfected with vector, FANCJWT, or FANCJS990A, expression was analyzed, and viability in response to MMC or UV was tested in survival assays (Supplementary Figure 8B). Transfection of FANCJS990A, as compared with FANCJWT or vector, enhanced UV and MMC resistance in the polη-complemented XPV cells, but not in the vector-complemented XPV cells (Supplementary Figure 8B). Altogether, the data strongly support that the BRCA1-interaction defective mutant, FANCJS990A promotes resistance to MMC and UV in a polη-dependent manner.
FANCJS990A MLH1 requires binding to promote ICL resistance
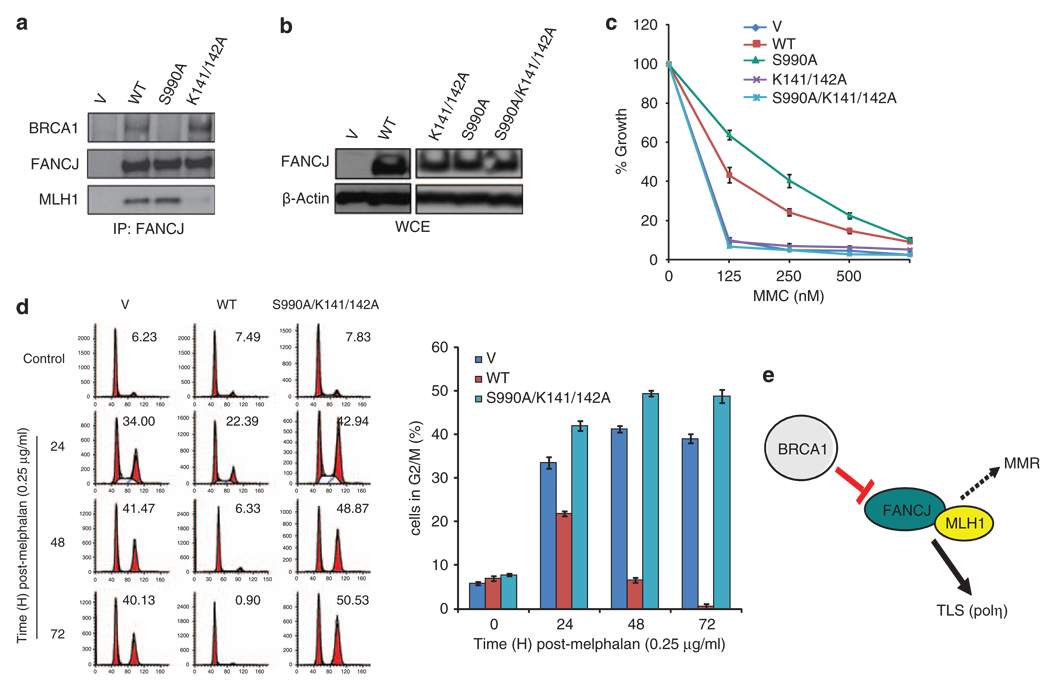
Previously, we identified that FANCJWT binds directly to MLH1, and this interaction is required for MMC resistance (Peng et al., 2007). MLH1 binds directly to the FANCJ helicase domain through lysines 141 and 142. To address whether the MLH1 interaction with FANCJS990A was also required for MMC resistance, we replaced lysines 141 and 142 of FANCJ with alanines (A), which ablates MLH1 binding, but does not alter FANCJ helicase function (Peng et al., 2007). FA-J cells were complemented with this triple mutant FANCJS990AK141/142A, FANCJS990A, FANCJK141/142A, vector, or FANCJWT. Expression and ablation of MLH1 and/or BRCA1 binding was confirmed by western blot (Figures 5a and b). In contrast to FANCJS990A, FANCJS990AK141/142A complemented FA-J cells were sensitive to MMC (Figure 5c). Furthermore, FA-J cells expressing FANCJS990AK141/142A underwent a prolonged G2/M accumulation in contrast to FA-J cells expressing FANCJWT or FANCJS990A (Figure 5d), suggesting that FANCJS990A promotes polη -dependent TLS in a MLH1-dependent manner (Figure 5e).
