Abstract
Ultraviolet (UV) irradiation from the sun adversely impacts skin health through complex, multiple molecular pathways. Premature skin aging (photoaging) is among the most widely appreciated harmful effects of chronic exposure to solar UV irradiation. Extensive damage to the dermal connective tissue is a hallmark of photoaged skin. Disruption of the normal architecture of skin connective tissue impairs skin function and causes it to look aged. UV irradiation induces expression of certain members of the matrix metalloproteinase (MMP) family, which degrade collagen and other extracellular matrix (ECM) proteins that comprise the dermal connective tissue. Although the critical role of MMPs in photoaging process is undeniable, important questions remain. This article summarizes our current understanding regarding the role of MMPs in the photoaging process and presents new data that 1) describe expression and regulation by UV irradiation of all members of the MMP family in human skin in vivo, and 2) quantify the relative contributions of the epidermis and dermis to expression of UV irradiation-induced MMPs in human skin in vivo.
Introduction
Human skin, is the only organ directly exposed to ultraviolet (UV) irradiation from the sun. Solar UV irradiation is a well-recognized, harmful environmental. Acute exposure of human skin to UV irradiation causes sunburn, altered pigmentation, inflammation, immune suppression, and dermal connective tissue damage (Fisher et al., 2002; Fisher et al., 1996; Fisher et al., 1997; Gilchrest and Yaar, 1992; Kaminer, 1995; Kripke, 1984). Chronic exposure to UV irradiation over many years disrupts normal architecture of the skin and ultimately causes premature skin aging (photoaging) and skin cancer.
Clinically, photoaging is recognizable by fine and coarse wrinkles, blotchy dyspigmentation, increased fragility, and rough skin texture (Berneberg et al., 2000; Kligman and Kligman, 1986; Scharffetter-Kochanek et al., 2000). Histological and ultrastructural studies have revealed miminal epidermal changes. In contrast, major alterations are seen in dermal connective tissue, characterized by damaged and disorganized collagen fibrils, which constitute the bulk (90% dry weight) of skin connective tissue, and massive accumulation of aberrant elastic material, referred to as “solar elastosis”. These observations indicate that UV irradiation causes qualitative alterations in extracellular matrix proteins and suggest that matrix degrading proteases participate in this process.
Type I collagen is the major structural protein in the dermal extracellular matrix (Nimni, 1983; Uitto and Bernstein, 1998). Collagen precursor molecules (procollagen) are synthesized by dermal fibroblasts. Procollagen is secreted into extracellular spaces, where it is enzymatically processed to mature collagen. Mature collagen spontaneously forms fibrils, which are stabilized by cross-links. Collagen fibrils are largely responsible for the strength and resilience of skin. Collagen fibrils have an estimated half life of 17 years. Therefore, fragmented collagen fibrils accumulate with the passage of time, and have long-lasting consequences on skin structure and function.
UV irradiation causes alternations of dermal collagen through two primary pathways: 1) stimulation of collagen breakdown, resulting in fragmented, disorganized collagen and 2) inhibition of procollagen biosynthesis, resulting in loss of collagen content. A single exposure to UV irradiation (2MED) causes near-complete loss of procollagen synthesis, which persists for 24 hours, followed by recovery 48–72 hours post exposure (Fisher et al., 2000). This reduction of procollagen synthesis is likely mediated by impairment of the TGF-β pathways, which controls procollagen expression. (Quan et al., 2002, 2004; Quan et al., 2001, 2005). Matrix-degrading metalloproteinases are key mediators of collagen degradation that is observed in photoaged skin (Fisher et al., 2002; Fisher et al., 1996; Fisher et al., 1998; Fisher and Voorhees, 1998; Fisher et al., 1997). This review article summarizes matrix metalloproteinase expression and regulation by UV irradiation in human skin in vivo.
