Abstract
Norepinephrine and ATP are co-released by periarterial sympathetic nerves. In mesenteric arteries (MA) from deoxycorticosterone-acetate (DOCA)-salt hypertensive rats, ATP, but not norepinephrine, release is impaired suggesting that their release may be regulated differently. We tested the hypothesis that different calcium channels contribute to ATP and norepinephrine release from sympathetic nerves in vitro in MA from normotensive and DOCA-salt hypertensive rats and that oxidative stress disrupts prejunctional regulation of co-transmission. Excitatory junction potentials (EJPs) were used to measure ATP release. Norepinephrine release was measured amperometrically with carbon-fiber microelectrodes. CdCl2 (30 μM) inhibited norepinephrine release in sham and DOCA-salt arteries by 78 and 85%, respectively. The N-type calcium channel antagonist, ω-conotoxin GVIA (CTX; 0.1 μM) inhibited norepinephrine release by 50 and 67% in normotensive and DOCA-salt arteries, respectively while CTX blocked EJPs. The P/Q-type calcium channel antagonist ω-agatoxin IVA (ATX; 0.03 μM) reduced norepinephrine release in sham but not DOCA-salt arteries and increased EJPs in sham but not DOCA-salt arteries. ATX did not increase EJPs in sham arteries in the presence of the α2-adrenergic receptor antagonist, yohimbine (1 μM). α2-Autoreceptor-sensitive EJP facilitation is impaired in DOCA-salt hypertension but this response is restored in DOCA-salt rats treated chronically with the antioxidant, apocynin. Apocynin restored α2-autoreceptor regulation of norepinephrine release. We conclude that ATP released from periarterial sympathetic nerves is controlled directly by N-type calcium channels. Norepinephrine release is controlled by N and P/Q type calcium channels. Norepinephrine release controlled by P/Q channels acts at α2-adrenergic receptors to inhibit norepinephrine release suggesting that there may be multiple pools of norepinephrine in periarterial sympathetic nerves. Regulation of norepinephrine release by α2-autoreceptors and P/Q-type channels is impaired in DOCA-salt hypertension and α2-autoreceptor function is disrupted by oxidative stress.
Keywords: amperometry, excitatory junction potential, oxidative stress, synaptic transmission, norepinephrine, purine receptors, α2-adrenergic receptors
Blood pressure is controlled in part by the activity of sympathetic nerves supplying the splanchnic circulation (King et al., 2007). Alterations in the function of sympathetic nerves innervating the splanchnic circulation are associated with increased blood pressure in animal models of hypertension (Iriuchijima et al., 1975; Masuyama et al., 1986; Matihias et al., 1991; Brock and vanHelden, 1995; Luo et al., 2003;). The splanchnic vasculature is densely innervated by sympathetic nerves that release norepinephrine, adenosine 5′-triphosphate (ATP) and neuropeptide Y (NPY) which all constrict vascular smooth muscle (Donoso et al., 1997). Norepinephrine constricts arteries by activating α1-adrenergic receptors (α1ARs) on vascular smooth muscle cells and norepinephrine release from sympathetic nerves is regulated by prejunctional α2-adrenergic receptors (α2ARs)(Msghina et al., 1991; Hill et al., 1993; Stärnje and Stärnje, 1995). Purinergic P2X1 type receptors are cation channels activated by ATP. P2X1 receptors mediate arterial constriction by causing a rapidly developing but short lived smooth muscle membrane depolarization (excitatory junction potential, EJP) leading to activation of L-type calcium channels and direct calcium entry through the P2X1 channel (Gitterman and Evans, 2001; Rummery et al., 2007). Ectonucleotidases released with ATP quickly degrade ATP to ADP, AMP and adenosine (Todorov et al., 1999; Westfall et al., 2002). Prejunctional inhibition of ATP release is caused in part by adenosine acting at A1 adenosine receptors (Morikawa et al., 2007). Norepinephrine can also inhibit ATP release via activation of prejunctional α2ARs on sympathetic nerves (Driessen et al., 1993; Stärnje and Stärnje, 1995). A1 adenosine receptors and α2ARs link to pertussis-toxin sensitive G-proteins to cause inhibition of nerve terminal calcium channels and inhibition of transmitter release from sympathetic nerves (Hill et al., 1993; Zhu and Ikeda, 1993).
The differential regulation of ATP and norepinephrine release from sympathetic nerves has been studied in several species and preparations with various outcomes. Studies done in the mouse vas deferens and rat tail artery show that ATP and norepinephrine release occur in parallel suggesting that they are co-stored and their release is regulated by the same mechanisms (Msghima et al., 1992, 1998). However, others have shown the ratio of norepinephrine to ATP release from sympathetic nerves is frequency dependent (Todorov et al., 1999) and is differentially sensitive to regulation of prejunctional α2ARs (Driessen et al., 1993; Brock and Tan, 2004). In addition, Tityus serrulatus toxin I (TsTX-I), a scorpion derived sodium channel antagonist, stimulates ATP, but not norepinephrine, release from sympathetic nerves supplying the rat vas deferens (Conceicao et al., 2005) again supporting a model where NE and ATP release from sympathetic nerves can be differentially regulated. Parasympathetic neurons supplying the bladder release acetylcholine and ATP as co-transmitters to contract bladder smooth muscle. In this tissue, acetylcholine release is selectively controlled by N-type calcium channels while ATP release is controlled predominately by P/Q- type channels (Waterman, 1996). This result shows that two transmitters released from autonomic nerves can be controlled by different sets of calcium channels. However, N-type channels are the predominant calcium channel found on sympathetic nerve terminals as selective blockers of this channel abolish transmission to target tissues particularly at low stimulation frequencies (Brock et al., 1989; Wright and Angus, 1996). In the mouse vas deferens, purinergic and adrenergic constrictions are equally sensitive to N-, P- and Q type calcium channel blockers (Waterman, 1997). In the rat mesenteric artery, guinea pig vas deferens and in the canine splenic artery, norepinephrine and ATP release are inhibited equally well by an N-type calcium channel blocker (Brock et al., 1989; Pruneau and Angus, 1990; Yang and Chiba, 2000). Based on these data, it appears that ATP and NE release from sympathetic nerves are controlled predominately by N-type calcium channels. However, in mesenteric arteries of DOCA-salt hypertensive rats EJP amplitude is reduced indicating that purinergic transmission is impaired (Demel and Galligan, 2008) while norepinephrine release from the same nerves is maintained (Westfall et al., 1987; Tsuda et al., 1989; Luo et al., 2003). These data provide some evidence that DOCA-salt hypertension may differentially affect ATP and norepinephrine storage or release mechanisms.
Norepinephrine is an electroactive molecule which can be detected using amperometry with microelectrodes in vitro. This technique has been used previously to study sympathetic neurotransmission to mesenteric arteries from normotensive and hypertensive rats (Dunn et al., 1999; Park et al., 2007). Norepinephrine is oxidized on the microelectrode surface and the oxidation current can be used as a measure of the number of norepinephrine molecules near the adventitial surface of the artery during and after nerve stimulation. In the present study, we tested the hypothesis that norepinephrine and ATP release are regulated differentially by calcium channels, and that this regulation is impaired in DOCA-salt hypertension. Furthermore we propose that disruption of prejunctional regulation of norepinephrine and ATP release is caused by oxidative stress that is known to occur in sympathetic nerves of DOCA-salt hypertensive rats (Dai et al., 2004). We maintained mesenteric arteries in vitro and used amperometry and intracellular electrophysiological methods to measure norepinephrine and EJPs, respectively. Calcium channel antagonists were used to assess the contribution of N and P/Q type calcium channels to norepinephrine and ATP release. The antioxidant drug, apocynin (Williams and Griendling, 2007) was administered chronically to DOCA-salt rats to reduce oxidative stress in an effort to restore prejunctional regulation of norepinephrine and ATP release.
