Summary
Foxo1, a member of the Fox0 subfamily of winged-helix forkhead transcription factors, is a target of insulin and insulin-like growth factor–1 (IGF-1) signal transduction pathways that activate protein kinase B (PKB) in pancreatic β cells. Foxo1 is a substrate for PKB, and its phosphorylation results in nuclear exclusion with concomitant alterations in gene expression that are important to cellular growth and differentiation. Because activation of PKB can require insulin receptor substrate proteins (IRS-1 and IRS-2) and phosphatidylinositol 3-kinase (PI3K), it is of interest to determine whether the activity of Foxo1 is also regulated by heterotrimeric G protein–coupled receptors (GPCRs) with IRS-1 or -2, PI3K, or PKB signaling potential. Indeed, studies of β cells have demonstrated that activation of a GPCR for the blood glucose–lowering hormone GLP-1 leads to major alterations of IRS-2, PI3K, and PKB activity. By promoting nuclear exclusion of Foxo1 in a PKB-mediated manner, GLP-1 may up-regulate the expression of a homeodomain transcription factor (PDX-1) that serves as a master regulator of β-cell growth and differentiation. This STKE Perspective summarizes signaling properties of GLP-1 that may explain its ability to increase β-cell mass, to increase pancreatic insulin secretory capacity, and to lower levels of blood glucose in type 2 diabetic subjects.
Introduction
Glucagon-like peptide-1-(7–36)-amide (GLP-1), a hormone secreted by endocrine L cells of the intestinal tract, has unique insulinotropic and growth factor–like signal transduction properties that indicate its usefulness as a new therapeutic agent for treatment of type 2 diabetes mellitus (adult-onset diabetes) (1). When administered to type 2 diabetic subjects, GLP-1 normalizes fasting levels of blood glucose while minimizing the increase of blood glucose concentration that occurs after ingestion of a meal. These beneficial actions of GLP-1 are rapid in onset and are attributable to its ability to potentiate glucose-dependent insulin secretion from pancreatic β cells located within the islets of Langerhans.
GLP-1 also exerts long-term effects on β-cell function (2). In vitro studies of insulin-secreting cell lines or isolated islets demonstrate that GLP-1 stimulates insulin gene transcription and proinsulin biosynthesis. Moreover, in vivo studies of rats demonstrate that GLP-1 increases β-cell mass—equivalent to the total number of pancreatic β cells per unit volume of pancreatic tissue multiplied by the pancreatic weight. GLP-1 accelerates the conversion of pancreatic ductal stem cells to new β cells, as well as stimulating the mitosis of existing β cells. These neogenic and proliferative actions of GLP-1 are complemented by its ability to protect against apoptotic β-cell death (3). Although it has yet to be demonstrated, such growth factor–like actions of GLP-1 may enable increased pancreatic insulin secretory capacity, thereby overcoming the peripheral insulin resistance and diminished glucose-dependent insulin secretion characteristic of type 2 diabetes mellitus.
Signal Transduction Properties of the GLP-1 Receptor in β Cells
The GLP-1 receptor (GLP-1-R) is a 62-kD class B heptahelical heterotrimeric GTP-binding protein (G protein)–coupled receptor (GPCR), the activation of which stimulates both β-cell cyclic adenosine monophosphate (cAMP) production and an increase in intracellular Ca2+ concentration ([Ca2+]i) (4). Downstream effectors of cAMP include protein kinase A (PKA) and the Epac family of cAMP-regulated guanine nucleotide exchange factors (cAMP-GEFs) (5). By activating PKA and Epac, GLP-1 sensitizes β cells to the stimulatory effects of blood glucose and so increases the efficacy (maximal effect) and potency (threshold concentration) of glucose as a stimulus for insulin secretion (6). This action of GLP-1 underlies its ability to amplify pulsatile insulin secretion in healthy subjects and also explains its ability to restore glucose-dependent insulin secretion in type 2 diabetic subjects (1).
