Anyone who has experienced an episode of viral or bacterial infection knows well the subjective feelings of sickness, in the form of malaise, lassitude, fatigue, numbness, coldness, muscle and joint aches, and reduced appetite. Because they are common, these symptoms usually are ignored by physicians. They are considered uncomfortable, but banal, components of the pathogen-induced debilitation process that affects sick individuals.
This simplistic view has turned out to be incorrect. The psychologic and behavioral components of sickness represent, together with fever response and associated neuroendocrine changes, a highly organized strategy of the organism to fight infection [1]. This strategy, referred to as “sickness behavior,” is triggered by the proinflammatory cytokines produced by activated cells of the innate immune system in contact with specific pathogen-associated molecular patterns (PAMP). These cytokines include mainly interleukin (IL) 1 (IL-1α and IL-1β), IL-6, and tumor necrosis factor α (TNF-α).
The mechanisms that mediate the behavioral effects of peripherally released cytokines have been elucidated during the past decade. IL-1 and other cytokines act on the brain via two communication pathways: (1) a neural route represented by the primary afferent neurons that innervate the body site where the infectious process takes place and (2) a humoral pathway that involves the production of proinflammatory cytokines by phagocytic cells in the circumventricular organs (CVOs) and choroid plexus in response to circulating PAMP or cytokines, followed by the propagation of these immune signals into the brain parenchyma (Fig. 1) [2]. The objective of this review is to present the current knowledge on the way this communication system is organized and regulated and the implications of these advances for understanding brain physiology and pathology.
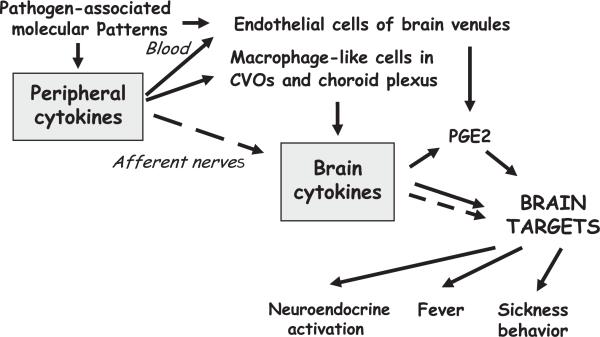
Fig. 1.
Mechanisms of brain actions of cytokines. Proinflammatory cytokines are released by activated innate immune cells at the periphery in response to PAMP. PAMP and circulating cytokines act on TLRs on macrophage-like cells in the CVOs and choroid plexus, leading to the production of brain cytokines that diffuse by volume propagation into the brain parenchyma. The action of peripheral proinflammatory cytokines also can be relayed to the brain by afferent nerves, resulting in the production of brain proinflammatory cytokines by microglial cells. In both cases, the action of brain proinflammatory cytokines can be mediated by prostaglandins that diffuse to brain targets or by activation of neural pathways within the brain, which enables the immune message to be transported far away from its site of origin. Prostaglandins can be synthesized only by endothelial cells of brain venules in response to circulating cytokines. Dotted arrows represent instances of neural transmission of the immune message from the periphery to the brain or within the brain itself.
Peripheral proinflammatory cytokines induce expression of cytokines in the brain
Origin of peripheral cytokines
Infectious microorganisms that invade the body encounter a first line of defense represented by monocytes, tissue macrophages, and liver Kupffer's cells. These phagocytic cells express Toll-like receptors (TLRs) that are geared to innately recognize specific PAMP. TLRs are defined by the presence of a conserved cytoplasmic signaling domain, the Toll/IL-1 receptor homology domain, that signals via the nuclear transcription factor, nuclear factor (NF)-κB [3]. Innate immune cells express TLR4s that recognize lipopolysaccharide (LPS), a component of the cell wall of gram-negative bacteria. The T-cell antigen, peptidoglycan, from gram-positive bacteria, is recognized by TLR2Rs, which are present on the same phagocytic cells. Binding of LPS to TLR4 results in the production of the proinflammatory cytokines, IL-1α and IL-1β. IL-1 then is able to induce its own synthesis and the synthesis of other cytokines potentiating its action (TNF-α and IL-6) or antagonizing it (the so-called “anti-inflammatory cytokines,” such as the specific antagonist of IL-1 receptors, IL-1Ra and IL-10). Proinflammatory cytokines do not act as hormones, because aside from IL-6, they are not transported in the circulation to distant cell targets. They act in an autocrine manner, on the same cells that have manufactured them, or in a paracrine manner, on adjacent cells within the same tissue. Cytokines usually are produced only when needed. Once released, cytokines are biologically active at nano- to picomolar concentrations, and they act on a limited number of receptors per cells that amplify their action via the activation of a large number of genes.
Peripherally produced proinflammatory cytokines induce sickness
Peripheral administration of a cytokine inducer, such as LPS, or of recombinant cytokines, such as IL-1β or TNF-α, mimics all nonspecific symptoms of sickness, including fever, activation of the hypothalamic-pituitary-adrenal (HPA) axis, reduction of food intake and other behavioral activities, and withdrawal from the physical and social environment [1]. Conversely, administration of cytokine antagonists abrogates the physiologic and behavioral effects of the cytokine inducer, LPS. In all these experiments, sickness behavior usually is assessed by reduction in food intake and decreased social investigation of a juvenile conspecific introduced into the home cage of the test animal. The findings of these experiments indicate that proinflammatory cytokines mediate the clinical signs of the host response to infection. The physiologic and behavioral changes that are characteristic of sickness are mediated in the central nervous system (CNS). Fever, for instance, represents a regulated rise in body temperature resulting from increased production of heat (thermogenesis) and decreased thermal loss (thermolysis) in response to an elevated set point for the regulation of body temperature. Given that the body temperature set point is controlled by temperature-sensitive neurons in the preoptic hypothalamus, pyrogenic cytokines, such as IL-1β and IL-6, need to act in the CNS to induce fever [4]. In the same manner, IL-1β acts on the paraventricular nucleus of the hypothalamus where the neurons that contain corticotropin releasing hormone (CRH) are located [5,6]. CRH is released in the portal blood, which leads to the release of corticotropin from the pituitary, which, in turn, increases the release and secretion of glucocorticoids by the adrenal cortex.
The proposed action of IL-1β in the CNS raises the question as to how this cytokine that is produced at the periphery signals the brain. Like other proinflammatoy cytokines, IL-1β is a large hydrophilic peptide that cannot cross the blood-brain barrier passively. In addition, as discussed previously, IL-1β and other cytokines are considered short-range communication molecules that act predominantly in an autocrine or paracrine manner rather than a hormonal manner. For this reason, several pathways of immune-to-brain communication are proposed for the action of these cytokines on the nervous system, from the induction of prostaglandins in those brain areas that are devoid of a functional blood-brain barrier to the existence of specific saturable transporters [2]. Early studies of the pyrogenic effects of cytokines were performed with intravenous injection of leukocytic pyrogens. Demonstration of the pyrogenic effects of IL-1β took place with the same mode of administration, and, therefore, it was logical to postulate that circulating cytokines act on brain areas that are devoid of a functional blood-brain barrier. The possibility of intervention of different mechanisms was raised only later, in the mid 1990s, when the brain effects of cytokines were investigated in animals in which afferent nerves from the abdominal cavity were severed.
Neural transmission of the cytokine message
The hypothesis that cytokines act indirectly on the CNS by activating afferent nerves was based on the recognition that two of the cardinal signs of inflammation, calor (heat) and dolor (pain), require sensory processing, which implies that inflammatory mediators released at the site of injury or infection are able to signal the brain. When LPS or cytokines are injected into the abdominal cavity, they induce inflammation of the peritoneum. One of the major routes of visceral sensibility is represented by the afferent branches of the vagus nerves. These branches contain in their perineural sheath macrophages and dendritic cells that express membrane TLRs and produce IL-1β in response to an intraperitoneal injection of LPS [7]. Sensory neurons of the vagus nerves express IL-1 receptors, and circulating IL-1β stimulates vagal sensory activity [8].