Figure 5.
FANCJS990A requires MLH1 binding to promote polη-dependent bypass. (a) FA-J cells stably expressing vector, FANCJWT, FANCJS990A, or FANCJK141/142A were collected and immunoprecipitated with anti-FANCJ Abs and blotted with the indicated Abs. (b) FA-J cells stably expressing vector, FANCJWT, FANCJS990A, FANCJK141/142A, or FANCJK141/142A/S990A were either collected for immunoblot with the indicated Abs or (c) treated with the indicated doses of MMC and allowed to grow for 5–8 days. The cells were then collected and counted to analyze percent growth. Data represent mean percent ± s.d. of growth from three independent experiments. (d) The FA-J cell lines were treated constitutively with 0.25 µg/ml melphalan, collected at the indicated times, and analyzed by FACS to determine the percentage of cells in G2/M. A representative experiment is shown. The bar graph represents mean ± s.d. from three independent experiments. (e) Model summarizes observations of this study. FANCJ when uncoupled from BRCA1 promotes polη-dependent TLS in a manner that requires MLH1 binding. Dotted line to MMR is added as a discussion point. To promote TLS, FANCJ could limit negative regulators of TLS, such as MMR.
Discussion
In this study, we explore the possibility that FANCJ binding to BRCA1 is important for ICL repair in mammalian cells. This possibility was proposed based on their direct binding and common functions in breast cancer suppression, HR, and ICL repair. We provide data that support this hypothesis by demonstrating that uncoupling FANCJ from BRCA1 alters the DNA damage response. Specifically, (1) cells expressing unbound FANCJ (FANCJS990A), unlike FANCJ that can bind BRCA1, are sensitive to DSBs, (2) have reduced RAD51-based HR, (3) survive cross-link DNA damage with a reduced G2/M accumulation, and (4) dependence on the TLS polymerase polη. Notably, depletion of polη sensitized FA-J cells complemented with FANCJS990A, but not FANCJWT, to MMC and reverted the hyper-UV resistance of FA-J or U2OS cells expressing FANCJS990A. Moreover, polη-null XPV cells transfected with FANCJS990A were not hyper-UV or MMC resistant unless polη was re-introduced. Together, these data suggest that FANCJ has anti-recombination and TLS functions that are normally regulated by BRCA1 binding. Moreover, we find that the BRCA1-bound or -unbound FANCJ requires its MLH1 interaction to promote ICL resistance.
FANCJ likely has a complex role in HR: contributes to HR when bound to BRCA1 and inhibits HR when unbound to BRCA1. If FANCJ functioned only as an anti-recombinase depletion of FANCJ would be expected to enhance HR. Instead, FANCJ depletion reduces HR, similar to BRCA1 depletion (Litman et al., 2005). Further indicating a positive role for FANCJ in HR, in response to DSBs induced by zeocin complementation of FANCJWT in FA-J cells enhanced the appearance of DNA damage induced RAD51 foci (Figures 2a and c). Whether the role of FANCJ in HR is direct is not clear. Conceivably, FANCJ could have an indirect role in HR as a ‘place-holder’ to prevent other proteins from disrupting HR, such as the anti-recombination helicases BLM or RTEL (Bugreev et al., 2007; Barber et al., 2008). Also consistent with a positive role in HR following ICLs, FANCJ deficiency enhanced recombination-independent repair (Shen et al., 2009). Intriguingly, polη also functions in HR to extend D-loop recombination intermediates (Kawamoto et al., 2005; McIlwraith et al., 2005). These dual functions in HR and TLS are likely regulated by the DNA damage response. Similar to polη, UV damage could link FANCJ to TLS. Here, the anti-recombination activity of FANCJ could be unleashed through loss of BRCA1 binding and gained helicase activity. This unleashed FANCJ activity could explain why BRCA1-deficient cells are defective in RAD51 foci formation and HR (Moynahan et al., 1999; Bhattacharyya et al., 2000).
