A substantial body of research has documented that various developmental characteristics early in childhood increase the risk of subsequent depression (e.g., Kessler et al., 1997; Reinherz et al., 1993; Reinherz et al., 1999). Perinatal complications, neurodevelopment problems, and atypical temperament represent one broad category of risk variables that may mirror physiological vulnerabilities to later psychological disorders. Perinatal problems have been defined as including preterm birth, delayed labor, atypical birth weight, caesarian section, and special care after birth (e.g., Allen et al., 1998). Neurodevelopmental difficulties have been operationalized as delayed standing, walking, speaking (e.g., van Os et al., 1997), and early difficult temperament as regulatory problems including persistent crying and atypical sleeping and/or feeding patterns (e.g., Keenen et al., 1998).
Focusing mostly on perinatal variables, studies of depressed adults have reported that mood disordered patients have higher rates of one or more obstetric complications than do various comparison groups (e.g., Guth et al., 1993; Preti et al., 2000). For example, according to Preti et al., (2000), adult patients with histories of mood disorder had significantly lower birth weight (for their gestational ages) than did matched normal controls. Guth et al. (1993) found that obstetric complications were more common among cases with early-onset mood disorder than among those with late-onset. And Vocisano et al., (1996) reported that inpatients with prolonged, severe, and functionally impairing MDD had higher frequencies of birth related problems and physical disorder in infancy than did less severely depressed outpatients.
Several studies with broader (e.g., community-based, birth cohort) sampling bases also have found relations between atypical early developmental features and affective psychopathology. For example, in a birth cohort study, Gale and Martyn (2004) found that low birth weight was associated with self-reported depressive symptoms in women during adulthood. Adults with childhood-onset affective disorders have been found to attain motor milestones later, score higher on perinatal insults and lower on gross motor skills, and be rated as more difficult babies compared to individuals with adult-onset depression (Jaffee et al., 2002; van Os et al., 1997). Early difficult temperament also has been associated with subsequent internalizing symptoms and disorders in childhood (e.g., Keenen et al., 1998; Maziade et al., 1989).
However, the literature on the relations of atypical development and mood disorder is far from consistent, given also reports of negative findings (e.g., Allen et al., 1998; Buka et al., 1993; Najman et al., 2005), findings of associations between perinatal problems and anxiety but not mood disorders (e.g., Allen et al., 1998; Cohen et al., 1989), and findings of positive relationships but lack of diagnostic specificity (e.g., Hirshfeld-Becker et al., 2004). Inconsistencies in the literature is not surprising, given not only sampling differences, but the various ways in which studies have defined depression (i.e., clinical diagnoses, operational criteria, self-rated scales), ascertained early developmental problems (e.g., retrospective reports of adults, pediatric records, contemporaneous ratings), and quantified key variables (e.g., event counts, severity scales).
Whereas the bulk of evidence appears to indicate some association between features of atypical early development and psychopathology, the clinician seeking to translate such findings into practical terms is faced with two questions: a) do atypical developmental features “matter” if the patient already developed mood disorder, and if so then how? and b) are the effects, if any, specific to depressive disorder? In the present study of a large sample of clinically depressed children, we sought to address these two questions. Given that age of onset and illness severity convey important clinical information and also can influence treatment decisions, we defined them as our dependent variables and examined their relations to indices of atypical neurodevelopment (during the first several years of life) as reported by mothers. Additionally, given that MDD often presents with comorbid dysthymic and anxiety disorders, and that the latter comorbidities generally emerge earlier than does MDD (e.g., Axelson and Birmaher, 2001; Kovacs et al., 1989), we examined whether our independent variables were related to these diagnoses as well. We used multiple informants and sources of information (including medical and related records) to date onset of disorders.
More specifically, we hypothesized that perinatal problems, developmental delay, and difficult infant temperament would render children vulnerable to earlier onset and more severe episodes of major depressive disorder. We also posited that the effects of risk factors may not be specific to MDD-onset, but would also relate to onset age of the first internalizing disorder (i.e., the age at which the first episode of MDD or comorbid dysthymia or anxiety disorder began). In line with suggestions that early risk factors should be studied in models that examine multiple and interactive effects (e.g., Goodman, 2002), we also investigated factors that could moderate associations between early childhood characteristics and our dependent variables. Because neonatal health or motor skill problems have been found to relate to depression or anxiety for boys but not for girls (Reinherz et al., 1999; Sigurdsson et al., 2002), we tested whether child’s sex serves as a moderator. Given some indications that marital partner changes early during a child’s life may be one factor in child depression (Najman et al., 2005), we predicted that having a stable, intact, two-parent family early on may act as a protective factor and attenuate the negative impact of atypical childhood characteristics. Finally, we considered as covariates: a) mother’s age at birth of the child, given indications that relatively younger (Jaffee et al., 2001) and older maternal ages are associated with increased rates of complications for offspring (van Katwijk and Peeters, 1998), and b) mother’s educational level, as a proxy for socioeconomic status.
METHOD
Participants
In the present article we report on 371 children (168 girls), aged 11.7 years on average (SD = 2.0 years, range: 7.3 - 14.9 years), who were enrolled by December 31, 2003 in a study of genetic and psychosocial risk factors in childhood-onset depression, had biological mothers as parental informants, and met diagnostic criteria for MDD (detailed below). Racial composition was 95.1% Caucasian, 0.3% African, 1.9% multiracial, and 2.7% Roma or other minorities, representative of the population of Hungary. The subjects in this article partially overlap with those in other papers that address different facets of children’s depressive illness (e.g., Liu et al., in press). A subset of children had comorbid disorders in addition to MDD (e.g., 34.5% had an anxiety disorder, 3.5% with conduct disorder; 6.2% with oppositional defiant disorder; and 15.6% with Attention Deficit/Hyperactivity Disorder).
At study entry, mothers’ ages ranged from 26 to 57 years, with a mean of 36.5 years (SD = 5.1). Mothers’ ages at their children’s birth ranged from 16 to 46 years (M = 25.3, SD = 5.1). The majority (88.7%) were 19 to 34 years old at child’s birth (the lowest 5.7% between 16-18 years, and the highest 5.7% between 35 - 46 years of age). Mothers’ years of education ranged from 6 to 21 years (M = 11.6 years, SD = 2.8). Most children (90.6%) lived in intact families of origin from birth to 4 years of age.
