Abstract
Recent breakthroughs in genomics have led to a critical reappraisal of factors once thought to initiate common complex forms of kidney disease. The tenet that diabetes mellitus and hypertension routinely initiate kidney disease whenever blood glucose concentrations or systemic blood pressures reach critical levels for prolonged periods is falling from favor, although it remains important to control hypertension and hyperglycemia to slow nephropathy progression and prevent cardiovascular disease. Many patients with systemic diseases that may potentially involve their kidneys never develop nephropathy. In addition, severe forms of several common kidney diseases cluster tightly in families. This manuscript discusses the existence of differential nephropathy susceptibility based on an individual's genetic make-up, in the context of environmental exposures. Novel genetic analysis methods and recently identified major kidney disease susceptibility genes are discussed, including novel perspectives for categorizing complex forms of nephropathy based on the expanding spectrum of MYH9-associated disease. Genetic screening, gene-environment and gene-gene interactions are also addressed.
Keywords: African American, chronic kidney disease, focal segmental glomerulosclerosis, hypertension, genetics, MYH9
Introduction
The application of genomic methods to more accurately characterize chronic kidney diseases (CKD) and end-stage renal disease (ESRD) was driven by the frequent absence of renal histology in patients, coupled with the widespread recognition of familial aggregation of diverse forms of kidney disease. The earliest reports of kidney disease clustering in families with multiple members having diabetes mellitus were published 20 years ago.1,2 It has since been demonstrated that African Americans display more impressive familial aggregation of ESRD than European Americans 3,4,5,6 and multiple causes of nephropathy may co-exist in African American families.3,7 A population-based case-control study demonstrated that socioeconomic status (SES) was not the sole cause of familial clustering of nephropathy 5 and a geocode analysis in nearly 24,000 incident ESRD patients confirmed the lack of effect of community-level characteristics reflecting SES on familial aggregation of ESRD.8 It was proposed that overarching renal failure susceptibility genes were present in select families, particularly African American.3,9 Genetic susceptibility to nephropathy in affected family members was postulated to be independent from systemic hyperglycemia and hypertension, HIV infection and anti-nuclear antibodies.
Although hypertension is listed as the cause of ESRD in 35% of incident African American patients (and nearly 25% of European American patients) in the United States Renal Data Systems, genetic analyses are clarifying the role of mild to moderate high blood pressure in initiation of nephropathy.10 Linear relationships are observed between blood pressure and severity of kidney disease in the general community.11 However, this finding does not appear to be reflective of the incident dialysis population. Questions exist as to the accuracy of the diagnosis of hypertensive nephropathy 9,12,13,14,15,16 and marked ethnic differences in the histology of this disease are reported.
Kidney biopsies in non-diabetic African American Study of Kidney Disease and Hypertension (AASK) participants with low-level proteinuria and elevated blood pressures revealed extensive focal global glomerulosclerosis, occasional segmental glomerulosclerosis and wide-spread interstitial fibrosis.17 Non-nephrotic FSGS has also been reported in patients with similar clinical presentations.18 The expected vascular changes, arteriolar nephrosclerosis, thought to reflect high blood pressure-induced injury, failed to correlate with systemic blood pressure in AASK.17 A subsequent report by Marcantoni et al. 19 revealed that European Americans given the diagnosis of hypertensive nephropathy had less solidified glomerulosclerosis and interstitial fibrosis than African Americans and mean arterial blood pressures were also not predictive of renal histology. The authors concluded that different mechanisms contribute to development of what is labeled "hypertensive" renal disease between ethnic groups and that genetic factors and intrinsic renal microvascular disease might be present in African Americans. This concept was recently supported by the failure of intensive hypertension treatment including with use of renin-angiotensin axis blockers to halt progression of non-diabetic nephropathy in AASK participants.20 In contrast, conventional cardiovascular disease risk factors including high blood pressure, hyperlipidemia and smoking appear to make greater contributions to arteriolar nephrosclerosis in European Americans given this clinical diagnosis, supporting a different pathogenesis based upon ethnic group.21,22 As will be discussed, the non-muscle myosin heavy chain 9 gene (MYH9) is associated with several etiologies of ESRD in African Americans, including the disease historically labeled "hypertensive nephropathy" (focal global glomerulosclerosis), as well as focal segmental glomerulosclerosis (FSGS) and CKD in European Americans.
Genetic epidemiologic designs for identifying genetic risk factors
Genetic epidemiology is one of the most rapidly growing fields of epidemiologic research. Nearly every human disease has a genetic component, from disorders such as cystic fibrosis which are caused by specific genetic mutations, to complex diseases such as kidney disease, which result from combinations of genes and/or exposures and lifestyles. The central paradigm for the identification of genes that contribute to kidney disease involves a set of questions and design/analytic strategies to answer those questions. First, is there evidence of familial clustering? There must be evidence that some proportion of disease variation or risk is due to genes. This can be addressed through migration, familial aggregation, adoption and twin studies, which aim to assess the heritability (the proportion of the phenotypic variation that is due to additive genetic effect) of the disease. Heritability of kidney function traits, such as estimated glomerular filtration rate (eGFR) and urine albumin:creatinine ratio (ACR) have been estimated to be approximately 30–40%.23,24
One may also want to assess whether a particular risk model fits the disease patterns well. Such studies are usually based on disease patterns among families, using a methodology called segregation analysis.25 Evidence for major and minor gene effects in kidney disease have previously been provided by segregation analyses of families with kidney disease.26,27 Once a genetic component to disease has been established, locating the disease gene in question - the gene responsible for ESRD - becomes more challenging. This question can be addressed with either linkage or association studies. These studies are usually dichotomized, according to the design and type of genetic properties exploited by that design, into studies for genetic linkage analyses and studies for genetic association analyses. Both of these employ marker-based approaches (referred to as indirect genetic analyses), while genetic association studies also encompass the study of candidate variants with previously known or hypothesized function, referred to as direct genetic analyses.
