Abstract
Skipped polyenes (i.e. 1,4-dienes and higher homologues) are stereodefined components of a vast array of biologically important natural products, including polyunsaturated fatty acids. While widespread in nature, these architectures are generally considered to represent significant barriers to efficient chemical synthesis. While partial reduction of skipped poly-ynes provides a pathway to a subset of such structures, general chemical methods for the preparation of skipped polyenes that contain varied stereochemistries and substitution patterns are lacking. Here, we describe a metal-promoted reductive cross-coupling reaction between vinylcyclopropanes and alkynes (or vinylsilanes) that provides stereoselective access to a diverse array of skipped polyenes through a process that establishes one C–C bond, generates up to three stereodefined alkenes, and can be used to introduce stereogenic centers at the central positions of the skipped polyene motif. We also demonstrate the significance of the present bond construction by preparing substituted and stereodefined polyunsaturated synthetic fatty acids.
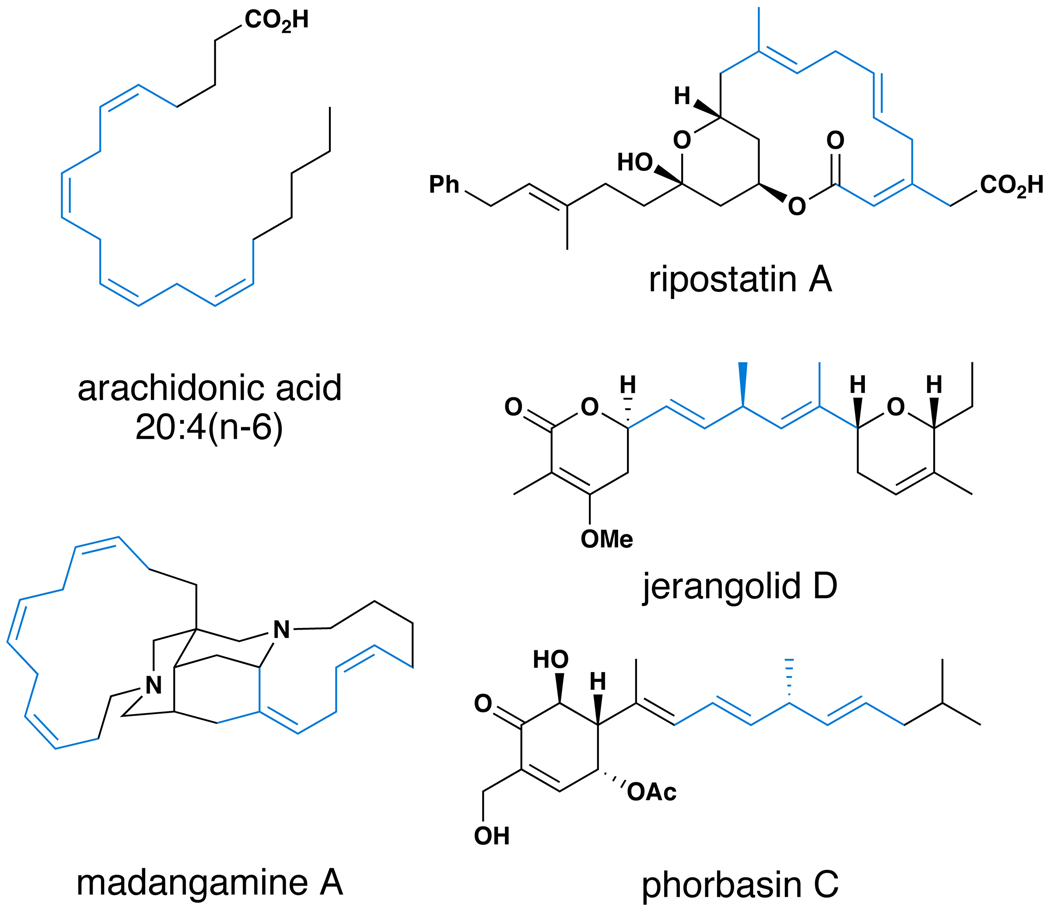
Fatty acids are a subset of small molecules that are essential for life, playing not only central roles in compartmentalization and membrane function but also impacting cellular pathways that regulate blood pressure, clotting and lipid levels as well as the immune response and inflammation.(1–3) Polyunsaturated fatty acids (i.e. arachidonic acid, α-linoleic acid, γ-linoleic acid and eicosapentaenoic acid) are a subset of this large class of biomolecules that are present in all higher organisms and play critical roles in human health (Figure 1A).(4,5) These ubiquitous biomolecules share a common structural motif that imparts their unique properties – methylene interrupted polyenes (highlighted in blue). This central architectural feature is a stereodefined motif that is encountered throughout nature, with examples including structurally complex bioactive natural products from polyketide, terpene and alkaloid biosynthetic pathways. These more architecturally intricate molecules, identified as potent antibiotic, antifungal and cytotoxic agents, house skipped polyenes that contain stereochemically diverse di- and tri-substituted alkenes (i.e. ripostatin A, madangamine A).(6–10) Notably, more complex examples include secondary metabolites that house 1,4-dienes with stereogenic sp3 carbons at the central position of the isolated non-conjugated diene (i.e. jerangolid D and phorbasin C).(11–14)
Figure 1. Stereodefined skipped polyenes are a structural motif found in diverse natural products.
