Abstract
Our previous work demonstrated that immunoproteasome is upregulated in the retina and brain in response to injury that does not involve an inflammatory response (Ferrington et al., 2008). These results suggest additional non-immune functions for the immunoproteasome in the cellular stress response pathway. The current study further investigates the potential involvement of the immunoproteasome in responding to the chronic stress of aging or oxidant exposure in the retina and cultured retinal pigment epithelial (RPE) cells from knock-out mice missing either one (lmp7−/−, L7) or two (lmp7−/−/mecl-1−/−, L7M1) immunoproteasome subunits. We show that aging and chronic oxidative stress upregulates immunoproteasome in the retina and RPE from WT mice. No upregulation of LMP2 was observed in retinas or RPE lacking MECL-1 and/or LMP7, suggesting that the full complement of immunoproteasome subunits is required to achieve maximal upregulation in response to stress. We also show that RPE deficient in immunoproteasome are more susceptible to oxidation-induced cell death, supporting a role for immunoproteasome in protecting from oxidative stress. These results provide key mechanistic insight into novel aspects of proteasome biology and are an important first step in identifying alternative roles for retinal immunoproteasome that are unrelated to its role in the immune response.
Keywords: Immunoproteasome, stress, retina, age, lmp7−/−, lmp7−/−/mecl-1−/−
INTRODUCTION
The proteasome is a multi-subunit proteolytic complex that is involved in the degradation of many cytosolic and nuclear proteins that regulate pathways critical for cell survival. The 20S proteasome, which makes up the catalytic core, is composed of four heptameric rings of α and β subunits that form the outer and inner rings, respectively, of the barrel-shaped structure. The 20S core contains three catalytically active β-subunits that have distinct cleavage specificities. These activities, referred to as caspase-like, trypsin-like and chymotrypsin-like, are associated with the β1, β2, and β5 standard subunits. Alternatively, the standard subunits can be replaced in nascent proteasomes by their immunoproteasome counterparts, LMP2, MECL-1, and LMP7.
Three types of core particles that are defined by the composition of their catalytic subunits have been described. The standard proteasome, containing β1, β2, and β5, is the predominant core in most tissues outside the immune system. The immunoproteasome, containing LMP2, MECL-1, and LMP7, is the major proteasome species in immune tissue but is also found in limited abundance outside the immune system (Eleuteri et al., 1997; Ferrington et al., 2008, Husom et al., 2004, Kapphahn et al., 2007). The third type of 20S core, referred to as the intermediate-type 20S, contains a mixture of both the standard and immunoproteasome subunits (Dahlmann et al., 2000; Klare et al., 2007).
Analysis of 20S cores containing different subunit compositions has shown a substantial difference in peptide hydrolysis and cleavage of model protein substrates (Dahlmann et al., 2000; Klare et al., 2007). Thus, the repertoire of peptides generated within a cell is determined by the population of proteasome subtypes. It is well established that the immunoproteasome is most efficient at generating immunogenic peptides for antigen presentation (Goldberg et al., 2002; Rock et al., 1994). However, the expression of immunoproteasome subunits in neurons (photoreceptor and Purkinje cells), glia (Mueller cells and astrocytes), and oligodendrocytes of the retina and brain implies other non-immune functions are possible (Díaz-Hernández et al., 2003; Ethen et al., 2007; Ferrington et al., 2008; Gavilán et al., 2009; Kapphahn et al., 2007; Louie et al., 2002; Mishto et al., 2006). Additionally, immunoproteasome upregulation in the central nervous system with acute injury, disease, and age suggests a role in responding to stress and injury (Díaz-Hernández et al., 2003; Ethen et al., 2007; Ferrington et al., 2008; Gavilán et al., 2009; Mishto et al., 2006). Taken together, these results lead to the hypothesis that immunoproteasome is performing functions unrelated to its role in the immune system.
To directly test the effect of immunoproteasome deficiency in the retina, we have utilized immunoproteasome knock-out mice, missing either one (lmp7 −/−) or two (lmp7 −/−/mecl-1 −/−) immunoproteasome subunits. Previous research on these mice has mainly focused on defining defects in their immune function using antigen-presenting tissue and cell lines (Basler et al., 2006; Caudill et al., 2006, Fehling et al., 1994; Stohwasser et al., 1996). The current work is the first to test whether the inability to make specific immunoproteasome subunits has a negative impact on retinal proteasome’s response to the chronic stresses of aging and oxidant exposure.
MATERIALS AND METHODS
Animals
C57BL/6 wild-type (WT) mice were purchased from the National Institute on Aging-maintained colony (Harlan Sprague Dawley, Indianapolis, IN). Breeders for mice deficient in one (lmp7 −/−, L7) or two (lmp7 −/−/mecl-1 −/−, L7M1) catalytic subunits of the immunoproteasome were generously donated by J. J. Monaco (University of Cincinnati). Descriptions of gene deletions and mouse characteristics have been previously published (Basler et al., 2006; Caudill et al., 2006; Fehling et al., 1994). All mice are on the C57BL/6 genetic background. Mice were housed in an animal facility maintained at 20 °C with a 12-hour light and dark cycle. Ages 2, 9, 15, 20 and 24 months were used in this study. Mice were handled according to the guidelines of the Institutional Animal Care and Use Committee of the University of Minnesota and the National Institutes of Health. Animals were sacrificed with CO2 and perfused with phosphate-buffered saline (PBS) with 2 U/mL heparin prior to tissue collection.
RNA isolation and quantitative RT-PCR
Total RNA was isolated from mouse retina using the RNeasy Mini Kit (Qiagen, Valencia, CA). Isolated RNA was quantified by spectrophotometry (λ = 260nm). cDNA was synthesized in a reverse transcription assay using 70 ng of total RNA. The RT reaction was performed in a GeneAmp PCR System 9700 (Applied Biosystems, Foster City, CA). RT-PCR conditions and primer sets are provided in the supplementary information (Table S1). Proteasome subunit expression was normalized to acidic ribosomal phosphoprotein P0 (ARBP) expression for each sample. Normalized gene expression was determined using the modified Livak method employed by the iQ5 software (BioRad).
Retinal protein processing
Retinas were processed as previously described (Ferrington et al., 2008; Kapphahn et al., 2006, 2007; Louie et al., 2002), using a homogenization buffer comprised of 20 mM Tris (pH 7.4), 20% wt/vol sucrose, 2 mM MgCl2, 10 mM glucose, and 2% wt/vol 3-[(3-cholamidopropyl) dimethylamino]-1-propanesulfonate (CHAPS). The supernatant containing the soluble retinal proteins from the final processing step was saved. Homogenates were stored at −80 °C. Protein concentrations were determined using the bicinchoninic acid (BCA) protein assay (Pierce, Rockford, IL) with bovine serum albumin as the standard.
