Abstract
Peripheral arterial disease (PAD) is a growing health problem for many Americans and often occurs along with other cardiovascular risk factors, including diabetes mellitus (DM), low-grade inflammation, hypertension, and lipid disorders. Intermittent claudication (IC), an early manifestation of PAD, commonly leads to reduced quality of life for patients who are limited in their ambulation. While recent wide adoption of percutaneous peripheral interventional (PPI) techniques has increased the number patients being aggressively treated for IC, the overall effectiveness of PPI for the treatment of IC is not well known, especially for DM patients who have both hemodynamic and functional obstacles to treatment success. This review is designed to illustrate how treatment outcomes for IC can be measured by different modalities and how diabetes and inflammation can influence those outcomes. In the setting of greater concern for health care resources and clinical accountability, better understanding of treatment outcomes and efficacy will help us manage these complex challenges.
Peripheral arterial disease (PAD) is a growing health problem for many Americans. The burden of PAD often occurs along with other cardiovascular risk factors, including diabetes mellitus (DM), low-grade inflammation, hypertension, and lipid disorders. DM, in particular, is an increasingly important cofactor because the number of PAD patients with DM continues to increase. Intermittent claudication (IC), an early manifestation of PAD, commonly leads to reduced quality of life (QOL) for patients who are limited in their ambulation. The recent wide adoption of percutaneous peripheral interventional (PPI) techniques has increased the number patients being aggressively treated for IC. However, the overall effectiveness of PPI for the treatment of IC is not well known, especially for DM patients who have both hemodynamic and functional obstacles to treatment success.
From a functional standpoint, DM patients frequently have coexisting impairments, such as neuropathy and obesity, that may negatively impact on walking rehabilitation and QOL after PPI. They also have a greater risk of cardiovascular-related morbidity and mortality, perhaps leading to more compromised benefit from PPI. Thus, the risk– benefit ratio for treatment of IC in DM patients is particularly complex and not well understood.
Recent attention has also focused on the prediabetic spectrum of impaired glucose metabolism, with a growing population of patients identified with insulin resistance (IR), impaired glucose tolerance, and obesity. Hypertriglyceridemia, IR, decreased high-density lipoprotein cholesterol, hypertension, and central obesity are clustered factors known as “metabolic syndrome,” which portends increased cardiovascular risk. Impaired fibrinolysis and low-grade inflammation are also associated with the metabolic syndrome and may act as comodifiers that predispose the patient to cardiovascular disease and affect hemodynamic outcomes after PPI, such as early failure or restenosis.
Patients with PAD have a higher prevalence of DM, IR, and inflammatory markers, and the interplay of these factors is currently under investigation. Their role as a predictor or effecter of treatment outcomes also needs to be analyzed using several different outcome measures to better capture their impact on the patients being treated.
BACKGROUND AND SIGNIFICANCE
Claudication
PAD, a chronic, progressive disease of arterial obliteration most commonly due to atherosclerosis, affects approximately 8 to 10 million Americans, up to half of whom have symptomatic disease.1,2 IC usually is the first symptomatic presentation of PAD. Patients with IC have limited ambulation distance or speed due to pain. This impairment in function results in a reduction in QOL.
Patients with IC have traditionally been treated with conservative medical management, including a walking program, smoking cessation, and risk factor reduction.3,4 Current pharmacologic therapies for IC have modest results. Pentoxifylline, a xanthine derivative, improves red cell deformity, lowers fibrinogen levels, and decreases platelet aggregation. Trial patients had a 50% improvement in the percentage change in absolute walking distance compared with 29% seen in placebo control patients, although this was not statistically significant.5 No patient questionnaires were used in this trial to assess clinical efficacy.
Cilostazol is a phosphodiesterase III inhibitor with antiplatelet and vasodilation effects. Controlled trials showed statistically improved walking distance in study patients (47% vs 13%).6 In these and other trials, the improvements seen in the control groups suggest that the placebo effect may be a contributor to outcomes. The current evidence is not sufficient to recommend the routine use of pharmacotherapy in all patients with IC.2
Traditionally, those who did not improve with these medical strategies or who were severely debilitated from their IC were revascularized with surgical bypass grafting.7 Conservative approaches to IC are based in part on data that suggested few patients progressed to worsening PAD, as evidenced by a minimal drop in the ankle-brachial index (ABI) over 5 years8 and the small number of IC patients who go on to develop critical limb ischemia (CLI).9 However, more recent data show that the natural history of elderly men with IC was a decline in walking and overall activity.10 These findings highlight a growing understanding that IC has socioeconomic impacts that were previously not well appreciated.11
Advancements in catheter-based techniques have added another therapeutic option in the treatment of IC. PPI are less invasive than open surgical bypass yet restore vessel patency, unlike pharmacotherapy. The use of PPI in the treatment of aortoiliac occlusive disease is well established.2 Outcomes for infrainguinal PPI for IC are less known, however, and their applicability to certain patient populations is controversial. Several issues have made the treatment of IC a more complex issue:
Although a monitored exercise rehabilitation program can result in improved walking, perceived QOL, and general physical activity,12,13 many patients cannot maintain an exercise program, and thus, their symptoms do not resolve.
IC is a symptom resulting from more than just decreased blood flow to the lower extremity. Disturbances in muscle metabolism,14 and specifically of glucose utilization,15 may also contribute to walking intolerance in patients with PAD.
Patients with lower extremity intervention have a 3.3% to 8.3% annual incidence of contralateral ischemia16; therefore, PAD is a two-limb problem and good clinical outcome requires function of both limbs.
Patients with IC are also at greater risk for mortality, with old age, lower ABI, DM, and stroke among risk factors.17
Several studies have yielded all-cause mortality rates of 30% at 5 years and 50% at 10 years, a prognosis equivalent to postresection of Duke’s B colon carcinoma.2 Thus, the long-term benefits of IC treatment may be diminished by the relatively high mortality rates in this cohort.
Arguably, the biggest questions in the treatment in IC is who, when, and how to treat, and if to treat at all. To best answer these questions, a series of large, multi-institutional, randomized trials would be needed. However, we have observed in our clinical experience that the treatment of IC with PPI seems to be driven by other factors in addition to published practice guidelines based on clinical studies. Economic factors, including first-mover advantage, market share growth, provider specialty competition, and industry involvement are affecting decisions in the treatment of IC and, specifically, the use of PPI in that treatment strategy.
Diabetes and insulin resistance in peripheral arterial disease
The prevalence of DM is rapidly increasing and is predicted to affect 221 million individuals worldwide by 2010.18 Overall, patients with DM have greater general disability, including decreased mobility.19 Patients with DM have an increased risk of developing PAD, and that risk increases with the duration of DM.20 Patients with DM and PAD have impaired mobility due to neuropathy, IC, and cardiovascular comorbidities.21
People with type 1 DM have autoimmune-mediated destruction of pancreatic islet cells that results in an absolute insulin deficiency. Type 2 DM is more prevalent is and characterized by IR or abnormal insulin secretion, or both. Although some type 2 DM patients may require exogenous insulin, many may not if glucose control can be achieved with diet alone or the addition of oral hypoglycemic agents. Thus, some patients may have a component of IR and be unaware because of the mildness of symptoms or poor understanding of infrequent symptoms.
