Abstract
Studies on the ability of bone marrow derived cells to adopt the morphology and protein expression of epithelial cells in vivo have expanded rapidly over the last decade, and hundreds of publications report that bone marrow derived cells can become epithelial cells of multiple organs including lung, liver, GI tract, skin, pancreas and others. In this review, we critically evaluate the literature related to engraftment of bone marrow derived cells as epithelial cells in the lung. Over 40 manuscripts focused on whether bone marrow cells can differentiate into lung epithelial cells have been published, nearly all of which claim to identify marrow derived epithelial cells. A few investigations have concluded that no such cells are present and that the phenomenon of marrow derived epithelial cells is based on detection artifacts. Here we discuss the problems that exist in published papers identifying marrow derived epithelial cells, and propose standards for detection methods that provide the most definitive data. Identification of BM derived epithelial cells requires reliable and sensitive techniques for their detection, which must include cell identification based on the presence of an epithelial marker and the absence of blood cell markers as well as a marker for donor BM origin. In order for these studies to be rigorous, they must also use approaches to rule out cell overlap by microscopy or single cell isolation. Once these stringent criteria for identification of marrow derived epithelial cells are used universally, then the field can move forward to address the critical questions regarding which bone marrow derived cells are responsible for engraftment as epithelial cells, the mechanisms by which this occurs, whether these cells play a role in normal tissue repair, and whether specific cell subsets can be used for therapeutic benefit.
Introduction
Stem cells in adult tissues were previously thought to differentiate exclusively into cells of their tissue of origin. However, a number of reports have shown that adult stem cells from the bone marrow have a greater potential than previously appreciated. Since 1998, there have been many exciting discoveries indicating that stem cells derived from the bone marrow (BM) can differentiate into mature, non-hematopoietic cells of multiple tissues, including epithelial cells of the lung [1–5]. These results suggest that stem cells derived from the bone marrow may become valuable tools for cell replacement strategies and regenerative medicine in the future. It is therefore of vital importance to study the physiologic role of bone marrow cell plasticity as well as the mechanisms underlying this phenomenon. However, extensive studies of BM derived epithelial cells cannot be undertaken without reliable and sensitive techniques for their detection. In this review, we discuss the problems with many of the approaches and techniques used in published papers to detect marrow derived epithelial cells. We define technical requirements and quality standards essential for obtaining definitive data on this phenomenon.
To date, there is no agreement in the field on the scientific terminology to be used when describing “marrow derived lung epithelial cells.” Until and unless it is demonstrated that a bone marrow cell can fully differentiate into a FUNCTIONAL epithelial cell, we define this phenomenon as “bone marrow cells adopting the morphology and protein expression of epithelial cells”. For simplicity, we use the term “marrow derived epithelial cell” (MDLE) in the following text.
Overview of controversies
Here we review some of the controversies regarding the ability of bone marrow derived cells to adopt the morphology and protein expression of epithelial cells, and suggest approaches for addressing each of these concerns.
In 2001, Krause et al showed that epithelial cells of the lung can be derived from a single bone marrow stem cell [1]. To date, there have been over 40 primary research papers investigating this phenomenon in the lung (Table 1). While nearly all of these publications conclude that bone marrow derived cells can differentiate into epithelial cells in vivo, the conclusions from different papers investigating the phenomenon of marrow derived lung epithelial cells (MDLE) are highly variable. Many different BM cell subpopulations have been studied in different contexts of tissue injury, and techniques for detection and enumeration of MDLE are highly variable as well. Some authors report very low numbers of lung epithelial cells that are marrow derived [1–4, 6–12], while others find a high percentage of MDLE [13–15]. In contrast, some authors were not able to identify any marrow derived epithelial cells [16–19]. Due to this variability in the current literature, researchers are not in agreement regarding whether this phenomenon occurs in vivo.
Table 1.
Published Research Papers On Marrow Derived Epithelial Cells
| BM Cell Type | Route of administration | Ref | MDLE reported |
|---|---|---|---|
| WBM, ELH | IV | Krause, Theise et al, Cell 2001 [1] | YES |
| WBM, Lin- | IV | Theise et al, Exp Hem 2002 [2] | YES |
| WBM | IV | Grove et al, AJRCMB [20] | YES |
| KTLS-HSC | IV | Wagers et al, Science 2002 [17] | NO |
| BM-MNC, SP | IV | Abe et al, Cytother 2003 [44] | YES |
| WBM | Iv | Yamada et al, JI 2004 [12] | YES |
| WBM | IV | Harris, Herzog et al, Science 2004 [7] | YES |
| SP-HSC, WBM | IV | Kotton et al, AJRCMB 2005 [16] | NO |
| SP-HSC | IV | MacPherson et al, J Cell Sci 2005 [45] | YES |
| SP-HSC, WBM | IV | MacPherson et al, Resp Res 2006 [4] | YES |
| WBM, LSK | IV | Aliotta et al, Exp Hem 2006 [3] | YES |
| WBM | IV | Herzog et al, Stem Cells 2006 [6] | YES |
| WBM | IV | Herzog et al, FASEB 2007 [6] | YES |
| WBM | IV | Spees et al, ARJCCM 2007 [9] | YES |
| WBM, LSK | IV | Reese et al, Stem Cells 2008 [21] | YES |
| WBM | Chimeric rats | Spees et al, FASEB 2008 [10] | YES |
| Lin- BM | IV | Rejman et al, ASGT 2009 [53] | YES |
| WBM | Intra-nasal | Fritzell et al, ASRCMB 2009 [19] | NO |
| MSC | IV | Kotton et al, Dev 2001 [14] | YES |
| MSC | IV | Ortiz et al, PNAS 2003 [22] | YES |
| MSC | IV | Rojas et al, AJRCMB 2005 [13] | YES |
| MSC, WBM | IV | Loi et al, AJRCCM 2005 [8] | YES |
| Human CB-MSC | IV | Sueblinvong et al, AJRCCM 2008 [11] | YES |
| MSC, CCSP+ adh BM | IT, IV | Wong et al, JCI 2009 [15] | YES |
| hBMT | IV | Suratt et al, AJRCCM 2003 [46] | YES |
| hBMT | IV | Mattson et al, Transplantation 2004 [47] | YES |
| hBMT | IV | Zander et al, Ann Clin Lab Sci 2006 [48] | YES |
| hLung txp | Kleeberger et al, Am J Path 2003 [49] | YES | |
| hLung txp | Spencer et al, Thorax 2004 [50] | YES | |
| hLung txp | Zander et al, Transplantation 2005 [51] | YES | |
| hLung txp | Albera et al, Tissue Eng 2005 [52] | YES |
Legend: ELH = elutriated, Lin-depleted, homed, WBM = whole bone marrow, KTLS = c-kit+ Thy1lo, Lin-Sca1+, BM-MNC = bone marrow mononuclear cells, LSK = Lin- Sca1+ c-kit+, HSC = hematopoietic stem cells, SP = side population, MSC = marrow stromal cells, CB-MSC = cord blood MSC, CCSP+ adh BM = clara cell specific protein positive, adherent BM cells, hBMT = human bone marrow transplantation, hLung txp = human lung transplantation, IV: intra-venous, IT: intra-tracheal
One of the major causes of this controversy is that the quality of data reporting findings on MDLE is extremely variable. Papers which definitively show that lung epithelial cells can be derived from the bone marrow are in the minority. Some data are less definitive due to 1) low quality images, 2) the omission of proper controls, 3) lack of staining for epithelial-specific markers, 4) lack of exclusion of hematopoietic cells and/or 5) lack of exclusion of cell overlay from their analysis. This is a great loss to the field. Some investigators argue against marrow derived cells adopting the phenotype of epithelial cells of the lung due to the fact that many published data on marrow derived cells are not definitive, and supported by publications reporting a lack of marrow derived epithelial cells [16, 17].