Conceivably, the anti-recombination activity of FANCJS990A could indirectly enhance TLS. For example, the yeast helicase Srs2 promotes TLS by binding PCNA and antagonizing HR and recombination bypass pathways. The ability of Srs2 to disrupt recombination and displace Rad51 requires its enzyme activity (Barbour and Xiao, 2003; Papouli et al., 2005). Similar to Srs2, FANCJ colocalizes at sites of replication arrest with PCNA and has been shown to translocate DNA, unwind D-loops and displace RAD51 (Gupta et al., 2005; Dupaigne et al., 2008; Sommers et al., 2009). Moreover, UvrD, which is structurally and functionally related to Srs2, binds the MLH1 homologue, MutL (Mechanic et al., 2000). MutL helps to load and activate the UvrD helicase. Perhaps, MLH1 helps to load or activate the FANCJ helicase to promote TLS or HR.
FANCJ could enhance TLS not only by limiting recombination, but also by potentiating TLS. For example, FANCJ could limit negative regulators of TLS, such as mismatch repair (MMR), which detect mismatches generated by mutagenic TLS processing. Mismatch-induced MMR checkpoint signaling could generate the MMC sensitivity in FA-J cells lacking the FANCJ/MLH1 interaction. Alternatively, FANCJS990A could directly enhance TLS by altering the structure of the stalled DNA replication fork. Failure to separate DNA strands or create a DNA loop at the ICL is thought to block NER-dependent incisions required for recombination-independent repair (Zheng et al., 2003). The unleashed FANCJS990A could enhance DNA strand separation in a manner distinct from the BRCA1-bound FANCJ to facilitate NER-dependent processing events and recombination-independent repair. Either scenario could explain how FANCJS990A promotes TLS without directly binding polη (data not shown).
Together, these findings imply that reduction or loss of BRCA1 binding to FANCJ could enable cells to survive toxic chemotherapies and provide a possible route to chemoresistance. If true, targeting FANCJ or polη bypass could reverse resistance to agents that induce ICLs in such cancers. Likewise, a possible route to cancer in BRCA1-mutation carriers could result from excess unbound FANCJ and mutagenic bypass. Perhaps, this is why FANCJ amplification is also linked to malignancy (Sinclair et al., 2003; Eelen et al., 2008). In fact, loss of BRCA1 binding to FANCJ could evolve from mutations in either gene, or from loss of DNA-damage signaling components that regulate the association of these two proteins. Future studies are needed to clarify the signaling pathways that participate in regulating the switch between BRCA1-bound and-unbound FANCJ.
Materials and methods
Cell culture
MCF7, U2OS, HeLa, XP30RO (XP-V)-PCDNA vector, and PCNDA-polη complemented (generous gift of Alan Lehmann) (Kannouche et al., 2001) cells were cultured in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum and antibiotics. Stable cells were selected with 20 mg/ml G418. FA-J (EUFA30-F) fibroblasts were cultured in DMEM supplemented with 15% fetal bovine serum and antibiotics. FA-J cells were infected with pOZ vectors and selected as before (Peng et al., 2007).
Cell growth and G2/M accumulation assays
FA-J cells infected with pOZ (Peng et al., 2007) vectors and U2OS cells were transfected with siRNA using Fugene or Lipofectamine. siRNA reagents for polη (siRNA Polη pool, antisense sequence of #1, AACCCUUCAAUGUAA GUGCUU, or antisense sequence of #2, UAGUUCCUGGG CUAAUUGCUU), Rev1 (siRNA Rev1 pool), Rad18 (siRNA Rad18 pool, antisense sequence of #1, UACCAGUUCAUC UAAUAUGUU, antisense sequence of #2, AAAUUAUCC AUUAACCUGCUU, antisense sequence of #3, UUACUG AGGUCAUAUUAUCUU, or antisense sequence of #4, UGACUCUAAAGCAAACUGCUU), Rad54 (siRNA Rad54 pool), and luciferase (Luc) were obtained from Dharmacon (Lafayette, CO, USA). The FANCJ siRNA reagent was described earlier (Litman et al., 2005). U2OS cells were infected with shRNA against pGIPZ non-silencing control, Rad18 #1 (mature antisense sequence, AAATA TATCCATGTGAGCT), or Rad18 #2 (mature antisense sequence, TTGGTCTTTGCAGCAGGGC). shRNAs were obtained from the UMMS shRNA core facility. Infected cells or XPV complemented with vector or polη were subsequently transfected with V, FANCJWT, or FANCJS990A. After transfection (24–48 h), 1500–3000 U2OS cells/well or 12 500 complemented FA-J cells/well were seeded into six-well plates, respectively. Complemented FA-J cells were seeded into six-well plates at 8000 cells/well. Seeded cells were incubated overnight and left untreated or treated with MMC (Sigma, St Louis, MO, USA) for 1 h, UV (Stratalinker, Spectronics Corporation, Westbury, NY, USA), cisplatin (Sigma) for 4 h, zeocin (Invitrogen, Carlsbad, CA, USA) for 1 h, 6-TG (Sigma) for 24 h, or MMS (Sigma) for 1 h. Cells were counted after 5–8 days using a hemocytometer. Percent growth was calculated as (treated cells/untreated cells) × 100. G2/M accumulation was assayed as described (Litman et al., 2005), but at 0.25 µg/ml melphalan.
