Abstract
Although evidence suggests that patients with left hemisphere strokes and nonfluent aphasia who receive 1 Hz repetitive transcranial magnetic stimulation (rTMS) over the intact right inferior frontal gyrus experience persistent benefits in naming, it remains unclear whether the effects of rTMS in these patients generalize to other language abilities. We report a subject with chronic nonfluent aphasia who showed stable deficits of elicited propositional speech over the course of five years, and received 1200 pulses of 1 Hz rTMS daily for 10 days at a site identified as being optimally responsive to rTMS in this patient. Consistent with prior studies there was improvement in object naming, with a statistically significant improvement in action naming. Improvement was also demonstrated in picture description at 2, 6, and 10 months after rTMS with respect to the number of narrative words and nouns, sentence length, and use of closed class words. Compared to his baseline performance, the patient showed significant improvement on the Western Aphasia Battery subscale for spontaneous speech. These findings suggest that manipulation of the intact contralesional cortex in patients with nonfluent aphasia may result in language benefits that generalize beyond naming to include other aspects of language production.
Keywords: Interhemispheric interactions, language, TMS, inferior frontal gyrus, discourse, propositional speech
Introduction
Persistent aphasia is a common and frequently devastating consequence of dominant hemisphere strokes, for which the effectiveness of clinical interventions is limited (e.g. Lincoln et al., 1984). Recent evidence suggests that exogenous magnetic and electrical manipulation of brain activity may facilitate recovery of various faculties after stroke (e.g. Alonso-Alonso et al., 2007, Schlaug et al., 2008). Repetitive transcranial magnetic stimulation (rTMS) has yielded encouraging results with respect to motor function (e.g. Mansur et al., 2005) and neglect (e.g. Brighina, 2003). In patients with chronic nonfluent aphasia this technique has been used to facilitate long-lasting improvements in naming ability (Naeser 2005a, 2005b; Martin et al., 2009).
Most prior studies of rTMS in patients with stroke-related deficits, including aphasia, have employed inhibitory low frequency (1 Hz) stimulation to the intact hemisphere of the brain. To date, investigations of the effects of contralesional rTMS on language recovery have focused on overt naming as the principle measure of performance, and indicate that patients with chronic nonfluent aphasia who receive 1 Hz rTMS over the posterior pars triangularis portion of the inferior frontal gyrus (IFG) of the nondominant hemisphere experience benefits in picture naming that persist for months to years (Naeser et al., 2005a, 2005b; Martin et al., 2009). The frequency of naming deficits in aphasia and the relative ease of assessing change in naming performance make it a useful measure. However, it remains unproven whether the benefits in naming that have been observed in response to rTMS generalize to other abilities that are critical to functional language recovery. In order to address this issue, we investigated the effect of contralesional rTMS of the right IFG on tasks that assess elicited propositional speech.
Impairment of propositional speech is a defining characteristic of non-fluent aphasias, and is commonly associated with lesions of Broca’s area and underlying deep white matter structures (Alexander et al., 1990). Picture description is one of the most useful and frequently employed measures of elicited propositional speech production (Coelho, 2007). Here we report a subject with a left hemisphere stroke and chronic aphasia who showed stable deficits of propositional speech over the course of five years prior to our investigation. Over six sessions, 1Hz rTMS (600 pulses at 90% motor threshold) was administered at six separate sites in the right frontal lobe (Figure 1a), in order to locate a site that was most responsive with respect to picture naming. These sites included the region of the motor cortex corresponding to the mouth, a site on Brodmann Area 44 (BA 44; pars opercularis), two separate sites on BA 45 (designated as the dorsal posterior and dorsal anterior pars triangularis), an intermediate site that by some accounts (e.g. Amunts et al., 2004) may either represent the anterior aspect of BA 44 or the posterior aspect of BA 45 (designated here as the anterior pars opercularis/ventral posterior pars triangularis; BA 44/45), and a site on BA 47 (pars orbitalis). Subsequently, 1Hz rTMS (1200 pulses at 90% motor threshold) was delivered to the identified optimal site for 10 daily sessions over 12 days. Because rTMS to the intact hemisphere is theorized to improve the function of frontal language regions, and possibly bilateral temporo-parietal language networks, we predicted that manipulation of the intact hemisphere at this site would result in persistent improvements in both naming and propositional speech.
