Abstract
Background
Though the dysfunction of central dopaminergic system has been proposed, the etiology or pathogenesis of schizophrenia is still uncertain partly due to limited accessibility to dopamine receptor. The purpose of this study was to define whether or not the easily accessible dopamine receptors of peripheral lymphocytes can be the peripheral markers of schizophrenia.
Results
44 drug-medicated schizophrenics for more than 3 years, 28 drug-free schizophrenics for more than 3 months, 15 drug-naïve schizophrenic patients, and 31 healthy persons were enrolled. Sequential reverse transcription and quantitative polymerase chain reaction of the mRNA were used to investigate the expression of D3 and D5 dopamine receptors in peripheral lymphocytes. The gene expression of dopamine receptors was compared in each group. After taking antipsychotics in drug-free and drug-naïve patients, the dopamine receptors of peripheral lymphocytes were sequentially studied 2nd week and 8th week after medication.
In drug-free schizophrenics, D3 dopamine receptor mRNA expression of peripheral lymphocytes significantly increased compared to that of controls and drug-medicated schizophrenics, and D5 dopamine receptor mRNA expression increased compared to that of drug-medicated schizophrenics. After taking antipsychotics, mRNA of dopamine receptors peaked at 2nd week, after which it decreases but the level was above baseline one at 8th week. Drug-free and drug-naïve patients were divided into two groups according to dopamine receptor expression before medications, and the group of patients with increased dopamine receptor expression had more severe psychiatric symptoms.
Conclusions
These results reveal that the molecular biologically-determined dopamine receptors of peripheral lymphocytes are reactive, and that increased expression of dopamine receptor in peripheral lymphocyte has possible clinical significance for subgrouping of schizophrenis.
Background
Schizophrenia, commonly developed in adolescents and young adults, is one of the most common mental disorders, but the pathophysiology and etiology of schizophrenia is still obscure. Numerous studies on dopamine and schizophrenia have suggested that the change in the dopamine system is related to schizophrenia, but there is little direct evidence for the "dopamine hypothesis in schizophrenia". Recent progress in molecular biology and imaging techniques has enabled new insight for schizophrenia research, but these methods are still limited by their availability and often reveal inconsistent results. The "dopamine hypothesis" is largely based on pharmacological manipulation of the dopamine system, either by mimicking [1,2] or reducing the symptoms of schizophrenia [3]. However changes in the dopamine system are influenced not only by dopamine itself, but also by dopamine receptors. Therefore, to elucidate the exact changes in the dopamine system researches about the relationship between dopamine and dopamine receptors are needed.
In the early 1980s, Le Fur reported the existence of high affinity binding sites for [3H]spiroperidol in lymphocyte of peripheral blood [4], but there has been long debate as to whether these sites are true dopamine receptors or nonspecific binding sites. Recent progress in molecular biology reveals the existence of mRNA of D3, D4, D5 dopamine receptors in peripheral lymphocytes, thereby suggesting that the binding sites for [3H]spiroperidol in peripheral lymphocytes may be true dopamine receptors [5,6,7]. However, the clinical significance of these findings, and whether or not these receptors reflect central dopamine receptors remains uncertain.
The purposes of this study were to examine if the mRNA of peripheral dopamine receptor is statically or dynamically changed in schizophrenia, and whether or not these receptors have some value as a potential peripheral marker reflecting central one in schizophrenia.
Materials and methods
Subjects
The total number of subjects was 87 schizophrenic patients. 44 of them were chronic schizophrenics who had been taking antipsychotic drugs for more than 3 years (drug-med patients), 28 were drug-free schizophrenics who had not taken antipsychotic drugs for more than 3 months (drug-free patients), and 15 were drug-naïve schizophrenics who had never taken antipsychotic drugs (drug-naïve patients). For the controls, age and sex matched 31 healthy persons were enrolled. All patients fulfilled the DSM-IV criteria and the patients with a previous history of neurologic, neuropsychologic, medical, and surgical disease were excluded. The demographic data of subjects were shown at the Table 1.
Table 1.
Demographic data of study groups
| Control | Drug-med pts | Drug-free | Drug-naïve | |||
| (N=31) | (N=44) | pts(N=28) | pts(N=15) | p-value** | ||
| Age* | 33.9 ± 9.5 | 37.3 ± 11.4 | 35.1 ± 9.1 | 30.8 ± 7.7 | NS*** | |
| Sex | Male | 17 | 33 | 6 | 16 | NS*** |
| Female | 14 | 11 | 9 | 15 | ||
| Age of symptom onset* | 22.5 ± 6.8 | 26.7 ± 9.2 | 28.1 ± 7.8 | NS*** | ||
| Duration of illness* | 12.7 ± 8.3 | 7.6 ± 5.2 | 2.5 ± 3.0 | <0.01 | ||
| TBPRS | 36.1 ± 12.9 | 41.9 ± 25.4 | 36.9 ± 18.2 | NS*** | ||
| Dose of antipsychotics**** | 1000.1 ± 130.4 | 769.6 ± 491.2 | 614.2 ± 234.7 | NS*** | ||
| Duration of no-medication | 10.61 ± 7.01 | |||||
| (mean months ± SD) | ||||||
* :mean ± standard deviation (years) ** :one-way ANOVA test, independent T-test, Chi-square test *** :NS; statistically non-significant **** :chlorpromazine equivalent dose (mg/cay)
Methods
Study design
Before taking antipsychotics, clinical scale and the amount of dopamine receptor mRNA were checked in the group of schizophrenics and the amount of dopamine receptor mRNA was checked in controls. At 2nd week and 8th week after taking antipsychotics, the the amount of dopamine receptor mRNA were re-checked in drug-free and drug-naïve patients.