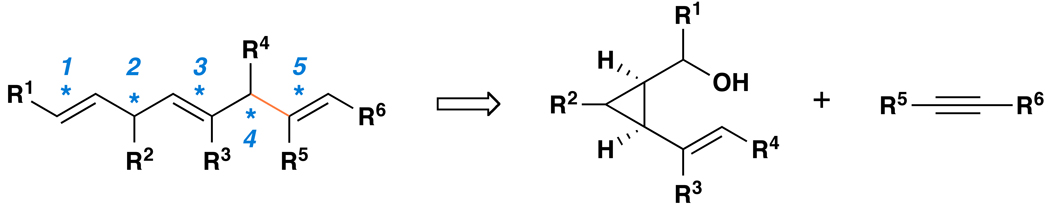
The stereoselective preparation of these structural motifs remains a significant challenge in organic chemistry.(15–18) While methods based on carbonyl olefination, alkylation and partial reduction of acetylenes have been employed in numerous campaigns in stereoselective synthesis, these often multi-step processes are each plagued by significant limitations in scope, selectivity and efficiency. Here, we describe a titanium-mediated stereoselective fragment coupling reaction that delivers a variety of complex skipped polyenes by a process that establishes five unique stereochemical relationships across the skipped polyene backbone in concert with C–C bond formation (Figure 2). While defining a unique stereoselective transformation in organic chemistry, this convergent coupling reaction illuminates a powerful solution to the synthesis of a complex stereodefined structural motif observed throughout nature. In addition to presenting the basic reaction scope and selectivity of the process, we demonstrate the application of this reaction to the preparation of complex synthetic polyunsaturated fatty acids (PUFAs).
Figure 2. A route to stereodefined skipped polyenes by titanium-mediated reductive cross-coupling of vinylcyclopropanes with alkynes.
 = Stereochemical relationships established in this skipped polyene synthesis;
= Stereochemical relationships established in this skipped polyene synthesis; 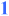 = stereochemistry set in a stereospecific fashion (E or Z);
= stereochemistry set in a stereospecific fashion (E or Z);  = stereochemistry retained from cyclopropane;
= stereochemistry retained from cyclopropane;  and
and 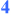 = stereochemistry set in a stereoselective fashion (≥20:1);
= stereochemistry set in a stereoselective fashion (≥20:1); 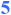 = stereochemistry is set as a function of the reaction mechanism.
= stereochemistry is set as a function of the reaction mechanism.  = carbon–carbon bond formed in Ti-mediated cross-coupling.
= carbon–carbon bond formed in Ti-mediated cross-coupling.
Results and Discussion
Reaction design
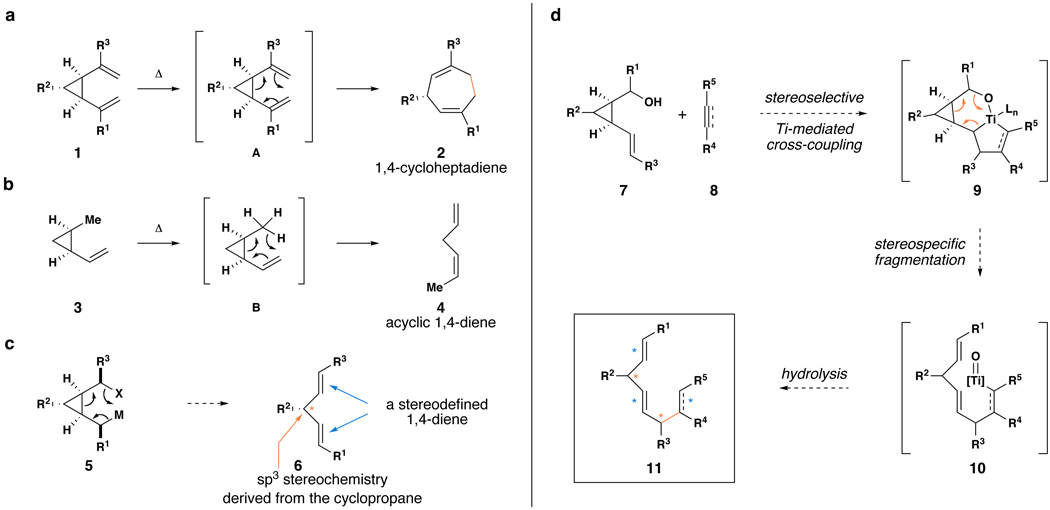
In designing a mode of reactivity suitable for the stereoselective construction of acyclic skipped polyenes, we were inspired by the well-established sigmatropic rearrangement chemistry of vinylcyclopropanes (Figure 3). In the case of cis-divinylcyclopropanes 1, Cope rearrangement (a) is driven by the release of ring strain associated with the cyclopropane, and a cyclic 1,4-diene 2 is produced (Figure 3a).(19,20) In a mechanistically related rearrangement, cis-disubstituted vinylcyclopropanes (3) can undergo 1,5-hydrogen migration (b) to deliver acyclic 1,4-dienes (4) (Figure 3b).(21–24)
Figure 3. The design of a convergent coupling reaction suitable for the stereoselective synthesis of complex skipped polyenes.