Although linkage analyses of chronic kidney diseases have been conducted 28,29,30, the detection of genes associated with kidney disease has been a daunting challenge especially because the age of onset is late and the genes confer only modest risk. These two features of renal disease, and in fact, of many other complex diseases, have limited the success of traditional linkage analysis in localizing disease genes. Therefore, association-based approaches have been suggested for identification of common alleles that confer modest risk to complex late-onset diseases.31 The success of genome-wide association studies in identifying susceptibility alleles for common diseases, including kidney quantitative phenotypes, such as eGFR 32 in the past few years has advanced the field of genetic epidemiology exponentially.
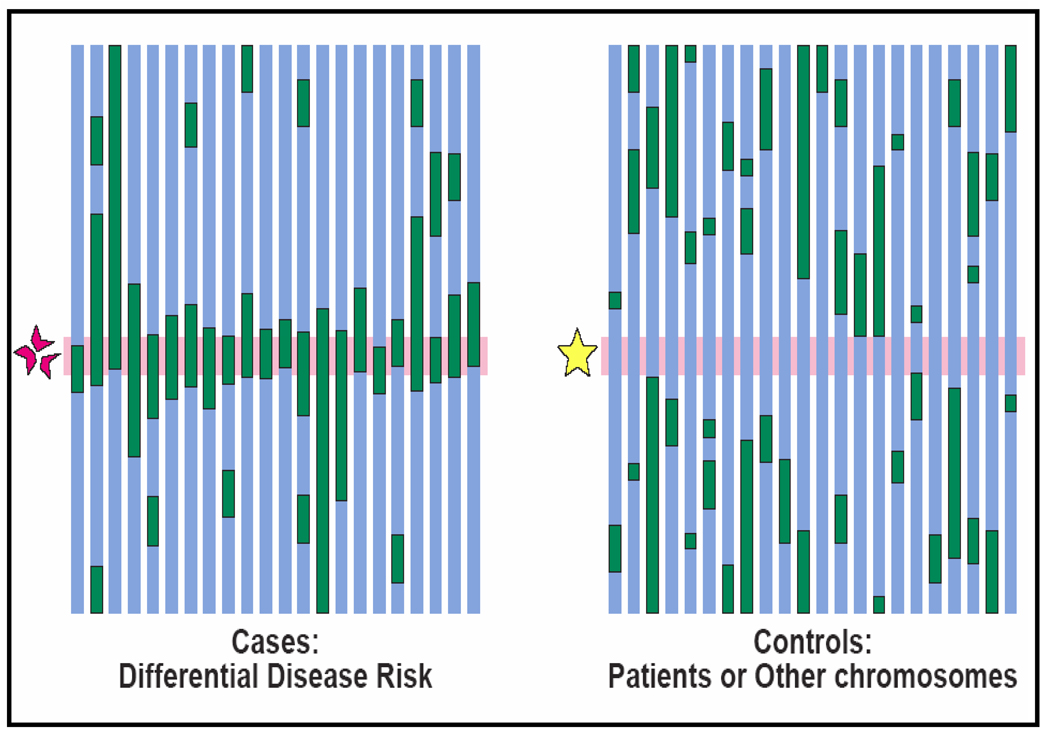
One specialized form of association study is called MALD (mapping by admixture linkage disequilibrium).33 It is a highly-efficient method requiring only 1500–3000 markers, compared to the much denser GWAS panels where at least 1 million markers are necessary. The basic principle of mapping by admixture linkage disequilibrium is straight forward. Although most human genetic variations exist across populations, some vary substantially across populations. Thus, when mixing occurs between genetically heterogeneous populations, the admixed offspring population inherits chromosomal regions of distinct ancestry. This generates association among alleles that are informative for ancestry. Over successive generations of random mating, initial association between linked loci, or linkage disequilibrium, persists longer than association between unlinked loci. MALD exploits admixture-generated linkage disequilibrium to map loci that explain phenotypic variation between the ancestral populations. Figure 1 presents a schematic representation of MALD analysis. The dark areas represent chromosomal segments from the parental population with the higher frequency of the risk allele, e.g. African population. Due to mixing of populations and recombination over several generations, originally large blocks of DNA from the African ancestry have become incorporated in smaller segments throughout the chromosome. Risk alleles that are inherited from the ancestral population are closely related to nearby ancestral markers (i.e., in linkage disequilibrium). A comparison of cases with ESRD and controls without disease will show an excess number of ancestral markers in cases near the disease locus. The central hypothesis of a MALD analysis of ESRD is that some susceptibility alleles are present at higher frequency in African than in European Americans. Thus, the identification of ESRD susceptibility alleles is possible by screening the genome in African Americans with ESRD, searching for regions of the genome where individuals with the disease have more (or less) African ancestry than their genome-wide average.
Figure 1.
Conceptual framework of Mapping by Admixture Linkage Disequilibrium (MALD) analysis using a case-control design
In 2008, two independent studies utilizing the MALD method to identify genes for ESRD 34 and FSGS 35 successfully identified MYH9 as a susceptibility gene for kidney disease.