In addition to frank DM, IR occurs in an estimated 22% of the United States (US) population.22 With IR, impaired insulin signaling results in decreased utilization of glucose and fatty acids in skeletal muscles. This results in hyperglycemia and increased levels of circulating free fatty acids. In response to the hyperglycemia, islet cells produce more insulin, which leads to hyperinsulinemia. The IR syndrome is the presence of three or more of the following: abdominal obesity, high triglyceride levels, low high-density lipoprotein cholesterol, hypertension, and hyperglycemia.22 The National Cholesterol Education Program suggested a similar definition for the metabolic syndrome.23
Although the mechanisms of diabetic vascular disease remain incompletely understood, considerable data suggest that hyperglycemia itself causes vascular damage. Several studies have linked both microvascular and macrovascular complications of DM to the level of glycemic control. Longstanding hyperglycemia can lead to nonenzymatic glycation and oxidation of proteins and lipids, resulting in the accumulation of advanced glycation end products (AGE). These AGE are thought to interact with receptors (RAGE) in the vasculature, resulting in activation of mechanisms leading to atherosclerosis. RAGE also occurs endogenously as a soluble form (sRAGE), which can act as a binding decoy for the cellular RAGE. A novel treatment strategy was developed using the soluble extracellular domain of the AGE receptor (sRAGE) to bind and inactivate AGE. In vivo studies showed sRAGE suppressed diabetic atherosclerosis in a glycemia-independent and lipid-independent manner.24 Low levels of sRAGE were also found to be associated with coronary artery disease in non-DM men.25
Although IC may represent mild PAD in non-DM patients, CLI26,27 and amputation28 are more likely to develop in IC patients with DM. Evidence also shows that PAD patients with DM are undertreated medically29; however, it is not known which is the cause and which the effect in these observations. Patients with DM are also less likely to present with IC as a herald for later CLI.30 Similarly, IR is associated with atherosclerosis and predicts cardiovascular events.31,32
Inflammation in peripheral arterial disease
The role of inflammation in PAD is becoming increasingly recognized, as is the synergism between inflammation, DM, and IR. Several inflammatory markers have been found to be associated with atherosclerosis and related events, including C-reactive protein (CRP), interleukin (IL)-6,33 tumor necrosis factor-α,34 P selectin,35 soluble intracellular adhesion molecule-1 (sICAM-1), soluble vascular cell adhesion molecule-1 (sVCAM-1), CD40 ligand, and E selectin.36
CD40 ligand is a transmembrane protein expressed on a variety of cells, including endothelial cells, vascular smooth muscle cells, and activated platelets. It is also found in plasma as a soluble protein, sCD40 ligand. CD40 ligand binding to its receptor, CD40, results in initiation of inflammatory responses.37 Elevated sCD40 ligand levels have been associated with increased risk for cardiovascular events, and intensive risk factor reduction has resulted in reduction of sCD40 ligand.38 Patients with DM also show increased CD40 ligand receptor– binding complex.39 One potential mechanism linking sCD40 ligand to atherosclerosis in DM patients is through hyperglycemia being an up-regulator of sCD40 ligand, resulting in endothelial activation and monocyte recruitment to the arterial wall.40
CRP is a long-described inflammatory marker belonging to the pentraxin family of immune response proteins. It has several functions that can lead to atherosclerotic progression, including induction of cell adhesion molecule expression and mediation of low-density lipoprotein uptake by endothelial bound macrophages.41 In prospective cohort studies, CRP was associated with progression of carotid atherosclerosis42 and severity of atherosclerosis at various anatomic sites.43 Patients with PAD have elevated inflammatory markers, including CRP, and their levels correspond to the level of disease44: control patients, 1.04 mg/L; IC patients, 3.4 mg/L; and CLI patients, 7.17 mg/L. Higher CRP is associated with greater walking disability as measured by walking tests and functional surveys.45 Inflammatory markers from asymptomatic patients have been showed to be predictors risk factors for the development of subsequent PAD.46 Inflammation is related to endothelial dysfunction, which in turn may contribute to worsening PAD.47 One mechanism for this effect may be the ability of CRP to reduce endothelial nitric oxide synthase (eNOS) expression.48
IL-6 is an acute inflammatory cytokine and the principal inducer of CRP synthesis by the liver. Several studies have linked levels of IL-6 with traditional cardiovascular risk factors, including hypertension, smoking, IR,49 and excess adipose tissue.50 In patients with acute myocardial infarction, IL-6 levels increase and peak at 36 hours, supporting the role of inflammation after myocardial injury.51 As a predictor among healthy patients, elevated IL-6 levels have also been shown to be related to increased risk of cardiovascular events or death independent of CRP and the presence of traditional cardiovascular risk factors.52
Evidence suggests that low-grade inflammation is linked to impairments of fibrinolysis.53 Elevated fibrinogen is associated with greater risk for cardiovascular events such as stroke and myocardial infarction.54 Tissue plasminogen activator (tPA) and its inhibitor, plasminogen activator inhibitor (PAI-1), play a key part in fibrinolysis. PAI-1 inhibits tPA from cleaving zymogen plasminogen into its active form and thus results in elevated fibrinogen levels. PAI-1 activity is elevated in patients with coronary artery disease, myocardial infarction, and stroke.55,56 Less is known about the role of fibrinolysis in PAD. Elevated PAI-1 is associated with PAD57 and with worsening degrees of claudication.58 Exercise has been shown to lower PAI-1 levels in patients with IC,59 though it is not clear whether increased blood flow from an intervention would lead to the same reduction.
The mechanism of interaction between DM and inflammation in PAD is controversial. DM may accelerate the progression of atherosclerosis or act through other separate pathways. Increased inflammatory markers in healthy patients may also be predictive of greater risk in the later development of DM or insulin-resistance syndrome.60 Insulin has been shown to have anti-inflammatory effects through mechanisms mediated by nitric oxide.61,62 Rosiglitazone, a thiazolidinedione used in the therapy of type 2 DM, also has anti-inflammatory effects.63 Patients with elevated CRP and hyperglycemia (as indicated by hemoglobin A1c) are at greater risk for the occurrence of major adverse cardiovascular events, which include myocardial infarction, percutaneous coronary interventions, coronary bypass graft, carotid revascularization, stroke, and death.64
Peripheral angioplasty and stenting
Advances in percutaneous interventional techniques have allowed clinicians to offer therapeutic treatment without the more invasive nature and longer recovery time of open bypass. In essence, the threshold of invasive treatment has been lowered because the new treatment modality is perceived to be less costly to the patient. The efficacy of aortoiliac angioplasty and stenting is well accepted, but the role of PPI in infrainguinal disease is less established.
Technical and anatomic considerations for catheter-based therapies are more challenging in the superficial femoral artery (SFA) and popliteal vessels compared with more proximal arteries. Initial reports of outcomes for balloon angioplasty alone in these vessels generally displayed inferior outcomes. Improvements in technology, including the use of nitinol stents and perhaps drug-eluting stent designs, as well as plaque debulking techniques, are under continuous evolution.
Early results of infrainguinal PPI showed low 2-year primary patency of 30% to 58%, although secondary patency rates were modestly better at 64% to 72%.65–67 No difference in outcomes was found for sirolimuseluting vs bare metal nitinol stents for SFA disease.68
The TransAtlantic Inter-Society Consensus (TASC) document is the result of collaborative efforts by multiple medical specialties to better define standards for management of PAD patients and the reporting of outcomes.2 The TASC classification of femoropopliteal disease is based on the severity, length, number, and location of lesions (Table). TASC treatment recommendations for IC are well defined for the extreme groups: endovascular treatment for TASC A and surgery for TASC D in appropriately selected candidates. The treatment for TASC B and C groups is not as well defined, however. For TASC A and B lesions, some have found SFA stenting to be comparable with surgical bypass.69 Stenting results for more severe SFA lesions showed lower long-term patency than surgery, but secondary interventions can be an adjunct in these patients.70 Recent retrospective studies looking at the combined use of PCI and open revascularization methods for IC show improved outcomes.71
Table.
TransAtlantic Inter-Society Consensus (TASC) morphologic stratification of femoropopliteal lesions
| Classification | Criteria |
|---|---|
| TASC A | Single stenosis ≤ 3 cm long |
| TASC B | Single stenosis or occlusion 3–10 cm long or Multiple stenoses or occlusions each <3 cm long |
| TASC C | Single stenosis or occlusion longer than 5cm or Multiple stenoses or occlusions each 3–5 cm long |
| TASC D | Complete CFA or SFA occlusion or Complete popliteal and trifurcation occlusion |
The need for reintervention after PPI should not be taken lightly, however, because PPI does have a risk of procedural complications, including access site thrombosis or hemorrhage, lesion thrombosis or embolization, and contrast nephropathy.72,73 Potential long-term complications, such as accelerated progression of disease, remain unclear.