To assess the psychiatric pathology of patients, the Brief Psychiatric Rating Scale (BPRS) was adopted [8] and Extrapyramidal Symptom Rating Scale (ESRS) was used to assess the drug side effects [9].
Quantitation of dopamine receptor mRNA
1) preparation of blood lymphocyte
Peripheral blood samples were placed on Ficoll-Paque (Pharmacia, Sweden) gradients and the centrifuged for 15 minutes. Separation of lymphocytes was done no later than 6 hours after drawing blood.
2) extraction of total RNA and reverse transcription
Total RNAs were extracted from lymphocytes by RNeasy Total RNA kit (Quiagen, Germany). 2 μg of total RNA extracted from lymphocytes was reverse transcribed into first-strand cDNA with 1 μg of random hexanucleotide and 200 units of Molony murine leukemia virus reverse transcriptase (GibcoBRL, Gaithersburg) in a 20 μl final volume.
3) oligonucleotide primer used for PCR amplification
The oligonucleotid primers used for PCR amplification of D3 dompamine receptor (D3R), D5 dopamine receptor (D5R), beta-actin (βA) as internal controls, and mutant template for competition of each products as external control were synthesized using a nucleotide synthesizer. Their sequences were shown at the Table 2.
Table 2.
Primer used to amplify the dopamine receptor, beta-actin and their competitive mutants
| product | |||
| Target | Primer | primer sequence | length (bp) |
| D3R | Upstream | 5'-ACG-ACA-TGG-CTG-GGC-TAC-G-3' | 155 |
| Downstream | 5' -GCC-AAC-AGC-CTG-GCG-CTA-G-3' | ||
| D5R | Upstream | 5'-GTC-GCC-GAG-GTG-GCC-GGT-TAC-3' | 362 |
| Downstream | 5'-GCT-GGA-GTC-AGA-ATT-CTC-TGC-AT-3' | ||
| BA | Upstream | 5'-CGT-GGG-CCG-CCC-TAG-GCA-CCA-3' | 260 |
| Downstream | 5'-TTG-GCC-TTA-GGG-TTC-AGG-GGG-G-3' | ||
| Mutant D3R | Upstream | 5' -TCA-AGA-GTT-CCC-TAT-CAC-TCT-CAA-GTT- | 474 |
| TCG-TGA-GCT-GAT -TG-3' | |||
| Down stream | 5'-GTC-GCC-GAG-GTG-GCC-GGT-TAC-CAA-GTT- | ||
| TCG-TGA-GCT-GAT -TG-3' | |||
| Mutant D5R | Upstream | 5'-GTC-GCC-GAG-GTG-GCC-GGT-TAC-CAA-GTT- | 435 |
| TCG-TGA-GCT-GAT -TG-3' | |||
| Downstream | 5'-GCT-GGA-GTC-AGA-ATT-CTC-TGC-ATA-TTT- | ||
| GAT-TCT-GGA-CCA-TGG-C-3' | |||
| Mutant βA | Upstream | 5'-TTG-GCC-TTA-GGG-TTC-AGG-GGG-GAT-TTG- | 429 |
| ATT-CTG-GAC-CAT-GGC-3' | |||
| Downstream | 5'-CGT-GGG-CCG-CCC-TAG-GCA-CCA-CAA-GTT- | ||
| TCG-TGA-GCT -GAT-TG-3' |
4) Quantitative RT-PCR
Mutant D3R primers were used in PCR amplification with the mimic DNA fragment provided in PCR MIMIC construction kit (Clontech, USA) to create PCR templates with gene-specific 5'and 3' ends for D3R, then these templates were amplified and purified. These products were quantified and diluted before being used as competitive external standard. With the same technique, mutant templates for D5R and βA were synthesized. At various concentrations of mutant template, PCR amplification was performed from 75 ng cDNA with 5 μl PCR buffer (PH8.5, 2.5 mM MgCl2), 4 μl 1.25 mM dNTP, 0.2 μl (5 U/μl) Taq DNA polymerase, 9.8 μl distilled water, 1 μl upstream and downstream D3 or D5 primer in 10 μl final volume. PCR was carried out in a DNA thermal cycler (GeneAmp PCR system 9600, Perkin Elmer, USA) after first denaturation at 94°C for 3 minutes, and each cycle consisted of denaturation at 94°C for 40 seconds, annealing at 59°C for 40 seconds, and extension at 72°C for 80 seconds. The number of total PCR cycle was 37. PCR for βA was carried out for 27 cycles and each cycle was consisted of denaturation at 94°C for 30 seconds, annealing at 59°C for 30 seconds, and extention at 72°C for 1 minute. For quantitative analysis, each 10 μl of PCR products were examined by electrophoresis on 1.5% agarose gel containing 0.5 μg/ml ethidium bromide, and optical density was quantitated by using densitometric scanning imaging system (CSC; chemicoluminence detection module 1.0 and TINA 2.0 program, Raytest, Belmont, CA, USA). Let the logarithmic value of concentration of D3 mutant template at x axis and logarithmic value of mutant-product ratio (optical density of D3R mutant template / optical density of D3R) at y axis, then regression equation was done. Same procedure was done for D5R and βA. Quantitative analysis of each sample was done if the coefficient value was more than 0.9. Expected concentration value of D3R, D5R, βA was the X value of 0 point in the Y axis (Figure 1, Figure 2). To confirm reliability of these methods, concentration of D3R was plotted on a semilogarthmic scale against the amount of cDNA and checked whether this curve was in linear relation. To confirm reproducibility, random samples of 14 patients were checked twice, and the results were compared with each other.
