Abstract
Macrophages play a central role in tissue homeostasis and the immune system. Their primary function is to internalize cellular debris and microorganisms for degradation within their phagosomes. In this context, their capacity to process and sequester lipids such as triacylglycerides and cholesteryl esters makes them key players in circulatory diseases such as atheroclerosis. To discover new inhibitors of lipolytic processing within the phagosomal system of the macrophage we have developed a novel, cell-based assay suitable for high-throughput screening. We employed particles carrying a fluorogenic triglyceride substrate and a calibration fluor to screen for inhibitors of phagosomal lipolysis. A panel of secondary assays were employed to discriminate between lipase inhibitors and compounds that perturbed general phagosomal trafficking events. This process enabled us to identify a new structural class of pyrazole-methanone compounds that directly inhibit lysosomal and lipoprotein lipase activity.
Keywords: phagosome, high throughput cell-based screen, lipase, hydrolase inhibitor, lysosomal acid lipase, endosomal lipase, lipid homeostasis
Introduction
Traditionally, drug discovery programs are target-based, revolving around defined screens for inhibitors of purified enzymes. These methods have been very successful, however, there are disadvantages because such approaches require an intimate knowledge of the cellular process in question. In contrast cell-based high-throughput screens (HTS) do not assume such knowledge, can interfere at multiple points in a complex cellular process and are, by their efficacy, bioavailable at least at the cellular level. Macrophages play key roles in the pathology of many diseases from infection to atherosclerosis, and in this current study we designed a whole cell HTS to identify inhibitors of the intraphagosomal lipolysis activities that play a critical role in the lipid metabolism of the phagocyte.
Phagocytosis enables internalization of particulate material by macrophages into membrane-bound compartments or phagosomes. Once formed, phagosomes are actively remodeled by vesicular fusion and fission events that modify both the phagosomal membrane and its lumenal contents, a process termed phagosome maturation (1). During maturation the phagosome vacuole becomes increasingly more acidic and acquires hydrolase enzymes before ultimately fusing with pre-existing lysosomal compartments (2,3). During maturation the membrane-bound Vo-ATPase is recruited to the phagosome facilitating acidification of the lumen environment. As the phagosome lumen steadily acidifies the phagosome preferentially interacts with late endosomal compartments and acquires markers associated with the lysosome such as Rab7, syntaxin-7, LAMPs and lysobisphosphatidic acid (4-6).
The primary function of a phagosome is to degrade its lumenal contents. To this end, the phagosome interior acquires and/or activates various hydrolytic enzymes such as proteases, nucleases, and lipases. Recently, we have developed a panel of fluorogenic assays that enables real-time quantitiation of the changes in the lumenal environment during this process. These assays measure pH, phagosome/lysosome fusion and the acquisition of proteinase, lipase and b-galactosidase activities, in addition to measuring the superoxide burst and the lipid peroxidation capacity of the phagosome (7-9). These assays are currently being exploited to generate a mechanistic understanding of how the phagosome is modulated to fulfill the complex functions of the phagocyte both in tissue homeostasis and as an immune effector cell (7,8)
Amongst the many substrates processed within the phagosome are lipids and cholesterol (9). In actuality, the macrophage is a key cell involved in lipid and cholesterol homeostasis (10). Uptake of cholesterol and lipid from cellular debris, and from serum derived LDL particles, is balanced with efflux of the lipid products. When this balance is upset through hypercholesterolemia or chronic pro-inflammatory stimulus the macrophages can convert into foam cells, a cell type characterized by abundant lipid droplets containing cholesterol esters and triacylglycerides (11). Macrophage foam cells contribute to the formation of atherosclerotic plaques and are a significant component in the progression to heart disease. The induction of foamy macrophages has also been implicated in the late stage pathology of infectious disease such as tuberculosis (12).
Hydrolysis of lipids occurs throughout the phagosome maturation process and this activity is commonly attributed to lysosomal acid lipase (LAL) which hydrolyzes cholesterol esters and triacylglycerol in acidic environments (13,14). Additionally, neutral lipase activity has been reported within endosomes and phagosomes indicating the presence of additional lipases that may have pH optima, intracellular trafficking pathways, or substrate specificities which are distinct from LAL (8,15-17). Therefore we took advantage of a whole cell-based screen to identify inhibitors of phagosomal lipase enzymes. Thus, we made no presumptions about the identity of the hydrolases involved, and probed the enzymes in the environment in which they are most active.
The assays exploited in this screen are constructed around a 2-3 μm diameter particle that is derivatized with an appropriate ligand for phagocytosis, a fluorochrome for calibration purposes, and a fluorogenic substrate specific to detect the enzymatic activity of interest. These assays were developed on a spectrofluorometer platform, which generates population-based measurements from approximately 3.5×104 cells. As the experimental particles carry a calibration fluor, the readout is dose-corrected facilitating comparison between samples. This design is ideal for a HTS platform because it facilitates rapid screening by fluorescent plate-reader. Furthermore, by utilizing a panel of secondary assays that measure different parameters of phagosome maturation, we can determine the specificity of any small molecule inhibitors identified in the primary screen. Here we describe application of this novel screening method to identify small molecule compounds that perturb phagosomal maturation events and inhibit intraphagosomal lipolysis.
Materials and Methods
Cells and materials
Macrophages were harvested from the bone marrow of C57BL/6 mice (Jackson Laboratories, Bar Harbor, ME, USA) and maintained in Dulbecco's modified Eagle's medium supplemented with 10% FCS, 2 mM L-glutamine, 1 mM sodium pyruvate and 20% L929-cell conditioned media maintained at 37°C and 7.0% CO2 (18). For fluorometric studies, fully differentiated macrophages were transferred to assay microplates or coverslips for 18 hr to establish confluent monolayers.