The outcome of PPI in DM patients is not well known. Some authors have found no difference in outcomes for DM patients undergoing femoropopliteal PPI,69,74 whereas others have found worse outcomes in DM patients. Long femoropopliteal stenting has poor mid-term patency results, especially in patients with DM.75 Patients with higher exogenous insulin levels (as measured by lower C-peptide/insulin ratio) were at greater risk for restenosis after femoral percutaneous transluminal angioplasty (PTA).76 A more substantial amount of similar evidence of worse outcomes can be found for DM patients undergoing percutaneous coronary intervention (PCI).77,78 Evidence also suggests that rosiglitazone reduces instent restenosis in DM patients undergoing PCI.79
Likewise, the effect of systemic inflammation on PPI outcomes is not well known. Stent placement induces neointimal proliferation through stent struts by means of local inflammation.80 Measurements of systemic inflammatory markers show conflicting results, however. Some authors have found increased CRP and IL-6 levels after renal artery stenting81 and coronary stenting,82 although the levels were not measured beyond 24 hours. Others found no evidence that the procedure itself causes significant inflammation, as measured by white blood cell and endothelial markers.83 It is not clear whether preprocedural levels of inflammatory markers are predictive of PPI outcomes.
Functional and quality of life outcomes in peripheral arterial disease
Initially, the measurement of success for percutaneous interventions was defined by anatomic criteria and vessel patency, but subsequent work has also focused on patient outcomes such as walking distance, walking speed,84 and QOL. Indeed, randomized controlled treatment of IC showed patients who received invasive treatment had better walking measurements and toe pressures after 1 year, whereas supervised exercise training was similar to untreated controls.85 For IC patients, vessel patency does not necessarily translate to improvements in walking or lifestyle, especially for patients who are already debilitated. Conversely, improvements in walking or lifestyle do not imply vessel patency, as demonstrated by a person who stops smoking. Thus, the measured benefit of percutaneous intervention for IC must be a combination of outcomes.
Patients with IC can present with a wide range of severity of symptoms. They can have greater perceived problems with energy, pain, emotional reactions, sleep, and physical mobility.86 As such, the effect of IC on a patient’s QOL depends on several baseline factors: walking distance and intensity, overall general activity, and the presence of other factors that reduce QOL. Two surveys, Walking Impairment Questionnaire (WIQ) and the EuroQol Group’s EQ-5D instrument have been widely used to measure functional and QOL outcomes for IC patients.
The WIQ was developed to measure patient-perceived walking performance for patients with PAD and IC specifically.87 It estimates IC severity, walking distance, walking speed, and stair climbing ability. Among its benefits is ease of use compared with treadmill testing. Furthermore, treadmill testing may not be reflective of actual daily walking activities that are more specifically captured in WIQ. The WIQ scores do, however, correlate with established walking tests, such as the 6-minute walking distances and 4-meter walking velocity.88
The EQ-5D survey measures health outcomes and has been validated in a variety of disease conditions and geographic populations, including patients with IC89 and US residents.90–92 Its advantage is it is simple, is a quick survey to administer, and results in a single score. The survey consists of five questions about mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. Scores on the EQ-5D were seen to increase (0.57 to 0.75) in patients undergoing revascularization93 and aortoiliac stenting.94 Transformation of patient results to health utilities can be made using known, published reference data appropriate to the study population.
Conservative management of IC has been shown to be effective. Monitored walking and exercise programs can result in symptomatic and functional improvements as measured by Short Form 36 Health Survey (SF-36) and WIQ.95 However, many patients do not complete monitored exercise programs because of logistic or medical limitations and therefore may not gain the QOL described in large studies.
The measurement of functional improvements as measured by walking distance, walking speed, and stair climbing in patients treated for IC has been well documented.95,96 The improvement of QOL is more difficult to measure, however, and cannot be directly inferred from standard vascular anatomic and physiologic results. For example, health-related QOL as measured by SF-36 and WIQ is not reflected well by ABI alone.97 Similarly, improvements in QOL after IC treatment of any kind did not correlate with changes in ABI.98 In some patients, stability in claudication symptoms may be due to decreased activity rather than to disease stability.99 Even at the initial evaluation point by vascular providers, correlation is lacking between the patient’s QOL and the physician’s impression of the symptoms and physical findings.100 This evidence further supports the need for the combined use of clinical and QOL measurements in assessing the impact of PAD and its treatment.
Cost-effectiveness outcomes in peripheral arterial disease
Cost-effectiveness analysis seeks to compare two or more strategies. The evaluation is based on identifying costs for each strategy and its resulting outcomes while comparing it to its benefits standardized to a single health unit, such as quality-adjusted life-years (QALY). Previous work in determining the cost-effectiveness of treatment strategies for IC has showed mixed results. De Vries et al101 found that revascularization, defined as surgical bypass or PPI, was more cost-effective than exercise rehabilitation.101 In contrast, Treesak et al102 found that exercise was more cost-effective than PTA, and Ambrosetti et al12 found that exercise programs meet acceptable cost-benefit figures.12 The outcomes of specific invasive treatment decisions are also complex. For the iliac arteries, Bosch et al103,104 found stenting to be more cost-effective than PTA alone.103,104 For the SFA, Greenberg et al105 found no cost-benefit increase for stenting compared to PTA alone, and Visser et al106 found PTA was as cost-effective as bypass.
Most cost-effectiveness analyses have used data from different institutions to build Markov models.101,103,107 Patients from different institutions are likely to differ in demographics, risk factors, and socioeconomic values, and are subject to regional or institutional practice patterns. Treatment costs also vary significantly with the frequency of procedure-related complications.108 The complication rate depends on institutional expertise and the case-risk mix of patients at the institution; thus, data from studies of different patient groups may not be transferable.
Cost-effective ratios largely depend on the health values of different conditions. These health values may be affected by the concomitant burden of disease from other comorbidities. Thus, a patient with IC as the sole disability is likely to value its treatment differently than a patient with IC among many other disabilities.
The applicability of cost-effectiveness analysis cannot be applied to all patients, because subsets of patients may have different risks and benefits from the same procedure. Cost-effective ratios represent a weighted average of different health states. Individual patients have specific risk factors that may not be well represented in the weighted averages. Hunink et al107 found that from a cost-effective standpoint, angioplasty is the preferred initial treatment for all patients with lower extremity ischemia, except for patients with CLI and femoropopliteal occlusion, where surgical bypass is the preferred treatment. The identification of significant patient factors or clinical scenarios that may have significantly different cost-benefit ratios is an important step in the practical applicability of cost-effectiveness analysis.
The applicability of cost-effectiveness analysis can vary for different institutions and time periods. Institutional size, patient volume, and treatment patterns vary significantly, so it follows that their costs do as well. Conclusions from older studies may no longer be as accurate because of advancements in technology, changes in costs, and changes in patient characteristics. Nevertheless, understanding relative cost differences between treatment strategies or patient groups can help practitioners and policy-makers better address resource allocation issues.
As a whole, cost-effectiveness analyses have increased our understanding of the implications of complex clinical treatment decisions. For a variety of reasons, however, actual clinical practice patterns do not usually comply with cost-effective recommendations. Conflicting cost-effectiveness analysis results, poor applicability, dated costs, and technologic innovation may be among the potential reasons why clinicians make decisions contrary to cost-effectiveness analysis results. As well, cost-effectiveness analysis is based on two or more treatment strategies rather than patient factors. The available choice of strategies is the focus of cost-effectiveness analysis.
