Abstract
Predominance of right-handedness has historically been considered as a hallmark of human evolution. Whether nonhuman primates exhibit population-level manual bias remains a controversial topic. Here we investigated the hypothesis that bimanual coordinated activities may be a key-behavior in our ancestors for the emergence and evolution of human population-level right-handedness. To this end, we collected data on hand preferences in 35 captive gorillas (Gorilla gorilla) during simple unimanual reaching and for bimanual coordinated feeding. Unimanual reaching consisted of grasping food on the ground while bimanual feeding consisted of using one hand for holding a food and processing the food item by the opposite hand. No population-level manual bias was found for unimanual actions but, in contrast, gorillas exhibited a significant population-level right-handedness for the bimanual actions. Moreover, the degree of right-handedness for bimanual feeding exceeds any other known reports of hand use in primates, suggesting that lateralization for bimanual feeding is robust in captive gorillas. The collective evidence is discussed in the context of potential continuity of handedness between human and nonhuman primates.
Keywords: handedness, hemispheric specialization, unimanual reaching, bimanual coordination, primates
A universal human behavioral trait is right-handedness (Perelle and Ehrman, 1994; Annett, 2002; Raymond and Pontier, 2004). Although there is some variation between different cultures, all human populations studied to date have been shown to display right hand preferences, particularly for complex motor actions (e.g., Fagard, 2001). Moreover, the archeological data suggest that right-handedness in tool use can be dated back at least 2.5 millions years ago (Corballis, 1991; Bradshaw and Rogers, 1993). Whether evidence of handedness can be dated back even further in Hominid evolution, particularly in our closest living relative, the great apes, remains a topic of intense debate (McGrew and Marchant, 1997; Hopkins, 2006).
Whereas historically population-level behavioral and hemispheric specialization have been considered hallmarks of human evolution (Warren, 1980; Ettlinger, 1988; Crow, 2004), there is a growing body of evidence of population-level behavioral and brain asymmetries in a host of vertebrates (Rogers and Andrew, 2002; Vallortigara and Rogers, 2005; Hopkins, 2007). For example, population-level limb preferences for motor actions have been found in some species of toads, rats and dogs, suggesting some phylogenetic continuity between animal species (Hook, 2004 for review). However, not all species show the same patterns and some have been critical of both the methods and interpretation of results in nonprimate species with respect to evolutionary models of handedness (MacNeilage et al., 1987; Crow, 2004). It is in this regard that studies of handedness in nonhuman primates have become increasingly important for testing and evaluating different evolutionary and genetic models of handedness (Hopkins, 2004, 2007; Vauclair and Meguerditchian, 2007).
Indeed, there is a growing body of evidence showing a predominance of right-handedness in nonhuman primates, particularly captive chimpanzees, for complex manual tasks such as bimanual feeding, coordinated bimanual actions, bipedal reaching, throwing, and gestural communication, etc. (for review, see Hopkins, 2006, 2007). However, some authors remain skeptical of these findings on both methodological and theoretical grounds (McGrew and Marchant, 1997; Palmer, 2002, 2003; Crow, 2004). For example, on the basis of some reviews of nonhuman primate handedness, some authors have argued that the overall results are inconsistent, particularly in reference to results obtained in wild compared to captive individuals (McGrew and Marchant, 1997; Papademetriou et al., 2005 for reviews). In fact, it has even been argued that right-handedness in captive chimpanzees is an artifact of being raised in a human environment (e.g., McGrew and Marchant, 1997). However, others have argued that inconsistent patterns of results, particularly between wild and captive settings, may reflect differences in the behavior measured, statistical analysis of the results and statistical power (Hopkins, 1999; Hopkins and Cantalupo, 2005). For instance, many studies of handedness focus on simple manual actions, such as reaching for food, a task that fails to elicit handedness at the individual level and therefore is likely a poor measure of handedness. When looking closely at the literature, the available studies in wild populations that have failed to report population manual bias have largely recorded simple measures of hand use, such as unimanual reaching (e.g. in chimpanzees: Marchant and McGrew, 1996; McGrew and Marchant, 1997, 2001) in relative small samples. In captive populations, it appears that the reports of population-level handedness have typically measured more complex manual activities such as bimanual coordinated actions (Hopkins, 2007). In contrast, within the same populations, simple behavioral measures of hand preferences (similar than the ones used in the field such as unimanual reaching) have usually revealed an absence or weaker right-handedness bias than bimanual tasks in humans (Fagard and Marks, 2000), chimpanzees (Hopkins, 1993, 1995), baboons (Vauclair et al., 2005) and capuchin monkeys (Spinozzi et al., 1998). Also, there is a large body of evidence of the effect of the task complexity on the direction, magnitude and consistency of the individual hand preferences in humans (e.g., Perelle and Ehrman, 1994; Marchant et al. 1995; Fagard, 2001), great apes (Boesch, 1991; McGrew, et al., 1999; O’Malley and McGrew, 2006; Hopkins, 2007) and monkeys (Fagot and Vauclair, 1988, 1991; Spinozzi et al., 1998; Blois-Heulin et al., 2006; Lilak and Phillips, 2007; Meunier and Vauclair, 2007; Schweitzer et al., 2007). Collectively, these results indicate that complex bimanual actions appear to be more sensitive for detecting individual differences in handedness than less complex tasks, suggesting that they are more appropriate for investigating manual asymmetries. Consequently, the contrast of results between wild versus captive samples seems less clear than the contrast between low-level tasks (e.g. unimanual reaching) versus high-level tasks (e.g. bimanual coordinated action). Thus the “task effect” may be a relevant factor for reconciling the contradictory reports of handedness in the literature from wild and captive populations of primates (Hopkins, 2006; Vauclair and Meguerditchian, 2007).