Figure 1.
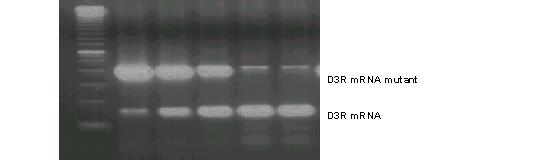
Examples of quantitative RT-PCR of D3R mRNA in one patient. Upper band represents the D3R mutant that competes with D3R cDNA (lower band). This figure shows lane 3 has approximately equal concentrations of D3R and D3R mutant.
Figure 2.
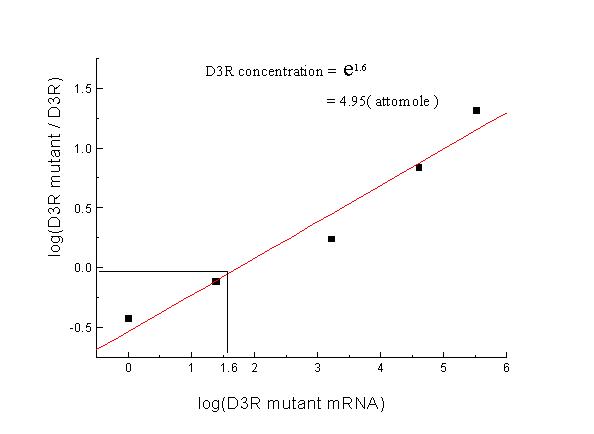
Analysis of actual D3R mRNA concentraion of Figure 1. The exact concentrations of the patient in Figure 1a. were shown. The 0 point on the Y axis represents equal concentrations of D3R and D3R mutant, and the corresponding point on the X axis represents the actual value of D3R (logarithmic value).
Statistical test
Statistical determinations were performed by using a statistical program (SPSS version 8.0). Mann-Whitney test, independent T-test, Chi-square test, Pearson correlation, multiple regression analysis. One-way ANOVA test, and repeated measure of ANOVA were chosen.
Results
Test for sensitivity and reproducibility of methodology
To confirm sensitivity and reproducibility, the D3R, D5R, βA products amplified by RT-PCR technique were electrophoresed on 1.5% agarose gels, and the OD was plotted on the X axis against the amount of cDNA used in PCR on the Y axis. This curve was linear (Figure 3).
Figure 3.
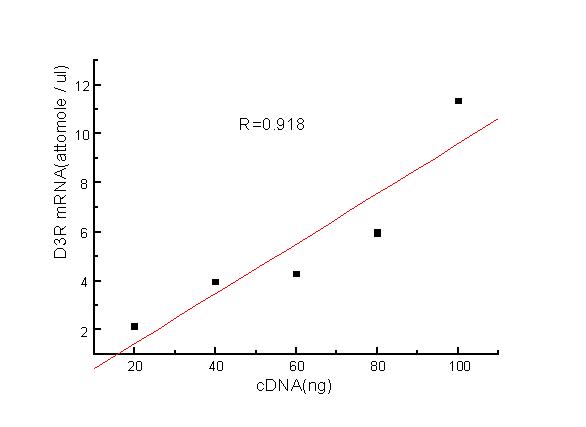
D3R mRNA standard curve. Linear correlation is shown between template cDNA and D3R mRNA. This finding represents the sensitivity and reproducibility of the methodological technique.
Concentrations of D3R randomly sampled in 14 patients were checked twice by the same technique for reproducibility. The results were statistically identical (Cronbach's alpha is 0.92). Therefore, RT-PCR was reliable tool for quantitation of mRNA.