Immunoprecipitation, western blot and antibodies
Cells were harvested and prepared for immunoprecipitation and western blot as described (Litman et al., 2005). Immunoprecipitation Abs included FANCJ (E67) or Myc (9e10). Antibodies for western blot analysis included BRCA1 (ms110) and FANCJ monoclonal (2G7 and 2C10) (Cantor et al., 2001) or polyclonal E67 (Cantor et al., 2004). In addition, β-actin (Sigma), Rad18 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), Rev1 (H-300, Santa Cruz), polη (Abcam, Cambridge, MA, USA), and Rad54 (Abcam) Abs were used.
DNA constructs
The FANCJWT, FANCJK52R, FANCJK141/142A, and FANCJS990A pCDNA-3myc-6xhis and pOZ vectors have been described earlier (Cantor et al., 2001; Peng et al., 2007). The eGFP-polη construct was described earlier (Kannouche et al., 2001). The FANCJK141/142AS990A pOZ vector was generated with the QuickChange site-directed mutagenesis kit (Stratagene, La Jolla, CA, USA) using our published primers (Cantor et al., 2001; Peng et al., 2007).
Immunofluorescence
FA-J cells were either left untreated or treated with 12.5 µg/ml zeocin and incubated 12 h and processed for immunofluorescence as described (Cantor et al., 2001). Antibodies included RAD51 (Santa Cruz 1:200) and γ-H2AX (Upstate 1:500, Upstate, Temecula, CA, USA). Visualization of eGFP-polη foci was as described (Kannouche et al., 2001). In brief, U2OS cells were transfected with eGFP-polη, incubated for overnight, seeded on cover slips, incubated overnight, and examined 4 h post-UV or for 24 and 48 h post-MMC. Foci counting experiments were conducted blind to the counter and in triplicate as the number of cells with 10 or more foci.
Homologous recombination
U2OS pDR-GFP cells were obtained from Maria Jasin (Pierce et al., 1999) and 1.8 × 105 cells were seeded per well in six-well plates and incubated overnight. The cells were transfected with 0.5 µg of pCDNA3, FANCJWT, FANCJS990A, or FANCJK52R and 2.0 µg of pBABE I-Sce1 using Fugene. Transfected cells were incubated for 72 h, collected by trypsinization, and analyzed by FACS. The percentage of green positive cells was calculated using Flow Jo software.