Cell culture
RPE cells from WT and L7M1 mice were harvested and immortalized as previously described (Ferrington et al., 2006). For chronic oxidative stress, cells were cultured in 100 mm petri dishes with growth medium containing Dulbecco’s Modified Eagle Medium (DMEM), 1X nonessential amino acids, 0.4 mM L-glutamine, 25 mM glucose, 50 U/mL penicillin, 50 U/mL streptomycin and 10% fetal calf serum. Confluent cells were treated daily with culture medium containing 2% fetal calf serum and 0, 0.3, 0.5, or 1.0 mM hydrogen peroxide. The responses from 0.3 and 0.5 mM peroxide did not differ from each other so were combined for the low-dose oxidative stress treatment group. Only WT RPE cells were treated with 1.0 mM peroxide (high dose oxidative stress). Cells were treated for a period of one to six days, harvesting one dish of cells each day. Cell viability assay following hydrogen peroxide exposure was performed in 96-well plates as previously described (Ferrington et al., 2006).
RPE homogenization
RPE were collected by rinsing the plates in PBS followed by the addition of the lysis buffer containing 25 mM Tris (pH=7.8), 10 mM KCl, 2.5 mM EDTA, 0.5% nonyl phenoxylpolyethoxylethanol-40 (NP-40), 10% glycerol, and 1 mM dithiothreitol (DTT). Cells were scraped from the plate and the lysate was stored at −20 °C for 16 hr. The lysate was thawed and centrifuged at 16,000 x g for 20 min at 4 °C. The final supernatant was collected and stored at −80 °C. Protein concentrations were determined using the BCA assay.
20S proteasome purified from spleen
20S proteasome was purified from WT or L7M1 mouse spleen as described previously using ammonium sulfate precipitation followed by sequential column separation (DEAE-5PW, Mini Q) of proteins (Husom et al., 2004). Each preparation used spleens from approximately ten animals. The final purified proteasome was suspended in a buffer containing 10mM Tris-HCl (pH 7.2) and stored at −80°C. Protein concentration was determined using the BCA assay.
Western blot analysis
Western blotting was performed as previously described (Kapphahn et al., 2006, 2007; Ferrington et al., 2008). Primary antibodies and Western blot conditions are provided in the supplementary material (Table S2). The presence of the third immunoproteasome subunit (MECL-1) was not evaluated due to an inadequate reaction with commercially available antibodies. Density values for the proteasome subunits, regulators, and ubiquitin-modified proteins were normalized to the mean value of 2 mo. WT mice. To obtain an estimate of how peroxide affects immunoproteasome content, the density of the control (no treatment) cells from each day was subtracted from the density of the hydrogen peroxide treated RPE.
Proteasome activity measurements
Proteasome activity was assayed using fluorogenic peptide substrates as previously described (Kapphahn et al., 2007, Ferrington et al., 2008). Proteasome activity measured in purified 20S and proteasome-enriched homogenate from spleen was normalized to the α7 content for each sample.
Statistical analysis
Differences between three or more groups were tested by 2-way or 1-way ANOVA. When there was significant interaction between groups in the 2-way ANOVA, data was analyzed as 1-way ANOVA tests for each strain and each age. When appropriate, a Tukey-Kramer multiple comparison post-hoc test was performed. A two-sample T-test was performed for comparison of two groups. To observe the time dependent increase of immunoproteasome in response to hydrogen peroxide, data were evaluated using linear regression. Statistical analysis was performed using NCSS 2001 and significance was set at p<0.05.
RESULTS
Proteasome subunit gene expression
Proteasome subunit gene expression was evaluated in 2 month-old mice to confirm the genetic disruption of the lmp7 and mecl-1 genes in the retinas of immunoproteasome KO mice and to determine whether ablation of these genes affected the expression of other proteasome subunits. PCR amplification of retinal mRNA confirmed the disruption of lmp7 in the L7 and L7M1 mice (Fig. S1A). Additionally, mecl-1 was not expressed in L7M1 retinas (Fig. S1A, B). Quantitative analysis of catalytic subunit expression using qRT-PCR showed no significant difference between strains (Fig. S1B,C). Expression of the constitutively expressed α7 subunit, used as a measure of total proteasome content, was also consistent between strains. These results indicate that the genetic ablation eliminated expression of lmp7 and mecl-1 genes, but does not alter the expression of other proteasome subunits in young mice.
Altered subunit content with age and between strains
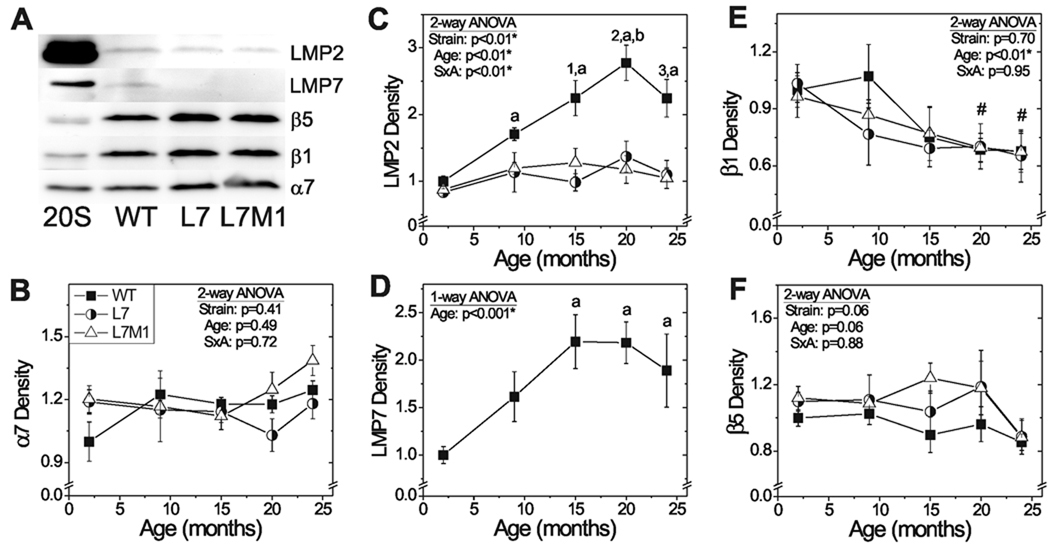
To determine how aging and ablation of specific immunoproteasome subunits affected retinal catalytic core subunit composition, Western blotting was performed using subunit-specific antibodies (Fig. 1A). Western blot data showed no significant difference in the α7 subunit, which is a measure of total proteasome content, with either age or between strains (Fig. 1B). At 2 months, catalytic subunit expression was equivalent among strains (Fig. 2C-F). With aging, all strains exhibited a 35-percent decrease in β1 standard subunit content by 24 months and no change in β5 content (Fig. 2E, F). With aging of WT mice, a significant age-dependent 2- and 3-fold increase in immunoproteasome subunits LMP7 and LMP2 was observed (Fig. 2C,D). In contrast, LMP2 content in L7 and L7M1 retinas was not altered with age.