Like studies in chimpanzees and other nonhuman primates, whether gorillas exhibit population-level right-handedness is still unclear and the findings in this species are not entirely consistent (see Hopkins and Morris, 1993; McGrew and Marchant, 1993 for reviews). As suggested above, this may be explained by the fact that (a) the type of measures of hand preferences used in these studies are not consistent across the literature, (b) only a few studies have investigated complex bimanual activities and (c) most of the studies had a very small sample of subjects that limits considerably the interpretation and determination of population-level handedness. Interestingly, whereas simple unimanual actions fail to elicit population-level handedness in gorillas (Fagot and Vauclair, 1988; Annett and Annett, 1991; Byrne and Byrne, 1991; but see Shafer, 1993), significant population-level right-handedness has been reported for bimanual food processing in wild gorillas (Byrne and Byrne, 1991) and a trend toward right-hand bias has been found for a coordinated bimanual tube task which consists of removing food with one hand from inside a PVC tube while holding it with the opposite hand in captive individuals (Hopkins et al., 2003; Begg-Reid and Schillaci, 2008). In fact, regarding the importance of bimanual activities in the feeding ecology of the gorillas (Byrne and Byrne, 1993), one would hypothesize that these manual actions would elicit significant biases at the individual and potentially the population-level.
To investigate whether bimanual coordinated activities may potentially constitute a key behavior for the measurement and assessment of handedness in gorillas, as has been reported in other primates, we studied the hand preferences for two spontaneous manual behaviors including simple unimanual reaching and bimanual coordinated feeding.
METHOD
Subjects
Data were collected on 35 captive gorillas (Gorilla gorilla) including 20 females and 15 males ranging in age from 1 to 48 years (Mean = 17.31, S.E. = 2.23). Twenty-three of the gorillas (12 females, 11 males) were housed at Zoo Atlanta located in Atlanta, Georgia and were living in 6 social groups ranging from 3 to 9 individuals. Twelve of the gorillas (8 females, 4 males) were housed at Lincoln Park Zoo (LPZ) and were living in two social groups. This sample constituted the total number of observed subjects but it should be noted that the sample size varied slightly according to the manual behaviors investigated.
Procedure
From September 2007 to July 2009, we opportunistically recorded hand use for both unimanual and bimanual behaviors by simple daily observations of the social groups of gorillas, particularly during feeding times but also during other daily activities. At both zoos, the observers chose opportunistically a subject when they were performing the manual behaviors of interest. If multiple gorillas were feeding, the experimenter(s) observed the subject which was located in the most visible area of the enclosure and for which the fewest data points were available because some gorillas foraged more frequently than others. In order to obtain a reasonable numbers of observations for each subject and increase our overall sample size, a concerted effort was made to focus on subjects that had the fewest observations whenever possible.
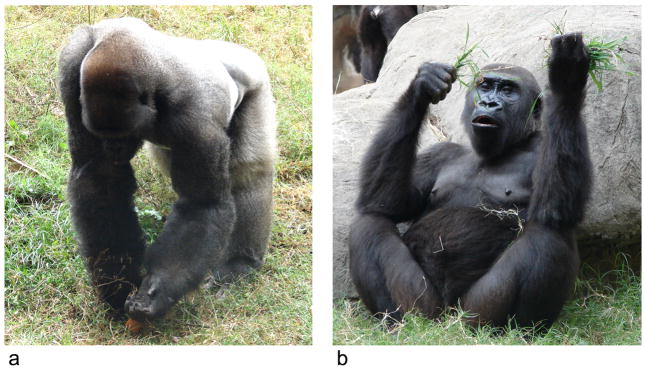
At Zoo Atlanta (ZA), hand use for unimanual reaching (Fig. 1a) was recorded when the observer saw the gorillas reaching for grass or for food on the ground (vegetables, fruits). This was often observed during feeding times when food was scattered in the outdoor viewing area at the zoo. In order to minimize postural biases in the choice of the hand, to be considered a valid reaching response, the subject had to be in a symmetrical posture, either seated or quadrupedal, with both hands available and able to grasp the food in front of them. A single unimanual response was recorded for a reaching response and the subjects had to reposition themselves and move to another location between reaching responses in order to obtain discrete responses. At LPZ, the criterion for recording unimanual reaching responses was identical to those used at ZA; however, at LPZ small food items such a raisins or peanuts were scattered throughout the subjects’ outdoor enclosure and the gorillas would move to different locations in the enclosure to grasp the food item. The experimenter would record their hand use as left or right during each discrete reaching response.
Fig. 1.
a. Unimanual reaching. A male gorilla reaches his left hand in other to grasp food on the group. b. Bimanual feeding. A female gorilla accumulated grass in the left hand and use the right hand for picking up the grass and put it in mouth.
(A color version of this figure can be found in the on-line version of the paper).
Bimanual feeding is a coordinated bimanual action frequently observed in gorillas when eating (Fig. 1b). When food (grass, bamboo, fruits, vegetables, seeds…) is held or accumulated in one hand (referred to the “frame” hand), bimanual feeding consists of using the opposite hand to pick up the food or extract pieces of food in order to manipulate it or bring it in mouth. At both AZ and LPZ, when a gorilla within a group performed bimanual feeding, the “active” hand for manipulating the food was considered and recorded as the dominant hand use (either left or right).
There is some debate in the literature about whether bouts or frequencies of hand use, such as those during feeding actions, constitute the best measure of hand preferences (McGrew and Marchant, 1997; Hopkins et al., 2001, 2005). Basically, some authors have criticized reports of handedness in nonhuman primates that recorded only frequencies in hand use (McGrew and Marchant, 1997). They pointed that a statistical bias may result from the possible dependence of the data between each hand use response. Bimanual feeding is one circumstance that applies to this circumstance because the subjects typically are holding multiple food items in the subordinate hand while feeding on each food item with the opposite hand.
