Abstract
Clathrin-coated vesicles are known to play diverse and pivotal roles in cells. The proper formation of clathrin-coated vesicles is dependent on, and highly regulated by, a large number of clathrin assembly proteins. These assembly proteins likely determine the functional specificity of clathrin-coated vesicles, and together they control a multitude of intracellular trafficking pathways—including those involved in embryonic development. In this study, we focus on two closely related clathrin assembly proteins, AP180 and CALM (clathrin assembly lymphoid myeloid leukemia protein), in the developing embryonic rat brain. We find that AP180 begins to be expressed at embryonic day 14 (E14), but only in postmitotic cells that have acquired a neuronal fate. CALM, on the other hand, is expressed as early as E12, by both neural stem cells and postmitotic neurons. In vitro loss-of-function studies using RNA interference (RNAi) indicate that AP180 and CALM are dispensable for some aspects of embryonic neurogenesis but are required for the growth of postmitotic neurons. These results identify the developmental staging of AP180 and CALM expression and suggest that each protein has distinct functions in neural development.
Keywords: intracellular trafficking, neural stem cells, postmitotic neurons, dopaminergic
Introduction
Clathrin-coated vesicles are evolutionarily conserved intracellular organelles that are necessary for the cells of species as diverse as unicellular yeast and multicellular plants and animals. At the plasma membrane, clathrin-mediated endocytosis selects and packages membrane-bound cargoes into vesicles and transports them to designated subcellular locations. This process serves a variety of purposes, from transmitting extracellular signals to cells, to preserving the constituents of intracellular organelles and the plasma membrane. The latter function is nowhere better studied than in the synapse of the neuron, which undergoes constant change yet requires instant restoration. It is well-known that clathrin-mediated endocytosis is essential to the prompt and precise retrieval of synaptic vesicle components during bouts of synaptic transmission (Morgan et al., 2002; Murthy and De Camilli, 2003; Ryan, 2006).
Over the past two decades, an understanding of the molecular make-up of the clathrin-coated vesicle has been greatly advanced by the discovery of a large set of proteins referred to as clathrin-associated sorting proteins (Brett and Traub, 2006) or clathrin assembly proteins (Lafer, 2002; Robinson, 2004; Schmid and McMahon, 2007; Ungewickell and Hinrichsen, 2007). Although the majority of these proteins are structurally unrelated to each other, they all have one function in common: regulating the construction or the function of the clathrin-coated vesicle (Lafer, 2002; Robinson, 2004; Brett and Traub, 2006; Schmid and McMahon, 2007; Ungewickell and Hinrichsen, 2007). The existence of this still-expanding list of clathrin assembly proteins, coupled with evidence that some of them are cell type- or subcellular location- specific (Sousa et al., 1992; Metzler et al., 2003; Yao et al., 2003, 2005, 2006), implies that specific combinations of assembly proteins underlie the ability of the clathrin-coated vesicle to shuttle many different cargoes and to operate in distinct cellular and physiological contexts.
We previously showed (Bushlin et al., 2008) that AP180 (Murphy et al., 1991; Morris et al., 1993; Zhou et al., 1993) and CALM (Dreyling et al., 1996; Tebar et al., 1999), clathrin assembly proteins expressed by the neuron (Sousa et al., 1992; Dreyling et al., 1996; Yao et al., 2003, 2005; Petralia and Yao, 2007), performed distinctively different functions in growing neurons. Reduction of the proteins by RNAi revealed that AP180 primarily influences axonal outgrowth whereas CALM has a profound effect on the growth and maintenance of dendrites (Bushlin et al., 2008). Our results support the idea that these assembly proteins may determine the subcellular functional specificity of clathrin-coated vesicles. Our findings also suggest that AP180 and CALM have important functions in neurons prior to synaptogenesis, which hints that clathrin-coated vesicles may have a much wider role in neurons. Surprisingly, however, little has been reported on clathrin-mediated trafficking in the neuron beyond its role in conventional synaptic communication of mature neurons. In this study, we extend our previous study to examine AP180 and CALM in early embryonic brain development during a time window when neural stem cells proliferate and then differentiate into neurons which grow axons and dendrites. We find that AP180 is expressed prominently and exclusively in postmitotic neurons, whereas CALM is broadly present in many cell types including postmitotic neurons, neural progenitors, and embryonic stem cells. By using RNAi to deplete AP180 or CALM from cultured neural progenitors and their neuronal progeny, we elucidate roles for AP180 and CALM in some aspects of embryonic brain development.
Materials and Methods
Animals
Timed pregnant Sprague-Dawley rats (Taconic, Rockville, MD) were used as a source of embryos and postnatal pups. Animal procedures were approved by the National Institute on Aging Animal Care and Use Committee, and followed the NIH Guidelines for the Care and Use of Laboratory Animals.
Antibody characterization
Primary antibodies used in this study are listed in Table 1. The AP180-I antibody (Sigma) stains a single band of ~180 kDa on immunoblots (Ahle and Ungewickell, 1986; Petralia and Yao, 2007). AP180 knockdown by RNAi completely abolishes the staining (immunoblots and immunofluorescent stained cells; Bushlin et al., 2008). The AP180 (clone 34; BD Transduction Lab) antibody shows an identical staining pattern as the AP180-I antibody on both immunoblots and immunofluorescent stained cells (Traub et al., 1999; Drake et al., 2000; Mishra et al., 2004; Petralia and Yao, 2007).
Table 1.
Antibodies used in this study
| Antigen | Immunogen | Host Isotype | Working dilution | Source/Catalog # |
|---|---|---|---|---|
| AP180 | A single protein fraction from purified bovine clathrin coated vesicles | Mouse IgG2b |
1:5,000 for blots 1:500 for staining |
Sigma A4825 (AP180-I) |
| AP180 | C-terminal peptide of rat AP180 (amino acids 706-896) | Mouse IgG1 |
1:500 for blots | BD Transduction Lab A610469 (clone 34) |
| CALM | C-terminal peptide of human CALM (amino acids 634-651) | Goat polyclonal |
1:250 for blots 1:100 for staining |
Santa Cruz sc6433 |
| CALM | C-terminal peptide of rat CALM (amino acids 606-622) | Goat polyclonal |
1:100 for staining | Santa Cruz sc5395 |
| β-actin | N-terminal peptide of the β-isoform of actin (DDDIAALVIDNGSGK) | Mouse IgG1 |
1:5,000 | Sigma A5441 |
| Tuj1 | Purified microtubules from rat brain | Rabbit IgG |
1:200 for sections 1:2000 for cells |
Covance MRB-435P |
| NeuN | Purified cell nuclei from mouse brain | Mouse IgG1 |
1:100 | Chemicon MAB377 |
| SOX2 | Recombinant human SOX2 (amino acids 135-317) | Mouse IgG2a |
1:200 | R&D Systems MAB2018 |
| Vimentin | Purified vimentin fro pig eye lens | Mouse IgG1 |
1:2,000 | Milipore MAB3400 |
| Ki67 | Recombinant human Ki67 | Mouse IgG1 |
1:200 | DakoCytomation M7248 |
| cleaved caspase 3 | N-terminal peptide of human caspases 3 (RGTELDCGIETD) | Rabbit polyclonal |
1:200 | Cell Signaling #9661 |
| TH | Purified tyrosine hydroxylase from rat pheochromocytoma | Rabbit polyclonal |
1:500 | Pel Freez P40101 |
| Nurr1 | C-terminal peptide of rat nurr1 (amino acid 538-598) | Rabbit polyclonal |
1:1,000 | Santa Cruz sc990 |
| pitx3 | KKQRRQRTHFTSQQLQE | Rabbit polyclonal |
1:1,000 | Affinity BioReagents PA1-17059 |
| Nanog | Recombinant human Nanog (amino acids 153-305) | Rat IgG2a |
1:50 | R&D Systems MAB1997 |
| Oct3/4 | N-terminal peptide of human Oct-4 (amino acids 1-134) | Rabbit polyclonal |
1:200 | Santa Cruz sc9081 |
| SSEA4 | Human embryonal carcinoma cell line 2102 Ep | Mouse IgG3 |
1:200 | DSHB MC-813-70 |
| GFAP | Bovine spinal cord homogentate | Mouse IgG2b |
1:200 | BD Pharmigen 556327 |
| S100β | Purified bovine S100β | Mouse IgG1 |
1:500 | Sigma S2532 |
Both CALM antibodies stain the same two bands of 72 and 62 kDa on immunoblots (Meyerholz et al., 2005; Petralia and Yao, 2007). CALM knockdown by RNAi reduces the staining on immunoblots and immunofluorescent stained cells (Bushlin et al., 2008; Harel et al., 2008).
The antibody to β-actin stains a single band of ~50 kDa on immunoblots; it has been characterized (Liao et al., 2000) thus widely used for monitoring protein loading in immunoblotting. This antibody does not cross react with other actin isoforms because it was produced against the N-terminal 15 amino acids peptide of the β-actin (Sigma), the region known to have a low sequence homology among actin isoforms (Lessard, 1988).
The antibody Tuj1 has been shown to react only with neuron-specific class III β-tubulin but not with β-tubulin in glial cells (Menezes and Luskin, 1994; Davies, 2007). The staining pattern we observed is indistinguishable from that reported in previous studies (Menezes and Luskin, 1994; Davies, 2007).