EXPERIMENTAL PROCEDURES
Animals
Sprague-Dawley rats were obtained from Charles River Laboratories (Portage, MI). Animal use procedures were approved by the Institutional Animal Care and Use Committee at Michigan State University and were done in accordance with the “Guiding Principles in the Care and Use of Animals” of the American Physiological Society. Rats were acclimated for 2–3 days before entry into experimental protocols. Rat chow (Harlan/Teklad 8640 Rodent Diet) and distilled water were provided ad libitum. Rats were housed in temperature- and humidity- controlled rooms with a 12:12-h light-dark cycle.
DOCA-salt hypertension
Male Sprague-Dawley rats weighing 175–200 grams were anesthetized via isoflurane inhalation. The skin over the left flank (lateral abdominal wall) was shaved and prepared with an iodine-based antiseptic. A 1.5 cm vertical incision was made through the skin and underlying muscle just caudal to the rib cage. The left kidney was exteriorized and removed after ligation of the renal artery, vein and ureter with 4-0 silk sutures. The muscle and skin layers were closed separately with 4-0 silk and 4-0 monofilament nylon sutures, respectively. A 3 × 1.5 cm rectangle area between the shoulder blades of the back was shaved and disinfected for subcutaneous DOCA implantation under a 1 cm incision. The skin was closed with 4-0 nylon sutures. DOCA implants (600 mg/kg) were prepared by mixing deoxycorticosterone acetate in silicone rubber resulting in a giving dose of 200 mg/kg. Sham-operated rats underwent left kidney removal only. Surgery was performed on a heated pad and rats recovered in a heated box. Antibiotics (enrofloxacin, 5 mg/kg subcutaneous) and an analgesic (butorphanol tartrate, 2 mg/kg, subcutaneous) were administered immediately after surgery. After recovery the rats were housed under standard conditions for 4 weeks. DOCA-implanted rats received standard pelleted rat chow and salt water (1 % NaCl + 0.2 % KCl) ad libitum, while sham rats received standard pelleted rat chow and tap water ad libitum. Systolic blood pressure was measured using the tail-cuff method four weeks after surgery. Rats with systolic blood pressure equal to or higher than 150 mmHg were considered hypertensive.
A group of 10 DOCA-salt rats was provided with apocynin (2 mM) in their drinking water beginning immediately after DOCA-salt surgery and throughout the subsequent 4 weeks prior to collecting tissues for in vitro study.
Tissue preparation
Four weeks after DOCA-salt surgeries, rats were deeply anesthetized with an intrapertioneal injection of sodium pentobarbital (50 mg/kg) and euthanized via pneumothorax and severing of the abdominal aorta. The mesentery was surgically removed and tertiary branches were dissected out, cleaned of adipose and connective tissue and pinned taut using stainless steel pins (50 μm diameter) in a perfusion chamber coated with Sylgard® (Dow Corning, Midland, MI). Tissues were superfused with Krebs’ solution of the following composition (mM): NaCl, 117; KCl, 4.7; CaCl2, 2.5; MgCl2, 1.2; NaHCO3, 25; NaH2PO4, 1.2; dextrose, 11. Nifedipine (1 μM) (L-type calcium channel antagonist) and prazosin (0.1 μM) (α1AR antagonist) were added to the buffer to attenuate vessel constriction during trains of electrical nerve stimulation. The buffer was heated to 37° C and bubbled with 95% O2 and 5% CO2. Tissues were allowed to equilibrate for 30 minutes before beginning experiments.
Electrophysiological recordings of EJPs
Intracellular recordings from individual arterial smooth muscle cells were obtained using glass microelectrodes filled with 2M KCl (100–200 M tip resistance). Impalements were accepted if the following criteria were satisfied: (1) cell penetration was abrupt (2) membrane potential was ≥ −50 mV and (3) the membrane potential was stable for at least 5 minutes. Recordings from a single cell lasted 20 – 120 minutes. A Dagan Instruments (Minneapolis, MN) IX2-700 amplifier was used to record membrane potential. EJPs were evoked using a Krebs’ solution-filled, bipolar, focal stimulating electrode containing two parallel Ag/AgCl wire electrodes connected to a Grass Instruments S88 stimulator (Grass Technologies, Astro-Med, Inc, West Warwick, RI). The stimulating electrode was positioned perpendicular to the tissue directly across from the recording electrode. Periarterial nerves were stimulated at the lowest voltage (50–120V) which produced a maximal amplitude EJP. Short trains (5 pulses at 0.5 Hz, 0.5 ms pulse duration) were used to study EJP facilitation. Signals were sampled at 5 kHz and filtered at 500 Hz using an analog to digital converter (Digidata 1200, Axon Instruments/Molecular Devices, Sunnyvale, CA) and Axoscope 9.0 software (Axon Instruments/Molecular Devices). A digital average of five sweeps was used to measure the amplitude of EJPs under control and treatment conditions. Data were analyzed using Clampfit 9.0 software (Axon Instruments/Molecular Devices).
Amperometric measurements
Tissue preparation and the amperometric technique used to make local measurements of norepinephrine release from periarterial sympathetic nerves have been described in detail previously (Park et al., 2007). Carbon fiber microelectrodes were soaked in distilled isopropyl alcohol (IPA) and affixed to a micromanipulator (MP-1, Narishige Instruments, Japan), which enabled its reproducible positioning against the side of a mesenteric artery. A platinum wire counter and a commercial no leak Ag–AgCl (3M KCl, model EE009, Cypress Systems Inc., USA) reference electrode were also mounted in the bath to complete the electrochemical cell. Electrochemical measurements were made with an Omni 90 analog potentiostat (Cypress Systems Inc.), an analog to digital converter (Digidata 1200) and Axoscope 9.0 software. Continuous amperometric i–t curves were recorded at a detection potential of 400 mV. Norepinephrine release was evoked using a train of nerve stimulation (10 Hz, 3 s 60–80V) and a bipolar focal stimulating electrode (see above). The analog voltage output from the potentiostat, a value that reflects the current flowing through the working electrode, was low-pass filtered at a time constant of 200 ms (5 Hz) before being digitized using an A/D converter at a sampling rate of 100 Hz. The data were then stored on a computer for further processing.
Drug application
Drugs were either added to the physiological buffer or applied directly to the recording site using a local drug application system (VC-8 Valve Controller, Warner Instruments, Hamden, CT). There was a 1 minute delay between the onset of drug application and drug effect. Drugs were applied for a minimum of 5 minutes before testing drug effects. When calcium channel toxins were applied in ascending concentration increments via the locally positioned flow tubes, each concentration was allowed to equilibrate for 5 minutes prior to testing EJP or NE oxidation current amplitude.