As detailed below, we believe that this controversy needs to be addressed by optimization of detection techniques and a concerted effort to publish only the highest quality, definitive data, including appropriate controls.
Problems associated with the detection of marrow derived lung epithelial cells
Detection of rare marrow derived epithelial cells presents a great challenge and requires extremely sensitive and specific detection techniques. Results can only be interpreted if all proper controls are performed and presented. In the following, we will discuss some of the problems that are apparent in some of the published research articles on marrow derived epithelial cells. Common problems are:
– Overlay of bone marrow derived cells with epithelial cells is not ruled out. This can be overcome using confocal microscopy and markers for hematopoietic cells and/or using isolated single cells (though this latter approach leaves open the question of whether the isolated single cell with epithelial characteristics was located in vivo within the normal tissue architecture).
– Marrow derived epithelial cells are not identified by use of lung epithelial specific markers (epithelial cells are often identified based on location or morphology alone).
– Low quality images with low magnification and/or high background are difficult to interpret
- – Lack of adequate positive and negative controls. Controls are needed to assess:
- ○Artifacts: autofluorescence, false positive signals
- ○Nonspecific staining
- ○False negative staining/signal due to tissue preparation (e.g. overfixation) or inconsistent marker expression (e.g. GFP transgenes)
- ○Lack of demonstration of specific signal and staining pattern on positive control: to be compared with signal detected in marrow derived cells
In the following, we address these concerns in greater detail in order to enhance awareness of these problems in the field, and to encourage ongoing discussion about improving methods for the detection of marrow derived epithelial cells.
Failure to rule out cell overlay or to perform costaining for hematopoietic cell antigens
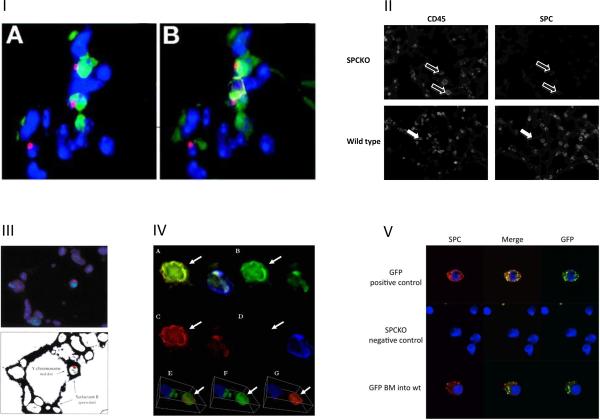
If a cell appears positive by microscopy for both an epithelial marker and a BM donor-specific marker, it is possible that overlay of a blood cell of donor origin and an epithelial cell has created signals that appear to be co-localized in one cell. It is therefore critical to rule out cell overlay. Herzog et al [6] show the importance of CD45 co-staining to exclude overlay of a donor derived blood cell with a lung epithelial cell. They show a clear example of a Y-chromosome (male donor-specific) signal colocalizing with a TTF1 positive epithelial cell nucleus, which makes this cell appear to be derived from the male donor. Staining for CD45, however, reveals cell overlay in this case (Fig 1.I). In addition, cell overlay should be assessed using confocal or deconvolution microscopy, as shown by Chang et al, who used deconvolution microscopy to detect overlay of a GFP positive BM derived cell and a surfactant protein C (SPC) positive lung epithelial cell, which appeared to be one double positive cell when using a conventional microscope [18]. Many published research articles do not exclude cell overlay by any of these methods. For example, in a manuscript from the Krause laboratory (we use an example from our laboratory purposely), in one image, we show cytokeratin (CK) positive lung epithelial cells that are donor BM derived based on GFP expression, but we do not show CD45 staining to exclude overlay of a donor derived blood cell with a CK positive epithelial cell [20]. Although such staining was performed, in retrospect, it is critical that it be shown in order to assure that the published data are definitive. Such controls may also reveal MDLE where they were otherwise not detected. For example, Kotton et al [16] used GFP to track cells derived from donor bone marrow and reported that the frequency of cells that appeared to be double-labeled for GFP fluorescence and type 2 pneumocyte-specific red immunostaining in the recipient lungs was not different from that in negative control sections, which had double positive cells based on background autofluorescence. They therefore concluded that marrow derived epithelial cells were not present. However, in their experiments, CD45 staining and confocal microscopy would have helped to identify autofluorescent macrophages and artifacts to exclude them from the analysis, which might have revealed the presence of true marrow derived type 2 pneumocytes. Multiple other publications present Y-chromosome and CK double-positive cells within the epithelia of female mice or humans that had undergone transplantation with male bone marrow derived cells. However, without showing co-staining for CD45, it is possible that these cells are tissue macrophages, either adjacent to CK positive epithelial cells or mimicking cytokeratin positive cells due to autofluorescence. We know from our own experience that especially tissue macrophages located within the epithelium, can be difficult to distinguish from epithelial cells if no CD45 staining is used. In Fig 1.II, the lower panel shows a paraffin section of a murine wild type lung stained for CD45. Many CD45 positive cells are located within the epithelium (white arrows). Their shape, localization and insufficient morphological detail make them difficult to distinguish from type 2 pneumocytes. This example shows the importance of staining for a blood specific marker in addition to a donor marker. It is unknown whether BM derived cells that are undergoing differentiation into epithelial cells may retain their CD45 expression. We have never included cells that co-express CD45 and epithelial markers in our analyses. However, other publications, such as that from McPherson et al [4], find that 19.3% of marrow derived cytokeratin positive cells in the trachea are also CD45 positive, and argue that these cells may be derived from cell fusion.