Patient factors are not immutable either, however. Although a physician cannot implicitly choose DM as a risk factor for a patient, the physician can choose to modify the severity of the risk factor, or the medical field as a whole can choose to focus more resources on the prevention or reduction of DM. Similarly, physicians do not intentionally choose to give their patients a postoperative complication, but it is important to recognize that patients with seemingly minor postoperative complications can have radically altered prognosis.109 Thus, cost analysis for the effect of specific patient factors, namely DM, may identify cost-critical points that have large impact on subsequent cost and patient outcomes.
Background summary
As the number of active elderly Americans increases, IC will become a more important factor in limiting lifestyle and affecting QOL. The treatment of IC has changed in recent years. It is likely that more IC patients are being treated with invasive techniques because the perceived risk-benefit threshold has lowered with the adoption of PPI techniques. Similarly, the number of patients with IR or frank DM is also increasing, and a subset of them will present initially with IC.
Some early evidence supports infrainguinal PPI for the treatment of IC, but outcomes for patients with DM are not as clear. Furthermore, the role of inflammation in PAD is becoming increasingly understood in its relationship with IR. In contrast to most PAD patients, additional biologic and functional obstacles to PAD treatment success may be present in DM patients. Accordingly, outcomes of PPI for DM patients should be measured using hemodynamic, functional, as well as QOL end points. These three broad outcome categories are dependent on each other but also have many nonvascular effectors. For example, QOL depends on hemodynamic and functional success, although patients who undergo repeated procedure to maintain hemodynamic success might have a lower QOL. Similarly, patients who are burdened with significant comorbidites might not enjoy the functional benefits of hemodynamic success. We see outcomes for PPI as a continuum of different measures that better quantify the benefit to the patient and the health care system. DM, IR, and inflammatory markers are just a few of the factors that influence these outcomes at several levels (Fig).
Fig.
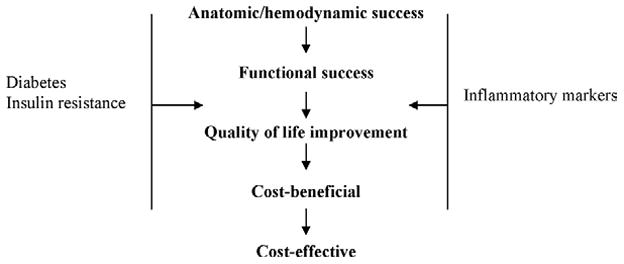
Levels of outcomes for percutaneous peripheral interventions.
Nationally, the treatment of IC with PPI may be increasing because of several factors, including steady development of new technology, practice of PPI among different competing medicine specialty groups, and a perceived better risk-benefit profile. The impact of widespread PPI use on patient outcomes and the health care system is not well known, however. The subgroup of DM or IR patients with elevated inflammatory markers may also have different outcomes than the general population. Therefore, current national practice patterns for IC should be examined with attention to critical risk factors and potential diminished outcomes.
ONGOING RESEARCH
To address some of the issues of the effect of DM and inflammation on outcomes of PPI, we have recently begun a National Institutes of Health–funded study of such patients at our institution. We hypothesize that DM and IR are cofactors that confer greater risk for PPI treatment failure for IC, as measured by hemodynamic, functional and QOL outcomes. To test this hypothesis, a prospective, longitudinal cohort study is being conducted to acquire anatomic, biomarker, medical, cost accounting, functional, and QOL data for patients undergoing PPI of the femoropopliteal segment for treatment of IC. Our specific aims are to analyze the association of DM, IR, and inflammatory markers with (1) “traditional” vascular anatomic outcomes (vessel patency, reintervention, limb loss), (2) functional walking performance (treadmill test), and (3) QOL (as measured by WIQ and EQ-5D). Complex multivariable modeling will be used to analyze relationships between risk factors and outcomes. Cost analysis will highlight critical cost-sensitive factors or events in the patient care process. Large national databases will be used to examine secular trends in the treatment of IC and extend the implications of the results seen in this study.
Although the individual outcome measures used in our study have been used elsewhere, we believe that the interrelationship between these different measures of treatment success has not been studied systematically, especially in the background of significant medical comorbidities such as DM and inflammation. Identifying correctable factors or understanding the influence of uncorrectable factors will allow us to improve treatment outcomes. At the same time, better understanding of how to measure those outcomes will allow us to improve their positive impact on the patient.
CONCLUSIONS
The growth of percutaneous vascular interventional techniques has resulted in many unanswered questions that require novel studies and analytic techniques. One group of questions deals with determining the outcomes of interventions and the influence of commonly prevalent risk factors. A second set of questions deals with measuring QOL and other socioeconomic outcomes that have not been well measured. For lifestyle-limiting conditions such as claudication, QOL and overall functional status are important outcomes not well captured by tradition measurements such as survival and limb salvage. Finally, with the rapid development and adoption of technology in all medical fields, the long-term impact to the health care system needs to be examined in light of decreasing national resources.
We believe that clinical trials should be complemented by more general inquiries into financial and policy implications. Likewise, policy research in isolation cannot entirely guide the care of patients because of the inherent individual complexity and variation of patients. Clinical physicians who are also trained in health services research can bridge the gap between innovative clinical practice and the effect of technology on the health care system. This “outcomes translational research,” analogous to translational research for the basic sciences, will extend the application of new technologies and techniques to better fit with the changing socioeconomic demands in health care.
Footnotes
Competition of interest: none.
References
- 1.Creager MA. Medical management of peripheral arterial disease. Cardiol Rev. 2001;9:238–45. doi: 10.1097/00045415-200107000-00010. [DOI] [PubMed] [Google Scholar]
- 2.Management of peripheral arterial disease (PAD) TransAtlantic Inter-Society Consensus (TASC) Eur J Vasc Endovasc Surg. 2000;19(suppl A):Si-xxviii, S1–250. [PubMed] [Google Scholar]