We addressed this issue by recording handedness for both bouts and frequency in hand use within a feeding event. Specifically, concerning the measure of the frequencies, hand use for each of the “pick up the food” responses was recorded within or between bouts of bimanual feeding. With respect to the measures of the bouts, a single bout was considered when a subject started a sequence of bimanual feeding after accumulating food in one hand (the “frame” hand). Then, when the subject used the opposite hand (referred to the “active” hand) without interruption, the whole sequence was considered as one single response of hand use regardless of the number of frequencies for “picking up the food” with the active hand. The bout was considered as finished when the subject stopped the sequence or switched the hands for bimanual feeding. In this latter case, another bout was counted for the following sequence.
Data analysis
For each category of manual action (i.e., unimanual and bimanual feeding), we used the following statistics for analysis of the data. First, the direction of hand preference for each subject was determined by calculating an individual z-score on the basis of their total left and right hand responses. Then, based on their z-score, the individual gorillas were classified as left-handed (z≤– 1.96), right-handed (z≥1.96) or ambiguously handed (−1.96 < z < 1.96). Second, the degree of hand asymmetries for a given subject was evaluated by calculating an individual handedness index score (HI) using the formula (R−L)/(R + L), where R and L represent the total right and left hand responses, respectively. The HI values varied on a continuum from −1.0 to 1.0 and the sign indicates the direction of hand preferences (positive = right hand preference; negative = left hand preference). The absolute values (ABS-HI) reflect the strength of individual hand preference. Concerning bimanual feeding, these statistical analyses were used for each type of hand use measure, i.e., bouts and frequencies.
RESULTS
Descriptive information
The frequencies of left- and right- hand use for unimanual reaching and bimanual feeding as well as associated HI and z-scores are shown in Table 1. For each of the behaviors, we only included subjects in the final analysis that produced a minimum of 15 responses. After excluding any subjects that did not produce at least 15 responses, 2682 responses were made from 32 subjects (the number of observations per subject varied from 15 to 186 responses, M = 83.81, S.E. = 8.17). Concerning bimanual feeding, including only those apes that had 8 or more bouts of feeding, 833 bouts have been recorded from 32 subjects (the number of bouts per subject varied from 8 to 51 responses, M = 26.03, S.E. = 1.92). With respect to the measures of frequencies of bimanual feeding, 3074 were included in the final analysis from the 32 gorillas who performed a minimum of 15 responses (the number of observations per subject varied from 23 to 299 responses, M = 96.06, S.E. = 12.44).
TABLE 1.
Individual hand preferences and frequencies of left- and right- hand responses for unimanual reaching and bimanual feeding.
| Bimanual Feeding | Unimanual Reaching | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subject | age | sex | # L | # R | HI | z | Hand | # L | # R | HI | z | Hand |
| Kudzoo | 14 | F | 48 | 251 | 0.68 | 11.74 | R | 75 | 109 | 0.18 | 2.51 | R |
| Sukari | 9 | F | 17 | 199 | 0.84 | 12.38 | R | 67 | 92 | 0.16 | 1.98 | R |
| Kiniani | 24 | F | 12 | 60 | 0.67 | 5.66 | R | 75 | 65 | −0.07 | −0.85 | A |
| Olympia | 11 | F | 64 | 140 | 0.37 | 5.32 | R | 103 | 27 | −0.58 | −6.67 | L |
| Choomba | 44 | F | 13 | 79 | 0.72 | 6.88 | R | 60 | 54 | −0.05 | −0.56 | A |
| Lulu | 8 | F | 39 | 140 | 0.56 | 7.55 | R | 57 | 52 | −0.05 | −0.48 | A |
| Kashata | 14 | F | 6 | 40 | 0.74 | 5.01 | R | 49 | 53 | 0.04 | 0.40 | A |
| Kuchi | 23 | F | 25 | 32 | 0.12 | 0.93 | A | 42 | 42 | 0.00 | 0.00 | A |
| Machi | 31 | F | 29 | 65 | 0.38 | 3.71 | R | 29 | 43 | 0.19 | 1.65 | A |
| Shamba | 48 | F | 1 | 22 | 0.91 | 4.38 | R | 15 | 17 | 0.06 | 0.35 | A |
| Macy B | 2 | F | 26 | 18 | −0.18 | −1.21 | A | 6 | 10 | 0.25 | 1.00 | A |
| Kali | 2 | F | 1 | 4 | - | - | - | 6 | 8 | - | - | - |
| Susie | 4 | F | 47 | 14 | −0.54 | −4.23 | L | 30 | 62 | 0.35 | 3.34 | R |
| Madini | 13 | F | 28 | 41 | 0.19 | 1.57 | A | 31 | 15 | −0.35 | −2.36 | L |
| Bulera | 20 | F | 16 | 46 | 0.48 | 3.81 | R | 29 | 40 | 0.16 | 1.32 | A |
| Bahati | 18 | F | 22 | 38 | 0.27 | 2.07 | R | 31 | 30 | −0.02 | −0.13 | A |
| Kowali | 31 | F | 13 | 53 | 0.61 | 4.92 | R | 21 | 32 | 0.21 | 1.51 | A |
| Makari | 22 | F | 14 | 53 | 0.58 | 4.76 | R | 6 | 59 | 0.82 | 6.57 | R |
| Tabibu | 17 | F | 0 | 63 | 1.00 | 7.94 | R | 6 | 16 | 0.45 | 2.13 | R |
| Rollie | 12 | F | 5 | 73 | 0.87 | 7.70 | R | 18 | 34 | 0.31 | 2.22 | R |
| Charlie | 11 | M | 40 | 115 | 0.48 | 6.02 | R | 95 | 91 | −0.02 | −0.29 | A |
| Taz | 18 | M | 56 | 24 | −0.40 | −3.58 | L | 67 | 79 | 0.08 | 0.99 | A |