Enrollment and Assessment Procedures
Children were recruited through 23 child psychiatric facilities (7 of which had both inpatient and outpatient units) across Hungary, serving both urban and rural areas. Based on information available for most of our sites for the year 2004, we estimate that they provided services to at least 80% of the newly registered child psychiatry cases, giving us access to a significant portion of the referred population nationwide. Children presenting at each site were scheduled for a research assessment if they met the following criteria: 7.0 years to 14.9 years old, not mentally retarded, no evidence of major systemic medical disorder, had available at least one biologic parent and a 7 – 17.9 year-old sibling (required by the study’s genetic component), and attained a predetermined cut-off score on one of various depressive symptom screens (e.g., the short version of the Children’s Depressive Inventory; Kovacs & MHS Staff, 2003; selected items from the Child Behavior Checklist, Achenbach, 1991). (Siblings are not included in this article.) Children meeting these initial criteria were scheduled for a 2-part evaluation, conducted on 2 separate occasions, about 6 weeks apart, by different clinicians. We obtained written consent for participation signed by both parents and the child, in accordance with the legal requirements in Hungary and the University of Pittsburgh, Pittsburgh, USA.
The first part of the evaluation entailed administration of the “Mood Disorder Module” of a diagnostic interview (described below), as well as the Intake General Information Sheet (IGIS), a comprehensive demographic and anamnestic data form. Participants also completed self-rated scales (not included in the present report). The IGIS is an event-focused structured interview with pre-coded item response choices covering, among others, demographic and family variables, as well as developmental, physical health, and psychosocial history, with the parent serving as informant. To set the proper framework and facilitate recall, evaluations started with a semistructured interview, designed to construct a “time line” for the patient from birth to the date of the assessment. The time-line anchors included major “public” events with the corresponding dates (e.g., Christmas, start of a school year) and personally relevant events (e.g., birth of a sibling, both positive and negative familial events, variables reflecting on adjustment). The time-line (“chronograph”) served to identify the times when the child’s symptoms became problematic and to date disorder onsets and offsets.
The second part of the evaluation involved the full diagnostic interview and the completion of additional self-rated scales, but was administered only if the child proband had met DSM criteria for mood disorder at the first evaluation. (If DSM criteria were not met, the child was assigned an “at-risk” status and entered a follow-up arm of the study). For our diagnostic interview, we used the Interview Schedule for Children and Adolescents - Diagnostic Version (ISCA-D), which is an extension of the Interview Schedule for Children and Adolescents (ISCA) (Sherrill and Kovacs, 2000). The interview, which covers the relevant Axis-I DSM-IV as well as some DSM-III disorders, was conducted by the same interviewer separately with the parent about the child, and the child about him/herself, yielding symptom ratings and diagnoses for “current” as well as “lifetime” disorders. Results of both the first and second parts of the assessments and associated documentation (e.g., psychiatric records) were subjected to a consensus diagnostic procedure (Maziade et al., 1992). Pairs of senior child psychiatrist, trained as Best Estimate Diagnosticians (BEDs), separately reviewed all material, and then together derived consensus diagnoses. “Caseness,” as well as onset dates of disorders, was based on best-estimate consensus. As described in connection with previous work (Kovacs et al, 1984 a, b), operational rules were used to define disorder onset and recovery, and “midpoint” rules were used to date onsets and offsets, if more exact dating was not possible.
The interviews were administered by child psychiatrists and psychologists who completed 3 months of didactic and practical training in the semi-structured interview technique. They were required to reach an average of 85% symptom-agreement on 5 consecutive videotaped interviews against “gold standard” interview ratings provided by the trainers. Routine monitoring and follow-up training sessions served to minimize rater drift. All interviews were audiotaped. Interrater reliability on ISCA-D symptoms was satisfactory (using audiotapes of interviews for n = 46 pairs of raters). For MDD symptoms, kappas ranged from .64 to .88, with 80% of the coefficients at or above .70. For DD symptoms (using DSM-IV criteria), kappas ranged from .38 to .93, with 80% at or above .70. For Generalized Anxiety Disorder symptoms (the most common DSM-IV anxiety diagnosis), kappas ranged from .53 to 1.00, with 62.5% at or above .70. Similar inter-rater reliability coefficients were obtained for other ISCA-D disorders as well.
Independent and Dependent Variables
Early atypical neurodevelopmental characteristics
Using IGIS items that pertain to the child proband’s early development history from his/her birth to toddler age, we created three indices of atypical development: Perinatal Problems (4 items), Developmental Delay (2 items), and Difficult Temperament (3 items). The construct of temperament includes multiple dimensions tapping emotional, biological, and behavioral reactivity and regulation (Rothbart & Bates, 1998). Our temperament index included a global question on how difficult it was to comfort the infant (similar to the single-item question included as part of Jaffee et al.’s investigation [2002]), and because we were particularly interested in physiological vulnerability, two items measuring biological irregularity (similar to Thomas and Chess’s [1977] temperament category of rhythmicity). Each index or scale reflects the number of “yes” responses to the corresponding items. See Table 1 for the specific items and their endorsement rates in our sample.
Table 1.
Early neurodevelopmental characteristics of clinically referred depressed children (N=367-371)
| Item endorsement | Score descriptives | |||
|---|---|---|---|---|
| Variables | N | % of sample | Mean (SD) | Range |
| Perinatal problem index | 0.72 (.98) | 0 – 4 | ||
| Premature/late delivery | 47 | 12.7% | ||
| Complications during deliverya | 79 | 21.3% | ||
| Very large/small at birth | 70 | 18.9% | ||
| Special care after birthb | 73 | 19.7% | ||
| Difficult temperament index | 0.87 (.97) | 0 – 3 | ||
| Recurrent feeding problems | 88 | 23.9% | ||
| Recurrent/chronic sleeping problems | 118 | 31.9% | ||
| Usually/often hard to comfort/soothe | 119 | 32.2% | ||
| Developmental delay index | 0.20 (.48) | 0 – 2 | ||
| Late for age when began to walk without help | 30 | 8.1% | ||
| Late to start to speak in sentences | 46 | 12.4% | ||
E.g., excessive bleeding, “cord” around the neck, Rh incompatibility
E.g., placed in incubator, under special observation
Intact family status
An entire section of the IGIS is dedicated to enumerating parental caregivers for each year of the child’s life. Using these items, we created a dichotomous summary variable to reflect whether or not a child was continuously taken care of by both biological parents from birth until 4 years of age (intact versus non-intact family). Altogether 35 children (9.4% of the sample) experienced broken homes early on.