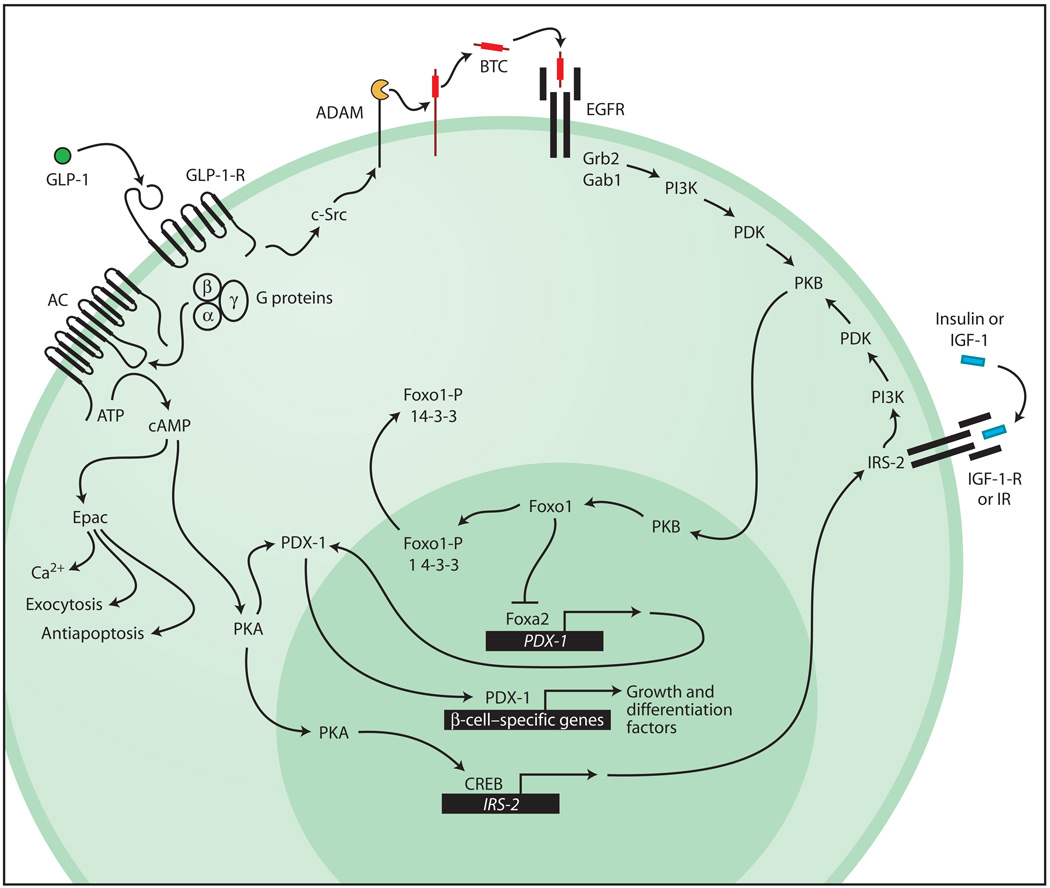
In addition to such classical GPCR signaling mechanisms, it is established that GLP-1 activates growth factor–like signaling pathways. Of particular importance to the discussion presented here is the ability of GLP-1 to activate protein kinase B (PKB, also known as Akt) (7–10). Because PKB is also activated upon exposure of β cells to insulin or insulin-like growth factor-1 (IGF-1) (11), it may serve as a locus for convergence of the GLP-1-R, insulin autoreceptor (IR), and IGF-1 receptor (IGF-1-R) signaling pathways (Fig. 1). Given that the IR and IGF-1-R signaling pathways are implicated in the maintenance of β-cell growth, differentiation, and survival (11), one would predict that GLP-1, an activator of PKB, should recapitulate the actions of insulin and IGF-1, but in a GPCR-mediated manner. Moreover, the activation of PKB by GLP-1 may explain its ability to up-regulate the expression of a key β-cell transcription factor (PDX-1) (12), and it is likely to be secondary to the ability of GLP-1 to stimulate phosphatidylinositol 3-kinase (PI3K) (13) and to up-regulate expression of the insulin receptor substrate-2 (IRS-2) (14) (Fig. 1).