Quantitation of dopamine receptor mRNA among study groups
1) Analysis of factors for quatitation of D3R/βA, D5R/βA
Multiple regression was done to confirm whether gender, age in normal healthy controls, and the symptom onset, prevalence period, dosage of antipsychotics, BPRS score in drug-med patients were influential factors. All the factors were not statistically significant. (Table 3).
Table 3.
Influence of clinical factors on D3R/βA, D5R/βA mRNA ratio in drug-med patients
| Sex | Age | Onset | duration | BPRS | dose | |
| D3R/βA | NS* | NS* | NS* | NS* | NS* | NS* |
| D5R/βA | NS* | NS* | NS* | NS* | NS* | NS* |
*: NS; statistically non-significant
2) Comparison of D3R/βA, D5R/βA among study groups before taking antipsychotics
D3R mRNA expression of peripheral lymphocytes was significantly increased in drug-free patients, compared to controls and drug-med patients. There was no difference between controls and drug-med patients. A few drug-naïve patients also revealed increased D3R and D5R mRNA expression, but without statistical significance compared to controls (Figure 4, Figure 5, Table 4).
Figure 4.
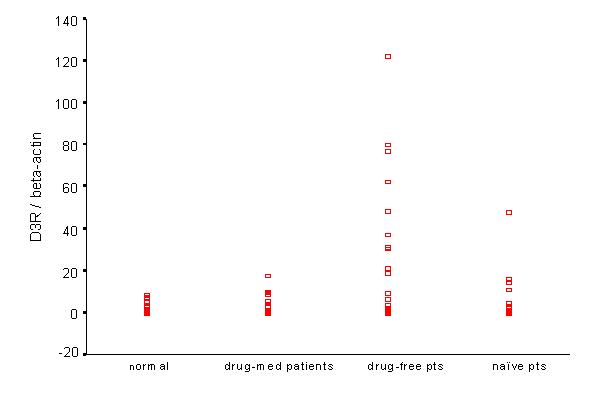
D3R/βA mRNA among study groups. In drug-free patients, D3R/βA was higher than that of normal or on-med patients. There was no difference between normal and on-med patients
Figure 5.
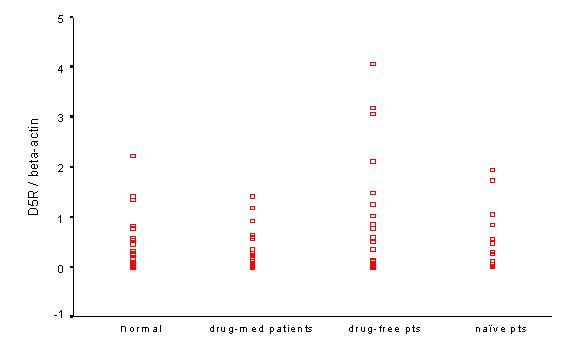
D5R/βA mRNA among study groups. There were similar findings with figure 3, but there is no significant difference among groups.
Table 4.
D3R/βA, D5R/βA mRNA ratio between normal and schizophrenic patients (mean ± standard error)
| Normal | Drug-med pts | Drug-free pts | Drug-naive pts | p-value* | |
| D3R/βA | 2.10 ± 0.47** | 2.09 ± 0.63** | 19.45 ± 5.57** | 7.75 ± 3.21 | p < 0.05 |
| D5R/βA | 0.37 ± 0.10 | 0.19 ± 0.05** | 0.78 ± 0.21** | 0.58 ± 0.16 | p < 0.05 |
* :One-way ANOVA test, Post-hoc test (Bonferroni test) ** :p < 0.05
The D5R/βA ratio was significantly elevated between drug-free patients and drug-med patients, but there was no statistical difference between drug-free patients and normal controls (Figure 5).
3) The relationship between D3R/βA, D5R/βA and drug free duration in drug-free patients
To analyze the relationship between elevated dopamine receptor mRNA and the drug-free duration in drug-free patients, the D3R/βA, D5R/βA ratio was plotted against the drug-free duration. Statistical test showed no significant difference between D3R/βA, D5R/βA ratio and drug-free duration (Figure 6, Figure 7).
Figure 6.
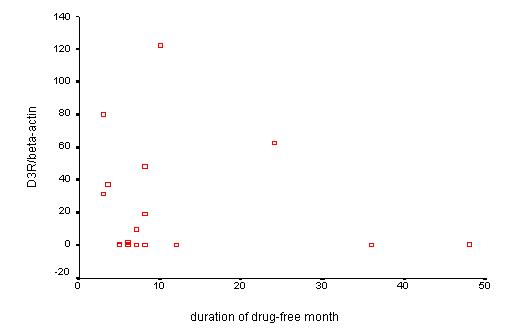
Relationship between D3R/βA and drug-free duration in drug-free patients. To analyze the cause of high dopamine receptor in drug-free patients, the relationship between D3R/βA and drug-free duration is shown. Though negative correlation may be suggested, it was not statistically significant.
Figure 7.