Figure 1.
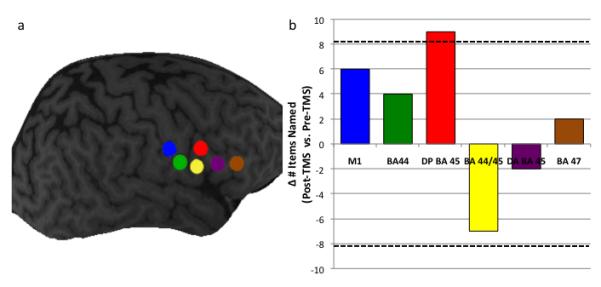
a) The six sites at which TMS was delivered in Phase 1 of the study: M1 corresponding to the mouth (blue), Brodmann area 44 (BA 44; green), dorsal posterior BA 45 (red), dorsal anterior BA 44/ventral posterior BA 45 (yellow), dorsal anterior BA 45 (purple), and BA 47 (brown).
Figure 1b) The difference in performance on a naming task before and after rTMS to the six right hemisphere sites. Hatched lines indicate significant differences.
Results
Phase 1: Identification of Optimal Site for Picture Naming
Across the six rTMS sessions there was no effect of session order on picture naming performance before or after rTMS, for either novel (F[4,9]=0.584; p=0.689) or repeated (F[4,9]=0.658; p=0.647) items, indicating that there was no effect of practice on performance. Repeated stimulus and novel stimulus trials were therefore collapsed during analysis.
Two procedures were employed to identify a site at which rTMS improved performance. First, to evaluate whether the effect of rTMS at each target site differed from its effect at other sites, one-sample t-tests were performed comparing the difference between pre-TMS and post-TMS performance (designated the Δ Naming score) at each site to the mean of the Δ Naming scores for the remaining sites (e.g. Δ Naming for BA 44 was compared to the mean of Δ Naming for all sites except BA 44; M1 Δ Naming was compared to Δ Naming for all sites except M1, etc.). Stimulation of the dorsal posterior BA 45 (DP45) was associated with a significantly greater increase in naming performance compared to the mean change in performance at all other sites (t[4]=−3.628, p = 0.022), while stimulation of anterior BA 44/ventral posterior BA 45 (BA 44/45) was associated with a significant decrease in naming performance (t[4]=−5.823, p = 0.004) (Figure 1b).
Second, to evaluate whether the change in naming performance induced by rTMS was attributable to day-to-day variability we calculated the mean variability for the six pre-TMS sessions; the standard deviation for the six pre-TMS sessions was 3.27. We reasoned that, for any given site, an improvement in naming that exceeded the pre-TMS score for that site by more than ±2.5 SDs was not likely to be attributable to chance. The only site at which the improvement with TMS met this criterion was the dorsal posterior aspect of BA 45 (Figure 2b). In light of the converging evidence from these analyses, this site was thus selected for Phase 2 of the investigation.
Figure 2.
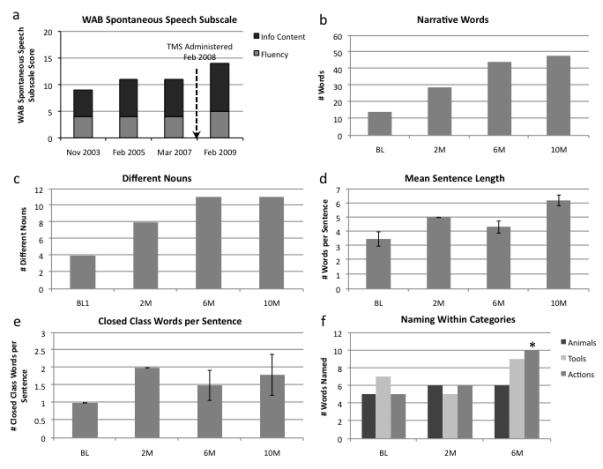
Performance on measured of propositional speech and naming. 2a) The Western Aphasia Battery spontaneous speech subscale. Black bars indicate the Information Content component of the Spontaneous Speech subscale; grey bars indicate the Fluency component. 2b-2e) Performance on the Cookie Theft Picture Description: b) total number of narrative words used, c) number of different nouns used, d) mean sentence length, and e) number of closed class words used per sentence. Vertical bars represent standard error. 2f) Performance on the Naming in Categories subtest of the BDAE. Improvement in action naming between baseline and six months is significant (asterisk).