Figure 1. Detection of marrow derived epithelial cells.
I) Visualizing cell overlay: A figure from Herzog et al [6] demonstrates that one can be fooled by microscopy artifact. Thyroid transcription factor-1 (TTF-1) (green), Y chromosome (red), and CD45 (yellow) fluorescent staining on sections from a female mouse that received male BM. Nuclei are stained blue with DAPI. In (A), there are two TTF-1□cells that appear to contain the Y chromosome. However, in the (B), with the addition of CD45 staining (yellow), it is clear that these cells are CD45. In this image, CD45 staining shows that even on 3-μm sections, cell overlay can occur. (Herzog EL, Van Arnam J, Hu B, Krause DS. Threshold of lung injury required for the appearance of marrow-derived lung epithelia. Stem Cells. 2006;24:1986–1992. Used with permission from Alpha Med Press). II) CD45 positive cells exhibit autofluorescence: In the upper panel, SPC-KO lung tissue was stained for SPC in the red channel. CD45 staining in the blue channel reveals macrophages, which exhibit autofluorescence and appear slightly positive for SPC (open arrows). The lower panel shows CD45 positive blood cells, which are located within the epithelial cell layer (white arrows). III) Marrow-derived epithelial cells in the lung detected by double FISH for surfactant B mRNA and Y chromosome: This figure from Krause et al [1] shows surfactant protein B-expressing alveolar type 2 cells derived from a single BM stem cell derived from a male donor mouse in the lung of a female recipient mouse. The upper image shows nuclei (blue DAPI stain), Surfactant protein B transcription centers (green), and Y chromosome (red). The lower image shows the autofluorescence of the cell bodies in order to clarify the tissue architecture within the alveoli (shown black in this schematic). This image was obtained by using the “find edges” command in Adobe Photoshop after increasing the gain to detect autofluorescence. (Krause DS, Theise ND, Collector MI, et al. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell. 2001;105:369–377. Used with permission from Cell Press). IV) Donor-derived pulmonary epithelial cells (Aliotta et al 2006): Aliotta et al [3] detected donor-derived cells that had the morphologic and phenotypic characteristics of pulmonary epithelial cells in the lungs of wild type mice that had undergone BM transplantation with BM cells from GFP donor mice. In this figure, lung tissue was labeled with anti-CD45 (blue) and anti-cytokeratin (red) antibodies and photographed using deconvolution fluorescence microscopy. The featured cells (arrow) are GFP+ (B: FITC filter), cytokeratin+ (C: rhodamine filter), and CD45- (D: DAPI filter), indicating that they represent donor-derived pulmonary epithelial cells (A: all filters). A three-dimensional view of the featured cells from the deconvolution image confirms colocalization of GFP and cytokeratin, but not CD45 (E,F,G). (Aliotta JM, Keaney P, Passero M, et al. Bone marrow production of lung cells: the impact of G-CSF, cardiotoxin, graded doses of irradiation, and subpopulation phenotype. Exp Hematol. 2006;34:230–241. Used with permission). V) Removal of blood and endothelial cells by cell sorting, confocal microcopy: Lungs from wild type mice that received BM transplantation from GFP-donor mice were digested und subjected to FACS-sorting to remove blood and endothelial cells. The resulting cell population was enriched for epithelial cells. Cells were attached to poly-L-lysine-coated coverslips and stained for SPC in red and the donor marker GFP in green. Analysis was done by confocal microscopy. The upper panel shows lung cells from a normal GFP-mouse that served as positive control for SPC and GFP staining. The middle panel shows lung cells from an SPC-KO mouse that are negative for both SPC and GFP. The bottom panel shows an SPC positive type 2 cell which is derived from the GFP+ donor bone marrow in a wild type mouse that received BM transplantation from a GFP donor.
Many published papers demonstrating MDLE would be more definitive if staining for hematopoietic cells was used to exclude overlay of marrow derived cell with an epithelial cell. Krause et al [1] used FISH for the Y-chromosome as a marker for epithelial cells derived from male donors in female bone marrow transplant recipients. Expression of mRNA for surfactant protein B (SPB), which is expressed in type 2 pneumocytes within the lung, was detected in the same nucleus as the Y-chromosome by double FISH (Fig 1.III). These data show that a marrow-derived cell adopted the phenotype of a type 2 pneumocyte. Control cells in the same field of view strengthen the data; there are nuclei that are positive for surfactant protein B mRNA and negative for the Y chromosome in the same field as the double positive cell providing additional controls for the staining approach. Because the SPB and Y-FISH signals were detected not only within the same cell, but within the same nucleus, these data seemed to be as definitive as possible at the time they were published. Also, by DAPI staining, there appears to be only one nucleus in this region of the 3 micron section shown. However, as demonstrated several years later by Herzog et al [6], there is a possibility that this signal could be derived from cell overlay. By today's standards, it becomes clear that the use of CD45 staining would have increased the conclusiveness of these data.
Lack of epithelial cell specific markers
If a donor marker (e.g. Y-chromosome or GFP) is found in a cell that is located within the lung epithelium and shaped like an epithelial cell, the location and shape of this cell alone are not sufficient to identify it as an epithelial cell. Characteristics like location within the tissue, size, shape, granularity and autofluorescence are important to identify a cell as epithelial cell. However, these characteristics are not exclusive to epithelial cells, and additional markers specific for the epithelial cell types that are being investigated need to be used to prove that a the marrow derived cell expresses proteins that are characteristic of an epithelial cell. In the previous paragraph, we discussed the importance of staining for hematopoietic markers in order to exclude donor-derived blood cells from the analysis when screening for marrow derived epithelial cells. Nevertheless, even if a donor derived cell in the epithelium of the lung does not express a hematopoietic cell marker, it is still necessary to prove that this cell has not only adopted the morphology but also the protein expression of a lung epithelial cell by demonstrating that it expresses lung epithelial specific markers. Some papers report the number of marrow derived epithelial cells based purely on the presence of a donor specific marker and lack of CD45 expression. This is insufficient evidence that the cells are epithelial, and thus the frequency reported is not valid. These cells may be CD45 negative bone marrow cells (e.g. marrow stromal cells), or the CD45 expression may have been missed due to lower level staining than is present on other blood cells.