a, Cope rearrangement of cis-divinylcyclopropanes. b, 1,5-hydrogen migrations in cis-vinylcyclopropanes. c, A plausible 6-electron process for acyclic 1,4-diene synthesis – alkene geometry is set as a function of the stereochemistry of the intermediate and the mechanistic course of the fragmentation. d, Design of a cross-coupling/fragmentation cascade for preparation of stereodefined skipped polyenes.  = stereochemistry of up to three alkenes is established;
= stereochemistry of up to three alkenes is established;  = this carbon–carbon bond forming process has the potential to establish 1,4-dienes bearing a central stereodefined C3-carbon;
= this carbon–carbon bond forming process has the potential to establish 1,4-dienes bearing a central stereodefined C3-carbon; 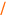 = carbon–carbon bond formed in Ti-mediated cross-coupling; M = metal.
= carbon–carbon bond formed in Ti-mediated cross-coupling; M = metal.
We speculated that a related six-electron process could ensue from an organometallic intermediate of general structure 5 (Figure 3c). Here, we reasoned that fragmentation would have the potential to proceed in a stereospecific manner, where the stereochemistry of the organometallic intermediate (5) could be translated directly to the stereochemistry of the skipped polyene product (6).
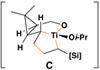
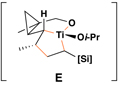
To prepare the required stereodefined metalated cyclopropanes (5), we speculated that a titanium-mediated, alkoxide-directed fragment union reaction between a substituted vinylcyclopropane 7 and a suitably functionalized coupling partner (i.e. 8), could deliver tricyclic titanacyclopentane intermediates of general structure 9 (Figure 3d).(25) In addition to overcoming the sluggish reactivity of substituted alkenes in carbometalation chemistry, the hydroxy group of 7 would orchestrate encapsulation of the titanium center while establishing a rigid organometallic intermediate 9 suitably functionalized for the planned fragmentation. If rupture of the tricycle 9 proceeds in a stereospecific fashion, then the stereoselective annulation process (7 + 8 → 10) would lead, after hydrolysis, to the establishment of a stereodefined skipped polyene 11. Overall, this reaction design would define a convergent coupling reaction that has the potential to establish up to three stereodefined alkenes and one new stereogenic center ( ), while generating a complex yet common stereochemically defined structural motif in nature. As synthetic approaches to stereodefined cyclopropanes of general structure 7 are readily available, we reasoned that the proposed coupling process would define a potentially powerful entry to skipped polyenes.
), while generating a complex yet common stereochemically defined structural motif in nature. As synthetic approaches to stereodefined cyclopropanes of general structure 7 are readily available, we reasoned that the proposed coupling process would define a potentially powerful entry to skipped polyenes.
Exploring the reactivity of the vinylcyclopropane component
The feasibility of this proposal was first examined in coupling reactions of chlorodimethylvinylsilane with substituted vinylcyclopropanes, themselves derived from well-established cyclopropanation chemistry of allylic diazoacetates (i.e. 12–16) (Table 1).(26,27) As depicted in entry 1, initial exploration provided support for the proposed stereoselective coupling process. Here, vinylcyclopropane 17 was converted to 1,4-diene 23 in 58% isolated yield (82% yield based on recovered starting material). While illustrating a useful reaction for the synthesis of a 1,4-diene bearing a central quaternary carbon, this reaction proceeded in a stereoselective manner and established a central (E)-disubstituted alkene (E:Z ≥ 20:1).
Table 1.