Phase 2: Response to rTMS at Optimal Site
Western Aphasia Battery
The patient’s performance on the Spontaneous Speech subtest of the WAB was stable over the course of the five years prior to his participation (Figure 2a). Ten months after administration of rTMS the score on this subtest improved significantly (one-sample t-test: t[2]=−5.5, p=0.032). This improvement on the composite subtest was comprised of improvements on both information content and fluency scales. These numeric changes represented substantive improvements in the patient’s propositional speech as observed by examiners. For instance, an increase from seven to nine on the information content scale equated to a change in performance from “mention of at least 6 items in the picture” to “an almost complete description of the picture” in which “at least 10 people, actions, or objects” were named. Similarly, an increase from four to five on the fluency scale represented a change from “halting, telegraphic speech” with “mostly single words” to “often telegraphic but more fluent speech with some grammatical organization.”
Cookie Theft Picture Description
A one-sample t-test comparing the mean number of narrative words employed to describe the Cookie Theft Picture before and after treatment with TMS was significant (t[2]=4.453, p=0.045), indicating more narrative words employed after stimulation than before (Figure 2b). A one-sample t-test comparing the mean number of different nouns uttered by the patient after receiving rTMS to his pre-TMS baseline was also significant (t[2]=6.000, p=0.027), indicating that more objects in the scene were described after stimulation than beforehand (Figure 2c). A one-way ANOVA in which mean sentence length was the dependent variable and session (baseline, 2 months, 6 months, and 10 months) was the independent variable demonstrated a significant effect of session (F[3,13]=5.519, p=0.017), indicating a significant increase in sentence length over time (Figure 2d). A subsequent post hoc t-test revealed a significant increase in mean sentence length between baseline and 10 months (t[5]=−3.972; p=0.011). Other comparisons of sentence length between timepoints were not significant. As a way to further quantify the complexity of the patient’s utterances, a t-test was used to compare the number of closed class words (the class of words that includes determiners, conjunctions, pronouns, and prepositions) used by the patient per sentence before rTMS to his use of these words after receiving rTMS, collapsed across post-TMS timepoints. This comparison revealed a trend toward significance (t[11]=−2.152; p=0.054; Figure 2e). Table 1 shows examples of the patient’s elicited speech on the Cookie Theft Picture description before and after receiving TMS.
Table 1.
Cookie Theft Picture Description at Baseline and 6 Months Following TMS
| Baseline | 6 Months |
|---|---|
| this is cookies kids chair almost fall (unintelligible) almost fall girls cookies too this is big girl |
food big man water holding water cause otherwise rain washing. guy chairs but not good cause ohhhhh! (pantomimes falling) cookies! far almost too far, but you know what? one hand, no, two hands also girl I like cookies too so nice nice outside I think not cold. girl, big girl washing clean |
Naming
Compared to performance at baseline, the patient’s performance on naming of animals, actions and tools, improved between baseline and 6-month follow-up (Figure 2f). A chi-square test used to compare performance in each category at baseline to 6-month follow-up revealed a statistically significant improvement (χ2=4.444; p=0.035) for naming of actions, increasing from 5 of 12 items named to 10 of 12 items. The numerical improvement in naming of animals (5 items to 6) and actions (7 items to 9), did not achieve statistical significance.
Discussion
In a subject with stroke and chronic aphasia, low frequency inhibitory rTMS was applied to several locations in the contralesional right hemisphere. Stimulation of a single site in the right IFG resulted in significant improvement in naming. After extended administration of low-frequency rTMS to that site, the subject showed sustained improvement, not only in naming, but also in elicited speech, as assessed by his ability to describe visual scenes. Importantly, the subject’s performance on measures of elicited propositional speech had been stable for years prior to undergoing rTMS. Consistent with our observations, both the patient and his wife also thought that there had been a clear but modest improvement in his speech output and willingness to speak. Our findings support a growing body of evidence that indicates that inhibition of the intact contralesional pars triangularis results in improved naming in patients with chronic nonfluent aphasia. Importantly, our findings also extend prior investigations by demonstrating that improvements in language following contralesional stimulation may generalize beyond naming to other abilities such as propositional speech.