| Stadi | 16 | M | 139 | 128 | −0.04 | −0.67 | A | 86 | 42 | −0.34 | −3.89 | L |
| Ivan | 45 | M | 0 | 0 | - | - | - | 56 | 47 | −0.09 | −0.89 | A |
| Kekla | 18 | M | 69 | 129 | 0.30 | 4.26 | R | 39 | 50 | 0.12 | 1.17 | A |
| M’bely | 5 | M | 3 | 49 | 0.88 | 6.38 | R | 37 | 48 | 0.13 | 1.19 | A |
| Kidogo | 9 | M | 5 | 30 | 0.71 | 4.23 | R | 7.00 | 38 | 0.69 | 4.62 | R |
| Jasiri | 9 | M | 28 | 44 | 0.22 | 1.89 | A | 2.00 | 28 | 0.87 | 4.75 | R |
| Ozoom | 46 | M | 21 | 73 | 0.55 | 5.36 | R | 11 | 4 | −0.47 | −1.81 | A |
| Gunther | 1 | M | 10 | 18 | 0.29 | 1.51 | A | 4 | 1 | - | - | - |
| Kazi | 2 | M | 0 | 3 | - | - | - | 3 | 2 | - | - | - |
| Kwan | 20 | M | 47 | 14 | −0.54 | −4.23 | L | 29 | 33 | 0.06 | 0.51 | A |
| Jojo | 29 | M | 44 | 18 | −0.42 | −3.30 | L | 25 | 49 | 0.32 | 2.79 | R |
| Azizi | 6 | M | 35 | 25 | −0.17 | −1.29 | A | 25 | 30 | 0.09 | 0.67 | A |
| Amare | 4 | M | 10 | 48 | 0.66 | 4.99 | R | 29 | 33 | 0.06 | 0.51 | A |
F: Female; M: Male; # L: number of left-hand responses; # R: number of right-hand responses; HI: Handedness Index score that corresponds to degree of manual asymmetry, the sign indicates the direction of the manual bias (negative value: left-hand bias, positive value: right-hand bias); z: individual z-score; Hand: hand preference; R: right-handed subject; L: left-handed subject; A: ambiguous handed subject. Italicized names indicate those gorillas from Lincoln Park Zoo.
Direction and strength of hand preferences
We initially examined the association between HI values for bouts and frequency of hand use to assess consistency between these two approaches to handedness assessment. A Pearson Product moment correlation between the HI measures for bouts and frequency of bimanual feeding was positive and significant r(31) = 0.935, p <0.001. Moreover, a paired samples t-test revealed no significant difference in the HI values computed based on bouts compared to frequency in hand use t(31) = 0.76, p>0.44. In fact, the mean HI values for the bouts and frequency of hand use in bimanual feeding revealed similar degrees of population-level right-handedness (see Table 2). Because the HI values for these two types of measures were nearly identical and strongly correlated with each other, we subsequently used only the HI values based on frequency of hand use in bimanual feeding in subsequent analyses.
TABLE 2.
Distribution of hand preferences and degree of population-level manual bias
| Manual behaviors | # L | # A | # R | N | M.HI | S.E. | t | p |
|---|---|---|---|---|---|---|---|---|
| Unimanual reaching | 3 | 20 | 9 | 32 | 0.11 | 0.06 | 1.99 | >0.05 |
| Females | 2 | 11 | 6 | 19 | 0.11 | 0.07 | ||
| Males | 1 | 9 | 3 | 13 | 0.12 | 0.10 | ||
| Bimanual feeding - Frequ. | 4 | 7 | 21 | 32 | 0.37 | 0.07 | 4.72 | <0.001 |
| Females | 1 | 3 | 15 | 19 | 0.49 | 0.09 | ||
| Males | 3 | 4 | 6 | 13 | 0.19 | 0.11 | ||
| Bimanual feeding - Bouts | 2 | 12 | 18 | 32 | 0.35 | 0.06 | 5.01 | <0.001 |
| Females | 1 | 5 | 13 | 19 | 0.48 | 0.08 | ||
| Males | 1 | 7 | 5 | 13 | 0.16 | 0.10 | ||
L: number of left-handed subjects; # R: number of right-handed subjects; # A: number of ambiguous handed subjects; N: sample of subjects; M.HI: Mean Handedness Index score of N individuals that corresponds to degree of population-level handedness, the sign indicates the direction of the manual bias (negative value: left-hand bias, positive value: right-hand bias); S.E.: Standard Error of the mean; t: value of the t resulting from a t-test; p: significance of p
One sample t-tests of the HI values indicated significant population-level right handedness for bimanual feeding t(31)=4.72, p<0.001 but not unimanual reaching t(31)=1.99, p>0.05 (see Fig. 2). Additionally, a paired samples t-tests indicated that the HI values for bimanual feeding were significantly more rightward compared to unimanual reaching t(31) = −2.62, p<0.02. An examination of the distribution of handedness based on the z-scores confirmed the t-test results. For bimanual feeding, there were 21 right-, 4 left- and 7 ambiguously-handed gorillas. For unimanual reaching, there were 9 right-, 3 left- and 20 ambiguously-handed subjects. Whereas, the distribution of hand preferences differs significantly for both unimanual reaching, χ2 (2, N=32) = 13.94, p<0.001, and bimanual feeding, χ2 (2, N=32) = 15.44, p<0.001, the number of right-handed subjects are significantly greater than left-handed subject only for bimanual feeding, χ2 (1, N=26) = 11.56, p<0.001. There were too few lateralized subjects for unimanual reaching to meet the assumptions of the chi-square test. The difference in sensitivity to variation in hand preference between unimanual reaching and bimanual feeding was further supported by the comparison the ABS-HI scores for the two behaviors. The mean ABS-HI for bimanual feeding (Mean = 0.50, S.E. = 0.05) was significantly higher than for unimanual reaching (Mean = 0.23, S.E. = 0.05), t(31) = −3.00, p<0.01.
Fig. 2.
