Abstract
A rapid liquid chromatography-tandem mass spectrometry (LC-MS-MS) assay was developed for the measurement of urinary 8-iso prostaglandin F2α (8-iso-PGF2α), a biomarker of lipid peroxidation. Since urine contains numerous F2 prostaglandin isomers, each of identical mass and similar mass spectrometric fragmentation patterns, chromatographic separation of 8-iso-PGF2α from its isomers is necessary for its quantitative analysis using tandem mass spectrometry. We were able to achieve this separation using an isocratic LC method with a run time under nine minutes which is at least three-fold faster than previous methods—while maintaining sensitivity, accuracy, precision and reliability. The limits of detection and quantitation were 53 and 178 pg/mL urine, respectively. We compared our method with a commercially available affinity purification and enzyme immunoassay kit and found both assays in agreement. Despite the high sensitivity of the enzyme immunoassay method, it is more expensive and has a narrower dynamic range than LC-MS-MS. Our method was optimized for rapid measurement of 8-iso-PGF2α in urine, and it is ideally suited for clinical sample analysis.
Keywords: Mass spectrometry, enzyme immunoassay, quantitative analysis, biomarker, 8-iso prostaglandin F2α
Introduction
The lipid peroxidation product 8-iso prostaglandin F2α (8-iso-PGF2α) forms from the nonenzymatic oxidation of arachidonic acid and is a biomarker of oxidative stress. Elevated 8-iso-PGF2α levels are linked with a variety of conditions associated with oxidative stress [1-5]. In particular, smoking is strongly associated with higher 8-iso-PGF2α levels [6]. Isoprostanes are present in all bodily tissues, blood and urine. In plasma, 8-iso-PGF2α concentration ranges between 40–100 pg/mL [7] and exists in esterified and free forms, which can be measured separately [8], or the esterified form may be hydrolyzed for total isoprostane measurement [9,10]. One of the major limitations of measuring isoprostanes in plasma or tissues is the potential for formation of artifacts from spontaneous oxidation of arachidonic acid. Morrow et al. [11] found that plasma samples stored at −20 °C contained more than 50-fold higher isoprostane levels than fresh plasma. Special precautions must be taken if plasma or tissue measurements are desired.
Once formed in the body, 8-iso-PGF2α is rapidly excreted in the urine [12]. Normal human urinary 8-iso-PGF2α concentrations vary from 180 to 500 pg/mg creatinine or more [11,13]. In studies evaluating dietary antioxidants, measurements of urinary 8-iso-PGF2α are often preferred to plasma or tissue measurements because there is no potential for artifactual formation, urine levels represent a time-averaged value reducing intra-subject variability, sampling is minimally invasive, and sample volume is abundant.
Because 8-iso-PGF2α is an important biomarker for many clinical studies investigating oxidative stress or the effects of antioxidants, assays are needed that are robust, accurate, rapid, and cost-effective. The earliest methods for isoprostane analysis in urine were based on GC-MS and required two stages of TLC purification and chemical derivitization [14]. Recognizing the need for a less cumbersome method, Ohashi et al. [8] developed an LC-MS assay with solid phase extraction followed by liquid-liquid extraction sample cleanup and high resolution selected ion monitoring measurement. Tandem mass spectrometry was found to increase the specificity of MS methods because many, though not all, isoprostane isomers with the same mass have different fragmentation patterns [15]. Since the type III F2 isoprostanes which include 8-iso-PGF2α have identical fragmentation patterns, chromatographic separation is essential for the quantitative analysis of 8-iso-PGF2α. Taylor et al. [10] used a 32-minute LC gradient to separate the various 8-iso-PGF2α isomers and their metabolites. Although their method used a one-step solid phase extraction sample cleanup which significantly reduced analysis time and cost, the long HPLC gradient still limited the number of samples that could be analyzed per day. Bastani et al. [16] shortened this gradient to 18 min, but the total run time was still 22 min plus an unspecified equilibration time.
