Abstract
Purpose
Androgen deprivation therapy (AD) has been shown to increase late ≥ grade 2 rectal toxicity when used concurrently with three-dimensional conformal radiotherapy (3DCRT). Intensity modulated radiotherapy (IMRT) has the potential to reduce toxicity by limiting the radiation dose received by the bowel and bladder. This study compares both genitourinary (GU) and gastrointestinal (GI) toxicity in men treated with 3DCRT+AD versus IMRT+AD.
Methods and Materials
From July 1992 to July 2004, 293 men received 3DCRT (n=170) or IMRT (n=123) with concurrent AD (< 6 months, n=123; ≥ 6 months, n =170). Median RT doses were 76 Gy for 3DCRT (ICRU) and 76 Gy for IMRT (95% to the PTV). Toxicity was assessed by a patient symptom questionnaire assessing toxicity completed at each visit and recorded using a modified late effects normal tissue task force radiation morbidity scale (LENT).
Results
Mean follow-up was 86 months (SD=29.3) for the 3DCRT group and 40 months (SD=9.7) for the IMRT group. Acute GI toxicity (OR=4, 95% CI: 1.6–11.7, p=0.005) was significantly higher with 3DCRT than with IMRT and was independent of AD duration (i.e. <6 vs. ≥6 months). Time to development of late GI toxicity was significantly longer in the IMRT group. The 5-year Kaplan-Meier estimates for ≥ grade 2 GI toxicity were 20% for 3DCRT versus 8% for IMRT (p=0.01). On MVA, ≥ grade 2 late GI toxicity (HR=2.1, 95% CI: 1.1–4.3, p=0.04) was more prevalent in 3DCRT patients.
Conclusions
Compared to 3DCRT, IMRT significantly decreased acute and late GI toxicity in patients treated with AD.
Keywords: Intensity Modulated Radiation Therapy, Gastrointestinal Toxicity, Androgen Deprivation Therapy, Prostate Cancer
Introduction
The definitive non-surgical management of prostate cancer has benefited from advances in radiation treatment planning and delivery techniques. Dose escalation using three-dimensional conformal radiotherapy (3DCRT) in men with intermediate to high-risk disease has been associated with significant improvements in freedom from biochemical and clinical progression (1–4). These improvements were, however, at the cost of increased gastrointestinal (GI) toxicity, and rates of ≥ grade 2 GI toxicity in the range of 26–35% have been associated with does of 78 Gy.
The addition of androgen deprivation (AD) therapy to 3DCRT for intermediate and high-risk prostate cancer has been shown to improve in local control, biochemical-free survival, distant metastases and overall survival (5–8). Some studies have suggested that the addition of AD increases the risk of GI and GU toxicity while others have not (7, 9–11). At Fox Chase Cancer Center, our experience is that the addition of long-term AD (LTAD) significantly increases grade 2 and higher GI and GU toxicity with in patients treated with 3DCRT for locally advanced prostate cancer (9).
Intensity modulated RT (IMRT), by way of inverse treatment planning and dose-volume constraints for normal tissues, allows for the delivery of highly conformal radiotherapy with greater conformality to the target volume compared to 3DCRT. Theoretical (12) and clinical (13) studies have demonstrated lower dose exposure and smaller volumes of normal tissue receiving higher doses using an IMRT approach when compared with 3DCRT. Zelefsky et al. (13) demonstrated the safety of delivering 81 Gy with an 8-year grade 2 or higher rectal bleeding rate of < 2% and, more importantly, no grade 4 or higher rectal complications in clinically localized prostate cancer. Al-Mamgani et al. (14) recently reported a subgroup of 78 men that received 78 Gy on the Dutch randomized trial showing IMRT reduced GI and GU toxicity compared to 3DCRT. At our institution, IMRT has been used exclusively in the definitive treatment of all patients with prostate cancer since July 2001. This study compares the incidence of GI and GU toxicity in 293 men treated with 3DCRT versus IMRT with adjuvant AD, which has not been addressed in the literature. Furthermore, unlike the patients reported by Al-Mamgani et al., the majority of the patients in the current study were treated to the regional lymph nodes having implications for GU and GI toxicity.
METHODS AND MATERIALS
Institutional Review Board approval was obtained prior to initiation of this study. Between July, 1992 and July, 2004, 293 men with clinical stage T1-3, NX/0, M0 adenocarcinoma of the prostate received RT and AD definitively. One hundred seventy patients were treated with 3DCRT (July 1992–June 2001) and 123 with IMRT (July 2001–July 2004). Mean follow-up was 86 months (SD=29.3) for the 3DCRT group and 40 months (SD=9.7) for the IMRT group.
Radiation Therapy
The techniques used for the 3D-CRT and IMRT treatments have been previously described (15, 16). Briefly, patients were immobilized in a thermoplastic cast and prostate localization was confirmed using a B-type acquisition ultrasound in all patients. Weekly portal imaging was also used to confirm treatment accuracy. The International Commission on Radiation Units and Measurements reference point doses were used (17). All patients were treated with megavoltage photon energies (10–18 MV). Dose was prescribed to the 95% isodose line and was then normalized so that the 95% isodose line covered the intended target volume. There were three possible fields for patients treated with 3DCRT (18): prostate alone with or without additional pelvic radiation, which was further classified as partial or whole pelvis. More advanced disease received radiation to the seminal vesicles, periprostatic lymph nodes (partial pelvis), or the whole pelvis (18). A 1 cm planning target volume margin was used for 3DCRT.
