Abstract
This study evaluated the relationship between sleep quality in late pregnancy and recurrence of postpartum major depression (PPMD) through 28 weeks postpartum. The Pittsburgh Sleep Quality Index (PSQI) at 36 weeks gestation was assessed in 51 non-depressed women with a history of PPMD; recurrence was determined by the 21-item Hamilton Rating Scale for Depression and the Schedule for Affective Disorders and Schizophrenia. Sleep quality in late pregnancy was not related to recurrence per se, but it was related to timing of recurrence (Kruskal–Wallace = 9.78, p = .008). Rapid recurrence (within 4 weeks post delivery) was preceded by fewer sleep complaints (mean PSQI for early recurrers = 4.8 vs. 7.3 for non-recurrers, p = .09). Recurrence after 4 weeks postpartum was preceded by more sleep complaints in late pregnancy (mean PSQI for late recurrers = 9.9 vs. 7.3 for non-recurrers, p = .02). Sleep quality in late pregnancy may help in identifying women at risk for a PPMD recurrence.
Women are at the highest risk during their lifetimes for depressive episodes during the child-bearing years (O’Hara, Zekoski, Philipps, & Wright, 1990). Up to 20% of women will have an initial major depressive episode within the first 3 months postpartum (Gavin et al., 2005), with the risk of suffering recurrent PPMD about 25% (Wisner, Perel, Peindl, Hanusa, Piontek, & Finding, 2004b). PPMD is a serious public health concern (Gaynes et al., 2005a; Wisner, Chambers, & Sit, 2006). The maternal role, which is vital to the infant’s safety, survival, and well-being (Logsdon, Wisner, & Pinto-Foltz, 2006), can be compromised by PPMD. Children of mothers with PPMD are at an increased risk of impaired mental and motor development, poor self-regulation, and behavior problems (Alder, Fink, Bitzer, Hosli, & Holzgreve, 2007).
A myriad of factors contribute to the etiology of both incident and recurrent PPMD (Beck, 2001; Bloch, Daly, & Rubinow, 2003; Gaynes et al., 2005b). Among the established risk factors, previous episodes of depression, family history of depression (O’Hara, Schlechte, Lewis, & Varner, 1991; Robertson, Grace, Wallington, & Stewart, 2004), and depressive symptomatology during pregnancy are the strongest predictors for both incident and recurrent episodes. Demographic variables, including marital status, race, age, and socioeconomic status (SES), have also been implicated as risk factors (Beck, 2001; Ross, Campbell, Dennis, & Blackmore, 2006). Unfortunately, these recognized risk factors have proven inadequate at predicting which women will recur. Another potential risk factor that may increase the risk of PPMD is disturbed sleep during late pregnancy. Several investigators report that disturbed sleep is a prodromal symptom of both first-onset and recurrent depressive symptoms or episodes outside of the postpartum (Ford & Kamerow, 1989; Perlis, Giles, Buysse, Tu, & Kupfer, 1997), as well as during the post-partum (Coble et al., 1994; Swain, O’Hara, Starr, & Gorman, 1997; Wolfson, Crowley, Anwer, & Bassett, 2003). Wolfson and colleagues noted that women reporting more sleep disturbances in late pregnancy were more likely to have clinically significant depressive symptomatology at 2 to 4 weeks postpartum than those with few sleep disturbances. Jomeen and Martin (2007) observed that poor sleep quality in the first trimester, as characterized by the PSQI, was associated with increased depressive symptoms. Taken together, these findings suggest that sleep disturbances in late pregnancy increase vulnerability to PPMD, particularly in women who are susceptible, such as those with a history of PPMD (Wisner, Parry, & Piontek, 2002).
The objective of this study was to conduct a secondary analysis to evaluate the relation between sleep quality in late pregnancy and recurrent PPMD. With data collected from a study on prevention of PPMD in high-risk women, we examined whether sleep complaints in late pregnancy, as characterized by the PSQI, were associated with a new depressive episode through 28 weeks post delivery. Our hypothesis was that higher PSQI scores in late pregnancy would be associated with increased PPMD recurrence.
METHOD
Participants
Pregnant women (N = 51) with past histories of an episode of PPMD, enrolled in a randomized clinical trial (RCT) designed to test the efficacy of prophylactic antidepressant medication use, were included in the analyses. A detailed description of the participants and the procedures have been published elsewhere (Wisner et al., 2001). All participants reported at least one postpartum episode of major depression within 5 years of enrollment, but none were depressed at gestational Week 36 as assessed by the Schedule for Affective Disorders and Schizophrenia (SADS; Endicott & Spitzer, 1978). Participants whose 21-item Hamilton Rating Scale for Depression (21–HRSD; Hamilton, 1960) score exceeded 15 at Week 36 were excluded from the prevention intervention studies. Therefore, women with sleep disturbance in the context of a depressive episode were by definition excluded.
Procedures
Approval was obtained from the Case Western Reserve University institutional review board, which was the setting in which the original research was conducted. Written informed consent was obtained from all participants. Participants were administered questionnaires in person during interview sessions at 36 weeks gestation and weekly during the first 20 weeks postpartum (Wisner et al., 2001). The RCT of pharmacotherapy prophylaxis (nortriptyline, n = 26 vs. placebo, n = 25) was conducted over the initial 17 weeks postpartum followed by a 3-week drug taper period. The aim of the initial study was to provide treatment for the 13-week postpartum period of high risk for recurrence (Kendell, Chalmers, & Platz, 1987; Munk-Olsen, Laursen, Pedersen, Mors, & Mortensen, 2006) and allow for drug tapering prior to study exit. Women who remained well at the end of the trial were contacted at Weeks 26 through 28 postpartum (Wisner et al., 2001).