The role of the vagus nerves in the transmission of information from the periphery to the brain is confirmed by vagotomy experiments in which the vagus nerves were sectioned under the diaphragm so as not to compromise cardiac and pulmonary function. Using this approach, vagal afferents are shown to mediate the activation of the brainstem, hypothalamus, and limbic structures in response to peripherally administered LPS, as demonstrated by the attenuation of the expression of the early activation gene c-fos in the primary and secondary projection areas of the vagus nerves [9]. Sickness behavior also was abrogated in vagotomized animals injected with LPS or IL-1β [10–12]. The decreased response of vagotomized animals to proinflamma-tory cytokines was not the result of an inability to mount a peripheral cytokine response to LPS, because vagotomy did not alter plasma levels of cytokines or the ability of peritoneal macrophages to produce cytokines [10]. Furthermore, vagotomized animals still were able to develop a full-blown episode of sickness in response to IL-1β injected by routes other than the intraperitoneal route, including the subcutaneous, intravenous, and intracerebroventricular routes [11,12]. Further evidence for the role of vagal afferents in the induction of sickness behavior was provided by reversible inactivation of the dorsal vagal complex, the primary projection area of the vagus nerves, by a local anesthetic agent. This intervention abrogated LPS-induced sickness behavior and c-Fos expression in downstream brain areas [13].
Other afferent nerves are solicited when the inflammatory response takes place in different parts of the body. For instance, inflammation in the oral cavity is found to give rise to fever via the glossopharyngeal nerves, because the transaction of this neural trunk abrogates the fever response to the injection of IL-1β or LPS into the soft palate and the enhanced expression of brain cytokines in response to this peripheral immune stimulus [14,15].
The importance of the neural pathway in the transmission of the immune message from the periphery to the brain is not the same for all components of sickness behavior. In particular, vagal afferents are less important for the cytokine-induced fever and activation of the HPA axis than for cytokine-induced sickness behavior, because vagotomized rats do not develop the behavioral alterations that are characteristic of sickness while they still are able to mount a fever [16]. These findings indicate that other pathways of communication function in parallel with the neural pathway.
Humoral transmission of the cytokine message
Besides the relatively fast neural pathway of immune-to-brain communication, there is a slower pathway that involves the action of PAMP or circulating cytokines on macrophage-like cells in circumventricular organs and endothelial cells of brain vessels. This results in the local production of cytokines and molecular intermediates, such as prostaglandins of the E2 series (PGE2) and nitric oxide. PGE2 represent the main mediators of cytokine-induced fever and activation of the HPA axis, because pretreatment with specific inhibitors of the prostaglandin synthesizing enzyme, cyclooxygenase 2 (COX-2), attenuates these responses [17,18]. The synthesis of PGE2is dependent on the induction of COX-2 and the enzyme, prostaglandin E synthase, both of which are expressed in endothelial cells of cerebral blood vessels and perivascular macrophages after intravenous IL-1β administration. PGE2 diffuse into the brain parenchyma and act on neuronal EP3 or EP4 receptors in the brainstem and hypothalamic neural structures that are involved in the control of the HPA axis activity and the regulation of body temperature. These brain areas include the catecholaminergic brainstem nuclei, the paraventricular nucleus of the hypothalamus, and the ventromedial preoptic area.
The reduction in social behavior and the anorexia that develop in response to peripheral LPS and IL-1 are mediated by brain IL-1, because these responses are attenuated by intracerebroventricular administration of the IL-1 receptor antagonist [19,20]. In response to peripheral LPS, IL-1β is synthesized by macrophage-like cells in the circumventricular organs and choroid plexus, where the blood-brain barrier is deficient [21,22]. This certainly is the result of the action of circulating LPS on TLR4 receptors that are present on the same cells. Another possibility is that IL-1 is produced in response to circulating cytokines and PAMP. IL-1 can act on neuronal IL-1 receptors in the area postrema, the circumventricular organ of the brainstem [23]. This results in the activation of a neuronal pathway projecting to the parabrachial nucleus and from there to the central amygdala and the bed nucleus of the stria terminalis. There is evidence that IL-1 also can propagate by volume transmission from the choroid plexus into the surrounding brain parenchyma to reach distant structures, such as the baso-lateral amygdala, that contains neurons expressing IL-1 receptors [24]. These two pathways, of which the respective importance remains to be elucidated, could be responsible for the behaviorally depressing effects of IL-1. In the same manner, diffusion of IL-1 from the median eminence to the arcuate nucleus could mediate IL-1–induced anorexia. The exact mechanisms of the depressive action of cytokines on food intake, however, are obscure, because lesions of the arcuate nucleus do not disrupt the effects of IL-1 on food intake [25].
The fast neural pathway and the slow humoral pathway converge in a manner, which remains unknown, to promote the brain expression of IL-1, because electrical stimulation of the vagus nerve induces the expression of brain IL-1, and vagotomy abrogates the induction of expression of brain IL-1 in response to intraperitoneal LPS and IL-1 [26–28]. A likely possibility is that the neural immune-to-brain communication pathway recruits various brain areas and sensitizes them to the action of the slowly propagating cytokine message [29].
The exact nature of the neurotransmitters that are responsible for the behavioral effects of IL-1 is unknown. The effects of proinflammatory cytokines on brain neurotransmitters are grossly similar to those of other stressors but this similarity breaks off at the regional level [30]. More detailed neuroanatomic studies are required to identify the neurotransmitter content of those neuronal structures that are activated directly or indirectly by cytokines, so that the role of the putative neurotransmitter mediator can be assessed by micropharmacology intervention techniques.
Molecular basis of sickness behavior
Role of interleukin 1
The availability of species-specific recombinant cytokines has allowed assessment of the range of physiologic and behavioral effects of the proinflammatory cytokines that are produced during an infectious episode. This has been done using pharmacologic approaches consisting of administering the cytokine under investigation alone or in combination with other cytokines to healthy animals. As discussed previously, IL-1β is an important cytokine for the induction of sickness behavior. Administration of IL-1β alone at the periphery or into the lateral ventricle of the brain induces all the central components of the acute phase reaction, including fever, HPA axis activation, and behavioral depression [31]. In contrast, IL-6 has only pyrogenic and corticotropic activities but no behavioral activity [32]. These findings do not suggest that IL-1β is the sole cytokine that mediates sickness behavior. In accordance with the concept of a cytokine network, a given cytokine never acts alone but in the context of other cytokines that potentiate or oppose its activity. IL-6, for instance, potentiates the behaviorally depressing effects of IL-1β [32]. Such complementary interactions between proinflammatory cytokines can be addressed more easily when one cytokine is missing from the cytokine network, because the gene for this cytokine or its receptor has been deleted by the technique of homologous recombination. IL-6 knockout (KO) mice, for example, are less sensitive to the behavioral effects of LPS or IL-1β injected peripherally or centrally [33]. In the same manner, type I IL-1 receptor (IL-1RI) KO mice are responsive to the behaviorally depressing effects of LPS, whereas they do not respond any longer to peripheral or central IL-1 [34]. In mice in which the gene coding for the IL-1RI is deleted, the blockade of another proinflammatory cytokine, TNF-α, by a fragment of its soluble receptor injected centrally abrogates the behaviorally depressing effects of LPS, whereas it has no effect in wild type mice [34]. These results show that deficiency in one cytokine can be compensated by another cytokine of the network.
As discussed previously, IL-1 seems to be the predominant mediator of sickness behavior in the brain, because blockade of its action by central administration of the IL-1 receptor antagonist attenuates cytokine-induced sickness behavior measured by either depression of social exploration [35] or reduction of food intake [19,35]. The involvement of brain IL-1 in the depressing effects of LPS on food intake is confirmed in an experiment using mice that are deficient in the IL-1β converting enzyme. This enzyme, also known as caspase 1, processes inactive pro–IL-1β into mature IL-1β. IL-1β converting-enzyme KO mice are less sensitive to the depressing effects of LPS on food intake when LPS is injected into the lateral ventricle of the brain, whereas they do not differ from controls in their response to intraperitoneal LPS [36].