Fig. 1.
GLP-1 acts by means of the GLP-1-R to stimulate adenylyl cyclase (AC), and thereby cAMP production, and to activate the cAMP-binding proteins PKA and Epac. PKA promotes translocation of PDX-1 to the nucleus, where it binds to regulatory elements within the enhancer or promoter sequences of genes important to β-cell growth and differentiation (13, 32). PKA also acts by means of CREB to up-regulate expression of IRS-2. Actions of Epac include a stimulation of intracellular Ca2+ signaling (5), secretory granule exocytosis (5), and the promotion of β-cell survival (33). Binding of GLP-1 to the GLP-1-R transactivates the EGFR in a manner mediated by c-Src and the ADAM (a disintegrin and metalloproteinase)–family metalloproteinase. Liberation of the soluble EGFR ligand betacellulin (BTC) stimulates EGFR autophosphorylation and promotes formation of an EGFR-Grb2-Gab1-PI3K complex with resultant activation of PKB. The IR and IGF-1-R signaling pathways acting by means of IRS-2 and PI3K converge with the GLP-1-R signaling pathway at PKB. Activated PKB translocates to the nucleus, where it acts at Foxo1 to disinhibit Foxa2-dependent PDX-1 gene promoter activity. Phosphorylated Foxo1 (Foxo1-P) associates with 14-3-3 proteins and is exported out of the nucleus to accumulate in the cytosol.
The stimulation of PI3K activity by GLP-1 is mediated by the GLP-1-R, but may require transactivation of the β-cell epidermal growth factor receptor (EGFR). It is proposed that occupancy of the GLP-1-R leads to c-Src–mediated activation of a membrane-bound metalloproteinase, with concomitant release of a soluble ligand (betacellulin) active at the EGFR (15). Recent studies provide a mechanistic explanation for how EGFR transactivation leads to activation of PKB. Studies of INS-1 insulin-secreting cells treated with GLP-1 demonstrate increased PI3K activity associated with Gab1 immunoreactivity in cell lysates (7). Gab1 is structurally related to IRS-1 and it interacts with Grb2, hence its designation as Grb2-associated binder-1. Because ligand binding to the EGFR promotes the formation of a complex consisting of the EGFR, Grb2, Gab1, and PI3K (16), transactivation of the EGFR by GLP-1 is expected to up-regulate PKB activity independently of IRS-1 and 2 (Fig. 1).
A more indirect mechanism by which PKB is activated by GLP-1 may also exist. The GLP-1-R agonist Exendin-4 increases levels of IRS-2 in MIN6 insulin-secreting cells (14). This action of Exendin-4 is mimicked by a cAMP-elevating agent (forskolin), is associated with phosphorylation of the cAMP response element–binding protein CREB, and is abrogated by overexpression of dominant-negative A-CREB. Evidently, activation of the GLP-1-R leads to CREB-mediated IRS-2 gene expression. By up-regulating the expression of IRS-2, GLP-1 may facilitate growth factor signaling pathways originating at the IR or IGF-1-R, which indirectly activate PKB (Fig. 1).
PKB May Mediate the Action of GLP-1 to Increase β-Cell Mass
The hyperglycemia that is characteristic of type 2 diabetes results from a combination of insulin resistance, insufficient pancreatic insulin secretion, and excess hepatic glucose production. To overcome insulin resistance, the pancreas exhibits compensatory islet hyperplasia with increased β-cell mass. GLP-1 may act by means of PKB to accelerate this process because the proliferative action of GLP-1 in a β-cell line (INS-1) is abrogated by overexpression of a dominant-negative kinase-dead PKB (10). In contrast, a constitutively active PKB mutant induces islet hyperplasia and increases β-cell mass in mice (17, 18). Manipulations that favor IR or IGF-1-R signaling by means of PKB are also growth-promoting: Selective regulated expression of IRS-2 stimulates an increase of β-cell mass and cures diabetes in mice lacking IRS-2 (IRS-2−/− mice) (19). Consistent with the role of PKB in promoting β-cell survival, GLP-1 protects against apoptosis (9, 10, 20), which provides an additional explanation for its ability to increase β-cell mass.