- 3.Amighi J, Sabeti S, Schlager O, Francesconi M, Ahmadi R, Minar E, et al. Outcome of conservative therapy of patients with severe intermittent claudication. Eur J Vasc Endovasc Surg. 2004;27:254–8. doi: 10.1016/j.ejvs.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 4.Beebe HG. Intermittent claudication: effective medical management of a common circulatory problem. Am J Cardiol. 2001;87:14D–18D. doi: 10.1016/s0002-9149(01)01672-1. [DOI] [PubMed] [Google Scholar]
- 5.Lindgarde F, Jelnes R, Bjorkman H, Adielsson G, Kjellstrom T, Palmquist I, et al. Conservative drug treatment in patients with moderately severe chronic occlusive peripheral arterial disease. Scandinavian Study Group. Circulation. 1989;80:1549–56. doi: 10.1161/01.cir.80.6.1549. [DOI] [PubMed] [Google Scholar]
- 6.Money SR, Herd JA, Isaacsohn JL, Davidson M, Cutler B, Heckman J, et al. Effect of cilostazol on walking distances in patients with intermittent claudication caused by peripheral vascular disease. J Vasc Surg. 1998;27:267–74. doi: 10.1016/s0741-5214(98)70357-x. discussion 274–5. [DOI] [PubMed] [Google Scholar]
- 7.Comerota AJ. Endovascular and surgical revascularization for patients with intermittent claudication. Am J Cardiol. 2001;87:34D–43D. doi: 10.1016/s0002-9149(01)01674-5. [DOI] [PubMed] [Google Scholar]
- 8.Smith FB, Lee AJ, Price JF, van Wijk MC, Fowkes FG. Changes in ankle brachial index in symptomatic and asymptomatic subjects in the general population. J Vasc Surg. 2003;38:1323–30. doi: 10.1016/s0741-5214(03)01021-8. [DOI] [PubMed] [Google Scholar]
- 9.Dormandy J, Heeck L, Vig S. The natural history of claudication: risk to life and limb. Semin Vasc Surg. 1999;12:123–37. [PubMed] [Google Scholar]
- 10.Gardner AW, Montgomery PS, Killewich LA. Natural history of physical function in older men with intermittent claudication. J Vasc Surg. 2004;40:73–8. doi: 10.1016/j.jvs.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 11.Brevetti G, Chiariello M. Peripheral arterial disease: the magnitude of the problem and its socioeconomic impact. Curr Drug Targets Cardiovasc Haematol Disord. 2004;4:199–208. doi: 10.2174/1568006043336140. [DOI] [PubMed] [Google Scholar]
- 12.Ambrosetti M, Salerno M, Boni S, Daniele G, Tramarin R, Pedretti RF. Economic evaluation of a short-course intensive rehabilitation program in patients with intermittent claudication. Int Angiol. 2004;23:108–13. [PubMed] [Google Scholar]
- 13.Gardner AW, Katzel LI, Sorkin JD, Bradham DD, Hochberg MC, Flinn WR, et al. Exercise rehabilitation improves functional outcomes and peripheral circulation in patients with intermittent claudication: a randomized controlled trial. J Am Geriatr Soc. 2001;49:755–62. doi: 10.1046/j.1532-5415.2001.49152.x. [DOI] [PubMed] [Google Scholar]
- 14.Barker GA, Green S, Walker PJ. Effect of carbohydrate supplementation on walking performance in peripheral arterial disease: a preliminary physiologic study. J Vasc Surg. 2004;40:932–8. doi: 10.1016/j.jvs.2004.07.047. [DOI] [PubMed] [Google Scholar]
- 15.Bylund AC, Hammarsten J, Holm J, Schersten T. Enzyme activities in skeletal muscles from patients with peripheral arterial insufficiency. Eur J Clin Invest. 1976;6:425–9. doi: 10.1111/j.1365-2362.1976.tb00538.x. [DOI] [PubMed] [Google Scholar]
- 16.de Vries SO, Donaldson MC, Hunink MG. Contralateral symptoms after unilateral intervention for peripheral occlusive disease. J Vasc Surg. 1998;27:414–21. doi: 10.1016/s0741-5214(98)70315-5. [DOI] [PubMed] [Google Scholar]
- 17.Muluk SC, Muluk VS, Kelley ME, Whittle JC, Tierney JA, Webster MW, et al. Outcome events in patients with claudication: a 15-year study in 2777 patients. J Vasc Surg. 2001;33:251–7. doi: 10.1067/mva.2001.112210. discussion 257–8. [DOI] [PubMed] [Google Scholar]
- 18.Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414:782–7. doi: 10.1038/414782a. [DOI] [PubMed] [Google Scholar]
- 19.Gregg EW, Beckles GL, Williamson DF, Leveille SG, Langlois JA, Engelgau MM, et al. Diabetes and physical disability among older U.S. adults. Diabetes Care. 2000;23:1272–7. doi: 10.2337/diacare.23.9.1272. [DOI] [PubMed] [Google Scholar]
- 20.Al-Delaimy WK, Merchant AT, Rimm EB, Willett WC, Stampfer MJ, Hu FB. Effect of type 2 diabetes and its duration on the risk of peripheral arterial disease among men. Am J Med. 2004;116:236–40. doi: 10.1016/j.amjmed.2003.09.038. [DOI] [PubMed] [Google Scholar]
- 21.Dolan NC, Liu K, Criqui MH, Greenland P, Guralnik JM, Chan C, et al. Peripheral artery disease, diabetes, and reduced lower extremity functioning. Diabetes Care. 2002;25:113–20. doi: 10.2337/diacare.25.1.113. [DOI] [PubMed] [Google Scholar]
- 22.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287:356–9. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 23.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 24.Park L, Raman KG, Lee KJ, Lu Y, Ferran LJ, Jr, Chow WS, et al. Suppression of accelerated diabetic atherosclerosis by the soluble receptor for advanced glycation endproducts. Nat Med. 1998;4:1025–31. doi: 10.1038/2012. [DOI] [PubMed] [Google Scholar]
- 25.Falcone C, Emanuele E, D’Angelo A, Buzzi MP, Belvito C, Cuccia M, et al. Plasma levels of soluble receptor for advanced glycation end products and coronary artery disease in nondiabetic men. Arterioscler Thromb Vasc Biol. 2005;25:1032–7. doi: 10.1161/01.ATV.0000160342.20342.00. [DOI] [PubMed] [Google Scholar]
- 26.Aquino R, Johnnides C, Makaroun M, Whittle JC, Muluk VS, Kelley ME, et al. Natural history of claudication: long-term serial follow-up study of 1244 claudicants. J Vasc Surg. 2001;34:962–70. doi: 10.1067/mva.2001.119749. [DOI] [PubMed] [Google Scholar]
- 27.Jonason T, Ringqvist I. Diabetes mellitus and intermittent claudication. Relation between peripheral vascular complications and location of the occlusive atherosclerosis in the legs. Acta Med Scand. 1985;218:217–21. doi: 10.1111/j.0954-6820.1985.tb08850.x. [DOI] [PubMed] [Google Scholar]
- 28.McDaniel MD, Cronenwett JL. Basic data related to the natural history of intermittent claudication. Ann Vasc Surg. 1989;3:273–7. doi: 10.1016/S0890-5096(07)60040-5. [DOI] [PubMed] [Google Scholar]
- 29.Lange S, Diehm C, Darius H, Haberl R, Allenberg JR, Pittrow D, et al. High prevalence of peripheral arterial disease and low treatment rates in elderly primary care patients with diabetes. Exp Clin Endocrinol Diabetes. 2004;112:566–73. doi: 10.1055/s-2004-830408. [DOI] [PubMed] [Google Scholar]
- 30.Matzke S, Lepantalo M. Claudication does not always precede critical leg ischemia. Vasc Med. 2001;6:77–80. [PubMed] [Google Scholar]
- 31.Isomaa B, Almgren P, Tuomi T, Forsen B, Lahti K, Nissen M, et al. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care. 2001;24:683–9. doi: 10.2337/diacare.24.4.683. [DOI] [PubMed] [Google Scholar]
- 32.Yip J, Facchini FS, Reaven GM. Resistance to insulin-mediated glucose disposal as a predictor of cardiovascular disease. J Clin Endocrinol Metab. 1998;83:2773–6. doi: 10.1210/jcem.83.8.5005. [DOI] [PubMed] [Google Scholar]