Preparation of fluorescent triglyceride substrate beads
3 μm diameter silica Nucleosil C18 reverse phase HPLC beads (Macherey-Nagel, Easton, PA, USA) were coated with a mixture of neutral lipids containing the fluorogenic substrate 1-trinitrophenyl-amino-dodecanoyl-2-pyrenedecanoyl-3-O-hexadecyl-glycerol as previously described by Yates et al (8). Briefly, 2.0 mg C18 HPLC beads in chloroform, 300 μg of 1,2-Dipalmitoyl-sn-Glycero-3-[Phospho-rac-(1-glycerol)] (Avanti Polar Lipids, Alabaster, AL, USA) in chloroform:methanol (2:1 v/v), 5 μg of 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine-N-(Cap Biotinyl) (Avanti Polar Lipids) chloroform:methanol (2:1 v/v), 50 μg cholesterol in chloroform, 25 μg trinitrophenyl-amino-dodecanoyl-2-pyrenedecanoyl-3-O-hexadecyl-glycerol chloroform:methanol (2:1 v/v), and 5 μg octadecyl rhodamine B chloride (Molecular Probes) in chloroform was evaporated under nitrogen. This lipid and bead mixture was resuspended in PBS by sonication at 40°C. The lipid coated beads were placed immediately on ice and washed with 1 mL ice-cold PBS by vortexing and centrifugation at 2000 × g for 1 min three times before incubation with mouse monoclonal anti-biotin IgG (Sigma) 30 min on ice. The IgG-opsonized lipid coated beads were washed three times with 1 mL ice-cold PBS by vortexing and centrifugation at 2000 × g for 1 min. Beads were resuspended in assay buffer (PBS pH 7.2, 1 mM CaCl, 2.7 mM KCl, 0.5 mM MgCl2, 5 mM Dextrose and 5% FCS).
High-throughput phagosomal lipolysis assays
Substrate coated beads in assay buffer were bound to macrophage monolayers in assay plates at a bead to cell ratio of 10 beads per macrophage. Synchronized phagocytosis was accomplished by incubating the cell monolayers with the bead suspension at 37°C for 10 min. Cells were then washed with PBS to remove unbound beads with a Biotek ELx40 plate washer and the cell media was replaced with assay buffer (PBS pH 7.2, 1 mM CaCl, 2.7 mM KCl, 0.5 mM MgCl2, 5 mM dextrose). Fluorescent intensities were recorded in real time at 37°C with a fluorescent plate reader. For endpoint screening of chemical libraries, after the addition of beads, cells were incubated in assay buffer at 37°C in a 7.0% CO2 incubator for 60 min. The cells were subsequently washed with PBS using a Biotek ELx40 plate washer and fixed with 4% paraformaldehyde before endpoint fluorescent measurements were recorded by plate reader. Kinetic experiments and assay development with 384 well plates was analyzed in a Molecular Devices Flexstation II fluorescent plate reader. A Molecular Devices Spectra Max Gemini EM unit was employed for plates in the 96 well format. For primary screening a Perkin Elmer Envision plate reader with automated stacker was used for endpoint evaluation. Hydrolyzed triglyceride substrate emits a fluorescent signal at 400 nm when excited at 342 nm and the rhodamine fluorescent signal was detected at 610 nm when excited at 555 nm.
Automated light microscopy
Compound treated cells were imaged on a Molecular Devices Discovery-1 automatic fluorescence microscope equipped with a Xenon-arc lamp (Perkin-Elmer), a Nikon 10x Plan Fluor objective, and a Photometrics CoolSnapHQ camera (1,392 × 1,040 pixels; Roper Scientific, Tucson, AZ).
Microscopy
Macrophage monolayers were established on glass bottom Petri dishes (MatTek, Ashland, MA, USA) 18 h before use. Images were acquired with a Leica SP5 confocal laser-scanning system with an inverted microscope (Leica Microsystems GmbH). UV excitation of pyrene was accomplished with a Stabilite 2017 argon laser system (Spectra-Physics, Mountain View, CA, USA). Imaging was performed with an HCX PL APO 40x 0.85 dry objective at zoom factor of 3.0. Both lipase reporter bead fluorescence signals were simultaneously acquired using the 351 nm and 561 nm excitation laser lines, and the emission signal was detected in the ranges 400–420 and 600–620 nm, respectively.
Preparation of phagosomal proteolysis beads
Carboxylated 3.0 μm diameter silica beads (Kisker Biotech, Steinfurt, Germany) were washed three times in 1 mL PBS by vortexing and centrifugation at 2000 × g for 1 min. Beads were resuspended in 1 mL PBS with 25 mg/mL carbodiimide and agitated for 15 min. Excess carbodiimide crosslinker was removed by washing the beads three times in 1 mL coupling buffer (0.1 M sodium borate pH 8.0) by vortexing and centrifugation at 2000 × g for 1 min. Beads were resuspended in 500 uL of coupling buffer containing 1.0 mg DQ-green-BSA (Molecular Probes, Eugene, OR, USA), and 0.1 mg human IgG (Sigma) for 12 hours as described (19). Following the coupling reaction, the coated beads were washed three times in quench buffer (PBS pH 7.2, 250 mM glycine) by vortexing and centrifugation at 2000 × g for 1 min to block unreacted carbodiimide. The beads were resuspended in 1 mL coupling buffer containing 50 μg Alexa Fluor 594 succidinimyl ester (Molecular Probes) and agitated for 1 hour. The beads were washed three times in 1 mL quench buffer and three times in 1 mL PBS by vortexing and centrifugation at 2000 × g for 1 min before suspending the beads in assay buffer.
Phagosomal proteolysis assays
DQ-green-BSA coated beads in reaction buffer were bound to macrophage monolayers in assay plates at a bead to cell ratio of 10 beads per macrophage. Synchronized phagocytosis of beads was accomplished by incubation of the cells with beads at 37°C for 10 min. Unbound beads were washed from the wells with PBS in a Biotek ELx40 plate washer. During the washing procedure the cell media was exchanged with assay buffer and the plate was analyzed with a Molecular Devices Flexstation II fluorescent plate reader at 37 °C. Degraded DQ-green-BSA emits a fluorescent signal at 515 nm when excited at 490 nm while the Alexa 594 fluorescent signal was detected at 620 nm when excited at 594 nm.