Whole pelvic radiation was defined by initial fields whose superior border extended to L5–S1 interspace. Prostate-alone fields were defined as having a maximum field size of 10 × 10 cm. Partial pelvic fields were larger than prostate-alone fields but smaller than whole pelvic fields. The median doses to the whole pelvic and partial pelvic fields were 45 Gy (range, 18–50.4 Gy) and 46 Gy (range, 12.6–72 Gy), respectively. The radiation doses to the prostate for patients treated to the prostate alone, partial pelvic fields, and whole pelvic fields were 72, 76, and 76 Gy, respectively. Median total dose to the prostate in this group was 76 Gy (range: 73–80 Gy).
Target volumes for patients in the IMRT group have been previously described and included prostate only, prostate and seminal vesicles, or prostate and seminal vesicle and pelvic lymph nodes (19). For IMRT planning, patients were immobilized and their prostate localized in a similar fashion to those in the 3DCRT group. Structures at risk were contoured and used to generate a clinical target volume. This was subsequently expanded by 8 mm in all directions except posteriorly, where the expansion was 3–5 mm (to account for the prostate-rectal interface). An isocentric multi-beam technique with inverse optimization was then used to deliver specified doses to the planning target volume. Dose was prescribed so that >95% of the PTV received 100% of the prescribed dose. Rectal volume receiving greater than 65 Gy and greater than 40 Gy was limited to less than 17% and less than 35%, respectively. Bladder volume receiving greater than 65 Gy and greater than 40 Gy was limited to less than 25% and less than 50%, respectively.
Androgen Deprivation Therapy
Androgen deprivation was used at the discretion of the physician or as described in RTOG 92-02 for patients treated on protocol (6). AD was classified as short term if administered for a period of ≤ 6 months and long term if greater than 6 months. AD consisted primarily of an oral antiandrogen (1–4 month course) and luteinizing hormone releasing hormone agonist administered as depot injections.
Toxicity Assessment
Generally, follow-up was performed initially at 3–4 months post-treatment and subsequently at six to 12 month intervals with serial PSA levels and physician-performed digital rectal examination. A patient symptom questionnaire assessing toxicity was completed at each visit and recorded in the Fox Chase Modified Late Effects Normal Tissue Task (LENT) Force radiation morbidity scales were used to grade late GI and GU toxicity (9). This scale is similar to the RTOG late morbidity scale but is thought to be more sensitive owing to the addition of coagulations for bleeding. Acute toxicity was defined as one occurring within three months of treatment completion, with late toxicity occurring anytime thereafter. Outcomes were measured from the initiation of RT to the date of onset of complication or date of last follow-up. Biochemical failure (BF) was defined as the nadir PSA+2 ng/ml.
An initial analysis of the obvious difference in follow-up periods had suggested a significant discordance between the two groups (mean: 86 mo for 3DCRT vs. 40 mo for IMRT, p < 0.0001). Follow-up in each category was sufficiently long, though, such that acute toxicity could be considered a binary event for which time to the event did not need to be incorporated into the analysis. However, because of loss to follow-up, censoring, and different follow-up rates between the groups, late toxicity was analyzed as a time-to-event outcome using Kaplan-Meier estimation and a Cox proportional hazard model.
Statistical Analyses
Acute toxicity was analyzed as a dichotomous (≥ grade 2 vs. otherwise) variable. Univariate analysis was performed using a chi-square test for discrete variables and the Wilcoxon test for continuous variables, and multivariable analysis (MVA) by logistic regression. Radiotherapy dose was analyzed using the two-sided t-test. Late toxicity was analyzed as a time-to-event variable. Any ≥ grade 2 late toxicity was coded as an event, otherwise as censored. Univariate analysis of late toxicity was performed via Kaplan-Meier estimation, and MVA by Cox proportional hazard modeling. The possible predictors for toxicity analyzed were, BF, time interval to BF (IBF) (20), AD duration (<6 vs. ≥6 months), diabetes, T-stage, iPSA, GS, RT dose and RT technique (IMRT vs. 3DCRT). A p value ≤0.05 was considered statistically significant. To account for the potential confounding effects of the size of the target volume on toxicity events, we also performed similar analyses on patients treated to the largest volume in each group (i.e. treatment to include the prostate, seminal vesicles, and pelvic lymph nodes).
RESULTS
Various patient and treatment related characteristics are shown in Table 1. Mean initial PSA value (iPSA) for the entire cohort was 19 ng/mL (SD=20.1), for 3DCRT+AD was 23 ng/mL (SD= 22.2) and IMRT+AD groups was 14 ng/mL (SD=15.3). Mean Gleason Scores were 7 in all three groups. The mean age in 3DCRT and IMRT groups was similar, 68 years. There was a greater proportion of patients in the 3DCRT+AD group with iPSA values > 20 ng/ml (39% vs. 19%, p < 0.0001) and with palpable ≥ T2 disease (86% vs. 51%, p < 0.0001).
Table 1.