- 33.Ridker PM, Rifai N, Stampfer MJ, Hennekens CH. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation. 2000;101:1767–72. doi: 10.1161/01.cir.101.15.1767. [DOI] [PubMed] [Google Scholar]
- 34.Ridker PM, Rifai N, Pfeffer M, Sacks F, Lepage S, Braunwald E. Elevation of tumor necrosis factor-alpha and increased risk of recurrent coronary events after myocardial infarction. Circulation. 2000;101:2149–53. doi: 10.1161/01.cir.101.18.2149. [DOI] [PubMed] [Google Scholar]
- 35.Ridker PM, Buring JE, Rifai N. Soluble P-selectin and the risk of future cardiovascular events. Circulation. 2001;103:491–5. doi: 10.1161/01.cir.103.4.491. [DOI] [PubMed] [Google Scholar]
- 36.Hwang SJ, Ballantyne CM, Sharrett AR, Smith LC, Davis CE, Gotto AM, Jr, et al. Circulating adhesion molecules VCAM-1, ICAM-1, and E-selectin in carotid atherosclerosis and incident coronary heart disease cases: the Atherosclerosis Risk In Communities (ARIC) study. Circulation. 1997;96:4219–25. doi: 10.1161/01.cir.96.12.4219. [DOI] [PubMed] [Google Scholar]
- 37.Vishnevetsky D, Kiyanista VA, Gandhi PJ. CD40 ligand: a novel target in the fight against cardiovascular disease. Ann Pharmacother. 2004;38:1500–8. doi: 10.1345/aph.1D611. [DOI] [PubMed] [Google Scholar]
- 38.Lim HS, Blann AD, Lip GY. Soluble CD40 ligand, soluble P-selectin, interleukin-6, and tissue factor in diabetes mellitus: relationships to cardiovascular disease and risk factor intervention. Circulation. 2004;109:2524–8. doi: 10.1161/01.CIR.0000129773.70647.94. [DOI] [PubMed] [Google Scholar]
- 39.Jinchuan Y, Zonggui W, Jinming C, Li L, Xiantao K. Upregulation of CD40–CD40 ligand system in patients with diabetes mellitus. Clin Chim Acta. 2004;339:85–90. doi: 10.1016/j.cccn.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 40.Cipollone F, Chiarelli F, Davi G, Ferri C, Desideri G, Fazia M, et al. Enhanced soluble CD40 ligand contributes to endothelial cell dysfunction in vitro and monocyte activation in patients with diabetes mellitus: effect of improved metabolic control. Diabetologia. 2005 doi: 10.1007/s00125-005-1750-2. [DOI] [PubMed] [Google Scholar]
- 41.Zwaka TP, Hombach V, Torzewski J. C-reactive protein-mediated low density lipoprotein uptake by macrophages: implications for atherosclerosis. Circulation. 2001;103:1194–7. doi: 10.1161/01.cir.103.9.1194. [DOI] [PubMed] [Google Scholar]
- 42.Schillinger M, Exner M, Mlekusch W, Sabeti S, Amighi J, Nikowitsch R, et al. Inflammation and Carotid Artery--Risk for Atherosclerosis Study (ICARAS) Circulation. 2005;111:2203–9. doi: 10.1161/01.CIR.0000163569.97918.C0. [DOI] [PubMed] [Google Scholar]
- 43.van der Meer IM, de Maat MP, Bots ML, Breteler MM, Meijer J, Kiliaan AJ, et al. Inflammatory mediators and cell adhesion molecules as indicators of severity of atherosclerosis: the Rotterdam Study. Arterioscler Thromb Vasc Biol. 2002;22:838–42. doi: 10.1161/01.atv.0000016249.96529.b8. [DOI] [PubMed] [Google Scholar]
- 44.Cassar K, Bachoo P, Ford I, Greaves M, Brittenden J. Markers of coagulation activation, endothelial stimulation and inflammation in patients with peripheral arterial disease. Eur J Vasc Endovasc Surg. 2005;29:171–6. doi: 10.1016/j.ejvs.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 45.McDermott MM, Guralnik JM, Greenland P, Green D, Liu K, Ridker PM, et al. Inflammatory and thrombotic blood markers and walking-related disability in men and women with and without peripheral arterial disease. J Am Geriatr Soc. 2004;52:1888–94. doi: 10.1111/j.1532-5415.2004.52514.x. [DOI] [PubMed] [Google Scholar]
- 46.Engstrom G, Site-Flondell D, Lindblad B, Janzon L, Lindgarde F. Risk of treatment of peripheral arterial disease is related to inflammation-sensitive plasma proteins: a prospective cohort study. J Vasc Surg. 2004;40:1101–5. doi: 10.1016/j.jvs.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 47.Brevetti G, Silvestro A, Di Giacomo S, Bucur R, Di Donato A, Schiano V, et al. Endothelial dysfunction in peripheral arterial disease is related to increase in plasma markers of inflammation and severity of peripheral circulatory impairment but not to classic risk factors and athero-sclerotic burden. J Vasc Surg. 2003;38:374–9. doi: 10.1016/s0741-5214(03)00124-1. [DOI] [PubMed] [Google Scholar]
- 48.Verma S, Kuliszewski MA, Li SH, Szmitko PE, Zucco L, Wang CH, et al. C-reactive protein attenuates endothelial progenitor cell survival, differentiation, and function: further evidence of a mechanistic link between C-reactive protein and cardiovascular disease. Circulation. 2004;109:2058–67. doi: 10.1161/01.CIR.0000127577.63323.24. [DOI] [PubMed] [Google Scholar]
- 49.Fernandez-Real JM, Vayreda M, Richart C, Gutierrez C, Broch M, Vendrell J, et al. Circulating interleukin 6 levels, blood pressure, and insulin sensitivity in apparently healthy men and women. J Clin Endocrinol Metab. 2001;86:1154–9. doi: 10.1210/jcem.86.3.7305. [DOI] [PubMed] [Google Scholar]
- 50.Mohamed-Ali V, Goodrick S, Rawesh A, Katz DR, Miles JM, Yudkin JS, et al. Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-alpha, in vivo. J Clin Endocrinol Metab. 1997;82:4196–200. doi: 10.1210/jcem.82.12.4450. [DOI] [PubMed] [Google Scholar]
- 51.Ikeda U, Ohkawa F, Seino Y, Yamamoto K, Hidaka Y, Kasahara T, et al. Serum interleukin 6 levels become elevated in acute myocardial infarction. J Mol Cell Cardiol. 1992;24:579–84. doi: 10.1016/0022-2828(92)91042-4. [DOI] [PubMed] [Google Scholar]
- 52.Harris TB, Ferrucci L, Tracy RP, Corti MC, Wacholder S, Ettinger WH, Jr, et al. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am J Med. 1999;106:506–12. doi: 10.1016/s0002-9343(99)00066-2. [DOI] [PubMed] [Google Scholar]
- 53.Speidl WS, Zeiner A, Nikfardjam M, Geppert A, Jordanova N, Niessner A, et al. An increase of C-reactive protein is associated with enhanced activation of endogenous fibrinolysis at baseline but an impaired endothelial fibrinolytic response after venous occlusion. J Am Coll Cardiol. 2005;45:30–4. doi: 10.1016/j.jacc.2004.09.052. [DOI] [PubMed] [Google Scholar]
- 54.Wilhelmsen L, Svardsudd K, Korsan-Bengtsen K, Larsson B, Welin L, Tibblin G. Fibrinogen as a risk factor for stroke and myocardial infarction. N Engl J Med. 1984;311:501–5. doi: 10.1056/NEJM198408233110804. [DOI] [PubMed] [Google Scholar]
- 55.Juhan-Vague I, Pyke SD, Alessi MC, Jespersen J, Haverkate F, Thompson SG. Fibrinolytic factors and the risk of myocardial infarction or sudden death in patients with angina pectoris. ECAT Study Group. European Concerted Action on Thrombosis and Disabilities. Circulation. 1996;94:2057–63. doi: 10.1161/01.cir.94.9.2057. [DOI] [PubMed] [Google Scholar]
- 56.Lindgren A, Lindoff C, Norrving B, Astedt B, Johansson BB. Tissue plasminogen activator and plasminogen activator inhibitor-1 in stroke patients. Stroke. 1996;27:1066–71. doi: 10.1161/01.str.27.6.1066. [DOI] [PubMed] [Google Scholar]