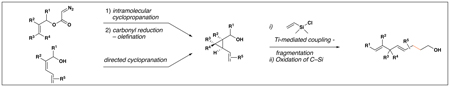 | |||||||
|---|---|---|---|---|---|---|---|
| entry | allylic alcohol or diazoacetate starting material |
vinylcyclopropane | Presumed metallacyclic intermediatea |
Yield (%)b |
(E:Z)c | drc | productd |
| 1 |  |
 |
 |
58 (82% brsm) |
20:1 | – | 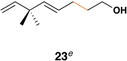 |
| 2 | 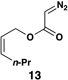 |
 |
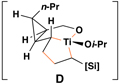 |
54 | 20:1 | – | 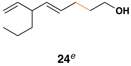 |
| 3 | 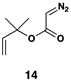 |
 |
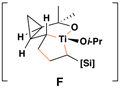 |
50 | 20:1 | – | 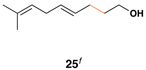 |
| 4 | 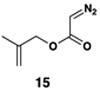 |
 |
 |
61 | 10:1 | – | 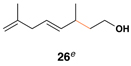 |
| 5 | 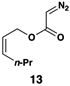 |
 |
 |
55 | 20:1 | 20:1 | 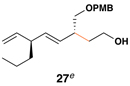 |
| 6 | 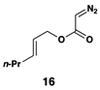 |
 |
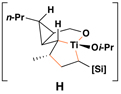 |
52 | 20:1 | 20:1 | 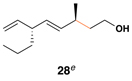 |
| 7 | 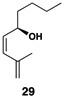 |
 |
 |
52 | 20:1 | 20:1 |  |
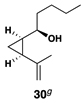
| 8 | 29 | 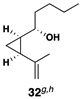 |
 |
42 | 20:1 | 20:1 | 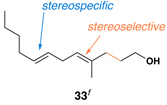 |
 = bonds formed in the coupling process.
= bonds formed in the coupling process.
While not computed for examples 2–8, as seen in entry 1, these reactions did not consume all of the vinylcyclopropane starting material.
No evidence was found for the production of stereoisomeric products.
Reaction conditions for cross-coupling: vinylsilane, ClTi(Oi-Pr)3, c-C5H9MgCl, Et2O (−78 to −50 °C), then add Li-alkoxide of vinylcyclopropane (−70 °C to rt over 3 h).
Oxidation conditions: TBHP, H2O, CsOH•H2O, TBAF, DMF, 70 °C.
Oxidation conditions: KF, KHCO3, H2O2, MeOH, THF.
ICH2Cl, Sm[Hg], THF, (85%, dr ≥ 20:1).
PDC, 4 Å sieves, CH2Cl2 (91%), then L-Selectride, THF (76% of desired isomer, dr = 6:1).
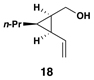
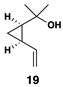
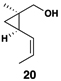
Similarly, conversion of vinylcyclopropane 18 to 1,4-diene 24 proceeded in 54% isolated yield (entry 2). Here, high E- selectivity (≥ 20:1) was accompanied by the generation of a 1 , 4-diene possessing a central chiral carbon. Entries 3 and 4 demonstrate that this coupling process is compatible with more highly substituted substrates. While conversion of 19 to the 1,4-diene 25 establishes a trisubstituted alkene, the trisubstituted vinylcyclopropane 20 is converted to a 1,4-diene that possesses a 1,1-disubstituted alkene and an allylic stereocenter (26). In both cases, high (E)-selectivity is observed in the formation of the central disubstituted alkene (≥ 10:1).
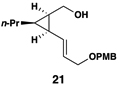
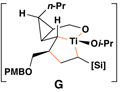
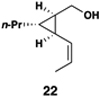
This coupling reaction can be used for the preparation of substituted skipped polyenes that house stereogenic centers at the 3-and 6-positions of the central 1,4-diene. As depicted in entries 5 and 6, vinylcyclopropanes 21 and 22 can be converted to stereodefined 1,4-dienes 27 and 28 in 55 and 52% yield, respectively. In each case, products were isolated as single isomers (dr ≥ 20:1, E:Z ≥ 20:1).
Probing stereospecificity
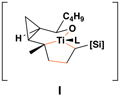
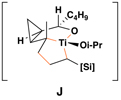
This complex coupling reaction appears to proceed in a stereospecific fashion. As depicted in entries 7 and 8 of Table 1, coupling of the isomeric vinylcyclopropanes 30 and 32 proceeds to deliver the skipped Z,E-diene 31 and E,E-diene 33. While the establishment of the central E-trisubstituted alkene occurs with apparently high levels of stereoselectivity (≥20:1), the disubstituted alkene of these products is generated in a stereospecific fashion. On close spectroscopic analysis of the crude products from these coupling reactions, no evidence could be found for the production of minor products derived from conversion of 30 to 33 or from 32 to 31. This intimate relationship between relative stereochemistry of the starting material and olefin geometry of the 1,4-diene product is consistent with a mechanistic proposal that follows from stereoselective formation of the tricyclic titanacyclopentanes I and J, followed by stereospecific fragmentation.