Supplementary Material
Acknowledgements
We thank Larry Thompson, Alan Lehmann, and Roger Greenberg for critical comments. We also thank Maria Jasin for the DR-U2OS cells and Alan Lehmann for the XPV-vector and polη-complemented lines as well as polη-GFP construct, Hans Joenje for FA-J cells, and Claire Baldwin for readership comments. This study was supported by NIH R01 CA129514-01A1 and from charitable contributions from Mr and Mrs Edward T Vitone Jr.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc)
References
- Albertella MR, Green CM, Lehmann AR, O’Connor MJ. A role for polymerase eta in the cellular tolerance to cisplatin-induced damage. Cancer Res. 2005;65:9799–9806. doi: 10.1158/0008-5472.CAN-05-1095. [DOI] [PubMed] [Google Scholar]
- Alt A, Lammens K, Chiocchini C, Lammens A, Pieck JC, Kuch D, et al. Bypass of DNA lesions generated during anticancer treatment with cisplatin by DNA polymerase eta. Science. 2007;318:967–970. doi: 10.1126/science.1148242. [DOI] [PubMed] [Google Scholar]
- Barbour L, Xiao W. Regulation of alternative replication bypass pathways at stalled replication forks and its effects on genome stability: a yeast model. Mutat Res. 2003;532:137–155. doi: 10.1016/j.mrfmmm.2003.08.014. [DOI] [PubMed] [Google Scholar]
- Barber LJ, Youds JL, Ward JD, McIlwraith MJ, O’Neil NJ, Petalcorin MI, et al. RTEL1 maintains genomic stability by suppressing homologous recombination. Cell. 2008;135:261–271. doi: 10.1016/j.cell.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya A, Ear US, Koller BH, Weichselbaum RR, Bishop DK. The breast cancer susceptibility gene BRCA1 is required for subnuclear assembly of Rad51 and survival following treatment with the DNA cross-linking agent cisplatin. J Biol Chem. 2000;275:23899–23903. doi: 10.1074/jbc.C000276200. [DOI] [PubMed] [Google Scholar]
- Bridge WL, Vandenberg CJ, Franklin RJ, Hiom K. The BRIP1 helicase functions independently of BRCA1 in the Fanconi anemia pathway for DNA crosslink repair. Nat Genet. 2005;37:953–957. doi: 10.1038/ng1627. [DOI] [PubMed] [Google Scholar]
- Bugreev DV, Yu X, Egelman EH, Mazin AV. Novel pro- and anti-recombination activities of the Bloom’s syndrome helicase. Genes Dev. 2007;21:3085–3094. doi: 10.1101/gad.1609007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantor S, Drapkin R, Zhang F, Lin Y, Han J, Pamidi S, et al. The BRCA1-associated protein BACH1 is a DNA helicase targeted by clinically relevant inactivating mutations. Proc Natl Acad Sci USA. 2004;101:2357–2362. doi: 10.1073/pnas.0308717101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantor SB, Andreassen PR. Assessing the link between BACH1 and BRCA1 in the FA pathway. Cell Cycle. 2006;5:164–167. doi: 10.4161/cc.5.2.2338. [DOI] [PubMed] [Google Scholar]
- Cantor SB, Bell DW, Ganesan S, Kass EM, Drapkin R, Grossman S, et al. BACH1, a novel helicase-like protein, interacts directly with BRCA1 and contributes to its DNA repair function. Cell. 2001;105:149–160. doi: 10.1016/s0092-8674(01)00304-x. [DOI] [PubMed] [Google Scholar]
- Delacote F, Deriano L, Lambert S, Bertrand P, Saintigny Y, Lopez BS. Chronic exposure to sublethal doses of radiation mimetic Zeocin selects for clones deficient in homologous recombination. Mutat Res. 2007;615:125–133. doi: 10.1016/j.mrfmmm.2006.11.028. [DOI] [PubMed] [Google Scholar]
- Dronkert ML, Kanaar R. Repair of DNA interstrand crosslinks. Mutat Res. 2001;486:217–247. doi: 10.1016/s0921-8777(01)00092-1. [DOI] [PubMed] [Google Scholar]