As an alternative to mass spectrometry, enzyme immunoassays (EIAs) have been developed to measure 8-iso-PGF2α, and commercially available affinity columns are used with these EIAs for sample purification. Advantages of affinity assays for the measurement of 8-iso-PGF2α include high sensitivity and microtiter plate formats for rapid measurements of large numbers of samples, but they are expensive and exhibit cross-reactivity with other isoprostane isomers. For example, the manufacturer of the EIA kit we evaluated in this investigation reports 20.6% cross-reactivity towards 8-iso-PGF3α and 4% to 2,3-dinor-8-iso-PGF2α. EIAs have generated inconsistent results when compared with validated GC measurements [17], and the requirement for extensive sample cleanup negates some of the advantages of the otherwise high-throughput format. In this study, a fast LC-MS-MS assay for 8-iso-PGF2α was developed, validated and cross-validated by comparison to EIA.
Materials and Methods
Reagents
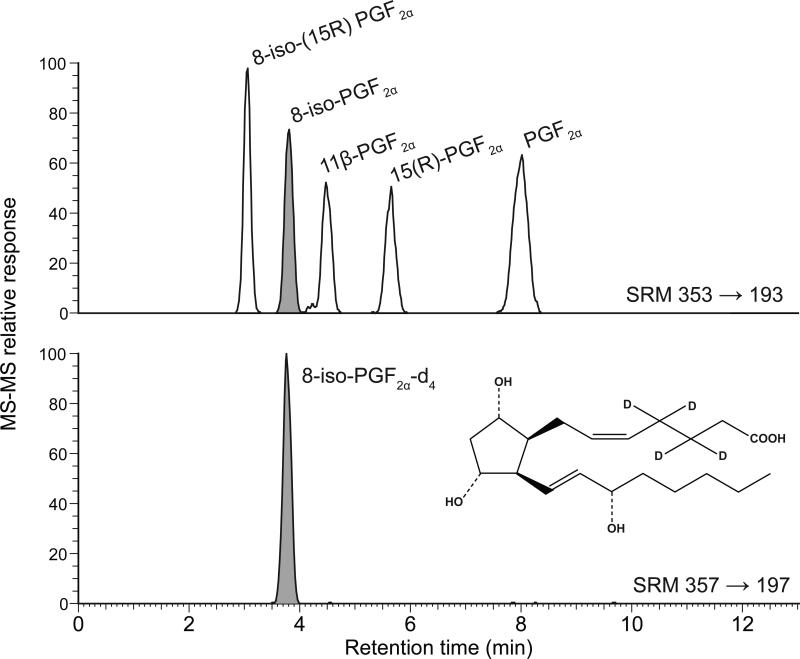
Standards, affinity columns (Cat. No. 10368), and immunoassay kits (Cat. No. 516351) were purchased from Cayman Chemical (Ann Arbor, MI). The standards included 8-iso-PGF2α, 8-iso-15(R)-PGF2α, PGF2α, 15(R)-PGF2α, 11β-PGF2α, and the surrogate standard 8-iso-PGF2α-d4 (see Figure 1 for structure and location of deuterium labels). All solvents and other chemicals were purchased from Fisher Scientific (Fair Lawn, NJ).
Figure 1.
LC-MS-MS analysis of standards showing chromatographic separation. The deuterium labeled surrogate standard is shown in the bottom chromatogram.