A Unifying Hypothesis to Explain How GLP-1 Stimulates β-Cell Growth
Available evidence indicates that the pancreatic and duodenal homeodomain transcription factor PDX-1 plays a pivotal role in the process by which GLP-1 exerts its stimulatory effects on β-cell growth and differentiation. Whereas PDX-1+/− haploinsufficiency in mice limits the compensatory islet hyperplasia that occurs in response to insulin resistance (21), activation of the GLP-1-R up-regulates expression of PDX-1 in rats and mice rendered disabetic by partial pancreatectomy, old age, or interbreeding (22–24). Given that PDX-1 plays an active role in developmental processes that lead to formation of new islets during fetal growth (25), such findings suggest that GLP-1 uses PDX-1 to activate coordinate gene expression that is latent in adult islets, but which is inducible and which may contribute to pancreatic regeneration in diabetic subjects.
How might GLP-1 activate this process? One clue is that PDX-1 expression is reduced in islets of IRS-2−/− mice (26). These mice develop diabetes but are rendered normoglycemic by transgenic expression of PDX-1 (26). Given that GLP-1 increases levels of PDX-1 mRNA in β-cell lines (12), it may use either IRS-2 or its downstream effectors (PI3K, PKB) to up-regulate PDX-1 expression. In fact, recent studies of Foxo1, a winged-helix forkhead transcription factor (27), provide the basis for a new hypothesis as to how this action of GLP-1 might be achieved. Because haploinsufficiency of Foxo1 reverses the loss of β-cell mass observed in IRS-2−/− mice (28), it appears that Foxo1 acts as a negative regulator of IR or IGF-1-R signaling pathways that support β-cell neogenesis and proliferation. In this scenario, Foxo1 acts as a repressor of PDX-1 gene expression by virtue of its ability to counteract stimulatory effects of Foxa2 (also known as HNF-3-β) at the PDX-1 promoter (Fig. 1). This scenario is consistent with the ability of Foxa2 to transactivate PDX-1 gene expression by means of its direct action at the PDX-1 gene promoter (29, 30). It is also consistent with the known ability of Foxo1 to bind the PDX-1 promoter and to inhibit the transactivation function of Foxa2 (28, 31). Given that PKB-mediated phosphorylation of Foxo1 promotes its nuclear exclusion (27), multiple growth factor signaling pathways may converge at the level of PKB activation to disinhibit PDX-1 gene expression. By adopting this signaling mechanism, GLP-1 may stimulate growth and differentiation of β cells.
Conclusion
Not surprisingly, the unique constellation of insulinotropic and growth factor–like signal transduction properties summarized above has prompted interest in the use of GLP-1 and its synthetic peptide or fusion protein analogs (Exenatide, NN2211, CJC-1131, Albugon) as novel blood glucose–lowering agents for treatment of type 2 diabetes mellitus (1). Similarly, attention has recently focused on the development of dipeptidyl peptidase IV (DPP-IV) inhibitors (LAF237, MK-0431, NN7201), which elevate circulating levels of endogenous GLP-1 by slowing its enzymatic degradation (1). Quite clearly, the future remains bright for GLP-1–based drug discovery efforts.
Footnotes
Note added in proof: Kodama et al. (34) have reported that the GLP-1 receptor agonist Exendin-4 promotes nuclear exclusion of Foxo1 and increases expression of PDX-1 in β cells of mouse islets.