Figure 1.
Age- and strain-related changes in proteasome subunit protein expression. (A) Representative Western blots of proteasome subunits in WT (■), L7 (○) and L7M1 (Δ) retina. Purified 20S proteasome from mouse spleen was used as a positive control. (B-F) Summary of Western blot densities for proteasome subunits. One-way ANOVA results are provided in panel D. Two-way ANOVA results are provided in panels B, C, E, and F. When there was significant interaction (SxA), one-way ANOVA comparisons were performed for each strain and each age. Results of Tukey-Kramer post-hoc comparisons are indicated by letters, numbers or symbols. (B) α7 content. No significant difference with age or between mouse strains. (C) LMP2 content. One-way ANOVA results by strain with age: (WT, p<0.001) a, different from WT 2 mo.; b, different from WT 9 mo. One-way ANOVA results by age between strains: (15 mo., p=0.009; 20 mo., p<0.001; 24 mo. p=0.003) 1, different from L7M1 and L7 at 15 mo.; 2, different from L7 and L7M1 at 20 mo.; 3, different from L7 at 24 mo. (D) LMP7 content in WT mice. a, different from WT 2 mo. (E) β1 content. #, different from 2 mo. (F) β5 content. No significant difference with age or between mouse strains. All values are mean ± SEM and are shown relative to WT 2 mo. WT n=3–15; L7 n=4; L7M1 n=5–10 per group.
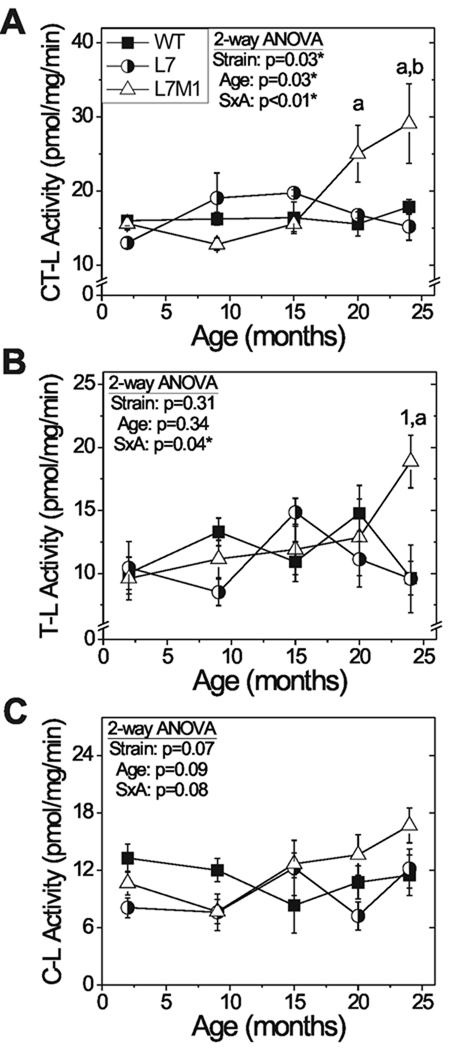
Figure 2.
Age- and strain-related changes in proteasome catalytic activity. Two-way ANOVA results are shown in each panel. When there was significant interaction (SxA), one-way ANOVA comparisons were performed for each strain and each age. Results of Tukey-Kramer post-hoc comparisons are indicated by letters or numbers. (A) Chymotrypsin-like (CT-L) activity of WT (■), L7 (○) and L7M1 (Δ) retina. One-way ANOVA results by strain with age: (L7M1, p=0.01) a, different from L7M1 9 mo.; b, different from L7M1 2 and 15 mo. One-way ANOVA results by age between strains showed no significant difference at any age. (B) Trypsin-like (T-L) activity. One-way ANOVA results by strain with age: (L7M1, p=0.04) a, different from L7M1 2mo. One-way ANOVA results by age between strains (24 mo., p=0.01) 1, different from WT and L7 at 24 mo. (C) Caspase-like (C-L) activity. Two-way ANOVA showed no difference between strains or with age. All values are mean ± SEM. WT n=3–8; L7 n=4–5; L7M1 n=4–12 per group.
Age- and strain-related changes in proteasome catalytic activity
To determine the effect of age and immunoproteasome-deficiency on proteasome activity, fluorogenic peptide substrates were used to monitor the caspase-, chymotrypsin- and trypsin-like activities that are associated with the β1/LMP2, β5/LMP7, and β2/MECL-1 subunits, respectively. All assays were performed in the presence and absence of the proteasome inhibitor, MG132, to distinguish proteolysis that was specific to the proteasome.
For all mouse strains, proteasome activity was essentially equal for each peptide from ages 2 to 15 months, suggesting that elimination of specific subunits did not significantly alter retinal proteasome function (Fig 2A,B,C). For both WT and L7 retinas, proteasome activity did not change with age for all peptides. In contrast, L7M1 retinas exhibited a significant increase at 20 and 24 months for the chymotrypsin-like activity and trypsin-like activity was significantly increased by 24 months. These results suggest inherent differences in the response to aging of retinal proteasome from L7M1 compared with L7.
The relative content of ubiquitin-modified proteins was evaluated by Western blot as an indirect measure of proteasome function. No difference in ubquitinated proteins was noted between strains or with age (Fig. S2). These data suggest that proteasome activity is adequate to degrade the ubiquitin-associated proteins.
Relative content of proteasome activators
The increase in proteasome activity in L7M1 could possibly be attributed to an increased content of the proteasome activators PA28 (11S) and PA700. These regulatory complexes are capable of significantly increasing proteasome activity (Dubiel et al., 1992; Ferrington et al., 2005). Data from Western blots showed there was no difference in content of either PA28 or PA700 with aging or between strains (Fig. S3). These results rule out differences in content of these two proteasome regulators as an explanation for the increased activity in aged L7M1 retinas.
Activity for standard and immunoproteasome
Another potential explanation for the age-related increase in L7M1 activity is differences in catalytic subunit composition. At 24 months, WT retinas contain more immunoproteasome and L7M1 retinas contain mainly the standard proteasome. Importantly, proteasome total content is not different between groups. Previous work has shown that the presence of the immunoproteasome subunits in the 20S core can significantly alter peptide hydrolysis (Boes et al., 1994; Dahlmann et al., 2000; Ehring et al., 1996; Nelson et al., 2000). However, the data is conflicting since both increases and decreases in chymotrypsin- and trypsin-like activity have been attributed to the immunoproteasome due to potential artifacts introduced by cytokine treatment or by comparing proteasomes from different tissues (Boes et al., 1994; Driscoll et al., 1993; Eleuteri et al., 1997; Gaczynska et al., 1994).