Measures and Instruments
The PSQI (Buysse, Reynolds, Monk, Berman, & Kupfer, 1989), a 19-item questionnaire, was used to measure habitual sleep quality over the previous month. It is comprised of seven subscales assessing habitual duration of sleep, nocturnal sleep disturbances, sleep latency, sleep quality, daytime dysfunction, sleep medication usage, and sleep efficiency. Each subscale has a possible score of 0 through 3, with an overall global score of 0 through 21. The PSQI and its psychometric properties have been validated in various psychiatric populations (Buysse et al., 1989), and recently in a study of pregnant women (Jomeen & Martin, 2007). In these reports, the sensitivity and specificity are favorable when using a cutoff of ≤ 5 (Buysse et al., 1989; Jomeen & Martin, 2007).
The 21–HRSD (Hamilton, 1960) is a commonly used measure of depressive symptomatology in clinical psychiatric research in both pregnant and postpartum populations (Wisner et al., 2001). Scores are continuous, with a range from 0 to 52.
Definition of Recurrence
Women were administered the 21–HRSD weekly during the first 20 weeks of the postpartum period to assist in identifying a recurrent episode (Wisner et al., 2001). If a woman endorsed symptoms of depression (21–HRSD ≥ 15), the participant was evaluated again within the following 7-day period. A second 21–HRSD ≥ 15 prompted a clinical evaluation by two board-certified psychiatrists that the clinical presentation met Diagnostic and Statistical Manual of Mental Disorders (4th ed., text rev. [DSM–IV–TR]; American Psychiatric Association, 2000) criteria for major depressive disorder. If both evaluators concurred, the participant was identified as having a depression recurrence.
Statistical Analyses
We examined the overall relation between PSQI scores in late pregnancy and PPMD recurrence. Because we previously reported a distinct pattern in the timing of postpartum recurrence (Wisner, Perel, Peindl, & Hanusa, 2004), we also performed post hoc analyses to examine the same question based on timing of recurrence. We divided our sample into three groups: women who did not recur (n = 32), women who recurred within 4 weeks after delivery (n = 5), and women who recurred between 5 to 28 weeks post delivery (n = 14). We dichotomized women as based on PSQI total scores using criteria indicated by Buysse et al. (1989). Although the sensitivity and specificity are favorable when using a cutoff of ≤ 5 in non-pregnant cohorts (Buysse et al., 1989), pregnant women endorse significantly more sleep complaints than do non-pregnant women (Lee, Zaffke, & McEnany, 2000). Furthermore, the question of sleep medication usage provides little utility in this population (Jomeen & Martin, 2007). In a careful examination of the PSQI scores in this sample, the distributions showed that 35 (69%) women had a global score > 5, and only 4 (7.9%) used sleeping medications. These data indicate that the majority had significant sleep complaints. In this group of pregnant women, the mean and median were both 7. These results suggested that an alternate cutpoint of > 7 was more appropriate to distinguish good and poor sleepers during late pregnancy. However, no empirical evidence supports a cutoff of > 7; therefore, to explore a more suitable cutoff in pregnant women we categorized the PSQI into three groups: ≤ 5, 6 to 7, and >7. PSQI scores were used as either a continuous or categorized measure where appropriate.
To compare sleep quality among the three recurrence groups we used Fisher’s exact (FE) tests for the dichotomized PSQI and exact Kruskal–Wallace (KW) tests for the continuous PSQI. For later recurrences (LRs; Weeks 5–28), we compared time to recurrence and the PSQI measure using exact Tarone and Ware (1977) extensions of logrank tests for differences in time to survival. In addition to the tests of the PSQI variables, we also tested the univariable significance of several traditional risk factors associated with risk of PPMD recurrence. These included age, race (Caucasian or not Caucasian), marital status (married or not married), SES using the Hollingshead four-factor definition for SES (Hollingshead & Redlich, 1958), and 21–HRSD at gestational Week 36. Because of the small number of women in the early recurrence group, multivariable analyses were not completed. For early recurrences, demographic variables with nearly significant associations (p < .10) led to stratified analyses to check whether the relation between PSQI variables and recurrence were maintained in each strata.
For LRs, univariate comparisons of the demographic variables were completed with logrank tests. Variables with significance levels less than p = .10 were included in multivariable models of time to recurrence with PSQI scores. For LRs, we also tested whether PSQI scores and 21–HRSD scores obtained at Week 4 postpartum were related to time to recurrence. Seven component scores of the PSQI were compared among the three recurrence groups with FE tests.
Although our previous study (Wisner et al., 2001) reported no effect of drug assignment on recurrence, we tested the significance of drug assignment and the interaction between drug assignment and PSQI scores.
SPSS (v. 14.0, SPSS Inc., Chicago), Stata (v. 8, College Station, TX), and StatXact (v. 8, Cytel Inc., Cambridge, MA) were used for analyses. Relations were deemed significant at p < .05.