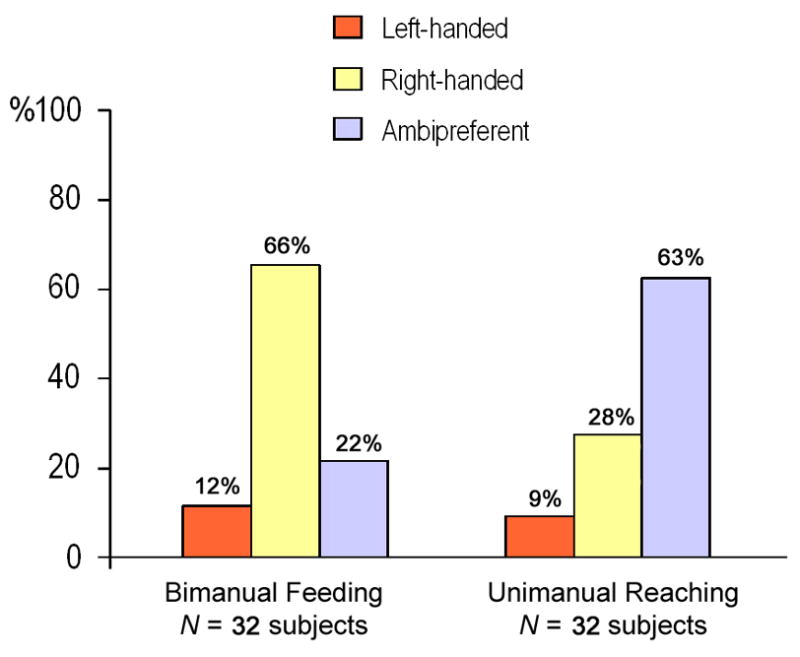
Distribution in percentages of right-, left- and ambiguously-handed gorillas for bimanual feeding (frequencies) and unimanual reaching.
(A color version of this figure can be found in the on-line version of the paper).
Potential effect of sample, sex and age
We initially compared the HI scores for the AZ and LPZ gorillas to assess whether directional biases in handedness differed between two the two samples. No significant difference in HI scores were found for either bimanual t(30) = 1.19, p > .210 or unimanual t(30) = 1.32, p > .198. Thus, the HI values were comparable between the two samples. The effects of the sex were assessed using an analysis of variance (ANOVA) with the HI score for each measure serving as the dependent measure. There was no significant difference in HI scores between females and males for unimanual reaching, F(1,30) = 0.05, p>0.98 or bimanual feeding F(1,30) = 3.33, p>0.06 (see Table 2). There was also no significant effect of sex in the strength of the manual bias (absolute values of HI) according to a t-test for unimanual reaching t(30) = 0.37, p > .10 or bimanual feeding, t(30) = 1.42, p > .10. The association between age and both directional and strength of handedness was performed using a Pearson Product Moment correlation. None of the correlations reached statistical significance.
Comparison of hand preferences with previous bimanual measures
As noted previously, measures of hand preferences for bimanual coordinated activities have been collected using an experimental tube task (see introduction) in 15 of the gorillas used in the present study (Hopkins et al., 2003) that were housed at Zoo Atlanta. Within the same 15 individuals, we assessed the correlation of measures of hand preferences (HI scores) between the tube task from the Hopkins et al. (2003) paper and our present set of data on bimanual feeding. The correlation is positive but not significant, r(15) = 0.33, p>0.10. When we statistically compare the HI scores, the gorillas were significantly more right-handed for the bimanual feeding (Mean HI = .44) compared to the TUBE task (Mean HI = .11) t(15) = 2.47, p < 0.03.
Because the patterns of the coordinated actions of the hands seem similar between the tube and bimanual feeding (a hand holds the food/tube while the other hand picks up this food inside the tube/other hand), the difference in hand preferences was unexpected but the comparison between the two measures is confounded with time. More than 5 years separated the collection of data for these two studies and maturational or developmental changes in hand preference may have occurred during this time, particularly in the young individuals. If we restrict the test-retest correlation in HI scores to the individuals that were adults in the two studies and exclude the gorillas that were under 10 years old when first tested on the tube task (Hopkins et al., 2003), a significant positive correlation was found in hand preferences within the 10 remaining adult subjects, r(10) = 0.66, p<0.05. Moreover, no significant difference was found in HI scores between the two bimanual measures within this cohort of 10 individuals.
DISCUSSION
The results of this study are straight-forward. Captive lowland gorillas showed significant population-level right handedness for bimanual feeding but not unimanual reaching. There were no significant sex differences in handedness for unimanual reaching and bimanual feeding; however, it is of note that the degree of right-handedness for bimanual feeding in females compared to males approached conventional levels of statistical significance. Furthermore, comparison of the results between two different samples of gorillas revealed no significant differences in the pattern of handedness.
The difference in patterns of handedness between unimanual reaching and bimanual coordinated activities, such as the feeding behavior recorded in this study, are consistent with other findings in nonhuman primates, such as capuchins monkeys, baboons and chimpanzees (Hopkins, 2007). In all of these different primate species, measures of handedness that require coordinated bimanual actions a) elicit stronger hand preference at the individual level of analysis and b) reveal population-level handedness at the species level of analysis. These collective findings support the view that, compared to bimanual coordinated activities, simple unimanual reaching measures are less sensitive measures of hemispheric specialization in nonhuman primates (e.g., Vauclair and Meguerditchian, 2007). Further support for this argument comes from recent studies showing that hand preferences for coordinated bimanual actions, and not simple reaching, correlate with neuroanatomical asymmetries in the motor-hand area of the precentral gyrus in both chimpanzees and capuchin monkeys (Hopkins and Cantalupo, 2004; Phillips and Sherwood, 2007; Sherwood et al., 2007).