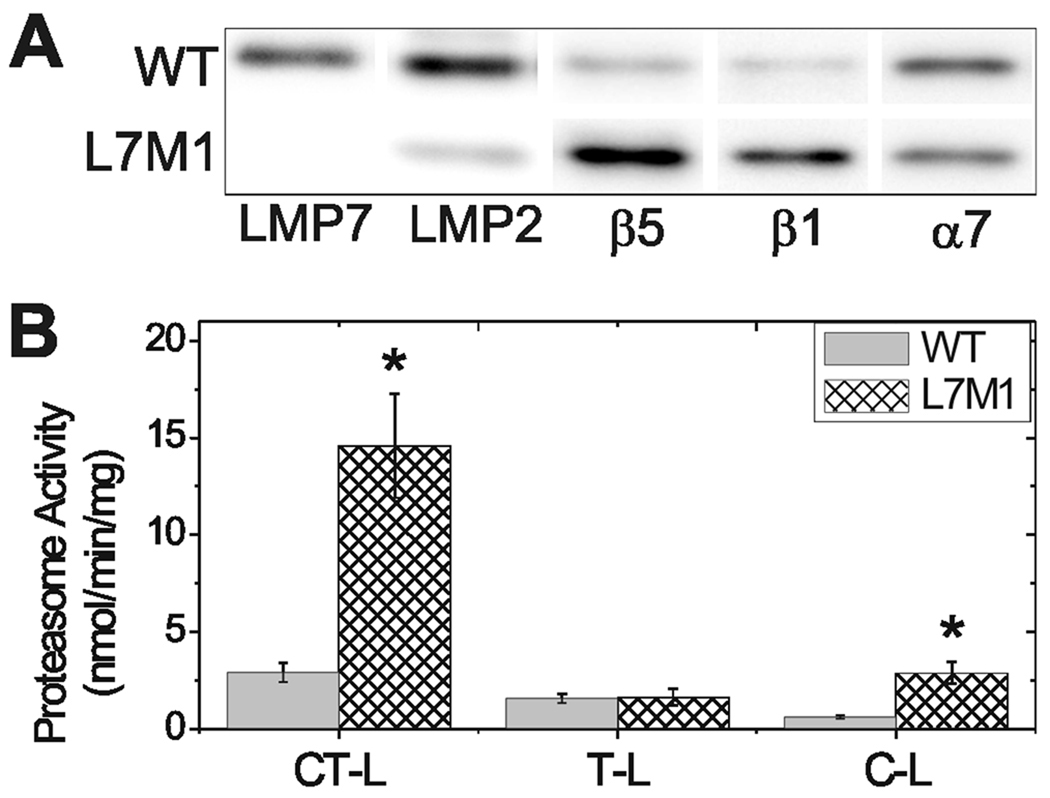
To eliminate potential confounding variables due to treatment or tissue-specific regulators, we used proteasome purified from the spleen of WT and L7M1 mice, which contain predominately immunoproteasome and standard proteasome, respectively (Fig. 3A). This experimental system allowed us to test whether the proteasome subunit composition could explain the differences in proteasome activity with age. Since both types of proteasome were isolated from spleen, variations in proteasome activity should depend primarily on the proteasome species.
Figure 3.
Catalytic activity of standard and immunoproteasome. (A) Representative Western blot of purified 20S proteasome from WT or L7M1 spleen. (B) Catalytic activity of purified 20S spleen proteasome from WT (solid) or L7M1 (cross-hatched) spleen using fluorogenic peptide substrates to test chymotrypsin-like (CT-L), trypsin-like (T-L), and caspase-like (C-L) activities. Two-sample T-tests were performed; *, indicates significance at p<0.05. All values are mean ± SEM, n=4 per group.
Standard proteasome from L7M1 mice showed higher chymotrypsin-like and caspase-like activities (Fig. 3B). Trypsin-like activity was not different between standard and immunoproteasome. Experiments performed with L7M1 and WT spleen homogenates produced essentially identical results (Fig. S4). Based on these data, we would predict an age-dependent decrease in activity for both L7M1 and WT mice since the β1 standard subunit is significantly decreased at 24 months. Additionally, the elevated content of immunoproteasome, which exhibits lower activity, in aged WT retinas also suggests activity should be decreased with aging. The discrepancy in our experimental data comparing spleen and retina suggests that factors other than subunit composition are involved in regulating proteasome activity in the retina with aging.
Proteasome response to oxidative stress in the RPE
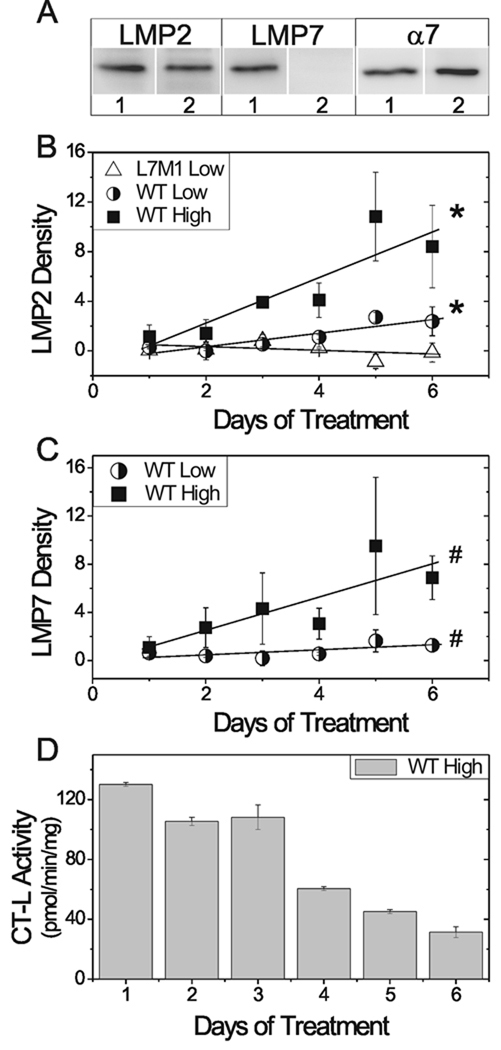
One of the prevalent factors associated with aging is increased oxidative stress, which has been shown to alter proteasome subunit composition and activity (Ding et al., 2003). To test the effect of chronic oxidative stress, we used cultured RPE cells derived from WT and L7M1 retinas. Prior to exposure to peroxide, both cell lines of cultured RPE contained approximately equivalent amounts of total proteasome (α7) and LMP2 (Fig. 4A). As expected, cells from L7M1 mice contain no LMP7. Following exposure to a daily, dose of peroxide, WT RPE showed a dose- and time-dependent increase in LMP2 and LMP7 subunits (Fig. 4B,C). In contrast, no change in LMP2 content was observed in L7M1 cells using the low dose conditions (Fig. 4B). Of note, the higher peroxide dose was not tolerated by the L7M1 cells.
Figure 4.