Dihydroethidium (DHE) staining
Inferior mesenteric ganglia (IMG) were surgically removed from euthanized rats in chilled Krebs-Ringers-HEPES (KRH) solution or the following composition (mM): NaCl 130, KCl 1.3, Ca2Cl2 2.2, MgSO4 1.2, KH2PO4 1.2, HEPES 1.0, glucose 0.09 (pH = 7.4). Freshly dissected tissues were allowed to sit at room temperature before incubation with 2 μM DHE solution in a light protected tube at 37° C for 60 min. Following DHE incubation, IMGs were 130 washed 3x with KRH solution and mounted with Fluoromount G (Southern Biotechnology Associates) mounting medium. Confocal fluorescent images were obtained (543 nm excitation; wavelengths > 560 were collected; Zeiss, LSM 5 Pascal). Neurons were counted and fluoresence intensity in each sample was measured offline using Image-Pro Plus 2. Paired ganglia from untreated and apocynin treated rats were processed and imaged under identical conditions. Fluorescence intensity in ganglia from apocynin treated rats was normalized to the fluorescence intensity measured in paired untreated ganglia.
Drugs
All drugs were obtained from Sigma chemical (St. Louis, MO USA) except ω-Conotoxin GVIA (CTX) and ω-agatoxin IVA (ATX) which were obtained from Alomone Laboratories (Jerusalem, Israel). All drugs were diluted in deionized water except for nifedipine and prazosin which were dissolved in 95% ethanol to make a concentrated stock solution. Working solutions of nifedipine and prazosin contained <0.01% ethanol. Final solutions were made in Krebs’ buffer on the day of the experiment.
Statistics
Data are presented as mean ± S.E.M. and “n” values are the number of animals from which the data were obtained. Concentration-response data were fitted using non-linear regression and the Hill equation (Graphpad Prism, San Diego, U.S.A). Data were analyzed using Student’s t-test or one-way ANOVA with Newman-Keuls post-hoc test as appropriate. Differences were considered significant when P < 0.05.
Results
Multiple calcium channels contribute to norepinephrine release from periarterial sympathetic nerves
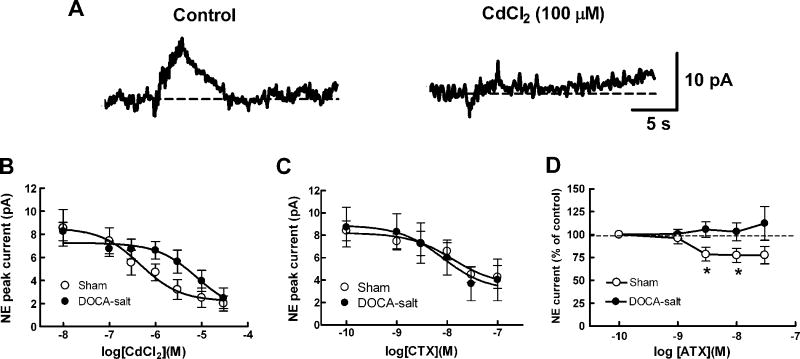
We measured norepinephrine release caused by a brief train of nerve stimulation (10 Hz, 3s) as an oxidation current and assessed the effects of CdCl2 (0.01 – 30 μM) on this response (Fig. 1A and B). CdCl2 produced a concentration dependent inhibition of the oxidation currents in arteries from sham and DOCA-salt rats. The negative log of the half maximum inhibitory concentration (pIC50) in sham arteries was 6.3 ± 0.3 (n=6) while in arteries from DOCA-salt rats this value was 5.1 ± 0.4 (n=6, P <0.05, Student’s t-test). Maximum inhibition was 78% and 85% in sham and DOCA-salt arteries, respectively. The rightward shift in the CdCl2 concentration response curve in DOCA-salt arteries suggests that are differences between sham and DOCA-salt arteries in calcium channel dependent release of norepinephrine from sympathetic nerves. The N-type calcium channel blocker, CTX (0.001–0.1 μM), also produced a concentration-dependent inhibition of norepinephrine oxidation currents in sham and DOCA-salt arteries (Figure 1C). The pIC50 value in sham arteries (7.9 ± 0.4, n = 7) was not different from that in DOCA-salt arteries (8.1 ± 1.0, n = 6 P > 0.05). The maximum inhibition caused by CTX (0.1 μM) was 50% and 67% in sham and DOCA-salt arteries, respectively.
Fig. 1.
CdCl2 and conotoxin GVIA (CTX) inhibit NE oxidation currents recorded from mesenteric arteries. A, NE oxidation currents are blocked by CdCl2. ns, nerve stimulation B, Concentration dependent inhibition of NE oxidation currents in sham (n=6) and DOCA-salt (n=6) mesenteric arteries. CdCl2 completely inhibited currents but the curve in DOCA-salt rats was shifted to the right compared to that in sham mesenteric arteries. C, CTX concentration response curves for inhibition of NE oxidation currents in sham (n=7) and DOCA-salt (n=6) mesenteric arteries. Sham and DOCA-salt mesenteric arteries were equally sensitive to CTX which inhibited the oxidation currents by a maximum of ~60%. Data are mean ± S.E.M.
CTX inhibits EJPs in mesenteric arteries from Sham and DOCA-salt rats
EJPs evoked using by short trains of focal stimulation were smaller in amplitude in DOCA-salt compared to sham arteries (Fig. 2A,B). CTX (0.001 – 0.1 μM) produced equivalent inhibition of the amplitude of the first EJP in a train of stimulation in sham and DOCA-salt arteries (Fig. 2C). CTX also reduced the amplitude of the 5th EJP in the stimulus train in sham and DOCA-salt arteries and reduced EJP facilitation in sham arteries (Fig. 2B).
Fig. 2.
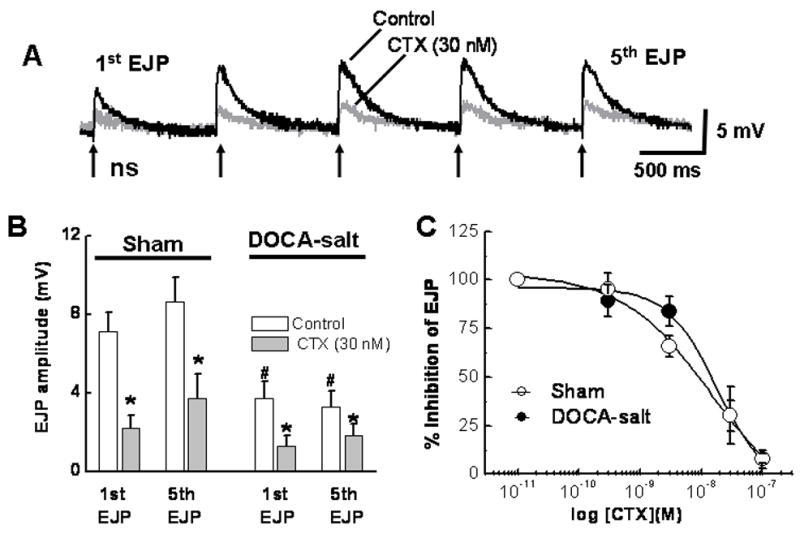
CTX inhibits EJPs. A, Representative recordings of EJPs from mesenteric arteries from sham mesenteric artery in the absence and presence of CTX. EJPs were evoked by a train of 5 stimuli (at the arrows) at 0.5 Hz. CTX inhibited the EJPs. B, CTX inhibits the first and fifth EJP in a train of stimulation (n=6, *P< 0.05). # indicates that EJPs recorded from DOCA-salt arteries were smaller than EJPs in sham arteries (P< 0.05). C, CTX concentration response curves for EJP inhibition are the same in sham (n=6) and DOCA-salt (n=6) arteries (n=6).