The antibody to neuron-specific nuclear protein NeuN labels specifically the nuclei of postmitotic neurons (Mullen et al., 1992). Consistently, we observe NeuN staining only in the nuclei of mature neurons, never in proliferating cells or newly born neuron. The same NeuN antibody (MAM377, Chemicon) is commonly used including many studies published in Journal of Comparative Neurology (see JCN Antibody Database).
The antibody to SOX2 labels neural progenitors in brain sections in a pattern of cellular morphology ad distribution consistent with previous reports (Graham et al., 2003). This antibody has been shown to recognize only the expected 35 kDa band in lysates from human F9 cells (Yuan et al., 1995).
The antibody to vimentin stains cells in a pattern consistent with previous reports (Osborn et al., 1984; Zerlin et al, 1995). According to the manufacturer, it recognizes a single 60 kDa band on immmunoblots;, and it does not cross react with other related intermediated filament proteins.
The antibody to Ki67 labels the nuclei of proliferating cells (G1, S, G2, and M phases of the cell cycle; Gerdes et al., 1984). According to the manufacturer and previous studies (Gerdes et al., 1984), the antibody staining is absent in resting cell (G0) cells.
The antibody to cleaved caspase 3 specifically stains cells that undergo apoptosis (Martin et al., 2007). According to the manufacturer and previous studies (Marin et al., 2007), this antibody recognizes only the 19 kDa and 17 kDa fragments of cleaved caspase 3 but not the full length caspase 3 (~32 kDa).
The antibody to tyrosine hydroxylase (TH) shows no background staining, but selectively and specifically stains dopaminergic neurons in the ventral mesencephalon; and in in vitro cell systems (Schwartz et al., 2005; Luo et al., 2006). Immunoblots show a single band at ~60 kDa from its immunoprecipitated stratum samples (Haycock, 1989).
The antibody to pitx3 labels the dopaminergic neurons in the ventral mesencephalon—a pattern consistent with previous reports (Smidt et al., 1997; Maxwel et al., 2005; Schwartz et al., 2005). According to the manufacturer, it recognizes only the expected 31.8 kDa band by immunoblotting.
The antibody to nurr1 stains only the cells in the ventral mesencephalon. The staining pattern coincides with the distribution of TH and pitx3.
The antibody to SSEA4 labels only pluripotent stem cells (Kannagi et al., 1983; Thomson et al., 1998; Schwartz et al., 2005). The specificity of this antibody is confirmed by the absence of the staining in differentiated cells (Schwartz et al., 2005; and the present study).
The antibody to Oct3/4 recognizes products of Oct3 (also known as Oct4). It stains the pluripotent stem cells expressing SSEA4, consistent with previous studies (Thomson et al., 1998; Looijenga et al., 2003; Schwartz et al., 2005; Luo et al., 2006). According to manufacturer, it recognizes only the expected ~45 kDa band on immunoblots of F9 cell lysate.
The antibody to Nanog stains pluripotent stem cells, a pattern consistent with previous studies (Chambers et al., 2003; Schwartz et al., 2005; Kerr et al., 2008); and a pattern coincides with the cellular staining with antibodies to SSEA4 and Oct3/4. The specificity of this antibody is confirmed by the absence of the staining in differentiated cells.
The antibody to GFAP has been shown to stain with the glial fibrillary acid protein in differentiated astrocytes, and it does not cross react with other intermediate filaments (Pegram et al., 1985; McLendon et al., 1986). The staining pattern we observed using this antibody coincides with the described distribution of immmunoreactivity obtained with other GFAP antisera (Debus et al., 1983).
The antibody to S100β reacts only with β-subunit of S100, not other members of the EF-hand family proteins (Namba et al., 2005). Immunoblots using this antibody reveal the expected single band at ~10 kDa (Tanga et al., 2006).
For all the above primary antibodies, patterns described as positive staining were not seen when the primary antibody was omit.
Secondary antibodies were obtained from Molecular Probes (Invitrogen) and Jackson ImmunoResearch (West Grove, PA).
Immunoblotting
Whole heads (E12), whole brains (E14), or cerebral cortical tissues (E18 and P2) were dissected and homogenized in ice-cold lysis buffer exactly as described previously (Petralia and Yao, 2007). SDS-PAGE, immunoblotting, and enhanced chemiluminescent detection were carried out using standard protocols.
RT-PCR
Immediately following the collection of tissues (rat E12 to P2) or cells (NTera2), total RNA was extracted using Trizol followed by cDNA synthesis with Superscript First-Strand Strand Synthesis System (Invitrogen). The polymerase chain reaction (PCR) was carried out with RedTaq (Sigma) following the manufacturer’s specifications: 1 μl of cDNA diluted 1:10 in DEPC water, 1 μl of 10 μM forward and reverse primers, 22 μl of DEPC water, 25 μl of RedTaq reagent. The thermal cycling parameters for the PCR reactions were: an initial denaturation for three minutes at 94°C followed by denaturation for 1 min at 94°C; annealing for 1 min at 55°C; extension for 1 min at 72°C and final extension for 7 min at 72°C, 30 cycles were used for reactions. To ensure that RNA samples were not contaminated with genomic DNA during RNA extraction, all samples were tested by running the reverse transcriptase reaction without SuperScript III, and PCR was carried out with GAPDH primers. The information for all PCR primers is listed in Supplementary Table 1.
Immunohistochemistry
Whole heads of E14 rat embryos were dissected and fixed in 4% paraformaldehyde overnight. The tissues were then cryopreserved in sucrose and embedded in Tissue-Tek embedding medium. Sagittal sequential sections of 10 μm were cut on a cryostat, collected onto charged glass slides, and stored at −20 °C for subsequent analysis. A minimum of four embryos were analyzed. Nonspecific staining was blocked by incubating tissue sections with 0.1% Triton X-100 and 10% normal serum (from the same species where the secondary antibody was derived from) in PBS (pH 7.4) for 60 min at room temperature. Sections were incubated with primary antibodies overnight at 4 °C. Following thorough washing in PBS, sections were incubated with appropriate fluorescently tagged secondary antibodies, counter-stained with DAPI, and mounted with PermaFluor Aqueous Mounting Medium (Thermo Fisher Scientific).
The labeled sections were examined using a Zeiss LSM 510 laser scanning confocal microscope. Images were acquired at a 1024 X 1024 pixel resolution and each image was an average of four scans on the same focal plane. The brightness and contrast of the images presented were adjusted in Adobe Photoshop (8.0). The Photoshop-processed images were compiled in Adobe Illustrator (10.0.3). No additional digital image processing was performed. Cell counting was carried out using ImageJ. Antibody immunoreactive cells in each subdivision (cortical plate, subventricular zone, and ventricular zone) of the neuroepithelium were counted and data was expressed as the percent of cells stained in each subdivision. Four embryos (at least three sections from each embryo) were analyzed and statistical analysis was performed using Student’s t test.
Embryonic cell culture and transfection
Telencephalon and mesencephalon of E14 rat embryos were dissected in PBS. Following dissociation using fire-polished glass pipettes, cells were cultured at a density of ~1,000 cells/mm2 on poly-L-lysine (Sigma) and Laminin I (Invitrogen) coated coverslips in Neurobasal medium (Invitrogen) supplemented with N2 (Invitrogen). EGF (20 ng/ml) and FGF (20 ng/ml) were also added to the culture medium to facilitate cell survival and optimize transfection efficiency. Six hours after plating, the cells were replaced with fresh medium and maintained in humidified incubators at 37 °C and 6% CO2·
Cells were transfected either at 6 hours and analyzed 3 days later, or transfected at 3 days analyzed 6 days later. Transfection was carried out using Fugene6 in accordance to the manufacturer’s protocol. Cells, in triplicate sets, were co-transfected with testing pSuper-shRNA (Harel et al., 2008; Bushlin et al., 2008) and EGFP at the ratio of 3:1 (total plasmid DNA was 1 μg per well in 12-well plates). The transfected cells were fixed with 4% paraformaldehyde and permeabilized in PBS containing 0.1% Triton X-100. The cells were then incubated with a primary antibody for a specific marker and followed by an appropriate Alexa 568 secondary antibody.
The transfection experiments were repeated 4–6 times and all three coverslips from each experiment were subjected to microscopic analysis. Microscopic fields that covered the entire coverslip were systematically surveyed and all of EGFP-expressing cells were scored regardless of the presence or absence of the immunostained marker. The number of EGFP-expressing cells positively stained for each marker was calculated.