Sample Preparation
Human urine samples stored at −80 °C were thawed, vortexed, and centrifuged for 3 min at 3500 × g to remove particulates. Sample extraction was based on the method by Taylor et al. [13], with modifications. Briefly, 2 mL of 50 mM Tris-HCl buffer (adjusted to pH 6.00 (±0.05) using 50 mM Tris-base) was added to each 2.5 mL urine sample. Then, 10 ng of the surrogate standard, 8-iso-PGF2α-d4, was added along with 750 μL of methanol. For quality control and method validation, the appropriate amount of standard was spiked into a pooled urine sample. Strata X-AW (60 mg, 3 mL) SPE cartridges (Phenomenex, Torrance, CA) were preconditioned with 2 mL methanol containing 2% formic acid followed by 2 mL water. Samples were then loaded and the cartridges washed sequentially with 2 mL water, 4 mL of 25% methanol in water, and then 2 mL of 100% acetonitrile. After washing, the column was dried under vacuum and eluted with 3 × 1 mL methanol with 15 s vacuum drying between portions. The methanol eluates were combined, evaporated to dryness, reconstituted in 50 μL of mobile phase (described below), and centrifuged to remove particulates prior to LC-MS-MS analysis.
LC-MS-MS
LC-MS-MS analysis was carried out using a Shimadzu (Kyoto, Japan) LC-20AD system interfaced to an Applied Biosystems (Foster City, CA) API 4000 triple quadrupole mass spectrometer controlled by Analyst version 1.5 software. A Shimadzu Shim-Pack XR-ODS column (2×50 mm, 2.2 μm) was used with an isocratic flow of 2 mM aqueous ammonium acetate/acetonitrile/methanol (81:18:1, v/v/v) at 0.45 mL/min and a temperature of 45 °C. Alternatively, an Agilent Zorbax Eclipse XDBC18 (2.1×50 mm, 3.5 μm) was used at 0.45 mL/min and ambient temperature. The mobile phase was freshly and accurately prepared using volumetric glassware to ensure optimum chromatographic separation and repeatability. Negative ion electrospray was used to form deprotonated molecules of the analyte and its surrogate standard followed by collision-induced dissociation and selected reaction monitoring of the transitions m/z 353 → 193 (8-iso-PGF2α) and m/z 357 → 197 (surrogate standard, 8-iso-PGF2α-d4). The mass spectrometer parameters were optimized as follows: source temperature 500 °C; spray voltage −4200 V; declustering potential −90 V; curtain gas 12; gas 1 45; entrance potential −10 V; capillary exit potential −19 V; collision energy −36 V; and a dwell time of 250 ms per ion transition.
Affinity purification and EIA
To compare the performance of the new LC-MS-MS method to a commercially available affinity purification and EIA kit, a set of identical urine samples was prepared and assayed by SPE followed by LC-MS-MS as above or by affinity purification followed by EIA. Sample preparation and analysis for EIA was carried out in accordance with the manufacturer's instructions. Briefly, aliquots of a pooled urine sample spiked with 0, 50, 100, 200, and 500 pg/mL were applied to a 20 mL size affinity column equilibrated with 4 mL buffer included in the kit. Next, columns were washed with 10 mL column buffer followed by 10 mL water. Columns were eluted with 2 × 5 mL elution solution (ethanol/water, 95:5, v/v), and the eluent was evaporated under a stream of nitrogen. Samples were reconstituted in 100 μL methanol and split into two equal portions. The first portion was dried and reserved for LC-MS-MS measurement to determine whether the affinity column retained any cross-reacting isoprostanes. The second portion was reconstituted and diluted 400-fold with EIA buffer provided in the immunoassay kit. Finally, 50 μL aliquots of the diluted samples were analyzed in duplicate using the EIA kit as directed. To test affinity purified samples for the presence of cross-reacting isoprostane isomers, the affinity purified pooled urine sample was analyzed by using LC-MS with negative ion electrospray with scanning of the range m/z 300 to 400, which is the expected mass range for F2 isoprostane isomers.
Validation
Method validation was carried out in accordance with FDA guidelines for bioanalytical method validation [18]. Since all urine contains isoprostanes, seven point calibration curves were prepared from pooled urine using the method of standard addition [19] over the range of 50 pg/mL to 10 ng/mL 8-iso-PGF2α spiked into urine. Intraday precision was determined by measuring four replicates each at low, intermediate, and high concentration, and interday precision was measured by comparing the same set of samples analyzed on three different days. Recovery was measured by spiking known amounts of 8-iso-PGF2α into pooled urine samples and comparing against unextracted standards representing 100% recovery. Absence of matrix effects was confirmed by injecting a prepared pooled urine sample onto the LC column and infusing a post-column addition of 2.5 μg/mL 8-iso-PGF2α at 100 μL/min.