Various Patient And Treatment Characteristics
| Characteristic | IMRT (n = 123) | 3DCRT (n = 170) | p-value |
|---|---|---|---|
| Age | 70 years (range: 45–86) | 69 years (range: 45–84) | 0.9 |
| Follow-up | 39 months (range: 25–60) | 89 months (range: 25–160) | <0.0001 |
| iPSA | <0.0001 | ||
| <10 ng/mL | 73 (59%) | 55 (32%) | |
| 10–20 | 27 (22) | 49 (29) | |
| >20 | 23 (19) | 66 (39) | |
| Gleason Score | 0.08 | ||
| 2–6 | 34 (28) | 66 (39) | |
| 7 | 42 (34) | 57 (33) | |
| 8–10 | 47 (38) | 47 (28) | |
| T-stage | <0.0001 | ||
| T1 | 60 (49) | 24 (14) | |
| T2 | 39 (32) | 100 (59) | |
| T3 | 24 (20) | 46 (27) | |
| AD | 0.0002 | ||
| STAD | 36 (29) | 87 (51) | |
| LTAD | 87 (71) | 83 (49) | |
| ≤ 1 year | 18 (15) | 20 (12) | |
| > 1 – 5 | 67 (54) | 59 (35) | |
| > 5 | 2 (2) | 4 (2) | |
| Diabetes | 23 (19) | 23 (14) | 0.23 |
| RT dose* | 76 Gy (range: 74–78) | 76 Gy (range:73–80) | <0.0001 |
| Fields | <0.0001 | ||
| Prostate alone | 31 (25) | 11 (6) | |
| Prostate + SV | 21 (17) | 3 (2) | |
| Prostate + SV + Lymph nodes | 71 (58) | 156 (92) | |
| Photon Energy | < 0.0001 | ||
| < 10 MV | 25 (20) | 0 (0) | |
| 10 – 14 | 58 (69) | 47 (28) | |
| > 14 | 13 (11) | 123 (72) |
Abbreviations: RT = radiotherapy; IMRT = intensity modulated radiotherapy; 3DCRT = three dimensional conformal radiotherapy; iPSA= initial prostate specific antigen; AD= androgen deprivation; STAD= short term androgen deprivation; LTAD= long-term androgen deprivation; SV= seminal vesicles
ICRU for 3DCRT
One hundred and seventy patients were treated with 3DCRT+AD and 123 with IMRT+AD. Mean RT dose was 76 Gy (range: 73–80; SD ± 1.9) in the 3DCRT+AD group and 77 Gy (range: 74–78; SD ± 1.4) in the IMRT +AD group. Ninety-two percent of patients in the 3DCRT group received pelvic nodal irradiation compared to 58% for IMRT.
One hundred twenty three patients received STAD (87 in the 3DCRT group and 36 in the IMRT group) and the remaining 170 received LTAD (83 in 3DCRT and 87 in the IMRT group). IMRT+AD patients were more likely to receive LTAD compared to 3DCRT patients (71% vs. 49%; p = 0.0002).
Toxicity
A late GI toxicity event was recorded in 136 patients (46%), forty-six of whom reported ≥ grade 2 GI toxicity (Table 2). Of those who reported late ≥ grade 2 toxicity, 10 patients (22%) received IMRT+AD and the rest received 3DCRT+AD (78%). Nineteen patients with ≥ grade 2 GI toxicity (41%) received STAD and 27 (59%) LTAD.
Table 2.
Crude Late GI Toxicity
| Characteristic | Late GI Toxicity* | p-value | |
|---|---|---|---|
| Grade 0–1 | ≥ grade 2 | ||
| Treatment | 0.002 | ||
| IMRT | 113 (46%) | 10 (22%) | |
| 3DCRT | 134 (54) | 36 (78) | |
| iPSA | 0.9 | ||
| <10 ng/mL | 109 (44) | 19 (32) | |
| 10–20 | 63 (26) | 13 (28) | |
| >20 | 75 (30) | 14 (30) | |
| Gleason Score | 0.97 | ||
| 2–6 | 85 (34) | 15 (33) | |
| 7 | 83 (34) | 16 (35) | |
| 8–10 | 79 (32) | 15 (33) | |
| T stage | 0.06 | ||
| T1 | 77 (31) | 7 (15) | |
| T2 | 111 (45) | 28(61) | |
| T3 | 59 (24) | 11 (24) | |
| AD | 0.9 | ||
| STAD | 104 (42) | 19 (41) | |
| LTAD | 143 (58) | 27 (59) | |
| Diabetes | 40 (16) | 6 (13) | 0.59 |
| Fields | 0.2 | ||
| Prostate alone | 38 (15) | 4 (9) | |
| Prostate + SV | 22 (9) | 2 (4) | |
| Prostate + SV + Lymph nodes | 187 (76) | 40 (87) | |
Abbreviations: GI = gastrointestinal; iPSA= initial prostate specific antigen; AD= androgen deprivation; STAD= short term androgen deprivation; LTAD= long-term androgen deprivation; SV= seminal vesicles
Fox Chase modified LENT scale
A late GU toxicity event was documented in 129 patients, though only 17 (13%) of them reported an event that was classified as ≥ grade 2 per the Fox Chase Cancer Center modified LENT scale. Eleven patients (65%) reporting a ≥ grade 2 GU toxicity event were treated with 3DCRT while the rest received IMRT. Nine patients (53%) underwent STAD and eight (47%) LTAD (Table 3). There was no difference in mean age (p = 0.4) or RT dose (p = 0.2) between patient experiencing < Grade 2 versus ≥ 2 GU toxicity.
Table 3.