NTera 2 cell culture
The NTera2 cell line was obtained from American Type Culture Collection (American Type Culture Collection; Manassas, VA) and cultured according to manufacturer’s specifications. Undifferentiated cells were maintained in Dulbecco’s Modified Eagle’s Medium (Invitrogen), supplemented with 10% fetal bovine serum. Cells were passaged once they were approximately 90% confluent at a 1:3 ratio. NTera2 cells were differentiated towards as previously described using PA6 conditioned medium (Schwartz et al., 2005). Undifferentiated NTera2 cells were initially plated on to 0.1% gelatin (Sigma) coated coverslips and differentiated in PA6 conditioned medium. Medium was changed 3 days following the initial plating and then every other day thereafter. Cells were maintained in humidified incubators at 37 °C and 5% CO2·
Results
Expression of AP180 and CALM in embryonic rat brains
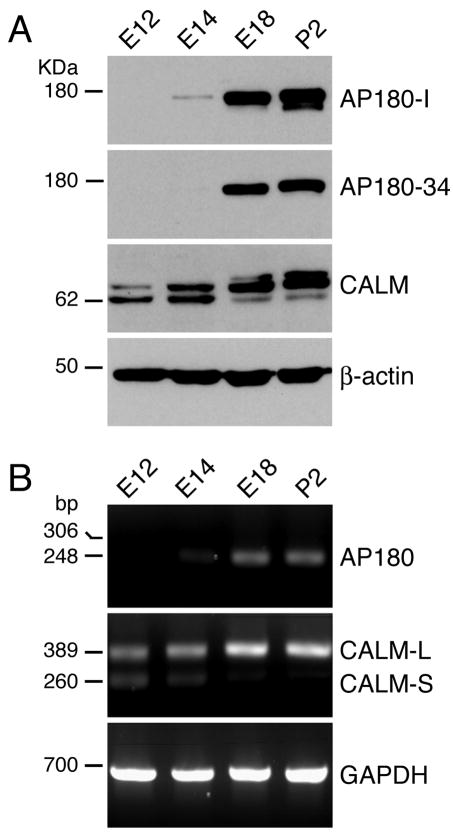
We began our study by examining changes in the expression levels of AP180 and CALM in developing rat brains between embryonic day 12 (E12) and postnatal day 2 (P2). We used two AP180 antibodies, AP180-I and AP180-34, both of which recognize AP180 specifically (Ahle and Ungewickell, 1986; Morris et al., 1993; Traub et al., 1999; Drake et al., 2000; Mishra et al., 2004; Yao et al., 2005)(Table 1). In P2 and E18 brains, AP180 was robustly expressed (Fig. 1A), consistent with a previous finding (Petralia and Yao, 2007). In E14 brains, the AP180 band was faint but clearly revealed by the AP180-I antibody (Fig. 1A, top panel). In E12 brains, no AP180 was detected by either antibody.
Figure 1. The expression levels of AP180 and CALM change during development of the rat brain.
A: Immunoblot analysis of brain tissue extracts from rats of the indicated embryonic (E) and postnatal (P) ages. AP180-I and AP180-34 were two different AP180 antibodies used (see Table 1 for more information).
B: RT-PCR analysis of the tissues from rats of the indicated ages. The PCR primers for AP180 were designed with the intention of revealing the expression of AP180 (GenBank: X68877) and AP180i (GenBank: X68878 or NM_031728) as 248-bp and 306-bp PCR products, respectively. The 306-bp product was not detected in the tissues of any age studied. The PCR primers for CALM were designed to detect the long-splice variant (GenBank: NM_053554) as a 389-bp product and the short variant (GenBank: AF041373) as a 260-bp product. Information on the PCR primers is listed in Supplementary Table 1.
CALM was detectable at an earlier stage in embryonic brain development than was AP180 (Fig. 1A, third panel). We previously confirmed that the two CALM immunoreactive bands (72 kDa and 62 KDa) detected by the CALM antibody correspond to the two CALM splice variants (Petralia and Yao, 2007). Here we found that both CALM splice variants were expressed as early as E12, however their expression levels from E12 to P2 exhibited opposite trends: the long variant displayed a progressive increase as the brain developed, whereas levels of the short variant decreased. We also noticed that, in the E18 and P2 tissues, there was a third band with a slightly higher molecular mass, which might be a glycosylated form of the protein.
RT-PCR analysis revealed that the expression patterns of AP180 and CALM mRNAs (Fig. 1B; Supplementary Fig. S1) in the developing rat brain were remarkably consistent with the patterns of the proteins; notably, (i) a low but clearly measurable level of AP180 mRNA began to appear at E14 and its level increased rapidly thereafter, and (ii) both CALM splice variants were expressed at E12, and their expression levels were opposed to each other—a pattern that mirrored the expression pattern of their protein counterparts. Moreover, there was no third CALM PCR product amplified in either E18 or P2 tissues (Fig. 1B; Supplementary Fig. S1), implying that the third (high molecular mass) band in the blot could be a posttranslationally modified form of CALM. Thus, AP180 and CALM are present in embryonic rat brains during the period in which extensive neurogenesis takes place, and CALM is expressed earlier than AP180 during development.
AP180 is selectively expressed in postmitotic neurons
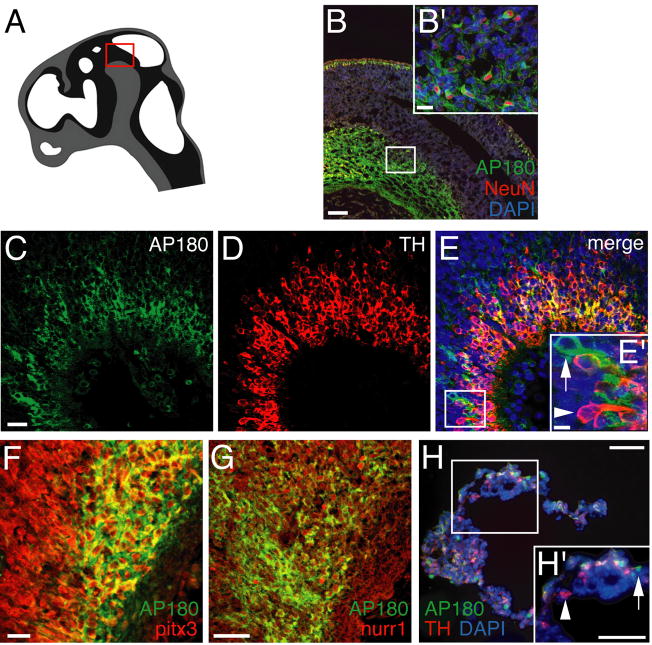
To assess the types of developing cells that express AP180, we performed immunostaining analysis of embryonic brain sections. We used the AP180-I antibody because it is the more sensitive of the two (Fig. 1A; and data not shown). We examined E14 because AP180 begins to be expressed at this age as shown by the immunoblot and RT-PCR analysis (Fig. 1). We focused on the mesencephalon (Fig. 2A) for two reasons: (i) the mesencephalon in E14 rat contains mixed populations of neural cells of both proliferating progenitors and postmitotic neurons, allowing us to study multiple cell types, and (ii) During the initial survey, we noticed that the AP180-expressing cells were enriched in the mesencephalon at E14.
Figure 2. Selective expression of AP180 in postmitotic neurons.
A: Schematic representation of a sagittal view of the E14 rat brain. The red box marks the portion of the mesencephalon where the immunostaining was analyzed. B-G: The distribution of AP180-positive cells (green) in E14 neuroepithelium was almost identical to that of Tuj1-positive cells (red). Most of the AP180-positive cells were located in the cortical plate (CP); only a few of them (arrowheads in E and F) were in the subventricular zone (SVZ) and ventricular zone (VZ). Quantification in G shows no difference in the distribution of AP180-positive and Tuj1-positive cells in each area. n=4 embryos (at least 3 sections from each embryo). H-J: Double labeling of AP180 (green) and NeuN (red) showing that NeuN-positive cells expressed AP180 (arrowheads). K-M: AP180 (green) and SOX2 (red) were expressed by mutually exclusive populations of cells. (More examples are included in Supplementary Figure S2). O-Q: No AP180-positive cells (green), including those in the SVZ and VZ (arrowheads), expressed the cell proliferation marker Ki67 (red). Scale bars: D, 40 μm; F, M and Q, 10 μm; J,5 μm.
We first examined the neuroepithelium of the mesencephalon. We used a classification system that divided the neuroepithelium into three subdivisions: cortical plate (CP), subventricular zone (SVZ), and ventricular zone (VZ). Most of the AP180-expressing cells were concentrated in the CP (Fig. 2B-D), and a few of them were occasionally found scattered in the SVZ or VZ (Fig. 2E,F). To determine the identity of AP180-expressing cells, we double-stained sections with antibodies against AP180 and one of several stage-specific cellular markers. We began with Tuj1, a well-established marker for newly developed neurons (Menezes and Luskin, 1994); we found that the distribution of AP180-expressing cells coincided with that of Tuj1-expressing cells (Fig. 2B-G). Quantitative analysis of doubly-stained cells showed that most of the Tuj1-expressing cells were positively stained with AP180 (78% ± 7; Table 2), and all of the AP180-expressing cells stained with Tuj1 (Fig. 2B-F). Double staining of AP180 with NeuN, a marker for mature postmitotic neurons (Mullen et al., 1992), showed that while all of the NeuN-expressing neurons stained positive for AP180 (Fig. 2H-J; Table 2), it was not the case that all AP180-expressing cells stained positive for NeuN, suggesting that AP180 was not present in both immature and mature neurons. We also co-stained sections with antibodies against AP180 and SOX2, a marker for neural progenitors (Graham et al., 2003), and noted that AP180 and SOX2 were not co-expressed in any cells (Fig. 2K-M; Supplementary Fig. S2). To rule out the possibility that the sporadic AP180-expressing cells located in the SVZ and VZ were mitotic cells, we stained tissues with AP180 and Ki67, a marker for proliferating cells (Gerdes et al., 1984); in no case did we observe a cell co-stained with AP180 and Ki67 (Fig. 2O-Q; Table 2). AP180 is therefore present in newly-generated and mature postmitotic neurons but absent in neural progenitors.