Receptor mechanisms of the effect of interleukin 1 in the brain
Interleukin 1 receptors
The effects of IL-1 on its cellular targets are mediated by several receptor subtypes, featuring an extracellular domain with three immunoglobulin-like domains, a single transmembrane domain, and an intracellular domain that involves adaptor proteins and kinase cascades. The IL-1RI that mediates all of the known biologic effects of IL-1 uses adaptor molecule MyD88 to mediate a complex pathway, involving a cascade of kinases organized by multiple adapter molecules into signaling complexes, leading to activation of the transcription factor, NF-κB [37]. The type II IL-1 receptor (IL-1RII) is a negative regulator of the IL-1 system and functions as a decoy receptor. Its intracellular domain is short and has no signaling function. The additional IL-1 receptor accessory protein (IL-1RacP) is necessary for IL-1 signal transduction, because binding of IL-1 to the IL-1RI leads to the formation of a heterodimeric complex with this accessory protein, whereas binding of IL-1Ra to the IL-1RI prevents the formation of this complex [38]. This allows understanding of why IL-1RacP KO mice behave like IL-1RI KO mice.
Brain interleukin 1 receptors
All the biochemistry techniques used to date to characterize IL-1 receptors on neurons, glial cells, and endothelial cells of brain venules show a striking similarity between brain IL-1 receptors and those found on peripheral immune and nonimmune cells. Most of the members of the IL-1Rs family are cloned from transformed lines of blood cells. The descriptive work on the type and localization of IL-1 receptors present in the brain is based on auto-radiographic detection of radioiodinated ligands, such as IL-1α, IL-1β,or IL-1Ra; polymerase chain reaction detection of a small fragment of the complementary DNA (cDNA)-encoding IL-1 receptors; and immunohistochemical detection with antibodies raised against epitopes of IL-1 receptors from peripheral blood cells. Because these techniques do not allow conclusive determination of whether or not brain IL-1Rs and peripheral IL-1Rs are the same, it can be assumed that the difficulty of cloning new brain-specific IL-1Rs is in favor of the identity between peripheral and brain receptors. In agreement with this assumption, detection of IL-1Rs in the brain using in situ hybridization or RNase protection assay with full-length cDNA or long riboprobes [23] indicates that the same IL-1RI messenger RNA (mRNA) is present in peripheral and central nervous tissue. Moreover, auto-radiography studies of KO mice for IL-1RI confirm that this receptor is responsible for all IL-1–binding sites in the mouse brain [34]. Yet, this last result was unexpected in view of the presence of IL-1RI and IL-1RII in the mouse brain. The most likely explanation for the lack of IL-1RII binding in the brain of IL-1RI KO mice is the low abundance of IL-1RII and the low sensitivity of in vitro binding techniques. In agreement with this interpretation, immunohistochemistry studies reveal the expression of IL-1RII but not IL-RI in the mouse hypothalamus, despite the fact that this expression is undetected with the use of binding techniques [39]. These results emphasize the need for multiple methods to study brain cytokine receptors to be able to confirm the presence or their absence in this organ.
Localization of brain interleukin 1 receptors
In the rat, the first study performed to localize IL-1 binding sites made use of quantitative autoradiography to show that IL-1 receptors are spread widely across the brain, with the highest level in the granular layer of the dentate gyrus, the granule cell layer of the cerebellum, the hypothalamus, and the pyramidal cell layer of the hippocampus [40]. These findings are interpreted as indicating a neuronal localization. Seven years later, Ericsson and coworkers [23] used in situ hybridization with a 1.35-kilobase cDNA probe to determine the distribution of IL-1RI in rat brain. IL-1RI mRNA expression was localized in non-neuronal cells in structures at the interface between the brain parenchyma and its fluid environments, such as the choroid plexus and the endothelial cells of the brain vasculature. Neuronal expression appeared mostly in the hippocampus and also was detected in a few cell groups of the basolateral nucleus of the amygdala and the basomedial nuclei of the hypothalamus. It was not possible to determine the exact nature of the few labeled cells observed in the arcuate nucleus and the area postrema.
Compared with IL-1RI, much less is known about the expression of IL-1RII mRNA in the rat brain. IL-1RII mRNA seems to be undetectable in the normal adult brain but is induced in the dentate gyrus, the hippocampus, and the basolateral amygdaloid nucleus in response to a systemic injection of kainic acid [41]. IL-1RII mRNA also can be observed 24 hours later in neurons of the medial and median preoptic area, dorsomedial and paraventricular hypothalamic nuclei, and various thalamic nuclei. When distinct inflammatory lesions are induced in the rat brain by localized injection of IL-1β, IL-1RII expression is found to be restricted to brain endothelial cells and infiltrating neutrophils [42].
In contrast to the localized expression of IL-1RI and IL-1RII mRNA, rat IL-1RacP mRNA detected by RNase protection assay is ubiquitous and expressed at high levels in many brain regions (hypothalamus, cortex, hippo-campus, and cerebellum) [43]. The presence of IL-1RacP in brain areas that are devoid of IL-1RI questions the exact role of this accessory protein in the rat brain.
In the mouse, the first evidence for the presence of IL-1 receptors in the brain was obtained by demonstration of the expression of IL-1RI and IL-1RII mRNA and by radioactive IL-1α binding affinity [44–46]. Later on, immunohistochemistry allowed confirmation of the protein expression and neuronal localization of the two subtypes of IL-1 receptors [39]. IL-1RI and IL-1RII were found to be expressed on neuronal soma in the granular cell layer of the dentate gyrus and the CA1-CA4 pyramidal cells fields of Ammon's horn of the hippocampus. Similarly, both IL-1R isoforms are expressed on ependymal epithelial cells, choroid plexus epithelial cells, and Purkinje's cells of the cerebellum. IL-RII, but not IL-RI, is detected on neuronal soma and proximal cell processes in the hypothalamic paraventricular gray matter. No clear evidence of immunolabeling on vascular endothelial cells and meninges has been found. Choroid plexus epithelial cells and ventricular ependidymal epithelial cells expressed easily detectable IL-1RI and IL-1RII, however, which is in accordance with their role in the entrance of IL-1 into the brain. Only limited immunoreactivity for IL-1RI and IL-1RII was detected on astroglial cells of normal adult mouse brain, although in mice previously infected with moloney murine leukemia, an abundant expression of IL-1RI was observed on reactive astrocytes. In the intact rat brain, IL-1RI expression is much more localized and restricted to nonneuronal cells, especially endothelial cells of brain venules, as assessed by immunohistochemistry with antibodies directed against the extracellular domain of IL-1RI or the p65 subunit of NF-κB [47,48].
IL-1RacP localization is similar in the mouse and rat brains [49,50]. Surprisingly, 125I-IL-1 binding studies in IL-1RacP KO mice do not reveal a lower affinity of the binding sites, indicating that IL-1RI and IL-1RacP do not necessarily form in this organ the heterodimeric complex that normally is required for high affinity binding. Again, these findings argue for a role of IL-1RacP in the rodent brain that is not yet defined.
Functionality of brain interleukin 1 receptors
Administration of IL-1β or IL-1α into the lateral ventricle of the brain or directly into the brain parenchyma induces the typical signs of sickness behavior. These effects are mediated by brain IL-1 receptors, because they are abrogated by local administration of IL-1Ra into the brain [1].
To determine which subtype of IL-1 receptors mediates the behavioral effects of IL-1, passive immunization experiments, antisense technology, and mouse KO strategies are used. Blockade of IL-1RI with a specific neutralizing antibody totally abrogates the behavioral effects of centrally and peripherally injected IL-1β in mice [51]. Blockade of IL-1RII potentiates the suppressing effect of IL-1β on food intake [51]. Blockade of brain IL-1RI by antisense oligonucleotides abrogates the anorexic but not the adipsic effects of intracerebroventricular IL-1β [52]. IL-1RI deficient mice no longer were responsive to the behaviorally depressing effects of IL-1β injected at the periphery or directly into the brain. This was not because of an inability to mount a sickness response, because they were responsive to LPS [34]. In the same manner, IL-1RacP KO mice did not respond any longer to IL-1β injected into the lateral ventricle of the brain [53].