Family Investigation of Nephropathy and Diabetes (FIND) African American MALD Results
Two recent publications demonstrate the strength of using MALD analyses in an enriched admixed population with predisposition to ESRD, subsequently identifying the MYH9 gene with progression to ESRD associated with kidney diseases other than diabetes.34,35 The FIND MALD study was based on the strength of the epidemiological evidence from population-based cohorts and clinical trial data among African Americans that progression to ESRD occurred at a faster rate and resulted in higher burden of ESRD among those of African ancestry compared to those of European ancestry.10,20
Furthermore, CKD progression to ESRD may occur via common pathways among diabetes- and non diabetes-associated kidney diseases. Processes such as epithelial mesenchymal transition, inflammation, and fibrosis lead to scarring of the glomerulus and tubulointerstitium, resulting in decreased kidney mass and ultimately reduced kidney function.36,37,38 These pathways may account for the variability in progression to ESRD among different ethnic groups, and thus led to the hypothesis of the genetic susceptibility for CKD progression.
The FIND African American MALD study was conducted among self-described African Americans derived primarily from the eastern coast of the United States, with the majority from Baltimore and surrounding regions. A total of 1,372 cases with ESRD and 806 non-kidney diseased controls were recruited. Cases had renal replacement therapy with either dialysis or a kidney transplant, as the phenotype was progression to ESRD. The partner controls from similar environments had normal serum creatinine concentration without albuminuria. The FIND MALD analyses revealed a significant genome-wide association between excess African ancestry and non diabetes-associated ESRD (log of the odds [lod] score 5.70) on chromosome 22q12, but not with diabetes-associated ESRD (lod score 0.47). It was also determined that the much of the total excess risk for non diabetes-associated ESRD in African Americans, compared to European Americans, was due to inheritance of the African allele at the chromosome 22 locus, accounting for approximately 70% of the prevalence of non-diabetes associated ESRD.
Further analyses demonstrated that MYH9 on chromosome 22 was the biological positional candidate gene associated with ESRD. Fourteen single nucleotide polymorphisms (SNPs) in MYH9 were genotyped. The 14 SNPs were all associated with non diabetic ESRD, with odds of disease ranging from 1.17 to 3.10 (11 SNPs had highly significant p values as low as 4.33×10−9), but not with diabetes-associated ESRD. The causes of ESRD that were MYH9-associated were primarily FSGS, HIV-FSGS and clinically diagnosed hypertension-associated ESRD. All cases with FSGS and HIV FSGS (collapsing variant of FSGS) were biopsy proven; however, biopsies were not available for all participants classified as having hypertension-associated ESRD. The odds of ESRD did differ by the cause of non-diabetic ESRD, with the highest risk for FSGS (rs482481 OR 4.34; 95% CI 2.61–6.07) and the lowest for hypertension-associated ESRD (OR 2.32; 95% CI 1.77–3.03). The lower odds of association with hypertensive ESRD may reflect heterogeneity for this phenotype. It is likely that the hypertensive ESRD phenotype contains some individuals with unidentified FSGS/focal global glomerulosclerosis, as well as some patients with primary vascular disease. Identification of MYH9 as the susceptible allele for non-diabetic forms of kidney disease across a spectrum of diseases lends credence to the hypothesis that common pathways lead to progression of nephropathy to ESRD.
MYH9 encodes the non muscle myosin IIA protein which is ubiquitous in the body. This protein is responsible for maintaining cell structure and function, as it acts in concert with actin. Glomerular, tubular and vascular cells all contain myosin; however, the glomerular podocyte may be the site of action considering the association with FSGS and the vulnerability of podocytes to conditions of stress. Recent murine studies of HIVAN demonstrate that the expression of podocyte genes, such as MYH9, can be altered by HIVAN conditions and may demonstrate gene-environment interactions that are necessary for development of kidney disease.39 Although the specific pathogenic mechanism of MYH9–induced genetic risk has not yet been established, a novel area of research that will improve the understanding of CKD progression has developed.
Other MYH9-associated kidney diseases
After identification of the major MYH9 association with idiopathic FSGS, HIVAN, and the disease historically labeled hypertensive nephropathy, the search for novel MYH9 associations and refinement of this spectrum of diseases began. The National Heart Lung and Blood Institute (NHLBI) HyperGEN Study recruited European American and African American sibling pairs concordant for mild-moderate hypertension, along with their normotensive offspring. SNPs in the MYH9 E1 haplotype were only weakly associated with albuminuria in African Americans, likely reflecting inclusion of small numbers with intrinsic renal disease, without evidence of association in European American families.40 Patients with CKD-induced secondary hypertension were occasionally enrolled in HyperGEN, as only presence of severe nephropathy (ESRD) was an exclusion criterion. Despite enrichment for essential hypertension, the frequency of MYH9 risk haplotypes and SNPs were similar to those reported in ethnically matched population-based controls not enriched for hypertension in the initial studies. This supported the concept that MYH9 was a nephropathy susceptibility gene that was unrelated to systemic hypertension.
This concept was solidified by a larger MYH9 association analysis in 696 African Americans with putative hypertensive-ESRD recruited at the Wake Forest University School of Medicine.41 Many cases lacked quantitative urinary protein excretion; however, all were listed as having hypertensive ESRD by treating nephrologists. Although heterogeneous renal disorders were likely to be included in this group, MYH9 demonstrated strong evidence of association. The SNP rs5756152 had an odds ratio (OR) of 3.47 (p=2×10−10; recessive) and the E1 haplotye OR was 2.23 (p=4.5×10−12; recessive). After adjustment for the E1 haplotype, independent associations were also detected for a second haplotype (L1) and another SNP rs5756152. This confirmed strong association between MYH9 and postulated hypertensive ESRD, as is seen in FSGS and HIVAN. It was likely that focal global glomerulosclerosis was an MYH9-associated primary kidney disease, the disease historically called hypertensive nephropathy. It was also possible that multiple MYH9 polymorphisms in different genomic regions could be disease associated, as opposed to one causative SNP.
