Abstract
Background
Deciding whether to employ intravenous fibrinolytic therapy for acute cerebral ischemia within 3 hours of onset is challenging for patients, family members, and healthcare providers.. Visual displays can permit individuals to rapidly understand response patterns to therapy. This study sought to evaluate, refine, and improve existing visual aids for stroke fibrinolytic decision-making.
Methods
Existing visual aids were identified by Medline search and querying of national guideline organizations, pharmaceutical manufacturers, and stroke specialists and rated on a formal 8 point quality rating scale (0 lowest, 8 highest). Based on in available instruments, new visual displays were developed to improve informed decision-making in routine practice.
Results
Two existing visual aids were identified, one from an emergency medicine society and one from a pharmaceutical company. Both were comparison visual displays of outcomes with and without treatment; no decision matrix visual aid was found. Both scored 4.0 on the quality scale, showing defects of effect size distortion, privileging less salient outcomes, dissimilar representation by treatment group, and limited stakeholder participation in generation. Revised versions of these graphics were developed with higher quality scores (6.75 and 7.75). In addition, a new decision matrix display with quality score 8.0 was developed that complements the numeric text of a national patient education tool developed jointly by US neurology, emergency medicine, and stroke patient organizations.
Conclusion
Existing visual aids for stroke fibrinolysis decision-making have deficiencies. New visual displays are now available to convey the health benefits and risks of fibrinolytic stroke therapy efficiently and informatively to patients and family members.
Keywords: Cerebral infarction, Thrombolysis, Decision-making, Acute Stroke, Treatment
Deciding whether to employ intravenous fibrinolytic therapy for acute cerebral ischemia within 3 hours of onset is challenging for patients, family members, and healthcare providers. Lay individuals must rapidly agree to or decline an interventional that carries substantial potential benefits but also substantial potential risks, for a condition that often first suddenly and unexpectedly appeared only tens of minutes earlier. Lengthy and iterative discussions, appropriate in nonacute settings, are potentially dangerous in acute stroke because of the high neuronal cost of deliberation. Every one minute in which therapy is delayed in the typical large artery ischemic stroke, 2 million more brain cells die. Every 10 minutes in which therapy is delayed, one less patient experiences a benefit from treatment.1 For this reason, the national target for the time interval from patient arrival in the Emergency Department to start of therapy is 60 minutes,2 and much of that time is consumed by diagnostic and initial stabilizing care, leaving only a brief interval for treatment decision counseling.
Graphical displays can permit individuals to rapidly understand response patterns to therapy.3 While understanding of quantitative risk is critical for informed consent and shared decision-making, lay individuals often fail to comprehend key aspects of numeric information that is presented simply as text. Universal human cognitive limitations cause biases in interpreting numerical probabilities that affect all individuals.3–5 Moreover, many patients have limited numeracy skills, discomfort with numerical expressions of risk, and analytic reasoning processes are impaired by age and by the stroke itself. Graphs are an appealing complement to numbers because they are visually interesting and exploit rapid, automatic visual perception skills. A well designed visual display can reduce the amount of mental computation by replacing it with automatic visual perception, help patients to personalise health risk information, to appreciate the scientific uncertainties inherent in the treatment choice, clarify the personal value or desirability of potential benefits relative to potential harms, and to communicate their values to their practitioners. Because of the extreme time urgency in acute stroke decision-making, graphic decision aids can play a critical role in facilitating informed consent and empowering patients and family to participate in shared decision-making.
However, the power of graphical decision aids to inform inevitably is accompanied by an equal power to mislead. The quality of graphical decision aids can vary. Presentational biases, including framing, axis distortion, and relative rather than absolute comparison, may distort the decision-making process and prevent patients and families from reaching an accurate appraisal of health risks and a well-informed selection of their therapy.
The quality of graphical decision aids for intravenous fibrinolytic therapy in acute stroke has not previously been formally investigated.
Methods
Existing visual aids for fibrinolytic stroke therapy decision-making were identified by Medline search and querying of national guideline organizations, pharmaceutical manufacturers, and stroke specialists (see Appendix for search strategy). Inclusion criterion: 1) Decision support graphic focused on benefits and risks of thrombolytic stroke therapy. Exclusion criteria: 1) illustration focused on pathophysiology or mechanism of action, 2) nonfigural (verbal or numeric) decision support instrument. One stroke neurologist (JJLS) identified suitable retrieved publications and extracted the data.
We constructed a formal rating scale to assess the quality of the visual displays using a modified Delphi process. Scale items were drawn from best practice recommendations regarding construction of visual figures to convey health benefits and risks in the medical decision-making, including relevant elements of the International Patient Decision Aid Standards Collaboration quality checklist.3, 5–7 The resulting Quality Scale for Emergency Clinical Decision Aid Graphics (QS-ECDAG) has 8 items and yields a score from 0 (worst) to 8 (best).(Table 1) Individual items address graph type, depiction of benefits and risks, uniform depiction of outcomes across treatment groups, effect size distortion, proportionality of display elements to underlying data, absolute rather than relative risk ratios, figural emphasis of noncomparable outcomes according to salience to patients, data sources, and stakeholder and patient participation in graph generation. In addition, when display elements were disproportionate to the quantities they depicted, the degree of distortion was quantified using the Tufte Lie Factor: Lie Factor = size of effect shown in graphic/size of effect in data.8 Following Tufte, values greater than 1.05 or less than 0.95 were considered to indicate substantial distortion. Quality scale scoring was performed by two senior investigators (JLS, BO). Concurrence at the total score level was assessed by correlation coefficient and at component item level by the kappa statistic. Discrepant total scores, should any have occurred, were to be handled by averaging.
Table 1.