MMP gene expression in non-irradiated and UV-irradiated human skin in vivo
Matrix metalloproteinases (MMPs) comprise a family of zinc-containing proteinases that are responsible for degradation of extracellular matrix (ECM) proteins, which form skin dermal connective tissue (Nelson et al., 2000; Sternlicht and Werb, 2001). To date, the MMP gene family consists of 25 members, 24 of which are expressed in mammals (Egeblad and Werb, 2002; Fu et al., 2008; Sternlicht and Werb, 2001). MMPs are classified as collagenases, gelatinases, stromelysins, and membrane-type MMPs according to their substrate specificities and whether they are secreted soluble proteins or bound to cell surface membrane (Fu et al., 2008). MMPs are involved in a wide range of proteolytic events in physiological and pathological circumstances including embryogenesis, wound healing, inflammation, angiogenesis, and cancer (Birkedal-Hansen et al., 1993; Egeblad and Werb, 2002; Kerkela and Saarialho-Kere, 2003; Sternlicht and Werb, 2001).
Studies conducted by us and others over the past several years have revealed that UV radiation elevates at least three different MMPs in human skin in vivo, i.e., interstitial collagenase (MMP-1), stromelysin-1 (MMP-3), and 92kDa gelatinase (MMP-9) (Brenneisen et al., 2002; Fisher et al., 2002; Fisher et al., 1996; Fisher and Voorhees, 1998; Fisher et al., 1997; Wang et al., 2008). These three MMPs are strongly regulated by transcription factor AP-1, which is rapidly induced and activated by UV irradiation in human skin in vivo (Birkedal-Hansen et al., 1993; Fisher and Voorhees, 1998; Gutman and Wasylyk, 1990; Mauviel, 1993). The combined actions of MMP-1, 3, and 9 have the capacity to degrade most of the proteins that comprise the dermal extracellular matrix. However, whether UV irradiation induces expression of other members of the MMP family in human skin has not been fully investigated. To address this question, we systemically investigated basal and UV irradiation-induced gene expression of each mammalian MMP in human skin in vivo.
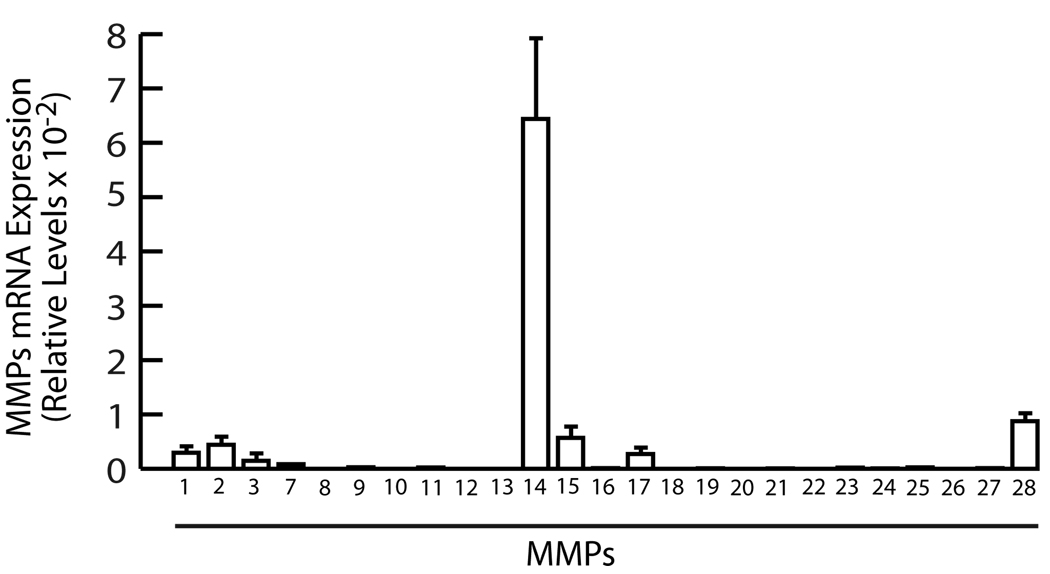
With the exception of MMP-14, basal mRNA expression levels of the MMP family members were extremely low in normal healthy, sun-protected, adult human skin (Fig 1). Transcripts for MMP-8, 10, 12, 20, and 26 were not detected. Transcripts for remaining MMPs were near the level of detection, approximately 1000-fold lower than internal control, housekeeping gene 36B4. Basal expression of MMP-14 mRNA was approximately 35-fold higher than other detectable MMPs.