The signaling pathways that mediate the behavioral effects of IL-1 recently have been investigated. Because IκBα is expressed strongly in the circumventricular organs and choroid plexus during peripheral immune stimulation [54], and this response is associated with NF-κB translocation, the effects of IL-1 at this level are likely to be dependent on the transcription factor, NF-κB. In accordance with this hypothesis, central administration of a NF-κB inhibitor peptide is found to block the somnogenic and pyrogenic effects of peripheral IL-1β in rabbits [55]. In the same manner, blockade of NF-κB activation by intracerebroventricular administration of a cell per-meant peptide that blocks the interaction of the NFKB essential modulator (NEMO) with the IκB kinase complex abrogates sickness behavior elicited by intraperitoneal IL-1β [56]. In contrast to the predominant role of NF-κB in IL-1 signaling at the blood-brain interface, activation of mitogen-activated protein kinases pathways seems to be involved in the neural effects of IL-1, including IL-1β–induced inhibition of long term potentiation in perfo-rant path-granule cell synapses and IL-1β–induced elevation in intracellular free calcium levels in rat cortical synaptosomes [57].
Endogenous anti-inflammatory molecules
Because of the tight regulation of the expression and action of cytokines at the periphery and in the brain, cytokine-induced sickness behavior normally is a fully reversible phenomenon. The molecular factors that are involved in this regulation include mainly anti-inflammatory cytokines that target IL-1 specifically, such as IL-1Ra and soluble IL-1RII, or have more generalized antagonist effects on a wide variety of proinflammatory cytokines, such as IL-10 and transforming growth factor-β. Molecules that do not belong to the network of cytokines include glucocorticoids, neuropep-tides (such as vasopressin and alpha-melanotropin), and growth factors (such as insulin-like growth factor I) [1]. Any alteration in the balance between proinflammatory and anti-inflammatory cytokines, in the sense of a predominance of proinflammatory cytokines over anti-inflammatory cytokines, results in an exaggerated sickness response to activation of the peripheral immune system or direct activation of the brain cytokine system. This is shown to be the case in aged mice [58] and in obese mice [59]. These two conditions are associated with a low-grade inflammation status that, because of the immune-to-brain communication, leads to priming or sensitization of brain microglial cells [60]. Superimposed on this low-grade inflammation status, a peripheral infectious episode leads to exaggerated synthesis of inflammatory cytokines and other mediators in the brain, which in turn have an impact on behavior and mood or exacerbate the progression of chronic neurodegenerative disease.
Pathophysiologic implications
Based on previous evidence, sickness behavior seems to be nothing else than the outward expression of a reversible episode of cytokine expression and action in the brain in response to peripheral immune stimulation. Sickness behavior, however, is not a passive response, in the form of a temporary disappearance of the usual activities of the host. The proinflammatory cytokines that are produced by activated innate immune cells serve as sensory signals that are recognized and interpreted by the brain. The brain representation of peripheral immune activation resets the organism's priorities to enable the subjects at risk to deal with infection in the most efficient way allowed to them. The expression of sickness behavior is not simply the result of the changes in internal state experienced by sick subjects but the joint function of the changes in their internal state and the environmental constraints to which they are exposed [1,61]. This is characteristic of motivated behavior. An experiment performed on sickness behavior in lactating mice, in which caring for their pups is the predominant motivation, provides a good example of the motivational conflict that can take place in sick individuals. Lactating mice were made sick by an appropriate dose of systemic LPS. They remained inactive and indifferent to the solicitations of their pups until the pups were dispersed in the cage and the nest was removed. In this situation, their maternal motivation took over their sickness motivation, and they engaged in pup retrieval. Furthermore, when provided with cotton wool, which they normally use to build a nest, they did not engage in nest building in addition to pup retrieval unless the ambient temperature dropped to 6° C [62].
Because cytokine-induced sickness behavior is the expression of a motivational state that is triggered by activation of the peripheral innate immune system, it is not pathologic per se but as normal as the fear response that occurs in individuals exposed to the threat of a predator (Fig. 2). Like fear, sickness behavior can become abnormal or pathologic when it occurs out of context (ie, in the absence of any inflammatory stimulus) or when it is exaggerated in intensity or duration. Several conditions can be responsible for this situation: (1) proinflammatory cytokines can be produced in higher quantities and for a longer duration than normal; (2) the regulatory molecules that normally downregulate activation of the molecular and cellular components of the sickness response are faulty; or (3) the neuronal circuits that are the targets of inflammatory mediators and organize sickness behavior become sensitized. Because of the close similarities between symptoms of sickness and clinical signs of depression, any of these conditions is likely a risk factor for the occurrence of major depressive disorders. Evidence for the possibility of a shift from sickness behavior to depression is available from two different sources, clinical research and experimental studies on animal models of depressive disorders.
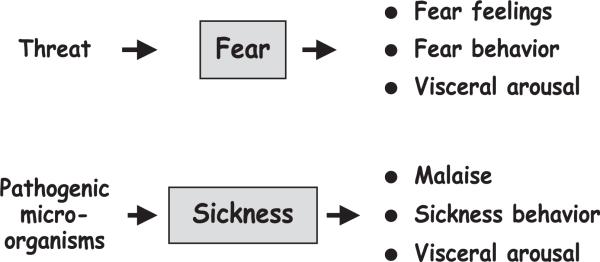
Fig. 2.
Motivational model of sickness. Like fear, sickness has motivational properties in the sense that it organizes the organism's functioning at three levels—subjective, behavioral, and visceral—so as to cope with the threat to which the organism is exposed.
At the clinical level, there is growing evidence that major depression is associated with significant elevations in circulating levels of proinflammatory cytokines, in particular IL-6 [63–68]. (See the article by Irwin and colleagues elsewhere in this issue for further discussion of the relationship between depression and immunity.) Conversely, chronic activation of the innate immune system can precipitate the development of depressive disorders, as exemplified by the psychopathologic alterations that occur in patients receiving repeated injections of recombinant cytokines, mainly IL-2 or interferon (INF)-α, for the treatment of viral infections (hepatitis C) or cancer. During the first stages of cytokine therapy, all patients usually develop a full-blown episode of sickness behavior, characterized by the symptoms of fever, malaise, anorexia, pain, and fatigue. At later stages of treatment, up to one third of patients develop alterations in mood that are characteristic of depression, including sadness, inability to feel, depressed mood, and even suicidal ideation [69]. The onset of depressive symptoms depends on the cytokine and treatment modalities (eg, dosage and administration route). The occurrence of depression can be prevented by pretreatment with paroxetine, a selective serotonin reuptake inhibitor with antidepressant properties. Nevertheless, pretreatment with paroxetine has a minimal or null effect on the development of neurovegetative symptoms of sickness, including fever, fatigue, and anorexia, confirming the dissociation between sickness behavior and depression [70].
These findings can be interpreted to suggest that depressive disorders develop from cytokine-induced sickness behavior only in vulnerable patients (Fig. 3). Vulnerability, in the present context, refers to an innate or acquired predisposition to develop a given pathology when causal factors are present. Dysfunction in genes controlling key proteins in serotoninergic neurotrans-mission (eg, activity of the serotonin transporter [71]) or childhood trauma [72] are identified as vulnerability factors for depression. Vulnerability to cytokine-induced depression can be revealed by psychologic features. Patients who have high scores on depression scales (including the Montgomery-Asberg Depression Rating Scale and the Hamilton Depression Rating Scale) at the start of cytokine treatment are more likely to develop depressive syndrome in response to immunotherapy than patients who have a low score at baseline [73,74]. Vulnerability also can be revealed by physiologic features. Patients who respond to the first injection of IFN-α by an exaggerated pituitary-adrenal response are more likely to become depressed in response to repeated administration of IFN-α than patients who display a lower pituitary-adrenal response [75]. These two different characteristics are markers of vulnerability. They can help to identify patients who are at risk, but they do not explain why patients who have these characteristics are more vulnerable than those who do not have them. In particular, it remains to be elucidated whether or not the risk factors for developing depression during cytokine therapy are the same as those accounting for depressive disorders in psychiatric patients or whether or not these risk factors are specific to cytokines and involve, for instance, polymorphisms in genes involved in cytokine production.