Most of the previous studies that have investigated hand use for coordinated bimanual actions in nonhuman primates have generally involved a structured handedness task referred to as the TUBE task (Hopkins, 2007). The TUBE task requires that subjects hold a baited poly-vinyl-chloride (PVC) with one hand and extract food with the opposite hand, and thus is not all that different from the behavior of the gorillas observed in this study. However, in our view, there is some confusion and inconsistent use of the words and operational definitions of “coordinated bimanual action”, “complex bimanual action” and “bimanual feeding” (e.g., Papademetriou et al., 2005 for an example of an over-generalization of the term “bimanual feeding”). In the present study, we defined “bimanual feeding” or “bimanual coordinated action” as manual activities that imply asymmetrical and coordinated use of the hands, (i.e. one hand fulfills the minor “frame” role (holding the food) while the other hand engages in the dominant “active” role in picking up or manipulating the food maintained in the “frame” hand). We do not, however, consider this bimanual behavior as being comparable to the descriptions of “complex bimanual tasks” such as nut-cracking behavior in the wild chimpanzees studied by Boesch (1991). Nut-cracking does not require similar coordinated bimanual actions, although both hand may be used in a sequential manner (one hand holds the hammer while the other brings the kernel to the anvil or mouth). Indeed, the two hands do not have to interact directly with each other and can perform these two different “active” actions independently of each other. A similar definitional argument can be made with regard to previous studies of bimanual feeding in bonobos and chimpanzees (Hopkins, 1994; Hopkins and deWaal, 1995) as they relate to the definition employed in this study. In the bonobos and chimpanzees studied by Hopkins and colleagues, bimanual feeding was defined as the active use of one hand for bringing food in mouth while the opposite hand was holding other food items, without the constraint that the two hands work in an asymmetrical coordinated manner. In light of the importance that some have placed on the role of asymmetrical, coordinated hand use on the evolution of complex behavior and cognition, such as tool use (van Schaik et al., 2003), it seems very important to clearly define what is meant by “coordinated bimanual actions”.
The pattern of handedness found when measuring bouts compared to frequency in hand use for bimanual feeding were nearly identical and the two measures strongly correlated with each other. This result is also consistent with findings in captive chimpanzees and baboons (Hopkins et al., 2001, 2005; Damerose and Hopkins, 2002) and contradicts the view that the evidence of right-hand bias in nonhuman primates may be related to a statistical bias induced by the use of the frequencies instead of the bouts (McGrew and Marchant, 1997).
To our knowledge, the present study in bimanual feeding reveals the strongest degree of population-level right handedness ever reported in apes for motor manipulative activities (Hopkins, 2006). In captive chimpanzees, the ratio of right-to-left handedness is about 2:1 for coordinated bimanual actions whereas in our sample, the ratio was more than 5:1, which is quite pronounced and rivals some reports of handedness in humans, particularly among individuals from non-westernized cultures (Perelle and Ehrmann, 1994). We would suggest that, given the importance of hierarchical, bimanual motor actions in the feeding ecology of gorillas (Byrne and Byrne, 1993), the strong degree of right-handedness observed in our sample may reflect an inherent adaptation for hemispheric specialization for bimanual actions. However, with the relatively small sample size in this study, it would be premature to speculate on this difference but, at a minimum, the results clearly warrant further investigation in additional samples of gorillas.
Lastly, these results are also consistent with the reports of population-level right handedness for bimanual food processing reported in wild mountain gorillas (Byrne and Byrne, 1991). Thus, in gorillas, there is some consistency in results between captive and wild settings with respect to hand preference for bimanual feeding. Taken together, the collective data suggest several conclusions. First, in contrast to unimanual reaching, bimanual coordinated feeding is a reliable and sensitive measure of hemispheric specialization in great apes. Second, this spontaneous coordinated behavior might be a fruitful area of investigation in future handedness studies, in wild populations of apes and monkeys. Finally, in agreement with Wundrum (1986), we suggest that the ability to coordinate the hands in an asymmetric manner may have proven to be an important requisite skill for the emergence of right-handedness in early hominids. It is important to emphasize that efficient asymmetric coordination of the hands to perform complex manipulation is not a requisite condition for the emergence of population-level handedness (see Vallortigara and Rogers, 2005; Hopkins, 2007). Indeed, asymmetric coordination of the hands can provide the needed adaptive functions to individual subjects without need for all the individuals having the same hand preferences. Thus, why the gorillas largely conform to using the right hand remains unclear and warrants further investigation.
Acknowledgments
NIH grants NS-42867 and HD-56232.
We are grateful to Charles Horton for allowing us to conduct this study at the Zoo Atlanta as well as the animal care staff such as Kris Krickbaum and Jodi Carrigan for their welcome and helpful assistance. At Lincoln Park Zoo, we are extremely grateful to Maureen Leahy, Dominic Calderisi, Vivian Vreeman, Marissa Milstein, the animal care staff, and our volunteer data collectors.
Contributor Information
Adrien Meguerditchian, Email: adrien.meguerditchian@univ-provence.fr.
William D. Hopkins, Email: whopkin@emory.edu.