Immunoproteasome response to chronic oxidative stress in the RPE. (A) Representative Western blot of proteasome subunits in cultured WT (1) and L7M1 (2) RPE cells. Cultured RPE were treated daily with either a high (WT (■)) or low (WT (○) and L7M1 (Δ)) dose of hydrogen peroxide. (B) LMP2 and (C) LMP7 content was measured by Western blot following one to six days of chronic oxidative stress. The density of the control (no treatment) cells from each day was subtracted from the density of the hydrogen peroxide treated RPE. Subunit density was analyzed using linear regression. *, p<0.05. #, p<0.10. Values are mean ± SEM, n=2–4 separate experiments per group. (D) Chymotrypsin-like (CT-L) activity in WT cultured RPE treated with a high dose of hydrogen peroxide for one to six days. Activity was measured in triplicate (mean ± SD).
These results highlight two important points. First, they suggest chronic oxidative stress as a potential mechanism for induction of immunoproteasome subunits in the WT retina. Second, the absence of an oxidation-induced increase in LMP2 in RPE from L7M1 cells as well as in the retina from aged L7M1 mice (Fig. 1) suggest LMP2 upregulation during chronic stress requires expression of the full complement of immunoproteasome subunits. Our results show that under conditions of minimal stress (i.e., in 2 month old mouse retina or untreated cells), a basal level of immunoproteasome is present. However, cells deficient in one or two immunoproteasome subunits are unable to respond to stress (i.e., aging or low-level oxidation) by upregulating LMP2. These in vivo and in vitro results provide corroborative evidence for a cooperative mechanism of co-incorporating immunoproteasome subunits into nascent 20S core particles under conditions of stress in WT cells.
To determine how the oxidation-induced upregulation in immunoproteasome content affects proteasome function, activity was measured in WT RPE exposed to a daily dose of peroxide (Fig. 4D). We observed a time dependent decrease in chymotrypsin-like activity that correlated with the increased content of both LMP2 (r = −0.86, p=0.03) and LMP7 (r = −0.79, p=0.06). The lower chymotrypsin-like activity measured in WT RPE expressing elevated immunoproteasome agrees with results in spleen where immunoproteasome exhibited lower chymotrypsin-like activity compared with standard proteasome (Fig. 3). Taken together, these results suggest that factors other than subunit composition are involved in regulating proteasome activity in the retina, which is composed of a complex network of neurons and glia.
Consequences of immunoproteasome deficiency
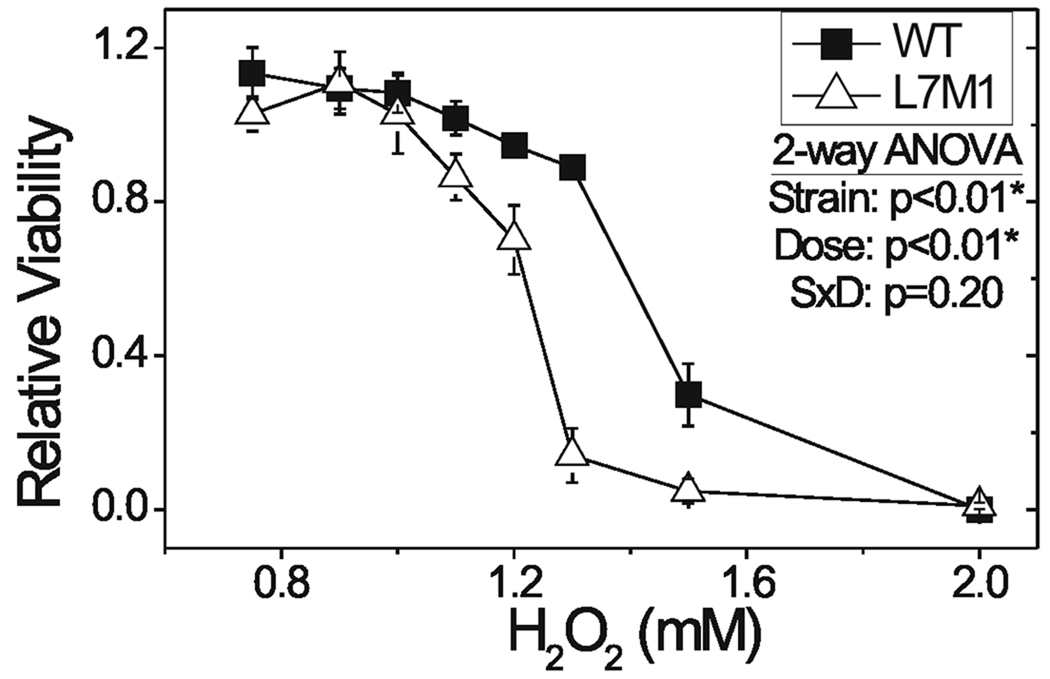
Immunoproteasome upregulation under conditions of chronic oxidative stress (i.e., aging and peroxide treatment) suggests immunoproteasome could provide protection from oxidative damage. To directly test this hypothesis, we compared the relative resistance of cultured RPE cell lines derived from WT and L7M1 mice to an oxidative challenge. Cell viability was measured 24 hours after exposure to varying doses of peroxide (Fig. 5). Exposure to peroxide caused a dose-dependent decrease in cell viability for both cell lines. However, the immunoproteasome-deficient cells were significantly more susceptible to oxidation-induced cell death as determined by the peroxide dose required to obtain a 50% reduction in cell viability (LD50). The mean LD50 was significantly lower in L7M1 1.26 ± 0.03 mM) compared to WT (1.44 ± 0.02 mM) RPE as determined from 5 separate experiments (p=0.002). These data suggest the absence of immunoproteasome makes cells more susceptible to oxidation-induced cell death and supports the hypothesis that immunoproteasome plays a role in protecting from oxidative damage.
Figure 5.
RPE cell viability after exposure to hydrogen peroxide. Cultured WT (■) and L7M1 (Δ) RPE cells were exposed to hydrogen peroxide at the indicated doses and cell viability was measured 24 hours later. Two-way ANOVA results showed lower cell viability in L7M1 compared with WT RPE (p=0.002). All values are mean ± SEM. WT n=5 and L7M1 n=4 separate experiments each with eight replicates.
DISCUSSION
Summary
This study investigated the effect of chronic stress, i.e., aging or exposure to peroxide, on the proteasome in WT and immunoproteasome-deficient retinas and cultured RPE. We showed that aging and oxidative stress upregulates immunoproteasome in the retina and RPE from WT mice. LMP2 was not upregulated in mice lacking MECL-1 and/or LMP7, suggesting that the full complement of immunoproteasome subunits is required to achieve maximal upregulation in response to stress. Furthermore, we showed that RPE cells deficient in immunoproteasome were more susceptible to peroxide-induced cell death. These results provide important novel information about proteasome biology and suggest immunoproteasome may play an essential role in protecting from oxidative stress in the immune-privileged retina.