Figure 1.
Basal gene expression of MMP family members in human skin in vivo. Full thickness (4mm) sun-protected buttock human skin was obtained from healthy adult human volunteers (eight subjects, average 36 years age), as previously described (Fisher, G.J, 1991. J Invest Dermatol 96:699–707; Quan, 2004, Amer J Pathol 169:482–490). Total RNA was extracted, using a commercial kit (RNeasy midikit, Qiagen, Chatsworth, CA) according to the manufacturer’s protocol. Reverse transcription was performed using Taqman Reverse Transcription kit (Applied Biosystems, Foster City, CA). Real-time RT-PCR was performed using a Taqman Universal PCR Master Mix kit (Applied Biosystems, Foster City, CA) and 7500 Real-Time PCR System (Applied Biosystems, Foster City, CA). All primers and probes were purchased from Applied Biosystems (Assays-on-Demand™ Gene Expression Products). Results are means±SEM of MMPs mRNA normalized to 36B4 (internal control) mRNA. All procedures involving human subjects were conducted in accord with the regulations set forth by the University of Michigan Institutional Review Board, and all subjects provided written informed consent.
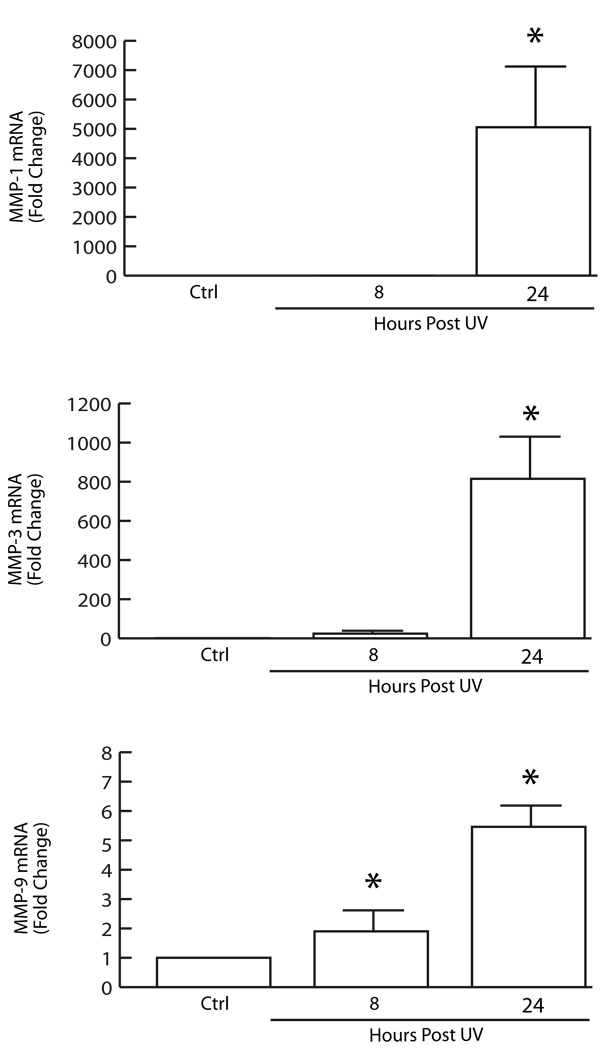
Interestingly, among the 19 MMPs expressed in normal human skin, only three were significantly induced in response to UV irradiation; MMP-1 (collagenase), MMP-3 (stromelysin-1), and MMP-9 (92kDa gelatinase)(Fig.2). MMP-1 and MMP-3 mRNA levels were induced several thousand-fold at 24 hours post UV irradiation, whereas MMP-9 was modestly induced (6-fold). MMP-1 initiates cleavage of fibrillar collagen, typically type I and III in skin, at a single site within its central triple helix. Once cleaved by MMP-1, collagen can be further degraded by elevated levels of MMP-3 and MMP-9. These data confirm and extend our previous findings (Fisher et al., 2002; Fisher et al., 1996; Fisher and Voorhees, 1998; Fisher et al., 1997; Wang et al., 2008). Interestingly, in contrast to UV-inducible MMPs, MMP-14 was reduced nearly 80% at 8 hours post UV irradiation, and remained decreased at least 24 hours (data not shown). The physiological function of MMP-14 in human skin remains to be determined.