Onset age of MDD and first internalizing disorder (MDD, dysthymic, or anxiety disorder)
The mean MDD onset age for the sample was 10.51 (SD = 2.28), with a range from 3.80 to 14.84. Of the 371 children, 51 (13.7%) also had dysthymic disorder (DD), and 128 (34.5%) had anxiety disorders (Overanxious Disorder and Generalized Anxiety Disorder were the most common, with all the other anxiety disorders being represented.) For 90 of the cases with anxiety comorbidity, the anxiety disorder onset earlier than MDD or DD. For our sample, the mean onset age of the first internalizing disorder was 9.72 (SD = 2.60, range: 2.58 - 14.74).
MDD episode severity
This index was computed for the first episode of MDD (as recorded in the ISCA-D), based on 15 symptoms, each rated on a 3-point severity scale: 0 = not present; 1 = subthreshold; and 2 = threshold/clinical. If only one item was missing, that item was pro-rated. Because all children in the sample had MDD, and the minimum of 5 symptoms rated at the “clinical” level was required for the diagnosis, the possible range for the severity score was 10 to 30. The actual range was 10 to 29 (M = 19.72, SD = 3.68).
Statistical Analyses
We used survival analysis to examine the effects of variables on onset age of MDD or internalizing disorder. Survival analysis is useful with outcomes or events that depend on elapsed time, and can estimate how predictors may be associated with time to the event. Kaplan-Meier survival curves were generated for subgroups; log-rank tests were used to test statistical significance.
To test for relations between the predictors and the age at which children’s first episodes occurred, we first conducted univariate Cox regression analyses with each risk index and covariate. We report hazard ratios and 95% confidence intervals to indicate the risk of the outcome in any given unit of time, with one unit increase of the predictor. We also checked the proportional hazards assumption about time-dependence for each predictor variables. Second, in an initial multiple regression model, we included the three risk scales, as well as covariates with p ≤ .05 in the univariate Cox models. All hypothesis-driven interaction terms were also included. We then used a backward elimination method, removing each (starting with the one with the largest p-value), and retaining covariates or interaction terms in the final model with p ≤ .05. Thus, the final multivariate Cox regression models reflect the impact of independent variables and significant interaction terms, while adjusting for demographic factors (where p ≤ .05).
To model the effects of early risk factors on the severity of the children’s first depressive episode, we used GLM procedure. We examined the associations between the perinatal, developmental, and temperament problems, as well as how the interaction terms and covariates related to the severity of the MDD symptomatology.
RESULTS
The specific variables, which comprised the 3 indices of early neurodevelopmental characteristics, had various rates in our sample (Table 1); in general, developmental delays were least common (from about 8% to 12%) while features of difficult temperament were reported for about 24% to 32% of the cases. The 3 indices were unrelated to each other (r-values ranged from .02 to .06, p > .24), and were unrelated to mothers’ age at child’s birth, mothers’ education level, and whether the child was reared in an intact vs. non-intact family early in life. However, boys scored higher on developmental delays (M = .25, SD = .52) than did girls (M = .15, SD = .38), t (369) = -2.11, p < .05.
Modeling Onset Age of MDD
A series of univariate Cox Regression models yielded significant effects for child’s sex, early intact family status, and maternal age at child’s birth. (See Table 2 for Hazard ratios.) At the onset of their MDD, boys (M = 10.08; SD = 2.12 years) were 1 year younger than girls (M = 11.03; SD = 2.37 years). Children exposed to changes in caregivers before age four were younger at the onset of their MDD (M = 9.68; SD = 1.96 years) than those from intact families (M = 10.60; SD = 2.30 years), and children whose mothers were 35 years and older when they gave birth had earlier onset of MDD (M = 9.14; SD = 1.89) than children with mothers in the normative age group (M = 10.57; SD = 2.30).
Table 2.
Modeling Age of Onset of first MDD Episode (N=367)
| Univariate Models | Final Multivariate Model | |
|---|---|---|
| Variables | Hazard Ratio (95% CI) | Hazard Ratio (95% CI) |
| Perinatal problems | 0.98 (0.88, 1.08) | 0.96 (.86, 1.06) |
| Developmental delay | 0.96 (0.77, 1.19) | 0.87 (.69, 1.10) |
| Difficult temperament | 1.11 (0.99, 1.23)+ | 5.88 (2.05, 16.83)** |
| Sex (male = 1) | 1.68 (1.36, 2.08)*** | 1.75 (1.41, 2.17)*** |
| Intact family until age 4 | 0.64 (0.45, 0.91)* | 0.93 (0.58, 1.47) |
| Mother’s education (years) | 0.98 (0.94, 1.01) | -- |
| Maternal age at birth (years) | ||
| 16-18 vs. 19-34 | 0.94 (0.60, 1.46) | -- |
| 35-46 vs. 19-34 | 1.73 (1.10, 2.72)* | -- |
| Temperament X Intact Family | -- | 0.65 (.45, .92)* |
| Temperament X Time | -- | 0.58 (.37, .92)* |
Note. MDD = major depressive disorder; CI = confidence interval; Cox regression analyses were used.
p < .07.
p < .05.
p < .01.
p < .001.
However, in the final multivariate model, mother’s age at child’s birth became nonsignificant, and only one interaction term was retained. The results indicate that having a difficult temperament and being a boy were associated with earlier onset of MDD (Table 2). Furthermore, the main effect of temperament was qualified by its interaction with intact family status, and is illustrated by Kaplan-Meier survival curves (separately for intact vs. not-intact families).