RESULTS
Table 1 shows the demographic and gestational information for these participants. Briefly, participants were healthy, pregnant, and between the ages of 21 to 39 years (M = 31.2 ± 4 years). The majority were married (96%), Caucasian (94%), and delivering their second child (77%). Thirty (58.8%) were categorized at low or low to middle class. Among this high-risk sample of 51 women, 19 (37%) developed PPMD, and 32 (63%) remained well at Week 28 postpartum. Of the 19 recurrences, 5 (26%) recurred within the first 4 weeks postpartum. The other 14 (74%) were distributed throughout Weeks 5 to 28. Specifics regarding actual timing of recurrence and results subsequent to 28 weeks postpartum are described in Wisner et al. (2001). Table 1 shows the means of the PSQI and 21–HRSD at 36 weeks gestation and at 4 weeks postpartum. There were significant differences in mean PSQI scores at 36 weeks gestation among the three recurrence groups (KW = 9.8, p < .05), but not in 21–HRSD scores at 36 weeks (p > .05). Table 2 shows the distribution of the global PSQI scores, as well as the subcomponent scores by recurrence group. Curiously, sleep quality was significantly better in late pregnancy in women with early recurrences compared to no recurrences (FE = 13.57, p = .001). Among the women with good sleep quality (PSQI scores ≤ 5), 5 out of 16 (31%) recurred within 4 weeks postpartum, whereas none of the participants with poor sleep quality (PSQI > 5) at 36 weeks gestation recurred within 4 weeks postpartum. PSQI scores did not significantly differ between women with LRs and women with no recurrences (post hoc p = .13).
TABLE 1.
Demographic and Gestational Information by Recurrence Group
| Variable | Total |
No Recurrence |
Early Recurrence (< 4 Weeks Postpartum) |
Later Recurrence (> 4 Weeks Postpartum) |
FE; p | ||||
|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | ||
| Age (in years) | |||||||||
| ≤ 30 | 24 | 47 | 16 | 50 | 0 | 0 | 8 | 57 | 5.0; .10 |
| > 30 | 27 | 53 | 16 | 50 | 5 | 100 | 6 | 43 | |
| SES | |||||||||
| Low and low middle | 30 | 59 | 18 | 56 | 2 | 40 | 10 | 71 | 1.8; .43 |
| Middle to upper | 21 | 41 | 14 | 44 | 3 | 60 | 4 | 29 | |
| Marital status | |||||||||
| Married or cohabitating | 48 | 94 | 30 | 94 | 5 | 100 | 13 | 93 | 0.5; 1.00 |
| Not married | 3 | 6 | 2 | 6 | 0 | 0 | 1 | 7 | |
| Race | |||||||||
| Non-White | 3 | 6 | 2 | 6 | 0 | 0 | 1 | 7 | 0.5; 1.00 |
| White | 48 | 94 | 30 | 94 | 5 | 100 | 13 | 93 | |
| Parity | |||||||||
| > 2nd baby | 13 | 26 | 9 | 28 | 0 | 0 | 4 | 29 | 1.5; .50 |
| 2nd baby | 38 | 75 | 23 | 72 | 5 | 100 | 10 | 71 | |
| RCT drug group | |||||||||
| Nortriptyline | 26 | 51 | 15 | 47 | 3 | 60 | 8 | 57 | 0.7; .77 |
| Placebo-nortriptyline | 25 | 49 | 17 | 53 | 2 | 40 | 6 | 43 | |
| C-section delivery | |||||||||
| No | 43 | 86 | 28 | 90 | 3 | 60 | 12 | 86 | 3.2; .19 |
| Yes | 7 | 14 | 3 | 10 | 2 | 40 | 2 | 14 | |
| Any baby problemsa | |||||||||
| No | 38 | 76 | 20 | 65 | 5 | 100 | 13 | 93 | 5.2; .06 |
| Yes | 12 | 24 | 11 | 35 | 0 | 0 | 1 | 7 | |
| M | SD | M | SD | M | SD | M | SD | KW; p | |
| Days from 36-week gestation to delivery | 30.82 | 12.49 | 32.41 | 12.69 | 29.40 | 11.67 | 27.71 | 12.51 | 1.3; .53 |
| Birth weight (kilograms) | 3.46 | 0.53 | 3.47 | 0.49 | 4.15 | 0.68 | 3.26 | 0.51 | 4.3; .11 |
| 21–HRSD (36 weeks) | 6.00 | 4.00 | 6.00 | 4.00 | 5.00 | 4.00 | 7.00 | 4.00 | 0.7; .71 |
| 21–HRSD (Week 4) | 6.04 | 4.41 | 6.09 | 4.26 | na | na | 6.36 | 5.27 | 0.4; .80 |
| PSQI (36 weeks) | 8.00 | 3.00 | 7.00 | 3.00 | 5.00 | 0.00 | 10.0 | 4.00 | 9.8; <.05 |
| PSQI (Week 4) | 7.00 | 3.00 | 6.00 | 3.00 | na | na | 8.00 | 3.00 | 5.2; <.05 |
Note. FE = Fischer’s exact test; SES = socioeconomic status; RCT = randomized clinical trial; KW = Kruskal–Wallis test; 21–HRSD = 21-item Hamilton Rating Scale for Depression.
Any baby problems includes gestational age < 37 or > 41 (n = 5), APGAR score < 6 at 1 min post delivery (n = 3), treatment for sepsis (n = 2), need for volume expansion (n = 1), and other unspecified problems (n = 3).
TABLE 2.