Figure 2.
UV irradiation induces MMP-1, MMP-3, and MMP-9 in human skin in vivo. Sun-protected buttock skin of healthy adult human volunteers was exposed to twice the minimum erythema dose of solar-simulated UV irradiation (SPEC 450W xenon arc solar simulator). Full thickness (4mm) biopsies were obtained 8 and 24 hours post irradiation. Total RNA was extracted and MMP-1, MMP-3, and MMP-9 mRNA levels were determined by real-time RT-PCR, as described in figure 1 legend. Results are means±SEM of MMPs mRNA normalized to 36B4 (internal control) mRNA. N=8, p<0.05.
We have previously reported that MMP-8 (neutrophil collagenase) (Fisher et al., 2001) and MMP-12 (macrophage elastase) (Chung et al., 2002) proteins are present in human skin, 24 hours following UV irradiation, as a result of influx of neutrophils and macrophages from the circulation. Skin neutrophils and macrophages are terminal differentiated cells, which no longer actually transcribe new mRNA. Residual MMP-8 and MMP-12 mRNA was below the limit of detection. We previously reported that the majority of MMP-8 protein remained in the inactive precursor form (Fisher et al., 2001) and accounted for little, if any, collagenolytic activity in skin following UV irradiation (Brennan et al., 2003). Furthermore, exposure of sun-protected human skin to purified human MMP-1 in organ culture causes collagen fragmentation, and alterations in the structure and organization of collagen fibrils in the dermis that resemble those observed in photoaged skin (Fligiel et al., 2003; Varani et al., 2008; Varani et al., 2001). Taken together, these studies suggest that MMP-1, MMP-3, and MMP-9 are primary UV-inducible collagenolytic enzymes, and MMP-1 is the major protease capable of initiating degradation of native fibrillar collagens in human skin in vivo.
Epidermis is the major source of UV irradiation-induced MMPs in human skin in vivo
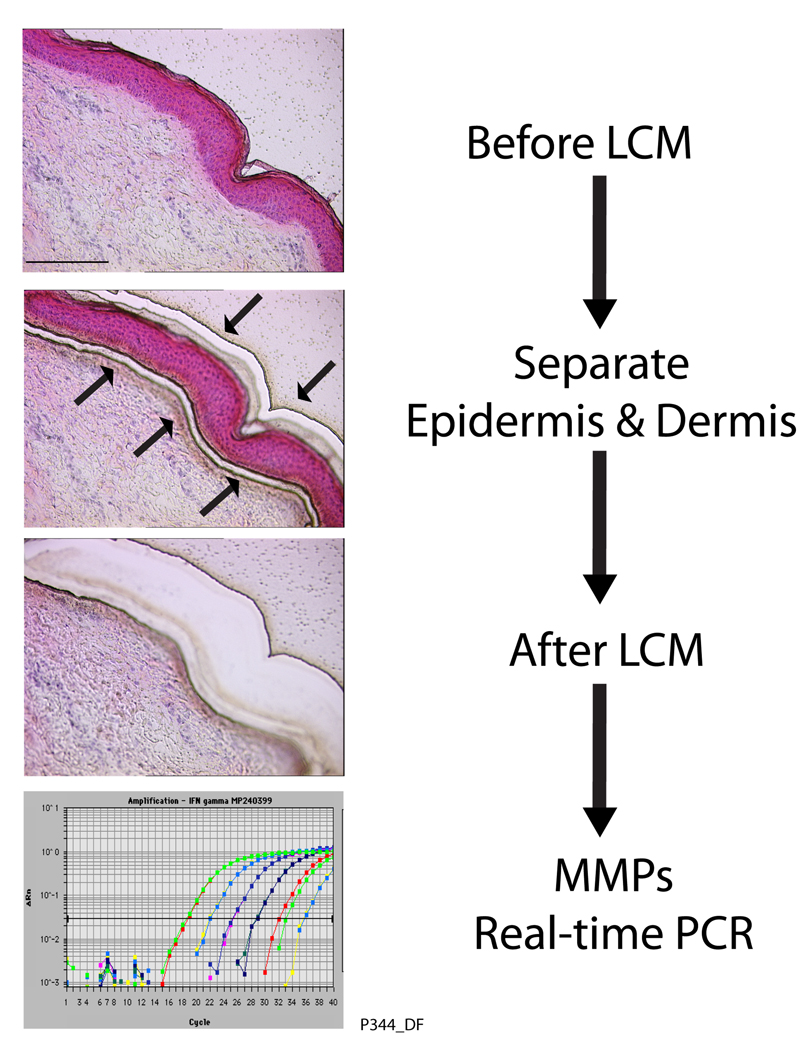
Based on in situ hybridization and immunohistology, we previously reported that keratinocytes are the major cellular source of MMPs in UV-irradiated human skin in vivo (Fisher and Voorhees, 1998; Fisher et al., 1997). To quantify the relative contributions of epidermis and dermis to UV irradiation-induced MMP expression, epidermis and dermis were separately analyzed by laser capture microdissection (LCM) coupled with real-time RT-PCR. Figure 3 shows human skin sections before and after LCM of epidermis and dermis. Total RNA was extracted from microdissected epidermis and dermis and UV irradiation-induced MMP mRNA levels were determined by real-time RT-PCR. In