In several published papers, the phenotype of marrow-derived epithelial cells in the lung is inferred entirely from their morphology and location. For example, Reese et al detected donor derived cells by staining for GFP using a horseradish peroxidase conjugated secondary antibody, and identified what they interpreted to be type 2 pneumocytes based on their morphology and their location in the alveolar walls [21]. However, given the controversial nature of the findings, it is essential that specific staining is also used to identify type 2 pneumocytes in order to conclude that the GFP positive, donor derived cells are epithelial. In another experiment in the same paper, in situ hybridization was used to detect surfactant protein-B mRNA, and immunohistochemistry for GFP was done on a serial section of the same tissue. This however increases the likelihood of detecting the signals from two adjacent cells. In a study published by Ortiz et al, [22], alveolar type 2 cells were isolated according to the method of Corti et al [23], which is based on enzymatic treatment and antibody depletion, and yields purities of 92–95%. Yet, Y-chromosome positive cells, which were detected on cytospins, were counted as alveolar type 2 cells derived from male donor MSC without staining for an epithelial cell specific marker to confirm that these Y-chromosome positive cells were type 2 cells. In multiple other reports as well, localization of donor derived (e.g. Y-chromosome or GFP positive) cells within lung tissue of recipient mice has been interpreted as engraftment of bone marrow derived cells in the lung, without using an epithelial marker to characterize these cells. Engraftment by definition is not identical with localization, which is all one can observe in these cases.
Lack of adequate controls
It is important to show appropriate staining controls when marrow derived cells are identified based on immunostaining. Artifacts in the tissue, which may be caused by fixation and tissue processing, may result in autofluorescence and false positive signals. To prove that a donor derived cell, which stains for an epithelial cell marker, is truly positive for this marker, the appropriate negative controls are needed to show the level of fluorescence and the pattern and intensity of a possible nonspecific signal in a negative control sample. There are at least three potential negative controls. Investigators should show the same cells (identical fixation, animal, etc) with no staining (native or auto-fluorescence) and, if possible, with control staining (e.g. isotype for antibodies, nonspecific DNA for FISH). Ideally, a third control should be images of cells or tissue sections from specimens lacking the marker of interest. For example, investigators should use female tissue as a negative control for Y chromosome, GFP-negative mouse tissue as a control for GFP signal detection, and, if available, antigen negative mice (knockout mice) as controls for antigen-specific immunodetection. In the Krause laboratory, we use mice lacking expression of surfactant protein C (SPC-KO) as recipients of bone marrow transplantation, and also as negative control tissues for immunostaining using anti-surfactant protein C antibody. A positive control is always needed to show the pattern and intensity of the specific signal in control cells and to compare it very carefully to the pattern and intensity of the signal observed in the possible marrow-derived epithelial cell. Some published data would be more conclusive if the proper controls were shown side by side with the results. In an example from the Krause laboratory, we identified donor derived lung epithelial cells by Y-chromosome FISH and immunofluorescence for cytokeratin, but did not show in the published manuscript either the positive or negative controls for CK staining [6]. While these controls were in fact performed, not showing them in the final manuscript leaves open the criticism that the fluorescent signal described as positive CK staining is not specific. Rojas et al show double immunofluorescence for donor derived GFP and aquaporin, SPC or smooth muscle actin, but do not show the positive or negative controls for any of these stainings [13]. Therefore, knowledgeable skeptics could say that the data do not definitively show that the donor BM MSC localizing to injured lung take on the phenotype of lung epithelial cells.
Different cell types within a tissue may exhibit different levels of autofluorescence. In the lung, especially tissue macrophages can have high levels of autofluorescence, which can appear vesicular and make these cells difficult to distinguish from the vesicular staining pattern of type 2 pneumocytes. Therefore, the level of autofluorescence present in different cell types of the tissue needs to be assessed using a negative control/unstained tissue from the same experiment. The autofluorescent signals have to be compared to specific signals very carefully to assure they can be distinguished from one another. For example, in Figure 1.II, the upper panel shows a lung tissue section from an SPC-KO mouse. CD45 positive macrophages exhibit autofluorescence in the red channel, which was used for SPC immunostaining (open arrows). This autofluorescence can appear similar to a specific SPC signal, if it's pattern and intensity is not compared very carefully to SPC positive type 2 pneumocytes in a wild type control. Autofluorescence can also be assessed by comparing fluorescence intensity in different channels, since autofluorescence is present throughout a broad region of the fluorescent spectrum, particularly within the wavelengths commonly used with FITC and rhodamine conjugated detection techniques. For instance, if a cell seems to be positively stained with a fluorophore that is detected in the “red” channel of the microscope (emission 550–600 nm), detection of an exactly overlapping signal in the “green” channel (500–550 nm) would indicate autofluorescence. It is therefore important to assure the presence of a specific signal in only one channel.
In addition to potential false positive signals due to autofluorescence, tissue preparation and processing can also lead to false negative signals. Fixation methods and conditions can strongly affect specific staining. For example, GFP native fluorescence usually fades after fixation with formaldehyde, depending on the type of tissue and the length of fixation, making it necessary to detect it with an anti-GFP antibody. Transgenes, like GFP, may also get silenced, or can be expressed by donor-derived cells at low levels that are below the threshold of detection [24, 25], especially if the GFP signal is not amplified with an anti-GFP antibody. In some cases, it may be necessary to amplify the signal using tyramide amplification in order to clearly detect GFP expressing cells of donor origin [25]. This may be a concern in a manuscript by Wagers et al [17] that reported the absence of MDLE. In this paper, GFP was used as a donor marker to track the fate of hematopoietic stem cells that were transplanted into wild type recipient mice, but only “in some cases” the GFP signal was amplified with an anti-GFP antibody, and the authors did not specify on which samples this was performed. Again, this leaves skeptics open to criticize the validity of the conclusions. Photomicrographs show very low numbers of GFP positive cells within different tissue sections from recipient mice, even though the hematopoietic system of the recipient mouse should be derived from the donor and thus, GFP+ cells should be abundant even after flushing intravascular blood cells while the heart is still beating, suggesting that cells expressing lower levels of GFP may have been missed. It is therefore very important to demonstrate to the reader that the techniques used to detect transgene expression are sensitive enough, when using these to detect marrow derived epithelial cells.
Furthermore, fixation may mask epitopes, making them inaccessible for immunodetection. Therefore, positive controls, which have been fixed and treated in the same way as experimental samples, must always be stained at the same time. At least some of these controls should be shown, and all should be indicated in the results section of publications.