Plasticity of proteasome subunit composition: response to chronic stress
In the current work, we showed that total proteasome (α7) content was not altered with aging (Fig. 1). However, aged WT mice exhibited increased levels of LMP2 and LMP7, suggesting transformation of a portion of the population from standard to immunoproteasome. This altered proteasome composition could be induced by age-related changes in the retinal environment, such as increased oxidative stress (Li et al., 2003; Louie et al., 2002) and inflammation (Chan-Ling et al., 2007; Xu et al., 2009). Consistent with this idea, induction of immunoproteasome was demonstrated in cultured RPE (Fig. 4) and neurons (Ding et al., 2003) following chronic oxidative stress. Additionally, induction of immunoproteasome subunits by cytokines is a well-established response in cultured immune cells (Akiyama et al., 1994), neurons (Díaz-Hernández et al., 2003), and the RPE (Gregerson et al., 2006). It is also possible that other signals, present only in the aged retina, are stimulating immunoproteasome expression since the promoter regions for lmp2, lmp7, mecl-1 contain putative consensus sequences for multiple transcription factors that are not associated with cytokine-induced stimulation (Hayashi et al., 1997; James et al., 2006; Zanelli et al., 1993; Zhou et al., 1993).
Altered subunit plasticity in KO mice under conditions of stress
In the current study, we found no difference in LMP2 incorporated into the 20S core under conditions of minimal stress, i.e., from retinas of 2 month-old WT, L7, and L7M1 mice (Fig. 1), or in cultured cells derived from WT and L7M1 mice prior to exposure to peroxide (Fig. 4). Additionally, LMP2 was observed in spleen proteasome from L7M1 mice, albeit at lower levels than found in WT spleen (Fig. 3). These results are consistent with reports from lymphoblastoids isolated from L7 and L7M1 mice where a slight decrease in LMP2 incorporation was observed (De et al., 2003). These data support the idea that even in the absence of MECL-1 and/or LMP7, a basal level of LMP2 can be incorporated into the 20S core.
Both the chronic stress of aging and oxidant exposure resulted in increased LMP2 incorporation in either retinas or cultured RPE from WT mice (Fig. 1,4). This response to stress was not replicated in the immunoproteasome-deficient tissue, suggesting that the full complement of immunoproteasome subunits is required to obtain the maximum induction of immunoproteasome in response to stress. These results support an important role for LMP7 in the incorporation of LMP2 into the 20S core under conditions of stress (De et al., 2003).
Activity of the standard and immunoproteasome
Significantly higher proteasome activity was observed in aged L7M1 retina containing nearly all standard proteasome as compared with aged WT retinas containing elevated levels of immunoproteasome. Importantly, an age-related decrease in the β1 standard subunit was observed in both WT and L7M1 retinas. We investigated whether difference in proteasome subunit composition could explain the strain-dependent variation in activity since proteasome subunit composition can significantly influence its catalytic activity (Boes et al., 1994; Dahlmann et al., 2000; Ehring et al., 1996; Nelson et al., 2000). However, the literature is wrought with conflicting results when comparing activities attributed to standard versus immunoproteasome in different tissues. This may be due to cell-specific differences in endogenous regulators. An additional complication is that peptide hydrolysis does not distinguish between proteasome subtypes. This is especially problematic in tissues containing a mixture of both standard and immunoproteasomes.
To obtain an unambiguous measure of activity for proteasome subtypes, we compared peptide hydrolysis from the 20S isolated from spleen from WT and L7M1 mice. These preparations contained nearly pure populations of either the immunoproteasome or standard proteasome (Fig. 3). Our results show that standard proteasomes have higher chymotrypsin- and caspase-like activities compared with immunoproteasome, but trypsin-like activity was not different between groups. Proteasome activity measured in RPE cells under chronic oxidative stress corroborates these results. We observed a decreased chymotrypsin-like activity correlated with immunoproteasome induction (Fig. 4). Our results are consistent with reports where activity in KO mice was compared in tissues (either spleen or virus infected liver) containing mainly the immunoproteasome (Basler et al., 2006; Stohwasser et al., 1996; Van Kaer et al., 1994). Taken together, these data unequivocally support the idea that the standard proteasome has increased peptide hydrolysis for the β1 and β5 subunits. However, these data also indicate that the strain-dependent difference in WT and L7M1 retina is due to factors other than subunit composition such as the presence of multiple cell types or endogenous regulators.
Potential alternative roles for immunoproteasome
We have shown that retinal cells respond to injury (Ferrington et al., 2008), disease (Ethen et al., 2007), oxidative stress (Fig. 4), and the chronic stress of aging (Fig. 1) by upregulating the immunoproteasome. We have also shown that cells deficient in immunoproteasome are more susceptible to peroxide-induced cell death (Fig. 5). These results imply a previously unrecognized role for immunoproteasome as a key component of the retinal stress response. A recent publication documenting a role for LMP2 in the cardioprotection afforded by ischemic preconditioning also supports the link between the immunoproteasome and the cell’s response to stress (Cai et al., 2008). These experiments, showing that ischemic preconditioning-induced degradation of phosphatase and tensin homologue deleted on chromosome 10 (PTEN) and the downstream activation of the kinase Akt is disrupted in LMP2−/− hearts, suggests immunoproteasome could participate in signaling that is critical for cell survival.
A putative role for LMP2 in NFκB activation, one of the primary stress response mechanisms, has also been suggested. In cell lines lacking LMP2, defects in proteolytic processing of NFκB precursors and IκB inhibitory protein were observed (Hayashi and Faustmann, 1999, 2001). Moreover, a recent report of enhanced processing of NFκB precursors and IκBα by immunoproteasome isolated from inflamed, diseased intestines provide corroborative evidence supporting a role for immunoproteasome in NFκB signaling (Visekruna et al., 2006). While a strict requirement for immunoproteasome in regulating the NFκB pathway is unlikely since immunoproteasome deletion is not lethal, it is possible that immunoproteasome’s action may influence the kinetics or termination of signals that are key for cell survival under stress.
Since standard and immunoproteasomes generate different peptides (Dahlmann et al., 2000), it is possible that some peptides uniquely produced by the immunoproteasome could be biologically active and regulate cell signaling (Yewdell, 2005). Upregulation of immunoproteasome under conditions of stress alters the spectrum of peptides within the cell, which could ultimately influence the cell survival. Peptides generated by immunoproteasome in immune-privileged tissue, such as the retina, are more than likely not produced for antigen presentation. They may instead be biologically active peptides that play a key role in retinal survival.
Summary and Conclusions
Data from this study showed that aging and chronic oxidative stress upregulates immunoproteasome in the retina and cultured RPE from WT mice. We also reported that the cells deficient in immunoproteasome were more susceptible to oxidation-induced cell death. These results suggest a previously unrecognized role for the immunoproteasome in protecting from oxidation-induced damage and provide key insight into novel aspects of proteasome biology. Defining the consequences associated with the inability to upregulate immunoproteasome under conditions of stress is an important first step in identifying alternative roles for immunoproteasome in the retina.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institutes of Health EY013623, AG032391 (DAF), T32-AG029796 (SH), an unrestricted grant from the Research to Prevent Blindness, and the Minnesota Lions Clubs.