agreement with our previous data, all three UV irradiation inducible MMPs, were primarily produced by the epidermis, rather than dermis (Fig.4). It should be noted that dermal samples contained some epidermal contamination (approximately 10%), based on measurement of keratin 14 mRNA, which is expressed exclusively by keratinocytes. Therefore, dermal contribution to UV irradiation-induced MMP expression cannot be greater than shown Figure 4.
Figure 3.
Laser capture microdisection (LCM) coupled real-time RT-PCR. Human skin punch biopsies were embedded in OCT, sectioned, and stained with H&E. Epidermis and dermis were captured using LCM (Leica ASLMD System, Leica Microsystems, Germany). Arrows indicate locations where epidermis and dermis were separated by laser. Total RNA was extracted from captured epidermis and dermis, and quantitative real-time RT-PCR was performed as described in figure 1 legend.
Figure 4.
UV irradiation induces gene expression of MMP-1, MMP-3, and MMP-9 primarily in human epidermis in vivo. Sun-protected buttock skin of healthy adult human volunteers was exposed to twice the minimum erythema dose of solar-simulated UV irradiation (SPEC 450W xenon arc solar simulator). Full thickness (4mm) biopsies were obtained 24 hours post irradiation as described in Figure 1 legend. Total RNA were extracted from epidermis and dermis, which were obtained by laser capture microdissection. MMP-1, MMP-3, and MMP-9 mRNA levels were determined by real-time RT-PCR, as described in Figure 1 legend. Results are means±SEM of MMPs mRNA normalized to 36B4 (internal control) mRNA. N=6, p<0.05.
In contrast to the results presented above, cell culture and skin equivalent model studies have concluded that dermal fibroblasts are the major source of MMPs that are expressed in response to UV irradiation (Fagot et al., 2002, 2004). The reasons for the discrepancies between responses of human skin cells in vivo and responses of cultured skin cells in vitro are not known. However, there are many other examples of cultured skin cells failing to mimic the behavior of cells in vivo. Obviously, it is important to compare, whenever possible, results obtained from simple model systems with those obtained from direct in vivo observations.
Although collagen-degrading MMP-1, 3 and 9 are primarily induced in the epidermis, the secreted enzymes diffuse into the dermis and degrade collagen, as shown by in situ zymography (Fig 5). This diffusion may be aided by direct binding of MMPs to the collagenous extracellular matrix (Knauper et al., 1997; Overall, 2001; Windsor et al., 1991).