References and Notes
- 1.Holz GG, Chepurny OG. Glucagon-like peptide-1 synthetic analogs: New therapeutic agents for use in the treatment of diabetes mellitus. Curr. Med. Chem. 2003;10:2471–2483. doi: 10.2174/0929867033456648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.List JF, Habener JF. Glucagon-like peptide 1 agonists and the development and growth of pancreatic β-cells. Am. J. Physiol. Endocrinol. Metab. 2004;286:E875–E881. doi: 10.1152/ajpendo.00007.2004. [DOI] [PubMed] [Google Scholar]
- 3.Drucker DJ. Glucagon-like peptide-1 and the islet β-cell: Augmentation of cell proliferation and inhibition of apoptosis. Endocrinology. 2003;144:5145–5148. doi: 10.1210/en.2003-1147. [DOI] [PubMed] [Google Scholar]
- 4.Thorens B. Expression cloning of the pancreatic β cell receptor for the gluco-incretin hormone glucagon-like peptide-1. Proc. Natl. Acad. Sci. U.S.A. 1992;89:8641–8645. doi: 10.1073/pnas.89.18.8641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holz GG. Epac: A new cAMP-binding protein in support of glucagon-like peptide-1 receptor-mediated signal transduction in the pancreatic β-cell. Diabetes. 2004;53:5–13. doi: 10.2337/diabetes.53.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holz GG, Kuhtreiber WM, Habener JF. Pancreatic beta-cells are rendered glucose-competent by the insulinotropic hormone glucagon-like peptide-1-(7–37) Nature. 1993;361:362–365. doi: 10.1038/361362a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trümper K, Trümper A, Trusheim H, Arnold R, Göke B, Hörsch D. Integrative mitogenic role of protein kinase B/Akt in β-cells. Ann. N.Y. Acad. Sci. 2000;921:242–250. doi: 10.1111/j.1749-6632.2000.tb06972.x. [DOI] [PubMed] [Google Scholar]
- 8.Buteau J, Foisy S, Rhodes CJ, Carpenter L, Biden TJ, Prentki M. Protein kinase C-ζ activation mediates glucagon-like peptide-1-induced pancreatic β-cell proliferation. Diabetes. 2001;50:2237–2243. doi: 10.2337/diabetes.50.10.2237. [DOI] [PubMed] [Google Scholar]
- 9.Buteau J, El-Assaad W, Rhodes CJ, Rosenberg L, Joly E, Prentki M. Glucagon-like peptide-1 prevents beta-cell glucolipotoxicity. Diabetologia. 2004;47:806–815. doi: 10.1007/s00125-004-1379-6. [DOI] [PubMed] [Google Scholar]
- 10.Wang Q, Li L, Xu E, Wong V, Rhodes C, Brubaker PL. Glucagon-like peptide-1 regulates proliferation and apoptosis via activation of protein kinase B in pancreatic INS-1 beta cells. Diabetologia. 2004;47:478–487. doi: 10.1007/s00125-004-1327-5. [DOI] [PubMed] [Google Scholar]
- 11.Dickson LM, Rhodes CJ. Pancreatic β-cell growth and survival in the onset of type 2 diabetes: A role for protein kinase B in the Akt? Am. J. Physiol. Endocrinol. Metab. 2004;287:E192–E198. doi: 10.1152/ajpendo.00031.2004. [DOI] [PubMed] [Google Scholar]
- 12.Wang X, Cahill CM, Pineyro MA, Zhou J, Doyle ME, Egan JM. Glucagon-like peptide-1 regulates the β cell transcription factor, PDX-1, in insulinoma cells. Endocrinology. 1999;140:4904–4907. doi: 10.1210/endo.140.10.7158. [DOI] [PubMed] [Google Scholar]
- 13.Buteau J, Roduit R, Susini S, Prentki M. Glucagon-like peptide-1 promotes DNA synthesis, activates phosphatidylinositol 3-kinase and increases transcription factor pancreatic and duodenal homeobox gene 1 (PDX-1) DNA binding activity in β (INS-1)-cells. Diabetologia. 1999;42:856–864. doi: 10.1007/s001250051238. [DOI] [PubMed] [Google Scholar]