Much of the evidence demonstrating that contralesional rTMS can be used to enhance language recovery has been reported by Naeser and colleagues. These investigators initially observed a significant but transient increase in accuracy and decrease in reaction time for subjects with stroke and nonfluent aphasia in naming pictures immediately following a single application of 1Hz rTMS to the right pars triangularis for 10 minutes (2002). Similar to the current study, Naeser and colleagues subsequently administered 10 20-minute sessions of 1 Hz rTMS to the right pars triangularis in four subjects (Naeser et al., 2005a). At two months follow-up, there was a significant improvement on the first 20 items of the Boston naming Test, the Animal Naming subtest of the BDAE, and the Tools/Implements measure on the BDAE. At 8 months, all three naming test scores continued to improve relative to pre-TMS testing, but only Tool/Implements was significant. Improvement was also observed in number of words per longest phrase length in elicited propositional speech for two of the patients when tested with the BDAE at 2 months post-rTMS.
A recent study by the same group of investigators (Martin et al., 2009) described two patients with chronic aphasia who received rTMS, one of whom responded well to stimulation and one who did not. They proposed that differences in the distribution of stroke may have accounted for differences in response to rTMS. Other investigators have suggested that the functional role of the right hemisphere in language recovery may differ between individuals, possibly accounting for differences in response to rTMS (e.g. Winhuisen et al., 2007). Martin and colleagues observed that in the patient who experienced improvement of naming ability, an increase in sentence length on a picture description test was noted. Our findings support and extend these findings, demonstrating that the propositional speech ability of a subject who had suffered from stable deficits over several years improved, not only in terms sentence length, but also with regard to the number of objects described within a complex scene and the complexity of the sentences employed.
One intriguing aspect of the current findings is the topographic specificity of rTMS in eliciting changes in language performance. Stimulating the dorsal posterior pars triangularis in our subject resulted in a 41% increase in naming ability. However, stimulation of the nearby anterior pars opercularis/ventral posterior pars triangularis resulted in a 23% decrease in naming. Naeser and colleagues also found substantial differences in naming ability based on small changes in the location of stimulation (c.f., Naeser et al, 2005A). While the mechanism underlying this topographical specificity of rTMS effects has not been fully elucidated, our results may have important implications for our understanding of interhemispheric interactions in patients with stroke. It has previously been proposed that some of the chronic deficits that accompany lateralized strokes may be related to disruption of mutually inhibitory inputs between the cerebral hemispheres (Fregni & Pascual-Leone, 2007). According to this model, unilateral injuries—for example a dominant hemisphere stroke— may lead to a reduction of inhibitory input from the damaged hemisphere onto the intact hemisphere. This release of inhibition may result in increased inhibitory input from the contralesional hemisphere on the damaged hemisphere, exacerbating the deficits initially incurred by the destruction of brain structures. This model has served as the central rationale for applying inhibitory rTMS to the intact contralesional hemisphere to treat a variety of symptoms associated with unilateral lesions, including paresis, neglect, and aphasia. Our finding that dramatic changes in performance are associated with small changes in coil position in the contralesional hemisphere suggests that a more nuanced model of the neural representation of language after stroke and of the role of interhemispheric relationships is needed.
One hypothesis that builds upon the existing model of interhemispheric inhibition is that the topographic specificity of right hemisphere stimulation may be related to anatomic distinctions in the left hemisphere for representing language. Evidence suggests that there are regional differences within the left IFG for processing different linguistic properties, including syntax, phonology, and semantics (e.g. Démonet et al., 1992, Kapur el al., 1994). Moreover, it has been shown that administration of rTMS to nearby regions of the left IFG can dissociate these linguistic elements (Gough et al., 2005). Extending this idea, administration of rTMS to different regions in the right IFG could result in manipulation of specific linguistic processes, which may affect object naming ability to varying degrees.
Another potential explanation for the effects of rTMS in the nondominant hemisphere, which is not mutually exclusive with other accounts, is the idea that language systems in patients with chronic aphasia may operate in stable but maladaptive states. By that account, rTMS may provide and exogenous signal that promotes plastic changes, thereby eroding the stability of maladaptive states and potentially allowing for the development more efficient states (c.f. Devlin & Watkins, 2007). While this model is speculative, it accommodates one aspect of the data that is not readily explained by other hypotheses, the finding that language function improves over the course of months following rTMS (e.g. Naeser et al 2005a, 2005b, Martin et al., 2009). This improvement may reflect the effects of further enhancement of connection strengths in these reset networks over time as they are utilized for language processing (e.g. Spatz, 1996; Bi & Poo, 2001).