Pittsburgh Sleep Quality Index and Component Scores by Recurrence Group
| Variable | No Recurrence |
Early Recurrence |
Later Recurrence |
FE; p | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Dichotomized total PSQI scorea | |||||||
| ≤ 5 | 10 | 63 | 5 | 31 | 1 | 6 | 13.57; .001 |
| > 5 | 22 | 63 | 0 | 0 | 13 | 37 | |
| Subjective sleep quality | |||||||
| Very good | 2 | 50 | 2 | 50 | 0 | 0 | 9.372; .030 |
| Fairly good | 17 | 68 | 3 | 12 | 5 | 20 | |
| Fairly bad | 13 | 59 | 0 | 0 | 9 | 41 | |
| Sleep latency | |||||||
| < 30 min | 18 | 62 | 3 | 10 | 8 | 28 | 0.153; 1.00 |
| > 30 min | 14 | 64 | 2 | 9 | 6 | 27 | |
| Sleep duration | |||||||
| > 7 hr | 13 | 77 | 3 | 18 | 1 | 6 | 8.379; .055 |
| 6–7 hr | 10 | 56 | 2 | 11 | 6 | 33 | |
| < 6 hr | 9 | 56 | 0 | 0 | 7 | 44 | |
| Sleep efficiency | |||||||
| > 85% | 12 | 67 | 4 | 22 | 2 | 11 | 9.917; .080 |
| 75%–84% | 12 | 75 | 1 | 6 | 3 | 19 | |
| 65%–74% | 4 | 44 | 0 | 0 | 5 | 56 | |
| < 65% | 4 | 50 | 0 | 0 | 4 | 50 | |
| Sleep disturbances | |||||||
| 0 | 7 | 78 | 1 | 11 | 1 | 11 | 4.676; .267 |
| 1–9 | 24 | 63 | 3 | 8 | 11 | 29 | |
| 10–18 | 1 | 25 | 1 | 25 | 2 | 50 | |
| Use of sleep medications | |||||||
| No | 31 | 63 | 5 | 10 | 13 | 27 | 1.191; .611 |
| Yes | 1 | 50 | 0 | 0 | 1 | 50 | |
| Daytime dysfunction | |||||||
| None | 9 | 82 | 1 | 9 | 1 | 9 | 4.750; .277 |
| Slight problem | 20 | 61 | 4 | 12 | 9 | 27 | |
| Somewhat problem | 3 | 43 | 0 | 0 | 4 | 57 | |
Note. PSQI based on 36 weeks gestation. There is a significant difference for dichotomized PSQI among recurrence groups (FE = 13.57, p = .001). Post hoc tests indicate that no recurrence = recurrence after 4 weeks (p = .13), no recurrence > recurrence within 4 weeks (p = .007), and recurrence within 4 weeks < recurrence after 4 weeks (p = .001). FE = Fisher’s exact test; PSQI = Pittsburgh Sleep Quality Index.
The hypothesis that PSQI scores predict PPMD recurrence was not supported in the entire sample. We subsequently examined post hoc the relationship between sleep quality in relation to early and LRs based on the rationale described previously regarding variability in timing to recurrence. We found that PSQI scores were related to timing of recurrence (KW = 9.78, p = .008). We observed that good sleep quality at 36 weeks gestation (lower PSQI scores) was modestly associated with early recurrence, χ2 = 4.14, p = .06; whereas poor sleep quality (higher PSQI scores) in late pregnancy was significantly associated with time to recurrence among LRs (2.13, p = .03).
In evaluating the seven component scores, early recurrers had significantly better sleep quality (FE = 9.4, p = .03), marginally more total sleep time (FE = 10.5, p = .058), and marginally better sleep efficiency (FE = 9.9, p = .08) than those who recurred later or not at all. There were no differences between the groups on sleep latency, sleep duration, nocturnal sleep disturbances, daytime dysfunction, or medication use (Table 2).
Participants’ race, marital status, parity, and SES were not significantly related to early recurrence (p > .3 for all). Only age was significantly related to early recurrence, χ2 = 4.9, p = .05. All women who recurred early were >30 years of age and had PSQI scores ≤ 5. Participants’ age, race, marital status, parity, and SES were not significantly related to LR (p > .5 for all). Neither the 21–HRSD score at 36 weeks gestation nor the 21–HRSD at Week 4 postpartum was significantly related to time to recurrence (p > .50 for both). PSQI at Week 4 postpartum was significantly correlated with PSQI at 36 weeks gestation (r = .49, p = .001), and PSQI at Week 4 postpartum (M = 6.7) was significantly lower than the PSQI at 36 weeks gestation (M = 8.1; paired t test = 2.80, p = .008). Women who had PSQI ≥ 8 at both times had the highest percentages of recurrences (9 out of 16; 60%).
Because the parent study was a prophylactic drug trial, we evaluated whether taking an antidepressant medication during the study period affected the timing of recurrence. Twenty-six participants were assigned to nortriptyline and 25 to placebo. Of the 19 women who recurred, 11 were assigned to nortriptyline and 8 to placebo. As reported in Wisner et al. (2001), there was no significant effect of drug on recurrence. There was also no significant difference in PSQI scores at 36 weeks for those assigned to either group (mean PSQI for the placebo group = 7.0, mean PSQI for the nortriptyline group = 8.5; t test = 1.52, p = .14). Results from the logistic regression for early recurrence and the Cox models for LRs indicate that neither drug assignment (for early recurrence, log likelihood [LL] ratio: χ2 = 0.3, p = .58; for LRs, Cox models LL ratio: χ2 = 0.01, p = .99) nor the interaction of drug assignment with PSQI scores was significantly associated with time to recurrence (for early recurrence, LL ratio: χ2 = 0.01, p = .97; for LRs, LL ratio: χ2 = 0.44, p = .51).