- Dupaigne P, Le Breton C, Fabre F, Gangloff S, Le Cam E, Veaute X. The Srs2 helicase activity is stimulated by Rad51 filaments on dsDNA: implications for crossover incidence during mitotic recombination. Mol Cell. 2008;29:243–254. doi: 10.1016/j.molcel.2007.11.033. [DOI] [PubMed] [Google Scholar]
- Eelen G, Vanden Bempt I, Verlinden L, Drijkoningen M, Smeets A, Neven P, et al. Expression of the BRCA1-interacting protein Brip1/BACH1/FANCJ is driven by E2F and correlates with human breast cancer malignancy. Oncogene. 2008;27:4233–4241. doi: 10.1038/onc.2008.51. [DOI] [PubMed] [Google Scholar]
- Gupta R, Sharma S, Sommers JA, Jin Z, Cantor SB, Brosh RM., Jr Analysis of the DNA substrate specificity of the human BACH1 helicase associated with breast cancer. J Biol Chem. 2005;280:25450–25460. doi: 10.1074/jbc.M501995200. [DOI] [PubMed] [Google Scholar]
- Hinz JM, Nham PB, Salazar EP, Thompson LH. The Fanconi anemia pathway limits the severity of mutagenesis. DNA Repair (Amst) 2006;5:875–884. doi: 10.1016/j.dnarep.2006.05.039. [DOI] [PubMed] [Google Scholar]
- Kannouche P, Broughton BC, Volker M, Hanaoka F, Mullenders LH, Lehmann AR. Domain structure, localization, and function of DNA polymerase eta, defective in xeroderma pigmentosum variant cells. Genes Dev. 2001;15:158–172. doi: 10.1101/gad.187501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannouche PL, Lehmann AR. Ubiquitination of PCNA and the polymerase switch in human cells. Cell Cycle. 2004;3:1011–1013. [PubMed] [Google Scholar]
- Kawamoto T, Araki K, Sonoda E, Yamashita YM, Harada K, Kikuchi K, et al. Dual roles for DNA polymerase eta in homologous DNA recombination and translesion DNA synthesis. Mol Cell. 2005;20:793–799. doi: 10.1016/j.molcel.2005.10.016. [DOI] [PubMed] [Google Scholar]
- Kim H, Chen J. New players in the BRCA1-mediated DNA damage responsive pathway. Mol Cells. 2008;25:457–461. [PMC free article] [PubMed] [Google Scholar]
- Levitus M, Waisfisz Q, Godthelp BC, Vries YD, Hussain S, Wiegant WW, et al. The DNA helicase BRIP1 is defective in Fanconi anemia complementation group J. Nat Genet. 2005;37:934–935. doi: 10.1038/ng1625. [DOI] [PubMed] [Google Scholar]
- Levran O, Attwooll C, Henry RT, Milton KL, Neveling K, Rio P, et al. The BRCA1-interacting helicase BRIP1 is deficient in Fanconi anemia. Nat Genet. 2005;37:931–933. doi: 10.1038/ng1624. [DOI] [PubMed] [Google Scholar]
- Litman R, Peng M, Jin Z, Zhang F, Zhang J, Powell S, et al. BACH1 is critical for homologous recombination and appears to be the Fanconi anemia gene product FANCJ. Cancer Cell. 2005;8:255–265. doi: 10.1016/j.ccr.2005.08.004. [DOI] [PubMed] [Google Scholar]
- McIlwraith MJ, Vaisman A, Liu Y, Fanning E, Woodgate R, West SC. Human DNA polymerase eta promotes DNA synthesis from strand invasion intermediates of homologous recombination. Mol Cell. 2005;20:783–792. doi: 10.1016/j.molcel.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Mechanic LE, Frankel BA, Matson SW. Escherichia coli MutL loads DNA helicase II onto DNA. J Biol Chem. 2000;275:38337–38346. doi: 10.1074/jbc.M006268200. [DOI] [PubMed] [Google Scholar]
- Moynahan ME, Chiu JW, Koller BH, Jasin M. Brca1 controls homology-directed DNA repair. Mol Cell. 1999;4:511–518. doi: 10.1016/s1097-2765(00)80202-6. [DOI] [PubMed] [Google Scholar]
- Moynahan ME, Pierce AJ, Jasin M. BRCA2 is required for homology-directed repair of chromosomal breaks. Mol Cell. 2001;7:263–272. doi: 10.1016/s1097-2765(01)00174-5. [DOI] [PubMed] [Google Scholar]