Low-resolution images, low magnification
In order to definitively show marrow derived epithelial cells, high quality, high-resolution images of adequate magnification should be provided. Confocal microscopy yields the most accurate results, and images from each fluorescent channel should be shown side by side in addition to the merged image. Three-dimensional projections of z-stacks are very helpful to show the morphology of the detected cell in the context of its environment, and to exclude cell overlay. Some published articles show images of low magnification and/or resolution, which are sometimes hard to interpret and in some cases entirely uninterpretable. The results of these papers would be more conclusive if high quality images were shown.
Definitive data on marrow derived epithelial cells
Examples of a few studies that definitively demonstrate bone marrow derived lung epithelial cells are described below. McPherson et al [4] demonstrated the presence of airway epithelial cells derived from male donor BM cells using Y-chromosome FISH concomitant with immunostaining for CD45 and cytokeratin with analysis by deconvolution microscopy. These marrow derived Y chromosome-positive, cytokeratin-positive, CD45-negative airway epithelial cells became apparent only after detergent induced damage, suggesting that tissue injury is required to promote the appearance of MDLE.
Aliotta et al [3] transplanted bone marrow cells from a GFP+ donor into wild type recipients, and subsequently used deconvolution microscopy and 3 dimensional projection to demonstrate that the donor marker GFP (green) and the epithelial marker cytokeratin (red) co- localize in the same cell, which does not express CD45 (blue), as shown in figure 1.IV. These carefully obtained and presented data definitively demonstrate that the phenomenon of marrow derived epithelial cells exists and can be detected using appropriate assays and methods of detection.
Criteria for definitive data on MDLE
As stated above, data showing MDLE must demonstrate that these cells are marrow derived, epithelial, nonhematopoietic, and not artifacts of cell overlay or autofluorescence.
This can be achieved by simultaneous staining for bone marrow-donor-specific, lung specific and blood specific markers. Detection of donor specific and epithelial specific markers within the same cell in tissue sections may be best shown using confocal or deconvolution microscopy, which should be combined with optical sectioning (z-stacks) to exclude cell overlay and demonstrate epithelial cell morphology. Great care has to be taken to exclude artifacts and autofluorescent signals from the analysis. Single cell analysis, for example, does not require confocal microscopy because cell overlay is not and issue, and has the advantage that there is no tissue background-fluorescence, which may interfere with the detection of specific signals. However, confocal microscopy can be very useful also on single cells to assess the intracellular distribution of proteins, the size of cytoplasmic vesicles, intranuclear staining and cell morphology.
Recent enhancements in detection of marrow derived lung epithelial cells
As in many studies requiring microscopy, fixation, staining protocols, and detection approaches need constant optimization and require great perseverance and accuracy for optimal specificity, efficiency and probability of detection. One of the improvements that we have made in our laboratory is to use fluorescence activated cell sorting of lung single cell suspensions to enrich for the epithelial cell population and to exclude most (if not all) macrophages, other blood cells and endothelial cells from the subsequent analysis. The sorted cells are then attached to coverslips and analyzed by immunofluorescence and confocal microscopy for expression of the donor marker GFP and alveolar type 2 cell specific surfactant protein C in intracellular vesicles (Fig 1.V).
Necessity for development of protocols for assessing epithelial cell functionality of marrow derived epithelial cells
Despite a number of excellent studies that clearly demonstrate MDLE, it has yet to be proven that marrow derived epithelial cells share the functional characteristics of lung epithelial cells, which would indicate that they can fully adopt the epithelial cell phenotype.
Expression of epithelial cell specific proteins or mRNA indicates that a marrow-derived cell has the gene expression of an epithelial cells and can imply specific function. Such studies are therefore a helpful approach when trying to assess functionality of marrow derived epithelial cells. Epithelial cell specific proteins that are not expressed by bone marrow cells and can be detected by immunofluorescence include surfactant proteins, cytokeratins, aquaporin, clara cell specific protein, and thyroid transcription factor 1. Single cell PCR or microarray analysis of isolated MDLE could be performed to assess whether the gene expression pattern matches that of an epithelial cell. Another approach that might be indicative of functionality of a marrow derived epithelial cell is ultrastructural characterization by electron microscopy (EM). For example, EM allows for detection of lamellar bodies in isolated type 2 pneumocytes, and immunogold labeling could be used to detect a donor specific marker like GFP.
While the approaches above are indicative of epithelial cell specific gene expression and/or morphology, none represents a definitive in vitro assay for alveolar epithelial cell function. To date, no optimal functional assays exist. However, there are some ways in which epithelial cell functionality can be assessed in vitro. For instance, Yu et al described a three-dimensional culture system in which primary type 2 pneumocytes formed alveolar like cysts, which consisted of a polarized monolayer of type 2 cells which secreted surfactant into the lumen [26]. For type 2 pneumocytes, in vitro cell behavior of isolated single cells can be studied by video microscopy using uptake of Lysotracker dye into lamellar bodies followed by active secretion of the dye [27, 28]. A better in vitro assay of functionality would be to demonstrate the biochemical behavior that is characteristic for the epithelial cell type in question. Fang et al [27] demonstrated expression of CFTR and it's contribution to cAMP-regulated apical-basolateral fluid transport in type 2 pneumocytes in vitro. Secretion of surfactant proteins into the culture medium can be detected by immunoblotting, and lipid production can be assessed as incorporation of 14C-choline or 14C-acetate into phospholipids [29, 30]. An enzyme immunoassay can detect the presence of the surfactant associated protein alveolin [31]. Analysis of type 1 (AT1) cell function in vitro has been limited by difficulties in isolating highly purified populations of AT1 cells and subsequently identifying them by other than morphological means following isolation. Cell flattening and expression of AT1 cell specific T1alpha can be observed during transition of cultured type 2 cells towards a type 1 cell phenotype [32]. With the use of this model, it has been demonstrated that alveolar epithelial cells, after several days in culture, exhibit active Na transport, which can be inhibited by both ouabain and amiloride [33], and express Na pump and Na channel proteins, consistent with the phenotype of AT1 cells in active transalveolar epithelial Na transport [34]. Analysis of these features would provide some information towards functionality of marrow derived type 1 cells. All these in vitro assays for analysis of functional properties would need to be adapted for extremely small cell numbers. Any analysis of cell function in vitro will be feasible and informative only when and if live MDLE can be isolated, specifically and absolutely without contaminating endogenous cells.