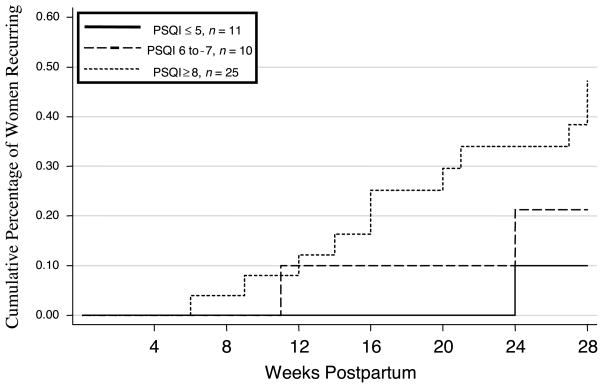
As stated, when considering women classified as good and poor sleepers based on criteria specified by Buysse et al. (1989), dichotomized PSQI scores were not significantly related to time to recurrence. Given that pregnant women endorse significant sleep complaints in late pregnancy (Okun & Coussons-Read, 2007), we explored whether alternate cutpoints would be more suitable in this sample. As indicated earlier, the median PSQI for women in this sample was 7. Approximately 70% of the women had a PSQI score ≥ 6. Ten (20%) scored 6 or 7, whereas 25 (49%) scored ≥ 8. We, therefore, trichotomized the PSQI at ≤ 5, 6 to 7, and ≥ 8. This post hoc analysis showed that the categorical PSQI was significantly related to time to recurrence (LR = 2.20, p = .03; see Figure 1).
FIGURE 1.
Time to recurrences of postpartum depression from Weeks 4 to 28 post delivery split by Pittsburgh Sleep Quality Index (PSQI) grouping. Note. Lines represent the cumulative proportion of participants who have recurrences of postpartum depression in Weeks 5 to 28 post delivery for groups with different PSQI scores. The groups represented by the lines have significantly different rates of recurrence—act Tarone and Ware statistics for comparison of survival functions: later recurrence = .20, p < .03.
DISCUSSION
In this study, our hypothesis that self-reported sleep quality predicts recurrence of PPMD was not supported. However, when the timing of recurrence was examined, we found that poor sleep, as indicated by the PSQI, was related to LR. Interestingly, the women who recurred early did not complain of poor sleep at 36 weeks. This finding, although intriguing, is difficult to interpret given the small sample size. Although speculative, these data suggest that sleep quality in late pregnancy may provide additional information regarding the timing of a recurrent event. In this sample, more women with uncharacteristically good sleep quality (PSQI ≤ 5) recurred rapidly (31.3% < 4 weeks vs. 7.1% > 4 weeks), whereas more women with poor sleep quality recurred after 4 weeks postpartum (44% > 4 weeks vs. 0% < 4 weeks). Further exploration of this relationship is warranted. Of clinical importance is distinguishing between postpartum depression and the “baby blues,” which occurs in the majority of new mothers. Baby blues, a transient mood syndrome that begins on Days 3 or 4 and lifts by 2 weeks post birth, was distinguished from PPMD in this study by using the SADS (Endicott & Spitzer, 1978) for definite major depression.
There is heterogeneity with regards to the timing of PPMD recurrence. Presently, a definitive time frame during which an episode can be labeled as postpartum depression has not been established. According to the DSM–IV–TR (American Psychiatric Association, 2000), the onset of the episode must occur within 4 weeks of birth. The Agency for Healthcare Research and Quality (Gaynes et al., 2005b) extends the period to the first 12 months after birth; however, this includes both minor and major depression; while a large-scale population study suggests that the critical window is the first 5 months postpartum, based on the evidence that unipolar depression diagnoses requiring hospitalization increased during this period compared to non-pregnant women (Munk-Olsen et al., 2006). Our findings, which are consistent with the notion of heterogeneity in timing to PPMD recurrence (Cox, Murray, & Chapman, 1993; Wisner, Perel, Peindl, & Hanusa, 2004), suggest that distinct differences in sleep quality could provide differential information with respect to not only who may be at risk for PPMD recurrence but also when the recurrence might be expected.
Among the 14 women who recurred after 4 weeks postpartum, poor sleep quality was a better predictor of PPMD recurrence than other risk factors including age, race, marital status, SES, and 21–HRSD scores at Week 36 gestation and 4 weeks postpartum (Beck, 2001; Robertson et al., 2004). Our finding is consistent with previous reports that disturbed sleep is a prodromal symptom of both first-onset and recurrent depressive episodes (Coble et al., 1994; Ford & Cooper-Patrick, 2001; Perlis et al., 1997; Perlman, Johnson, & Mellman, 2006; Swain et al., 1997; Wolfson et al., 2003; Jomeen & Martin, 2007). Perlis et al. reported that sleep disturbances represent vulnerability to depression. We speculate that persistent sleep problems accumulate over time and interact with individual vulnerability. This interaction may explain the variability in recurrence times observed in this study.