The finding that our patient experienced greater improvement in naming of actions relative to animals or tools also contributes to a growing body of evidence that suggests that the prefrontal cortex may play a critical role in action naming. Lesion study evidence points to an association between action naming impairment and lesions involving the left premotor and prefrontal areas (Tranel et al., 2001). Moreover, rTMS of the left dorsolateral prefrontal cortex in nonaphasic individuals has been shown to affect both action naming (Cappa et al., 2002) and processing of verbs (Cappelletti et al., 2008). The finding that stimulation of the right pars triangularis results in preferential improvement in action naming in patients with chronic nonfluent aphasia suggests that connections to the prefrontal cortex of the right hemisphere may also play a role in mediating action naming in individuals with chronic injury to the left hemisphere.
Our findings add to the relatively small number of reported cases in which rTMS has been used to improve chronic aphasia. Because we report results from a single subject, future investigations are necessary to determine the degree to which our findings can be generalized to other patients with nonfluent aphasia receiving noninvasive brain stimulation. Moreover, additional measures would be useful for assessing improvement in other domains of language and the impact of these changes on daily function. Despite these limitations, our findings support the claim that noninvasive brain stimulation may improve chronic aphasia and suggest that not only anomia but other aspects of language as well.
Methods
Clinical History
The patient is a 61-year-old previously healthy right-handed (Edinburgh Handedness Inventory Score = +100 = strongly right handed; Oldfield, 1971) man with 18 years of education who worked as a management consultant prior to his stroke. Seven years prior to participation in the current investigation (2000), he experienced sudden right hemiplegia and global aphasia, with severe deficits in fluency and comprehension; he was subsequently found to have an ischemic stroke encompassing the territory of the left middle cerebral artery (Figure 3). He exhibited persistent symptoms of right paresis and mild nonfluent aphasia (~3 word phrase length).
Figure 3.
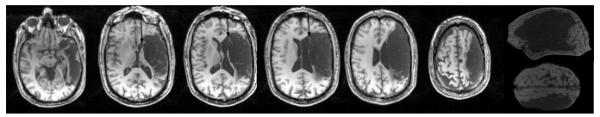
T1-weighted MRI (1mm slice images with field of view 192 × 256 × 80; axial images depicted are—from inferior to superior—slices 124, 100, 90, 84, 78, and 66). Extensive cortical lesion is present in middle and inferior frontal gyrus areas (includes Broca’s area), and in most of the middle and superior temporal gyrus areas (includes Wernicke’s area). There is some sparing of deep white matter, adjacent to inferior frontal horn (arrows, medial subcallosal fasciculus), deep to Broca’s area. Extensive sensorimotor cortex and parietal lobe lesion is present, with sparing of parts of BA 37 and 39 (angular gyrus).
In accord with the Declaration of Helsinki, the patient gave informed consent as approved by the Institutional Review Boards of both the University of Pennsylvania and the Moss Rehabilitation Research Institute.
Language Measures
Propositional Speech
On three separate occasions over the course of five years preceding his participation in the present investigation (November 2003, February 2005, and March 2007) the patient’s language ability was evaluated with the Western Aphasia Battery (WAB) (Shewan & Kertesz, 1980), which includes a subscale for spontaneous speech. This subscale is comprised of two 10-point measures rating information content and fluency. An experienced examiner scored these dimensions based on the patient’s ability to answer a series of six autobiographical questions (e.g. “What is you address?”) and his ability to describe a complex scene. For the scene description, he was presented with the picture and asked to describe everything occurring in the scene. Examiner interruptions were kept to a minimum and limited to general prompting (e.g. “anything else?”). The patient’s speech was transcribed to facilitate scoring and analysis. He underwent identical follow-up testing with the WAB 10 months after completing rTMS.