For children in intact families (see Figure 1), temperament was unrelated to age at MDD onset (χ2 (3) = 3.95, p = .27). However, for non-intact families (see Figure 2), children with a more difficult temperament had an earlier MDD onset age than did children with fewer difficulties (χ2 (3) = 13.57, p < .01). Temperament also interacted with elapsed time, suggesting that its effect is not constant. Specifically, as indicated by the parameter estimate of the interaction term (-.540), the effect is attenuated across time. For example, comparing children at age 14 to children at age 7, the hazard ratio for temperament problems is decreased by exp{-.540*[log(14) − log (7)]} = 0.69.
Figure 1.
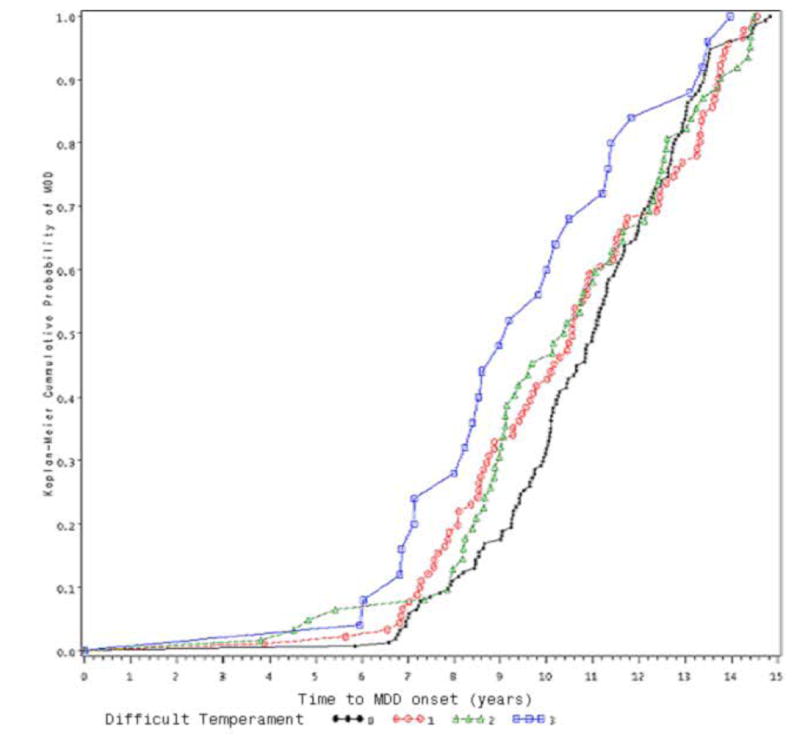
Effect of Early Temperament on MDD-onset among children from Intact Families
0: no temperament difficulty
1: minimal temperament difficulty
2: moderate temperament difficulty
3: severe temperament difficulty
Figure 2.
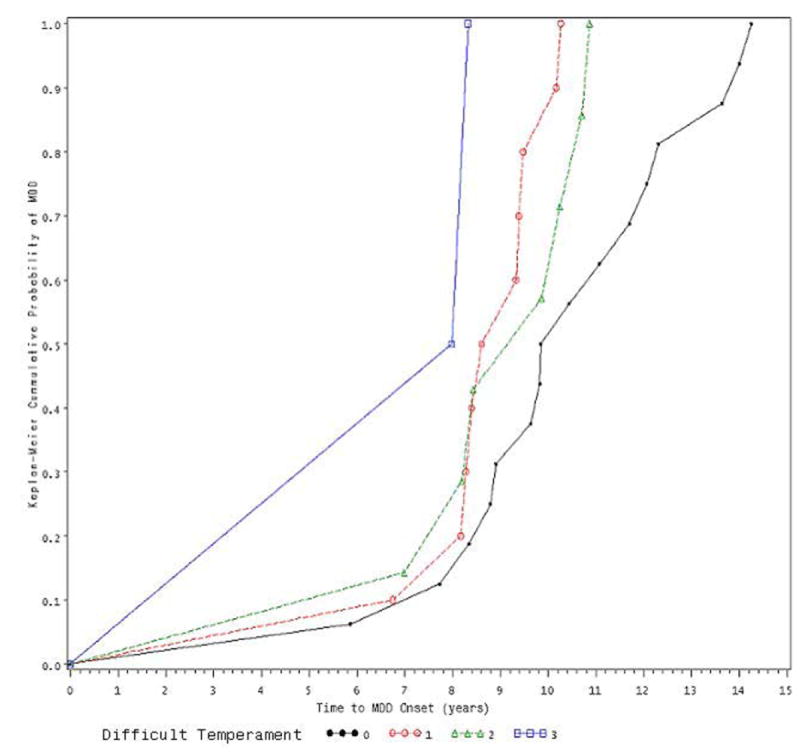
Effect of Early Temperament on MDD-onset among children from Non-Intact Families
0: no temperament difficulty
1: minimal temperament difficulty
2: moderate temperament difficulty
3: severe temperament difficulty
Modeling Severity of First MDD Episode
In a series of univariate general linear models, we found no significant associations between perinatal problems, developmental delay, difficult temperament and the severity of the first MDD episode. Only an association to child’s sex was found, F (1, 369) = 4.32, p < .05, with girls showing more severe symptoms (M = 20.15) than boys (M = 19.36). In multivariate GLM analyses, the interactions of the three developmental indices with sex and with family status were not statistically significant and were dropped from the final model. The final model, including just the three indices and child sex, was not significant, F (4, 362) = 1.60, p = .17.
Modeling Onset Age of MDD/Dysthymic/Anxiety Disorders
We first examined if children, who had developed dysthymic and/or anxiety disorder (Anx) in addition to MDD (n = 158), differed from children with MDD only (n = 213) in early risk factors. The groups did not differ in perinatal problems or developmental delays (p= .95, p= .065, respectively). However, children with comorbid DD or Anx were rated as having had a more difficult early temperament (M = .99, SD = 1.02) than were those without DD or Anx (M = .78, SD = .91), t (365) = -2.10, p < .05.
Univariate Cox regression models revealed two significant effects: boys (M = 9.50, SD = 2.51) had an earlier onset of MDD/DD/Anx than did girls (M = 9.98, SD = 2.69), and more difficult temperament was associated with earlier disorder onset. In the final model (see Table 3), child’s temperament and sex remained significant, and a significant interaction between Temperament and Intact Family was found, in the same direction as with MDD Onset-Age. Also, Temperament interacted with elapsed time, with the parameter estimate once again indicating that temperament better predicted onset-age among younger than older children.