Our findings are also consistent with studies examining sleep in pregnancy. Jomeen and Martin (2007) observed that higher PSQI scores in early pregnancy were associated with higher depressive symptoms in the postpartum. They are also consistent with Wolfson and colleagues (2003), who indicate that women with significantly different sleep schedules at the end of their pregnancies (suggestive of poor sleep quality) have elevated depressive symptoms in the postpartum. Although the overall conclusions are similar between our study and that by Wolfson et al., there are distinct differences that deserve attention. First, they examined quantitative sleep measures, such as total sleep time and number of disruptions, in a prospective manner using sleep diaries and the association with depressive symptoms in the postpartum. Sleep quality was not ascertained. Conversely, we assessed retrospective sleep quality using the PSQI to evaluate the relationship between sleep and depression in the postpartum. Second, the characteristics of participants were different and, therefore, less comparable. They recruited first-time mothers from the community with no apparent psychopathology or sleep disturbances, whereas our study purposely recruited women who were multiparous with a history of PPMD. Finally, the timing of sleep data collection was variable in the Wolfson et al. study (27–40 weeks gestation), whereas all the participants in our study were seen at 36 weeks. Because both quantitative and qualitative sleep change across pregnancy (Okun & Coussons-Read, 2007), it is difficult to presume that sleep quality would be similar between women observed at 27 and 40 weeks gestation. Whether it is sleep disturbances or the perceptions of disturbed sleep that is the risk factor for PPMD is not clear. Nonetheless, the current findings are, to the best of our knowledge, the first to show a significant association between subjective sleep quality in late pregnancy and timing of clinically diagnosed PPMD recurrence. In order to confirm these results, a prospective evaluation of sleep in late pregnancy and PPMD recurrence must be done.
A possible contributor to those who experienced a rapid recurrence may be different physiological processes and vulnerabilities (Bloch, Rotenberg, Koren, & Klein, 2006; Wisner, Perel, Peindl, & Hanusa, 2004). Although a sample of 5 women is insufficient to generalize to all women with a history of PPMD, there is empirical evidence that suggests early postpartum recurrence is associated with individual neurobiological sensitivity to the large scale hormonal withdrawal that accompanies childbirth (Bloch et al., 2003). It is conceivable that this group benefited the most from the hormonal state of late pregnancy via improved sleep (Baker, Mitchell, & Driver, 2001; Soderpalm, Lindsey, Purdy, Hauger, & Wit, 2004; Suzuki et al., 1993; Yamaoka, 1980) and mood (Bloch et al., 2003; Epperson, Wisner, & Yamamoto, 1999) given that late pregnancy is associated with 100-fold estradiol increases (relative to mean menstrual cycle concentrations), 10-fold increases in progesterone (relative to mid-luteal levels; Wilson & Parsons, 1996), and increased allopregnanolone concentrations. Progesterone and allopregnanolone are likewise associated with increased sleepiness via modulation of GABAA receptors (Lancel, Faulhaber, Holsboer, & Rupprecht, 1996; Lancel et al., 1997). This phenomenon could explain the few sleep complaints expressed by these women. We speculate, as have other investigators (Bloch et al., 2003; O’Hara et al., 1991), that a major contributor to the etiology of early recurring PPMD is a biological response to the rapid decline in hormones (Bloch et al., 2003).
Certain limitations are associated with secondary analyses. The design of the parent study was to test the efficacy of prophylactic antidepressant medication in the prevention of PPMD recurrence, not assess sleep quality as a risk factor (Wisner et al., 2001). Thus, our ability to extrapolate from these findings is limited. Participation in a clinical trial can introduce unexpected biological or psychological effects (Haour, 2005). Taking either an active medication or a placebo could have affected symptom endorsement differentially, thereby influencing recurrence rates. In this sample of high-risk women, 37% recurred within 7 months. This estimate is higher than previously reported (Gavin et al., 2005; Gaynes et al., 2005b; Wisner, Perel, Peindl, & Hanusa, 2004), but may be due to selection bias. Generalization of these findings is another limitation. Women who participate in RCTs may be distinctly different than women who choose not to participate. Furthermore, the small number of women who recurred within 4 weeks postpartum prohibits the generalization to all rapid recurrences. Also, we cannot extrapolate to all pregnant women, women without a history of depression, or women with a history of depression outside of the postpartum period. Finally, the age of the participants and the age of the child at home may differentially influence sleep patterns in late pregnancy and into the postpartum, thereby affecting outcomes. Nonetheless, these findings provide initial support for the assessment of sleep quality in late pregnancy as an additional piece of information in the prediction of recurrent PPMD.
We found that sleep quality in late pregnancy is a relevant risk factor in the timing of PPMD recurrence. These findings could have clinical implications for intervention and prevention opportunities. Knowledge of a woman’s sleep quality in late pregnancy may supplement other clinical information in identifying women at high risk for recurrence.
Acknowledgments
This is not an industry-supported study, and we indicate no financial conflicts of interest.
Contributor Information
Michele L. Okun, Department of Psychiatry, School of Medicine, University of Pittsburgh
Barbara H. Hanusa, Department of Psychiatry, School of Medicine, University of Pittsburgh
Martica Hall, Department of Psychiatry, School of Medicine, University of Pittsburgh.