Crude Late GU Toxicity
| Characteristic | Late GI Toxicity* | p-value | |
|---|---|---|---|
| Grade 0–1 | ≥ grade 2 | ||
| Treatment | 0.6 | ||
| IMRT | 117 (42%) | 6 (35%) | |
| 3DCRT | 159 (58) | 11 (65) | |
| iPSA | 0.2 | ||
| <10 ng/mL | 121 (44) | 7 (41) | |
| 10–20 | 74 (27) | 2 (12) | |
| >20 | 81 (29) | 8 (47) | |
| Gleason Score | 0.9 | ||
| 2–6 | 95 (34) | 5 (29) | |
| 7 | 93 (34) | 6 (35) | |
| 8–10 | 88 (32) | 6 (35) | |
| T stage | 0.1 | ||
| T1 | 81 (29) | 3 (18) | |
| T2 | 133 (48) | 6 (35) | |
| T3 | 62 (22) | 8 (47) | |
| AD | 0.3 | ||
| STAD | 114 (41) | 9 (53) | |
| LTAD | 162 (59) | 8 (47) | |
| Diabetes | 43 (16) | 3 (18) | 0.8 |
| Fields | 0.2 | ||
| Prostate alone | 41 (15) | 1 (6) | |
| Prostate + SV | 24 (9) | 0 (0) | |
| Prostate + SV + Lymph nodes | 211 (76) | 16 (94) | |
Abbreviations: GI = gastrointestinal; iPSA= initial prostate specific antigen; AD= androgen deprivation; STAD= short term androgen deprivation; LTAD= long-term androgen deprivation; SV= seminal vesicles
Fox Chase modified LENT scale
Acute GI toxicity (OR= 4, 95% CI: 1.6–11.7, p=0.005) was significantly higher in the 3DCRT group than the IMRT group. Actuarial rates and univariate results of late GI toxicity are shown in Table 4. Kaplan-Meier analysis of late ≥ grade 2 GI toxicity, when plotted against time from completion of therapy revealed a significant difference in toxicity between 3DCRT and IMRT groups (Fig. 1). The 5-yr Kaplan Meier estimates for ≥ grade 2 GI toxicity were 20% for 3DCRT versus 8% for IMRT (p = 0.01). 3DCRT had an odds ratio of 2.2 (CI: 1.1–4.5; p = 0.01) of being associated with a ≥ grade 2 GI toxicity event compared with IMRT.
Table 4.
Actuarial Late ≥ grade 2 GI Toxicity* and Univariate Analysis
| Variable | 3-year KM | 5-year KM | p-value | HR (95% CI) |
|---|---|---|---|---|
| AD duration | ||||
| STAD | 14% | 15% | 0.8 | 0.9 (0.5–1.6) |
| LTAD | 15 | 15 | 1 | |
| ≤ 1 year | 11 | 11 | ||
| > 1 – 5 | 17 | 17 | ||
| > 5 | 0 | 0 | ||
| T-stage | ||||
| T1 | 8 | 8 | 0.1 | 1 |
| T2 | 18 | 19 | 2.4 (1.1–5.6) | |
| T3 | 14 | 14 | 2 (0.8–5.1) | |
| Diabetes | ||||
| Yes | 13 | 13 | 0.7 | 1 |
| No | 15 | 15 | 1 (0.4–2.4) | |
| Gleason Score | Gi | |||
| 2–6 | 14 | 14 | 0.9 | 1 |
| 7 | 16 | 16 | 1.2 (0.6–2.4) | |
| 8–10 | 13 | 15 | 1.2 (0.6–2.5) | |
| iPSA | ||||
| <10 ng/mL | 13 | 13 | 0.9 | 1 |
| 10–20 | 13 | 15 | 1.0 (0.4–2.1) | |
| >20 | 18 | 18 | 1.2 (0.6–2.3) | |
| Treatment | ||||
| IMRT | 8 | 8 | 0.01 | 1 |
| 3DCRT | 19 | 20 | 2.2(1.1–4.5) | |
| BF status | ||||
| Yes | 16 | 16 | 0.8 | 1 |
| No | 15 | 15 | 0.9 (0.4–1.8) | |
| Increasing Age (continuous) | NA | NA | 0.4 | |
| Increasing RT Dose (continuous) | NA | NA | 0.08 | |
| Photon Energy | 0.2 | |||
| < 10 MV | 8 | 8 | 1 | |
| 10 – 14 | 12 | 13 | 1.3 (0.3–5.7) | |
| > 14 | 18 | 18 | 2.2 (0.5–9.3) |
Abbreviations: PSA= prostate specific antigen; AD= androgen deprivation; STAD= short term androgen deprivation; LTAD= long-term androgen deprivation; iPSA = initial prostate specific antigen; BF= biochemical failure; KM= Kaplan-Meier; HR = hazard ratio; CI = confidence interval
Fox Chase modified LENT scale
Figure 1.