Table 2.
Percent of cells co-expressing one of the following stage-specific cellular markers and AP180 in E14 neuroepithelium.
| Marker | % of positive cells |
|---|---|
| Tuj1 | 78 ± 7 |
| NeuN | 100 ± 2 |
| SOX2 | 0 |
| Ki67 | 0 |
Values are mean percent ± SEM from 4 embryos (at least 3 sections per embryo).
AP180 is present in young dopaminergic neurons
In addition to its presence in the neuroepithelium, AP180 was visibly abundant in cells spatially clustered in the ventral portion of the E14 mesencephalon (Fig. 3A and 3B). It is well established that developing midbrain dopaminergic neurons are situated in the ventral mesencephalon and many of the young neurons have acquired their dopaminergic phenotype by E14 (Altman and Bayer, 1981). To ascertain whether AP180 was expressed by these dopaminergic neurons, we co-stained brain sections with antibodies against AP180 and tyrosine hydroxylase (TH). AP180 was present in most, but not all TH-positive cells (Fig. 3C-E). The proportion of AP180-positive but TH-negative cells varied among tissue sections. Sections from the lateral portion of the ventral mesencephalon had more AP180-positive and TH-negative cells than did sections from the medial portion (compare Fig. 3C-E and Supplementary Fig. S3A-C). High-magnification images further revealed that there were also some TH-positive neurons lacking AP180 (insert in Fig. 3E).
Figure 3. AP180 is present in young dopaminergic neurons.
A: The red box in this schematic representation marks the ventral mesencephalon that houses developing dopaminergic neurons. B: Double stain for AP180 (green) and NeuN (red) showing the presence of AP180-expressing neurons in the ventral mesencephalon. B′ is a high-magnification image of the boxed area in B. Scale bar: B, 50 μm; B′, 10 μm. C-E: AP180 (green) is co-expressed with TH (red) in most neurons, although some neurons contain either AP180 only (arrow in E′) or TH only (arrowhead in E′). Scale bar: C, 20 μm; E′, 5 μm. F, G: Cells in the ventral mesencephalon stain intensely with antibody to pitx3 (red in F) and nurr1 (red in G), and many of pitx3- and nurr1-positive cells express AP180. Scale bars in F and G, 20 μm. H: Differentiated NTear2 cells doubly stained for AP180 and TH. While some cells co-express AP180 and TH, other cells express either AP180 only (arrow in H′) or TH only (arrowhead in H′). Scale bars: H and H′, 10 μm.
The cellular identity of AP180-negative TH-positive cells is unclear. We speculated that the converse set—cells that were AP180-positive TH-negative—may represent a set of young dopaminergic neurons that have yet to express TH at detectable levels. To test this idea, we doubly stained sections with AP180 antibody, and antibodies against pitx3 or nurr1, two transcription factors known to be expressed by developing dopaminergic neurons prior to TH expression (Law et al., 1992; Smidt et al., 1997; Maxwell et al., 2005). Figures 3F and 3G show examples of rat E14 ventral mesencephalon with many cells brightly stained with pitx3 and nurr1 antibodies. AP180 was clearly present in many of the pitx3- and nurr1-positive neurons.
We also examined NTera2 cells, a human embryonic carcinoma cell line used as a pluriponent embryonic stem cell system (Schwartz et al., 2005). When differentiated in culture, NTera2 cells become neuronal precursors that display many features of dopaminergic neurons including the expression of TH and have therefore been used for studying the development of dopaminergic neurons (Schwartz et al., 2005). We double-stained differentiated NTera2 cells with AP180 and TH antibodies and observed a pattern similar to that seen in the rat brain tissue: AP180 was present in some TH-positive cells, although there was a fraction of cells which expressed either AP180 or TH only (Figure 3H and Supplementary Figure S3D).
CALM is present not only in differentiated neurons but also in neural progenitors
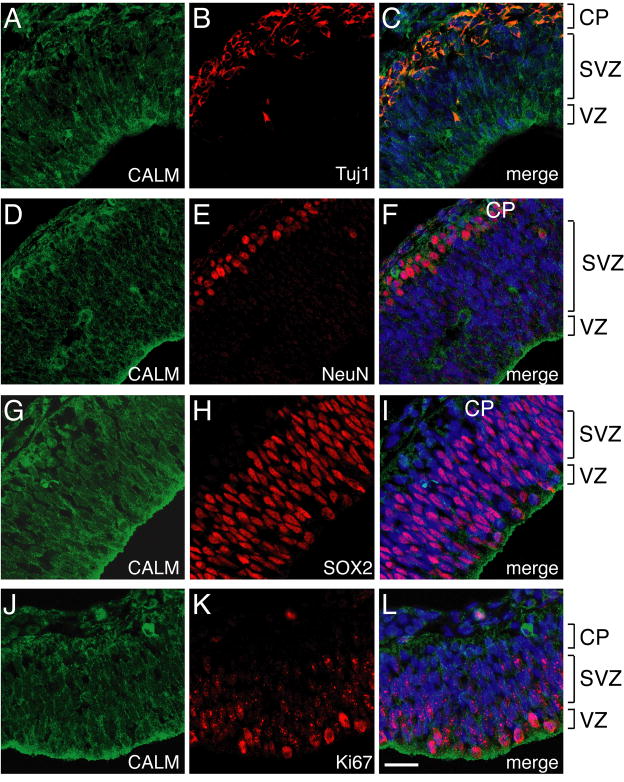
The distribution of CALM in E14 rat brains was distinctively different from that of AP180. CALM expression was more widespread than AP180: CALM was present in numerous cell types and widely distributed throughout the entire neuroepithelium (Fig. 4). This difference suggested that CALM might be present in neural progenitors as well as in mature neurons. To test this, we double-stained CALM with the same set of cell type- and stage-specific markers as AP180 (Fig. 4). Similar results were obtained using a different CALM antibody (sc5395; Table 1)(Supplementary Figure S4). Like AP180, CALM was present in the Tuj1-positive cells (Fig. 4A-C) and NeuN-positive cells (Fig. 4D-F), indicating that CALM is expressed in both young and mature postmitotic neurons. Unlike AP180, CALM was also present in SOX2-positive neural progenitors (Fig. 4G-I). Furthermore, labeling with Ki67 revealed CALM in Ki67-positive proliferating cells throughout the neuroepithelium, from the VZ and to the pia surface (Fig. 4J-L). Thus, CALM is present in neurons and neural progenitors.
Figure 4. CALM is present in differentiated neurons and also in neural progenitors.
A-F: Double staining of CALM (antibody sc6433) with Tuj1 (A-C) and NeuN (D-F) showing CALM’s presence in young and mature neurons. G-L: CALM was also present in SOX2-positive neural progenitors (G-I) and Ki67-positive proliferating cells (J-L). Scale bar: 20 μm (applies all images). Results of CALM staining using a different CALM antibody (antibody sc5395) are shown in Supplementary Figure S4. CALM’s diffuse staining pattern did not permit quantitative analysis as used in Figure 2.
CALM, but not AP180, is present in embryonic stem cells
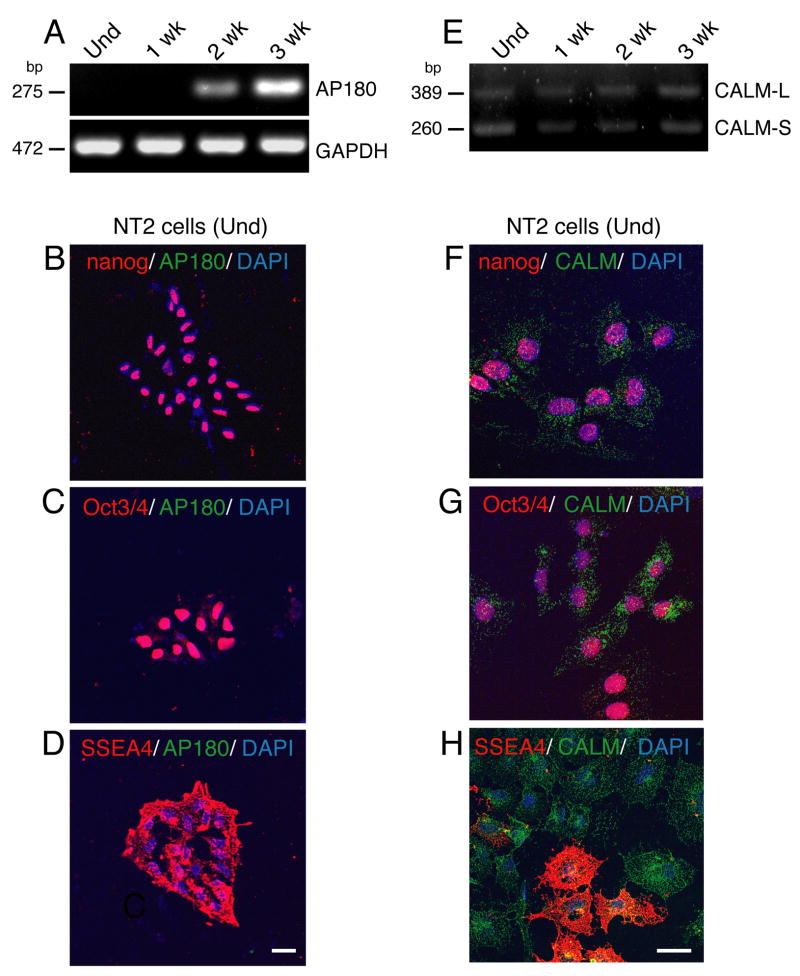
As already mentioned, undifferentiated NTera2 cells possess the phenotype of pluripotent embryonic stem cells, whereas differentiated NTera2 cells lose their pluripotent characteristics and develop into dopaminergic neurons (Schwartz et al., 2005). To extend the analysis of AP180 and CALM to a stage earlier than neural development, we examined undifferentiated and differentiated NTera2 cells. RT-PCR analysis shows that undifferentiated and cells differentiated in culture for only one week were completely devoid of AP180 mRNA expression (Fig. 5A). The absence of AP180 in the undifferentiated NTera2 cells was confirmed by co-staining for AP180 and pluripotent stem cell-markers, Nanog (Chambers et al., 2003), Oct3/4 (Thomson et al., 1998; Looijenga et al., 2003), and SSEA4 (Thomson et al., 1998). The cells positively stained for any of these markers showed no trace of AP180 staining (Fig. 5B-D). After two weeks differentiation, NTera2 cells began to express AP180 mRNA and its level increased in cells with a longer differentiation period—three weeks (Fig. 5A). As assessed by immunostaining, the NTera2 cells differentiated for two weeks expressed Tuj1, and AP180 was evident in the Tuj1-positive cells (Supplementary Figure S5), consistent with mRNA expression. Thus, pluripotent embryonic stem cells do not express AP180, but, during the process of differentiation and as soon as these cells acquire a neuronal identity, AP180 appears.