AASK enrolled African Americans in a clinical trial charged with determining the effects of blood pressure lowering and medications in "hypertensive nephrosclerosis". Enrollment criteria were felt to be diagnostic of this syndrome: non-diabetic subjects with moderate CKD, proteinuria < 2.5 gm/day and high blood pressure. A small number of subjects underwent kidney biopsy and the reviewing pathologists felt that hypertension was likely to have caused the kidney disease. DNA from 497 AASK subjects was genotyped at 4 MYH9 SNPs, including rs4821481 in the E1 haplotype.42 Despite the small sample size, rs4821481 was strongly associated in AASK cases (OR 1.63; p=6.5 × 10−5, recessive), with even stronger association in the subset of 161 patients whose serum creatinine increased ≥ 3 mg/dl during the study (rs4281481 OR 2.33; p=2.4 × 10−6 [recessive]; rs11912763 OR 2.69; p=0.008 [recessive]; and rs1005570 OR 1.57; p=0.027 [recessive]). These results conclusively demonstrate that MYH9 plays a major role in susceptibility to clinically diagnosed "hypertensive nephrosclerosis", with increasing effect in those with progressive kidney failure. Focal global glomerulosclerosis clearly belongs with FSGS (typified by podocyte depletion) and collapsing FSGS (HIVAN with podocyte proliferation) in a single spectrum of kidney disease. Like FSGS, global glomerulosclerosis is an inherited podocytopathy; a primary kidney disease that develops independently from systemic hypertension.
The Kopp et al. report failed to detect significant association between MYH9 and type 2 diabetes-associated ESRD (T2DM-ESRD) in African Americans, although OR for SNPs associated with non-diabetic ESRD were greater than 1.0.35 Similar trends toward weak association between T2DM-ESRD were also observed in FIND.34 A sample of 751 African Americans with T2DM-ESRD recruited at Wake Forest was then evaluated. Significant associations were detected with several MYH9 SNPs.43 Approximately 16% of cases of clinically diagnosed T2DM-ESRD in African Americans are attributable to MYH9; however, it remains unclear whether these individuals have FSGS and coincident type 2 diabetes or classic histologic changes of diabetic nephropathy. Renal biopsy studies remain to be performed. Many African Americans clinically diagnosed with T2DM-ESRD have close relatives with non-diabetic form of ESRD, so FSGS may be common in these multiplex families 3,7 and 20–30% of T2DM-ESRD cases are felt to be incorrectly classified when the diagnosis is made solely on clinical grounds.44
Additional genes underlying non-diabetic ESRD and regulation of GFR
Despite the significant association between MYH9 and non-diabetic forms of ESRD, genetic susceptibility from additional pathways appears likely. Other genes may initiate kidney disease, interact with causative genes and vary in frequency and strength of association by ethnic group. A recent meta-analysis of genome wide association studies discovered additional genes associated with early forms of CKD. The meta-analyses contained 4 population-based cohorts in the CHARGE Consortium: Atherosclerotic Risk in Communities (ARIC), Cardiovascular Health Study (CHS), Framingham Heart Study (FHS) and Rotterdam Study (RS), in which the kidney disease phenotype was based on estimated glomerular filtration rate (eGFR) derived from serum creatinine and cystatin C concentrations.32 Early CKD was described as an eGFR (creatinine) < 60 ml/min/1.73 m2. The initial discovery results were validated in 2 additional population-based cohorts: the Age Gene/Environment Susceptibility-Reykjavik Study (AGES) and the Women’s Genome Health Study (WGHS). Among the approximately 20,000 participants, there were a total of 2,388 cases with CKD. Six associated genes were identified across the spectrum of eGFR and CKD phenotypes; then replicated in 21,466 participants including 1,932 cases with CKD. Newly identified CKD genes included uromodulin (UMOD), shroom family member 3 (SHROOM3), spermatogenesis associated 5-like 1 - glycine amidinotransferase (SPATA5L-GATM), jagged 1 (JAG1), stanniocalcin 1 (STC1) and cystatin C (CST). It appears likely that the SPATA5L-GATM association reflects creatinine biosynthesis and CST association reflects cystatin C biosynthesis, not renal functional decline. Associations with these 6 genes, except for JAG1, did not replicate. Only UMOD had a significant association (p<5 ×10−8) for the CKD and eGFR (creatinine) traits and a highly suggestive association (p<4 ×10−7) among all 3 kidney disease phenotypes. The rs12917707 UMOD SNP was associated with CKD at a genome-wide significant level with the T allele conferring a 20% reduced risk of CKD (OR 0.80; p=2×10−12). This association was consistent across the strata of age, gender, hypertension, and diabetes status. UMOD encodes for the most abundant protein in the urine, although its specific function is not well understood. This novel discovery elucidates another pathway potentially leading to CKD. Additional genetic effects were seen in the genes SHROOM3 and STC1, where the direction of effect was consistent among the various CKD traits; however, these additional genes were not significantly associated across all 3 CKD traits. Importantly, this study demonstrates that the genetic effects for all of the significant loci explain only between 0.7% and 3.2% of the variance in eGFR (depending on the marker) and the CKD prevalence ranged up to 12.1% for those carrying all six risk alleles. The results suggested that additional genetic variants influence CKD. This GWAS was important since it identified genes potentially associated with the complex phenotype of CKD, as well as biomarkers defining the trait, and had the ability to replicate across various CKD phenotypes in different populations.