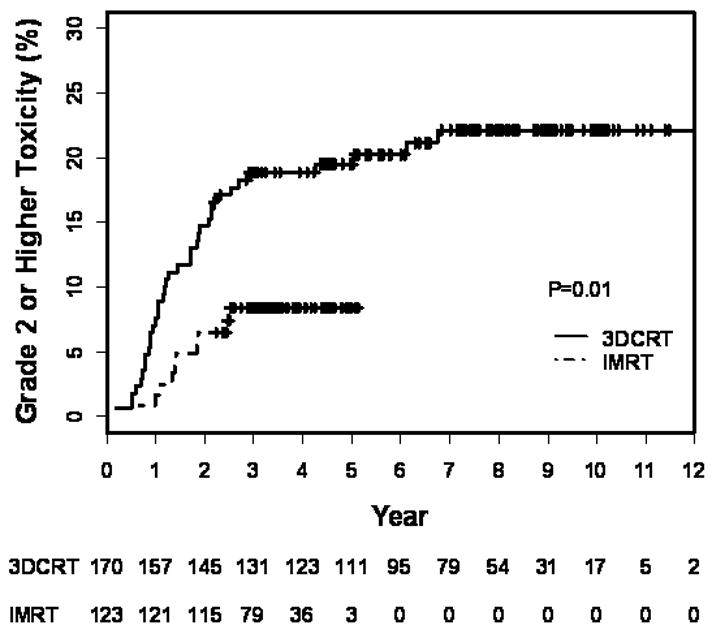
Kaplan-Meier estimation of late ≥ grade 2 GI toxicity in all patients treated with 3DCRT+AD or IMRT+AD as a function of years from treatment completion. Numbers at risk are indicated below the graph.
Multivariable analysis (Table 5) confirmed the reduced toxicity in patients receiving IMRT compared with the 3DCRT patients (HR = 2.1, CI: 1.1–4.3, p = 0.04). No other tested variables were found to significantly predict for ≥ grade 2 late GI toxicity.
Table 5.
Multivariable Analysis Of Late ≥ Grade 2 GI Toxicity*
| Variable | HR | 95% CI | p-value |
|---|---|---|---|
| 3DCRT vs IMRT | 2.1 | 1.1–4.3 | 0.04 |
| Increasing RT Dose (continuous) | 0.99 | 0.997–1.0 | 0.1 |
Abbreviations: 3DCRT= 3D conformal radiotherapy, IMRT = intensity modulated radiotherapy, HR = hazard ratio, CI = confidence interval
Fox Chase modified LENT scale
To investigate the effect of treatment to the pelvic lymph nodes, a similar analysis was performed. One hundred fifty six men in the 3DCRT group received pelvic lymph treatment and 71 for IMRT. A ≥ grade 2 GI toxicity event was recorded in 40 patients, 35 (88%) of whom received 3DCRT with the remaining 5 (12%) receiving IMRT.
Actuarial rates of late GI toxicity in the subset of patients treated to the pelvic lymph nodes, as well as results of univariate and multivariate analyses for this sub-population are outlined in Tables 6. The 5-year Kaplan-Meier estimates for ≥ grade 2 GI toxicity were 21% for 3DCRT versus 7% for IMRT (p = 0.02). On multivariable analysis (Table 7), the odds ratio of a late GI toxicity for 3DCRT was significant at 2.1 (CI: 1.1–4.3; p = 0.04) when compared with IMRT.
Table 6.
Actuarial Late ≥ grade 2 GI Toxicity* and Univariate Analysis in Subgroup Treated to Pelvic Lymph Nodes
| Variable | 3-year KM | 5-year KM | p-value | HR (95% CI) |
|---|---|---|---|---|
| AD duration | ||||
| STAD | 15% | 17% | 0.8 | 0.9 (0.5–1.7) |
| LTAD | 16 | 16 | 1 | |
| ≤ 1 year | 13 | 13 | ||
| > 1 – 5 | 18 | 18 | ||
| > 5 | 0 | 0 | ||
| T-stage | ||||
| T1 | 9 | 9 | 0.2 | 1 |
| T2 | 19 | 21 | 2.4 (0.8–7) | |
| T3 | 15 | 15 | 1.9 (0.6–5.9) | |
| Diabetes | ||||
| Yes | 16 | 16 | 0.9 | 1 |
| No | 16 | 17 | 1 (0.4–2.2) | |
| Gleason Score | ||||
| 2–6 | 17 | 17 | 1.0 | 1 |
| 7 | 18 | 18 | 1.0 (0.5–2.2) | |
| 8–10 | 13 | 15 | 1.0 (0.5–2.2) | |
| iPSA | ||||
| <10 | 14 | 14 | 0.9 | 1 |
| 10–20 | 16 | 19 | 1.1 (0.5–2.6) | |
| >20 | 18 | 18 | 1.1 (0.5–2.2) | |
| Treatment | ||||
| IMRT | 7 | 7 | 0.02 | 1 |
| 3DCRT | 20 | 21 | 2.5(1.1–6.5) | |
| BF status | ||||
| Yes | 20 | 20 | 0.6 | 1 |
| No | 17 | 17 | 0.8 (0.4–1.7) | |
| Increasing Age (continuous) | NA | NA | 0.4 | |
| Increasing RT Dose (continous) | NA | NA | 0.09 | |
| Photon Energy | 0.3 | |||
| < 10 MV | 9 | 9 | 1 | |
| 10 – 14 | 12 | 15 | 1.1 (0.1–8.2) | |
| > 14 | 19 | 19 | 1.7 (0.2–12.7) |
Abbreviations: iPSA= initial prostate specific antigen; AD= androgen deprivation; STAD= short term androgen deprivation; LTAD= long-term androgen deprivation; SV= seminal vesicles; BF= biochemical failure; KM= Kaplan-Meier
Fox Chase modified LENT scale
Table 7.