Table 3.
Modeling Age of Onset of First Internalizing Disorder Episode (MDD/DD/Anx)
| Univariate Models | Final Multivariate Model | |
|---|---|---|
| Variables | Hazard Ratio (95% CI) | Hazard Ratio (95% CI) |
| Perinatal problems | 0.98 (0.89, 1.08) | 0.95 (0.85, 1.05) |
| Developmental delay | 1.11 (0.89, 1.38) | 1.06 (0.84, 1.33) |
| Difficult temperament | 1.22 (1.09, 1.36)*** | 4.07 (1.80, 9.20)*** |
| Sex (male = 1) | 1.30 (1.06, 1.60)* | 1.36 (1.10, 1.68)** |
| Intact family until age 4 | 0.77 (0.54, 1.09) | 1.01 (.63, 1.60) |
| Mother’s education (years) | 0.99 (.96, 1.02) | -- |
| Maternal age at birth (years) | ||
| 16-18 vs. 19-34 | 0.92 (0.59, 1.43) | -- |
| 35-46 vs. 19-34 | 1.58 (1.00, 2.52)+ | -- |
| Temperament X Intact Family | -- | 0.65(.45, .93)* |
| Temperament X Time | -- | 0.70 (.49, 1.00)* |
Note. MDD = major depressive disorder; DD = dysthymia; Anx = anxiety disorder; CI = confidence interval; Cox regression analyses were used.
p < .10.
p < .05.
p < .01.
p < .001.
DISCUSSION
To our knowledge, the present study is the first to investigate, in a very large clinical sample of youngsters with MDD, the possible impact of early neurodevelopmental difficulties on features of major depressive and related disorders. We were particularly interested in developmental characteristics that may mirror physiological vulnerability because such factors could be helpful in the early identification of cases at risk. Overall, our results complement a growing body of literature, which suggests that various atypical early childhood characteristics may affect both the risk and timing of psychopathology.
Of the three types of early developmental characteristics we examined, only difficult temperament was related to the age of onset of depression. Children with difficult early temperaments, indexed by mother-reported problems with feeding, sleeping, or soothability, had earlier onset of their depression than did children with milder or no temperamental difficulties. Notably, Jaffee et al. (2002) have reported that infant temperament (having been a “difficult baby”) distinguished young adults with childhood-onset and those with adult-onset depression. Our findings extend those results by suggesting that, even among clinically depressed young patients, problematic infant temperament does convey information about MDD onset age.
However, having had a difficult temperament also was associated with earlier DD, or anxiety disorder, as well as MDD (whichever emerged first), indicating a lack of specificity to MDD. Thus, atypical infant temperament may presage vulnerability to a range of mood-related psychiatric problems later on, underscoring that risk factors should be examined in relation to a range of disorders rather than a single condition (Kessler et al., 1997). Additionally, children who had comorbid dysthymic or anxiety disorder reportedly had more difficult temperaments than children without DD or anxiety.
If a difficult temperament prognosticates earlier onset of emotional disorder, what could be the mechanisms? Temperament, which is considered to be a relatively stable style of reactivity, is believed to reflect neurophysiological regulatory capacities (e.g., Rothbart and Bates, 1998). Toddlers with difficult temperaments may be compromised on some neurophysiologic parameter related to emotionality or emotion regulation (e.g., Fox, 1994), which may interfere with the development of effective coping responses, and render them susceptible to earlier onset of disorders. Findings that depressed children, or those at risk for depression, differ from comparison peers in their neuroendocrine or physiological responses to negative experimental mood induction, do suggest the existence of physiological or biological dimensions of vulnerability to depression (e.g., Forbes, et al., 2006; Luby et al., 2003). Genes may contribute to individual differences in both temperament and psychopathology. For example, some emerging research suggests that genetic variations associated with the phenotype of a difficult temperament may be the same that predispose an individual to develop a psychiatric disorder (e.g., Pezawas et al., 2005).
Notably, however, we found that early caregiver stability may mitigate some of the ramification of an infant having difficulties in rhythmicity or in being soothed, which is consistent with the buffering effects of a positive environment (e.g., Rothbart and Bates, 1998). Intact families may have available more of the emotional or material resources needed to take care of a “difficult” child. But because parent-infant relationships are influenced by the infant’s temperament as well (e.g., Kochanska et al., 2004), future research should examine whether parents from non-intact families experience more deleterious effects of having a difficult baby, and how this may impact offspring’s psychopathology.
Interestingly, the effect of temperament on disorder onset was attenuated across time in the sample. This finding may reflect that our anamnestic assessment focused on the period of infancy and toddlerhood. But, it is also possible that, across development, disorder parameters, such as age of onset, are subject to a variety and varying influences (other than individual characteristics).
We failed to confirm our hypotheses regarding the effects of perinatal problems and motor skill delay on age of onset of depressive and related disorders. Although clinical or population based studies have found that such characteristics distinguish childhood onset from adult onset affective disorders (Guth et al., 1993; Jaffee et al., 2002; van Os et al., 1997), differences in methodologies and samples may partly account for the inconsistent results. In our sample, early childhood characteristics also were unrelated to the severity of first MDD episode, despite Vocisano et al. (1996) finding a link between obstetric complications and severity of affective illness in adulthood.
Several other findings are of note. First, consistent with a large body of literature on the greater vulnerability of male infants to a variety of problems (e.g., Halpern, 1997), boys in our sample had higher scores on developmental delay than did girls, and their first episode of MDD, DD, or anxiety disorder occurred at a younger age than did girls’. But once an episode of MDD had onset, girls displayed more severe symptoms than boys, consistent with findings reported for adolescents (Reinhertz et al., 1999). Thus, in our sample, sex emerged as a main effect and not as a moderator variable as we had predicted. Additionally, diagnostic comorbidity in our patients was associated with reports of more difficult infantile temperaments. Notably, we reconfirmed prior reports (e.g., Kovacs et al., 1989) that, if depressed juveniles have comorbid anxiety disorders, the anxiety disorders will tend to onset earlier than the depressive disorder.