In addition, the patient underwent baseline evaluation for the present study seven years after his stroke (September 2007). The Cookie Theft Picture Description subtest of the Boston Diagnostic Aphasia Examination (BDAE 3rd Ed., Goodglass, Kaplan, Baressi, 2001) was employed as the principle measure of propositional speech and fluency. As with the WAB picture description, the patient was presented with the scene and asked to describe everything occurring in the picture. The Cookie Theft Picture Description was administered at baseline (September 2007) and at two, six, and ten months following administration of rTMS. Scoring of performance included measurement of the number of narrative words and sentences or topic comments used to describe the scene, the mean length of sentences, and the number of unique nouns, verbs, and closed class words employed.
Naming
The Naming in Categories subtest of the BDAE was administered (Goodglass, Kaplan, Baressi, 2001). This subtest consists of three categories: Actions, Animals, and Tools/Implements. Each category consisted of 12 black-and-white line drawing stimuli. The examiner directed the patient to name the action, animal, or implement depicted. The task was self-paced. Answers that contained one phonemic error were counted as correct to account for the patient’s dyspraxia of speech.
Phase 1: Identification of Optimal Site
MRI Methods
MRI data was collected on a 3.0 Tesla Siemens Trio Scanner. High-resolution whole-brain T1 -weighted images were acquired (TR=1620ms, TE=3.87ms, FOV=192×256 mm, 1×1×1 mm voxels) using a Siemens 8-channel head coil.
TMS Methods
Stimulation was administered with a Magstim Rapid transcranial magnetic stimulator, connected to a 70mm diameter figure-of-eight coil (Magstim, Whitland, UK). The Brainsight system (Rogue Research, Montreal) was used to co-register MRI data with the location of the subject and coil in both phases of the study. The patient’s motor threshold was established according to published criteria (Rossini et at, 1994). On six separate days over the course of two weeks, he received 1Hz stimulation at 90% of motor threshold for 10 minutes (600 pulses), delivered to six sites on the right IFG (Figure 2a): the motor cortex (M1), pars opercularis (BA 44), dorsal posterior pars triangularis (DP BA 45), dorsal anterior pars triangularis (DA BA 45), anterior pars opercularis/ventral posterior pars triangularis (BA 44/45), and pars orbitalis (BA 47).
Snodgrass and Vanderwart Items
Prior to and immediately following stimulation at each candidate site, the patient was shown sets of 40 line drawing stimuli taken from the Snodgrass and Vanderwart picture database (1980). Each picture was presented with a laptop computer for 10s and was preceded by a 120 ms tone and a 1s ISI. The patient was instructed to name the picture as soon as it appeared on the screen. To assess practice effects, 20 of the 40 items on each list were repeated in each of the twelve testing blocks (six blocks immediately before rTMS to each of the six candidate sites; six post-TMS blocks), while the other 20 items in each block were novel. Lists were controlled for frequency and visual complexity. All words were 1–2 syllables in length. No two sequential words within a list belonged to the same semantic category or had the same initial phoneme. Responses that differed from the target word by one phoneme were counted as correct.
Phase 2: Administration of rTMS at Optimal Site and Follow-Up
TMS Methodology
The patient received rTMS for 10 days in a 12-day period (Monday — Friday, weekend off, Monday – Friday) at the site (dorsal posterior pars triangularis) shown to produce the greatest improvement in naming. Each session consisted of 1200 pulses at a frequency of 1 Hz at an intensity of 90% of motor threshold.
Follow-Up
The patient underwent follow-up evaluation with the Cookie Theft Picture Description and BDAE Naming in Special Categories two months and six months after the 10 sessions of rTMS. In addition he completed the spontaneous speech subtest of the Western Aphasia Battery and the Cookie Theft Picture Description 10 months after administration of the rTMS.