LITERATURE CITED
- Annett M. Handedness and Brain Asymmetry: The Right Shift Theory. Hove: Psychology Press; 2002. [Google Scholar]
- Annett M, Annett J. Handedness for feeding in gorillas. Cortex. 1991;17:269–276. doi: 10.1016/s0010-9452(13)80131-1. [DOI] [PubMed] [Google Scholar]
- Begg-Reid C, Schillaci MA. Infant cradling in a captive mother gorilla. Zoo Biol. 2008;27:420–426. doi: 10.1002/zoo.20197. [DOI] [PubMed] [Google Scholar]
- Blois-Heulin C, Guitton JS, Nedellec-Bienvenue D, Ropars L, Vallet E. Hand preference in unimanual and bimanual tasks and postural effect on manual laterality in captive red-capped mangabeys (Cercocebus torquatus torquatus) Am J Primatol. 2006;68:429–444. doi: 10.1002/ajp.20239. [DOI] [PubMed] [Google Scholar]
- Boesch C. Handedness in wild chimpanzees. Int J Primatol. 1991;12:541–558. [Google Scholar]
- Bradshaw JL, Rogers LJ. The evolution of lateral asymmetries, language, tool-use and intellect. San Diego: Academic Press; 1993. [Google Scholar]
- Byrne RW, Byrne JE. Hand preferences in the skilled gathering tasks of mountain gorillas (Gorilla g. beringei) Cortex. 1991;27:521–546. doi: 10.1016/s0010-9452(13)80003-2. [DOI] [PubMed] [Google Scholar]
- Byrne RW, Byrne JE. Complex leaf-gathering skills of Mountain Gorillas (Gorilla g. beringei): variability and standardization. Am J Primatol. 1993;31:241–261. doi: 10.1002/ajp.1350310402. [DOI] [PubMed] [Google Scholar]
- Corballis MC. The lopsided ape: Evolution of the generative mind. New York: Oxford University Press; 1991. [Google Scholar]
- Crow T. Directional asymmetry is the key to the origin of modern Homo sapiens (the Broca-Annett axiom): A reply to Rogers’ review of The Speciation of Modern Homo Sapiens. Laterality. 2004;9:233–242. [Google Scholar]
- Damerose E, Hopkins WD. A comparison of scan and focal sampling procedures in the assessment of laterality for maternal cradling and infant nipple preferences in olive baboons (Papio anubis) Anim Behav. 2002;63:511–518. [Google Scholar]
- Ettlinger GF. Hand preference, ability and hemispheric specialization. How far are these factors related in the monkey? Cortex. 1988;24:389–398. doi: 10.1016/s0010-9452(88)80002-9. [DOI] [PubMed] [Google Scholar]
- Fagard J. Le développement de la latéralité manuelle. In: Fagard J, editor. Le développement des habiletés de l’enfant. Coordination bimanuelle et latéralité. Paris: CNRS Editions; 2001. pp. 221–234. [Google Scholar]
- Fagard J, Marks A. Unimanual and bimanual tasks and the assessment of handedness in toddlers. Dev Sci. 2000;3:137–147. [Google Scholar]
- Fagot J, Vauclair J. Handedness and bimanual coordination in the lowland gorilla. Brain Behav Evol. 1988;32:89–95. doi: 10.1159/000116536. [DOI] [PubMed] [Google Scholar]
- Fagot J, Vauclair J. Manual laterality in nonhuman primates: a distinction between handedness and manual specialization. Psychol Bull. 1991;109:76–89. doi: 10.1037/0033-2909.109.1.76. [DOI] [PubMed] [Google Scholar]
- Hook MA. The evolution of lateralized motor functions. In: Rogers LJ, Kaplan G, editors. Comparative Vertebrate Cognition. New York: Kluwer Academic/Plenum Publishers; 2004. pp. 325–370. [Google Scholar]
- Hopkins WD. Posture and reaching in chimpanzees (Pan troglodytes) and orangutans (Pongo pygmaeus) J Comp Psychol. 1993;107:162–168. doi: 10.1037/0735-7036.107.2.162. [DOI] [PubMed] [Google Scholar]
- Hopkins WD. Hand preferences for bimanual feeding in 140 captive chimpanzees (Pan troglodytes): Rearing and ontogenetic determinants. Dev Psychobiol. 1994;27:395–407. doi: 10.1002/dev.420270607. [DOI] [PubMed] [Google Scholar]
- Hopkins WD. Hand preferences for a coordinated bimanual task in 110 chimpanzees: Cross-sectional analysis. J Comp Psychol. 1995;109:291–297. doi: 10.1037/0735-7036.109.3.291. [DOI] [PubMed] [Google Scholar]
- Hopkins WD. On the other hand: statistical issues in the assessment and interpretation of hand preference data in nonhuman primates. Int J Primatol. 1999;20:851–866. [Google Scholar]
- Hopkins WD. Laterality in maternal cradling and infant positional biases: Implications for the evolution and development of hand preferences in nonhuman primates. Int J Primatol. 2004;25:1243–1265. doi: 10.1023/B:IJOP.0000043961.89133.3d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD. Comparative and familial analysis of handedness in great apes. Psychol Bull. 2006;132:538–559. doi: 10.1037/0033-2909.132.4.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD. Evolution of hemispheric specialization in primates. Oxford: Academic Press; 2007. [Google Scholar]
- Hopkins WD, Cantalupo C. Handedness in chimpanzees is associated with asymmetries in the primary motor cortex but not with homologous language areas. Behav Neurosci. 2004;118:1176–1183. doi: 10.1037/0735-7044.118.6.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Cantalupo C. Individual and setting differences in the hand preferences of chimpanzees (Pan troglodytes): A critical analysis and some alternative explanations. Laterality. 2005;10:65–80. doi: 10.1080/13576500342000301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Cantalupo C, Freeman H, Russell JL, Kachin M, Nelson E. Chimpanzees are right handed when recording bouts of hand use. Laterality. 2005;10:149–159. doi: 10.1080/13576500342000347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, deWaal F. Behavioral laterality in captive bonobos (Pan paniscus): Replication and extension. Int J Primatol. 1995;16:261–276. [Google Scholar]