Figure 5.
Collagenase activity induced by solar-simulated UV irradiation in human skin in vivo. Sun-protected buttock skin of healthy adult human volunteers was exposed to twice the minimum erythema dose of solar-simulated UV irradiation (SPEC 450W xenon arc solar simulator). Full thickness (4mm) biopsies were obtained 24 hours post irradiation as described in Figure 1 legend. Collagenase activity was detected by in situ zymography, using FITC-labeled type I collagen as substrate (green fluorescence). Collagenease-catalyzed collagen cleavage causes loss of green fluorescence, resulting in darkened areas, which are most noticeable in the epidermis and upper dermis of UV-irradiated human skin. Images are representative of five experiments.
Taken together with previous findings, data presented above indicate that epidermal keratinocytes are the major cellular source of MMPs that are produced in response to exposure of human skin to solar UV irradiation. However, it is possible that dermal cells may also play a role in epidermal production of MMPs, through indirect paracrine mechanisms, by release of growth factors or cytokines, which in turn modulate MMP production by epidermal keratinocytes.
References
- Berneberg M, Plettenberg H, Krutmann J. Photoaging of human skin. Photodermatol Photoimmunol Photomed. 2000;16:239–244. doi: 10.1034/j.1600-0781.2000.160601.x. [DOI] [PubMed] [Google Scholar]
- Birkedal-Hansen H, Moore W, Bodden M, Windsor L, Birkedal-Hansen B, DeCarlo A, et al. Matrix metalloproteinases: a review. Crit Rev Oral Bio Med. 1993;4:197–250. doi: 10.1177/10454411930040020401. [DOI] [PubMed] [Google Scholar]
- Brennan M, Bhatti H, Nerusu K, Bhagavathula N, Kang S, Fisher G, et al. Matrix mettalloproteinase-1 is the collagenolytic enzyme responsible for collagen damage in UV-irradiated human skin. Photochem Photobiol. 2003;78:43–48. doi: 10.1562/0031-8655(2003)078<0043:mmitmc>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Brenneisen P, Sies H, Scharffetter-Kochanek K. Ultraviolet-B irradiation and matrix metalloproteinases: from induction via signaling to initial events. Ann N Y Acad Sci. 2002;973:31–43. doi: 10.1111/j.1749-6632.2002.tb04602.x. [DOI] [PubMed] [Google Scholar]
- Chung JH, Seo JY, Lee MK, Eun HC, Lee JH, Kang S, et al. Ultraviolet modulation of human macrophage metalloelastase in human skin in vivo. J Invest Dermatol. 2002;119:507–512. doi: 10.1046/j.1523-1747.2002.01844.x. [DOI] [PubMed] [Google Scholar]
- Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nature Rev. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- Fagot D, Asselineau D, Bernerd F. Direct role of human dermal fibroblasts and indirect participation of epidermal keratinocytes in MMP-1 production after UV-B irradiation. Arch Dermatol Res. 2002;293:576–583. doi: 10.1007/s00403-001-0271-1. [DOI] [PubMed] [Google Scholar]
- Fagot D, Asselineau D, Bernerd F. Matrix metalloproteinase-1 production observed after solar-simulated radiation exposure is assumed by dermal fibroblasts but involves a paracrine activation through epidermal keratinocytes. Photochem Photobiol. 2004;79:499–505. doi: 10.1562/yg-03-11-r1.1. [DOI] [PubMed] [Google Scholar]
- Fisher G, Datta S, Wang Z, Li X, Quan T, Chung J, et al. c-Jun dependent inhibition of cutaneous procollagen transcription following ultraviolet irradiation is reversed by all-trans retinoid acid. J Clin Invest. 2000;106:661–668. doi: 10.1172/JCI9362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher G, Kang S, Varani J, Bata-Csorgo Z, Wan Y, Datta S, et al. Mechanisms of photoaging and chronological skin aging. Arch Dermatol. 2002;138:1462–1470. doi: 10.1001/archderm.138.11.1462. [DOI] [PubMed] [Google Scholar]