Lysosomal extract isolation
T75 flasks with confluent macrophage monolayers (5 × 106 cells) were incubated with 5 mL of iron-dextran in H2O (~40 mg/mL iron-dextran) mixed 1:1 with supplemented macrophage media. Cells were pulsed for 2 hr with iron-dextran before rinsing in warm medium, and the iron-dextran was chased into lysosomes for 1 hr. Macrophages were harvested by scraping into homogenization buffer (100 mM MES pH 5.5, 250 mM sucrose, 0.5 mM EGTA, 0.1% gelatin) and lysed by several passages through a 25-gauge needle. Cell debris and unbroken cells were removed by centrifugation at 500 × g for 10 min. The resulting supernatant was applied to a MiniMACS column (Miltenyi Biotech, Auburn, CA) pre-placed in a magnetic stand to retain iron-loaded lysosomes. After two washes with solublization buffer (100 mM MES pH 5.5, 10 mM MgCl2, 10 mM CaCl2) the column was removed from the magnetic stand and lysosomes were eluted with solublization buffer. To prepare lysosomal extracts the column purified material was sonicated on ice with a probe sonicator for 30 seconds. The lysate was centrifuged at 27,000 × g for 10 min at 4°C to remove cellular debris. Final protein concentration of the lysosomal extract was determined using the using the bicinchoninic acid (BCA) protein assay (20)
Fluorescent in vitro lipolysis assays
Evaluation of LPL activity in vitro with the fluorescent triglyceride substrate activity was done largely as described above for the cell based phagosomal lipolysis assays. Briefly, substrate coated beads were added to LPL assay buffer (PBS pH 7.2, 5.0% BSA, 10 mM MgCl2, 10 mM CaCl2) containing bovine milk LPL (Sigma) in 96 well assay plates, 100 μL final assay volume. Final LPL enzyme concentration was 100 U/mL (1 U is reported to release 1.0 nmol of p-nitrophenol per minute at pH 7.2 at 37°C using p-nitrophenyl butyrate as substrate). For the lysosomal acid lipase inhibition assay, substrate coated beads were added to solublization buffer containing 15 μg of the lysosomal extract in 96 well assay plates, 100 μL final assay volume. The assay plates were analyzed in a Molecular Devices Flexstation II fluorescent plate reader at 37 °C. Hydrolyzed triglyceride substrate emits a fluorescent signal at 400 nm when excited at 342 nm and the rhodamine fluorescent signal was detected at 610 nm when excited at 555 nm.
Preparation of the 3H-triacylglycerol hydrolysis beads
To coat beads with [3H]triolein, a solution of 2 mg of 3 μm diameter silica Nucleosil C18 reverse phase HPLC (Macherey-Nagel) particles in chloroform, 1.37 nmol of fatty acid labeled [3H]triolein in toluene, 300 μg of 1,2-Dipalmitoyl-sn-Glycero-3-[Phospho-rac-(1-glycerol)] (Avanti Polar Lipids) in chloroform/methanol (2:1, v/v), and 50 μg cholesterol in chloroform was evaporated under nitrogen. This lipid and bead mixture was resuspended in PBS by sonication at 40° for 2 minutes. These lipid coated particles were placed immediately on ice and washed twice with ice-cold PBS by vortexing and centrifugation at 2000 × g for 1 min and resuspended in assay buffer.
3H-triacylglycerol hydrolysis assay
Lipase activity was measured by the release of [3H]oleic acid from fatty acid labeled [3H]triolein (91 Ci/mmol; American Radiolabeled Chemicals) with a modified method from Belfrage and Vaughan (21). Briefly, 10.0 units bovine milk LPL (Sigma) or 0.2 units human pancreatic lipase (Sigma) was suspended in 100 μL LPL assay buffer containing 6.5 pmol fatty acid labeled [3H]triolein coated silica C18 reverse phase beads and incubated at 37 °C for 1 hr. The assay was terminated by adding of 1 mL of chloroform:methanol:hexane (1.25:1.41:1, v/v/v)and 200 μl of 0.1 M NaHCO3 pH 10.5. Hydrolyzed free fatty acids that partitioned into the aqueous phase were measured by liquid scintillation counting. Enzymatic hydrolysis was confirmed by extracting the reaction mixture with 1 mL of chloroform:methanol (2:1, v/v) and separating the free lipids on silica TLC plates developed in toluene:acetone (95:1, v/v).
Phagosomal lipolysis inhibitor evaluation
Macrophage monolayers were pre-established on sterile glass cover slips and for 18 h. Compounds in DMSO and DMSO alone were added to the cells 10 min prior to bead addition at specified concentrations. Substrate labeled beads in reaction buffer were incubated with the macrophage monolayers at room temperature for 3 min to achieve an average of 1–2 beads internalized per macrophage. Synchronized binding and uptake of the beads was accomplished by washing the cover slips to remove unbound beads before loading the cover slips into quartz cuvettes containing reaction buffer with appropriate compound at specified concentrations or DMSO at 37 °C in a thermostat-regulated QMSE4 spectrofluorometer (Photon Technologies International, Lawrenceville, NJ, USA). Fluorescent emissions were obtained from approximately 20 mm2 of the macrophage monolayer or approximately 3.5 × 104 cells. At the conclusion of the experiment each cover slip was examined via light microscopy to ensure cell viability and internalization of the beads.
Results
We developed a phagosomal lipase activity assay that monitors conversion of the triglyceride substrate (1-trinitrophenyl-amino-dodecanoyl-2-pyrenedecanoyl-3-O-hexadecyl-glycerol) into a fluorescent product within macrophage phagosomes (8). Hydrolysis of this triglyceride substrate liberates the pyrenedecanoic acid from the adjacent trinitrophenyl quencher liberating pyrene fluorescence (22). We deliver this triglyceride substrate to macrophage phagosomes on IgG opsonized, 3 μm silica-C18 reverse phase HPLC beads co-coated with neutral lipids and the calibration fluor, acyl-rhodamine. Phagosomal hydrolysis of the triglyceride substrate allows pyrene fluorescence at 400 nm, following excitation at 342 nm, while the constant fluorescent emission from the rhodamine is measured simultaneously facilitating ratiometric normalization. The kinetics of phagosomal triglyceride hydrolysis is reported as a ratio of pyrene fluorescence to rhodamine fluorescence.