- 14.Jhala US, Canettieri G, Screaton RA, Kulkarni RN, Krajewski S, Reed J, Walker J, Lin X, White M, Montminy M. cAMP promotes pancreatic β-cell survival via CREB-mediated induction of IRS2. Genes Dev. 2003;17:1575–1580. doi: 10.1101/gad.1097103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buteau J, Foisy S, Joly E, Prentki M. Glucagon-like peptide 1 induces pancreatic β-cell proliferation via transactivation of the epidermal growth factor receptor. Diabetes. 2003;52:124–132. doi: 10.2337/diabetes.52.1.124. [DOI] [PubMed] [Google Scholar]
- 16.Mattoon DR, Lamothe B, Lax I, Schlessinger J. The docking protein Gab 1 is the primary mediator of EGF-stimulated activation of the PI-3K/Akt cell survival pathway. BMC Biol. 2004;2:24. doi: 10.1186/1741-7007-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bernal-Mizrachi E, Wen W, Stahlhut S, Welling CM, Permutt MA. Islet β cell expression of constitutively active Akt1/PKBα induces stiking hypertrophy, hyperplasia, and hyperinsulinemia. J. Clin. Invest. 2001;108:1631–1638. doi: 10.1172/JCI13785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tuttle RL, Gill NS, Pugh W, Lee J-P, Koeberlein B, Furth EE, Polonsky KS, Naji A, Birnbaum MJ. Regulation of pancreatic β-cell growth and survival by the serine/threonine protein kinase Akt1/PKBα. Nat. Med. 2001;7:1133–1137. doi: 10.1038/nm1001-1133. [DOI] [PubMed] [Google Scholar]
- 19.Hennige AM, Burks DJ, Ozcan U, Kulkarni RN, Ye J, Park S, Schubert M, Fisher TL, Dow MA, Leshan R, Zakaria M, Mossa-Basha M, White MF. Upregulation of insulin receptor substrate-2 in pancreatic β cells prevents diabetes. J. Clin. Invest. 2003;112:1521–1532. doi: 10.1172/JCI18581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Y, Hansotia T, Yusta B, Ris F, Halban PA, Drucker DJ. Glucagon-like peptide-1 receptor signaling modulates β cell apoptosis. J. Biol. Chem. 2003;278:471–478. doi: 10.1074/jbc.M209423200. [DOI] [PubMed] [Google Scholar]
- 21.Kulkarni RN, Jhala US, Winnay JN, Krajewski S, Montminy M, Kahn CR. PDX-1 haploinsufficiency limits the compensatory islet hyperplasia that occurs in response to insulin resistance. J. Clin. Invest. 2004;114:828–836. doi: 10.1172/JCI21845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu G, Stoffers DA, Habener JF, Bonner-Weir S. Exendin-4 stimulates both beta-cell replication and neogenesis, resulting in increased beta-cell mass and improved glucose tolerance in diabetic rats. Diabetes. 1999;48:2270–2276. doi: 10.2337/diabetes.48.12.2270. [DOI] [PubMed] [Google Scholar]
- 23.Stoffers DA, Kieffer TJ, Hussain MA, Drucker DJ, Bonner-Weir S, Habener JF, Egan JM. Insulinotropic glucagon-like peptide 1 agonists stimulate expression of homeodomain protein IDX-1 and increase islet size in mouse pancreas. Diabetes. 2000;49:741–748. doi: 10.2337/diabetes.49.5.741. [DOI] [PubMed] [Google Scholar]
- 24.Perfetti R, Zhou J, Doyle ME, Egan JM. Glucagon-like peptide-1 induces cell proliferation and pancreatic-duodenum homeobox-1 expression and increases endocrine cell mass in the pancreas of old, glucose-intolerant rats. Endocrinology. 2000;141:4600–4605. doi: 10.1210/endo.141.12.7806. [DOI] [PubMed] [Google Scholar]