- Fisher GJ, Choi HC, Bata-Csorgo Z, Shao Y, Datta S, Wang ZQ, et al. Ultraviolet irradiation increases matrix metalloproteinase-8 protein in human skin in vivo. J Invest Dermatol. 2001;117:219–226. doi: 10.1046/j.0022-202x.2001.01432.x. [DOI] [PubMed] [Google Scholar]
- Fisher GJ, Datta SC, Talwar HS, Wang ZQ, Varani J, Kang S, et al. Molecular basis of sun-induced premature skin ageing and retinoid antagonism. Nature. 1996;379:335–339. doi: 10.1038/379335a0. [DOI] [PubMed] [Google Scholar]
- Fisher GJ, Talwar HS, Lin JY, Lin PP, McPhillips F, Wang ZQ, et al. Retinoic acid inhibits induction of c-Jun protein by ultraviolet irradiation that occurs subsequent to activation of mitogen-activated protein kinase pathways in human skin in vivo. J Clin Invest. 1998;101:1432–1440. doi: 10.1172/JCI2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher GJ, Voorhees JJ. Molecular mechanisms of photoaging and its prevention by retinoic acid: Ultraviolet irradiation induces MAP kinase signal transduction cascades that induce AP-1-regulated matrix metalloproteinases that degrade human skin in vivo. J Invest Dermatol. 1998;3:61–68. [PubMed] [Google Scholar]
- Fisher GJ, Wang ZQ, Datta SC, Varani J, Kang S, Voorhees JJ. Pathophysiology of premature skin aging induced by ultraviolet light. New Eng J Med. 1997;337:1419–1428. doi: 10.1056/NEJM199711133372003. [DOI] [PubMed] [Google Scholar]
- Fligiel S, Varani J, Datta S, Kang S, Fisher G, Voorhees J. Collagen degradation in aged/photodamaged skin in vivo and after exposure to matrix metalloproteinase-1 in vitro. J Invest Dermatol. 2003;120:842–848. doi: 10.1046/j.1523-1747.2003.12148.x. [DOI] [PubMed] [Google Scholar]
- Fu X, Parks WC, Heinecke JW. Activation and silencing of matrix metalloproteinases. Semin Cell Dev Biol. 2008;19:2–13. doi: 10.1016/j.semcdb.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Gilchrest BA, Yaar M. Ageing and photoageing of the skin: observations and the cellular and molecular level. Brit J Dermatol. 1992;127:25–30. doi: 10.1111/j.1365-2133.1992.tb16984.x. [DOI] [PubMed] [Google Scholar]
- Gutman A, Wasylyk B. The collagenase gene promoter contains a TPA and oncogene-responsive unit encompassing the PEA3 and AP-1 binding sites. EMBO J. 1990;9:2241–2246. doi: 10.1002/j.1460-2075.1990.tb07394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminer MS. Photodamage: magnitude of the problem. In: Gilchrest BA, editor. Photodamage. Cambridge: Blackwell Science; 1995. pp. 1–11. [Google Scholar]
- Kerkela E, Saarialho-Kere U. Matrix metalloproteinases in tumor progression: focus on basal and squamous cell skin cancer. Exp Dermatol. 2003;12:109–125. doi: 10.1034/j.1600-0625.2003.120201.x. [DOI] [PubMed] [Google Scholar]
- Kligman LH, Kligman AM. The nature of photoaging: its prevention and repair. Photoderm. 1986;3:215–227. [PubMed] [Google Scholar]
- Knauper V, Cowell S, Smith B, Lopez-Otin C, O'Shea M, Morris H, et al. The role of the C-terminal domain of human collagenase-3 (MMP-13) in the activation of procollagenase-3, substrate specificity, and tissue inhibitor of metalloproteinase interaction. J Biol Chem. 1997;272:7608–7616. doi: 10.1074/jbc.272.12.7608. [DOI] [PubMed] [Google Scholar]