- 57.Koksch M, Zeiger F, Wittig K, Siegemund A, Reininger CB, Pfeiffer D, et al. Coagulation, fibrinolysis and platelet P-selectin expression in peripheral vascular disease. Eur J Vasc Endovasc Surg. 2001;21:147–54. doi: 10.1053/ejvs.2000.1294. [DOI] [PubMed] [Google Scholar]
- 58.Killewich LA, Gardner AW, Macko RF, Hanna DJ, Goldberg AP, Cox DK, et al. Progressive intermittent claudication is associated with impaired fibrinolysis. J Vasc Surg. 1998;27:645–50. doi: 10.1016/s0741-5214(98)70229-0. [DOI] [PubMed] [Google Scholar]
- 59.Killewich LA, Macko RF, Montgomery PS, Wiley LA, Gardner AW. Exercise training enhances endogenous fibrinolysis in peripheral arterial disease. J Vasc Surg. 2004;40:741–5. doi: 10.1016/j.jvs.2004.07.030. [DOI] [PubMed] [Google Scholar]
- 60.Di Benedetto A, Russo GT, Corrado F, Di Cesare E, Alessi E, Nicocia G, et al. Inflammatory markers in women with a recent history of gestational diabetes mellitus. J Endocrinol Invest. 2005;28:34–8. doi: 10.1007/BF03345527. [DOI] [PubMed] [Google Scholar]
- 61.Fernandez-Real JM, Ricart W. Insulin resistance and chronic cardiovascular inflammatory syndrome. Endocr Rev. 2003;24:278–301. doi: 10.1210/er.2002-0010. [DOI] [PubMed] [Google Scholar]
- 62.Dandona P, Aljada A, Chaudhuri A, Mohanty P, Garg R. Metabolic syndrome: a comprehensive perspective based on interactions between obesity, diabetes, and inflammation. Circulation. 2005;111:1448–54. doi: 10.1161/01.CIR.0000158483.13093.9D. [DOI] [PubMed] [Google Scholar]
- 63.Mohanty P, Aljada A, Ghanim H, Hofmeyer D, Tripathy D, Syed T, et al. Evidence for a potent antiinflammatory effect of rosiglitazone. J Clin Endocrinol Metab. 2004;89:2728–35. doi: 10.1210/jc.2003-032103. [DOI] [PubMed] [Google Scholar]
- 64.Schillinger M, Exner M, Amighi J, Mlekusch W, Sabeti S, Rumpold H, et al. Joint effects of C-reactive protein and glycated hemoglobin in predicting future cardiovascular events of patients with advanced atherosclerosis. Circulation. 2003;108:2323–8. doi: 10.1161/01.CIR.0000095267.24234.00. [DOI] [PubMed] [Google Scholar]
- 65.Cheng SW, Ting AC, Wong J. Endovascular stenting of superficial femoral artery stenosis and occlusions: results and risk factor analysis. Cardiovasc Surg. 2001;9:133–40. doi: 10.1016/s0967-2109(00)00109-5. [DOI] [PubMed] [Google Scholar]
- 66.Gordon IL, Conroy RM, Arefi M, Tobis JM, Stemmer EA, Wilson SE. Three-year outcome of endovascular treatment of superficial femoral artery occlusion. Arch Surg. 2001;136:221–8. doi: 10.1001/archsurg.136.2.221. [DOI] [PubMed] [Google Scholar]
- 67.Pozzi Mucelli F, Fisicaro M, Calderan L, Malacrea M, Mazzone C, Cattin L, et al. Percutaneous revascularization of femoropopliteal artery disease: PTA and PTA plus stent. Results after six years’ follow-up. Radiol Med (Torino) 2003;105:339–49. [PubMed] [Google Scholar]
- 68.Duda SH, Bosiers M, Lammer J, Scheinert D, Zeller T, Tielbeek A, et al. Sirolimus-Eluting versus Bare Nitinol Stent for Obstructive Superficial Femoral Artery Disease: The SIROCCO II Trial. J Vasc Interv Radiol. 2005;16:331–8. doi: 10.1097/01.RVI.0000151260.74519.CA. [DOI] [PubMed] [Google Scholar]
- 69.Surowiec SM, Davies MG, Eberly SW, Rhodes JM, Illig KA, Shortell CK, et al. Percutaneous angioplasty and stenting of the superficial femoral artery. J Vasc Surg. 2005;41:269–78. doi: 10.1016/j.jvs.2004.11.031. [DOI] [PubMed] [Google Scholar]
- 70.Cheng SW, Ting AC, Ho P. Angioplasty and primary stenting of high-grade, long-segment superficial femoral artery disease: is it worthwhile? Ann Vasc Surg. 2003;17:430–7. doi: 10.1007/s10016-003-0028-8. [DOI] [PubMed] [Google Scholar]
- 71.Jamsen TS, Manninen HI, Tulla HE, Jaakkola PA, Matsi PJ. Infrainguinal revascularization because of claudication: total long-term outcome of endovascular and surgical treatment. J Vasc Surg. 2003;37:808–15. doi: 10.1067/mva.2003.148. [DOI] [PubMed] [Google Scholar]
- 72.Lasic Z, Nikolsky E, Kesanakurthy S, Dangas G. Vascular closure devices: a review of their use after invasive procedures. Am J Cardiovasc Drugs. 2005;5:185–200. doi: 10.2165/00129784-200505030-00005. [DOI] [PubMed] [Google Scholar]
- 73.Liistro F, Falsini G, Bolognese L. The clinical burden of contrast media-induced nephropathy. Ital Heart J. 2003;4:668–76. [PubMed] [Google Scholar]
- 74.Costanza MJ, Queral LA, Lilly MP, Finn WR. Hemodynamic outcome of endovascular therapy for TransAtlantic InterSociety Consensus type B femoropopliteal arterial occlusive lesions. J Vasc Surg. 2004;39:343–50. doi: 10.1016/j.jvs.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 75.Sabeti S, Mlekusch W, Amighi J, Minar E, Schillinger M. Primary patency of long-segment self-expanding nitinol stents in the femoropopliteal arteries. J Endovasc Ther. 2005;12:6–12. doi: 10.1583/04-1359.1. [DOI] [PubMed] [Google Scholar]
- 76.Maca T, Schillinger M, Hamwi A, Mlekusch W, Sabeti S, Wagner O, et al. Insulin, C-peptide, and restenosis after femoral artery balloon angioplasty in type II diabetic and nondiabetic patients. J Vasc Interv Radiol. 2005;16:31–5. doi: 10.1097/01.RVI.0000136030.26074.33. [DOI] [PubMed] [Google Scholar]
- 77.Mehran R, Dangas GD, Kobayashi Y, Lansky AJ, Mintz GS, Aymong ED, et al. Short- and long-term results after multivessel stenting in diabetic patients. J Am Coll Cardiol. 2004;43:1348–54. doi: 10.1016/j.jacc.2003.04.004. [DOI] [PubMed] [Google Scholar]
- 78.Gilbert J, Raboud J, Zinman B. Meta-analysis of the effect of diabetes on restenosis rates among patients receiving coronary angioplasty stenting. Diabetes Care. 2004;27:990–4. doi: 10.2337/diacare.27.4.990. [DOI] [PubMed] [Google Scholar]
- 79.Choi D, Kim SK, Choi SH, Ko YG, Ahn CW, Jang Y, et al. Preventative effects of rosiglitazone on restenosis after coronary stent implantation in patients with type 2 diabetes. Diabetes Care. 2004;27:2654–60. doi: 10.2337/diacare.27.11.2654. [DOI] [PubMed] [Google Scholar]
- 80.Versaci F, Gaspardone A. Prevention of restenosis after stenting: the emerging role of inflammation. Coron Artery Dis. 2004;15:307–11. doi: 10.1097/00019501-200409000-00002. [DOI] [PubMed] [Google Scholar]
- 81.Li JJ, Fang CH, Jiang H, Huang CX, Hui RT, Chen MZ. Time course of inflammatory response after renal artery stenting in patients with atherosclerotic renal stenosis. Clin Chim Acta. 2004;350:115–21. doi: 10.1016/j.cccn.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 82.Aggarwal A, Schneider DJ, Sobel BE, Dauerman HL. Comparison of inflammatory markers in patients with diabetes mellitus versus those without before and after coronary arterial stenting. Am J Cardiol. 2003;92:924–9. doi: 10.1016/s0002-9149(03)00971-8. [DOI] [PubMed] [Google Scholar]
- 83.Danielsson P, Danielsson G, Truedsson L, Norgren L. White blood cell and endothelial cell response to endovascular procedures in the leg. Int Angiol. 2004;23:122–7. [PubMed] [Google Scholar]