Multivariable Analysis Of Late ≥ Grade 2 GI Toxicity* in Subgroup Treated to Prostate, Seminal Vesicles and Pelvic Lymph Nodes
| Variable | HR | 95% CI | p-value |
|---|---|---|---|
| 3DCRT vs IMRT | 2.1 | 1.1–4.3 | 0.04 |
| Increasing RT Dose (continuous) | 0.99 | 0.997–1.0 | 0.1 |
Abbreviations: 3DCRT = 3D conformal radiotherapy, IMRT = intensity modulated radiotherapy, HR = hazard ratio, CI = confidence interval
Fox Chase modified LENT scale
DISCUSSION
Our analysis shows a statistically significant reduction in ≥ grade 2 GI toxicity with IMRT in intermediate- and high-risk prostate cancer treated with concurrent AD. Multivariable analysis showed that patients treated with 3DCRT were more than twice as likely to suffer a late grade ≥ 2 GI toxicity than patients treated with IMRT (HR = 2.1, CI: 1.1–4.3, p = 0.04) independent of other factors such as AD duration and diabetes. Al-Mamgani et al. (1) have recently demonstrated that IMRT reduced toxicity compared to 3DCRT in patients treated with RT alone on the Dutch randomized dose escalation trial. This study also demonstrats a reduction in toxicity with IMRT when AD is used.
Several studies have shown a relationship between AD and GI toxicity. In the RTOG 92-02 study, a prospective randomized trial that evaluated the benefit of LTAD with advanced prostate cancer, all patients received STAD (2 months before and 2 months during radiotherapy) and were randomized to receive 2 further years of AD vs. no further therapy. This trial demonstrated a significant increase in late Grade 3 and higher toxicity associated with RT and LTAD (p = 0.037). Grade 3 or higher GI toxicity was 10% for STAD and 25% for LTAD (6, 7).
Initial reports from the RTOG 94-13 trial, a four arm study evaluating the optimal timing of STAD (2 months before and 2 months during radiation vs. 4 months starting after radiotherapy) and the role of elective nodal radiation for patients with a >15% risk of pelvic lymph nodes, had also demonstrated a trend toward increased GI toxicity at 2 years (p = 0.09) in patients receiving whole pelvis RT with concurrent ADT (21). A recently published update of the same study demonstrated statistically significant differences in toxicity between the four arms with the neoadjuvant hormone therapy and whole pelvis RT group having the highest rate of late GI toxicity (11). On the other had, a pooled analysis of the RTOG studies 85-31, 86-10 and 92-02 suggested a protective role for AD in terms of GI and GU toxicity (10). It unclear as to whether these conflicting results is related to categorization of toxicity. While these studies largely report ≥ grade 3 toxicities, some have suggested the more important outcome is grade 2 or higher toxicity as being a critical outcome (9, 22, 23). In any case, the results of Al-Mamgani et al. (1) and the current study suggest that GI toxicity is reduced with IMRT.
Genitourinary toxicity has traditionally been more difficult to predict aside from clinical factors such as age and chronic obstructive uropathy. There was no apparent increase in GU toxicity associated with higher prescribed doses in the MD Anderson or Dutch randomized trials. While in general dose-volume effects likely exist, bladder dose-volume constraints that limit toxicity are not well established. One study in over 1,400 patients explored various dose-volume parameters as potential predictors of late GU toxicity (24). Two methods were used to define the bladder: the entire bladder wall and it contents or the bladder wall alone. The authors concluded that increasing dose received by the entire volume or a higher proportion of the volume receiving 70 Gy were independent predictors irrespective of the contouring method used. At Fox Chase, IMRT bladder constraints have been to limit the volume of the bladder (and contents) receiving 65 Gy to 25%. Despite using these constraints with IMRT, our analysis was not able to demonstrate an improvement in GU toxicity with IMRT.
Our GI toxicity findings are consistent with the observations of a large retrospective series from the Memorial Sloan-Kettering Cancer Center in which an analysis of 1571 patients (of all risk groups, with or without concurrent AD) treated with either 3DCRT or IMRT was performed to assess incidence of and identify predictors for toxicity (25). The 10-year incidence of ≥ grade 2 GI toxicity for 3DCRT 13% and for IMRT was 5% (p<0.001). The authors also noted that while increasing dose was associated with increased toxicity, there was a reduction in reported events at higher doses in the range of 75.6 to 81 Gy, which coincided with the implementation of treatment with IMRT. In the current study, the 5-year actuarial rate of ≥ grade 2 GI toxicity of 20% with 3DCRT and 8% with IMRT. These rates are promising given the high-risk population, universal use of concurrent AD, generally larger treated volumes, and larger fraction size (2 Gy vs. 1.8 Gy at Memorial).
Limitations of this study include the usual shortcomings associated with retrospective reviews. For example, treatment (3DCRT vs. IMRT and STAD vs. LTAD) was not randomized. A randomized comparison between 3DCRT and IMRT is unlikely to occur due to physician bias evidenced by the widespread adoption of IMRT. Despite the sequential nature of this study, the mean RT dose, an important factor in terms of toxicity, was very similar between the two groups (76 Gy for 3DCRT and 77 Gy for IMRT). But, treatment volumes were larger in the 3DCRT group with a greater proportion of patients receiving treatment to the regional lymph nodes. Furthermore, the PTV for all 3DCRT patients usually extended posteriorly by approximately 10 mm while the corresponding margin for IMRT was 3 – 5 mm. While this may favor IMRT, when considering only patients treated to the lymph nodes (3DCRT or IMRT), ≥ grade 2 GI toxicity was lower in the IMRT group (7% for IMRT, 21% for 3DCRT, p = 0.02). Furthermore, in multivariable analysis, the odds ratio of a late GI toxicity for 3DCRT was 2.3 (CI: 0.9–5.9; p = 0.09) compared with IMRT. In terms of AD, there was a greater proportion of patients in the IMRT group receiving LTAD, which based on our experience with 3DCRT+LTAD (9) suggests this would bias results in favor of 3DCRT, and strengthens our finding that IMRT reduces toxicity. Last, a time-to-event analysis with the Cox method was used to account for loss of follow-up, censoring, and different follow-up rates. One disadvantage to this method is the assumption that loss to follow-up was unrelated to the outcome. One might reasonably assume that a patient with a late toxicity might not choose to return and seek care elsewhere.