Figure 5.
CALM is present in human embryonic stem cells, whereas AP180 is expressed only in neurons differentiated from the embryonic stem cells. A: AP180 mRNA was undetectable by RT-PCR in undifferentiated (Und) NTear2 cells and cells differentiated in culture for one week (1week), but became detectable in cells differentiated for two or three weeks. B-D: The undifferentiated NTear2 cells stained positively for embryonic stem cell markers (red), Nanog, Oct3/4, and SSEA4. None of these cells showed AP180 staining (green). Scale bar: 20 μm. E: Unlike AP180, CALM mRNA was expressed in undifferentiated and differentiated NT2 cells. F-H: Double immunostaining of CALM (green) and embryonic stem cell markers (red) in undifferentiated NTear2 cells. Scale bar: 20 μm.
Figure 5E shows that CALM mRNA, on the other hand, was clearly detectable in both undifferentiated and differentiated cells. The duration of differentiation did not seem to affect the level of CALM mRNA (Fig. 5E). Figure 5F-H shows examples of undifferentiated NTera2 cells double-stained for CALM and the above pluripotent stem cell markers. CALM, as revealed in its characteristic punctate staining pattern, was prominent in undifferentiated NTera2 cells that expressed Nanog, Oct3/4 or SSEA 4. Therefore, CALM is expressed in embryonic stem cells.
Reduction of AP180 and CALM does not affect cell specification but causes defective morphology in young neurons
The above analysis shows that AP180 and CALM in rat embryonic brain are expressed in different, but overlapping, stages of development. As a first step toward addressing the question of whether AP180 or CALM is important for embryonic neurogenesis, we carried out experiments using an in vitro cell system (Gauthier-Fisher et al., 2009; Wu et al., 2009). We dissociated E14 (AP180 begins to appear; Fig. 1) cortical cells, depleted AP180 or CALM using RNAi, and then stained the cells with cell type-specific markers. We wanted to know whether the lack or reduction of AP180 or CALM influenced the development of neural cell subtypes.
We used two experimental paradigms: (i) transfecting the cells 6 hours after plating and analyzing them 3 days later (0–3 paradigm), and (ii) transfecting the cells 3 days after plating and analyzing them 6 days later (3-3 paradigm). For the 0–3 paradigm, we focused on examining cell proliferation and cell death by staining the cells with Ki67 and a marker for apoptotic cells, cleaved caspase 3. For the 3-3 paradigm, we focused on cell subtype differentiation by examining cells stained with Tuj1, and Vimentin, a marker for multipotent progenitors (Zerlin et al., 1995). To establish a baseline, we stained the cells with the above markers before RNAi (day 0 for the 0–3 paradigm and day 3 for the 3-3 paradigm); Supplementary Figure S6A and S6B shows the percentage of cells expressing each marker. As expected, the number of Ki67-positive proliferating cells decreased as these cells matured in culture from day 0 to day 3, whereas the number of Tuj1-positive differentiating cells increased over the same period. We also stained the cells with astrocyte markers, GFAP and S100β. Because a negligible number of cells were stained with these markers, we did not study this subset of cells.
Having assessed the cell compositions, we proceeded with the RNAi experiments. We used a vector-based shRNA (pSuper-shRNA; Brummelkamp et al., 2002). The specificity and efficacy of both AP180- and CALM-shRNA and their corresponding control-shRNAs have been characterized and described in our previous studies (Bushlin et al., 2008; Harel et al., 2008). We transfected dissociated cells with AP180- or CALM-shRNA, together with EGFP to visualize transfected cells. The percentage of transfected cells was 1–2% in the 0–3 paradigm and ~5% in the 3-3 paradigm. Low transfection efficiency precluded an analysis of the subsets of the cells that are marginal at this stage, i.e. NeuN-expressing cells, but we can still ask: for the majority cell types at this stage in this uniform setting, does the knockdown of AP180 or CALM affect the number of progenitors and young postmitotic neurons?
We made additional efforts to overcome the low transfection efficiency by plating the cells at high density (~1,000 cells/mm2), repeating each experiment 4–6 times, systematically surveying all microscopic fields, and scoring all EGFP-cells (that is, shRNA-transfected cells). Figure 6A shows the percentage of EGFP-cells positively stained for Ki67. The CALM-shRNA did not change the number of Ki67-positive cells compared to the empty vector or the CALM-control-shRNA (Fig. 6A). Because AP180 is absent in Ki67-positive cells (Figure 2O-Q), we used the AP180-shRNAs as an additional control for this experiment. As expected, neither the AP180-shRNA nor the AP180-control-shRNA had an effect on the number of Ki67-cells. We also immunostained the cells with an antibody to cleaved caspase 3; we detected no difference in the number of cleaved caspase 3-positive cells among the transfected cells (Fig. 6B), indicating that the reduction of AP180 or CALM did not induce apoptosis.
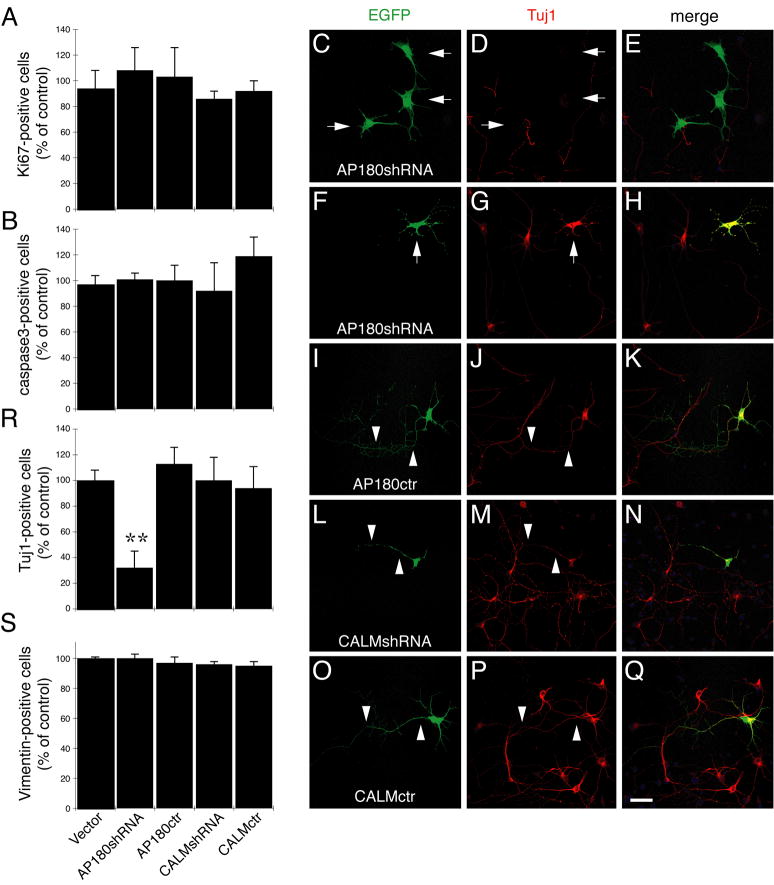
Figure 6.