Katherine L. Wisner, Departments of Psychiatry and Obstetrics and Gynecology and Reproductive Sciences, School of Medicine University of Pittsburgh
References
- Alder J, Fink N, Bitzer J, Hosli I, Holzgreve W. Depression and anxiety during pregnancy: A risk factor for obstetric, fetal and neonatal outcome? A critical review of the literature. Journal of Maternal Fetal Neonatal Medicine. 2007;20:189–209. doi: 10.1080/14767050701209560. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: Author; 2000. text rev. [Google Scholar]
- Baker FC, Mitchell D, Driver HS. Oral contraceptives alter sleep and raise body temperature in young women. Pflugers Archives. 2001;442:729–737. doi: 10.1007/s004240100582. [DOI] [PubMed] [Google Scholar]
- Beck CT. Predictors of postpartum depression: An update. Nursing Resources. 2001;50:275–285. doi: 10.1097/00006199-200109000-00004. [DOI] [PubMed] [Google Scholar]
- Bloch M, Daly RC, Rubinow DR. Endocrine factors in the etiology of postpartum depression. Comprehensive Psychiatry. 2003;44:234–246. doi: 10.1016/S0010-440X(03)00034-8. [DOI] [PubMed] [Google Scholar]
- Bloch M, Rotenberg N, Koren D, Klein E. Risk factors for early postpartum depressive symptoms. General Hospital Psychiatry. 2006;28:3–8. doi: 10.1016/j.genhosppsych.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Research. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Coble PA, Reynolds CF, Kupfer DJ, Houck PR, Day NL, Giles DE. Childbearing in women with and without a history of affective disorder. II. Electroencephalographic sleep. Comprehensive Psychiatry. 1994;35:215–224. doi: 10.1016/0010-440x(94)90194-5. [DOI] [PubMed] [Google Scholar]
- Cox JL, Murray D, Chapman G. A controlled study of the onset, duration and prevalence of postnatal depression. British Journal of Psychiatry. 1993;163:27–31. doi: 10.1192/bjp.163.1.27. [DOI] [PubMed] [Google Scholar]
- Endicott J, Spitzer RL. A diagnostic interview: The Schedule for Affective Disorders and Schizophrenia. Archives of General Psychiatry. 1978;35:837–844. doi: 10.1001/archpsyc.1978.01770310043002. [DOI] [PubMed] [Google Scholar]
- Epperson CN, Wisner KL, Yamamoto B. Gonadal steroids in the treatment of mood disorders. Psychosomatic Medicine. 1999;61:676–697. doi: 10.1097/00006842-199909000-00010. [DOI] [PubMed] [Google Scholar]
- Ford DE, Cooper-Patrick L. Sleep disturbances and mood disorders: An epidemiologic perspective. Depression and Anxiety. 2001;14:3–6. doi: 10.1002/da.1041. [DOI] [PubMed] [Google Scholar]
- Ford DE, Kamerow DB. Epidemiologic study of sleep disturbances and psychiatric disorders. An opportunity for prevention? Journal of the American Medical Association. 1989;262:1479–1484. doi: 10.1001/jama.262.11.1479. [DOI] [PubMed] [Google Scholar]
- Gavin NI, Gaynes BN, Lohr KN, Meltzer-Brody S, Gartlehner G, Swinson T. Perinatal depression: A systematic review of prevalence and incidence. Obstetrics and Gynecology. 2005;106:1071–1083. doi: 10.1097/01.AOG.0000183597.31630.db. [DOI] [PubMed] [Google Scholar]
- Gaynes BN, Gavin N, Meltzer-Brody S, Lohr KN, Swinson T, Gartlehner G, et al. Evidence Report/Technology Assessment No. 119 (Rep. No. AHRQ Publication 05-E006-1) Rockville, MD: Agency for Healthcare Research and Quality; 2005a. Perintal depression: Prevalence, screening accuracy, and screening outcomes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaynes BN, Gavin N, Meltzer-Brody S, Lohr KN, Swinson T, Gartlehner G, et al. Perinatal depression: Prevalence, screening accuracy, and screening outcomes. Evidence Report/Technology Assessment. 2005b;119:1–8. doi: 10.1037/e439372005-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery and Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haour F. Mechanisms of the placebo effect and of conditioning. Neuroimmunomodulation. 2005;12:195–200. doi: 10.1159/000085651. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB, Redlich FC. Social class and mental illness: A community study. New York: Wiley; 1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jomeen J, Martin CR. Assessment and relationship of sleep quality to depression in early pregnancy. Journal of Reproductive and Infant Psychology. 2007;25:97–99. [Google Scholar]
- Kendell RE, Chalmers JC, Platz C. Epidemiology of puerperal psychoses. British Journal of Psychiatry. 1987;150:662–673. doi: 10.1192/bjp.150.5.662. [DOI] [PubMed] [Google Scholar]
- Lancel M, Faulhaber J, Holsboer F, Rupprecht R. Progesterone induces changes in sleep comparable to those of agonistic GABAA receptor modulators. American Journal of Physiology. 1996;271:E763–E772. doi: 10.1152/ajpendo.1996.271.4.E763. [DOI] [PubMed] [Google Scholar]
- Lancel M, Faulhaber J, Schiffelholz T, Romeo E, Di Michele F, Holsboer F, et al. Allopregnanolone affects sleep in a benzodiazepine-like fashion. Journal of Pharmacology and Experimental Therapeutics. 1997;282:1213–1218. [PubMed] [Google Scholar]
- Lee KA, Zaffke ME, McEnany G. Parity and sleep patterns during and after pregnancy. Obstetrics and Gynecology. 2000;95:14–18. doi: 10.1016/s0029-7844(99)00486-x. [DOI] [PubMed] [Google Scholar]