Our finding that older maternal age at the child’s birth (compared to maternal age between 19 and 34 years at childbirth) conferred earlier onset of MDD to their offspring, partly confirm those of Reinhertz et al. (1993). Reinhertz et al. (1993) found that older parental age at childbirth was associated with an increased risk of depression in female adolescent offspring. Maternal age at childbirth, however, was unrelated in our analyses to any of the early risk factors and failed to enter the final predictive models. This finding suggests that older maternal age affects offspring’s psychopathology through other variables not examined in this study.
Limitations
Our study has several limitations. Because the anamnestic data on our patients were obtained retrospectively from their mothers, inaccuracies and biases in recall are of concern. In spite of its drawbacks, however, the retrospective reporting of perinatal and early developmental events has been an important component of various clinically oriented investigations (e.g., Buka et al., 2004; Foley et al., 2001; Lewis and Murray, 1987; Sanderson et al., 1998). Research has shown that the reproducibility and validity of maternal recall of perinatal events can vary from very good to poor (e.g., Foley et al., 2001; Launer et al., 1992; Tomeo et al., 1999) and is affected by the type of the data being sought and the method of acquisition (Buka et al., 2004). Our data gathering procedures had been designed with several features in mind, which have been recently recognized as facilitating (although not guaranteeing) the accuracy of retrospective recall (Buka et al., 2004), including face-face-interviews by clinically trained assessors, use of “common” rather than medical terms and phrases, and focusing on fairly frequently occurring events. Our finding of an interaction effect between child temperament and family status also argues against an overall bias in maternal recall because the association was evident only for a subgroup of participants. Nevertheless, given our temperament index was retrospective and based on just a subset of dimensions that contribute to early temperament, our findings should be replicated with a more comprehensive measure of temperament, or validated using other means (e.g., observational indices).
A second limitation is that mothers reported on both their children’s early development and psychiatric history, introducing shared method (within-reporter) variance. However, this source of bias was reduced by the fact that a child’s final psychiatric diagnosis was: a) based both on parental and child report, b) determined on two occasions by different clinical interviewers, and c) subjected to two “best estimate” child psychiatrists independently, who also had access to psychiatric and mental health records. While questions could also be raised about the accuracy of dating the onsets of disorders, two features of our design support our findings. First, our method of obtaining clinical history and onset dates (including the use of “time-lines” with culturally standard and personally meaningful marker events, visual aids, verbal summaries, and cross-links of information) has been shown to be the preferred approach for collecting various types of retrospective data (e.g., Caspi et al., 1996). And, second, our clinically referred sample did not have protracted illness, which is likely to reduce errors in dating; the average time elapsed between the age of onset of MDD and the date of the psychiatric evaluation was 1.14 years (SD = 1.34 years), and for about 67% of the sample, it was within one year. It could be argued that the portion of our youths who were not raised by both biological parents between birth and 4 years of age (9.4%) constitutes a very small segment of the sample. Although high rates of intact families have also been found in other pediatric samples, including those of Najman et al., 2005 (82% intact) and Hirshfeld-Becker et al., (2004) (86% intact), it would be informative to replicate our study with a sample that includes more single-parent or blended families.
Clinical Implications
Our findings highlight that, even in a vulnerable sample, the putative negative effects of early infant characteristics are not immutable, but can be ameliorated by family resources. Further, the impact of some early child characteristics on features of juvenile psychopathology seems to be attenuated by the passage of time. In clinical practice, psychiatrists typically have access only to parents’ reports of early child characteristics and are unlikely to have documents of early development. Based on our findings, careful interviewing of parents can yield data that may illuminate some aspects of children’s clinical history.
Acknowledgments
Members of the International Consortium for Childhood-Onset Mood Disorders:
István Benák, Emilia Kaczvinszky M.D., Viola Kothencné Osváth, Szeged University Medical Faculty, Department of Child and Adolescent Psychiatry, Szeged; Ildikó Baji M.D., Márta Besny̋o M.D., Julia Gádoros M.D., Vadaskert Hospital, Budapest; Judit Székely M.D. Semmelweis University I. Pediatric Department Budapest; Edit Dombovári M.D., Heim Pál Hospital for Sick Children Outpatient Unit of Child Psychiatry.
Participating physicians across various cities in Hungary:
Zsuzsa Bánk M.D., Katalin Bense M.D., Ferenc Dics̋o M.D., Em̋oke Endreffy Ph.D., Edina Farkas M.D., Gyöngyi Farkas M.D., Zsuzsanna Fekete M.D., Márta Fohn M.D., Magdolna Gácser M.D., Eszter Gyenge M.D., Éva Gyulai M.D., Mária Gyurcsó M,D., Rózsa Hasuly M.D., Ágnes Horváth M.D., Enik̋o Juhász M.D., Mária Károlyfalvi M.D., Dénes Kövendy M.D., Mária Mojzes M.D., Ilona Mógor M.D., Róza Oláh M.D., Mária Palaczky M.D., Mária Révhelyi M.D., Ilona Riegler MD., Sörf̋oz̋o Zsuzsanna, M.D., Péter Steiner M.D., Zsuzsa Takács M.D, Mariann Vados M.D.