CONCLUSIONS
IMRT significantly reduces the risk of late GI toxicity in men receiving concurrent radiotherapy and androgen deprivation therapy for prostate cancer, supporting its routine use and advantages for treating complex target volumes to with a high degree of conformality.
Figure 2.
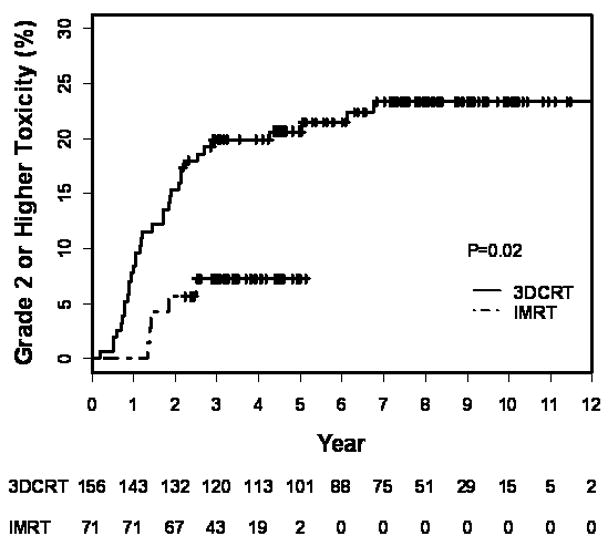
Kaplan-Meier estimation of late ≥ grade 2 GI toxicity in patients treated with 3DCRT+AD or IMRT+AD including treatment to the pelvic lymph nodes as a function of years from treatment completion. Numbers at risk are indicated below the graph.
Footnotes
Conflicts of Interest Notification
Navesh Sharma None
Tianyu Li None
Eric Horwitz Departmental Varian research funding
David Chen None
Alan Pollack None
Mark Buyyounouski None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Al-Mamgani A, Heemsbergen WD, Peeters ST, et al. Update of Dutch multicententer dose-escalation trial of radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2008;72:980–88. doi: 10.1016/j.ijrobp.2008.02.073. [DOI] [PubMed] [Google Scholar]
- 2.Kuban DA, Tucker SL, Dong L, et al. Long-term results of the M. D. Anderson randomized dose-escalation trial for prostate cancer. Int J Radiat Oncol Biol Phys. 2008;70:67–74. doi: 10.1016/j.ijrobp.2007.06.054. [DOI] [PubMed] [Google Scholar]
- 3.Pollack A, Zagars GK, Starkschall G, et al. Prostate cancer radiation dose response: results of the M. D. Anderson phase III randomized trial. Int J Radiat Oncol Biol Phys. 2002;53:1097–1105. doi: 10.1016/s0360-3016(02)02829-8. [DOI] [PubMed] [Google Scholar]
- 4.Zelefsky MJ, Leibel SA, Gaudin PB, et al. Dose escalation with three-dimensional conformal radiation therapy affects the outcome in prostate cancer. Int J Radiat Oncol Biol Phys. 1998;41:491–500. doi: 10.1016/s0360-3016(98)00091-1. [DOI] [PubMed] [Google Scholar]
- 5.Bolla M, Collette L, Blank L, et al. Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): a phase III randomised trial. Lancet. 2002;360:103–106. doi: 10.1016/s0140-6736(02)09408-4. [DOI] [PubMed] [Google Scholar]
- 6.Hanks GE, Pajak TF, Porter A, et al. Phase III trial of long-term adjuvant androgen deprivation after neoadjuvant hormonal cytoreduction and radiotherapy in locally advanced carcinoma of the prostate: the Radiation Therapy Oncology Group Protocol 92-02. J Clin Oncol. 2003;21:3972–3978. doi: 10.1200/JCO.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 7.Horwitz EM, Bae K, Hanks GE, et al. Ten-year follow-up of radiation therapy oncology group protocol 92-02: a phase III trial of the duration of elective androgen deprivation in locally advanced prostate cancer. J Clin Oncol. 2008;26:2497–2504. doi: 10.1200/JCO.2007.14.9021. [DOI] [PubMed] [Google Scholar]
- 8.Pilepich MV, Winter K, John MJ, et al. Phase III radiation therapy oncology group (RTOG) trial 86-10 of androgen deprivation adjuvant to definitive radiotherapy in locally advanced carcinoma of the prostate. Int J Radiat Oncol Biol Phys. 2001;50:1243–1252. doi: 10.1016/s0360-3016(01)01579-6. [DOI] [PubMed] [Google Scholar]
- 9.Feigenberg SJ, Hanlon AL, Horwitz EM, et al. Long-term androgen deprivation increases Grade 2 and higher late morbidity in prostate cancer patients treated with three-dimensional conformal radiation therapy. Int J Radiat Oncol Biol Phys. 2005;62:397–405. doi: 10.1016/j.ijrobp.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 10.Lawton CA, Bae K, Pilepich M, et al. Long-term treatment sequelae after external beam irradiation with or without hormonal manipulation for adenocarcinoma of the prostate: analysis of radiation therapy oncology group studies 85-31, 86-10, and 92-02. Int J Radiat Oncol Biol Phys. 2008;70:437–441. doi: 10.1016/j.ijrobp.2007.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lawton CA, DeSilvio M, Roach M, 3rd, et al. An update of the phase III trial comparing whole pelvic to prostate only radiotherapy and neoadjuvant to adjuvant total androgen suppression: updated analysis of RTOG 94-13, with emphasis on unexpected hormone/radiation interactions. Int J Radiat Oncol Biol Phys. 2007;69:646–655. doi: 10.1016/j.ijrobp.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dong L, Antolak J, Salehpour M, et al. Patient-specific point dose measurement for IMRT monitor unit verification. Int J Radiat Oncol Biol Phys. 2003;56:867–877. doi: 10.1016/s0360-3016(03)00197-4. [DOI] [PubMed] [Google Scholar]