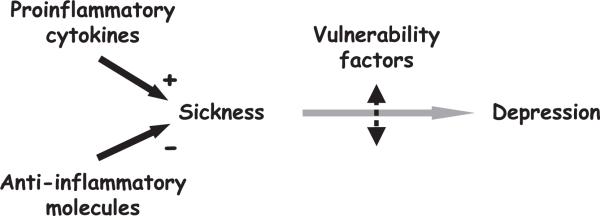
Fig. 3.
The two-hit model of cytokine-induced depression. Production of proinflammatory cytokines induces sickness behavior that usually is terminated by endogenous anti-inflammatory molecules. Sustained production of proinflammatory cytokines in the context of insufficient production of anti-inflammatory molecules can lead to depression in vulnerable individuals. Many factors, acquired or genetic, can contribute to vulnerability.
The model of cytokine-induced depression has the advantage of providing clinicians with the possibility of observing development of depressive symptoms over time in a large number of patients who can be monitored closely from the time they start receiving immunotherapy. Furthermore, patients who develop depression can be compared transversally to patients who remain free of any mood disorder. The model of cytokine-induced depression, therefore, provides valuable insights into the relationship between cytokines and depression. At the clinical level, there is evidence that symptoms of mood disorder are more polymorphic than just depression. A recent study of patients who had hepatitis C and were treated with IFN-α shows, for instance, that dysphoria and mixed states dominate the clinical presentation of patients, with increases in irritability and anxiety as the main symptoms [76]. The reasons for the differences between patients who have hepatitis C and patients who have cancer who present mainly with depressed mood are not known. They could be because of medical context (cytokine immunotherapy is palliative only for patients who have cancer, whereas it usually is curative for patients who have hepatitis C), immunologic context (immune responses of patients infected by a virus are different from those of patients who have cancer), or simply variations in treatment modalities (high doses of IFN-α administered intravenously and daily to patients who have malignant melanoma versus low doses of pegylated IFN-α administered once a week to patients who have chronic hepatitis C). These variations also could be related to differences in affective and psychiatric background, because many patients who have chronic hepatitis C have a history of substance abuse.
At the pathophysiologic level, an insight into the chain of events linking cytokines to mood alterations has emerged from the observation that patients who have cancer and are treated with cytokines develop a drastic decrease in plasma tryptophan levels that correlates with depression scores at 4 weeks of treatment [77]. This decrease in plasma trytophan levels previously was noted but in a qualitative rather than quantitative manner [78]. These findings are important, because bioavailability of tryptophan is the limiting factor for the synthesis of serotonin. The acute depletion of tryptophan produced by feeding excess amounts of large neutral aminoacids that compete with tryptophan for entry into the brain results in the development of depressed mood in subjects at risk for depression. A likely candidate for this decrease in plasma tryptophan in patients submitted to cytokine immunotherapy is the enzyme, indoleamine 2,3-dioxygenase (IDO), that degrades tryptophan into kynurenine and quinolinic acid (Fig. 4). IDO is present in macrophages and monocytes, endothelial cells, and brain glial cells. It is potently activated by proinflammatory cytokines, such as TNF-α and IFN-γ, both at the periphery and in the brain [79]. Its activation results in a decrease in tryptophan bioavailability for the synthesis of serotonin and in the formation of neuroactive compounds, such as kynurenine and quinolinic acid, that act, respectively, as antagonist and agonist of glutamate receptors. The interference of proinflammatory cytokines with serotoninergic neurotransmission can explain some of the clinical signs, such as impulsivity and depressed mood, that develop in vulnerable patients. It does not account, however, for the anhedonia, fatigue, and psychomotor retardation observed in patients treated with cytokine [69]. These symptoms probably reflect a decrease in dopaminergic neurotransmission. This hypothesis is supported by neuroimaging studies showing alterations in the activity of basal ganglia during cytokine therapy [80,81].
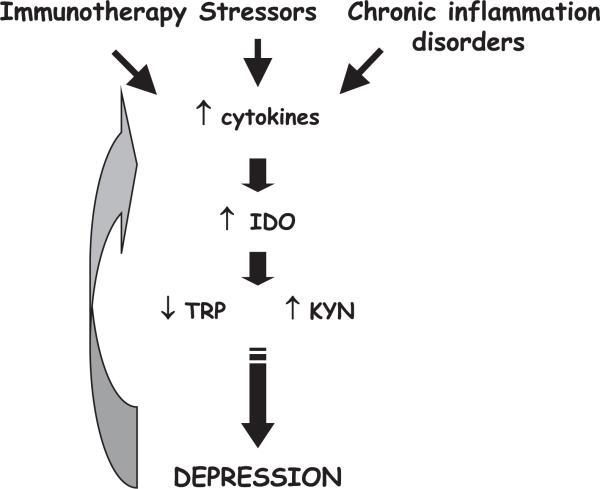
Fig. 4.
Mechanisms of the depressing effects of cytokines on mood. Activation of the innate immune system is triggered by cytokine immunotherapy or psychosocial stressors (via a β2adrenergic receptor). It results in the overproduction of proinflammatory cytokines. The same condition occurs during chronic inflammation. Cytokines, such as TNF-α and IFN-γ, increase activity of the enzyme, IDO, that degrades tryptophan along the kynurenine/quinolinic acid metabolic pathway, resulting in a decrease in tryptophan and an increase in kynurenine. The decreased tryptophan bioavailability leads to decreased serotoninergic neurotransmission and depressed mood. Depression itself can be accompanied by altered immunity, including activation of the innate immune system, further increasing the proinflammatory cytokine load.
Despite its heuristic value, the clinical relevance of the model of cytokine-induced depression could be questioned, because it is a rather extreme situation. There is evidence, however, that overproduction of proinflammatory cytokines also is associated with mood disorders in chronic inflammatory medical conditions, including coronary heart disease. Of course, the relationship between cytokines and depression is less easy to reveal, because patients who have such medical conditions are examined at different stages of their disease process. This may result in much higher interindividual variability. Despite these constraints, it is possible to observe higher levels of myocardial cytokines and higher antibody titers against microbial pathogens possibly involved in the pathophysiology of coronary heart disease in patients suffering from vital exhaustion at the time of coronary bypass [82]. In the same manner, depressive disorders that develop over time in postischemic coronary patients are associated with endothelial cell activation and probably inflammation, although the therapeutic use of statins that have anti-inflammatory actions attenuates differences betweens cases and controls [83].
Summary
Sufficient evidence is now available to accept the concept that the brain recognizes cytokines as molecular signals of sickness. Clarifying the way the brain processes information generated by the innate immune system is accompanied by a progressive elucidation of the cellular and molecular components of the intricate system that mediates cytokine-induced sickness behavior. We are still far, however, from understanding the whole. Among the hundreds of genes that proinflammatory cytokines can induce in their cellular targets, only a handful has been examined functionally. In addition, a dynamic view of the cellular interactions that occur at the brain sites of cytokine production and action is missing, together with a clarification of the mechanisms that favor the transition toward pathology.
Acknowledgments
Supported by Institut National de la Recherche Agronomique, Centre National de la Recherche Scientifique, University of Bordeaux 2, and National Institutes of Health (MH 71349).