ATX increases EJP facilitation
EJPs were elicited using a short train of stimulation (see Fig. 2A) in the absence and presence of ATX (0.01 μM). ATX did not affect the amplitude of the first EJP in the train, but it did increase the amplitude of the 5th EJP in arteries from sham rats (Fig. 3A,B; P < 0.05). EJP facilitation was concentration dependent with a maximum effect occurring at 0.01 μM (Figure 3C). ATX did not affect the amplitude of EJPs recorded from DOCA-salt arteries (Fig. 3B,C).
Fig. 3.
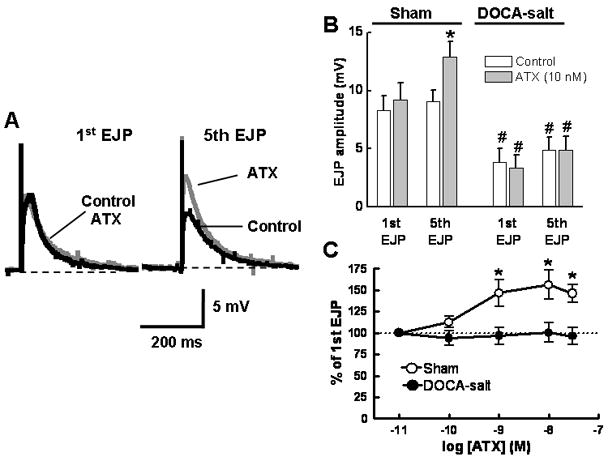
ATX increases EJPs in sham but not DOCA-salt arteries. A, Representative recordings of the first and 5th EJPs evoked by a 0.5 Hz stimulus train in the absence and presence of ATX (10 nM). ATX increased the 5th but not first EJP. B, Pooled data from studies similar to those shown in A. ATX increased the 5th EJP in the train in sham (n=10) but not DOCA-salt (n=6) arteries. *Indicates significantly larger than the first EJP while #indicates significantly smaller than EJPs recorded from sham arteries (P < 0.05). C, Concentration-response curves for ATX-induced facilitation of the 5th EJP in sham (n=12) but not DOCA-salt (n=6) arteries. Data the amplitude of the 5th EJP expressed as percentage of the 1st EJP in a stimulus train. *Indicates significantly different from DOCA-salt arteries. D, ATX inhibits NE oxidation currents in mesenteric arteries from sham (n=6) but not DOCA-salt (n=7) rats. Data are mean ± S.E.M.
It is possible that ATX increases EJP amplitude by inhibiting NE release eliminating negative feedback produced by prejunctional α2AR activation. To test this possibility, NE oxidation currents were measured in the absence and presence of ATX. ATX inhibited NE oxidation currents in arteries from sham but not DOCA-salt rats (Fig. 3D).
P/Q type calcium channel link α2ARs to inhibition of ATP release
EJPs were evoked by short trains of stimulation as described above. The amplitude of the first EJP in the stimulus train was not altered by the α2AR antagonist, yohimbine (1 μM), ATX (0.03 μM), or combined application of yohimbine and ATX (Fig. 4). However, yohimbine, ATX and yohimbine plus ATX increased the amplitude of the 5th EJP in the stimulus train (Fig. 4). EJP amplitudes in the presence of yohimbine, ATX or yohimbine plus ATX were not different from each other suggesting that these drugs acted via a shared mechanism to increase EJP amplitude.
Fig. 4. ATX and yohimbine increase EJP amplitude via a shared mechanism in sham mesenteric arteries.
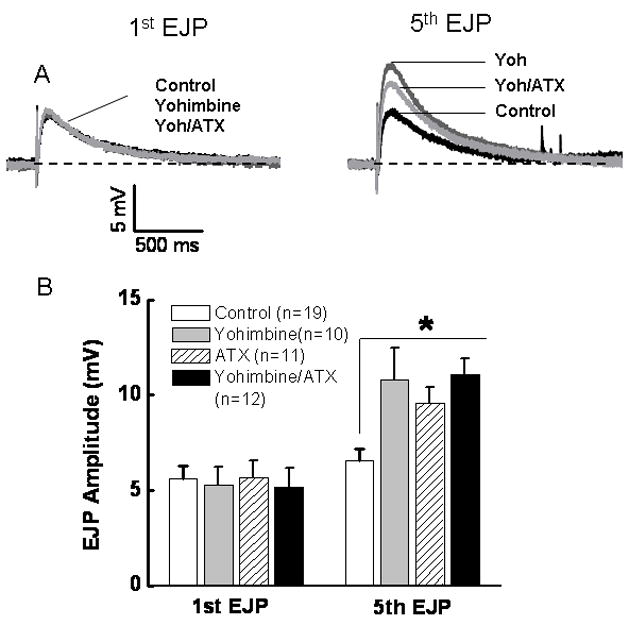
A, Representative recordings of the 1st and 5th EJPs evoked by a 5 pulse train of stimuli at 0.5 Hz. The 1st EJP was unchanged by either drug. The 5th EJP was increased in amplitude by yohimbine (1 μM) and ATX (0.03 μM). The response caused by combined application of both drugs was not different from that caused by either drug alone (P > 0.05). Data are mean ± S.E.M.
DOCA-salt hypertension does not alter calcium sensitivity of purinergic transmission
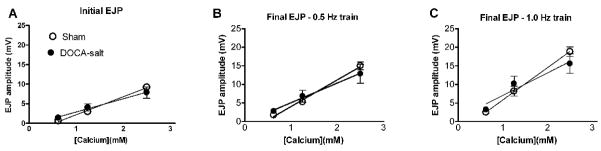
The data presented above indicates that calcium regulation of transmitter release by sympathetic nerves is altered in DOCA-salt hypertension. In order to determine if calcium sensitivity of ATP release was altered we measured EJP amplitude during short trains of stimulation in the presence of different extracellular calcium concentrations (0.625, 1.25 and 2.5 mM). Reducing extracellular calcium also reduced EJP amplitude in a concentration dependent manner. However, there was no difference in calcium sensitivity of EJP amplitude in arteries from sham and DOCA-salt rats (Fig. 5).
Fig. 5. Calcium sensitivity of EJP amplitude is not altered in DOCA-salt hypertension.
Calcium in the physiological buffer was reduced from 2.5–0.625 mM and responses to nerve stimulation were assessed by measuring the first and last EJP amplitude in the presence of yohimbine (1 μM). The responses were similar for control and DOCA-salt animals for the first EJP in a train (A) and 5th EJP in a 0.5 Hz (B) and 1.0 (C) Hz 3 second duration train of stimulation.
Apocynin restores α2-adrenergic receptor regulation of ATP release from periarterial sympathetic nerves of DOCA-salt rats
EJPs were recorded from arteries from untreated and apocynin treated DOCA-salt rats. In the presence of yohimbine, short trains of stimulation (0.5 Hz 10 s) evoked EJPs that did not increase significantly in amplitude in arteries from utreated DOCA-salt rats (Fig. 6). With yohimbine present, EJPs recorded from arteries from apocynin-treated rats showed substantial facilitation (Fig. 6).
Fig. 6. Chronic apocynin treatment restores α2-adrenergic autoreceptor regulation of ATP release from periarterial sympathetic nerves in DOCA-salt hypertension.