While defining a unique entry to stereodefined 1,4-dienes, to the best of our knowledge, this coupling reaction represents a unique transformation in organic chemistry. Alkoxide-directed metal centered [2+2+1] annulation enables stereoselective access to a fleeting organometallic intermediate (a configurationally defined cyclopropylcarbinyl anion) whose subsequent fragmentation occurs in a stereospecific manner.(28–34)
Skipped trienes from the reductive coupling of vinylcyclopropanes with alkynes
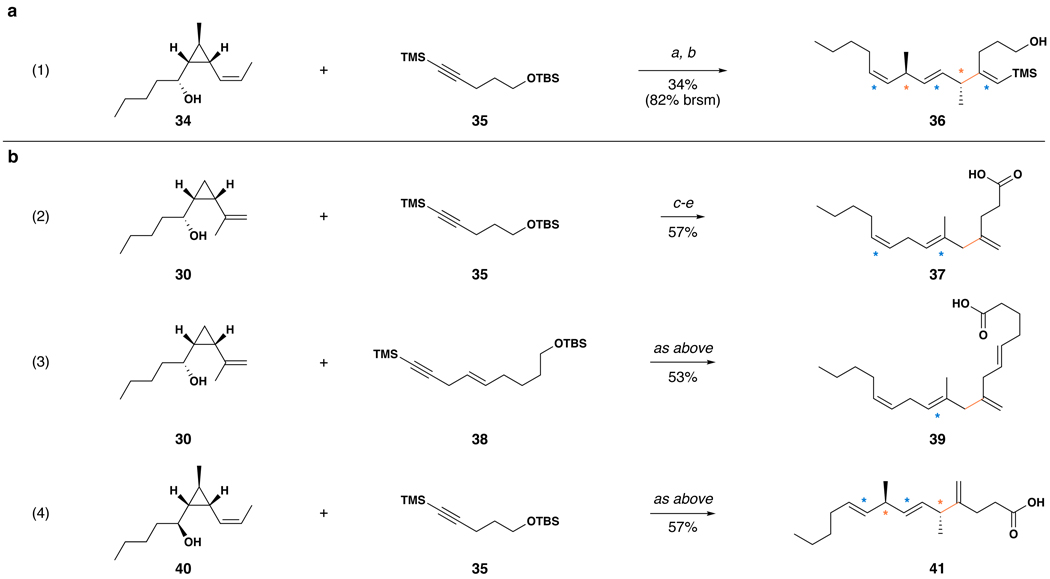
The titanium-mediated cross-coupling reaction of vinylcyclopropanes with alkynes enables direct stereoselective access to skipped trienes. As depicted in Figure 4a, reaction of vinylcyclopropane 34 with TMS-alkyne 35, followed by desilylation (TBAF, THF) delivers complex triene 36 in 34% isolated yield over the two-step process (82% based on recovered starting material in the T i-mediated coupling reaction). While regioselective functionalization of the alkyne proceeds in a manner where C–C bond formation occurs distal to the TMS-substituent,(35) the sense of regio- and stereoselective functionalization of vinylcyclopropane 34 is consistent with the observations made during study of the vinylcyclopropane–vinylsilane coupling. Overall, this convergent coupling reaction establishes one C–C bond, sets three stereodefined alkenes, one sp3 stereogenic center, and delivers a complex skipped triene in a single step. Notably, each sp3 stereogenic center in this product is positioned between the alkenes of the skipped polyene, defining a product that contains five contiguous stereogenic relationships that span eight contiguous carbons.
Figure 4. Synthesis of skipped polyenes by the coupling of vinylcyclopropanes with TMS-alkynes.
a, Direct synthesis of complex skipped trienes by reductive cross-coupling of vinylcyclopropanes with alkynes. b, Stereoselective preparation of complex synthetic polyunsaturated fatty acids (PUFAs). Reaction conditions employed: (a) ClTi(Oi-Pr)3, c-C5H9MgCl, 35 (−78 to −30 °C), PhMe, then lithium alkoxide of 34 in Et2O (−70 °C to rt over 3 h). (b) TBAF, THF. (c) ClTi(Oi-Pr)3, c-C5H9MgCl, alkyne (−78 to −30 °C), PhMe, then lithium alkoxide of the vinylcyclopropane in Et2O (−70 °C to rt over 3 h). (d) HF•pyr, CH3CN, CH2Cl2, (e) PDC, DMF, H2O. Yields reported are over the three-step sequence (c–e) and adjusted based on the quantity of recovered starting material (vinylcyclopropane). Isolated yields for each reaction sequence are 38% (eq 2), 36% (eq 3) and 23% (eq 4) over the three-step process (corresponding to average yields of 60–70% per step).  = up to three stereodefined alkenes are generated in concert with C–C bond formation;
= up to three stereodefined alkenes are generated in concert with C–C bond formation;  = 1,4-dienes are generated that house central C3-stereochemistry;
= 1,4-dienes are generated that house central C3-stereochemistry;  = carbon–carbon bond formed in Ti-mediated cross-coupling.
= carbon–carbon bond formed in Ti-mediated cross-coupling.