This study was supported by the NIMH Program Project Grant #MH56193, HHSA, Washington, DC, USA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Krisztina Kapornai, Department of Child and Adolescent Psychiatry, University of Szeged, Szeged, Hungary
Amy L. Gentzler, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania
Ping Tepper, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania
Enikő Kiss, Department of Child and Adolescent Psychiatry, University of Szeged, Szeged, Hungary
László Mayer, Department of Child and Adolescent Psychiatry, University of Szeged, Szeged, Hungary
Zsuzsanna Tamás, Vadaskert Hospital, Budapest, Hungary
Maria Kovacs, Department of Psychiatry,University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania
Ágnes Vetró, Department of Child and Adolescent Psychiatry, University of Szeged, Szeged, Hungary
References
- Achenbach TM. Manual for the Child Behavior Checklist 4-18 and 1991 Profile. Burlington: University of Vermont, Department of Psychiatry; 1991. [Google Scholar]
- Allen NB, Lewinsohn PM, Seeley JR. Prenatal and perinatal influences on risk for psychopathology in childhood and adolescence. Dev Psychopathol. 1998;10:513–529. doi: 10.1017/s0954579498001722. [DOI] [PubMed] [Google Scholar]
- Axelson DA, Birmaher B. Relation between anxiety and depressive disorders in childhood and adolescence. Depress Anxiety. 2001;14:67–78. doi: 10.1002/da.1048. [DOI] [PubMed] [Google Scholar]
- Buka SL, Goldstein JM, Spartos E, Tsuang MT. The retrospective measurement of prenatal and perinatal events: Accuracy of maternal recall. Schizophr Res. 2004;71:417–426. doi: 10.1016/j.schres.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Buka SL, Tsuang MT, Lipsitt LP. Pregnancy/delivery complications and psychiatric diagnosis: A prospective study. Arch Gen Psychiatry. 1993;50:151–156. doi: 10.1001/archpsyc.1993.01820140077009. [DOI] [PubMed] [Google Scholar]
- Caspi A, Moffitt TE, Thornton A, Freedman D, Amell JW, Harrington H, Smeijers J, Silva PA. The life history calendar: A research and clinical assessment method for collecting retrospective event-history data. Int J Methods in Psychiatr Res. 1996;6:101–114. [Google Scholar]
- Cohen P, Velez CN, Brook J, Smith J. Mechanisms of the relation between perinatal problems, early childhood illness, and psychopathology in late childhood and adolescence. Child Dev. 1989;60:701–709. doi: 10.1111/j.1467-8624.1989.tb02750.x. [DOI] [PubMed] [Google Scholar]
- Foley DL, Thacker LR, II, Aggen SH, Neale MC, Kendler KS. Pregnancy and perinatal complications associated with risks for common psychiatric disorders in a population-based sample of female twins. Am J Med Genet B Neuropsychiatr Genetics. 2001;105:426–431. [PubMed] [Google Scholar]
- Forbes EE, Fox NA, Cohn JF, Galles SF, Kovacs M. Children’s affect regulation during a disappointment: Psychophysiological responses and relation to parent history of depression. Biol Psychol. 2006;71:264–277. doi: 10.1016/j.biopsycho.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Fox NA, editor. Monographs of the Society for Research in Child Development. Vol. 59. 1994. The development of emotion regulation: Biological and behavioral considerations; pp. 2–3. Serial No 240. [PubMed] [Google Scholar]
- Gale CR, Martyn CN. Birth weight and later risk of depression in a national birth cohort. Br J Psychiatry. 2004;184:28–33. doi: 10.1192/bjp.184.1.28. [DOI] [PubMed] [Google Scholar]
- Goodman SH. Depression and early adverse experiences. In: Gotlieb I, Hammen C, editors. Handbook of depression. Guilford Press; NY: 2002. pp. 245–267. [Google Scholar]
- Guth C, Jones P, Murray R. Familial psychiatric illness and obstetric complications in early-onset affective disorder: A case-control study. Br J Psychiatry. 1993;163:492–498. doi: 10.1192/bjp.163.4.492. [DOI] [PubMed] [Google Scholar]
- Halpern DF. Sex differences in intelligence. Am Psychol. 1997;52:1091–1102. doi: 10.1037//0003-066x.52.10.1091. [DOI] [PubMed] [Google Scholar]
- Hirshfeld-Becker DR, Biederman J, Faraone SV, Robin JA, Friedman D, Rosenthal JM, Rosenbaum JF. Pregnancy complications associated with childhood anxiety disorders. Depress Anxiety. 2004;19:152–162. doi: 10.1002/da.20007. [DOI] [PubMed] [Google Scholar]
- Jaffee S, Caspi A, Moffitt TE, Belsky J, Silva P. Why are children born to teen mothers at risk for adverse outcomes in young adulthood? Results from a 20-year longitudinal study. Dev Psychopathol. 2001;13:377–397. doi: 10.1017/s0954579401002103. [DOI] [PubMed] [Google Scholar]
- Jaffee SR, Moffitt TE, Caspi A, Fombonne E, Poulton R, Martin J. Differences in early childhood risk factors for juvenile-onset and adult-onset depression. Arch Gen Psychiatry. 2002;58:215–222. doi: 10.1001/archpsyc.59.3.215. [DOI] [PubMed] [Google Scholar]
- Keenan K, Shaw D, Delliquadri E, Giovannelli J, Walsh B. Evidence for the continuity of early problem behaviors: Application of a developmental model. J of Abnorm Child Psychol. 1998;26:441–452. doi: 10.1023/a:1022647717926. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Davis CG, Kendler KS. Childhood adversity and adult psychiatric disorder in the US National Comorbidity Survey. Psychol Med. 1997;27:1101–1119. doi: 10.1017/s0033291797005588. [DOI] [PubMed] [Google Scholar]
- Kochanska G, Friesenborg AE, Lange LA, Martel MM. Parents’ personality and infants’ temperament as contributors to their emerging relationship. J Pers Soc Psychol. 2004;86:744–759. doi: 10.1037/0022-3514.86.5.744. [DOI] [PubMed] [Google Scholar]
- Kovacs M MHS Staff. Children’s Depression Inventory (CDI): Technical Manual Update. North Tonawanda, NY: Multi-Health Systems, Inc.; 2003. [Google Scholar]