Linkage analyses among 3 European cohorts demonstrated genetic regions on chromosomes 7p14, 9p21, 10p11, 11p15, 15q15-21, 16p13 and 18p11 that were significantly associated with serum creatinine concentration.45 More detailed genotyping was not done to delineate which specific genes were associated with kidney disease and linked regions do not appear to overlap UMOD and SHROOM3, but did overlap with other candidate genes including insulin-like growth factor binding protein 1 (IFGBP1) and the replicated diabetic nephropathy gene engulfment and cell motility 1 (ELMO1) on chromosome 7.46,47,48 Further genome wide studies will be necessary to explore genetic effects in other populations and ethnic groups.
A number of candidate gene analyses have been performed in CKD and non-diabetic forms of ESRD. Most of these studies lacked replication in additional populations or known causative variants associated with CKD. Recently, chromogranin A gene polymorphisims (CHGA) have been associated with ESRD in 2 independent cohorts of African Americans with hypertension-associated ESRD, compared to healthy controls.49 SNPs in the promoter region and 3’ end were associated with twofold higher odds of kidney disease and lower levels of the encoded protein catestatin were detected. The gene is specifically associated with neurotransmission in the sympathetic nervous system and may play an important role in blood pressure regulation, especially in African Americans.50 This study did not address interactions between MYH9 and CHGA and future studies in African Americans will need to adjust for MYH9 risk variants to detect additional genetic risk. Candidate gene analyses have studied genes in plausible biological pathways such as the renin-angiotensin system, lipid metabolism with APOE and inflammatory pathways, podocyte genes, all of which can lead to kidney disease progression;51,52,53 however, all studies require confirmation in large population-based studies to validate the magnitude and strength of association.
Future directions
The novel discovery of MYH9 and the validation in additional populations reflects a new spectrum of kidney disorders and merits a new term of MYH9-associated nephropathies. Related disorders in the African American population includes idiopathic FSGS, collapsing FSGS (e.g., HIVAN), focal global glomerulosclerosis and likely some misclassified type 2 diabetic nephropathy. "Hypertensive (arteriolar) nephrosclerosis" and "hypertension-associated ESRD" often reflects different disease processes in African Americans and European Americans. It now appears that high blood pressure per se does not often cause ESRD in young to middle aged non-diabetic African Americans with low level proteinuria.20 These patients appear more likely to have hypertension secondary to the presence of idiopathic focal global glomerulosclerosis.17 MYH9 is also strongly associated with FSGS and CKD in European Americans 35 and Europeans45, respectively.
Disease terminology would be improved if patients were genotyped and labeled as having "MYH9-associated nephropathy" or "CHGA-associated nephropathy" and with improved understanding of the roles of these genes in the pathogenesis of kidney disease. However, widespread MYH9 screening for nephropathy susceptibility in African Americans does not appear appropriate at present, even among those with high blood pressure. This relates to high frequency of the major risk haplotype (at risk SNPs) which is present in 60% of African Americans, 36% of whom are homozygous for two copies of the risk haplotype. Given the high frequency in African Americans, it may be prudent to limit screening for MYH9 risk alleles to close relatives of African Americans with FSGS and focal global glomerulosclerosis in order to detect those at differential risk for subsequent nephropathy and CKD progression. This might assist in determining which relatives are less likely to develop nephropathy after kidney donation. However, studies of this type remain to be performed.
The high frequency of MYH9 risk alleles in the general population reveals that "second hits", either MYH9 gene-second gene or MYH9 gene-environment interactions, are likely necessary for initiation of kidney disease. Clearly, the 36% of African Americans homozygous for MYH9 E1 risk haplotypes will not develop nephropathy. It is estimated that 4–5% of African American MYH9 homozygotes develop idiopathic FSGS. In contrast, approximately 20% of those with HIV infection and two E1 risk haplotypes will develop HIVAN (personal communication, Dr. Jeffrey Kopp). This fivefold increase in nephropathy risk due to HIV1 virus infection reflects an example of an additional environmental second hit necessary to develop MYH9-related disease. Other non-HIV viral infections (and/or toxin exposures) as well as additional MYH9 gene-gene interactions (e.g., podocin NPHS2 or uromodulin UMOD) may interact with MYH9 to cause kidney disease in genetically susceptible hosts. These areas remain under intense investigation.
Table 1.