Acknowledgements
We wish to thank Adelyn Brecher and Dr. Marianna Stark for their invaluable assistance in the preparation of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander MP, Naeser MA, Palumbo CL. Broca’s area aphasias: Aphasia after lesions including the frontal operculum. Neurology. 1990;40:353–362. doi: 10.1212/wnl.40.2.353. [DOI] [PubMed] [Google Scholar]
- Alonso-Alonso M, Fregni F, Pascual-Leone A. Brain stimulation in poststroke rehabilitation. Cerebrovascular Diseases. 2007;24(Suppl. 1):157–166. doi: 10.1159/000107392. [DOI] [PubMed] [Google Scholar]
- Amunts K, Weiss PH, Mohlberg H, Pieperhoff P, Eickhoff S, Gurd JM, Marshall JC, Shah NJ, Fink GR, Zilles K. Analysis of neural mechanisms underlying verbal fluency in cytoarchitectonically defined stereotaxic space: The roles of Brodmann areas 44 and 45. Neuroimage. 2004;22(1):42–56. doi: 10.1016/j.neuroimage.2003.12.031. [DOI] [PubMed] [Google Scholar]
- Bi G, Poo M. Synaptic modification by correlated activity: Hebb’s postulate revisited. Annual Review of Neuroscience. 2001;24:3–7. doi: 10.1146/annurev.neuro.24.1.139. [DOI] [PubMed] [Google Scholar]
- Brighina F, Bisiach E, Oliveri M, Piazza A, La Bua V, Daniele O, Fierro B. 1 Hz repetitive transcranial magnetic stimulation of the unaffected hemisphere ameliorates contralesional visuospatial neglect in humans. Neuroscience Letters. 2002;16(2):131–133. doi: 10.1016/s0304-3940(02)01283-1. [DOI] [PubMed] [Google Scholar]
- Cappa SF, Sandrini M, Rossini PM, Sosta K, Miniussi C. The role of the left frontal lobe in action naming: rTMS evidence. Neurology. 2002;59(5):720–723. doi: 10.1212/wnl.59.5.720. [DOI] [PubMed] [Google Scholar]
- Cappelletti M, Fregni F, Shapiro K, Pascual-Leone A, Caramazza A. Processing nouns and verbs in the left frontal cortex: A transcranial magnetic stimulation study. Journal of Cognitive Neuroscience. 2008;20(4):707–720. doi: 10.1162/jocn.2008.20045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho CA. Management of discourse deficits following traumatic brain injury: Progress, caveats, and needs. Seminars in Speech and Language. 2007;28(2):122–135. doi: 10.1055/s-2007-970570. [DOI] [PubMed] [Google Scholar]
- Damasio H, Grabowski TJ, Tranel D, Ponto LL, Hichwa RD, Damasio AR. Neural correlates of naming actions and of naming spatial relations. Neuroimage. 2001;13(6):1053–1064. doi: 10.1006/nimg.2001.0775. [DOI] [PubMed] [Google Scholar]
- Démonet J, Fiez J, Paulesu E, Petersen S, Zatorre R. PET studies of phonological processing: A critical reply to Poeppel. Brain and Language. 1992;55(3):352–379. doi: 10.1006/brln.1996.0109. [DOI] [PubMed] [Google Scholar]
- Devlin JT, Watkins KE. Stimulating language: Insights from TMS. Brain. 2007;130:610–622. doi: 10.1093/brain/awl331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fregni F, Pascual-Leone A. Technology insight: Noninvasive brain stimulation in neurology-perspectives on the therapeutic potential of rTMS and tDCS. Nature Clinical Practice Neurology. 2007;3(7):383–393. doi: 10.1038/ncpneuro0530. [DOI] [PubMed] [Google Scholar]
- Goodglass H, Kaplan E, Barresi B. The Assessment of Aphasia and Related Disorders. 3rd ed Lippincott, Williams & Wilkins; Philadelphia, PA: 2001. [Google Scholar]
- Gough PM, Nobre AC, Devlin JT. Dissociating linguistic processes in the left inferior frontal cortex with transcranial magnetic stimulation. Journal of Neuroscience. 2005;25(35):8010–8016. doi: 10.1523/JNEUROSCI.2307-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur S, Rose R, Liddle PF, Zipursky RB, Brown GM, Stuss D, Houle S, Tulving E. The role of the left prefrontal cortex in verbal processing: Semantic processing or willed action? NeuroReport. 1994;5(16):2193–2196. doi: 10.1097/00001756-199410270-00051. [DOI] [PubMed] [Google Scholar]