Effects of AP180 and CALM knockdown in dissociated E14 cortical cells. Cells were co-transfected with the vector or the shRNAs along with EGFP and then were stained for subset markers. All EGFP-cells were scored and the EGFP-cells positively stained for each marker were calculated. Data was expressed as percentage of vector control. A, B: The number of Ki67-positive cells (A) and cleaved caspase 3-positive cells (B) was unaffected by any of the siRNAs. n=4 experiments. C-Q: Microscopic images of representative cortical cells expressed the indicated shRNA (green) and stained for Tuj1 (red). Arrows in C,D indicate that cells expressing AP180-shRNA were not stained for Tuj1. Arrows in F,G indicate an AP180-shRNA cell positively stained for Tuj1. Arrowheads in I-Q mark the axons. Scale bar: 20 μm. R: The number of Tuj1-positive cells was reduced in cells expressing AP180-shRNA. **p=0.007 AP180shRNA versus Vector; p=0.005 AP180shRNA versus AP180ctr. n=6 experiments. S: The number of Vimentin-positive cells was not changed. AP180ctr: control AP180-shRNA; CALMctr: control CALM-shRNA. Error bars in A, B, R, and S represent SEM.
We next asked whether the knockdown of AP180 or CALM affected the subset of young neurons expressing Tuj1. Supplementary Figure S6C shows examples of the cells transfected with the vector; some of the transfected cells were Tuj1 negative and others were Tuj1 positive. However, among AP180-shRNA transfected cells (Fig. 6C-E), while the total number of transfected cells did not change, the number of transfected cells that were also stained positively for Tuj1 was significantly lower than either the vector control or the AP180-control-shRNA cells (Fig. 6R; AP180-shRNA 32%±13% versus vector 100%±8%, p=0.007; AP180-shRNA 32%±13% versus control AP180-shRNA 113%±13%, p=0.005; n=6). Furthermore, among the very few AP180-shRNA-expressing cells that were detectably stained for Tuj1, their appearance was different from a typical neuron: the single long neurite (the presumptive axon; Mattson et al., 1988) was missing, as exemplified in Figure 6F-H. This change was likely specifically shRNA-induced, because AP180-control-shRNA-expressing cells were not only stained for Tuj1 but also continued to display the typical polarized neuronal morphology (Fig. 6I-K).
Unlike AP180-shRNA, CALM-shRNA did not reduce the number of Tuj1-positive cells (Fig. 6R). However, these CALM-shRNA-expressing Tuj1-positive cells also exhibited a change in their morphology that was distinct from the AP180-shRNA-induced change; the cells expressing CALM-shRNA always had the single long neurite, but the rest of shorter neurites, the presumptive dendrites, became rigidly stubby or were even missing (Fig. 6L-N). This morphology was not seen in cells expressing CALM-control-shRNA (Fig. 6O-Q), indicating that it was a specific effect of CALM deficiency.
The observation that the AP180-shRNA reduces the number of Tuj1-positive cells raises the question of whether the knockdown of AP180 prevents neuronal progenitors from becoming postmitotic young neurons. If so, there should be a correlated increase in the number of progenitors because the total cell number did not seem to be affected by AP180 knockdown (judged by unchanged cell proliferation or cell death shown in Figure 6A-B). To address this question, we immunostained for Vimentin—a marker for progenitors (Zerlin et al, 1995). Figure 6S shows that there was no difference in the number of Vimentin-stained cells between AP180-shRNA expressing cells and control cells. Together, these results suggest that the reduction of AP180 and CALM does not exert obvious effects on the proliferation or cell fate of embryonic neural progenitor cells; instead AP180 appears critical for the growth of axons, whereas CALM facilitates dendrite outgrowth of newly generated neurons.
Discussion
We present a comparison of AP180 and CALM—two structurally related clathrin assembly proteins—in the embryonic rat brain. We come to three main conclusions. First, AP180 is expressed in embryonic brains as early as E14 where it is confined to cells that have committed to the neuronal lineage. Second, while CALM is present in neuronal cells that express AP180, it is also present in unspecified progenitors cells (and embryonic stem cells) that do not express AP180. Third, selective reduction of AP180 and CALM protein levels produces different defects on the growth of axons and dendrites in newly developed neurons. Thus, despite the remarkable similarities in the structural motifs and biochemical properties of AP180 and CALM, their functions in the developing nervous system are distinct.
There are a number of studies showing that AP180 has a specific role in the process of clathrin-mediated synaptic vesicle recycling release (Zhang et al., 1998; Morgan et al., 1999; Nonet et al., 1999). Our previous observations of readily detectable AP180 in immature neurons—before they even possess classical synaptic vesicles (Petralia and Yao, 2007; Bushlin et al., 2008)—indicate additional roles for AP180 in young growing neurons. The current study makes an additional contribution to our understanding of AP180 by showing that AP180 is expressed very early in the process of neuronal differentiation. We found that AP180 is widely expressed in different subtypes of neurons and that the appearance of AP180 correlates strikingly well with the birth of young neurons throughout the developing brain. For example, AP180 is highly expressed in the early born dopaminergic neurons located in the rat ventral mesencephalon at E14; the immunostaining pattern of AP180 coincides with the pattern of early markers for dopaminergic neurons. In the neuroepithelium of the E14 brain, the existence of AP180 is noticeably more apparent in the mesencephalon than in the later-developing telencephalon. In addition, the expression of AP180 highly correlates with the expression of Tuj1, a widely-used early neuronal marker. To our knowledge, AP180 is the first clathrin assembly protein that marks newly generated neurons.
Although CALM shares similar structural and biochemical features with AP180 (Hao et al., 1997; Kay et al., 1999; Ford et al., 2001; Mao et al., 2001; Lafer, 2002), these two proteins do not possess redundant functions. The expression of CALM is far more widespread than AP180. At the cellular level, CALM is expressed in all cell types examined (Dreyling et al., 1996; Tebar et al., 1999), whereas AP180 appears to be expressed exclusively in neurons (Sousa et al., 1992; Yao et al., 2003, 2005; Petralia and Yao, 2007). At the subcellular level in neurons, CALM does not appear to be enriched in a particular subcellular compartment, whereas AP180 is concentrated in axons and presynaptic terminals (Sousa et al., 1992; Yao et al., 2002; 2005; Petralia and Yao, 2007). A new finding of this study is that CALM is present in neural progenitors and pluripotent stem cells. For many types of stem cells, communication with the surrounding microenvironment or niche is responsible for specific stem cell activities. Therefore it is likely that the downstream signaling for these interactions requires well-controlled intracellular trafficking. The presence of CALM in these cells implies that CALM serves as one such traffic controller. Interestingly, the two CALM splice variants are differentially regulated during embryonic brain development, with the long variant being up-regulated and the short variant down-regulated between E12 and E18. Because the vast majority of cells are progenitor cells at E12, whereas most cells are postmitotic neurons at E18, the two CALM variants may play different roles in these two cell types.
Our study raises the obvious questions of what AP180 and CALM are for in embryonic neurogenesis, and what functional differences exist between AP180 and CALM that warrant their different expression patterns. To elucidate the functions of AP180 and CALM in regulating the behaviors of neural stem cells and newly generated neurons, we employed RNAi to selective deplete AP180 or CALM in cultured E14 cortical cells. Based upon the results of immunostaining cells with a maker of cell proliferation (Ki67), neither AP180 nor CALM influence the proliferation of neural progenitors. Levels of a cell death marker (cleaved caspase-3) were also not affected by AP180 or CALM knockdown, suggesting that neither AP180 nor CALM affect cell survival. However, we did find that AP180 knockdown significantly reduced the differentiation of cells into Tuj1-positive neurons. In addition, we found that axon growth is impaired in embryonic cortical neurons that lack AP180, whereas dendrite growth is suppressed in neurons lacking CALM. The latter results are similar to those of a previous study of embryonic hippocampal neurons (Bushlin et al., 2008). At this point, too little is known about the cellular mechanisms of AP180 and CALM in axon and dendrite outgrowth. If we assume that the sole function of AP180 or CALM is to regulate clathrin-coated vesicles, these observations (Bushlin et al., 2008; and the present study) suggest that clathrin-mediated intracellular trafficking determines and directs signaling pathways for precise and proper cellular polarization and growth of axons and dendrites. It is possible that different clathrin assembly proteins, or different combinations of these proteins provide the specificity for clathrin-coated vesicles involved in different cellular processes in neurons.
Supplementary Material
Acknowledgments
We thank Drs. Justin D. Lathia and Fengbai Wu for help with immunohistochemistry in the initial stage of the study. We also thank Wayne Rasband for assistance with the ImageJ software.
This work was supported by the Intramural Research Program of the National Institute on Aging of the NIH, and in part by the R&D contract with the MedStar Research Institute.