Synthesis of novel polyunsaturated fatty acids (PUFAs)
As a test of our methodology, we targeted application to the synthesis of novel stereochemically complex polyunsaturated fatty acids. While polyunsaturated fatty acids define a region of natural product chemical space that is rich in biological function, the dearth of methods available for the efficient synthesis of stereodefined skipped polyenes has generally limited medicinal investigation. The targets selected to explore the utility of our vinylcyclopropane-based skipped polyene synthesis were fatty acids that display complex stereochemical features for which a general synthetic method has not been available. Specifically, we aimed to access polyunsaturated fatty acids that contain: 1) di- and tri-substituted alkenes, 2) E- and Z- olefins, and 3) contain stereochemistry at the central positions of the 1,4-diene motifs. Such features represent the most complex examples of skipped polyenes observed in bioactive natural products.
As illustrated in Figure 4b, a sequence involving 1) reductive cross-coupling of suitably substituted vinylcyclopropanes with TMS alkynes, 2) desilylation, and 3) oxidation provides polyunsaturated fatty acids in a highly stereoselective fashion. While equation 2 demonstrates this process is effective for the preparation of a simple C-14 skipped triene-containing fatty acid, equations 3 and 4 confirm that this synthetic sequence is equally effective for the generation of higher homologues that contain stereodefined tetra-enes (39) and branched alkyl substitution (41).
While serving as a platform to challenge our chemical technology, this exercise has delivered complex stereodefined synthetic fatty acids. Likely due to the difficulties associated with preparing such architectures with existing chemical methods, such structures have never been reported. The mode of chemical reactivity described here provides a unique and powerful means to initiate investigations aimed at elucidating the physical and biological properties of complex and diverse polyunsaturated fatty acids.
Conclusion
Skipped polyenes are structural motifs present in organic molecules observed throughout nature and biology. Natural products that possess this motif are known to play key roles in compartmentalization and signal transduction, while others possess potent anticancer and antibiotic properties. Although recognized as a central stereodefined architectural feature of a variety of biologically significant natural products, skipped polyenes have remained as significant challenges in stereoselective chemical synthesis. We have described a chemical process that enables direct and stereoselective access to complex and diverse skipped polyenes by the direct union of vinylcyclopropanes with alkynes and vinylsilanes. While appearing complex, the vinylcyclopropane substrates for this process are available in a few steps from allylic diazoacetates by well-known cyclopropanation chemistry. Due to the previously established barriers associated with the preparation of this type of stereodefined architecture, and the relative ease with which the current method delivers these complex systems, we are looking forward to scientific advances that follow from this initial report.
Methods
General procedure for the reductive cross-coupling of vinylcyclopropanes with alkynes
To a round bottom flask (RBF) containing alkyne (2 mmol) and ClTi(Oi-Pr)3 (2 – 2.5 mmol, 1.0 M in hexanes) in toluene (0.1 M) at −78 °C is added c-C5H9MgCl (4 – 5 mmol) by syringe and the mixture is warmed to −30 °C and stirred for 1 h (becoming deep brown in color). In a separate RBF containing a vinylcyclopropane carbinol (1 mmol) in diethyl ether (Et2O) (<0.3 M) at −78 °C is added n-BuLi (1.2 mmol) by syringe and the mixture is warmed to 0 °C over 20 min and then added by cannula to the titanium complex that has been re-cooled to −70 °C. The mixture is then slowly warmed to RT over 2–3 h and treated with 1N HCl (~5 mL / mmol of vinylcyclopropane used) with rapid stirring. After 10 min the now colorless mixture is further diluted with ethyl acetate (EtOAc) and then filtered through a pad of silica rinsing with additional EtOAc. After concentration in vacuo, the non-polar product fractions are separated from the unreacted vinylcyclopropane by flash column chromatography (0 to 5% Et2O in hexanes followed by flushing with EtOAc).
In the examples presented, subsequent functionalization by desilylation and/or oxidation was accomplished by the following protocols: To this crude product in a plastic vial dissolved in acetonitrile (MeCN) / dichloromethane (CH2Cl2) (5 mL, 1:1) at 0 °C is added HF • pyridine by plastic syringe in 1 mL / 5 min increments. When the reaction is complete (indicated by TLC), the reaction mixture is poured slowly into a plastic beaker containing stirred saturated aqueous NaHCO3 (100 mL) at 0 °C, then diluted with Et2O. The layers are separated, the aqueous phase extracted with Et2O, organic extracts combined and filtered through MgSO4, concentrated in vacuo, and purified by flash column chromatography (10 – 15% EtOAc in hexanes). The resultant alcohol, which could contain small amounts of reduction side products, was used directly in the following oxidation. The purified skipped polyene alcohol (ie 0.5 mmol) in dimethylformamide (DMF) (3 mL) is added pyridinium dichromate (PDC) (2.0 mmol) and two drops of water at RT and the reaction is stirred for 12 h. The reaction mixture is then poured into brine (15 mL) and extracted three times with Et2O, extracts combined, washed with brine, filtered through MgSO4, concentrated in vacuo, and purified by flash column chromatography (20% EtOAc in hexanes) to yield the pure product.