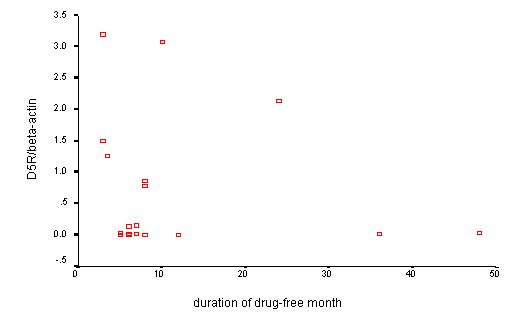
Relationship between D5R/βA and drug-free duration in drug-free patients. In D5R/βA, similar findings were shown with D3R/βA.
4) Relationship between amount of D3R/βA and clinical profile
According to the amount of D3R/βA, the drug-free and drug-naïve patients were divided into two groups. The high dopamine receptor group had higher D3R/βA ratio than that of normal controls (more than 2 standard deviation), while other patients were classified in the normal dopamine receptor group. The two groups were compared with BPRS scale and ESRS scale. 10 of 28 drug-free patients, and 3 of 15 naïve patients were included in the high dopamine receptor group. The patients of the high dopamine receptor group had statistically significant high BPRS compared to the patients in the normal dopamine receptor group, but there was no difference between the two group in ESRS (Table 5).
Table 5.
Comparison of clinical profiles according to the level of dopamine receptor mRNA before medication (mean ± SD)
| High dopamine R | Normal dopamine R | p-value* | |
| (N=13) | (N=30) | ||
| BPRS | 54.08 ± 34.70 | 34.47 ± 13.43 | <0.05 |
| ESRS | 1.58 ± 2.78 | 3.25 ± 7.09 | 0.561 |
* :Mann-Whitne test
5) Change of dopmaine receptor mRNA after antipsycotics
After taking antipsychotics, dopamine receptor mRNA peaked at 2nd week, after which it decreased, but the level of this was above baseline one at 8th week (Table 6).
Table 6.
Sequential studies of off-medication patients after antipsychotic medication (mean ± standard error)
| 0 week | 2 week | 8 week | ||
| Drug-free Pts | D3R/βA | 19.45 ± 5.57 | 26.74 ± 6.41 | 22.01 ± 6.19 |
| D5R/βA | 0.78 ± 0.21 | 1.88 ± 0.90 | 1.62 ± 0.82 | |
| Drug-naïve | D3R/βA | 7.75 ± 3.21 | 19.42 ± 5.17 | 14.11 ± 7.23 |
| pts | ||||
| D5R/βA | 0.58 ± 0.16 | 1.21 ± 0.51 | 0.74 ± 0.50 | |
Discussion
Dopamine and Dopaminergic neurons are localized in certain parts of the central nervous system and involved in the pathophysiology of schizophrenia. However, there is no direct evidence of dysfunction of brain dopaminergic systems in schizophrenic patients because no pathognomic change in the dopaminergic system has been found at autopsy and the direct assessment of brain dopaminergic systems in vivo is almost impossible at present. In addition, abnormal function of the dopamine system results not only from the dopamiergic neuron, but from dopamine receptors and these dopamine receptors can be changed secondly by dopamine. Therefore, the importance of the physiological and pathological roles of dopamine receptors is emerging.
Recent progress in molecular biology has revealed 5 distinct subtypes of dopamine receptors, and their structure and physiological functions have been identified. Owing to this advance, receptors other than D2 dopamine receptors, which is traditionally considered to be important in pathophysiology of schizophrenia, have been issued in relation to schizophrenia [10,11,12]. Among these other dopamine receptors, the D3 dopamine receptor is primarily localized in the limbic area, has a high affinity for antipsychotics, and it has been found to be elevated in postmortem study of schizophrenic patients [13]. As a result, the D3 dopamine receptor is considered to be important in the pathophysiology of schizophrenia. Therefore, the notion that the effect of antipsychotic medication is more closely related to D3 dopamine receptors, while extrapyramidal drug side effects are related to the D2 dopamine receptor, is one of the modified dopamine hypotheses [14,15].
The D5 dopamine receptor, belongs to the Dl dopamine receptor subfamily, has a similar nucleotide sequence to the Dl receptor [16], and a high affinity for dopamine more than the Dl dopamine receptor [17]. The D5 dopamine receptor, which is sparse in area of the Dl dopamine receptor, is mainly localized in the hippocampus, thalamus, and its physiological role is still uncertain [18]. However, some report suggest that the Dl dopamine receptor may be related in negative symptom [19,20] and the fact that the D5 dopamine receptor in the prefrontal cortex is down-regulated by antipsychotics in animal experiment [21] may suggest that the D5 dopamine receptor is also related to schizophrenia.