- Hopkins WD, Fernandez-Carriba S, Wesley MJ, Hostetter A, Pilcher D, Poss S. The use of bouts and frequencies in the evaluation of hand preferences for a coordinated bimanual task in chimpanzees (Pan troglodytes): An empirical study comparing two different indices of laterality. J Comp Psychol. 2001;115:294–299. doi: 10.1037//0735-7036.115.3.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Morris RD. Handedness in great apes: A review of findings. Int J Primatol. 1993;14:1–15. [Google Scholar]
- Hopkins WD, Stoinski T, Lukas K, Ross S, Wesley MJ. Comparative assessment of handedness for a coordinated bimanual task in chimpanzees (Pan), gorillas (Gorilla), and orangutans (Pongo) J Comp Psychol. 2003;117:302–308. doi: 10.1037/0735-7036.117.3.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilak AL, Phillips KA. Consistency of hand preference across low-level and high-level tasks in Capuchin monkeys (Cebus apella) Am J Primatol. 2007;70:254–60. doi: 10.1002/ajp.20485. [DOI] [PubMed] [Google Scholar]
- MacNeilage PF, Studdert-Kennedy MG, Lindblom B. Primate handedness reconsidered. Behav Brain Sci. 1987;10:247–303. [Google Scholar]
- Marchant LF, McGrew WC. Laterality of limb function in wild chimpanzees of Gombe National Park: comprehensive study of spontaneous activities. J Hum Evol. 1996;30:427–443. [Google Scholar]
- Marchant LF, McGrew WC, Eibl-Eibesfeldt I. Is human handedness universal? Ethological analyses from three traditional cultures. Ethology. 1995;101:239–258. [Google Scholar]
- McGrew WC, Marchant LF. Are gorillas right-handed or not? Hum Evol. 1993;8:17–23. [Google Scholar]
- McGrew WC, Marchant LF. On the other hand: current issues in and meta-analysis of the behavioral laterality of hand function in non-human primates. Yrb Phys Anthropol. 1997;40:201–232. [Google Scholar]
- McGrew WC, Marchant LF. Ethological study of manual laterality in the chimpanzees of the Mahale mountains, Tanzania. Behaviour. 2001;138:329–358. [Google Scholar]
- McGrew WC, Marchant LF, Wrangham RW, Kleinm H. Manual laterality in anvil use: Wild chimpanzees cracking Strychnos fruits. Laterality. 1999;4:79–87. doi: 10.1080/03069887600760101. [DOI] [PubMed] [Google Scholar]
- Meunier H, Vauclair J. Hand preferences on unimanual and bimanual tasks in whiteface capuchins (Cebus capucinus) Am J Primatol. 2007;69:1064–1069. doi: 10.1002/ajp.20437. [DOI] [PubMed] [Google Scholar]
- O’Malley RC, McGrew WC. Hand preferences in captive orangutans (Pongo pygmaeus) Primates. 2006;47:279–283. doi: 10.1007/s10329-006-0180-1. [DOI] [PubMed] [Google Scholar]
- Palmer AR. Chimpanzee right-handedness reconsidered: evaluating the evidence with funnel plots. Am J Phys Anthropol. 2002;118:191–199. doi: 10.1002/ajpa.10063. [DOI] [PubMed] [Google Scholar]
- Palmer AR. Reply to Hopkins and Cantalupo: chimpanzee right-handedness reconsidered-sampling issues and data presentation. Am J Phys Anthropol. 2003;121:382–384. [Google Scholar]
- Papademetriou E, Sheu CF, Michel GF. A meta-analysis of primate hand preferences, particularly for reaching. J Comp Psychol. 2005;119:33–48. doi: 10.1037/0735-7036.119.1.33. [DOI] [PubMed] [Google Scholar]
- Perelle IB, Ehrman L. An international study of human handedness: the data. Behav Gen. 1994;24:217–227. doi: 10.1007/BF01067189. [DOI] [PubMed] [Google Scholar]
- Phillips KA, Sherwood CC. Cerebral petalias and their relationship to handedness in capuchin monkeys (Cebus apella) Neuropsychologia. 2007;45:2398–2401. doi: 10.1016/j.neuropsychologia.2007.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond M, Pontier D. Is there geographical variation in human handedness? Laterality. 2004;9:35–51. doi: 10.1080/13576500244000274. [DOI] [PubMed] [Google Scholar]
- Rogers LJ, Andrew JR. Comparative vertebrate lateralization. Cambridge: Cambridge University Press; 2002. [Google Scholar]
- Schweitzer C, Bec P, Blois-Heulin C. Does the Complexity of the Task Influence Manual Laterality in De Brazza’s Monkeys (Cercopithecus neglectus)? Ethology. 2007;113:983–994. [Google Scholar]
- Shafer DD. Patterns of hand preference in gorillas and children. In: Ward JP, Hopkins WD, editors. Primate laterality: Current behavioral evidence of primate asymmetries. New-York: Springer-Verlag; 1993. pp. 267–283. [Google Scholar]
- Sherwood CC, Wahl E, Erwin JM, Hof PR, Hopkins WD. Histological asymmetries or primary motor cortex predicts handedness in chimpanzees (Pan trologytes) J Comp Neuro. 2007;503:525–537. doi: 10.1002/cne.21399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinozzi G, Castornina MG, Truppa V. Hand preferences in unimanual and coordinated-bimanual tasks by tufted capuchin monkeys (Cebus apella) J Comp Psychol. 1998;112:183–191. [Google Scholar]
- Vallortigara G, Rogers LJ. Survival with an asymmetrical brain: Advantages and disadvantages of cerebral lateralization. Behav Brain Sci. 2005;28:575–589. doi: 10.1017/S0140525X05000105. [DOI] [PubMed] [Google Scholar]
- van Schaik CP, Ancrenaz M, Borgen G, Galdikas B, Knott CD, Singleton I, Suzuki A, Utami SS, Merrill M. Orangutan Cultures and the Evolution of Material Culture. Science. 2003;299:102–105. doi: 10.1126/science.1078004. [DOI] [PubMed] [Google Scholar]
- Vauclair J, Meguerditchian A. Perceptual and motor lateralization in two species of baboons. In: Hopkins WD, editor. Evolution of Hemispheric Specialization in Primates. Oxford: Academic Press; 2007. pp. 177–198. [Google Scholar]
- Vauclair J, Meguerditchian A, Hopkins WD. Hand preferences for unimanual and coordinated bimanual tasks in baboons (Papio anubis) Cog Brain Res. 2005;25:210–216. doi: 10.1016/j.cogbrainres.2005.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren JM. Handedness and laterality in humans and other animals. Physiol Psychol. 1980;8:351–359. [Google Scholar]
- Wundrum IJ. Cortical motor asymmetries and Hominid feeding strategies. Hum Evol. 1986;1:183–188. [Google Scholar]