References
- 1.Dantzer R. Cytokine-induced sickness behavior: where do we stand? Brain Behav Immun. 2001;15:7–24. doi: 10.1006/brbi.2000.0613. [DOI] [PubMed] [Google Scholar]
- 2.Konsman JP, Parnet P, Dantzer R. Cytokine-induced sickness behaviour: mechanisms and implications. Trends Neurosci. 2002;25:154–9. doi: 10.1016/s0166-2236(00)02088-9. [DOI] [PubMed] [Google Scholar]
- 3.Barton GM, Medzhitov R. Toll-like receptor signaling pathways. Science. 2003;300:1524–5. doi: 10.1126/science.1085536. [DOI] [PubMed] [Google Scholar]
- 4.Romanovsky A, Almeida MC, Aronoff DM, et al. Fever and hypothermia in systemic inflammation: recent discoveries and revisions. Front Biosci. 2005;10:2193–216. doi: 10.2741/1690. [DOI] [PubMed] [Google Scholar]
- 5.Berkenbosch F, van Oers J, del Rey A, et al. Corticotropin-releasing factor-producing neurons in the rat activated by interleukin-1. Science. 1987;238:524–6. doi: 10.1126/science.2443979. [DOI] [PubMed] [Google Scholar]
- 6.Ericsson A, Kovacs KJ, Sawchenko PE. A functional anatomical analysis of central pathways subserving the effects of interleukin-1 on stress-related neuroendocrine neurons. J Neurosci. 1994;14:897–913. doi: 10.1523/JNEUROSCI.14-02-00897.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goehler LE, Gaykema RP, Nguyen KT, et al. Interleukin-1beta in immune cells of the abdominal vagus nerve: a link between the immune and nervous systems? J Neurosci. 1999;19:2799–806. doi: 10.1523/JNEUROSCI.19-07-02799.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ek M, Kurosawa M, Lundeberg T, et al. Activation of vagal afferents after intravenous injection of interleukin-1beta: role of endogenous prostaglandins. J Neurosci. 1998;18:9471–9. doi: 10.1523/JNEUROSCI.18-22-09471.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wan W, Wetmore L, Sorensen CM, et al. Neural and biochemical mediators of endotoxin and stress-induced c-fos expression in the rat brain. Brain Res Bull. 1994;34:7–14. doi: 10.1016/0361-9230(94)90179-1. [DOI] [PubMed] [Google Scholar]
- 10.Bluthe RM, Walter V, Parnet P, et al. Lipopolysaccharide induces sickness behaviour in rats by a vagal mediated mechanism. C R Acad Sci III. 1994;317:499–503. [PubMed] [Google Scholar]
- 11.Bluthe RM, Michaud B, Kelley KW, et al. Vagotomy attenuates behavioural effects of interleukin-1 injected peripherally but not centrally. Neuroreport. 1996;7:1485–8. doi: 10.1097/00001756-199606170-00008. [DOI] [PubMed] [Google Scholar]
- 12.Bluthe RM, Michaud B, Kelley KW, et al. Vagotomy blocks behavioural effects of interleukin-1 injected via the intraperitoneal route but not via other systemic routes. Neuroreport. 1996;7:2823–7. doi: 10.1097/00001756-199611040-00083. [DOI] [PubMed] [Google Scholar]
- 13.Marvel FA, Chen CC, Badr N, et al. Reversible inactivation of the dorsal vagal complex blocks lipopolysaccharide-induced social withdrawal and c-Fos expression in central autonomic nuclei. Brain Behav Immun. 2004;18:123–34. doi: 10.1016/j.bbi.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 14.Romeo HE, Tio DL, Taylor AN. Effects of glossopharyngeal nerve transection on central and peripheral cytokines and serum corticosterone induced by localized inflammation. J Neuroimmunol. 2003;136:104–11. doi: 10.1016/s0165-5728(03)00033-x. [DOI] [PubMed] [Google Scholar]
- 15.Romeo HE, Tio DL, Rahman SU, et al. The glossopharyngeal nerve as a novel pathway in immune-to-brain communication: relevance to neuroimmune surveillance of the oral cavity. J Neuroimmunol. 2001;115:91–100. doi: 10.1016/s0165-5728(01)00270-3. [DOI] [PubMed] [Google Scholar]
- 16.Konsman JP, Luheshi GN, Bluthe RM, et al. The vagus nerve mediates behavioural depression, but not fever, in response to peripheral immune signals; a functional anatomical analysis. Eur J Neurosci. 2000;12:4434–46. doi: 10.1046/j.0953-816x.2000.01319.x. [DOI] [PubMed] [Google Scholar]
- 17.Rivest S, Lacroix S, Vallieres L, et al. How the blood talks to the brain parenchyma and the paraventricular nucleus of the hypothalamus during systemic inflammatory and infectious stimuli. Proc Soc Exp Biol Med. 2000;223:22–38. doi: 10.1046/j.1525-1373.2000.22304.x. [DOI] [PubMed] [Google Scholar]
- 18.Romanovsky AA. Thermoregulatory manifestations of systemic inflammation: lessons from vagotomy. Auton Neurosci. 2000;85:39–48. doi: 10.1016/S1566-0702(00)00218-6. [DOI] [PubMed] [Google Scholar]
- 19.Laye S, Gheusi G, Cremona S, et al. Endogenous brain IL-1 mediates LPS-induced anorexia and hypothalamic cytokine expression. Am J Physiol Regul Integr Comp Physiol. 2000;279:R93–8. doi: 10.1152/ajpregu.2000.279.1.R93. [DOI] [PubMed] [Google Scholar]
- 20.Kent S, Bluthe RM, Dantzer R, et al. Different receptor mechanisms mediate the pyrogenic and behavioral effects of interleukin 1. Proc Natl Acad Sci U S A. 1992;89:9117–20. doi: 10.1073/pnas.89.19.9117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Dam AM, Brouns M, Louisse S, et al. Appearance of interleukin-1 in macrophages and in ramified microglia in the brain of endotoxin-treated rats: a pathway for the induction of non-specific symptoms of sickness? Brain Res. 1992;588:291–6. doi: 10.1016/0006-8993(92)91588-6. [DOI] [PubMed] [Google Scholar]
- 22.Konsman JP, Kelley K, Dantzer R. Temporal and spatial relationships between lipopolysaccharide-induced expression of Fos, interleukin-1beta and inducible nitric oxide synthase in rat brain. Neuroscience. 1999;89:535–48. doi: 10.1016/s0306-4522(98)00368-6. [DOI] [PubMed] [Google Scholar]
- 23.Ericsson A, Liu C, Hart RP, et al. Type 1 interleukin-1 receptor in the rat brain: distribution, regulation, and relationship to sites of IL-1-induced cellular activation. J Comp Neurol. 1995;361:681–98. doi: 10.1002/cne.903610410. [DOI] [PubMed] [Google Scholar]
- 24.Vitkovic L, Konsman JP, Bockaert J, et al. Cytokine signals propagate through the brain. Mol Psychiatry. 2000;5:604–15. doi: 10.1038/sj.mp.4000813. [DOI] [PubMed] [Google Scholar]
- 25.Reyes TM, Sawchenko PE. Involvement of the arcuate nucleus of the hypothalamus in interleukin-1-induced anorexia. J Neurosci. 2002;22:5091–9. doi: 10.1523/JNEUROSCI.22-12-05091.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laye S, Bluthe RM, Kent S, et al. Subdiaphragmatic vagotomy blocks induction of IL-1 beta mRNA in mice brain in response to peripheral LPS. Am J Physiol. 1995;268(5 Pt 2):R1327–31. doi: 10.1152/ajpregu.1995.268.5.R1327. [DOI] [PubMed] [Google Scholar]
- 27.Hansen MK, Nguyen KT, Goehler LE, et al. Effects of vagotomy on lipopolysaccharide-induced brain interleukin-1beta protein in rats. Auton Neurosci. 2000;85:119–26. doi: 10.1016/s1566-0702(00)00230-7. [DOI] [PubMed] [Google Scholar]
- 28.Hosoi T, Okuma Y, Nomura Y. Electrical stimulation of afferent vagus nerve induces IL-1beta expression in the brain and activates HPA axis. Am J Physiol Regul Integr Comp Physiol. 2000;279:R141–7. doi: 10.1152/ajpregu.2000.279.1.R141. [DOI] [PubMed] [Google Scholar]