Results and Discussion
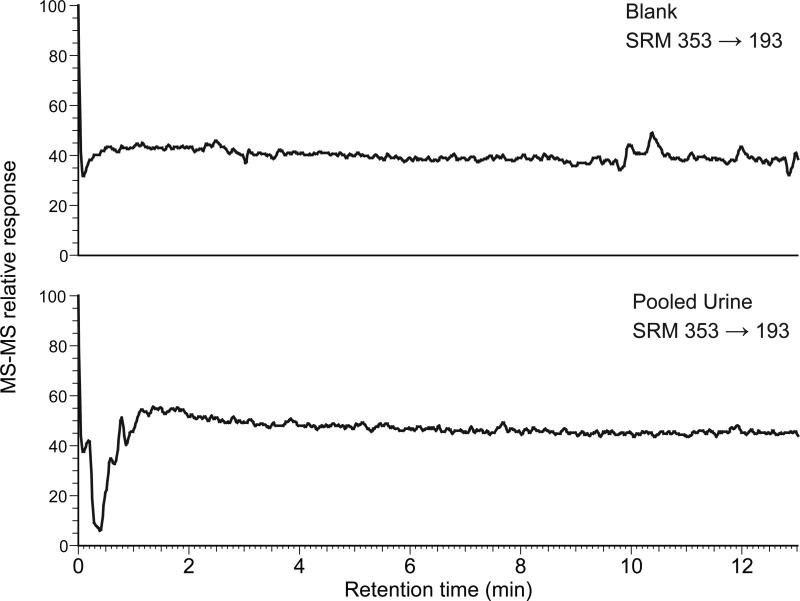
A rapid, sensitive, and accurate LC-MS-MS assay was developed for the determination of 8-iso-PGF2α in urine. Calibration curves were linear over the range of 50 pg/mL to 10 ng/mL 8-iso-PGF2α spiked into urine (r2 > 0.99). A typical calibration curve is shown in the supplementary data. The sensitivity and reproducibility of our method are comparable with published LC-MS-MS methods [13,16]. The limit of detection (signal-to-noise of 3:1) was 53 pg/mL8-iso-PGF2α in urine and the lower limit of quantitation (signal-to-noise of 10:1) was 178 pg/mL in urine. The inter-day precision and intra-day precision as well as accuracy were tested at three concentrations, and the results are reported in Table 1. The recoveries at low, intermediate, and high concentrations (0.1, 1.0 and 10 ng/mL) were 90%, 83%, and 79%, respectively. 8-iso-PGF2α was found to be stable in urine by Morrow et al.[21] who found no change in 8-iso-PGF2α concentration after a one week incubation of samples at 37 °C. To test the stability of the analyte after extraction, standard solutions of 8-iso-PGF2α in the HPLC mobile phase were measured after being allowed to stand at room temperature for one week. Measured concentrations were ≥ 95% of the starting concentration, indicating stability at room temperature for at least one week. Nevertheless, sample preparation and analysis was usually carried out within 24 h. As shown in Figure 3, absence of a matrix effect was confirmed.
Table 1.
Interday and intraday reproducibility of the LC-MS-MS quantitation of 8-iso-PGF2α in urine
| Concentration (ng/mL) |
Intraday(n=4) |
Interday (n=12) |
|||
|---|---|---|---|---|---|
| Expected | Measured (means, n=12) | %CV | Accuracy% | %CV | Accuracy % |
| 0.1 | 0.11 | 15.8 | 108 | 16.1 | 112 |
| 1.0 | 0.98 | 4.7 | 96.8 | 7.8 | 98.9 |
| 10.0 | 10.12 | 5.3 | 103 | 4.4 | 101 |
Figure 3.