Odds ratios (OR) for association in MYH9-nephropathy
| Nephropathy type | Cohort – Ethnicity | OR (95% CI) | P-value | Ref |
|---|---|---|---|---|
| Idiopathic FSGS | NIDDK/NCI – AA | 4.65 (3.11–7.02)* | 9 × 10−16 | 35 |
| Idiopathic FSGS | NIDDK/NCI – EA | 7.66 (0.75–380.02)* | 0.05 | 35 |
| HIVAN | NIDDK/NCI – AA | 5.92 (2.89–12.85)* | 7 × 10−8 | 35 |
| Non-diabetic ESRD | FIND – AA | 3.10 (2.15–4.47)+ | 1.5 × 10−9 | 34 |
| Diabetic ESRD | FIND – AA | 1.51 (1.01–2.27)+ | 0.04 | 34 |
| Non-diabetic ESRD | Wake Forest – AA | 2.23 (1.78–2.80)* | 4.5 × 10−12 | 41 |
| Diabetic ESRD | Wake Forest – AA | 1.27 (1.04–1.56)* | 0.02 | 43 |
| [Serum creatinine] | Eurospan -European | continuous trait (n/a) | 0.009 | 45 |
| Urine ACR | HyperGEN – AA | continuous trait (n/a) | 0.01 | 40 |
| Urine ACR | HyperGEN – EA | continuous trait (n/a) | 0.49 | 40 |
| "Hypertensive CKD" | AASK – AA | 2.69 (1.30–5.58) + | 0.008 | 42 |
AA = African American; EA = European American; ACR = albumin:creatinine ratio
reflects E1 haplotype;
reflects most associated SNP;
n/a = not applicable
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Ferguson R, Grim CE, Opgenorth TJ. A familial risk of chronic renal failure among blacks on dialysis? J Clin Epidemiol. 1988;41(12):1189–1196. doi: 10.1016/0895-4356(88)90023-6. [DOI] [PubMed] [Google Scholar]
- 2.Seaquist ER, Goetz FC, Rich S, Barbosa J. Familial clustering of diabetic kidney disease. Evidence for genetic susceptibility to diabetic nephropathy. N Engl J Med. 1989;320(18):1161–1165. doi: 10.1056/NEJM198905043201801. [DOI] [PubMed] [Google Scholar]
- 3.Freedman BI, Spray BJ, Tuttle AB, Buckalew VM., Jr The familial risk of end-stage renal disease in African Americans. Am J Kidney Dis. 1993;21:387–393. doi: 10.1016/s0272-6386(12)80266-6. [DOI] [PubMed] [Google Scholar]
- 4.Spray BJ, Atassi NG, Tuttle AB, Freedman BI. Familial risk, age at onset, and cause of end-stage renal disease in white Americans. J Am Soc Nephrol. 1995;5(10):1806–1810. doi: 10.1681/ASN.V5101806. [DOI] [PubMed] [Google Scholar]
- 5.Lei HH, Perneger TV, Klag MJ, Whelton PK, Coresh J. Familial aggregation of renal disease in a population-based case-control study. J Am Soc Nephrol. 1998;9(7):1270–1276. doi: 10.1681/ASN.V971270. [DOI] [PubMed] [Google Scholar]
- 6.Freedman BI, Volkova NV, Satko SG, Krisher J, Jurkovitz C, Soucie JM, et al. Population-based screening for family history of end-stage renal disease among incident dialysis patients. Am J Nephrol. 2005;25(6):529–535. doi: 10.1159/000088491. [DOI] [PubMed] [Google Scholar]
- 7.Bergman S, Key BO, Kirk KA, Warnock DG, Rostant SG. Kidney disease in the first-degree relatives of African-Americans with hypertensive end-stage renal disease. Am J Kidney Dis. 1996;27(3):341–346. doi: 10.1016/s0272-6386(96)90356-x. [DOI] [PubMed] [Google Scholar]
- 8.Song EY, McClellan WM, McClellan A, Rajyalakshmi G, Hadley AC, Krisher J, et al. Effect of community characteristics on familial clustering of end-stage renal disease. Am J Nephrol. 2009:805. doi: 10.1159/000243716. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freedman BI, Iskandar SS, Appel RG. The link between hypertension and nephrosclerosis. Am J Kidney Dis. 1995;25:207–221. doi: 10.1016/0272-6386(95)90001-2. [DOI] [PubMed] [Google Scholar]
- 10.U.S. Renal Data System USRDS. 2008 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. Bethesda, MD: 2008 2009.
- 11.Klag MJ, Whelton PK, Randall BL, Neaton JD, Brancati FL, Ford CE, et al. Blood pressure and end-stage renal disease in men. N Engl J Med. 1996;334:13–18. doi: 10.1056/NEJM199601043340103. [DOI] [PubMed] [Google Scholar]
- 12.Schlessinger SD, Tankersley MR, Curtis JJ. Clinical documentation of end-stage renal disease due to hypertension. Am J Kidney Dis. 1994;23:655–660. doi: 10.1016/s0272-6386(12)70275-5. [DOI] [PubMed] [Google Scholar]
- 13.McClellan W, Tuttle E, Issa A. Racial differences in the incidence of hypertensive end-stage renal disease (ESRD) are not entirely explained by differences in the prevalence of hypertension. Am J Kidney Dis. 1988;12:285–290. doi: 10.1016/s0272-6386(88)80221-x. [DOI] [PubMed] [Google Scholar]
- 14.Freedman BI, Tuttle AB, Spray BJ. Familial predisposition to nephropathy in African-Americans with non-insulin-dependent diabetes mellitus. Am J Kidney Dis. 1995;25(5):710–713. doi: 10.1016/0272-6386(95)90546-4. [DOI] [PubMed] [Google Scholar]
- 15.Zarif L, Covic A, Iyengar S, Sehgal AR, Sedor JR, Schelling JR. Inaccuracy of clinical phenotyping parameters for hypertensive nephrosclerosis. Nephrol Dial Transplant. 2000;15:1801–1807. doi: 10.1093/ndt/15.11.1801. [DOI] [PubMed] [Google Scholar]