- Niedernhofer LJ, Lalai AS, Hoeijmakers JH. Fanconi anemia (cross)linked to DNA repair. Cell. 2005;123:1191–1198. doi: 10.1016/j.cell.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Niedzwiedz W, Mosedale G, Johnson M, Ong CY, Pace P, Patel KJ. The Fanconi anaemia gene FANCC promotes homologous recombination and error-prone DNA repair. Mol Cell. 2004;15:607–620. doi: 10.1016/j.molcel.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Nojima K, Hochegger H, Saberi A, Fukushima T, Kikuchi K, Yoshimura M, et al. Multiple repair pathways mediate tolerance to chemotherapeutic cross-linking agents in vertebrate cells. Cancer Res. 2005;65:11704–11711. doi: 10.1158/0008-5472.CAN-05-1214. [DOI] [PubMed] [Google Scholar]
- Papouli E, Chen S, Davies AA, Huttner D, Krejci L, Sung P, et al. Crosstalk between SUMO and ubiquitin on PCNA is mediated by recruitment of the helicase Srs2p. Mol Cell. 2005;19:123–133. doi: 10.1016/j.molcel.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Patel KJ, Joenje H. Fanconi anemia and DNA replication repair. DNA Repair (Amst) 2007;6:885–890. doi: 10.1016/j.dnarep.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Peng M, Litman R, Xie J, Sharma S, Brosh RM, Jr, Cantor SB. The FANCJ/MutLalpha interaction is required for correction of the cross-link response in FA-J cells. EMBO J. 2007;26:3238–3249. doi: 10.1038/sj.emboj.7601754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce AJ, Johnson RD, Thompson LH, Jasin M. XRCC3 promotes homology-directed repair of DNA damage in mammalian cells. Genes Dev. 1999;13:2633–2638. doi: 10.1101/gad.13.20.2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash S, Johnson RE, Prakash L. Eukaryotic translesion synthesis DNA polymerases: specificity of structure and function. Annu Rev Biochem. 2005;74:317–353. doi: 10.1146/annurev.biochem.74.082803.133250. [DOI] [PubMed] [Google Scholar]
- Seal S, Thompson D, Renwick A, Elliott A, Kelly P, Barfoot R, et al. Truncating mutations in the Fanconi anemia J gene BRIP1 are low-penetrance breast cancer susceptibility alleles. Nat Genet. 2006;38:1239–1241. doi: 10.1038/ng1902. [DOI] [PubMed] [Google Scholar]
- Shen X, Do H, Li Y, Chung WH, Tomasz M, de Winter JP, et al. Recruitment of fanconi anemia and breast cancer proteins to DNA damage sites is differentially governed by replication. Mol Cell. 2009;35:716–723. doi: 10.1016/j.molcel.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair CS, Rowley M, Naderi A, Couch FJ. The 17q23 amplicon and breast cancer. Breast Cancer Res Treat. 2003;78:313–322. doi: 10.1023/a:1023081624133. [DOI] [PubMed] [Google Scholar]
- Sommers JA, Rawtani N, Gupta R, Bugreev DV, Mazin AV, Cantor SB, et al. FANCJ uses its motor ATPase to disrupt protein-DNA complexes, unwind triplexes, and inhibit rad51 strand exchange. J Biol Chem. 2009;284:7505–7517. doi: 10.1074/jbc.M809019200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W. Emergence of a DNA-damage response network consisting of Fanconi anaemia and BRCA proteins. Nat Rev Genet. 2007;8:735–748. doi: 10.1038/nrg2159. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Tateishi S, Kawasuji M, Tsurimoto T, Inoue H, Yamaizumi M. Rad18 guides poleta to replication stalling sites through physical interaction and PCNA monoubiquitination. EMBO J. 2004;23:3886–3896. doi: 10.1038/sj.emboj.7600383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Chini CC, He M, Mer G, Chen J. The BRCT domain is a phospho-protein binding domain. Science. 2003;302:639–642. doi: 10.1126/science.1088753. [DOI] [PubMed] [Google Scholar]
- Zheng H, Wang X, Warren AJ, Legerski RJ, Nairn RS, Hamilton JW, et al. Nucleotide excision repair- and polymerase etamediated error-prone removal of mitomycin C interstrand crosslinks. Mol Cell Biol. 2003;23:754–761. doi: 10.1128/MCB.23.2.754-761.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.