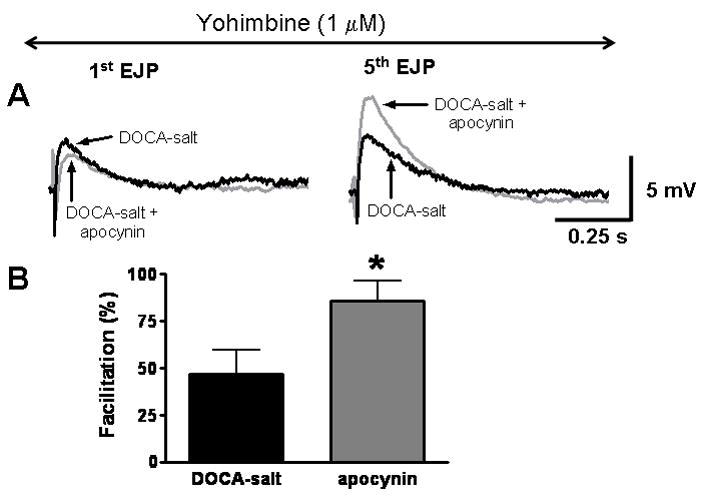
A, The 1st and 5th EJPs evoked by a short train of stimulation (0.5 Hz) in an artery from a DOCA-salt rat. Yohimbine does not cause an increase in EJP amplitude in untreated DOCA-salt tissues but it does increase EJP amplitude in arteries from apocynin treated DOCA-salt rats. B, Apocynin treatment restores yohimbine induced facilitation of EJP amplitude in DOCA-salt arteries (*significantly greate6 than the facilitation in arteries from untreated DOCA-salt rats, P <0.05, Student’s t-test).
Apocynin restores α2-adrenergic receptor regulation of norepinephrine release from periarterial sympathetic nerves of DOCA-salt rats
We also measured norepinephrine oxidation currents from mesenteric arteries of untreated and apocynin treated DOCA-salt rats. In untreated rats, the α2-adrenergic receptor antagonist idazoxan (1 μM) did not change the amplitude of the oxidation currents significantly (Fig. 7A,C). However, idazoxan did increase the norepinephrine oxidation currents in arteries from apocynin treated DOCA-salt rats (Fig. 7B,C).
Fig. 7. Apocynin restores α2-adrenergic receptor regulation of norepinephrine oxidation currents in DOCA-salt hypertension.
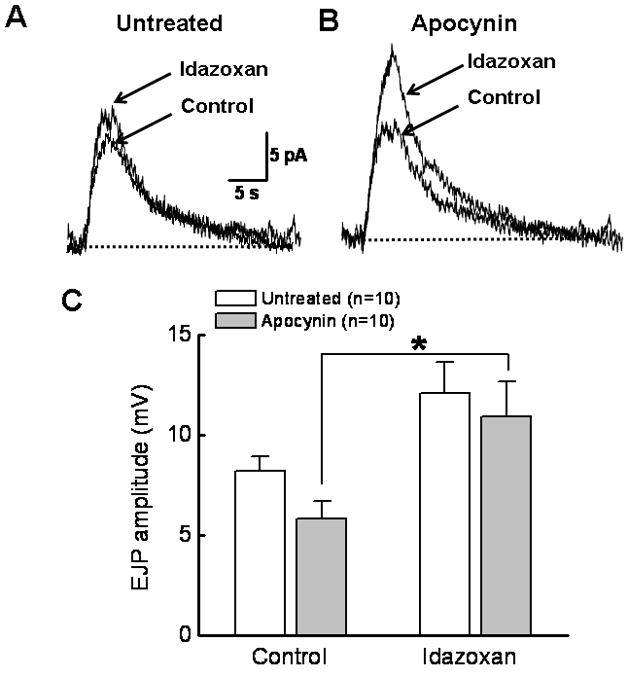
A, The α2 antagonist idazoxan (1 μM) does not change the oxidation current in the untreated artery. B, Idazoxan treatment increases NE oxidation currents in arteries from apocynin treated DOCA-salt rat. C. Pooled data from experiments shown in A and B. *Significantly different from untreated DOCA-salt arteries in the presence of idazoxan (P<0.05, One way ANOVA, Tukey’s multiple comparison).
Apocynin treatment reduced O2− levels in sympathetic ganglia
We verified that apocynin treatment was effective in reducing oxidative stress in sympathetic ganglia by measuring dihyrdoethidium (DHE) induced fluorescence in the inferior mesenteric ganglion from untreated and apocynin treated DOCA-salt rats. DHE induced fluorescence was reduced by about 50% in ganglia from apocynin treated rats (Fig. 8).
Fig. 8. Chronic apocynin treatment reduced O2− in sympathetic ganglia of DOCA-salt rats.
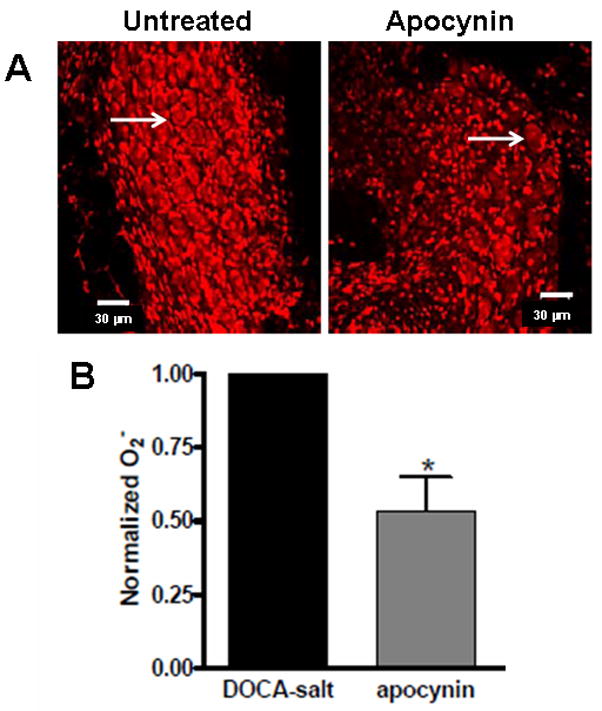
A, Representative photomicrographs of DHE induced fluorescence in sympathetic ganglia from an untreated and an apocynin treated DOCA-salt rat. Arrows indicate examples of sympathetic neurons. Apocynin reduced DHE fluorescence primarily in neurons. B. Semiquantitative analysis of DHE induced fluorescence in untreated and apocynin treated rats. (*Significantly different from untreated DOCA-salt ganglia, P<0.05).
Discussion
The results of these studies indicate that: 1) ATP and norepinephrine release from perivascular sympathetic nerves is differentially regulated by N and P/Q-type calcium channels where ATP release is controlled directly by N-type channels while norepinephrine release is controlled by N and P/Q-type channels; 2) P/Q-type calcium channels may selectively regulate release of a pool of norepinephrine that activates prejunctional α2ARs, this mechanism is disrupted in DOCA-salt hypertension; and 3) oxidative stress may be responsible for disruption of the mechanisms linked to prejunctional α2AR regulation of ATP release from sympathetic nerve in DOCA-salt hypertension.