Injections of blank (top) and a prepared pooled urine sample (bottom) with LC-MS-MS analysis and post-column infusion of 8-iso-PGF2α confirm the absence of matrix effect.
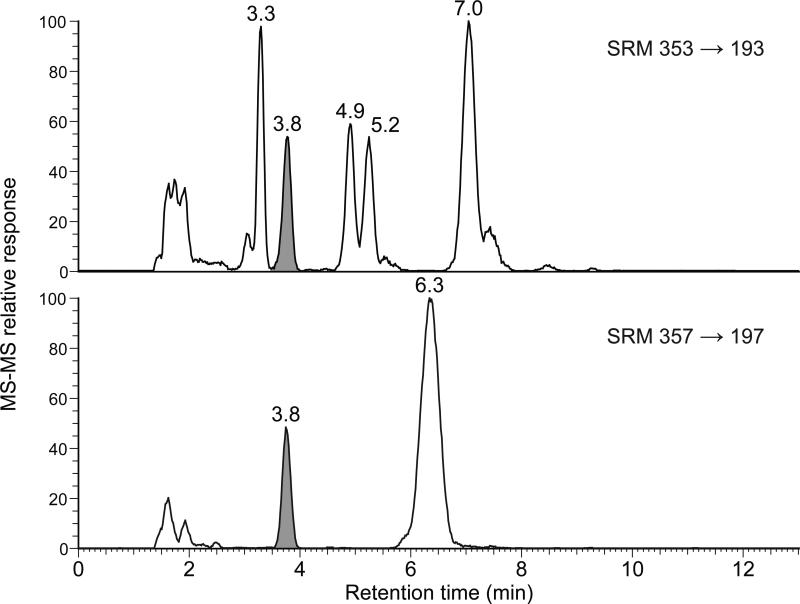
Because long gradient separations would be cost-prohibitive for large numbers of samples such as those collected during clinical trials, our method offers significant advantages over previous mass spectrometry-based methods without sacrificing chromatographic resolution. Using a chromatography column packed with 2.2 μm particles instead of the more typical 3.5 to 5 μm particles used in previous methods, the separation efficiency was improved and the run time reduced by use of a carefully selected combination of mobile phase, column length, temperature, and flow rate. In comparison to the method of Taylor et al., [10, 13] which uses a 32 min gradient followed by 5 min column equilibration, our method fully resolves the major F2 isoprostane peaks in less than 10 min (Figure 2). There is no column equilibration in our method because HPLC separations use an isocratic mobile phase. Our method does not require ultra-high performance liquid chromatography (UHPLC) equipment and can be run on standard HPLC hardware.
Figure 2.
LC-MS-MS chromatogram of a typical urine sample. Top: 8-iso-15(R)-PGF2α, 3.3 min; 8-iso-PGF2α, 3.8 min; 15(R)-PGF2α, 4.9 min; PGF2α, 7.0 min; and bottom: 8-iso-PGF2α-d4, 3.8 min. The peaks were identified by using co-injection with authentic standards.
In addition to 8-iso-PGF2α, LC-MS-MS methods have been developed to measure related species such as 2,3-dinor-8-iso-PGF2α with the hypothesis that such measurements might offer additional information about lipid peroxidation levels. Although detailed measurements of many isoprostane isomers and their metabolites have been made [10,15,20], only 8-iso-PGF2α has gained wide acceptance as a lipid peroxidation biomarker. There are no commercially available stable isotope labeled standards for many of these metabolites, and chromatographic separation of the full spectrum of metabolites would have to be made at the expense of speed and efficiency of 8-iso-PGF2α, which is the target of most antioxidant investigations. For expediency, we have optimized our LC-MS-MS method for 8-iso-PGF2α measurement.