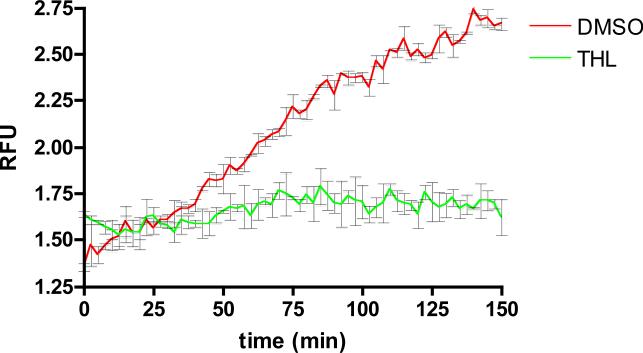
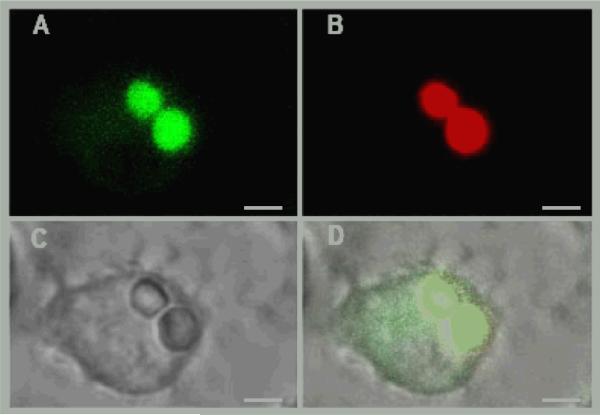
For screening purposes this phagosomal lipolysis assay was adapted to a 384 well plate format using primary murine macrophage cells as the phagocytic cell. In this assay, substrate coated beads were fed to macrophages in 384 well plates and rinsed to facilitate synchronized phagocytosis. During and after phagocytosis the pyrene and rhodamine emission signals were acquired every 2 minutes by alternating excitation and emission wavelengths. The kinetics of substrate hydrolysis was calculated as the ratio of pyrene to rhodamine signal and these data were plotted as a function of time. Kinetic data obtained from these analyses revealed continued hydrolysis of triglyceride substrate exhibiting a near linear increase in pyrene fluorescence for 150 min before the assay reached substrate limitation (Figure 1). The unquenched pyrene fluorescence signal and rhodamine fluorescence remains bead associated over the entire time course of the assay (Figure 2).
Figure 1. Macrophage phagosomal triglyceride lipolysis.
The kinetics of triglyceride substrate hydrolysis was plotted as relative fluorescence units or RFU. RFU is the ratio of pyrene fluorescence to rhodamine fluorescence. RFU was calculated using the equation RFU = PF/RF, where PF is the background subtracted pyrene fluorescence and RF is the background subtracted rhodamine fluorescence. 384 well plate measurements were taken every 2.5 min for 150 minutes. THL was added 10 min prior to bead addition at a concentration of 10 μM. The trace represents the averaged values over two repetitions, error bars represent SD.
Figure 2. Pyrene fluorescence signal remains bead associated within a macrophage phagosome.
Image of lipolysis reporter beads acquired 45 minutes following phagocytosis. Bead associated pyrene fluorescence (A.) rhodamine fluorescence (B.) brightfield (C.) and merged images (D.). Pyrene fluorescent signal is pseudocolored green and the scale bar represents 3 μm.
Based on these 384 well microplate results, a screening endpoint was fixed at 60 min within the linear portion of the assay to enable identification of compounds that impacted lipolytic activity either negatively or positively. The assay was terminated by washing the cells with PBS and fixing with 4 % paraformaldehyde before fluorometric analysis. To establish a screening coefficient or Z-factor, a pilot screen was conducted against approximately 4,000 compounds alongside 10 μM tetrahydrolipstatin (THL) and DMSO controls. THL is a potent general lipase inhibitor (23), and at 10 μM it significantly inhibits phagosomal lipase activity; it therefore served as a positive control throughout our experimentation (Figure 1). The Z-factor was calculated using the equation Z = 1-[3σc++3σc-]/|μc+-μc-| where σc+ and σc- represent the standard deviations of the positive and negative controls, respectively μc+ and μc- represent the mean values of the positive and negative controls, respectively (24). In this validation screen the signal-to-background range and well-to-well variance were acceptable with a Z-factor of 0.7-0.8 per plate indicating that the screen will have a strong probability of being reproducible and yield quality hit compounds.
High-throughput Screening
The 384 well phagosomal lipolysis endpoint assay was used to screen against approximately 80,000 annotated chemical compounds from combinatorial libraries, known drugs, experimental bioactive compounds, and pure natural products at the Cornell-Rockefeller University High Throughput Screening Center. For screening, compounds in DMSO were added to the macrophages for 4 hr prior to bead phagocytosis at a concentration of 10 μM in single wells. THL and DMSO controls were introduced into all plates throughout the screen to calculate percent inhibition. Percent inhibition was calculated with the equation PI = x-s/[(x-μc+)/100] where x is the median of the normalized pyrene signal from the entire plate, s represents the normalized pyrene signal from the compound treated well, and μc+ is the mean of the normalized pyrene signal from the THL treated wells.
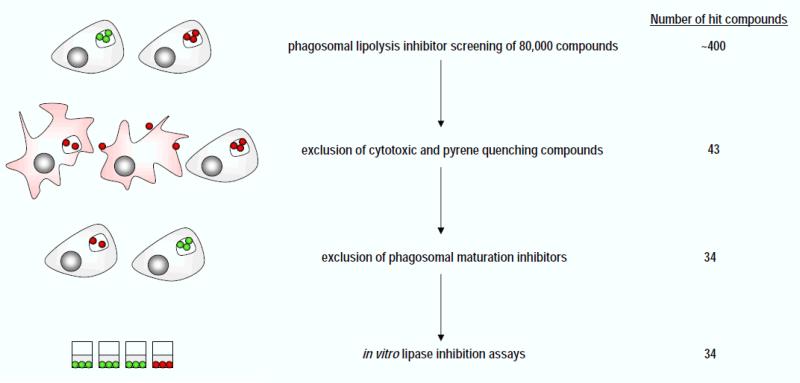
Screening against the entire library identified approximately ~400 compounds that reduced the pyrene substrate signal by at least 80% compared to the THL control. Images of cells and beads treated with each of these ~400 putative hits were acquired via automated microscopy with a Molecular Devices Discovery-1 microscopy platform. For each well the focal plane was established at the bottom of each well by automated laser-based scanning. Both brightfield and fluorescent TRITC (535 nm excitation, 610 nm emission) images were automatically captured. Manual analysis of brightfield and fluorescence images verified that a reduction in both the pyrene and rhodamine signals frequently correlated with visible signs of cytotoxicity and/or reduced phagocytosis. Signs of cytotoxicity were frequently observed as macrophage lysis or cell detachment while reduced phagocytosis was observed when beads were bound to the macrophage surface without internalization. Based on these manual analyses approximately 325 compounds were excluded from further analysis due to apparent cytotoxicity (data not shown). Of the remaining compounds, 32 were excluded due to their inherent capacity to quench pyrene fluorescence. Pyrene quenching was assessed by adding hit compounds to assay plates containing pre-hydrolyzed substrate beads and measuring pyrene fluorescence (data not shown). In total, primary screening identified 43 putative hits that appeared to inhibit phagosomal lipase activity. The general scheme of our phagosomal lipolysis inhibitor screening strategy is summarized in (Figure 3).