Demonstration of cell functionality in vivo would be the ultimate proof that a marrow-derived cell can fully adopt the phenotype of an epithelial cell, for example, if the MDLE would be able to restore function after loss of endogenous epithelial cells. There are studies showing therapeutic effects of marrow derived cells after induction of lung disease in animals, but these ameliorative effects can nearly always be explained by indirect effects due to downregulation of the inflammatory response, as is known to occur with marrow stromal cells, rather than due to engraftment of functional MDLE [35–38]. The Krause laboratory approached this in the past by transplanting wild type BM cells into surfactant protein C null mice. However, while MDLE cells that expressed surfactant protein C were identified, the actual phenotype of the knock out mice was quite subtle, such that there were no statistically significant differences between transplanted WT and KO mice, which precluded our ability to assess for in vivo function.
Functionality of marrow-derived cells has been successfully demonstrated in organs other than the lung. In the liver, transplantation of wild type bone marrow restores liver function in FAH knockout mice, an animal model of tyrosinemia type I, due to fusion of marrow derived cells with hepatocytes [39, 40]. In the gastrointestinal (GI) tract, transplantation of cystic fibrosis transmembrane conductance regulator (CFTR)-positive BM-derived cells gave rise to rare BM-derived GI and airway epithelial cells which provided CFTR a measurable level of CFTR-dependent chloride secretion in the GI tract and nasal epithelium of myeloablated recipient CFTR−/− cystic fibrosis mice [41]. Similar approaches and suitable models would need to be developed for the lung in order to demonstrate functionality of marrow derived epithelial cells in this organ. Ongoing efforts to produce transgenic mice with inducible suicide of specific lung epithelial subtypes may provide the best models for future studies to assess in vivo function of MDLE, if marrow derived cells would at least partially replace the ablated cell type. However, in most situations, marrow derived epithelial cells of the lung are exceedingly rare, which might make it difficult to detect functional effects in vivo. Once we have a better understanding of which cells are capable of engraftment as epithelial cells and the mechanisms by which this occurs, then efforts can be focused on increasing the levels of such cells for analysis of their function and, ultimately, potential therapeutic benefit.
Isolation of MDLE
For functional assays as well as in order to further characterize and study marrow derived lung epithelial cells, the ability to specifically isolate these cells will be important. Marrow derived epithelial cells are extremely rare and hard to separate from the endogenous epithelial cells. Identification of donor-derived cells requires antibody labeling for epithelial markers and donor markers, which nearly always involves fixation. Fixation, however, eliminates the possibility of any functional assessment. Instead, approaches in which marrow derived epithelial cells can be identified and isolated as live cells will be crucial. This could be achieved, for instance, by introducing bone marrow from a transgenic donor in which a fluorescent protein is expressed from an epithelial cell specific promoter. This would allow isolation of live MDLE based on expression of the fluorescent protein by FACS sorting of single cells.
Unresolved Questions
Here, we have focused on requirements for definitive data. But, identifying the cells is only the beginning of what we need to do in studies of marrow derived epithelial cells. Unresolved questions include
BM cell source: It has not been demonstrated so far which type of bone marrow cell is able to “adopt the morphology and protein expression of an epithelial cell”. There are no data that definitively prove whether hematopoietic cells are able to adopt the phenotype of epithelial cells in the lung. Wagers et al demonstrated that this is not the case, but their data are not conclusive, as stated above. Theoretically, other, non-hematopoietic BM cells, like MSC, might also be capable of differentiating into epithelial cells, but this has not been definitively proven, either.
Mechanism: possible processes that may lead to the appearance of marrow derived epithelial cells are a) transdifferentiation or plasticity, b) differentiation of a precursor, and c) fusion followed by reprogramming. `Transdifferentiation' or `Plasticity' refers to one committed cell becoming another type of committed cell, and this has not been definitively proven to date. It is likely that there are different mechanisms underlying this phenomenon, some of which may involve `plasticity”, others `differentiation' of a progenitor or stem cell, yet others may involve cell fusion. While marrow derived lung epithelial cells can appear without any evidence of cell fusion [7], Herzog et al [42] have demonstrated that they can be derived in part from cell fusion events. Another possible mechanism underlying differentiation of bone marrow derived cells into epithelial cells may be uptake of pulmonary epithelial cell-specific RNA-containing microvesicles by bone marrow cells [43].
In conclusion, there is definitive proof that marrow derived epithelial cells exist in vivo. Since they are very rare, sensitive and stringent detection techniques are needed for their analysis. Future research needs to elucidate the functionality of these cells as well as the bone marrow cell source and the mechanisms underlying their appearance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosure No financial interest/relationships with financial interest relating to the topic of this article have been declared.
References
- [1].Krause DS, Theise ND, Collector MI, et al. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell. 2001;105:369–377. doi: 10.1016/s0092-8674(01)00328-2. [DOI] [PubMed] [Google Scholar]
- [2].Theise ND, Henegariu O, Grove J, et al. Radiation pneumonitis in mice: a severe injury model for pneumocyte engraftment from bone marrow. Exp Hematol. 2002;30:1333–1338. doi: 10.1016/s0301-472x(02)00931-1. [DOI] [PubMed] [Google Scholar]