- Kripke ML. Immunological unresponsiveness induced by ultraviolet radiation. Immunol Rev. 1984;80:87. doi: 10.1111/j.1600-065x.1984.tb00496.x. [DOI] [PubMed] [Google Scholar]
- Mauviel A. Cytokine regulation of metalloproteinase gene expression. J Cell Biochem. 1993;53:288–295. doi: 10.1002/jcb.240530404. [DOI] [PubMed] [Google Scholar]
- Nelson AR, Fingleton B, Rothenberg ML, Matrisian LM. Matrix metalloproteinases: biologic activity and clinical implications. J Clin Oncol. 2000;18:1135–1149. doi: 10.1200/JCO.2000.18.5.1135. [DOI] [PubMed] [Google Scholar]
- Nimni ME. Collagen: structure, function, and metabolism in normal and fibrotic tissues. Semin Arthritis Rheum. 1983;13:1–86. doi: 10.1016/0049-0172(83)90024-0. [DOI] [PubMed] [Google Scholar]
- Overall CM. Matrix metalloproteinase substrate binding domains, modules and exosites. Overview and experimental strategies. Methods Mol Biol. 2001;151:79–120. [PubMed] [Google Scholar]
- Quan T, He T, Kang S, Voorhees J, Fisher G. Ultraviolet irradiation alters transforming growth factor β/Smad pathway in human skin in vivo. J Invest Dermatol. 2002;119:499–506. doi: 10.1046/j.1523-1747.2002.01834.x. [DOI] [PubMed] [Google Scholar]
- Quan T, He T, Kang S, Voorhees J, Fisher G. Solar ultraviolet irradiation reduces collagen in photoaged human skin by blocking transforming growth factor-β type II receptor/Smad signaling. Amer J Pathol. 2004;165:741–751. doi: 10.1016/s0002-9440(10)63337-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan T, He T, Voorhees J, Fisher G. Ultraviolet irradiation blocks cellular responses to transforming growth factor-β by down-regulating its type-II receptor and inducing Smad7. J Biol Chem. 2001;276:26349–26356. doi: 10.1074/jbc.M010835200. [DOI] [PubMed] [Google Scholar]
- Quan T, He T, Voorhees J, Fisher G. Ultraviolet irradiation induces Smad7 via induction of transciption factor AP-1 in human skin fibroblasts. J Biol Chem. 2005;280:8079–8085. doi: 10.1074/jbc.M409647200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharffetter-Kochanek K, Brenneisen P, Wenk J, Herrmann G, Ma W, Kuhr L, et al. Photoaging of the skin from phenotype to mechanisms. Exp Gerontol. 2000;35:307–316. doi: 10.1016/s0531-5565(00)00098-x. [DOI] [PubMed] [Google Scholar]
- Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uitto J, Bernstein E. Molecular mechanisms of cutaneous aging: connective tissue alterations in the dermis. J Invest Dermatol Symp Proc. 1998;3:41–44. [PubMed] [Google Scholar]
- Varani J, Perone P, Warner RL, Dame MK, Kang S, Fisher GJ, et al. Vascular tube formation on matrix metalloproteinase-1-damaged collagen. Br J Cancer. 2008;98:1646–1652. doi: 10.1038/sj.bjc.6604357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varani J, Spearman D, Perone P, Fligiel S, Datta S, Wang Z, et al. Inhibition of type I procollagen synthesis by damaged collagen in photoaged skin and by collagenase-degraded collagen in vitro. Amer J Pathol. 2001;158:931–942. doi: 10.1016/S0002-9440(10)64040-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Garza LA, Cho S, Kafi R, Hammerberg C, Quan T, et al. Effect of increased pigmentation on the antifibrotic response of human skin to UV-A1 phototherapy. Arch Dermatol. 2008;144:851–858. doi: 10.1001/archderm.144.7.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windsor LJ, Birkedal-Hansen H, Birkedal-Hansen B, Engler JA. An internal cysteine plays a role in the maintenance of the latency of human fibroblast collagenase. Biochemistry. 1991;30:641–647. doi: 10.1021/bi00217a008. [DOI] [PubMed] [Google Scholar]