Comparison to Immunoassay Method
Commercial enzyme immunoassays, alone or in combination with affinity purification, are available for 8-iso-PGF2α measurement. For urine samples, the manufacturer of the EIA kit used for comparison in this study recommends either TLC followed by solid phase extraction for sample cleanup or affinity column purification. Here, affinity purification was used followed by EIA to cross-validate our LC-MS-MS based method. A set of pooled urine samples spiked with 0, 50, 100, 200, and 500 pg/mL were analyzed by either SPE followed by LC-MS-MS or affinity purification followed by EIA, and the results are compared in Table 2. The values we obtained by EIA are in agreement with the LC-MS-MS values within the measurement error.
Table 2.
Comparison of SPE-purified urine analyzed by LC-MS-MS or affinity-purified urine analyzed by EIA for measurement of 8-iso-PGF2α. Values are the mean of two replicates.
| 8-iso-PGF2α spiked (pg/mL) | LC-MS-MS | EIA |
|---|---|---|
| 0 | 382 | 369 |
| 50 | 460 | 477 |
| 100 | 517 | 580 |
| 200 | 652 | 793 |
| 500 | 960 | 1025 |
8-iso-PGF2α affinity sorbents have been reported to cross-react with other isoprostane isomers. To determine whether the affinity purification or EIA we used could be affected by cross-reactivity, we analyzed the affinity purified samples by LC-MS for the presence of various cross-reacting isoprostane isomers. Using LC-MS, no signals were observed corresponding to the deprotonated molecules of PGF2α, 8-iso-PGF3α (m/z 351), PGF1α (m/z 355) or 2,3-dinor-8-iso-PGF2α (m/z 325) which are reported by the immunoassay manufacturer to cross react with 8-iso-PGF2α. These observations indicate that, for the pooled urine sample tested, known cross-reacting isoprostanes did not interfere with the EIC, even though 2,3-dinor-8-iso PGF2α and PGF2α, for example, are normally present in urine. It should be noted that these cross-reacting isoprostane isomers do not interfere with our LC-MS-MS assay because the masses, retention times, or both did not overlap with the analyte 8-iso-PGF2α. The possibility remains that samples might sometimes contain high enough levels of these cross-reacting isoprostanes to cause interference with the EIA.
Despite the potential for cross-reactivity, the 8-iso-PGF2α affinity columns were effective in removing contaminants from the urine samples. Our LC-MS-MS analysis of affinity column purified samples showed low background and only a single peak in the chromatogram. If affinity columns were used in place of the usual weak anion exchange SPE, then the sensitivity and speed of the subsequent LC-MS-MS assay could be dramatically improved. However, the cost of affinity columns is several-fold higher than SPE columns, and this cost disadvantage will probably prohibit their use for the analysis of large numbers of samples.
Measurement of urinary 8-iso-PGF2α using LC-MS-MS has several advantages when compared to EIA. Although both methods require sample preparation, the weak anion exchange SPE columns used in our new LC-MS-MS method are less expensive than the affinity purification columns used for EIA. Additionally we found that SPE columns could be used up to four times each without carryover or loss of column capacity while the manufacturer of the 8-iso-PGF2α affinity columns recommends only single use for urine samples. Although sensitive, the linear portion of the standard curve for the EIA is narrower than its usable range of 2–500 pg/mL. Therefore, careful dilution is required to bring the sample concentration into this range. Stable isotope labeled surrogate standards may be used with SPE to correct for sample loss, matrix effect, or instrument variability whereas such standards interfere with affinity methods. Lastly, the cross-reactivity of the affinity reagents might lead to an overestimation of 8-iso-PGF2α concentration in the EIA method. Our LC-MS-MS method addresses these limitations of the EIA assay.