- 25.McKinnon CM, Docherty K. Pancreatic duodenal homeobox-1, PDX-1, a major regulator of β cell identity and function. Diabetologia. 2001;44:1203–1214. doi: 10.1007/s001250100628. [DOI] [PubMed] [Google Scholar]
- 26.Kushner JA, Ye J, Schubert M, Burks DJ, Dow MA, Flint CL, Dutta S, Wright CVE, Montminy MR, White MF. Pdx1 restores β cell function in Irs2 knockout mice. J. Clin. Invest. 2002;109:1193–1201. doi: 10.1172/JCI14439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arden KC, Biggs WH., III Regulation of the FoxO family of transcription factors by phosphatidylinositol-3 kinase-activated signaling. Arch. Biochem. Biophys. 2002;403:292–298. doi: 10.1016/s0003-9861(02)00207-2. [DOI] [PubMed] [Google Scholar]
- 28.Kitamura T, Nakae J, Kitamura Y, Kido Y, Biggs WH, III, Wright CVE, White MF, Arden KC, Accili D. The forkhead transcription factor Foxo1 links insulin signaling to Pdx1 regulation of pancreatic β cell growth. J. Clin. Invest. 2002;110:1839–1847. doi: 10.1172/JCI200216857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu KL, Gannon M, Peshavaria M, Offield MF, Henderson E, Ray M, Marks A, Gamer LW, Wright CV, Stein R. Hepatocyte nuclear factor 3β is involved in pancreatic β-cell-specific transcription of the pdx-1 gene. Mol. Cell. Biol. 1997;17:6002–6013. doi: 10.1128/mcb.17.10.6002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee CS, Sund NJ, Vatamaniuk MZ, Matschinsky FM, Stoffers DA, Kaestner KH. Foxa2 controls Pdx1 gene expression in pancreatic β-cells in vivo. Diabetes. 2002;51:2546–2551. doi: 10.2337/diabetes.51.8.2546. [DOI] [PubMed] [Google Scholar]
- 31.Nakae J, Biggs WH, III, Kitamura T, Cavenee WK, Wright CVE, Arden KC, Accili D. Regulation of insulin action and pancreatic β-cell function by mutated alleles of the gene encoding forkhead transcription factor Foxo1. Nat. Genet. 2002;32:245–253. doi: 10.1038/ng890. [DOI] [PubMed] [Google Scholar]
- 32.Wang X, Zhou J, Doyle ME, Egan JM. Glucagon-like peptide-1 causes pancreatic duodenal homeobox-1 protein translocation from the cytoplasm to the nucleus of pancreatic β-cells by a cyclic adenosine monophosphate/protein kinase A-dependent mechanism. Endocrinology. 2001;142:1820–1827. doi: 10.1210/endo.142.5.8128. [DOI] [PubMed] [Google Scholar]
- 33.Kwon G, Pappan KL, Marshall CA, Schaffer JE, McDaniel ML. cAMP dose-dependently prevents palmitate-induced apoptosis by both protein kinase A- and cAMP-guanine nucleotide exchange factor-dependent pathways in β-cells. J. Biol. Chem. 2004;279:8938–8945. doi: 10.1074/jbc.M310330200. [DOI] [PubMed] [Google Scholar]
- 34.Kodama S, Toyonaga T, Kondo T, Matsumoto K, Tsuruzoe K, Kawashima J, Goto H, Kume K, Kume S, Sakakida M, Araki E. Enhanced expression of PDX-1 and Ngn3 by exendin-4 during beta cell regeneration in STZ-treated mice. Biochem. Biophys. Res.Commun. 2005;327:1170–1178. doi: 10.1016/j.bbrc.2004.12.120. [DOI] [PubMed] [Google Scholar]
- 35.G.G.H. acknowledges the support of the NIH (R01-DK45817) and the American Diabetes Association (Research Grant Award). Special thanks to J. Buteau for critical reading of the manuscript.