Previous studies showed that multiple calcium channel subtypes contribute to sympathetic nerve transmission in the mesenteric artery (Tanaka et al., 1999) and that norepinephrine and ATP release from sympathetic nerves may be regulated differently (Dunn et al., 1999; Conceicao et al., 2005; Smyth et al., 2009). These data suggest that norepinephrine and ATP may be stored and released from separate nerve terminals or from separate vesicle populations within the same nerve terminal. Other studies using optical measurements of smooth muscle calcium transients as a measure of P2X receptor activation of smooth muscle cells in the vas deferens suggest that norepinephrine and ATP are co-released from the same nerve terminals and possibly from the same vesicles (Brain, 2009). Our data extend these findings by showing that ATP and norepinephrine may be stored and released differently and also show that there may be multiple pools of norepinephrine that are also differentially regulated by N and P/Q type calcium channels. CTX inhibited norepinephrine oxidation currents equally well in arteries from sham and DOCA-salt rats. These data indicate the N-type calcium channel function is not altered in DOCA-salt hypertension as shown previously (Demel and Galligan, 2008). However, CTX produce a maximum inhibition of 50–60% suggesting that non-N-type calcium channels also contribute to norepinephrine release from periarterial sympathetic nerves. We found that ATX inhibited norepinephrine oxidation currents by 25–30% suggesting that P/Q-type channels contribute to CTX-resistant norepinephrine release. In contrast to N-type calcium channels, P/Q-type calcium channel function or expression is reduced in DOCA-salt hypertension as ATX did not alter norepinephrine oxidation currents in DOCA-salt mesenteric arteries.
Previous work has shown that ATX produces a modest inhibition of EJPs evoked by a train of stimulation in the rat tail artery (Brock and Cunnane, 1999). We found that ATX did not change the amplitude of EJPs evoked by single stimuli in the rat mesenteric artery. It is possible the prejunctional regulation of ATP release in the mesenteric and tail arteries differs or that calcium channel regulation of ATP release caused by single versus multiple action potentials differs. Our data in the mesenteric artery suggest that there is not complete co-storage of norepinephrine and ATP in sympathetic nerves supplying rat mesenteric arteries. There is a co-stored pool of norepinephrine/ATP coupled to N-type calcium channels. This pool may represent a population of vesicles containing both transmitters or two separate populations of vesicles that are closely linked to N-type calcium channels. However, there is a separate pool of norepinephrine-containing vesicles that is selectively coupled to P/Q type calcium channels. Separate stores of norepinephrine and ATP in periarterial sympathetic nerves are also supported by the observation that EJPs recorded from DOCA-salt arteries were smaller in amplitude than those recorded from sham arteries (Demel and Galligan, 2008). This result indicates that DOCA-salt hypertension is associated with impaired ATP storage and/or release in periarterial sympathetic nerves.
The ATX-sensitive release of norepinephrine may be responsible for activation of prejunctional α2ARs that link to autoinhibition of ATP release. This conclusion is based on the data showing that the α2AR antagonist, yohimbine did not alter the first EJP in the stimulus train but it did potentiate the 5th EJP in the stimulus train. Combined application of ATX and yohimbine did not produce an additive increase in EJP amplitude suggesting that these drugs acted via a common mechanism. NE acts at prejunctional α2ARs to modulate both norepinephrine and ATP release from sympathetic nerves (Stjärne and Stjärne, 1995). The first EJP in a stimulus train is not altered by α2AR antagonists because there is no norepinephrine present near the neuroeffector junction to activate α2ARs (Dunn et al., 1999). However, multiple stimuli cause norepinephrine to accumulate near the neuroeffector junction and under these conditions α2ARs are activated and inhibit further norepinephrine and ATP release. Our results support this model as the first EJP in the train was unaffected by yohimbine while the 5th EJP was increased. Our data also indicate that action potential-dependent release of the pool of norepinephrine responsible for activating α2ARs is controlled selectively by P/Q type calcium channels.
Norepinephrine release and overflow are increased in some human hypertensives and in some animal models of hypertension (Bouvier and de Champlain, 1985; Masuyama et al., 1986; Esler et al., 1986). Increased norepinephrine overflow is due in part to impaired prejunctional α2AR function in DOCA-salt hypertension (Luo et al., 2003; Westfall et al., 1987). α2AR agonist-induced suppression of electrically-evoked release of norepinephrine and ATP is reduced in mesenteric arteries from DOCA-salt rats indicating that α2AR levels are reduced or that they do not couple well to inhibition of transmitter release. The data presented here indicate that an additional mechanism may contribute to impaired α2AR function in DOCA-salt hypertension. In normotensive rats, P/Q type calcium channels couple to the release of a pool of norepinephrine-containing vesicles which in turn activates α2ARs to inhibit norepinephrine and ATP release. However in DOCA-salt rats, P/Q channel expression or function is reduced and these channels no longer couple to modulation of norepinephrine release. This would account for reduced α2AR function in sympathetic nerves supplying mesenteric arteries of DOCA-salt rats as reported previously (Moreau et al., 1995; Luo et al., 2003). However, the loss of α2AR function should be associated with an increase in EJP amplitude and ATP release. This paradox may be explained by the decreased ATP availability in the nerve terminal as EJP amplitude is reduced in mesenteric arteries from DOCA-salt rats (Demel and Galligan, 2008). Another point to consider when discussing different mechanisms controlling norepinephrine and ATP release is the different stimulation parameters that were used. EJPs were evoked with single stimuli or short trains of low frequency stimuli (5 pulses at 0.5 Hz). Norepinephrine oxidation currents were evoked with longer trains (3 s) of high frequency stimulation (10 s). The different stimulation parameters may recruit different sets of nerve terminal calcium channels and alter sensitivity ATP or norepinephrine release to blockade by different calcium channel antagonists.
Oxidative stress, particularly O2− is elevated in sympathetic nerves and mesenteric arteries of DOCA-salt hypertensive rats. While O2− can be generated from a number of sources in DOCA-salt rats (Callera et al., 2006), NADPH oxidase is one source (Li et al., 2003). In addition, we have shown recently that p22phox and p47phox, two NADPH oxidase subunits are localized to sympathetic nerve endings associated with mesenteric arteries (Cao et al., 2009). Apocynin is an antioxidant drug that can inhibitor of NADPH oxidase and it can also inhibit other O2− generating enzymes and it may directly quench O2− (Williams and Griendling, 2007) which is an enzyme that produces O2−. In this study we tested the hypothesis that O2− may be responsible for disruption of sympathetic neurotransmission to mesenteric arteries in DOCA-salt hypertension by treating DOCA-salt rats chronically with apocynin. Apocynin treatment reduced O2− levels in sympathetic ganglia as shown by reduced DHE-induced fluorescence. This was associated with an improvement in prejunctional α2-adrenergic receptor regulation of ATP release. The molecular targets of O2− in the sympathetic nerve terminal are unclear. This target does not appear to be the calcium channels as calcium sensitivity of ATP release was not altered in DOCA-salt rats. Other potential targets include G-proteins linking α2-adrenergic receptors to calcium channels (Marcil et al., 1998), vesicular stores of ATP or perhaps the SNARE proteins that mediate synaptic vesicle docking, fusion and recycling during bursts of nerve terminal activity (Keating, 2008).
Summary and Conclusion
These data indicate that N and P/Q type calcium channels couple to norepinephrine release from sympathetic nerves supplying rat mesenteric arteries. Only N-type calcium channels couple to ATP release. The pool of norepinephrine coupled to P/Q type calcium channels is linked to α2AR regulation of ATP release from periarterial sympathetic nerves. These data also indicate that there may be separate stores of norepinephrine and ATP in sympathetic nerve terminals and these stores are coupled differently to nerve terminal calcium channel subtypes. DOCA-salt hypertension is associated with a loss of P/Q- but not N-type, calcium channel function and a disruption in α2AR mediated regulation of ATP release. Disruption in α2AR function is caused by oxidative stress, most likely elevated O2− in or near sympathetic nerve endings in the arterial wall. These studies highlight the complex regulation of vasoconstrictor transmitters from periarterial sympathetic nerves. They also indicate that P/Q type calcium channels and α2ARs are molecular targets for the pathophysiological changes in sympathetic nerve function known to occur in salt-sensitive hypertension.