- 13.Zelefsky MJ, Chan H, Hunt M, et al. Long-term outcome of high dose intensity modulated radiation therapy for patients with clinically localized prostate cancer. J Urol. 2006;176:1415–1419. doi: 10.1016/j.juro.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 14.Al-Mamgani A, Heemsbergen WD, Peeters ST, et al. Role of intensity-modulated radiotherapy in reducing toxicity in dose escalation for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2009;73:685–691. doi: 10.1016/j.ijrobp.2008.04.063. [DOI] [PubMed] [Google Scholar]
- 15.Horwitz EM, Hanlon AL, Pinover WH, et al. Defining the optimal radiation dose with three-dimensional conformal radiation therapy for patients with nonmetastatic prostate carcinoma by using recursive partitioning techniques. Cancer. 2001;92:1281–1287. doi: 10.1002/1097-0142(20010901)92:5<1281::aid-cncr1449>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 16.Price RA, Murphy S, McNeeley SW, et al. A method for increased dose conformity and segment reduction for SMLC delivered IMRT treatment of the prostate. Int J Radiat Oncol Biol Phys. 2003;57:843–852. doi: 10.1016/s0360-3016(03)00711-9. [DOI] [PubMed] [Google Scholar]
- 17.Monti AF, Ostinelli A, Frigerio M, et al. An ICRU 50 radiotherapy treatment chart. Radiother Oncol. 1995;35:145–150. doi: 10.1016/0167-8140(95)01541-n. [DOI] [PubMed] [Google Scholar]
- 18.Jacob R, Hanlon AL, Horwitz EM, et al. Role of prostate dose escalation in patients with greater than 15% risk of pelvic lymph node involvement. Int J Radiat Oncol Biol Phys. 2005;61:695–701. doi: 10.1016/j.ijrobp.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 19.Pollack A, Hanlon AL, Horwitz EM, et al. Dosimetry and preliminary acute toxicity in the first 100 men treated for prostate cancer on a randomized hypofractionation dose escalation trial. Int J Radiat Oncol Biol Phys. 2006;64:518–526. doi: 10.1016/j.ijrobp.2005.07.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buyyounouski MK, Hanlon AL, Horwitz EM, et al. Interval to biochemical failure highly prognostic for distant metastasis and prostate cancer-specific mortality after radiotherapy. Int J Radiat Oncol Biol Phys. 2008;70:59–66. doi: 10.1016/j.ijrobp.2007.05.047. [DOI] [PubMed] [Google Scholar]
- 21.Roach M, 3rd, DeSilvio M, Lawton C, et al. Phase III trial comparing whole-pelvic versus prostate-only radiotherapy and neoadjuvant versus adjuvant combined androgen suppression: Radiation Therapy Oncology Group 9413. J Clin Oncol. 2003;21:1904–1911. doi: 10.1200/JCO.2003.05.004. [DOI] [PubMed] [Google Scholar]
- 22.Sanguineti G, Marcenaro M, Franzone P, et al. Neoadjuvant androgen deprivation and prostate gland shrinkage during conformal radiotherapy. Radiother Oncol. 2003;66:151–157. doi: 10.1016/s0167-8140(03)00031-8. [DOI] [PubMed] [Google Scholar]
- 23.Schultheiss TE, Lee WR, Hunt MA, et al. Late GI and GU complications in the treatment of prostate cancer. Int J Radiat Oncol Biol Phys. 1997;37:3–11. doi: 10.1016/s0360-3016(96)00468-3. [DOI] [PubMed] [Google Scholar]
- 24.Alcantara P, Schultheiss TE, Ruth K, et al. Dose-Volume Determinants of Late Genitourinary Toxicity After External Beam Radiotherapy for Prostate Cancer. International journal of radiation oncology, biology, physics. 2005;63:S294. [Google Scholar]
- 25.Zelefsky MJ, Levin EJ, Hunt M, et al. Incidence of late rectal and urinary toxicities after three-dimensional conformal radiotherapy and intensity-modulated radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2008;70:1124–1129. doi: 10.1016/j.ijrobp.2007.11.044. [DOI] [PubMed] [Google Scholar]