Supplementary Material
Acknowledgments
We gratefully acknowledge financial support of this work by the American Cancer Society (RSG-06-117-01), the Arnold and Mabel Beckman Foundation, Boehringer Ingelheim, Eli Lilly & Co. and the National Institutes of Health – NIGMS (GM80266). The authors also acknowledge early demonstrations of this mode of reactivity by Mr. Richard Hughes and Mr. Jason Abbott while junior graduate students in the Micalizio laboratory.
Footnotes
Author contributions
T.K.M. planned and carried out the experimental work. G.C.M. initiated and directed the project. The manuscript was written jointly by both authors.
Additional information
The authors declare no competing financial interests.
Supplemantary information and chemical compound information accompany this paper at www.nature.com/naturechemistry.
Reprints and permission information is available online at http://npg.nature.com/reprintsandpermissions/.
References
- 1.Krey G, et al. Fatty acids, eicosanoids, and hypolipidemic agents identified as ligands of peroxisome proliferator-activated receptors by coactivator-dependent receptor ligand assay. Mol. Endocrinol. 1997;11:779–791. doi: 10.1210/mend.11.6.0007. [DOI] [PubMed] [Google Scholar]
- 2.Ringbom T, et al. COX-2 inhibitory effects of naturally occurring and modified fatty acids. J. Nat. Prod. 2001;64:745–749. doi: 10.1021/np000620d. [DOI] [PubMed] [Google Scholar]
- 3.Hamilton JA. How fatty acids bind to proteins: the inside story from protein structures. Prostag. Leukotr. Ess. 2002;62:65–72. doi: 10.1054/plef.2002.0400. [DOI] [PubMed] [Google Scholar]
- 4.Yaqoob P. Fatty acids as gatekeepers of immune cell regulation. Trends Immunol. 2003;24:639–645. doi: 10.1016/j.it.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 5.Lie Ken Jie MSF, Pasha MK, Syed-Rahmatullah MSK. Fatty acids, fatty acid analogues and their derivatives. Nat. Prod. Rep. 1997;14:163–189. [Google Scholar]
- 6.Irschik H, Augustiniak H, Gerth K, Hoefle G, Reichenback H. The ripostatins, novel inhibitors of eubacterial RNA polymerase isolated from myxobacteria. J. Antibiot. 1995;48:787–792. doi: 10.7164/antibiotics.48.787. [DOI] [PubMed] [Google Scholar]
- 7.Cimino G, De Stefano S, Scognamiglio G, Sodano G, Trivellone E, Sarains A new class of alkaloids from the marine sponge Reniera sarai. Bull. Soc. Chim. Belg. 1986;95:783–800. [Google Scholar]
- 8.Cimino G, Scognamiglio G, Spinella A, Trivellone E. Structural studies on saraine A. J. Nat. Prod. 1990;53:1519–1525. [Google Scholar]
- 9.Becker MH, et al. Total synthesis of (−)-Sarain A. J. Am. Chem. Soc. 2007;129:11987–12002. doi: 10.1021/ja074300t. [DOI] [PubMed] [Google Scholar]
- 10.Kong F, Andersen RJ. Madangamine A, a novel cytotoxic alkaloid from the marine sponge Xestospongia ingens. J. Am. Chem. Soc. 1994;116:6007–6008. [Google Scholar]
- 11.Gerth K, Washausen P, Hofle G, Irschik H, Reichenback H. Antibiotics from gliding bacteria. The jerangolids: a family of new antifungal compounds from Sorangium cellulosum (Myxobacteria): production, physico-chemical and biological properties of jerangolid. J. Antibiot. 1996;49:71–75. doi: 10.7164/antibiotics.49.71. [DOI] [PubMed] [Google Scholar]
- 12.Pospisil J, Marko IE. Total synthesis of jerangolid D. J. Am. Chem. Soc. 2007;129:3516–3517. doi: 10.1021/ja0691728. [DOI] [PubMed] [Google Scholar]
- 13.McNally M, Capon RJ. Phorbasin B and C: Novel diterpenes from a southern Australian marine sponge, Phorbas species. J. Nat. Prod. 2001;64:645–647. doi: 10.1021/np0005614. [DOI] [PubMed] [Google Scholar]
- 14.Macklin TK, Micalizio GC. Total synthesis and structure elucidation of (+)- phorbasin C. J. Am. Chem. Soc. 2009;131:1392–1393. doi: 10.1021/ja809491b. [DOI] [PubMed] [Google Scholar]