Nigrostriatal and mesolimbic dopaminergic pathways are major dopaminergic systems in the brain. D3 dopamine receptor has a high affinity for dopamine [22], and is preferentially localized in mesolimbic dopamine system, and it projects to the ventral striatum. This receptor is considered to have a major role in cognition and emotion [23]. Therefore, changes of D3 dopamine receptors mediate the changes in the striato-pallidal-thalamo-cortical limbic loop, and these changes are considered to be involved in the symptoms and pathophysiology of schizophrenia [24]. While the nigrostriatal system and D2 dopamine receptors have a major role in pathophysiology in Parkinson's disease, the mesolimbic system and D3 dopamine receptor may be more involved in the pharmacological responsiveness and pathophysiology of schizophrenia.
A major limitation of the dopamine hypothesis in schizophrenia is that there are no suitable in vivo methods for direct assessment of functional changes of the dopamine system in the brain. Though positron emission tomography (PET) can assess D2 dopamine receptors in striatum [25], PET has limited usability other than striatum, and limbic area and cortex related to schizophrenia is difficult to assess by PET. To assess the changes in dopamine receptors, an easily accessible peripheral marker is needed, and the dopamine receptor of peripheral lymphocyte may be a possible candidate. However, small quantity of mRNA of dopamine receptors in the peripheral lymphocyte is a major limitation to research assessing clinical significance. Methods to quantitate gene expression are Northern blot, RNase protection assay, in situ hybridization, and RT-PCR. Though Northern blot and RNase protection assay are classical tools, the more sensitive RT-PCR is useful in detecting small quantities. In RT-PCR, as the reaction cycle increases, product is exponentially increased. While this exponential increase causes high sensitivity, if not adequately controlled, the initial amount is not precisely assessed. To solve this problem, PCR is done with a primer for a non-competitive endogenous target (βA) as an internal control (multiplex PCR) or with competitive external strands as an external control. For a more accurate assessment, each βA, D3, D5 was quantitated by the addition of a competitive external strand, then the dopamine receptor/βA ratio was done. Since all dopamine receptors and most other receptors related to the G-protein have a similar structure, designing a sensitive and specific primer is critical in performing quantitative RT-PCR. The D3 dopamine receptor especially has a splicing variant without physiological function [6]. Therefore, a sensitive and specific primer for quantitation of the intact form of D3 dopamine receptors is important.
Considering the changes in dopamine receptors after taking antipsychotics, these receptors may have a physiological role, but the relationship between peripheral and central dopamine receptors is still uncertain. In animal experiment, Shenkman concluded that cholinergic muscarine receptors in peripheral lymphocytes are useful markers for cholinergic muscarine bindings in the brain [26]. Dopamine receptors and cholinergic muscarine receptors are all typical G-protein related receptors, and there was a report that D3 mRNA in lymphocyte is decreased in Parkinson's disease, and that the degree of decrease was correlated with the severity of this disease [27]. Considering all the above findings, dopamine receptors in peripheral lymphocyte can be a peripheral marker of central nervous system dopamine receptors. However, there are various expressions of subtypes of dopamine receptors and these show various responses to antipsychotics according to the anatomic substrate in the central nervous system [13, 28]. D3 dopamine receptors in lymphocytes can reflect particular anatomy in the brain, not merely the whole brain, and considering the previous result from a report on D3 dopamine receptor [13] and a dense population of D3 receptor in the limbic striatum, limbic striatum may be a plausible corresponding site.
In this study, D3 dopamine receptor mRNA was increased in drug-free patients compared to drug-med patients and controls, while somewhat lower levels of D3 dopamine receptor mRNA were found in chronically medicated patients compared to normal controls before taking antipsychotics. The fact that D3 dopamine receptor mRNA is elevated in medicated schizophrenic patients produced similar findings to the report of Gureivch (1997). But after taking antipsychotics, dopamine receptor mRNA peaked at 2nd week, while at 8th week dopamine receptor mRNA had decreased, though the level was still found to be above normal controls. Though long term follow up was not done, considering that dopamine receptor mRNA decreased in chronically medicated patients, dopamine receptor mRNA might decrease to lower levels than that of the control patients.
The difference between drug-free patients and naïve patients is interesting. While dopamine receptor mRNA in drug-free patients is higher than in normal controls, the level of drug-naïve patients, though it increased, is not a statistically higher one. This may be interpreted in two ways:
First, the residual drug effect of antipsychotics due to a short withdrawal period may influence drug-free patients. We analyzed it by correlation with drug withdrawal periods and dopamine receptor mRNA in drug-free patients. The coefficient value is not statistically significant. It is not exactly known how rapidly D2-like receptors return to normal after neuroleptic treatment, but PET study showed that there is no residual increase of dopamine receptor in striatum within a month period of drug withdrawal [29]. Therefore this assumption may be less possible.
Second, there is the possibility of in-born elevation of lymphocyte dopamine receptors in certain schizophrenic patients and the possibility of subgrouping of schizophrenia. To elucidate this possibility, we analyzed drug-free and drug-naïve patients in two groups according to levels of dopamine receptors and compared these two groups with their clinical character. The patients in the high dopamine receptor group had statistically significant high BPRS compared to that of patients in the normal dopamine receptor group. But there was no difference between the two group in ESRS before taking antipsychotics (Table 5). There has been a long debate about the subgrouping of schizophrenia [30] and the lack of biological markers for this hypothesis is a major limitation. The peripheral dopamine receptor may be a possible candidate for this hypothesis. But due to the small number of study groups, and the limitations of other confounding factors, somewhat cautious conclusions are needed.