Acknowledgments
This work was supported by P01HL070687 and R01HL84258 from the National Institutes of Health. SLD was supported by an American Heart Association predoctoral fellowship (0615576Z).
List of abbreviations
- α2AR
alpha2 adrenergic receptor
- ATP
adenosine 5′-triphosphate
- ATX
ω-agatoxin IVA
- CTX
ω-conotoxin GVIA
- DOCA
deoxycorticosterone acetate
- EJP
excitatory junction potential
- KCl
potassium chloride
- MA
mesenteric artery
- NADPH oxidase
nicotinamide adenine dinucleotide phosphate-oxidase
- NE
norepinephrine
- NPY
neuropeptide Y
- TsTX-I
Tityus serrulatus toxin I
- TTX
tetrodotoxin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bouvier M, de Champlain J. Increased apparent norepinephrine release rate in anesthetized DOCA-salt hypertensive rats. Clin Exp Hypertens A. 1985;7:1629–1645. doi: 10.3109/10641968509073614. [DOI] [PubMed] [Google Scholar]
- Brain KL. Neuroeffector Ca2+ transients for the direct measurement of purine release and indirect measurement of co-transmitters in rodents. Exp Physiol. 2009;94:25–30. doi: 10.1113/expphysiol.2008.043679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock JA, Cunnane TC. Effects of Ca2+ concentration and Ca2+ channel blockers on noradrenaline release and purinergic neuroeffector transmission in rat tail artery. Br J Pharmacol. 1999;126:11–18. doi: 10.1038/sj.bjp.0702256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock JA, Van Helden DF. Enhanced excitatory junction potentials in mesenteric arteries from spontaneously hypertensive rats. Pflugers Arch. 1995;430:901–908. doi: 10.1007/BF01837403. [DOI] [PubMed] [Google Scholar]
- Brock JA, Tan JH. Selective modulation of noradrenaline release by alpha2-adrenoceptor blockade in the rat-tail artery in vitro. Br J Pharmacol. 2004;142:267–274. doi: 10.1038/sj.bjp.0705779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock JA, Cunnane TC, Evans RJ, Ziogas J. Inhibition of transmitter release from sympathetic nerve endings by omega-conotoxin. Clin Exp Pharmacol Physiol 1989. 1996;16:333–339. doi: 10.1111/j.1440-1681.1989.tb01568.x. [DOI] [PubMed] [Google Scholar]
- Callera GE, Tostes RC, Yogi A, Montezano AC, Touyz RM. Endothelin-1-induced oxidative stress in DOCA-salt hypertension involves NADPH-oxidase-independent mechanisms. Clin Sci (Lond) 2006;110:243–53. doi: 10.1042/CS20050307. [DOI] [PubMed] [Google Scholar]
- Cao X, Demel SL, Quinn MT, Galligan JJ, Kreulen D. Localization of NADPH oxidase in sympathetic and sensory ganglion neurons and perivascular nerve fibers. Auton Neurosci. 2009;151:90–97. doi: 10.1016/j.autneu.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conceicao I, Jurkiewicz A, Fonseca D, Opperman AR, Freitas TA, Lebrun I, Garcez-do-Carmo L. Selective release of ATP from sympathetic nerves of rat vas deferens by the toxin TsTX-I from Brazilian scorpion Tityus serrulatus. Br J Pharmacol. 2005;144:519–527. doi: 10.1038/sj.bjp.0706062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai X, Galligan JJ, Watts SW, Fink GD, Kreulen DL. Increased O2−- production and upregulation of ETB receptors by sympathetic neurons in DOCA-salt hypertensive rats. Hypertension. 2004;43:1048–54. doi: 10.1161/01.HYP.0000126068.27125.42. [DOI] [PubMed] [Google Scholar]
- Demel SL, Galligan JJ. Impaired purinergic neurotransmission to mesenteric arteries in deoxycorticosterone acetate-salt hypertensive rats. Hypertension. 2008;52:322–329. doi: 10.1161/HYPERTENSIONAHA.108.110353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoso MV, Steiner M, Huidobro-Toro JP. BIBP 3226, suramin and prazosin identify neuropeptide Y, adenosine 5′-triphosphate and noradrenaline as sympathetic cotransmitters in the rat arterial mesenteric bed. J Pharmacol Exp Ther. 1997;282:691–698. [PubMed] [Google Scholar]
- Driessen B, von Kugelgen I, Starke K. Neural ATP release and its alpha 2-adrenoceptor-mediated modulation in guinea-pig vas deferens. Naunyn Schmiedebergs Arch Pharmacol. 1993;348:358–366. doi: 10.1007/BF00171334. [DOI] [PubMed] [Google Scholar]
- Dunn WR, Brock JA, Hardy TA. Electrochemical and electrophysiological characterization of neurotransmitter release from sympathetic nerves supplying rat mesenteric arteries. Br J Pharmacol. 1999;128:174–180. doi: 10.1038/sj.bjp.0702760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esler M, Jennings G, Biviano B, Lambert G, Hasking G. Mechanism of elevated plasma noradrenaline in the course of essential hypertension. J Cardiovasc Pharmacol. 1986;8(Suppl 5):S39–43. doi: 10.1097/00005344-198608005-00008. [DOI] [PubMed] [Google Scholar]
- Gitterman DP, Evans RJ. Nerve evoked P2X receptor contractions of rat mesenteric arteries; dependence on vessel size and lack of role of L-type calcium channels and calcium induced calcium release. Br J Pharmacol. 2001;132:1201–1208. doi: 10.1038/sj.bjp.0703925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill CE, Powis DA, Hendry IA. Involvement of pertussis toxin-sensitive and -insensitive mechanisms in alpha-adrenoceptor modulation of noradrenaline release from rat sympathetic neurones in tissue culture. Br J Pharmacol. 1993;110:281–288. doi: 10.1111/j.1476-5381.1993.tb13806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iriuchijima J, Mizogami S, Sokabe H. Sympathetic nervous activity in renal and DOCA hypertensive rats. Jpn Heart J. 1975;16:36–43. doi: 10.1536/ihj.16.36. [DOI] [PubMed] [Google Scholar]
- Keating DJ. Mitochondrial dysfunction, oxidative stress, regulation of exocytosis and their relevance to neurodegenerative diseases. J Neurochem. 2008;104:298–305. doi: 10.1111/j.1471-4159.2007.04997.x. [DOI] [PubMed] [Google Scholar]
- King AJ, Osborn JW, Fink GD. Splanchnic circulation is a critical neural target in angiotensin II salt hypertension in rats. Hypertension. 2007;50:547–556. doi: 10.1161/HYPERTENSIONAHA.107.090696. [DOI] [PubMed] [Google Scholar]
- Li L, Fink GD, Watts SW, Northcott CA, Galligan JJ, Pagano PJ, Chen AF. Endothelin-1 increases vascular superoxide via endothelin(A)-NADPH oxidase pathway in low-renin hypertension. Circulation. 2003;107:1053–8. doi: 10.1161/01.cir.0000051459.74466.46. [DOI] [PubMed] [Google Scholar]
- Luo M, Hess MC, Fink GD, Olson LK, Rogers J, Kreulen DL, Dai X, Galligan JJ. Differential alterations in sympathetic neurotransmission in mesenteric arteries and veins in DOCA-salt hypertensive rats. Auton Neurosci. 2003;104:47–57. doi: 10.1016/S1566-0702(02)00287-4. [DOI] [PubMed] [Google Scholar]