- 15.Durand S, Parrain J-L, Santelli M. Construction of (Z,Z) skipped 1,4-dienes. Application to the synthesis of polyunsaturated fatty acids and derivatives. J. Chem. Soc., Perkin Trans. 2000;1:253–273. [Google Scholar]
- 16.Fürstner A, Larionou O, Flügge S. What is amphidinolide V? Report on a likely conquest. Angew. Chem. Int. Ed. 2007;46:5545–5548. doi: 10.1002/anie.200701640. [DOI] [PubMed] [Google Scholar]
- 17.Aïssa C. Mechanistic manifold and new developments of the Julia–Kocienski reaction. Eur. J. Org. Chem. 2009:1831–1844. [Google Scholar]
- 18.Demeunier R, Marko IE. In: Modern Carbonyl Olefination. Takeda T, editor. Wiley–VCH: Weinheim; 2004. p. 104. [Google Scholar]
- 19.Vogel E. Kleine kohlenstoff-ringe. Angew. Chem. 1960;72:4–26. [Google Scholar]
- 20.Hudlicky T, Fan R, Reed JW, Gadamasetti KG. Divinylcyclopropane–cycloheptadiene rearrangement. Org. React. 1992;41:1–133. [Google Scholar]
- 21.Ellis RJ, Frey HM. The thermal isomerisation of cis-1-methyl-2-vinylcyclopropane. P. Chem. Soc. 1964;221 [Google Scholar]
- 22.Parziale PA, Berson JA. Stereochemistry of the thermal homodienyl hydrogen shift reverse ene reaction. Stereoelectronic control of stereogenicity transfer through the anisotropic influence of a cyclopropane ring. J. Am. Chem. Soc. 1990;112:1650–1652. [Google Scholar]
- 23.Parziale PA, Berson JA. Remote control of stereogenicity transfer by ring-generated anisotropic orbital overlap. Stereochemistry of hydrogen shift in the intramolecular reverse ene reaction of a cis-2-alkyl-1-alkenylcyclopropane. J. Am. Chem. Soc. 1991;113:4595–4606. [Google Scholar]
- 24.Lin Y-L, Turos E. Studies of silyl-accelerated 1,5-hydrogen migrations in vinylcyclopropanes. J. Org. Chem. 2001;66:8751–8759. doi: 10.1021/jo0103221. [DOI] [PubMed] [Google Scholar]
- 25.Reichard HA, Micalizio GC. A site- and stereoselective intermolecular alkene–alkyne coupling process. Angew. Chem. Int. Ed. 2007;46:1440–1443. doi: 10.1002/anie.200603515. [DOI] [PubMed] [Google Scholar]
- 26.Doyle MP, Protopopoua MN. New aspects of catalytic asymmetric cyclopropanation. Tetrahedron. 1998;54:7919–7946. [Google Scholar]
- 27.Lebel H, Marcoux J-F, Molinaro C, Charette A. Stereoselective cyclopropanation reactions. Chem. Rev. 2003;103:977–1050. doi: 10.1021/cr010007e. [DOI] [PubMed] [Google Scholar]
- 28.Lansbury PT, Pattison VA, Clement WA, Sidler JD. Preparation and properties of cyclopropylcarbinyllithium. J. Am. Chem. Soc. 1964;86:2247–2251. [Google Scholar]
- 29.Patel DJ, Hamilton CL, Roberts JD. Small-ring compounds. XLIV. Interconversions of cyclopropylcarbinyl and allylcarbinyl Grignard reagents. J. Am. Chem. Soc. 1965;87:5144–5148. [Google Scholar]
- 30.Maercker A, Roberts JD. Small-ring compounds. XLVI. Stabilized cyclopropylcarbinyl anions. A retro cyclopropylcarbinyl-allylcarbinyl rearrangement. J. Am. Chem. Soc. 1966;88:1742–1759. [Google Scholar]
- 31.Charette AB, Naud J. Regioselective opening of substituted (cyclopropylmethyl)lithiums derived from cyclopropylmethyl iodides. Tetrahedron Lett. 1998;39:7259–7262. [Google Scholar]
- 32.Hoffmann RW, Koberstein R. Chiral organometallic reagents. Part XXV. The stereochemistry of the ring opening of cyclopropylallyllithium compounds. J. Chem. Soc., Perkin Trans. 2000;2:595–602. [Google Scholar]
- 33.Hoffmann RW, Koberstein R. The stereochemistry of a retro-carbolithiation reaction. Chem. Commun. 1999:33–34. [Google Scholar]
- 34.Danishefsky S. Electrophilic cyclopropanes in organic synthesis. Acc. Chem. Res. 1979;12:66–72. [Google Scholar]
- 35.Reichard HA, McLaughlin M, Chen MZ, Micalizio GC. Regioselective reductive cross-coupling reactions of unsymmetrical alkynes. Eur. J. Org. Chem. 2010:391–409. doi: 10.1002/ejoc.200901094. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.