- 16.Kestenbaum B, Rudser KD, de B I, Peralta CA, Fried LF, Shlipak MG, et al. Differences in kidney function and incident hypertension: the multi-ethnic study of atherosclerosis. Ann Intern Med. 2008;148:501–508. doi: 10.7326/0003-4819-148-7-200804010-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fogo A, Breyer JA, Smith MC, Cleveland WH, Agodoa L, Kirk KA, et al. Accuracy of the diagnosis of hypertensive nephrosclerosis in African Americans: a report from the African American Study of Kidney Disease (AASK) Trial. AASK Pilot Study Investigators. Kidney Int. 1997;51:244–252. doi: 10.1038/ki.1997.29. [DOI] [PubMed] [Google Scholar]
- 18.Freedman BI, Iskander SS, Buckalew VM, Jr, Burkart JM, Appel RG. Renal biopsy findings in presumed hypertensive nephrosclerosis. Am J Nephrol. 1994;14:90–94. doi: 10.1159/000168695. [DOI] [PubMed] [Google Scholar]
- 19.Marcantoni C, Ma LJ, Federspiel C, Fogo AB. Hypertensive nephrosclerosis in African Americans versus Caucasians. Kidney Int. 2002;62:172–180. doi: 10.1046/j.1523-1755.2002.00420.x. [DOI] [PubMed] [Google Scholar]
- 20.Appel LJ, Wright JT, Jr, Greene T, Kusek JW, Lewis JB, Wang X, et al. Long-term Effects of Renin-Angiotensin System-Blocking Therapy and a Low Blood Pressure Goal on Progression of Hypertensive Chronic Kidney Disease in African Americans. Archives of Internal Medicine. 2008;168:832–839. doi: 10.1001/archinte.168.8.832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bleyer AJ, Chen R, D'Agostino RB, Jr, Appel RG. Clinical correlates of hypertensive end-stage renal disease. Am J Kidney Dis. 1998;31:28–34. doi: 10.1053/ajkd.1998.v31.pm9428448. [DOI] [PubMed] [Google Scholar]
- 22.Bleyer AJ, Appel RG. Risk factors associated with hypertensive nephrosclerosis. Nephron. 1999;82:193–198. doi: 10.1159/000045402. [DOI] [PubMed] [Google Scholar]
- 23.Fox CS, Yang Q, Cupples LA, Guo CY, Larson MG, Leip EP, et al. Genomewide linkage analysis to serum creatinine, GFR, and creatinine clearance in a community-based population: the Framingham Heart Study. J Am Soc Nephrol. 2004;15(9):2457–2461. doi: 10.1097/01.ASN.0000135972.13396.6F. [DOI] [PubMed] [Google Scholar]
- 24.Hunt SC, Coon H, Hasstedt sj, cawthon rm, camp nj, wu ll, et al. Linkage of serum creatinine and glomerular filtration rate to chromosome 2 in Utah pedigrees. Am J Hypertens. 2004;17:511–515. doi: 10.1016/j.amjhyper.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 25.Jarvik G, Larson EB, Goddard K, Schellenberg GD, Wijsman EM. Influence of apolipoprotein E genotype on the transmission of Alzheimer disease in a community-based sample. Am J Hum Genet. 1996;58:191–200. [PMC free article] [PubMed] [Google Scholar]
- 26.Fogarty DG, Rich SS, Hanna L, Warram JH, Krolewski AS. Urinary albumin excretion in families with type 2 diabetes is heritable and genetically correlated to blood pressure. Kidney Int. 2000;57:250–257. doi: 10.1046/j.1523-1755.2000.00833.x. [DOI] [PubMed] [Google Scholar]
- 27.Imperatore G, Knowler WC, Pettitt DJ, Kobes S, Bennett PH, Hanson RL. Segregation analysis of diabetic nephropathy in Pima Indians. Diabetes. 2000;49(6):1049–1056. doi: 10.2337/diabetes.49.6.1049. [DOI] [PubMed] [Google Scholar]
- 28.Freedman BI, Beck SR, Rich SS, Heiss G, Lewis CE, Turner S, et al. A genome-wide scan for urinary albumin excretion in hypertensive families. Hypertension. 2003;42(3):291–296. doi: 10.1161/01.HYP.0000087890.33245.41. [DOI] [PubMed] [Google Scholar]
- 29.Chung KW, Ferrell RE, Ellis D, Barmada M, Moritz M, Finegold DN, et al. African American hypertensive nephropathy maps to a new locus on chromosome 9q31-q32. Am J Hum Genet. 2003;73:420–429. doi: 10.1086/377184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gharavi AG, Yan Y, Scolari F, Schena FP, Frasca GM, Ghiggeri GM, et al. IgA nephropathy, the most common cause of glomerulonephritis, is linked to 6q22-23. Nat Genet. 2000;26(3):354–357. doi: 10.1038/81677. [DOI] [PubMed] [Google Scholar]
- 31.Risch NJ. Searching for genetic determinants in the new millennium. Nature. 2000;405:847–856. doi: 10.1038/35015718. [DOI] [PubMed] [Google Scholar]
- 32.Kottgen A, Glazer NL, Dehghan A, Hwang SJ, Katz R, Li M, et al. Multiple loci associated with indices of renal function and chronic kidney disease. Nat Genet. 2009 doi: 10.1038/ng.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith MW, O'Brien SJ. Mapping by admixture linkage disequilibrium: advances, limitations and guidelines. Nat Rev Genet. 2005;6:623–632. doi: 10.1038/nrg1657. [DOI] [PubMed] [Google Scholar]
- 34.Kao WH, Klag MJ, Meoni LA, Reich D, Berthier-Schaad Y, Li M, et al. MYH9 is associated with nondiabetic end-stage renal disease in African Americans. Nat Genet. 2008;40:1185–1192. doi: 10.1038/ng.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kopp JB, Smith MW, Nelson GW, Johnson RC, Freedman BI, Bowden DW, et al. MYH9 is a major-effect risk gene for focal segmental glomerulosclerosis. Nat Genet. 2008;40:1175–1184. doi: 10.1038/ng.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roxburgh SA, Murphy M, Pollock CA, Brazil DP. Recapitulation of embryological programmes in renal fibrosis--the importance of epithelial cell plasticity and developmental genes. Nephron Physiol. 2006;103:139–148. doi: 10.1159/000092453. [DOI] [PubMed] [Google Scholar]