- Lincoln NB, McGuirk E, Mulley GP, Lendrem W, Jones AC, Mitchell JR. Effectiveness of speech therapy for aphasic stroke patients: A randomised controlled trial. The Lancet. 1984;1(8388):1197–1200. doi: 10.1016/s0140-6736(84)91690-8. [DOI] [PubMed] [Google Scholar]
- Lincoln NB, Mulley GP, Jones AC, Mcguirk E, Lendrem W, Mitchell JRA. Effectiveness of Speech Therapy for Aphasic Stroke Patients : A Randomised Controlled Trial. The Lancet. 1984;324(8403):644–645. doi: 10.1016/s0140-6736(84)91690-8. [DOI] [PubMed] [Google Scholar]
- Mansur CG, Fregni F, Boggio PS, Riberto M, Gallucci-Neto J, Santos CM, Wagner T, Rigonatti SP, Marcolin MA, Pascual-Leone A. A sham stimulation-controlled trial of rTMS of the unaffected hemisphere in stroke patients. Neurology. 2005;64(10):1802–1804. doi: 10.1212/01.WNL.0000161839.38079.92. [DOI] [PubMed] [Google Scholar]
- Martin PI, Naeser MA, Ho M, Doron KW, Kurland J, Kaplan J, Wang Y, Nicholas M, Baker E, Fregni F, Pascual-Leone A. Overt naming fMRI pre- and post-TMS: Two nonfluent aphasia patients, with and without improved naming post-TMS. Brain and Language. 2009;111(1):20–35. doi: 10.1016/j.bandl.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naeser MA, Martin PI, Nicholas M, Baker EH, Seekins H, Helm-Estabrooks N, Cayer-Meade C, Kobayashi M, Theoret H, Fregni F, Tormos JM, Kurland J, Doron KW, Pascual-Leone A. Improved naming after TMS treatments in a chronic, global aphasia patient--case report. Neurocase. 2005;11(3):182–193. doi: 10.1080/13554790590944663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naeser MA, Martin PI, Nicholas M, Baker EH, Seekins H, Kobayashi M, Theoret H, Fregni F, Maria-Tormos J, Kurland J, Doron KW, Pascual-Leone A. Improved picture naming in chronic aphasia after TMS to part of right Broca’s area: An open-protocol study. Brain and Language. 2005;93(1):95–105. doi: 10.1016/j.bandl.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Naeser MA, Theoret H, Kobayashi M, Martin P, Nicholas M, Baker E, Pascual-Leone A. Modulation of cortical areas with repetitive transcranial magnetic stimulation to improve naming in nonfluent aphasia [Abstract #133]. In proceedings of the 8th International Conference on Functional Mapping of the Human Brain; Sendai, Japan. June 2-6, 2002.2002. [Google Scholar]; NeuroImage. 16(2) Available on CD-Rom in. [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh Inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Barker AT, Berardelli A, Caramia MD, Caruso G, Cracco RQ, Dimitrijevic MR, Hallett M, Katayama Y, Lücking CH, Timmer J, Schelter B. Noninvasive electrical and magnetic stimulation of the brain, spinal cord and roots: Basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalography and Clinical Neurophysiology. 1994;91(2):79–92. doi: 10.1016/0013-4694(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Schlaug G, Renga V, Nair D. Transcranial direct current stimulation in stroke recovery. Archives of Neurology. 2008;65(12):1571–1576. doi: 10.1001/archneur.65.12.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shewan CM, Kertesz A. Reliability and validity characteristics of the Western Aphasia Battery (WAB) Journal of Speech and Hearing Disorders. 1980;45(3):308–324. doi: 10.1044/jshd.4503.308. [DOI] [PubMed] [Google Scholar]
- Snodgrass JG, Vanderwart MA. Standardized set of 260 pictures: Norms for name agreement, image agreement, familiarity, and visual complexity. Journal of Experimental Psychology: Human Learning & Memory. 1980;6(2):174–215. doi: 10.1037//0278-7393.6.2.174. [DOI] [PubMed] [Google Scholar]
- Spatz HC. Hebb’s concept of synaptic plasticity and neuronal cell assemblies. Behavioural Brain Research. 1996;78(1):3–7. doi: 10.1016/0166-4328(95)00221-9. [DOI] [PubMed] [Google Scholar]
- Winhuisen L, Thiel A, Schumacher B, Kessler J, Rudolf J, Haupt WF, Heiss WD. The right inferior frontal gyrus and poststroke aphasia: A follow-up investigation. Stroke. 2007;38(4):1286–1292. doi: 10.1161/01.STR.0000259632.04324.6c. [DOI] [PubMed] [Google Scholar]