Figure 3. Experimental pipeline for identifying inhibitors of macrophage phagosomal lipolysis.
In a primary screen, 80,000 compounds were assayed directly for inhibition of phagosomal lipolysis. Secondary screens were employed to exclude cytotoxic compounds, pyrene quenchers, and off-target compounds that inhibit phagosomal maturation. Lastly, in vitro assays with LPL and lysosomal extracts verified lipase inhibition by the hit compounds.
Phagosome Maturation Inhibitors
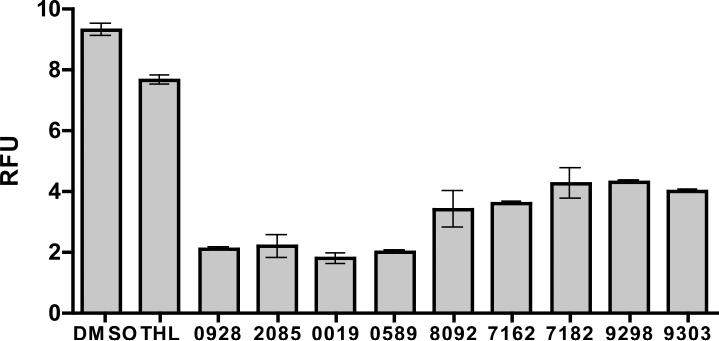
Delivery and activation of phagosomal hydrolases is an important property linked to maturation events of the phagosome. To exclude compounds that perturb phagosomal maturation, the 43 putative lipolysis inhibitors were evaluated with an assay that monitors phagosomal protease activity, an alternative and reliable indicator of phagosome maturation (8). As the phagosome matures the compartment acquires proteases usually as pro-enzymes, that become processed and activated in the increasingly acidic milieu of the maturing phagosome. To monitor phagosomal proteolysis, beads coated with DQ-Green bovine serum albumin (DQ-Green-BSA) were fed to macrophages and the extent of phagosomal proteolysis was determined as previously described (19). Briefly, DQ-Green-BSA is heavily derivatized with dipyrromethene boron difluoride (BODIPY) which exhibits reduced fluorescence due to self-quenching. Upon proteolytic digestion the DQ-Green-BSA becomes dequenched releasing fluorescent BODIPY labeled peptides. Of the 43 putative lipolysis inhibitors, 9 compounds inhibited phagosomal proteolysis indicating that phagosome maturation was altered by these compounds therefore they were excluded from further study (Figure 4). The remaining 34 compounds had no influence on the phagosomal proteolysis profile (data not shown) indicating that their activity in the primary assay was likely due to inhibition of lipase enzymes.
Figure 4. Macrophage phagosomal proteolysis/maturation inhibitors.
Phagosomal proteolysis of bead associated DQ-green-BSA expressed as RFU. RFU was calculated as the ratio of BODIPY to Alexa 594 fluorescence. RFU was calculated using the equation RFU = BF/RF, where BF is the background subtracted BODIPY fluorescence and AF is the background subtracted Alexa594 fluorescence. Endpoint measurements were acquired from 96 well plates at 150 minutes. Compounds were added 10 min prior to bead addition at a concentration of 10 μM. The graph represents the averaged values over two repetitions, error bars represent SD.
Phagosomal Lipolysis Inhibitors
Of the remaining 34 hit compounds, 25 possessed a distinct pyrazole-methanone core structure and 13 of these pyrazole-methanone compounds were purchased from Albany Medical Research, Inc in milligram quantities for further characterization (Figure 5A). In addition, 7 other putative lipase inhibitor compounds identified in the screen but possessing unrelated structures (data not shown).
Figure 5. Whole cell pyrazole-methanone phagosomal lipase inhibitors.
A. Chemical structures of the 13 pyrazole-methanone hit compounds studied in detail. B. Endpoint analysis of phagosomal lipolysis plotted as RFU. RFU was calculated using the equation RFU = PF/RF, where PF is the background subtracted pyrene fluorescence and RF is the background subtracted rhodamine fluorescence. Endpoint measurements were taken at 180 minutes in a 96 well assay format. Pyrazole-methanone compounds and THL were added to cells 10 min prior to bead addition at a concentration of 10 μM. The graph represents the averaged values over two repetitions, error bars represent SD.
The 13 pyrazole-methanone compounds were re-evaluated in our macrophage phagosomal lipase inhibition assay in a 96 assay format. Each of these 13 compounds displayed greater than 50% inhibition of phagosomal lipolysis at a concentration of 10 μM (Figure 5B). Previous analyses of phagosomal lipolytic activity have indicated that multiple different lipases may be active within the phagosome at different stages of the maturation process (8), therefore it is unclear which of the lipolytic enzymes present in the maturing phagosome may be impacted by the putative inhibitors. To establish lipase specificity the 13 pyrazole-methanone compounds were next tested for inhibitory activity against a model neutral lipase and acid lipase containing lysosomal extracts.
We first tested the 13 pyrazole-methanone compounds’ ability to directly inhibit bovine milk lipoprotein lipase (LPL) in a cell free assay. LPL is a model neutral lipase of the hepatic lipase family which has been reported to reside within early endosome compartments (15,17). Fluorescent substrate coated beads were added to 100U/mL LPL in the presence of compounds at 37°C. The 13 pyrazole-methanone compounds all displayed greater than 25% direct inhibition of LPL activity (data not shown). Of these the most effective bovine LPL inhibitors were the compounds A3, E4, F2, H2, and H4 (Figure 6A). These five pure compounds inhibited bovine LPL in a dose dependent manner in vitro in the concentration range of 1.0-100 μM. Interestingly, compounds A3, E4, H2, and H4 all contain a distinct nitrophenyl pyrazole group. Compound F2 also possesses a nitro group located on the distal end of the pyrazole-methanone scaffold.