Conclusions
We have developed an LC-MS-MS assay for measurement of 8-iso-PGF2α in urine, which is the most common lipid peroxidation biomarker used in clinical antioxidant studies. Compared with existing 8-iso-PGF2α assays, our new assay increases the speed and lowers the cost per analysis while maintaining accuracy, precision, and sensitivity. By chromatographically resolving 8-iso-PGF2α from its isomers in less than ten minutes, this new assay provides more accurate measurements than LC-MS-MS assays that do not resolve 8-iso-PGF2α from all of its isomers. Our assay is more than three-fold faster than previously published LC-MS-MS methods which require 32-minute and 22-minute gradients in addition to the time necessary for column equilibration. The use of isocratic chromatography during our LC-MS-MS method saves time by eliminating the need for column re-equilibration between runs, and yet complete chromatographic separation of 8-iso-PGF2α from its isomers is achieved in less than 10 minutes. Although we did not detect in our samples any cross-reacting isoprostanes that could lead to an overestimation of 8-iso-PGF2α when using EIA, our LC-MS-MS assay eliminates this risk by chromatographically resolving these compounds and distinguishing them by mass and by MS-MS. We are applying this new assay to measure changes in urinary 8-iso-PGF2α in 150 human subjects in an ongoing clinical intervention with lycopene, the tomato carotenoid and antioxidant thought to offer protection from prostate cancer. With this new 8-iso-PGF2α assay, this important lipid peroxidation biomarker can be measured accurately, quickly, and cost effectively.
Supplementary Material
Acknowledgement
Funding for this research was provided by grant number 5R01CA101052 from the National Cancer Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Laight D, Desai K, Gopaul N, Änggård E, Carrier M. F2 isoprostane evidence of oxidant stress in the insulin resistant, obese zucker rat: effects of vitamin E. Eur. J. Pharmacol. 1999;377:89–92. doi: 10.1016/s0014-2999(99)00407-0. [DOI] [PubMed] [Google Scholar]
- 2.Greco A, Minghetti L, Levi G. Isoprostanes, novel markers of oxidative injury, help understanding the pathogenesis of neurodegenerative diseases. Neurochem. Res. 2000;25:1357–1364. doi: 10.1023/a:1007608615682. [DOI] [PubMed] [Google Scholar]
- 3.Devaraj S, Hirany S, Burk R, Jialal I. Divergence between LDL oxidative susceptibility and urinary F2-isoprostanes as measures of oxidative stress in type 2 diabetes. Clin. Chem. 2001;47:1974–1979. [PubMed] [Google Scholar]
- 4.Pratico D, Lee VM-Y, Trojanowski J, Rokach J, Fitzgerald G. Increased F2-isoprostanes in alzheimer's disease: evidence for enhanced lipid peroxidation in vivo. FASEB J. 1998;12:1777–1783. doi: 10.1096/fasebj.12.15.1777. [DOI] [PubMed] [Google Scholar]
- 5.Basu S, Helmersson J. Factors regulating isoprostane formation in vivo. Antioxid. Redox. Signal. 2005;7:221–235. doi: 10.1089/ars.2005.7.221. [DOI] [PubMed] [Google Scholar]
- 6.Chehne F, Oguogho A, Lupattelli G, Palumbo B, Sinzinger H. Effect of giving up cigarette smoking and restarting in patients with clinically manifested atherosclerosis. Prostaglandins Leukot. Essent. Fatty Acids. 2002;67:333–339. doi: 10.1054/plef.2002.0438. [DOI] [PubMed] [Google Scholar]
- 7.Wang Z, Ciabattoni G, Creminon C, Lawson J, Fitzgerald G, Patrono C, Maclouf J. Immunological characterization of urinary 8-epiprostaglandin F2 alpha excretion in man. J. Pharmacol. Exp. Ther. 1995;275:94. [PubMed] [Google Scholar]