Abbreviations
- AMC
7-amino-4-methylcoumarin
- ARBP
acidic ribosomal phosphoprotein P0
- BCA
bicinchoninic acid
- CHAPS
3-[(3-cholamidopropyl)dimethylamino]-1-propanesulfonate
- DTT
dithiothreitol
- KO
knock-out L7, lmp7−/−
- L7M1
lmp7−/−mecl-1−/−; mo., month
- PVDF
polyvinylidene difluoride
- RPE
retinal pigment epithelium
- WT
wild-type
References
- Akiyama K, Yokota K, Kagawa S, Simbara N, Tamura T, Akioka H, Nothwang HG, Noda C, Tanaka K, Ichihara A. cDNA cloning and interferon gamma down-regulation of proteasomal subunits X and Y. Science. 1994;265:1231–1234. doi: 10.1126/science.8066462. [DOI] [PubMed] [Google Scholar]
- Basler M, Moebius J, Elenich L, Groettrup M, Monaco JJ. An altered T cell repertoire in MECL-1-deficient mice. J. Immunol. 2006;176:6665–6672. doi: 10.4049/jimmunol.176.11.6665. [DOI] [PubMed] [Google Scholar]
- Boes B, Hengel H, Ruppert T, Multhaup G, Koszinowski UH, Kloetzel PM. Interferon gamma stimulation modulates the proteolytic activity and cleavage site preference of 20S mouse protesomes. J. Exp. Med. 1994;179:901–909. doi: 10.1084/jem.179.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai ZP, Shen Z, Van Kaer L, Becker LC. Ischemic preconditioning-induced cardioprotection is lost in mice with immunoproteasome subunit low molecular mass polypeptide-2 deficiency. FASEB J. 2008;22:4248–4257. doi: 10.1096/fj.08-105940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caudill CM, Jayarapu K, Elenich L, Monaco JJ, Colbert RA, Griffin TA. T cells lacking immunoproteasome subunits MECL-1 and LMP7 hyperproliferate in response to polyclonal mitogens. J. Immunol. 2006;176:4075–4082. doi: 10.4049/jimmunol.176.7.4075. [DOI] [PubMed] [Google Scholar]
- Chan-Ling T, Hughes S, Baxter L, Rosinova E, McGregor I, Morcos Y, van Nieuwenhuyzen P, Hu P. Inflammation and breakdown of the blood-retina barrier during “physiological aging” in the rat retina: a model for CNS aging. Microcirculation. 2007;14:63–76. doi: 10.1080/10739680601073451. [DOI] [PubMed] [Google Scholar]
- Dahlmann B, Ruppert T, Kuehn L, Merforth S, Kloetzel PM. Different proteasome subtypes in a single tissue exhibit different enzymatic properties. J. Mol. Biol. 2000;303:643–653. doi: 10.1006/jmbi.2000.4185. [DOI] [PubMed] [Google Scholar]
- De M, Jayarapu K, Elenich L, Monaco JJ, Colbert RA, Griffin TA. β2 subunit propeptides influence cooperative proteasome assembly. J. Biol. Chem. 2003;278:6153–6159. doi: 10.1074/jbc.M209292200. [DOI] [PubMed] [Google Scholar]
- Díaz-Hernández M, Hernández F, Martín-Aparicio E, Gómez-Ramos P, Morán MA, Castaño JG, Ferrer I, Avila J, Lucas JL. Neuronal induction of immunoproteasome in Huntington’s disease. J. Neurosci. 2003;23:11653–11661. doi: 10.1523/JNEUROSCI.23-37-11653.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Q, Reinacker K, Dimayuga E, Nukala V, Drake J, Butterfield DA, Dunn JC, Martin S, Bruce-Keller AJ, Keller JN. Role of the proteasome in protein oxidation and neural viability following low-level oxidative stress. FEBS Lett. 2003;546:228–232. doi: 10.1016/s0014-5793(03)00582-9. [DOI] [PubMed] [Google Scholar]
- Driscoll J, Brown MG, Finley D, Monaco JJ. MHC-linked LMP gene products specifically alter peptidase activities of the proteasome. Nature. 1993;365:262–264. doi: 10.1038/365262a0. [DOI] [PubMed] [Google Scholar]
- Dubiel W, Pratt G, Ferrell K, Rechsteiner M. Purification of an 11 S regulator of the multicatalytic protease. J. Biol. Chem. 1992;267:22369–22377. [PubMed] [Google Scholar]
- Ehring B, Meyer TH, Eckerskorn C, Lottspeich F, Tampe R. Effects of major-histocompatibility-complex-encoded subunits on the peptidase and proteolytic activities of human 20S proteasomes: Cleavage of proteins and antigenic peptides. Eur. J. Biochem. 1996;235:404–415. doi: 10.1111/j.1432-1033.1996.00404.x. [DOI] [PubMed] [Google Scholar]
- Eleuteri AM, Kohanski RA, Cardozo C, Orlowski M. Bovine spleen multicatalytic proteinase complex (proteasome) J. Biol. Chem. 1997;272:11824–11831. doi: 10.1074/jbc.272.18.11824. [DOI] [PubMed] [Google Scholar]
- Ethen CM, Hussong SA, Reilly C, Feng X, Olsen TW, Ferrington DA. Transformation of the proteasome with age-related macular degeneration. FEBS Lett. 2007;581:885–890. doi: 10.1016/j.febslet.2007.01.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehling HJ, Swat W, Laplace C, Kuhn R, Rajewsky K, Muller U, von Boehmer H. MHC class I expression in mice lacking the proteasome subunit LMP7. Science. 1994;265:1234–1237. doi: 10.1126/science.8066463. [DOI] [PubMed] [Google Scholar]
- Ferrington DA, Husom AD, Thompson LV. Altered proteasome structure, function, and oxidation in aged muscle. FASEB J. 2005;19:644–646. doi: 10.1096/fj.04-2578fje. [DOI] [PubMed] [Google Scholar]
- Ferrington DA, Tran TN, Lew KL, Van Remmen H, Gregerson DS. Different death stimuli evoke apoptosis via multiple pathways in retinal pigment epithelial cells. Exp. Eye Res. 2006;83:638–650. doi: 10.1016/j.exer.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Ferrington DA, Hussong SA, Roehrich H, Kapphahn RJ, Kavanaugh SM, Heuss ND, Gregerson DS. Immunoproteasome responds to injury in the retina and brain. J. Neurochem. 2008;106:158–169. doi: 10.1111/j.1471-4159.2008.05345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaczynska M, Rock KL, Spies T, Goldberg AL. Peptidase activities of proteasomes are differentially regulated by the major histocompatibility complex-encoded genes for LMP2 and LMP7. Proc. Natl. Acad. Sci. USA. 1994;91:9213–9217. doi: 10.1073/pnas.91.20.9213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavilán MP, Castaño A, Torres M, Portavella M, Caballero C, Jiménez S, García-Martínez A, Parrado J, Vitorica J, Ruano D. Age-related increase in the immunoproteasome content in rat hippocampus: Molecular and functional aspects. J. Neurochem. 2009;108:260–272. doi: 10.1111/j.1471-4159.2008.05762.x. [DOI] [PubMed] [Google Scholar]