Literature cited
- Ahle S, Ungewickell E. Purification and properties of a new clathrin assembly protein. EMBO J. 1986;5:3143–3149. doi: 10.1002/j.1460-2075.1986.tb04621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman J, Bayer SA. Development of the brain stem in the rat. V. Thymidine-radiographic study of the time of origin of neurons in the midbrain tegmentum. J Comp Neurol. 1981;198:677–716. doi: 10.1002/cne.901980409. [DOI] [PubMed] [Google Scholar]
- Brett TJ, Traub LM. Molecular structures of coat and coat-associated proteins: function follows form. Curr Opin Cell Biol. 2006;18:395–406. doi: 10.1016/j.ceb.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Brummelkamp TR, Bernards R, Agami R. A system for stable expression of short interfering RNAs in mammalian cells. Science. 2002;296:550–553. doi: 10.1126/science.1068999. [DOI] [PubMed] [Google Scholar]
- Bushlin I, Petralia RS, Wu F, Harel A, Mughal MR, Mattson MP, Yao PJ. Clathrin assembly protein AP180 and CALM differentially control axogenesis and dendrite outgrowth in embryonic hippocampal neurons. J Neurosci. 2008;28:10257–10271. doi: 10.1523/JNEUROSCI.2471-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers I, Colby D, Robertson M, Nichols J, Lee S, Tweedie S, Smith A. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell. 2003;113:643–655. doi: 10.1016/s0092-8674(03)00392-1. [DOI] [PubMed] [Google Scholar]
- Davies D. Temporal and spatial regulation of alpha6 integrin expression during the development of the cochlear-vestibular ganglion. J Comp Neurol. 2007;502:673–682. doi: 10.1002/cne.21302. [DOI] [PubMed] [Google Scholar]
- Debus E, Weker K, Osborn M. Monoclonal antibodies specific for glial fibrillary acidic (GFA) protein and for each of the neurofilament triplet polypeptides. Differentiation. 1983;25:193–203. doi: 10.1111/j.1432-0436.1984.tb01355.x. [DOI] [PubMed] [Google Scholar]
- Drake MT, Downs MA, Traub LM. Epsin binds to clathrin by associating directly with the clathrin-terminal domain. Evidence for cooperative binding through two discrete sites. J Biol Chem. 2000;275:6479–6489. doi: 10.1074/jbc.275.9.6479. [DOI] [PubMed] [Google Scholar]
- Dreyling MH, Martinez-Climent JA, Zheng M, Mao J, Rowley JD, Bohlander SK. The t(10;11)(p13;q14) in the U937 cell line results in the fusion of the AF10 gene and CALM, encoding a new member of the AP-3 clathrin assembly protein family. Proc Natl Acad Sci. 1996;93:4804–4809. doi: 10.1073/pnas.93.10.4804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford MGJ, Pearse BMF, Higgins MK, Vallis Y, Owen DJ, Gibson A, Hopkins CR, Evans PR, McMahon HT. Simultaneous binding of PtdIns(4,5)P2 and clathrin by AP180 in the nucleation of clathrin lattices on membranes. Science. 2001;291:1051–1055. doi: 10.1126/science.291.5506.1051. [DOI] [PubMed] [Google Scholar]
- Gauthier-Fisher A, Lin DC, Greeve M, Kaplan DR, Rottapel R, Miller FD. Lfc and Tctex-1 regulate the genesis of neurons from cortical precursor cells. Nature Neurosci. 2009;12:735–744. doi: 10.1038/nn.2339. [DOI] [PubMed] [Google Scholar]
- Gerdes J, Lemke H, Baisch H, Wacker HH, Schwab U, Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol. 1984;133:1710–1715. [PubMed] [Google Scholar]
- Graham V, Khudyakov J, Ellis P, Pevny L. SOX2 Functions to maintain neural progenitor identity. Neuron. 2003;39:749–765. doi: 10.1016/s0896-6273(03)00497-5. [DOI] [PubMed] [Google Scholar]
- Hao W, Tan Z, Prasad K, Reddy KK, Chen J, Prestwich GD, Falck JR, Shears SB, Lafer EM. Regulation of AP-3 function by inositides. Identification of phosphatidylinositol 3,4,5-trisphosphate as a potent ligand. J Biol Chem. 1997;272:6393–6398. doi: 10.1074/jbc.272.10.6393. [DOI] [PubMed] [Google Scholar]
- Harel A, Wu F, Mattson MP, Morris CM, Yao PJ. Evidence for CALM in directing VAMP2 trafficking. Traffic. 2008;9:417–429. doi: 10.1111/j.1600-0854.2007.00694.x. [DOI] [PubMed] [Google Scholar]
- Kannagi R, Cochran NA, Ishigami F, Hakomori S, Andrews PW, Knowles BB, Solter D. Stage-specific embryonic antigens (SSEA-3 and -4) are epitopes of a unique globo-series ganglioside isolated from human teratocarcinoma cells. EMBO J. 1983;2:2355–2361. doi: 10.1002/j.1460-2075.1983.tb01746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay BK, Yamabhai M, Wendland B, Emr SD. Identification of a novel domain shared by putative components of the endocytic and cytoskeletal machinery. Protein Sci. 1999;8:435–438. doi: 10.1110/ps.8.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Kerr CL, Hill CM, Blumenthal PD, Gearhart JD. Expression of pluripotent stem cell markers in the human fetal testis. Stem Cells. 2008;26:412–421. doi: 10.1634/stemcells.2007-0605. [DOI] [PubMed] [Google Scholar]
- Lafer EM. Clathrin-protein interactions. Traffic. 2002;3:513–520. doi: 10.1034/j.1600-0854.2002.30801.x. [DOI] [PubMed] [Google Scholar]
- Law SW, Conneely OM, DeMayo FJ, O’Malley BW. Identification of a new brain specific transcription factor, Nurr1. Mol Endocrinol. 1992;6:2129–2135. doi: 10.1210/mend.6.12.1491694. [DOI] [PubMed] [Google Scholar]
- Lessard JL. Two monoclonal antibodies to actin: one muscle selective and one generally reactive. Cell Motil Cytoskeleton. 1988;10:349–362. doi: 10.1002/cm.970100302. [DOI] [PubMed] [Google Scholar]
- Liao J, Xu X, Wargovich MJ. Direct reprobing with anti--actin antibody as an internal control for Western blot analysis. Biotechniques. 2000;28:216–218. doi: 10.2144/00282bm05. [DOI] [PubMed] [Google Scholar]
- Looijenga LHJ, Stoop H, de Leeuw HPJC, de Gouveia Brazao CA, Gillis AJM, van Roozendaal KEP, van Zoelen EJJ, Weber RFA, Wolffenbuttel KP, van Dekken H, Honecker F, Bokemeyer C, Perlman EJ, Schneider DT, Kononen J, Sauter G, Wolter J. OosterhuisPOU5F1 (OCT3/4) Identifies Cells with Pluripotent Potential in Human Germ Cell Tumors. Cancer Res. 2003;63:2244–2250. [PubMed] [Google Scholar]
- Luo Y, Schwartz C, Shin S, Zeng X, Chen N, Wang Y, Yu X, Rao MS. A focused microarray to assess dopaminergic and glial cell differentiation from fetal tissue or embryonic stem cells. Stem Cells. 2006;24:865–875. doi: 10.1634/stemcells.2005-0392. [DOI] [PubMed] [Google Scholar]
- Martin LJ, Liu Z, Chen K, Price AC, Pan Y, Swaby JA, Golden WG. Motor neuron degeneration in amyotrophic lateral sclerosis mutant superoxide dismutase-1 transgenic mice: Mechanisms of mitochondriopathy and cell death. J Comp Neuro. 2007;500:20–46. doi: 10.1002/cne.21160. [DOI] [PubMed] [Google Scholar]
- Maxwell SL, Ho HY, Kuehner E, Zhao S, Li M. Pitx3 regulates tyrosine hydroxylase expression in the substantia nigra and identifies a subgroup of mesencephalic dopaminergic progenitor neurons during mouse development. Dev Biol. 2005;282:467–479. doi: 10.1016/j.ydbio.2005.03.028. [DOI] [PubMed] [Google Scholar]
- Mao Y, Chen J, Maynard JA, Zhang B, Quiocho FA. A novel all helix fold of the AP180 amino-terminal domain for phosphoinositide binding and clathrin assembly in synaptic vesicle endocytosis. Cell. 2001;104:433–440. doi: 10.1016/s0092-8674(01)00230-6. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Guthrie PB, Hayes BC, Kater SB. Roles for mitotic history in the generation and degeneration of hippocampal neuroarchitecture. J Neurosci. 1988;8:1704–1711. doi: 10.1523/JNEUROSCI.09-04-01223.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLendon RE, Burger PC, Pegram CN, Eng LF, Bigner DD. The immunohistochemical application of three anti-GFAP monoclonal antibodies to formalin-fixed, paraffin-embedded, normal and neoplastic brain tissues. J Neuropathol Exp Neurol. 1986;45:692–703. doi: 10.1097/00005072-198611000-00007. [DOI] [PubMed] [Google Scholar]
- Menezes JR, Luskin MB. Expression of neuron-specific tubulin defines a novel population in the proliferative layers of the developing telencephalon. J Neurosci. 1994;14:5399–5416. doi: 10.1523/JNEUROSCI.14-09-05399.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzler M, Li B, Gan L, Georgiou J, Gutekunst CA, Wang Y, Torre E, Devon RS, Oh R, Legendre-Guillemin V, Rich M, Alvarez C, Gertsenstein M, McPherson PS, Nagy A, Wang YT, Roder JC, Raymond LA, Hayden MR. Disruption of the endocytic protein HIP1 results in neurological deficits and decreased AMPA receptor trafficking. EMBO J. 2003;22:3254–3266. doi: 10.1093/emboj/cdg334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerholz A, Hinricchsen L, Groos S, Esk PC, Brandes G, Ungewickell EJ. Effect of clathrin assembly lymphoid myeloid leukemia protein depletion on clathrin coat formation. Traffic. 2005;6:1225–1234. doi: 10.1111/j.1600-0854.2005.00355.x. [DOI] [PubMed] [Google Scholar]