- 29.Dantzer R, Konsman JP, Bluthe RM, et al. Neural and humoral pathways of communication from the immune system to the brain: parallel or convergent? Auton Neurosci. 2000;85:60–5. doi: 10.1016/S1566-0702(00)00220-4. [DOI] [PubMed] [Google Scholar]
- 30.Dunn AJ, Wang J, Ando T. Effects of cytokines on cerebral neurotransmission. Comparison with the effects of stress. Adv Exp Med Biol. 1999;461:117–27. doi: 10.1007/978-0-585-37970-8_8. [DOI] [PubMed] [Google Scholar]
- 31.Anforth HR, Bluthe RM, Bristow A, et al. Biological activity and brain actions of recombinant rat interleukin-1alpha and interleukin-1beta. Eur Cytokine Netw. 1998;9:279–88. [PubMed] [Google Scholar]
- 32.Lenczowski MJ, Bluthe RM, Roth J, et al. Central administration of rat IL-6 induces HPA activation and fever but not sickness behavior in rats. Am J Physiol. 1999;276(3 Pt 2):R652–8. doi: 10.1152/ajpregu.1999.276.3.R652. [DOI] [PubMed] [Google Scholar]
- 33.Bluthe RM, Michaud B, Poli V, et al. Role of IL-6 in cytokine-induced sickness behavior: a study with IL-6 deficient mice. Physiol Behav. 2000;70:367–73. doi: 10.1016/s0031-9384(00)00269-9. [DOI] [PubMed] [Google Scholar]
- 34.Bluthe RM, Laye S, Michaud B, et al. Role of interleukin-1beta and tumour necrosis factor-alpha in lipopolysaccharide-induced sickness behaviour: a study with interleukin-1 type I receptor-deficient mice. Eur J Neurosci. 2000;12:4447–56. [PubMed] [Google Scholar]
- 35.Konsman JP, Tridon V, Dantzer R. Diffusion and action of intracerebroventricularly injected interleukin-1 in the CNS. Neuroscience. 2000;101:957–67. doi: 10.1016/s0306-4522(00)00403-6. [DOI] [PubMed] [Google Scholar]
- 36.Burgess W, Gheusi G, Yao J, et al. Interleukin-1beta-converting enzyme-deficient mice resist central but not systemic endotoxin-induced anorexia. Am J Physiol. 1998;274(6 Pt 2):R1829–33. doi: 10.1152/ajpregu.1998.274.6.R1829. [DOI] [PubMed] [Google Scholar]
- 37.Li X, Qin J. Modulation of Toll-interleukin 1 receptor mediated signaling. J Mol Med. 2005;83:258–66. doi: 10.1007/s00109-004-0622-4. [DOI] [PubMed] [Google Scholar]
- 38.Cullinan EB, Kwee L, Nunes P, et al. IL-1 receptor accessory protein is an essential component of the IL-1 receptor. J Immunol. 1998;161:5614–20. [PubMed] [Google Scholar]
- 39.French RA, VanHoy RW, Chizzonite R, et al. Expression and localization of p80 and p68 interleukin-1 receptor proteins in the brain of adult mice. J Neuroimmunol. 1999;93:194–202. doi: 10.1016/s0165-5728(98)00224-0. [DOI] [PubMed] [Google Scholar]
- 40.Katsuura G, Gottschall PE, Arimura A. Identification of a high-affinity receptor for inter-leukin-1 beta in rat brain. Biochem Biophys Res Commun. 1988;156:61–7. doi: 10.1016/s0006-291x(88)80805-2. [DOI] [PubMed] [Google Scholar]
- 41.Nishiyori A, Minami M, Takami S, et al. Type 2 interleukin-1 receptor mRNA is induced by kainic acid in the rat brain. Brain Res Mol Brain Res. 1997;50:237–45. doi: 10.1016/s0169-328x(97)00195-2. [DOI] [PubMed] [Google Scholar]
- 42.Docagne F, Campbell SJ, Bristow AF, et al. Differential regulation of type I and type II interleukin-1 receptors in focal brain inflammation. Eur J Neurosci. 2005;21:1205–14. doi: 10.1111/j.1460-9568.2005.03965.x. [DOI] [PubMed] [Google Scholar]
- 43.Ilyin SE, Plata-Salaman CR. In vivo regulation of the IL-1 beta system (ligand, receptors I and II, receptor accessory protein, and receptor antagonist) and TNF-alpha mRNAs in specific brain regions. Biochem Biophys Res Commun. 1996;227:861–7. doi: 10.1006/bbrc.1996.1597. [DOI] [PubMed] [Google Scholar]
- 44.Takao T, Tracey DE, Mitchell WM, et al. Interleukin-1 receptors in mouse brain: characterization and neuronal localization. Endocrinology. 1990;127:3070–8. doi: 10.1210/endo-127-6-3070. [DOI] [PubMed] [Google Scholar]
- 45.Ban E, Milon G, Prudhomme N, et al. Receptors for interleukin-1 (alpha and beta) in mouse brain: mapping and neuronal localization in hippocampus. Neuroscience. 1991;43:21–30. doi: 10.1016/0306-4522(91)90412-h. [DOI] [PubMed] [Google Scholar]
- 46.Parnet P, Amindari S, Wu C, et al. Expression of type I and type II interleukin-1 receptors in mouse brain. Brain Res Mol Brain Res. 1994;27:63–70. doi: 10.1016/0169-328x(94)90185-6. [DOI] [PubMed] [Google Scholar]
- 47.Konsman JP, Vigues S, Mackerlova L, et al. Rat brain vascular distribution of interleukin-1 type-1 receptor immunoreactivity: relationship to patterns of inducible cyclooxygenase expression by peripheral inflammatory stimuli. J Comp Neurol. 2004;472:113–29. doi: 10.1002/cne.20052. [DOI] [PubMed] [Google Scholar]
- 48.Nadjar A, Combe C, Laye S, et al. Nuclear factor kappaB nuclear translocation as a crucial marker of brain response to interleukin-1. A study in rat and interleukin-1 type I deficient mouse. J Neurochem. 2003;87:1024–36. doi: 10.1046/j.1471-4159.2003.02097.x. [DOI] [PubMed] [Google Scholar]
- 49.Gabellec MM, Jafarian-Tehrani M, et al. Interleukin-1 receptor accessory protein transcripts in the brain and spleen: kinetics after peripheral administration of bacterial lipopolysaccharide in mice. Neuroimmunomodulation. 1996;3:304–9. doi: 10.1159/000097284. [DOI] [PubMed] [Google Scholar]
- 50.Liu C, Chalmers D, Maki R, et al. Rat homolog of mouse interleukin-1 receptor accessory protein: cloning, localization and modulation studies. J Neuroimmunol. 1996;66:41–8. doi: 10.1016/0165-5728(96)00016-1. [DOI] [PubMed] [Google Scholar]
- 51.Cremona S, Goujon E, Kelley KW, et al. Brain type I but not type II IL-1 receptors mediate the effects of IL-1 beta on behavior in mice. Am J Physiol. 1998;274(3 Pt 2):R735–40. doi: 10.1152/ajpregu.1998.274.3.R735. [DOI] [PubMed] [Google Scholar]
- 52.Sonti G, Flynn MC, Plata-Salaman CR. Interleukin-1 (IL-1) receptor type I mediates anorexia but not adipsia induced by centrally administered IL-1beta. Physiol Behav. 1997;62:1179–83. doi: 10.1016/s0031-9384(97)80019-4. [DOI] [PubMed] [Google Scholar]
- 53.Liege S, Laye S, Li KS, et al. Interleukin 1 receptor accessory protein (IL-1RAcP) is necessary for centrally mediated neuroendocrine and immune responses to IL-1beta. J Neuroimmunol. 2000;110:134–9. doi: 10.1016/s0165-5728(00)00331-3. [DOI] [PubMed] [Google Scholar]
- 54.Quan N, Whiteside M, Kim L, et al. Induction of inhibitory factor kappaBalpha mRNA in the central nervous system after peripheral lipopolysaccharide administration: an in situ hybridization histochemistry study in the rat. Proc Natl Acad Sci U S A. 1997;94:10985–90. doi: 10.1073/pnas.94.20.10985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kubota T, Kushikata T, Fang J, et al. Nuclear factor-kappaB inhibitor peptide inhibits spontaneous and interleukin-1beta-induced sleep. Am J Physiol Regul Integr Comp Physiol. 2000;279:R404–13. doi: 10.1152/ajpregu.2000.279.2.R404. [DOI] [PubMed] [Google Scholar]