- Goldberg AL, Cascio P, Saric T, Rock KL. The importance of the proteasome and subsequent proteolytic steps in the generation of antigenic peptides. Mol. Immunol. 2002;39:147–164. doi: 10.1016/s0161-5890(02)00098-6. [DOI] [PubMed] [Google Scholar]
- Gregerson DS, Lew KL, McPherson SW, Heuss ND, Ferrington DA. RPE cells resist bystander killing by CTLs, but are highly susceptible to antigen-dependent CTL killing. Invest. Ophthalmol. Vis. Sci. 2006;47:5385–5394. doi: 10.1167/iovs.06-0636. [DOI] [PubMed] [Google Scholar]
- Hayashi M, Ishibashi T, Tanaka K, Kasahara M. The mouse genes encoding the third pair of β-type proteasome subunits regulated reciprocally by IFN-γ. J. Immunol. 1997;159:2760–2770. [PubMed] [Google Scholar]
- Hayashi T, Faustmann D. NOD mice are defective in proteasome production and activation of NF-κB. Mol. Cell Biol. 1999;19:8646–8659. doi: 10.1128/mcb.19.12.8646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Faustmann D. Selected contribution: Association of gender-related LMP2 inactivation with autoimmune pathogenesis. J. Appl. Physiol. 2001;91:2804–2815. doi: 10.1152/jappl.2001.91.6.2804. [DOI] [PubMed] [Google Scholar]
- Husom AD, Peters EA, Kolling EA, Fugere NA, Thompson LV, Ferrington DA. Altered proteasome function and subunit composition in aged muscle. Arch. Biochem. Biophys. 2004;421:67–76. doi: 10.1016/j.abb.2003.10.010. [DOI] [PubMed] [Google Scholar]
- James AB, Conway AM, Morris BJ. Regulation of the neuronal proteasome by Zif268 (Egr1) J. Neurosci. 2006;26:1624–1634. doi: 10.1523/JNEUROSCI.4199-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapphahn RJ, Giwa BM, Berg KM, Roehrich H, Feng X, Olsen TW, Ferrington DA. Retinal proteins modified by 4-hydroxynonenal: Identification of molecular targets. Exp. Eye Res. 2006;83:165–175. doi: 10.1016/j.exer.2005.11.017. [DOI] [PubMed] [Google Scholar]
- Kapphahn RJ, Bigelow EJ, Ferrington DA. Age-dependent inhibition of proteasome chymotrypsin-like activity in the retina. Exp. Eye Res. 2007;84:646–654. doi: 10.1016/j.exer.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klare N, Seeger M, Janek K, Jungblut PR, Dahlmann B. Intermediate 20 S proteasomes in HeLa cells: “Asymmetric” subunit composition, diversity and adaptation. J. Mol. Biol. 2007;373:1–10. doi: 10.1016/j.jmb.2007.07.038. [DOI] [PubMed] [Google Scholar]
- Li D, Sun F, Wang K. Caloric restriction retards age-related changes in rat retina. Biochem. Biophys. Res. Commun. 2003;309:457–463. doi: 10.1016/j.bbrc.2003.08.020. [DOI] [PubMed] [Google Scholar]
- Louie JL, Kapphahn RJ, Ferrington DA. Proteasome function and protein oxidation in the aged retina. Exp. Eye Res. 2002;75:271–284. [PubMed] [Google Scholar]
- Mishto M, Bellavista E, Santoro A, et al. Immunoproteasome and LMP2 polymorphism in aged and Alzheimer’s disease brains. Neurobiol. Aging. 2006;27:54–66. doi: 10.1016/j.neurobiolaging.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Nelson JE, Altschuller-Felberg C, Loukissa A, Cardozo C. Proteasome from cytokine-treated human cells shows stimulated BrAAP activity and depressed PGPH activity. Biochem. Cell Biol. 2000;78:115–118. [PubMed] [Google Scholar]
- Rock KL, Gramm C, Rothstein L, Clark K, Stein R, Dick L, Hwang D, Goldberg AL. Inhibitors of the proteasome block the degradation of most cell proteins and the generation of peptides presented on MHC class I molecules. Cell. 1994;78:761–771. doi: 10.1016/s0092-8674(94)90462-6. [DOI] [PubMed] [Google Scholar]
- Stohwasser R, Kuckelkorn U, Kraft R, Kostka S, Kloetzel P-M. 20S proteasome from LMP7 knock out mice reveals altered proteolytic activities and cleavage site preferences. FEBS Lett. 1996;383:109–113. doi: 10.1016/0014-5793(96)00110-x. [DOI] [PubMed] [Google Scholar]
- Van Kaer L, Ashton-Rickardt PG, Eichelberger M, Gaczynska M, Nagashima K, Rock KL, Goldberg AL, Doherty PC, Tonegawa S. Altered peptidase and viral-specific T cell response in LMP2 mutant mice. Immunity. 1994;1:533–541. doi: 10.1016/1074-7613(94)90043-4. [DOI] [PubMed] [Google Scholar]
- Visekruna A, Joeris T, Seidel D, Kroesen A, Loddenkemper C, Zeitz M, Kaufmann SH, Schmidt-Ullrich R, Steinhoff U. Proteasome-mediated degradation of IkappaBalpha and processing of p105 in Crohn disease and ulcerative colitis. J. Clin. Invest. 2006;116:3195–3203. doi: 10.1172/JCI28804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Chen M, Forrester JV. Para-inflammation in the aging retina. Prog. Retin. Eye Res. 2009;28:348–368. doi: 10.1016/j.preteyeres.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Yewdell JW. Immunoproteasomes: regulating the regulator. Proc. Natl. Acad. Sci. USA. 2005;102:9089–9090. doi: 10.1073/pnas.0504018102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanelli E, Zhou P, Cao H, Smart MK, David CS. Genomic organization and tissue expression of the mouse proteasome gene Lmp-7. Immunogenetics. 1993;38:400–407. doi: 10.1007/BF00184520. [DOI] [PubMed] [Google Scholar]
- Zhou P, Zanelli E, Smart M, David C. Genomic organization and tissue expression of mouse proteasome gene Lmp-2. Genomics. 1993;16:664–668. doi: 10.1006/geno.1993.1245. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.