- Mishra SK, Hawryluk MJ, Brett TJ, Keyel PA, Dupin AL, Jha A, Heuser JE, Fremont DH, Traub LM. Dual engagement regulation of protein interactions with the AP-2 adaptor alpha appendage. J Biol Chem. 2004;279:46191–46203. doi: 10.1074/jbc.M408095200. [DOI] [PubMed] [Google Scholar]
- Morgan JR, Augustine GJ, Lafer EM. Synaptic vesicle endocytosis: the races, places, and molecular faces. Neuromolecular Med. 2002;2:101–114. doi: 10.1385/NMM:2:2:101. [DOI] [PubMed] [Google Scholar]
- Morgan JR, Zhao X, Womack M, Prasad K, Augustine GJ, Lafer EM. A role for the clathrin assembly domain of AP180 in synaptic vesicle endocytosis. J Neurosci. 1999;19:10201–10212. doi: 10.1523/JNEUROSCI.19-23-10201.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris SA, Schroder S, Plessmann U, Weber K, Ungewickell E. Clathrin assembly protein AP180: primary structure, domain organization and identification of a clathrin binding site. EMBO J. 1993;12:667–675. doi: 10.1002/j.1460-2075.1993.tb05700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen RJ, Buck CR, Smith AM. NeuN, a neuronal specific nuclear protein in vertebrates. Development. 1992;116:201–211. doi: 10.1242/dev.116.1.201. [DOI] [PubMed] [Google Scholar]
- Murphy JE, Pleasure IT, Puszkin S, Prasad K, Keen JH. Clathrin assembly protein AP-3. The identity of the 155K protein, AP 180, and NP185 and demonstration of a clathrin binding domain. J Biol Chem. 1991;266:4401–4408. [PubMed] [Google Scholar]
- Murthy VN, De Camilli P. Cell biology of the presynaptic terminal. Annu Rev Neurosci. 2003;26:701–728. doi: 10.1146/annurev.neuro.26.041002.131445. [DOI] [PubMed] [Google Scholar]
- Namba T, Mochizuki H, Onodera M, Mizuno Y, Namiki H, Seki T. The fate of neural progenitor cells expressing astrocytic and radial glial markers in the postnatal rat dentate gyrus. Eur J Neurosci. 2005;22:1928–1941. doi: 10.1111/j.1460-9568.2005.04396.x. [DOI] [PubMed] [Google Scholar]
- Nonet ML, Holgado AM, Brewer F, Serpe CJ, Norbeck BA, Holleran J, Wei L, Hartwieg E, Jorgensen EM, Alfonso A. UNC-11, a Caenorhabditis elegans AP180 homologue, regulates the size and protein composition of synaptic vesicles. Mol Biol Cell. 1999;10:2343–2360. doi: 10.1091/mbc.10.7.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn M, Debus E, Weber K. Monoclonal antibodies specific for vimentin. Eur J Cell Biol. 1984;34:137–143. [PubMed] [Google Scholar]
- Pegram CN, Eng LF, Wikstrand CJ, McComb RD, Lee YL, Bigner DD. Monoclonal antibodies reactive with epitopes restricted to glial fibrillary acidic proteins of several species. Neurochem Pathol. 1985;3:119138. doi: 10.1007/BF02834285. [DOI] [PubMed] [Google Scholar]
- Petralia RS, Yao PJ. AP180 and CALM in the developing hippocampus: expression at the nascent synapse and localization to trafficking organelles. J Comp Neurol. 2007;504:314–327. doi: 10.1002/cne.21454. [DOI] [PubMed] [Google Scholar]
- Robinson MS. Adaptable adaptors for coated vesicles. Trends Cell Biol. 2004;14:167–174. doi: 10.1016/j.tcb.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Ryan TA. A pre-synaptic to-do list for coupling exocytosis to endocytosis. Curr Opin Cell Biol. 2006;18:416–421. doi: 10.1016/j.ceb.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Schmid EM, McMahon HT. Integrating molecular and network biology to decode endocytosis. Nature. 2007;448:883–888. doi: 10.1038/nature06031. [DOI] [PubMed] [Google Scholar]
- Schwartz CM, Spivak CE, Baker SC, McDaniel TK, Loring JF, Nguyen C, Chrest FJ, Wersto R, Arenas E, Zeng X, Freed WJ, Rao MS. NTera2: a model system to study dopaminergic differentiation of human embryonic stem cells. Stem Cells Dev. 2005;14:517–534. doi: 10.1089/scd.2005.14.517. [DOI] [PubMed] [Google Scholar]
- Smidt MP, van Schaick HS, Lanctot C, Tremblay JJ, Cox JJ, van der Kleij AA, Wolterink G, Drouin J, Burbach JP. A homeodomain gene Ptx3 has highly restricted brain expression in mesencephalic dopaminergic neurons. Proc Natl Acad Sci. 1997;94:13305–13310. doi: 10.1073/pnas.94.24.13305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa R, Tannery NH, Zhou S, Lafer EM. Characterization of a novel synapse-specific protein. I. Developmental expression and cellular localization of the F1-20 protein and mRNA. J Neurosci. 1992;12:2130–2143. doi: 10.1523/JNEUROSCI.12-06-02130.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanga FY, Raghavendra V, Nutile-McMenemy N, Marks A, Deleo JA. Role of astrocytic S100beta in behavioral hypersensitivity in rodent models of neuropathic pain. Neuroscience. 2006;140:1003–1010. doi: 10.1016/j.neuroscience.2006.02.070. [DOI] [PubMed] [Google Scholar]
- Tebar F, Bohlander SK, Sorkin A. Clathrin assembly lymphoid myeloid leukemia (CALM) protein: localization in endocytic-coated pits, interactions with clathrin, and the impact of overexpression on clathrin-mediated traffic. Mol Biol Cell. 1999;10:2687–2702. doi: 10.1091/mbc.10.8.2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- Traub LM, Downs M, Westrich JL, Fremont DH. Crystal structure of the a appendage of AP-2 reveals a recruitment platform for clathrin-coat assembly. Proc Natl Acad Sci. 1999;96:8907–8912. doi: 10.1073/pnas.96.16.8907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungewickell EJ, Hinrichsen L. Endocytosis: clathrin-mediated membrane budding. Curr Opin Cell Biol. 2007;19:417–425. doi: 10.1016/j.ceb.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Wu C, Qiu R, Wang J, Zhang H, Murai K, Lu Q. ZHX2 Interacts with Ephrin-B and Regulates Neural Progenitor Maintenance in the Developing Cerebral Cortex. J Neurosci. 2009;29:7404–7412. doi: 10.1523/JNEUROSCI.5841-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Tam M, Anderson SA. Fate mapping Nkx2.1-lineage cells in the mouse telencephalon. J Comp Neurol. 2008;506:16–29. doi: 10.1002/cne.21529. [DOI] [PubMed] [Google Scholar]
- Yao PJ, Bushlin I, Petralia RS. Partially overlapping distribution of epsin1 and HIP1 at the synapse: analysis by immunoelectron microscopy. J Comp Neurol. 2006;494:368–79. doi: 10.1002/cne.20810. [DOI] [PubMed] [Google Scholar]
- Yao PJ, Coleman PD, Calkins DJ. High-resolution localization of clathrin assembly protein AP180 in the presynaptic terminals of mammalian neurons. J Comp Neurol. 2002;447:152–162. doi: 10.1002/cne.10217. [DOI] [PubMed] [Google Scholar]
- Yao PJ, Petralia RS, Bushlin I, Wang Y, Furukawa K. Synaptic distribution of the endocytic accessory proteins AP180 and CALM. J Comp Neurol. 2005;481:58–69. doi: 10.1002/cne.20362. [DOI] [PubMed] [Google Scholar]
- Yao PJ, Zhang P, Mattson MP, Furukawa K. Heterogeneity of endocytic proteins: distribution of clathrin adaptor proteins in neurons and glia. Neurosci. 2003;121:25–37. doi: 10.1016/s0306-4522(03)00431-7. [DOI] [PubMed] [Google Scholar]
- Yuan H, Corbi N, Basilico C, Dailey L. Developmental-specific activity of the FGF-4 enhancer requires the synergistic action of Sox2 and Oct-3. Genes Dev. 1995;9:2635–2645. doi: 10.1101/gad.9.21.2635. [DOI] [PubMed] [Google Scholar]
- Zerlin M, Levison SW, Goldman JE. Early patterns of migration, morphogenesis, and intermediate filament expression of subventricular zone cells in the postnatal rat forebrain. J Neurosci. 1995;15:7238–7249. doi: 10.1523/JNEUROSCI.15-11-07238.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Koh YH, Beckstead RB, Budnik V, Ganetzky B, Bellen HJ. Synaptic vesicle size and number are regulated by a clathrin adaptor protein required for endocytosis. Neuron. 1998;21:1465–1475. doi: 10.1016/s0896-6273(00)80664-9. [DOI] [PubMed] [Google Scholar]
- Zhou S, Tannery NH, Yang J, Puszkin S, Lafer EM. The synapse-specific phosphoprotein F1-20 is identical to the clathrin assembly protein AP-3. J Biol Chem. 1993;268:12655–12662. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.