We believe a latter interpretation is more plausible but stricter and more prudent prospective study design is necessary before reaching more convincing conclusions. This study indicated that dopamine receptors in peripheral lymphocytes are elevated in certain populations of schizophrenic patients compared to controls before taking antipsychotics, and dynamically changed after taking antipsychotics. Therefore dopamine receptor in peripheral lymphocyte may be an easily accessible candidate for a biological marker and studying a pharmacodynamic study in a future.
Conclusion
This study reveals that the molecular biologically-determined dopamine receptors of peripheral lymphocytes are reactive after taking antipsychotics, and that increased expression of dopamine receptor in peripheral lymphocyte has possible clinical significance for subgrouping of schizophrenis.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Contributor Information
Yong T Kwak, Email: ytkwak@netsgo.com.
Min-Seong Koo, Email: drkooms@hanmail.net.
Chul-Hee Choi, Email: chulhee@yumc.ac.kr.
IN Sunwoo, Email: Sunwooin@yumc.ac.kr.
References
- Janowsky DS, el-Yousel MK, Davis JM, Sekerke HJ. Provocation of schizophrenic symptoms by intravenous administration of methylphenidate. Arch Gen Psychiatry. 1973;28:185–191. doi: 10.1001/archpsyc.1973.01750320023004. [DOI] [PubMed] [Google Scholar]
- Angrist B, Gershon S. Clinical response to several dopamine agonists in schizophrenic and nonschizophrenic subjects. Adv Biochem Psychopharmacol. 1977;16:667–680. [PubMed] [Google Scholar]
- Matthysse S. Antipsychotic drug actions: a clue to the neuropathology of schizophrenia. Federation Proc. 1973;32:200–208. [PubMed] [Google Scholar]
- Le Fur G, Phan T, Uzan A. Identification of stereospecific [3H]spiroperidol binding sites in mammalian lymphocytes. Life Sci. 1980;26:1139–1148. doi: 10.1016/0024-3205(80)90653-0. [DOI] [PubMed] [Google Scholar]
- Takahashi N, Nagai Y, Ueno S, Saeki Y, Yanagihara T. Human peripheral blood lymphocytes expression D5 dopamine receptor gene and transcribe the two pseudogenes. FEES Lett. 1992;314:23–25. doi: 10.1016/0014-5793(92)81452-R. [DOI] [PubMed] [Google Scholar]
- Nagai Y, Ueno S, Saeki Y, Soga F, Yanagihara T. Expression of the D3 dopamine receptor gene and a novel variant transcript generated by alternative splicing in human peripheral blood lymphocytes. Biochem Biophys Res Commun. 1993;194:368–374. doi: 10.1006/bbrc.1993.1829. [DOI] [PubMed] [Google Scholar]
- Bondy B, de Jonge S, Pander S, Primbs J, Ackenheil M. Identification of dopamine D4 mRNA in circulating lymphocytes using nested polymerase chain reaction. J Neuroimmunol. 1996;71:139–144. doi: 10.1016/S0165-5728(96)00148-8. [DOI] [PubMed] [Google Scholar]
- Overall J, Gorham D. The brief psychiatric rating scale. Psychol Rep. 1962;10:799–812. [Google Scholar]
- Chouinard A, Ross-Chouinard G. Extrapyramidal symptom rating scale. Can J Neurol Sci. 1980;7:233. [Google Scholar]
- Sokoloff P, Giros B, Martres MP, Bouthenet ML, Schwartz JC. Molecular cloning and a characterization of a novel dopamine receptor (D3) as a target for neuroleptics. Nature. 1990;347:146–151. doi: 10.1038/347146a0. [DOI] [PubMed] [Google Scholar]
- Crocq MA, Mant R, Asherson P, Williams J, Hode Y, Mayerova A, Collier D, Lannfelt L, Sokoloff P, Schwartz JC, et al. Association between schizophrenia and homozygosity at the dopamine D3 receptor gene. J Med Genet. 1992;29:858–860. doi: 10.1136/jmg.29.12.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arinami T, Itokawa M, Enguchi H, Tagaya H, Yano S, Shimizu H, Hamaguchi H, Tom M. Association of dopamine D2 receptor molecular variant with schizophrenia. Lancet. 1994;343:703–704. doi: 10.1016/S0140-6736(94)91581-4. [DOI] [PubMed] [Google Scholar]
- Gurevich EV, Bordelon Y, Shapiro RM, Arnold SE, Gur RE, Joyce JN. Mesolimbic dopamine D3 receptors and use of antipsychotics in patients with schizophrenia. Arch Gen Psychiatry. 1997;54:225–232. doi: 10.1001/archpsyc.1997.01830150047009. [DOI] [PubMed] [Google Scholar]