- 56.Nadjar A, Bluthe RM, May MJ, et al. Inactivation of the cerebral NFkappaB pathway inhibits interleukin-1beta-induced sickness behavior and c-Fos expression in various brain nuclei. Neuropsychopharmacology. 2005;30:1492–9. doi: 10.1038/sj.npp.1300755. [DOI] [PubMed] [Google Scholar]
- 57.Kelly A, Vereker E, Nolan Y, et al. Activation of p38 plays a pivotal role in the inhibitory effect of lipopolysaccharide and interleukin-1 beta on long term potentiation in rat dentate gyrus. J Biol Chem. 2003;278:19453–62. doi: 10.1074/jbc.M301938200. [DOI] [PubMed] [Google Scholar]
- 58.Godbout JP, Chen J, Abraham J, et al. Exaggerated neuroinflammation and sickness behavior in aged mice following activation of the peripheral innate immune system. FASEB J. 2005;19:1329–31. doi: 10.1096/fj.05-3776fje. [DOI] [PubMed] [Google Scholar]
- 59.O'Connor JC, Satpathy A, Hartman ME, et al. IL-1beta-mediated innate immunity is amplified in the db/db mouse model of type 2 diabetes. J Immunol. 2005;174:4991–7. doi: 10.4049/jimmunol.174.8.4991. [DOI] [PubMed] [Google Scholar]
- 60.Perry VH. The influence of systemic inflammation on inflammation in the brain: implications for chronic neurodegenerative disease. Brain Behav Immun. 2004;18:407–13. doi: 10.1016/j.bbi.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 61.Hart BL. Biological basis of the behavior of sick animals. Neurosci Biobehav Rev. 1988;12:123–37. doi: 10.1016/s0149-7634(88)80004-6. [DOI] [PubMed] [Google Scholar]
- 62.Aubert A, Goodall G, Dantzer R, et al. Differential effects of lipopolysaccharide on pup retrieving and nest building in lactating mice. Brain Behav Immun. 1997;11:107–18. doi: 10.1006/brbi.1997.0485. [DOI] [PubMed] [Google Scholar]
- 63.Lutgendorf SK, Garand L, Buckwalter KC, et al. Life stress, mood disturbance, and elevated interleukin-6 in healthy older women. J Gerontol A Biol Sci Med Sci. 1999;54:M434–9. doi: 10.1093/gerona/54.9.m434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kiecolt-Glaser JK, Glaser R. Depression and immune function: central pathways to morbidity and mortality. J Psychosom Res. 2002;53:873–6. doi: 10.1016/s0022-3999(02)00309-4. [DOI] [PubMed] [Google Scholar]
- 65.Alesci S, Martinez PE, Kelkar S, et al. Major depression is associated with significant diurnal elevations in plasma interleukin-6 levels, a shift of its circadian rhythm, and loss of physiological complexity in its secretion: clinical implications. J Clin Endocrinol Metab. 2005;90:2522–30. doi: 10.1210/jc.2004-1667. [DOI] [PubMed] [Google Scholar]
- 66.Maes M, Bosmans E, Meltzer HY. Immunoendocrine aspects of major depression. Relationships between plasma interleukin-6 and soluble interleukin-2 receptor, prolactin and cortisol. Eur Arch Psychiatry Clin Neurosci. 1995;245:172–8. doi: 10.1007/BF02193091. [DOI] [PubMed] [Google Scholar]
- 67.Maes M, Bosmans E, De Jongh R, et al. Increased serum IL-6 and IL-1 receptor antagonist concentrations in major depression and treatment resistant depression. Cytokine. 1997;9:853–8. doi: 10.1006/cyto.1997.0238. [DOI] [PubMed] [Google Scholar]
- 68.Brambilla F, Maggioni M. Blood levels of cytokines in elderly patients with major depressive disorder. Acta Psychiatr Scand. 1998;97:309–13. doi: 10.1111/j.1600-0447.1998.tb10005.x. [DOI] [PubMed] [Google Scholar]
- 69.Capuron L, Ravaud A, Miller AH, et al. Baseline mood and psychosocial characteristics of patients developing depressive symptoms during interleukin-2 and/or interferon-alpha cancer therapy. Brain Behav Immun. 2004;18:205–13. doi: 10.1016/j.bbi.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 70.Capuron L, Gumnick JF, Musselman DL, et al. Neurobehavioral effects of interferon-alpha in cancer patients: phenomenology and paroxetine responsiveness of symptom dimensions. Neuropsychopharmacology. 2002;26:643–52. doi: 10.1016/S0893-133X(01)00407-9. [DOI] [PubMed] [Google Scholar]
- 71.Jones I, Craddock N. Candidate gene studies of bipolar disorder. Ann Med. 2001;33:248–56. doi: 10.3109/07853890108998753. [DOI] [PubMed] [Google Scholar]
- 72.Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatry. 2001;49:1023–39. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- 73.Capuron L, Ravaud A. Prediction of the depressive effects of interferon alfa therapy by the patient's initial affective state. N Engl J Med. 1999;340:1370. doi: 10.1056/NEJM199904293401716. [DOI] [PubMed] [Google Scholar]
- 74.Miyaoka H, Otsubo T, Kamijima K, et al. Depression from interferon therapy in patients with hepatitis C. Am J Psychiatry. 1999;156:1120. doi: 10.1176/ajp.156.7.1120. [DOI] [PubMed] [Google Scholar]
- 75.Capuron L, Neurauter G, Musselman DL, et al. Interferon-alpha-induced changes in tryptophan metabolism. Relationship to depression and paroxetine treatment. Biol Psychiatry. 2003;54:906–14. doi: 10.1016/s0006-3223(03)00173-2. [DOI] [PubMed] [Google Scholar]
- 76.Constant A, Castera L, Dantzer R, et al. Mood alterations during interferon-alfa therapy in patients with chronic hepatitis C: evidence for an overlap between manic/hypomanic and depressive symptoms. J Clin Psychiatry. 2005;66:1050–7. doi: 10.4088/jcp.v66n0814. [DOI] [PubMed] [Google Scholar]
- 77.Capuron L, Ravaud A, Neveu PJ, et al. Association between decreased serum tryptophan concentrations and depressive symptoms in cancer patients undergoing cytokine therapy. Mol Psychiatry. 2002;7:468–73. doi: 10.1038/sj.mp.4000995. [DOI] [PubMed] [Google Scholar]
- 78.Maes M, Bonaccorso S, Marino V, et al. Treatment with interferon-alpha (IFN alpha) of hepatitis C patients induces lower serum dipeptidyl peptidase IV activity, which is related to IFN alpha-induced depressive and anxiety symptoms and immune activation. Mol Psychiatry. 2001;6:475–80. doi: 10.1038/sj.mp.4000872. [DOI] [PubMed] [Google Scholar]
- 79.Lestage J, Verrier D, Palin K, et al. The enzyme indoleamine 2,3-dioxygenase is induced in the mouse brain in response to peripheral administration of lipopolysaccharide and super-antigen. Brain Behav Immun. 2002;16:596–601. doi: 10.1016/s0889-1591(02)00014-4. [DOI] [PubMed] [Google Scholar]
- 80.Juengling FD, Ebert D, Gut O, et al. Prefrontal cortical hypometabolism during low-dose interferon alpha treatment. Psychopharmacology (Berl) 2000;152:383–9. doi: 10.1007/s002130000549. [DOI] [PubMed] [Google Scholar]
- 81.Capuron L, Pagnoni G, Fornwalt FB, et al. Brain metabolic changes and neurovegetative symptoms in medically ill patients undergoing interferon-alpha therapy. Soc Neurosci Abstr. 2005;660:23. [Google Scholar]
- 82.Appels A, Bar FW, Bar J, et al. Inflammation, depressive symptomtology, and coronary artery disease. Psychosom Med. 2000;62:601–5. doi: 10.1097/00006842-200009000-00001. [DOI] [PubMed] [Google Scholar]
- 83.Lesperance F, Frasure-Smith N, Theroux P, et al. The association between major depression and levels of soluble intercellular adhesion molecule 1, interleukin-6, and C-reactive protein in patients with recent acute coronary syndromes. Am J Psychiatry. 2004;161:271–7. doi: 10.1176/appi.ajp.161.2.271. [DOI] [PubMed] [Google Scholar]