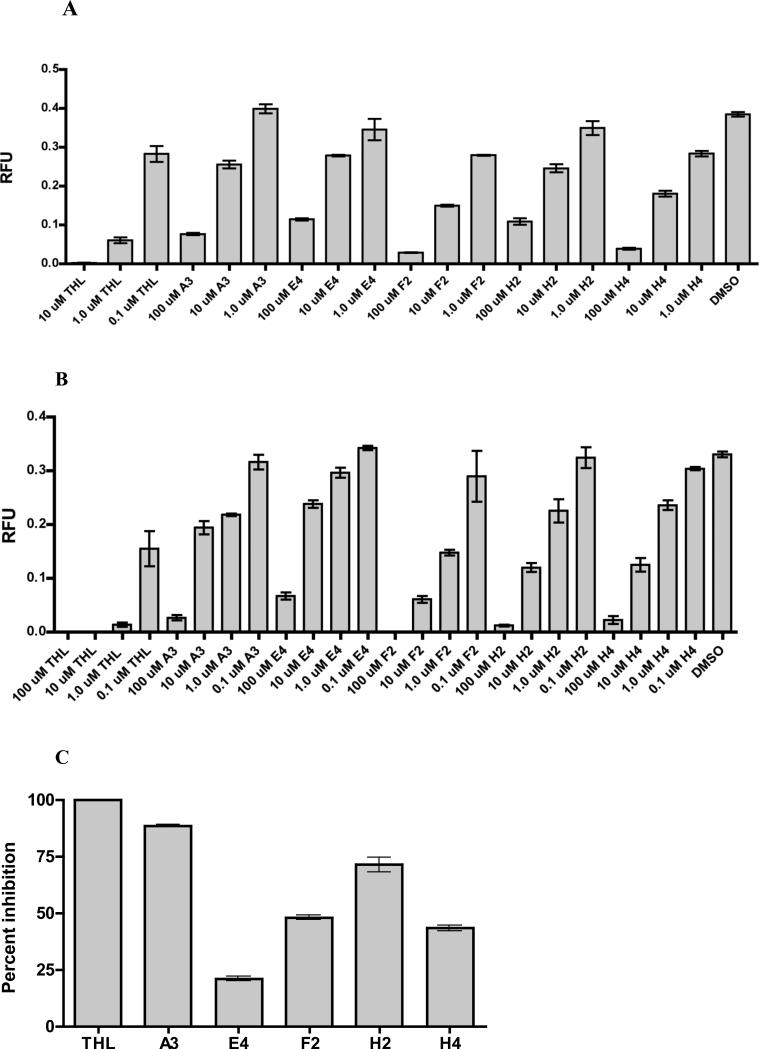
Figure 6. Pyrazole-methanone lipoprotein lipase and lysosomal acid lipase inhibitors.
A. In vitro inhibition of lipoprotein lipase mediated lipolysis plotted as RFU. RFU was calculated using the equation RFU = PF/RF, where PF is the background subtracted pyrene fluorescence and RF is the background subtracted rhodamine fluorescence. Endpoint measurements were taken at 180 minutes in a 96 well assay format. B. Inhibition of lysosomal extract derived acid lipolysis plotted as RFU. Endpoint measurements were taken at 180 minutes in a 96 well assay format. C. Inhibition of lipoprotein lipase catalyzed [3H]oleic acid release from [3H]triolein expressed as percent inhibition compared to THL. The graph represents the averaged values over two repetitions, error bars represent SD.
The compounds were tested against macrophage-derived lysosomal extracts. To obtain lysosomal extracts, macrophages were pulsed with fluid phase iron dextran, which was chased into lysosomal compartments for a 2 hr incubation. Cells with iron loaded lysosomes were harvested, mechanically lysed, and the lysosomal compartments were isolated with a magnetic separation column as described (25,26). A pH of 5.5 was maintained throughout the isolation procedure to ensure that the lysosomal enzymes with acidic pH optima were maintained in an active state. The final lysosomal extracts were generated by probe sonicating the column purified lysosomes in a pH 5.5 buffer. These lysosomal extracts are enriched with lysosomal hydrolases including one or more lysosomal lipases with acidic pH optima. These lysosomal extracts served as the enzyme source of LAL and were tested against the compounds A3, E4, F2, H2, and H4. The compounds A3, E4, F2, H2, and H4 inhibited lysosomal extract lipase activity in a dose dependent manner in vitro across the concentration range of 1.0-100 μM (Figure 6B). At a concentration of 10 μM these inhibitors displayed inhibition of lysosomal extract lipase activity similar to what was observed in the whole cell assays at a concentration of 10 μM.
We next verified the above findings using a authentic triacylglycerol substrate in a bovine LPL hydrolysis assay (21). This assay monitors LPL catalyzed release of [3H]-labeled fatty acids from lipid labeled [3H]-triolein. For this assay, released [3H]-labeled fatty acids were quantified by extracting the free fatty acids into alkaline organic solvent and scintillation counting. For experimental continuity we coated the [3H]-triolein substrate onto reverse phase HPLC beads similar to the substrate coating for the fluorescent lipolysis assays. In the [3H]-fatty acid hydrolysis assay, the compounds A3, E4, F2, H2, and H4 displayed 20-80 percent inhibition of bovine LPL activity at a concentration of 100 μM (Figure 6C). In summary the compounds A3, E4, F2, H2, and H4 are all able to directly inhibit LPL and LAL but do not inhibit human pancreatic lipase (data not shown).
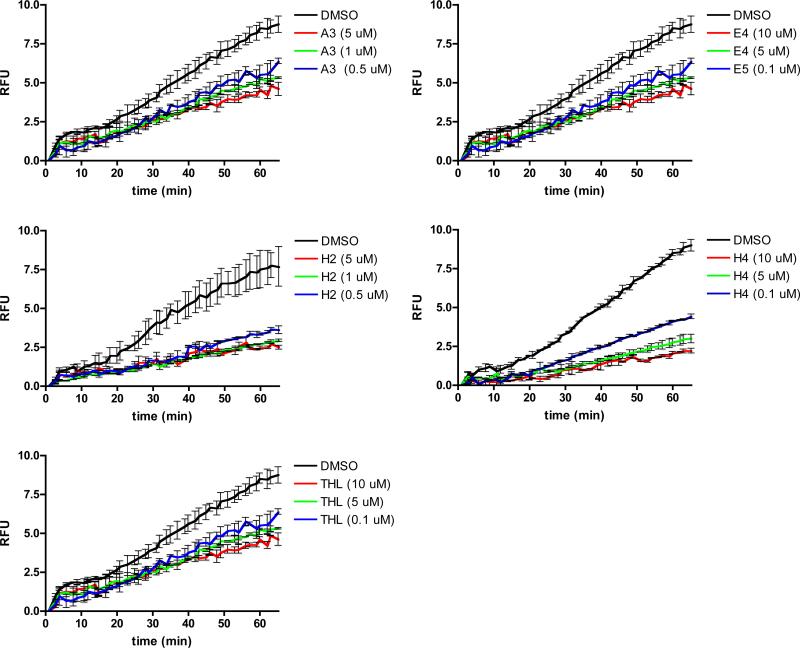
Lastly we evaluated the potency of these compounds in the biological context of our whole cell assay. For these analyses we employed a more sensitive spectrofluorometer assay. In this assay we observed inhibition of macrophage phagosomal lipolysis by compounds A3, E4, H2, and H4 across the concentration range of 0.1-10 μM (Figure 7). These effective concentrations compare favorably to the experimentally determined effective concentrations of THL in our whole cell phagosomal lipolysis inhibition assays.