- 84.Manfredini F, Conconi F, Malagoni AM, Manfredini R, Mascoli F, Liboni A, et al. Speed rather than distance: a novel graded treadmill test to assess claudication. Eur J Vasc Endovasc Surg. 2004;28:303–9. doi: 10.1016/j.ejvs.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 85.Gelin J, Jivegard L, Taft C, Karlsson J, Sullivan M, Dahllof AG, et al. Treatment efficacy of intermittent claudication by surgical intervention, supervised physical exercise training compared to no treatment in unselected randomised patients I: one year results of functional and physiological improvements. Eur J Vasc Endovasc Surg. 2001;22:107–13. doi: 10.1053/ejvs.2001.1413. [DOI] [PubMed] [Google Scholar]
- 86.Khaira HS, Hanger R, Shearman CP. Quality of life in patients with intermittent claudication. Eur J Vasc Endovasc Surg. 1996;11:65–9. doi: 10.1016/s1078-5884(96)80136-5. [DOI] [PubMed] [Google Scholar]
- 87.Hiatt WR, Hirsch AT, Regensteiner JG, Brass EP. Clinical trials for claudication. Assessment of exercise performance, functional status, and clinical end points. Vascular Clinical Trialists. Circulation. 1995;92:614–21. doi: 10.1161/01.cir.92.3.614. [DOI] [PubMed] [Google Scholar]
- 88.McDermott MM, Liu K, Guralnik JM, Martin GJ, Criqui MH, Greenland P. Measurement of walking endurance and walking velocity with questionnaire: validation of the walking impairment questionnaire in men and women with peripheral arterial disease. J Vasc Surg. 1998;28:1072–81. doi: 10.1016/s0741-5214(98)70034-5. [DOI] [PubMed] [Google Scholar]
- 89.de Vries SO, Kuipers WD, Hunink MG. Intermittent claudication: symptom severity versus health values. J Vasc Surg. 1998;27:422–30. doi: 10.1016/s0741-5214(98)70316-7. [DOI] [PubMed] [Google Scholar]
- 90.Johnson JA, Luo N, Shaw JW, Kind P, Coons SJ. Valuations of EQ-5D health states: are the United States and United Kingdom different? Med Care. 2005;43:221–8. doi: 10.1097/00005650-200503000-00004. [DOI] [PubMed] [Google Scholar]
- 91.Kulkarni AS, Balkrishnan R, Richmond D, Pearce DJ, Feldman SR. Medication-related factors affecting health care outcomes and costs for patients with psoriasis in the United States. J Am Acad Dermatol. 2005;52:27–31. doi: 10.1016/j.jaad.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 92.Wolfe F, Michaud K. Severe rheumatoid arthritis (RA), worse outcomes, comorbid illness, and sociodemographic disadvantage characterize RA patients with fibromyalgia. J Rheumatol. 2004;31:695–700. [PubMed] [Google Scholar]
- 93.Bosch JL, Hunink MG. Comparison of the Health Utilities Index Mark 3 (HUI3) and the EuroQol EQ-5D in patients treated for intermittent claudication. Qual Life Res. 2000;9:591–601. doi: 10.1023/a:1008929129537. [DOI] [PubMed] [Google Scholar]
- 94.Bosch JL, van der Graaf Y, Hunink MG. Health-related quality of life after angioplasty and stent placement in patients with iliac artery occlusive disease: results of a randomized controlled clinical trial. The Dutch Iliac Stent Trial Study Group. Circulation. 1999;99:3155–60. doi: 10.1161/01.cir.99.24.3155. [DOI] [PubMed] [Google Scholar]
- 95.Menard JR, Smith HE, Riebe D, Braun CM, Blissmer B, Patterson RB. Long-term results of peripheral arterial disease rehabilitation. J Vasc Surg. 2004;39:1186–92. doi: 10.1016/j.jvs.2004.01.034. [DOI] [PubMed] [Google Scholar]
- 96.Nehler MR, McDermott MM, Treat-Jacobson D, Chetter I, Regensteiner JG. Functional outcomes and quality of life in peripheral arterial disease: current status. Vasc Med. 2003;8:115–26. doi: 10.1191/1358863x03vm483ra. [DOI] [PubMed] [Google Scholar]
- 97.Long J, Modrall JG, Parker BJ, Swann A, Welborn MB, 3rd, Anthony T. Correlation between ankle-brachial index, symptoms, and health-related quality of life in patients with peripheral vascular disease. J Vasc Surg. 2004;39:723–7. doi: 10.1016/j.jvs.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 98.Currie IC, Wilson YG, Baird RN, Lamont PM. Treatment of intermittent claudication: the impact on quality of life. Eur J Vasc Endovasc Surg. 1995;10:356–61. doi: 10.1016/s1078-5884(05)80057-7. [DOI] [PubMed] [Google Scholar]
- 99.McDermott MM, Liu K, Greenland P, Guralnik JM, Criqui MH, Chan C, et al. Functional decline in peripheral arterial disease: associations with the ankle brachial index and leg symptoms. JAMA. 2004;292:453–61. doi: 10.1001/jama.292.4.453. [DOI] [PubMed] [Google Scholar]
- 100.Pell JP. Impact of intermittent claudication on quality of life. The Scottish Vascular Audit Group. Eur J Vasc Endovasc Surg. 1995;9:469–72. doi: 10.1016/s1078-5884(05)80018-8. [DOI] [PubMed] [Google Scholar]
- 101.de Vries SO, Visser K, de Vries JA, Wong JB, Donaldson MC, Hunink MG. Intermittent claudication: cost-effectiveness of revascularization versus exercise therapy. Radiology. 2002;222:25–36. doi: 10.1148/radiol.2221001743. [DOI] [PubMed] [Google Scholar]
- 102.Treesak C, Kasemsup V, Treat-Jacobson D, Nyman JA, Hirsch AT. Cost-effectiveness of exercise training to improve claudication symptoms in patients with peripheral arterial disease. Vasc Med. 2004;9:279–85. doi: 10.1191/1358863x04vm570oa. [DOI] [PubMed] [Google Scholar]
- 103.Bosch JL, Haaring C, Meyerovitz MF, Cullen KA, Hunink MG. Cost-effectiveness of percutaneous treatment of iliac artery occlusive disease in the United States. AJR Am J Roentgenol. 2000;175:517–21. doi: 10.2214/ajr.175.2.1750517. [DOI] [PubMed] [Google Scholar]
- 104.Bosch JL, Tetteroo E, Mali WP, Hunink MG. Iliac arterial occlusive disease: cost-effectiveness analysis of stent placement versus percutaneous transluminal angioplasty. Dutch Iliac Stent Trial Study Group. Radiology. 1998;208:641–8. doi: 10.1148/radiology.208.3.9722840. [DOI] [PubMed] [Google Scholar]
- 105.Greenberg D, Rosenfield K, Garcia LA, Berezin RH, Lavelle T, Fogleman S, et al. In-hospital costs of self-expanding nitinol stent implantation versus balloon angioplasty in the femoropopliteal artery (the VascuCoil Trial) J Vasc Interv Radiol. 2004;15:1065–9. doi: 10.1097/01.RVI.0000136293.18041.88. [DOI] [PubMed] [Google Scholar]
- 106.Visser K, de Vries SO, Kitslaar PJ, van Engelshoven JM, Hunink MG. Cost-effectiveness of diagnostic imaging work-up and treatment for patients with intermittent claudication in The Netherlands. Eur J Vasc Endovasc Surg. 2003;25:213–23. doi: 10.1053/ejvs.2002.1838. [DOI] [PubMed] [Google Scholar]
- 107.Hunink MG, Wong JB, Donaldson MC, Meyerovitz MF, de Vries J, Harrington DP. Revascularization for femoropopliteal disease. A decision and cost-effectiveness analysis. JAMA. 1995;274:165–71. [PubMed] [Google Scholar]
- 108.Jansen RM, de Vries SO, Cullen KA, Donaldson MC, Hunink MG. Cost-identification analysis of revascularization procedures on patients with peripheral arterial occlusive disease. J Vasc Surg. 1998;28:617–23. doi: 10.1016/s0741-5214(98)70085-0. [DOI] [PubMed] [Google Scholar]
- 109.Silber JH, Rosenbaum PR, Trudeau ME, Chen W, Zhang X, Kelz RR, et al. Changes in prognosis after the first postoperative complication. Med Care. 2005;43:122–31. doi: 10.1097/00005650-200502000-00005. [DOI] [PubMed] [Google Scholar]