- Marcil J, de Champlain J, Anand-Srivastava MB. Overexpression of Gi-proteins precedes the development of DOCA-salt-induced hypertension: relationship with adenylyl cyclase. Cardiovasc Res. 1998;39:492–505. doi: 10.1016/s0008-6363(98)00111-4. [DOI] [PubMed] [Google Scholar]
- Masuyama Y, Tsuda K, Kuchii M, Nishio I. Peripheral neural mechanism of hypertension in rat models--peripheral sympathetic neurotransmission in hypertension. J Hypertens. 1986;4(Suppl 4):S189–192. [PubMed] [Google Scholar]
- Mathias CJ. Role of sympathetic efferent nerves in blood pressure regulation and in hypertension. Hypertension. 1991;18(5 Suppl):III22–30. doi: 10.1161/01.hyp.18.5_suppl.iii22. [DOI] [PubMed] [Google Scholar]
- Moreau P, Drolet G, Yamaguchi N, de Champlain J. Alteration of prejunctional alpha 2-adrenergic autoinhibition in DOCA-salt hypertension. Am J Hypertens. 1995;8:287–293. doi: 10.1016/0895-7061(94)00211-s. [DOI] [PubMed] [Google Scholar]; Hypertension. 18:III22–30. doi: 10.1161/01.hyp.18.5_suppl.iii22. [DOI] [PubMed] [Google Scholar]
- Morikawa T, Tanaka N, Kubota Y, Mizuno H, Nakamura K, Kunitomo M, Shinozuka K. ATP modulates the release of noradrenaline through two different prejunctional receptors on the adrenergic nerves of rat prostate. Clin Exp Pharmacol Physiol. 2007;34:601–605. doi: 10.1111/j.1440-1681.2007.04627.x. [DOI] [PubMed] [Google Scholar]
- Msghina M, Gonon F, Stjarne L. Paired pulse analysis of ATP and noradrenaline release from sympathetic nerves of rat tail artery and mouse vas deferens: effects of K+ channel blockers. Br J Pharmacol. 1998;125:1669–1676. doi: 10.1038/sj.bjp.0702246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Msghina M, Mermet C, Gonon F, Stjarne L. Electrophysiological and electrochemical analysis of the secretion of ATP and noradrenaline from the sympathetic nerves in rat tail artery: effects of alpha 2-adrenoceptor agonists and antagonists and noradrenaline reuptake blockers. Naunyn Schmiedeberg’s Arch Pharmacol. 1992;346:173–186. doi: 10.1007/BF00165299. [DOI] [PubMed] [Google Scholar]
- Park J, Galligan JJ, Fink GD, Swain GM. Differences in sympathetic neuroeffector transmission to rat mesenteric arteries and veins as probed by in vitro continuous amperometry and video imaging. J Physiol. 2007;584:819–834. doi: 10.1113/jphysiol.2007.134338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruneau D, Angus JA. Omega-conotoxin GVIA is a potent inhibitor of sympathetic neurogenic responses in rat small mesenteric arteries. Br J Pharmacol. 1990;100:180–184. doi: 10.1111/j.1476-5381.1990.tb12073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rummery NM, Brock JA, Pakdeechote P, Ralevic V, Dunn WR. ATP is the predominant sympathetic neurotransmitter in rat mesenteric arteries at high pressure. J Physiol. 2007;582:745–754. doi: 10.1113/jphysiol.2007.134825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth LM, Yamboliev IA, Mutafova-Yambolieva VN. N-type and P/Q-type calcium channels regulate differentially the release of noradrenaline, ATP and β-NAD in blood vessels. Neuropharmacology. 2009;56:368–78. doi: 10.1016/j.neuropharm.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stjärne L, Stjärne E. Geometry, kinetics and plasticity of release and clearance of ATP and noradrenaline as sympathetic co-transmitters: roles for the neurogenic contraction. Prog Neurobiol. 1995;47:45–94. doi: 10.1016/0301-0082(95)00018-q. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Mochizuki Y, Tanaka H, Shigenobu K. Significant role of neuronal non-N-type calcium channels in the sympathetic neurogenic contraction of rat mesenteric artery. Br J Pharmacol. 1999;128:1602–1608. doi: 10.1038/sj.bjp.0702954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorov LD, Mihaylova-Todorova ST, Bjur RA, Westfall DP. Differential co-transmission in sympathetic nerves: role of frequency of stimulation and prejunctional autoreceptors. J Pharmacol Exp Ther. 1999;290:241–246. [PubMed] [Google Scholar]
- Tsuda K, Tsuda S, Nishio I, Masuyama Y. Inhibition of norepinephrine release by presynaptic alpha 2-adrenoceptors in mesenteric vasculature preparations from chronic DOCA-salt hypertensive rats. Jpn Heart J. 1989;30:231–239. doi: 10.1536/ihj.30.231. [DOI] [PubMed] [Google Scholar]
- Waterman SA. Multiple subtypes of voltage-gated calcium channel mediate transmitter release from parasympathetic neurons in the mouse bladder. J Neurosci. 1996;16:4155–4161. doi: 10.1523/JNEUROSCI.16-13-04155.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterman SA. Role of N-, P- and Q-type voltage-gated calcium channels in transmitter release from sympathetic neurones in the mouse isolated vas deferens. Br J Pharmacol. 1997;120:393–398. doi: 10.1038/sj.bjp.0700948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westfall TC, Meldrum MJ, Carpentier S, Naes L, Zhang SQ. Alterations in the release of norepinephrine at the vascular neuroeffector junction in hypertension. Blood Vessels. 1987;24:94–99. doi: 10.1159/000158677. [DOI] [PubMed] [Google Scholar]
- Westfall DP, Todorov LD, Mihaylova-Todorova ST. ATP as a cotransmitter in sympathetic nerves and its inactivation by releasable enzymes. J Pharmacol Exp Ther. 2002;303:439–444. doi: 10.1124/jpet.102.035113. [DOI] [PubMed] [Google Scholar]
- Williams HC, Griendling KK. NADPH oxidase inhibitors: new antihypertensive agents? J Cardiovasc Pharmacol. 2007;50:9–16. doi: 10.1097/FJC.0b013e318063e820. [DOI] [PubMed] [Google Scholar]
- Wright CE, Angus JA. Effects of N-, P- and Q-type neuronal calcium channel antagonists on mammalian peripheral neurotransmission. Br J Pharmacol. 119:49–56. doi: 10.1111/j.1476-5381.1996.tb15676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XP, Chiba S. Effects of omega-conotoxin GVIA and diltiazem on double peaked vasoconstrictor responses to periarterial electric nerve stimulation in isolated canine splenic artery. Br J Pharmacol. 2000;129:47–52. doi: 10.1038/sj.bjp.0702989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Ikeda SR. Adenosine modulates voltage-gated Ca2+ channels in adult rat sympathetic neurons. J Neurophysiol. 1993;70:610–620. doi: 10.1152/jn.1993.70.2.610. [DOI] [PubMed] [Google Scholar]