Figure 7. Compound potency in phagosomal lipolysis assay.
A. Inhibition of phagosomal lipolysis of bead associated triacylglyceride fluorogenic substrate plotted as RFU. RFU was calculated using the equation RFU = PF/RF, where PF is the background subtracted pyrene fluorescence and RF is the background subtracted rhodamine fluorescence. Kinetic measurements were taken every minute for 70 minutes. Compounds were added 10 minutes prior to bead addition at specified concentrations. The graph represents the averaged values over two repetitions, error bars represent SD.
Discussion
In this study we describe an accessible screening methodology with primary and secondary, cell-based assays that facilitate identification of phagosomal hydrolase inhibitors while eliminating compounds with off-target effects on phagosome maturation. With this screening pipeline we have identified a new class of pyrazole-methanone lipase inhibitors. Lipase inhibition by these compounds was validated using the whole cell phagosomal lipolysis assays and in vitro lipolysis assays with both bovine LPL and LAL containing macrophage lysosomal extracts. The potency of the pyrazole-methanones inhibitors was established in a cell based assay for phagosomal lipolysis. Our studies demonstrated an effective concentration range between 0.1-10 μM in our most sensitive cell based assay. Within this concentration range the pyrazole-methanones inhibitors displayed potency that compares directly to the potent lipase inhibitor THL.
The high degree of structural similarities found in the compounds we identified by screening is remarkable. Of the 80,000 annotated compounds screened, the pyrazole-methanone scaffold was represented in 41 total compounds; we experimentally identified 25 of these in our primary screening. Of the 13 pyrazole-methanone compounds that we studied in detail the most potent phagosomal lipase inhibitors were of the nitrophenyl pyrazole-methanone compound subset. The nitrophenyl pyrazole-methanone compounds A3, E4, H2, and H4 have not yet been reported in the PubChem BioAssay database (27). To our knowledge these compounds have not been previously identified as lipase inhibitors. Ongoing studies are now designed to understand the structural features of these pyrazole-methanone compounds that are responsible for LPL and LAL inhibition. A benefit to cell based screening is that non-toxic compounds with reasonable pharmacokinetic properties are selected with relative ease. Lastly, we did not notice any apparent macrophage cytotoxicity associated with the pyrazole-methanone class of compounds.
Our cell-based screening pipeline has proven to be reliable at identifying specific inhibitors of phagosomal lipolytic enzymes. Considering the dynamic and multifactorial nature of the phagosome, one would predict that inhibitors of many classes could alter phagosomal enzyme activities. Because of this, our cell-based screening pipeline includes the necessary secondary assays to identify compounds that exert off target effects by altering overall phagosome function. Much of our pipeline is automated sans, the visual inspection of the primary hit treated cells. Visual inspection was conducted to identify obvious cytotoxic compounds. This manual step could be avoided in future screens by multiplexing our reporter beads to assay multiple different phagosomal parameters simultaneously. Multiplexing our assay would allow us to identify cytotoxic and off-target compounds in a single automated assay step. Alternatively, cytotoxic compounds could be identified based on live/dead staining of the treated cells. This approach would require compound cherry-picking and an additional assay step.
The fact that we have a panel of assays that can record the changing environments within the phagosomal milieu clears the way to the development of a range of HTS assays to identify inhibitors of specific phagosomal functions. These assays have proven to be extremely robust and highly flexible, facilitating analysis by plate reader, spectrofluorometer, confocal microscope and flow cytometry (28). We predict that these screens will identify compounds that modulate antigen presentation (proteolysis) (7), inflammatory responses (oxidative burst) (29), in addition to modulators of lipid metabolism. Because the macrophage, and other professional phagocytes, lie at the fulcrum of many homeostatic and pathological processes these novel, real-time readouts offer unique opportunities for the identification of new lead compounds for drug development.
Acknowledgments
We thank Charles Karan and Ronald Realubit of the Rockefeller-Cornell HTS facility for assistance in conducting the initial compound screens. This work was supported by US Public Health Services grants AI 057086 and AI080651 to DGR.