- Kovacs M, Feinberg TL, Crouse-Novak MA, Paulauskas P, Finkelstein R. Depressive disorders in childhood: I. A longitudinal prospective study of characteristics and recovery. Arch Gen Psychiatry. 1984a;41:229–237. doi: 10.1001/archpsyc.1984.01790140019002. [DOI] [PubMed] [Google Scholar]
- Kovacs M, Feinberg TL, Crouse-Novak MA, Paulauskas P, Pollock M, Finkelstein R. Depressive disorders in childhood: II. A longitudinal study of the risk for a subsequent major depression. Arch Gen Psychiatry. 1984b;41:643–649. doi: 10.1001/archpsyc.1984.01790180013001. [DOI] [PubMed] [Google Scholar]
- Kovacs M, Gatsonis C, Paulauskas SL, Richards C. Depressive disorder in childhood. IV. A longitudinal study of comorbidity with and risk for anxiety disorders. Arch Gen Psychiatry. 1989;46:776–782. doi: 10.1001/archpsyc.1989.01810090018003. [DOI] [PubMed] [Google Scholar]
- Launer LJ, Forman MR, Hundt GL, Sarov B, Chang D, Berendes HW, Naggan L. Maternal recall of infant feeding is accurate. J Epidemiol Community Health. 1992;46:203–206. doi: 10.1136/jech.46.3.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis SW, Murray RM. Obstetric complications, neurodevelopmental deviance, and risk of schizophrenia. J Psychiatr Res. 1987;21:413–421. doi: 10.1016/0022-3956(87)90088-4. [DOI] [PubMed] [Google Scholar]
- Liu X, Gentzler AL, Tepper P, Kiss E, Kothencné V, Tamás Z, Vetró Á, Kovacs M. Clinical features of depressed children and adolescents with various forms of suicidality. Journal of Clinical Psychiatry. doi: 10.4088/jcp.v67n0917. in press. [DOI] [PubMed] [Google Scholar]
- Luby JL, Heffelfinger A, Mrakotsky C, Brown K, Hessler M, Spitznagel E. Alterations in stress cortisol reactivity in depressed preschooler relative to psychiatric and no-disorder comparison groups. Arch Gen Psychiatry. 2003;60:1248–1255. doi: 10.1001/archpsyc.60.12.1248. [DOI] [PubMed] [Google Scholar]
- Maziade M, Côté R, Bernier H, Boutin P, Thivierge J. Significance of extreme temperament in infancy for clinical status in pre-school years. I: Value of extreme temperament at 4-8 months for predicting diagnosis at 4.7 years. Br J Psychiatry. 1989;154:535–543. doi: 10.1192/bjp.154.4.535. [DOI] [PubMed] [Google Scholar]
- Maziade M, Roy MA, Fournier JP, Cliché D, Merette C, Caron C, Garneau Y, Montgrain N, Shriqui C, Dion C. Reliability of best-estimate diagnosis in genetic linkage studies of major psychoses: Results from the Quebec pedigree studies. Am J Psychiatry. 1992;149:1674–1686. doi: 10.1176/ajp.149.12.1674. [DOI] [PubMed] [Google Scholar]
- Najman JM, Hallam D, Bor W, O’Callaghan M, Williams GM, Shuttlewood G. Predictors of depression in very young children: A prospective study. Soc Psychiatry Psychiatr epidemiol. 2005;40:367–374. doi: 10.1007/s00127-005-0895-0. [DOI] [PubMed] [Google Scholar]
- Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, Egan MF, Mattay VS, Hariri AR, Weinberger DR. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: A genetic susceptibility mechanism for depression. Nat Neurosci. 2005;8:828–834. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- Preti A, Cardascia L, Zen T, Pellizzari P, Marchetti M, Favaretto G, Miotto P. Obstetric complications in patients with depression - a population-based case-control study. J of Affect Dis. 2000;61:101–106. doi: 10.1016/s0165-0327(99)00185-8. [DOI] [PubMed] [Google Scholar]
- Reinherz HZ, Giaconia RM, Pakiz B, Silverman AB, Frost AK, Lefkowitz ES. Psychosocial risks for major depression in late adolescence: A longitudinal community study. J Am Acad Child Adolesc Psychiatry. 1993;32:1155–1163. doi: 10.1097/00004583-199311000-00007. [DOI] [PubMed] [Google Scholar]
- Reinherz HZ, Giaconia RM, Hauf AMC, Wasserman MS, Silverman AB. Major depression in the transition to adulthood: Risks and impairments. J Abnorm Psychol. 1999;108:500–510. doi: 10.1037//0021-843x.108.3.500. [DOI] [PubMed] [Google Scholar]
- Rothbart MK, Bates JE. Temperament. In: Damon W, Eisenberg N, editors. Handbook of Child Psychology: Social, Emotional, and Personality Development. 5. New York: Wiley; 1998. pp. 105–176. [Google Scholar]
- Sanderson M, Williams MA, White E, Daling JR, Holt VL, Malone KE, Self SG, Moore DE. Validity and reliability of subject and mother reporting of perinatal factors. Am J Epidemiol. 1998;147:136–140. doi: 10.1093/oxfordjournals.aje.a009425. [DOI] [PubMed] [Google Scholar]
- Sherrill JT, Kovacs M. Interview Schedule for Children and Adolescents (ISCA) J Am Acad Child Adolesc Psychiatry. 2000;39:67–75. doi: 10.1097/00004583-200001000-00018. [DOI] [PubMed] [Google Scholar]
- Sigurdsson E, van Os J, Fombonne E. Are impaired childhood motor skills a risk factor for adolescent anxiety? Result from the 1958 U.K. Birth Cohort and the National Child Development Study. Am J Psychiatry. 2002;159:1044–1046. doi: 10.1176/appi.ajp.159.6.1044. [DOI] [PubMed] [Google Scholar]
- Thomas A, Chess S. Temperament and development. New York: Brunner/Mazel; 1977. [Google Scholar]
- Tomeo CA, Rich-Edwards JW, Michels KB, Berkey CS, Hunter DJ, Frazier AL, Willett WC, Buka SL. Reproducibility and validity of maternal recall of pregnancy-related events. Epidemiology. 1999;10:774–777. [PubMed] [Google Scholar]
- van Katwijk C, Peeters LLH. Clinical aspects of pregnancy after the age of 35 years: A review of the literature. Hum Reprod Update. 1998;4:185–194. doi: 10.1093/humupd/4.2.185. [DOI] [PubMed] [Google Scholar]
- van Os J, Jones P, Lewis G, Wadsworth M, Murray R. Developmental precursors of affective illness in a general population birth cohort. Arch Gen Psychiatry. 1997;54:625–631. doi: 10.1001/archpsyc.1997.01830190049005. [DOI] [PubMed] [Google Scholar]
- Vocisano C, Klein DN, Keefe RS, Dienst ER, Kincaid MM. Demographics, family history, premorbid functioning, developmental characteristics, and course of patients with deteriorated affective disorder. Am J Psychiatry. 1996;153:248–255. doi: 10.1176/ajp.153.2.248. [DOI] [PubMed] [Google Scholar]