- 37.Brosius FC., III New insights into the mechanisms of fibrosis and sclerosis in diabetic nephropathy. Rev Endocr Metab Disord. 2008;9:245–254. doi: 10.1007/s11154-008-9100-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Woroniecki RP, Schnaper HW. Progression of glomerular and tubular disease in pediatrics. Semin Nephrol. 2009;29:412–424. doi: 10.1016/j.semnephrol.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Papeta N, Chan KT, Prakash S, Martino J, Kiryluk K, Ballard D, et al. Susceptibility loci for murine HIV-associated nephropathy encode trans-regulators of podocyte gene expression. J Clin Invest. 2009;119:1178–1188. doi: 10.1172/JCI37131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Freedman BI, Kopp JB, Winkler CA, Nelson GW, Rao DC, Eckfeldt JH, et al. Polymorphisms in the nonmuscle myosin heavy chain 9 gene (MYH9) are associated with albuminuria in hypertensive African Americans: the HyperGEN study. Am J Nephrol. 2009;29:626–632. doi: 10.1159/000194791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Freedman BI, Hicks PJ, Bostrom MA, Cunningham ME, Liu Y, Divers J, et al. Polymorphisms in the non-muscle myosin heavy chain 9 gene (MYH9) are strongly associated with end-stage renal disease historically attributed to hypertension in African Americans. Kidney Int. 2009;75:736–745. doi: 10.1038/ki.2008.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lipkowitz MS, Iyengar S, Molineros J, Langefeld CD, Comeau ME, Klotman PE, Bowden DW, Freeman RG, Khitrov G, Zhang W, Kao WHL, Parekh RS, Choi M, Kopp JB, Winkler CA, Nelson G, Freedman BI, Bottinger EP the AASK Investigators. Association analysis of the non-muscle myosin heavy chain 9 gene (MYH9) in hypertensive nephropathy: African American Study of Kidney Disease and Hypertension (AASK) J Am Soc Nephol. 2009 In Press. [Google Scholar]
- 43.Freedman BI, Hicks PJ, Bostrom MA, Comeau ME, Divers J, Bleyer AJ, et al. Non-muscle myosin heavy chain 9 gene MYH9 associations in African Americans with clinically diagnosed type 2 diabetes mellitus-associated ESRD. Nephrol Dial Transplant. 2009 doi: 10.1093/ndt/gfp316. doi:10.1093/ndt/gfp316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pham TT, Sim JJ, Kujubu DA, Liu IL, Kumar VA. Prevalence of nondiabetic renal disease in diabetic patients. Am J Nephrol. 2007;27(3):322–328. doi: 10.1159/000102598. [DOI] [PubMed] [Google Scholar]
- 45.Pattaro C, Aulchenko YS, Isaacs A, Vitart V, Hayward C, Franklin CS, et al. Genome-wide linkage analysis of serum creatinine in three isolated European populations. Kidney Int. 2009;76:297–306. doi: 10.1038/ki.2009.135. [DOI] [PubMed] [Google Scholar]
- 46.Shimazaki A, Kawamura Y, Kanazawa A, Sekine A, Saito S, Tsunoda T, et al. Genetic variations in the gene encoding ELMO1 are associated with susceptibility to diabetic nephropathy. Diabetes. 2005;54:1171–1178. doi: 10.2337/diabetes.54.4.1171. [DOI] [PubMed] [Google Scholar]
- 47.Leak TS, Perlegas PS, Smith SG, Hicks PJ, Li L, Langefeld CD, et al. Variants in the ELMO1 gene are associated with diabetes and nephropathy in African Americans. J Am Soc Nephol. 2007 In press (Abstract) [Google Scholar]
- 48.Pezzolesi MG, Katavetin P, Kure M, Poznik GD, Skupien J, Mychaleckyj JC, et al. Confirmation of Genetic Associations at ELMO1 in the GoKinD Collection Support its Role as a Susceptibility Gene in Diabetic Nephropathy. Diabetes. 2009 doi: 10.2337/db09-0641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Salem RM, Cadman PE, Chen Y, Rao F, Wen G, Hamilton BA, et al. Chromogranin A polymorphisms are associated with hypertensive renal disease. J Am Soc Nephrol. 2008;19:600–614. doi: 10.1681/ASN.2007070754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen Y, Mahata M, Rao F, Khandrika S, Courel M, Fung MM, et al. Chromogranin A regulates renal function by triggering Weibel-Palade body exocytosis. J Am Soc Nephrol. 2009;20:1623–1632. doi: 10.1681/ASN.2008111148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rao F, Zhang L, O'Connor DT. Complex trait genetics the role of mechanistic "intermediate phenotypes" and candidate genetic loci. J Am Coll Cardiol. 2008;52:166–168. doi: 10.1016/j.jacc.2008.02.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hsu CC, Kao WH, Coresh J, Pankow JS, Marsh-Manzi J, Boerwinkle E, et al. Apolipoprotein E and progression of chronic kidney disease. JAMA. 2005;293:2892–2899. doi: 10.1001/jama.293.23.2892. [DOI] [PubMed] [Google Scholar]
- 53.Kottgen A, Kao WH, Hwang SJ, Boerwinkle E, Yang Q, Levy D, et al. Genome-wide association study for renal traits in the Framingham Heart and Atherosclerosis Risk in Communities Studies. BMC Med Genet. 2008;9:49. doi: 10.1186/1471-2350-9-49. [DOI] [PMC free article] [PubMed] [Google Scholar]