- [3].Aliotta JM, Keaney P, Passero M, et al. Bone marrow production of lung cells: the impact of G-CSF, cardiotoxin, graded doses of irradiation, and subpopulation phenotype. Exp Hematol. 2006;34:230–241. doi: 10.1016/j.exphem.2005.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].MacPherson H, Keir PA, Edwards CJ, Webb S, Dorin JR. Following damage, the majority of bone marrow-derived airway cells express an epithelial marker. Respir Res. 2006;7:145. doi: 10.1186/1465-9921-7-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Grove JE, Bruscia E, Krause DS. Plasticity of bone marrow-derived stem cells. Stem Cells. 2004;22:487–500. doi: 10.1634/stemcells.22-4-487. [DOI] [PubMed] [Google Scholar]
- [6].Herzog EL, Van Arnam J, Hu B, Krause DS. Threshold of lung injury required for the appearance of marrow-derived lung epithelia. Stem Cells. 2006;24:1986–1992. doi: 10.1634/stemcells.2005-0579. [DOI] [PubMed] [Google Scholar]
- [7].Harris RG, Herzog EL, Bruscia EM, Grove JE, Van Arnam JS, Krause DS. Lack of a fusion requirement for development of bone marrow-derived epithelia. Science. 2004;305:90–93. doi: 10.1126/science.1098925. [DOI] [PubMed] [Google Scholar]
- [8].Loi R, Beckett T, Goncz KK, Suratt BT, Weiss DJ. Limited restoration of cystic fibrosis lung epithelium in vivo with adult bone marrow-derived cells. Am J Respir Crit Care Med. 2006;173:171–179. doi: 10.1164/rccm.200502-309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Spees JL, Pociask DA, Sullivan DE, et al. Engraftment of bone marrow progenitor cells in a rat model of asbestos-induced pulmonary fibrosis. Am J Respir Crit Care Med. 2007;176:385–394. doi: 10.1164/rccm.200607-1004OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Spees JL, Whitney MJ, Sullivan DE, et al. Bone marrow progenitor cells contribute to repair and remodeling of the lung and heart in a rat model of progressive pulmonary hypertension. FASEB J. 2008;22:1226–1236. doi: 10.1096/fj.07-8076com. [DOI] [PubMed] [Google Scholar]
- [11].Sueblinvong V, Loi R, Eisenhauer PL, et al. Derivation of lung epithelium from human cord blood-derived mesenchymal stem cells. Am J Respir Crit Care Med. 2008;177:701–711. doi: 10.1164/rccm.200706-859OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Yamada M, Kubo H, Kobayashi S, et al. Bone marrow-derived progenitor cells are important for lung repair after lipopolysaccharide-induced lung injury. J Immunol. 2004;172:1266–1272. doi: 10.4049/jimmunol.172.2.1266. [DOI] [PubMed] [Google Scholar]
- [13].Rojas M, Xu J, Woods CR, et al. Bone marrow-derived mesenchymal stem cells in repair of the injured lung. Am J Respir Cell Mol Biol. 2005;33:145–152. doi: 10.1165/rcmb.2004-0330OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kotton DN, Ma BY, Cardoso WV, et al. Bone marrow-derived cells as progenitors of lung alveolar epithelium. Development. 2001;128:5181–5188. doi: 10.1242/dev.128.24.5181. [DOI] [PubMed] [Google Scholar]
- [15].Wong AP, Keating A, Lu WY, et al. Identification of a bone marrow-derived epithelial-like population capable of repopulating injured mouse airway epithelium. J Clin Invest. 2009;119:336–348. doi: 10.1172/JCI36882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kotton DN, Fabian AJ, Mulligan RC. Failure of bone marrow to reconstitute lung epithelium. Am J Respir Cell Mol Biol. 2005;33:328–334. doi: 10.1165/rcmb.2005-0175RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Wagers AJ, Sherwood RI, Christensen JL, Weissman IL. Little evidence for developmental plasticity of adult hematopoietic stem cells. Science. 2002;297:2256–2259. doi: 10.1126/science.1074807. [DOI] [PubMed] [Google Scholar]
- [18].Chang JC, Summer R, Sun X, Fitzsimmons K, Fine A. Evidence that bone marrow cells do not contribute to the alveolar epithelium. Am J Respir Cell Mol Biol. 2005;33:335–342. doi: 10.1165/rcmb.2005-0129OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Fritzell JA, Jr., Mao Q, Gundavarapu S, et al. Fate and effects of adult bone marrow cells in lungs of normoxic and hyperoxic newborn mice. Am J Respir Cell Mol Biol. 2009;40:575–587. doi: 10.1165/rcmb.2008-0176OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Grove JE, Lutzko C, Priller J, et al. Marrow-derived cells as vehicles for delivery of gene therapy to pulmonary epithelium. Am J Respir Cell Mol Biol. 2002;27:645–651. doi: 10.1165/rcmb.2002-0056RC. [DOI] [PubMed] [Google Scholar]
- [21].Reese JS, Roth JC, Gerson SL. Bone marrow-derived cells exhibiting lung epithelial cell characteristics are enriched in vivo using methylguanine DNA methyltransferase-mediated drug resistance. Stem Cells. 2008;26:675–681. doi: 10.1634/stemcells.2007-0803. [DOI] [PubMed] [Google Scholar]
- [22].Ortiz LA, Gambelli F, McBride C, et al. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci U S A. 2003;100:8407–8411. doi: 10.1073/pnas.1432929100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Corti M, Brody AR, Harrison JH. Isolation and primary culture of murine alveolar type II cells. Am J Respir Cell Mol Biol. 1996;14:309–315. doi: 10.1165/ajrcmb.14.4.8600933. [DOI] [PubMed] [Google Scholar]
- [24].Swenson ES, Price JG, Brazelton T, Krause DS. Limitations of green fluorescent protein as a cell lineage marker. Stem Cells. 2007;25:2593–2600. doi: 10.1634/stemcells.2007-0241. [DOI] [PubMed] [Google Scholar]
- [25].Toth ZE, Shahar T, Leker R, et al. Sensitive detection of GFP utilizing tyramide signal amplification to overcome gene silencing. Exp Cell Res. 2007;313:1943–1950. doi: 10.1016/j.yexcr.2007.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Yu W, Fang X, Ewald A, et al. Formation of cysts by alveolar type II cells in three-dimensional culture reveals a novel mechanism for epithelial morphogenesis. Mol Biol Cell. 2007;18:1693–1700. doi: 10.1091/mbc.E06-11-1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Fang X, Song Y, Hirsch J, et al. Contribution of CFTR to apical-basolateral fluid transport in cultured human alveolar epithelial type II cells. Am J Physiol Lung Cell Mol Physiol. 2006;290:L242–249. doi: 10.1152/ajplung.00178.2005. [DOI] [PubMed] [Google Scholar]
- [28].Haller T, Ortmayr J, Friedrich F, Volkl H, Dietl P. Dynamics of surfactant release in alveolar type II cells. Proc Natl Acad Sci U S A. 1998;95:1579–1584. doi: 10.1073/pnas.95.4.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Augustin-Voss HG, Schoon HA, Gerull A, Ueberschar S. Biochemical and metabolic properties of bovine type II pneumocytes in primary culture. Lung. 1989;167:343–350. doi: 10.1007/BF02714962. [DOI] [PubMed] [Google Scholar]