- Logsdon MC, Wisner KL, Pinto-Foltz MD. The impact of postpartum depression on mothering. Journal of Obstetrics, Gynecology, and Neonatal Nursing. 2006;35:652–658. doi: 10.1111/j.1552-6909.2006.00087.x. [DOI] [PubMed] [Google Scholar]
- Munk-Olsen T, Laursen TM, Pedersen CB, Mors O, Mortensen PB. New parents and mental disorders: A population-based register study. Journal of the American Medical Association. 2006;296:2582–2589. doi: 10.1001/jama.296.21.2582. [DOI] [PubMed] [Google Scholar]
- O’Hara MW, Schlechte JA, Lewis DA, Varner MW. Controlled prospective study of postpartum mood disorders: Psychological, environmental, and hormonal variables. Journal of Abnormal Psychology. 1991;100:63–73. doi: 10.1037//0021-843x.100.1.63. [DOI] [PubMed] [Google Scholar]
- O’Hara MW, Zekoski EM, Philipps LH, Wright EJ. Controlled prospective study of postpartum mood disorders: Comparison of childbearing and nonchildbearing women. Journal of Abnormal Psychology. 1990;99:3–15. doi: 10.1037//0021-843x.99.1.3. [DOI] [PubMed] [Google Scholar]
- Okun ML, Coussons-Read ME. Sleep disruption during pregnancy: How does it influence serum cytokines? Journal of Reproductive Immunology. 2007;73:158–165. doi: 10.1016/j.jri.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Perlis ML, Giles DE, Buysse DJ, Tu X, Kupfer DJ. Self-reported sleep disturbance as a prodromal symptom in recurrent depression. Journal of Affective Disorders. 1997;42:209–212. doi: 10.1016/s0165-0327(96)01411-5. [DOI] [PubMed] [Google Scholar]
- Perlman CA, Johnson SL, Mellman TA. The prospective impact of sleep duration on depression and mania. Bipolar Disorder. 2006;8:271–274. doi: 10.1111/j.1399-5618.2006.00330.x. [DOI] [PubMed] [Google Scholar]
- Robertson E, Grace S, Wallington T, Stewart DE. Antenatal risk factors for postpartum depression: A synthesis of recent literature. General Hospital Psychiatry. 2004;26:289–295. doi: 10.1016/j.genhosppsych.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Ross LE, Campbell VL, Dennis CL, Blackmore ER. Demographic characteristics of participants in studies of risk factors, prevention, and treatment of postpartum depression. Canadian Journal of Psychiatry. 2006;51:704–710. doi: 10.1177/070674370605101107. [DOI] [PubMed] [Google Scholar]
- Soderpalm AH, Lindsey S, Purdy RH, Hauger R, Wit DH. Administration of progesterone produces mild sedative-like effects in men and women. Psychoneuroendocrinology. 2004;29:339–354. doi: 10.1016/s0306-4530(03)00033-7. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Dennerstein L, Greenwood KM, Armstrong SM, Sano T, Satohisa E. Melatonin and hormonal changes in disturbed sleep during late pregnancy. Journal of Pineal Research. 1993;15:191–198. doi: 10.1111/j.1600-079x.1993.tb00904.x. [DOI] [PubMed] [Google Scholar]
- Swain AM, O’Hara MW, Starr KR, Gorman LL. A prospective study of sleep, mood, and cognitive function in postpartum and nonpostpartum women. Obstetrics and Gynecology. 1997;90:381–386. doi: 10.1016/s0029-7844(97)89252-6. [DOI] [PubMed] [Google Scholar]
- Tarone RE, Ware J. On distribution-free tests for equality of survival distributions. Biometrika. 1977;72:156–160. [Google Scholar]
- Wilson L, Parsons M. Endocrinology of human gestation. In: Adashi EY, Rock JA, Rosenwaks Z, editors. Reproductive endocrinology, surgery, and technology. Lippincott/Raven; 1996. pp. 452–475. [Google Scholar]
- Wisner KL, Chambers C, Sit DK. Postpartum depression: A major public health problem. Journal of the American Medical Association. 2006;296:2616–2618. doi: 10.1001/jama.296.21.2616. [DOI] [PubMed] [Google Scholar]
- Wisner KL, Parry BL, Piontek CM. Clinical practice. Postpartum depression. New England Journal of Medicine. 2002;347:194–199. doi: 10.1056/NEJMcp011542. [DOI] [PubMed] [Google Scholar]
- Wisner KL, Perel JM, Peindl KS, Hanusa BH. Timing of depression recurrence in the first year after birth. Journal of Affective Disorders. 2004;78:249–252. doi: 10.1016/S0165-0327(02)00305-1. [DOI] [PubMed] [Google Scholar]
- Wisner KL, Perel JM, Peindl KS, Hanusa BH, Findling RL, Rapport D. Prevention of recurrent postpartum depression: A randomized clinical trial. Journal of Clinical Psychiatry. 2001;62:82–86. doi: 10.4088/jcp.v62n0202. [DOI] [PubMed] [Google Scholar]
- Wisner KL, Perel JM, Peindl KS, Hanusa BH, Piontek CM, Findling RL. Prevention of postpartum depression: A pilot randomized clinical trial. American Journal of Psychiatry. 2004;161:1290–1292. doi: 10.1176/appi.ajp.161.7.1290. [DOI] [PubMed] [Google Scholar]
- Wolfson AR, Crowley SJ, Anwer U, Bassett JL. Changes in sleep patterns and depressive symptoms in first-time mothers: Last trimester to 1-year postpartum. Behavioral Sleep Medicine. 2003;1:54–67. doi: 10.1207/S15402010BSM0101_6. [DOI] [PubMed] [Google Scholar]
- Yamaoka S. Modification of circadian sleep rhythms by gonadal steroids and the neural mechanisms involved. Brain Research. 1980;185:385–398. doi: 10.1016/0006-8993(80)91076-8. [DOI] [PubMed] [Google Scholar]