- 8.Ohashi N, Yoshikawa M. Rapid and sensitive quantification of 8-isoprostaglandin F2α in human plasma and urine by liquid chromatography–electrospray ionization mass spectrometry. J. Chromatogr. B. 2000;746:17–24. doi: 10.1016/s0378-4347(00)00201-2. [DOI] [PubMed] [Google Scholar]
- 9.Roberts L, Morrow J. Measurement of F2-isoprostanes as an index of oxidative stress in vivo. Free Radic. Biol. Med. 2000;28:505–513. doi: 10.1016/s0891-5849(99)00264-6. [DOI] [PubMed] [Google Scholar]
- 10.Taylor A, Bruno R, Frei B, Traber M. Benefits of prolonged gradient separation for high-performance liquid chromatography–tandem mass spectrometry quantitation of plasma total 15-series F2-isoprostanes. Anal. Biochem. 2006;350:41–51. doi: 10.1016/j.ab.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 11.Morrow J, Harris T, Jackson Roberts L. Noncyclooxygenase oxidative formation of a series of novel prostaglandins: analytical ramifications for measurement of eicosanoids. Anal. Biochem. 1990;184:1–10. doi: 10.1016/0003-2697(90)90002-q. [DOI] [PubMed] [Google Scholar]
- 12.Roberts L, Moore K, Zackert W, Oates J, Morrow J. Identification of the major urinary metabolite of the F2-isoprostane 8-iso-prostaglandin F2 alpha in humans. J. Biol. Chem. 1996;271:20617–20620. doi: 10.1074/jbc.271.34.20617. [DOI] [PubMed] [Google Scholar]
- 13.Taylor A, Bruno R, Traber M. Women and smokers have elevated urinary F2-isoprostane metabolites: A novel extraction and LC-MS methodology. Lipids. 2008;43:925–936. doi: 10.1007/s11745-008-3222-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morrow J, Roberts L. Mass spectrometry of prostanoids: F2 isoprostanes produced by non-cyclooxygenase free radical-catalyzed mechanism. Methods Enzymol. 1994;233:163–174. doi: 10.1016/s0076-6879(94)33019-0. [DOI] [PubMed] [Google Scholar]
- 15.Li H, Lawson J, Reilly M, Adiyaman M, Hwang S, Rokach J, FitzGerald G. Quantitative high performance liquid chromatography/tandem mass spectrometric analysis of the four classes of F2 isoprostanes in human urine. Proc. Natl. Acad. Sci. U.S.A. 1999;96:13381. doi: 10.1073/pnas.96.23.13381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bastani N, Gundersen T, Blomhoff R. Determination of 8-epi-PGF2alpha concentrations as a biomarker of oxidative stress using triple-stage liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2009;23:2885–2890. doi: 10.1002/rcm.4197. [DOI] [PubMed] [Google Scholar]
- 17.Liang Y, Wei P, Duke R, Reaven P, Harman S, Cutler R, Heward C. Quantification of 8-iso-prostaglandin-F2a and 2,3-dinor 8-iso-prostaglandin-F2a in human urine using liquid chromatography-tandem mass spectrometry. Free Radic. Biol. Med. 2003;34:409–418. doi: 10.1016/s0891-5849(02)01018-3. [DOI] [PubMed] [Google Scholar]
- 18.FDA . Guidance for industry: Bioanalytical method validation. United States Food and Drug Administration; 2001. Tech. rep. [Google Scholar]
- 19.Harris D. Quantitative Chemical Analysis. Macmillan; 2003. [Google Scholar]
- 20.Davies S, Zackert W, Luo Y, Cunningham C, Frisard M, Roberts L. Quantification of dinor, dihydro metabolites of F2-isoprostanes in urine by liquid chromatography/tandem mass spectrometry. Anal. Biochem. 2006;348:185–191. doi: 10.1016/j.ab.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 21.Morrow J, Hill K, Burk R, Nammour T, Badr K, Roberts L. A series of prostaglandin F2-like compounds are produced in vivo in humans by a non-cyclooxygenase, free radical-catalyzed mechanism. Proc. Natl. Acad. Sci. U.S.A. 1990;87:9383–9387. doi: 10.1073/pnas.87.23.9383. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.