- [30].Uhal BD, Hess GD, Rannels DE. Density-independent isolation of type II pneumocytes after partial pneumonectomy. Am J Physiol. 1989;256:C515–521. doi: 10.1152/ajpcell.1989.256.3.C515. [DOI] [PubMed] [Google Scholar]
- [31].Kumar RK, Truscott JY, Rhodes GC, Lykke AW. Type 2 pneumocyte responses to cyclophosphamide-induced pulmonary injury: functional and morphological correlation. Br J Exp Pathol. 1988;69:69–80. [PMC free article] [PubMed] [Google Scholar]
- [32].Borok Z, Danto SI, Lubman RL, Cao Y, Williams MC, Crandall ED. Modulation of t1alpha expression with alveolar epithelial cell phenotype in vitro. Am J Physiol. 1998;275:L155–164. doi: 10.1152/ajplung.1998.275.1.L155. [DOI] [PubMed] [Google Scholar]
- [33].Cheek JM, Kim KJ, Crandall ED. Tight monolayers of rat alveolar epithelial cells: bioelectric properties and active sodium transport. Am J Physiol. 1989;256:C688–693. doi: 10.1152/ajpcell.1989.256.3.C688. [DOI] [PubMed] [Google Scholar]
- [34].Borok Z, Liebler JM, Lubman RL, et al. Na transport proteins are expressed by rat alveolar epithelial type I cells. Am J Physiol Lung Cell Mol Physiol. 2002;282:L599–608. doi: 10.1152/ajplung.00130.2000. [DOI] [PubMed] [Google Scholar]
- [35].Lee SH, Jang AS, Kim YE, et al. Modulation of cytokine and nitric oxide by mesenchymal stem cell transfer in lung injury/fibrosis. Respir Res. 11:16. doi: 10.1186/1465-9921-11-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Lee JW, Fang X, Gupta N, Serikov V, Matthay MA. Allogeneic human mesenchymal stem cells for treatment of E. coli endotoxin-induced acute lung injury in the ex vivo perfused human lung. Proc Natl Acad Sci U S A. 2009;106:16357–16362. doi: 10.1073/pnas.0907996106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].van Haaften T, Byrne R, Bonnet S, et al. Airway delivery of mesenchymal stem cells prevents arrested alveolar growth in neonatal lung injury in rats. Am J Respir Crit Care Med. 2009;180:1131–1142. doi: 10.1164/rccm.200902-0179OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Aslam M, Baveja R, Liang OD, et al. Bone marrow stromal cells attenuate lung injury in a murine model of neonatal chronic lung disease. Am J Respir Crit Care Med. 2009;180:1122–1130. doi: 10.1164/rccm.200902-0242OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Lagasse E, Connors H, Al-Dhalimy M, et al. Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat Med. 2000;6:1229–1234. doi: 10.1038/81326. [DOI] [PubMed] [Google Scholar]
- [40].Wang X, Willenbring H, Akkari Y, et al. Cell fusion is the principal source of bone-marrow-derived hepatocytes. Nature. 2003;422:897–901. doi: 10.1038/nature01531. [DOI] [PubMed] [Google Scholar]
- [41].Bruscia EM, Price JE, Cheng EC, et al. Assessment of cystic fibrosis transmembrane conductance regulator (CFTR) activity in CFTR-null mice after bone marrow transplantation. Proc Natl Acad Sci U S A. 2006;103:2965–2970. doi: 10.1073/pnas.0510758103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Herzog EL, Van Arnam J, Hu B, et al. Lung-specific nuclear reprogramming is accompanied by heterokaryon formation and Y chromosome loss following bone marrow transplantation and secondary inflammation. FASEB J. 2007;21:2592–2601. doi: 10.1096/fj.06-7861com. [DOI] [PubMed] [Google Scholar]
- [43].Aliotta JM, Sanchez-Guijo FM, Dooner GJ, et al. Alteration of marrow cell gene expression, protein production, and engraftment into lung by lung-derived microvesicles: a novel mechanism for phenotype modulation. Stem Cells. 2007;25:2245–2256. doi: 10.1634/stemcells.2007-0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Abe S, Lauby G, Boyer C, Rennard SI, Sharp JG. Transplanted BM and BM side population cells contribute progeny to the lung and liver in irradiated mice. Cytotherapy. 2003;5:523–533. doi: 10.1080/14653240310003576. [DOI] [PubMed] [Google Scholar]
- [45].Macpherson H, Keir P, Webb S, et al. Bone marrow-derived SP cells can contribute to the respiratory tract of mice in vivo. J Cell Sci. 2005;118:2441–2450. doi: 10.1242/jcs.02375. [DOI] [PubMed] [Google Scholar]
- [46].Suratt BT, Cool CD, Serls AE, et al. Human pulmonary chimerism after hematopoietic stem cell transplantation. Am J Respir Crit Care Med. 2003;168:318–322. doi: 10.1164/rccm.200301-145OC. [DOI] [PubMed] [Google Scholar]
- [47].Mattsson J, Jansson M, Wernerson A, Hassan M. Lung epithelial cells and type II pneumocytes of donor origin after allogeneic hematopoietic stem cell transplantation. Transplantation. 2004;78:154–157. doi: 10.1097/01.tp.0000132326.08628.74. [DOI] [PubMed] [Google Scholar]
- [48].Zander DS, Cogle CR, Theise ND, Crawford JM. Donor-derived type II pneumocytes are rare in the lungs of allogeneic hematopoietic cell transplant recipients. Ann Clin Lab Sci. 2006;36:47–52. [PubMed] [Google Scholar]
- [49].Kleeberger W, Versmold A, Rothamel T, et al. Increased chimerism of bronchial and alveolar epithelium in human lung allografts undergoing chronic injury. Am J Pathol. 2003;162:1487–1494. doi: 10.1016/S0002-9440(10)64281-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Spencer LT, Paone G, Krein PM, Rouhani FN, Rivera-Nieves J, Brantly ML. Role of human neutrophil peptides in lung inflammation associated with alpha1-antitrypsin deficiency. Am J Physiol Lung Cell Mol Physiol. 2004;286:L514–520. doi: 10.1152/ajplung.00099.2003. [DOI] [PubMed] [Google Scholar]
- [51].Zander DS, Baz MA, Cogle CR, Visner GA, Theise ND, Crawford JM. Bone marrow-derived stem-cell repopulation contributes minimally to the Type II pneumocyte pool in transplanted human lungs. Transplantation. 2005;80:206–212. doi: 10.1097/01.tp.0000165095.39320.50. [DOI] [PubMed] [Google Scholar]
- [52].Albera C, Polak JM, Janes S, et al. Repopulation of human pulmonary epithelium by bone marrow cells: a potential means to promote repair. Tissue Eng. 2005;11:1115–1121. doi: 10.1089/ten.2005.11.1115. [DOI] [PubMed] [Google Scholar]
- [53].Rejman J, Colombo C, Conese M. Engraftment of bone marrow-derived stem cells to the lung in a model of acute respiratory infection by Pseudomonas aeruginosa. Mol Ther. 2009;17:1257–1265. doi: 10.1038/mt.2009.96. [DOI] [PMC free article] [PubMed] [Google Scholar]