- Meltzer HY. The mechanism of action of novel antipsychotic drugs. Schizophr Bull. 1991;17:263–287. doi: 10.1093/schbul/17.2.263. [DOI] [PubMed] [Google Scholar]
- Sigmundson HK. Pharmacotherapy of schizophrenia: a review. Can J Psychiatry. 1994;supple 39:70–75. [PubMed] [Google Scholar]
- Sunahara RK, Guan HC, O'Dowd BF, Seeman P, Laurier LG, Ng G, George SR, Torchia J, Van Tol HH, Niznik HB. Cloning of the gene for a human dopamine D5 receptor with high affinity for dopamine than Dl. Nature. 1991;350:614–619. doi: 10.1038/350614a0. [DOI] [PubMed] [Google Scholar]
- Weinshank RL, Adham N, Macchi M, Oisen MA, Branchek TA, Hartig PR. Molecular cloning and characterization of a high affinity dopamine receptor (Dl beta) and its pseudogene. JBiol Chem. 1991;266:22427–22435. [PubMed] [Google Scholar]
- Sedvall G, Farde L. Chemical brain anatomy in schizophrenia. Lancet. 1995;340:743–749. doi: 10.1016/S0140-6736(95)91508-7. [DOI] [PubMed] [Google Scholar]
- Davidson M, Harvey PD, Bergman RL, Powchik P, Kaminsky R, Losonczy MF, Davis KL. Effects of the Dl agonist SKF-38393 combined with haloperidol in schizophrenic patients. Arch Gen Psychiatry. 1990;47:190–191. doi: 10.1001/archpsyc.1990.01810140090014. [DOI] [PubMed] [Google Scholar]
- Den Boer JA, van Megen HJ, Fleischhacker WW, Louwerens JW, Slaap BR, Westenberg HG, Burrows GD, Srivastava ON. Differential effects of the Dl-DA receptor antagonist SCH39166 on positive and negative symptoms of schizophrenia. Psychopharmacology. 1995;121:317–322. doi: 10.1007/BF02246069. [DOI] [PubMed] [Google Scholar]
- Lidow MS, Elsworth JD, Goldman-Rakic PS. Down-regulation of the Dl and D5 dopamine receptors in the primate prefrontal cortex by chronic treatment with antipsychotic drugs. J Pharmacol Exp Ther. 1997;281:597–603. [PubMed] [Google Scholar]
- Strange PG. New insights into dopamine receptors in the central nervous system. Neurochem Int. 1993;22:223–236. doi: 10.1016/0197-0186(93)90050-F. [DOI] [PubMed] [Google Scholar]
- Meador-Woodruff JH, Mansour A, Saul J, Watson SJ., Jr Neuroanatomical distribution of dopamine receptor messenger RNAs: In Niznik HB, ed Dopamine receptors and transporters New York, Marcel Dekker, 1994. pp. 401–415.
- Csemansky JG, Murphy GM, Faustman WO. Limbic/mesolimbic connections and the pathogenesis of schizophrenia. Biol Psychiatry. 1991;30:383–400. doi: 10.1016/0006-3223(91)90295-W. [DOI] [PubMed] [Google Scholar]
- Farde L, Hall H, Ehrin E, Sedvall G. Quantitative analysis of D2 dopamine receptor binding in the living human brain by PET. Science. 1986;231:258–261. doi: 10.1126/science.2867601. [DOI] [PubMed] [Google Scholar]
- Shenkman L, Rabey JM, Gilad GM. Cholinergic muscarinic binding by rat lymphocytes: effect of antagonist treatment, strain and aging. Brain Res. 1986;380:303–308. doi: 10.1016/0006-8993(86)90226-X. [DOI] [PubMed] [Google Scholar]
- Nagai Y, Ueno S, Saeki Y, Soga F, Hirano M, Yanagihara T. Decrease of the D3 dopamine receptor mRNA expression in lymphocytes from patients with Parkinson's disease. Neurology. 1996;46:791–795. doi: 10.1212/wnl.46.3.791. [DOI] [PubMed] [Google Scholar]
- Schmauss C, Haroutunian V, Davis KL, Davidson M. Selective loss of dopamine D3-type receptor mRNA expression in parietal and motor cortices of patients with chronic schizophrenia. Proc Nati Acad Sci USA. 1993;90:8942–8946. doi: 10.1073/pnas.90.19.8942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron JC, Martinet JL, Cambon H, Boulenger JP, Poirier MF, Caillard V, Blin J, Huret JD, Loc'h C, Maziere B. Striatal dopamine receptor occupancy during and following neuroleptic treatment: correlative evaluation by positron emission tomography and plasma prolactin levels. Psychopharmacology. 1989;99(4):463–472. doi: 10.1007/BF00589893. [DOI] [PubMed] [Google Scholar]
- Crow TJ. The two-syndrome concept: origins and current status. Schizophr Bull. 1985;11:471–486. doi: 10.1093/schbul/11.3.471. [DOI] [PubMed] [Google Scholar]