Footnote: Supported by the National Institutes of Health grants AI057086 and AI080651
Abbreviations
- EGTA
ethylene glycol-tetraacetic acid
- FCS
fetal calf serum
- IgG
immunoglobulin-G
- LAL
lysosomal acid lipase
- LPL
lipoprotein lipase
- MES
2-(4-morpholino)-ethane sulfonic acid
- THL
tetrahydrolipstatin
Literature Cited
- 1.Huynh KK, Kay JG, Stow JL, Grinstein S. Fusion, fission, and secretion during phagocytosis. Physiology (Bethesda) 2007;22:366–72. doi: 10.1152/physiol.00028.2007. [DOI] [PubMed] [Google Scholar]
- 2.Rohde K, Yates RM, Purdy GE, Russell DG. Mycobacterium tuberculosis and the environment within the phagosome. Immunol Rev. 2007;219:37–54. doi: 10.1111/j.1600-065X.2007.00547.x. [DOI] [PubMed] [Google Scholar]
- 3.Scott CC, Botelho RJ, Grinstein S. Phagosome maturation: a few bugs in the system. J Membr Biol. 2003;193(3):137–52. doi: 10.1007/s00232-002-2008-2. [DOI] [PubMed] [Google Scholar]
- 4.Desjardins M, Huber LA, Parton RG, Griffiths G. Biogenesis of phagolysosomes proceeds through a sequential series of interactions with the endocytic apparatus. J Cell Biol. 1994;124(5):677–88. doi: 10.1083/jcb.124.5.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Desjardins M, Nzala NN, Corsini R, Rondeau C. Maturation of phagosomes is accompanied by changes in their fusion properties and size-selective acquisition of solute materials from endosomes. J Cell Sci. 1997;110(Pt 18)(Pt 18):2303–14. doi: 10.1242/jcs.110.18.2303. [DOI] [PubMed] [Google Scholar]
- 6.Luzio JP, Rous BA, Bright NA, Pryor PR, Mullock BM, Piper RC. Lysosomeendosome fusion and lysosome biogenesis. J Cell Sci. 2000;113(Pt 9)(Pt 9):1515–24. doi: 10.1242/jcs.113.9.1515. [DOI] [PubMed] [Google Scholar]
- 7.Yates RM, Hermetter A, Taylor GA, Russell DG. Macrophage activation downregulates the degradative capacity of the phagosome. Traffic. 2007;8(3):241–50. doi: 10.1111/j.1600-0854.2006.00528.x. [DOI] [PubMed] [Google Scholar]
- 8.Yates RM, Hermetter A, Russell DG. The kinetics of phagosome maturation as a function of phagosome/lysosome fusion and acquisition of hydrolytic activity. Traffic. 2005;6(5):413–20. doi: 10.1111/j.1600-0854.2005.00284.x. [DOI] [PubMed] [Google Scholar]
- 9.Ikonen E. Mechanisms for cellular cholesterol transport: defects and human disease. Physiol Rev. 2006;86(4):1237–61. doi: 10.1152/physrev.00022.2005. [DOI] [PubMed] [Google Scholar]
- 10.Schmitz G, Grandl M. Lipid homeostasis in macrophages - implications for atherosclerosis. Rev Physiol Biochem Pharmacol. 2008;160:93–125. doi: 10.1007/112_2008_802. [DOI] [PubMed] [Google Scholar]
- 11.Bobryshev YV. Monocyte recruitment and foam cell formation in atherosclerosis. Micron. 2006;37(3):208–22. doi: 10.1016/j.micron.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 12.Russell DG, Cardona PJ, Kim MJ, Allain S, Altare F. Foamy macrophages and the progression of the human tuberculosis granuloma. Nat Immunol. 2009;10(9):943–8. doi: 10.1038/ni.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson RA, Sando GN. Cloning and expression of cDNA encoding human lysosomal acid lipase/cholesteryl ester hydrolase. Similarities to gastric and lingual lipases. J Biol Chem. 1991;266(33):22479–84. [PubMed] [Google Scholar]
- 14.Goldstein JL, Dana SE, Faust JR, Beaudet AL, Brown MS. Role of lysosomal acid lipase in the metabolism of plasma low density lipoprotein. Observations in cultured fibroblasts from a patient with cholesteryl ester storage disease. J Biol Chem. 1975;250(21):8487–95. [PubMed] [Google Scholar]
- 15.Heeren J, Grewal T, Jackle S, Beisiegel U. Recycling of apolipoprotein E and lipoprotein lipase through endosomal compartments in vivo. J Biol Chem. 2001;276(45):42333–8. doi: 10.1074/jbc.M107461200. [DOI] [PubMed] [Google Scholar]
- 16.Hornick CA, Thouron C, DeLamatre JG, Huang J. Triacylglycerol hydrolysis in isolated hepatic endosomes. J Biol Chem. 1992;267(5):3396–401. [PubMed] [Google Scholar]
- 17.Verges M, Bensadoun A, Herz J, Belcher JD, Havel RJ. Endocytosis of hepatic lipase and lipoprotein lipase into rat liver hepatocytes in vivo is mediated by the low density lipoprotein receptor-related protein. J Biol Chem. 2004;279(10):9030–6. doi: 10.1074/jbc.M312908200. [DOI] [PubMed] [Google Scholar]
- 18.Sturgill-Koszycki S, Schaible UE, Russell DG. Mycobacterium-containing phagosomes are accessible to early endosomes and reflect a transitional state in normal phagosome biogenesis. Embo J. 1996;15(24):6960–8. [PMC free article] [PubMed] [Google Scholar]
- 19.Yates RM, Russell DG. Real-time spectrofluorometric assays for the lumenal environment of the maturing phagosome. Methods Mol Biol. 2008;445:311–25. doi: 10.1007/978-1-59745-157-4_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 21.Belfrage P, Vaughan M. Simple liquid-liquid partition system for isolation of labeled oleic acid from mixtures with glycerides. J Lipid Res. 1969;10(3):341–4. [PubMed] [Google Scholar]
- 22.Duque M, Graupner M, Stutz H, Wicher I, Zechner R, Paltauf F, Hermetter A. New fluorogenic triacylglycerol analogs as substrates for the determination and chiral discrimination of lipase activities. J Lipid Res. 1996;37(4):868–76. [PubMed] [Google Scholar]
- 23.Hadvary P, Lengsfeld H, Wolfer H. Inhibition of pancreatic lipase in vitro by the covalent inhibitor tetrahydrolipstatin. Biochem J. 1988;256(2):357–61. doi: 10.1042/bj2560357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang JH, Chung TD, Oldenburg KR. A Simple Statistical Parameter for Use in Evaluation and Validation of High Throughput Screening Assays. J Biomol Screen. 1999;4(2):67–73. doi: 10.1177/108705719900400206. [DOI] [PubMed] [Google Scholar]
- 25.Alonso S, Pethe K, Russell DG, Purdy GE. Lysosomal killing of Mycobacterium mediated by ubiquitin-derived peptides is enhanced by autophagy. Proc Natl Acad Sci U S A. 2007;104(14):6031–6. doi: 10.1073/pnas.0700036104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pethe K, Swenson DL, Alonso S, Anderson J, Wang C, Russell DG. Isolation of Mycobacterium tuberculosis mutants defective in the arrest of phagosome maturation. PNAS. 2004;101(37):13642–13647. doi: 10.1073/pnas.0401657101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sayers EW, Barrett T, Benson DA, Bryant SH, Canese K, Chetvernin V, Church DM, DiCuccio M, Edgar R, Federhen S, others Database resources of the National Center for Biotechnology Information. Nucleic Acids Res. 2009;37(Database issue):D5–15. doi: 10.1093/nar/gkn741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Russell DG, VanderVen BC, Glennie S, Mwandumba H, Heyderman RS. The macrophage marches on its phagosome: dynamic assays of phagosome function. Nat Rev Immunol. 2009;9(8):594–600. doi: 10.1038/nri2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.VanderVen BC, Yates RM, Russell DG. Intraphagosomal measurement of the magnitude and duration of the oxidative burst. Traffic. 2009;10(4):372–8. doi: 10.1111/j.1600-0854.2009.00877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]