Quality Scale for Emergency Clinical Decision Aid Graphics (QS-ECDAG)
| Scale Items | Points |
|---|---|
| Graph/figure type is appropriate to the information being conveyed (e.g. bar charts for comparisons among groups, line graphs for trends over time, pie charts for proportions) |
1 |
| Both benefits and risks are shown | 1 |
| Similar outcomes are shown in similar manner across treatment groups | 1 |
| Graphic display elements are proportional to the quantities depicted | 1 |
| Graph displays absolute indices, not relative indices, of benefit and harm, and compares outcome probabilities using the same denominator |
1 |
| Outcomes generally valued by patients as differentially important are differentially emphasized graphically (e.g. by color, font size, etc) |
1 |
| Data sources are identified and figure is based on highest grade available evidence (e.g. randomized controlled trials rather than uncontrolled trials) |
1 |
| Process of figure generation included input from disease-specific experts (0.25); multiple medical specialties (0.25); health methodologists (0.25); patients/public (0.25) |
1 |
| Total Score | |
If deficiencies were noted in available graphic displays, revised displays were constructed, removing the deficiencies to the extent possible within the original general framework of the figure. The revised displays were then rescored on the quality scale.
Treatment decision icon displays can generally be categorized as 1) outcome distribution comparisons, and 2) choice consequence matrices. In outcome distribution comparisons, the full range of outcomes under treatment choice A and treatment choice B are shown adjacent to each other. In choice consequence matrices, the graphic takes the outcomes under treatment choice A as a given and shows the changes in these outcomes that would result from treatment choice B. When literature review found only outcome distribution comparisons in the literature, we generated and scored choice consequence matrix displays. These figures were created by a panel that included stroke neurologists, an internist, an emergency physician, a health outcomes researcher and a biostatistician, based on published data,9–11 and informed by qualitative interview feedback regarding alternative graphical presentations from stroke survivors from the Southern California Stroke Association.
Results
Two existing visual aids were identified. Both were comparison visual displays of outcomes with and without treatment.
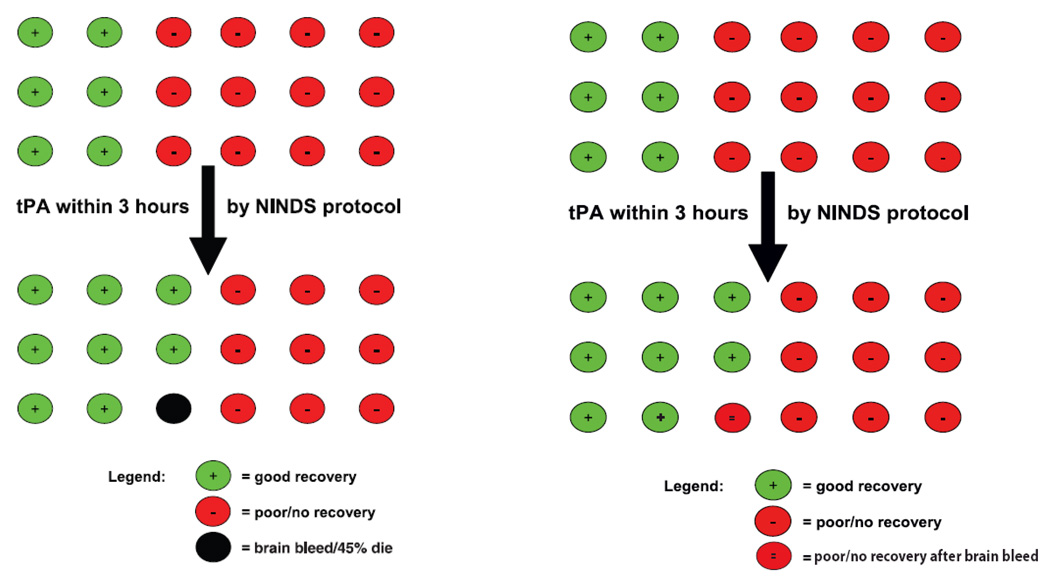
One display, from the American Academy of Emergency Medicine (AAEM), scored 4.0 on the quality rating scale.(Figure 1A)12 Defects included 1) distortion, 2) visual mis-privileging, 3) dissimilar representation by treatment group, and 4) no indication of inclusion of multiple specialties, methodologists, and lay individuals in figure generation.
Effect size distortion: The placebo good recovery rate is displayed as 6/18 (33%) but the actual average of good outcomes on all 4 endpoint scales is 29%.13 The resulting Tufte Lie factor is 1.14 (33/29), indicating overestimation of the rate of good outcomes without treatment.
Visual mis-privileging: The figure hues give the greatest color emphasis to the single black circle (strong figure/ground psychophysical relationship), but the black color represents a less important short-term outcome (bleeding with early worsening) than the red-green final functional outcomes, the endpoint that is of greatest importance to patients and families.
Dissimilar representation by treatment group: The figure shows deaths only in relation to symptomatic hemorrhage in the treatment group, not all cause mortality across both treatment groups. In the NINDS study, there actually was no statistically significant between group differences in the death rate, and deaths were numerically more frequent in the placebo than in the TPA group.
Participants in figure generation: The AAEM aid does not indicate who participated in figure generation. Besides emergency physicians, who were presumably involved, it is not clear if physicians from other specialties, stroke disease experts, health methodologists, or patients/lay individuals participated.
A revised figure was produced that corrected the visual mis-privileging and dissimilar representation by treatment group, and partially corrected limited inclusiveness in stakeholder participation, but did not alter the distortion, with a resulting quality score of 6.75.(Figure 1B).
Figure 1.
A) Left figure from an emergency medicine society shows defects of effect size distortion, privileging less salient outcomes, and dissimilar representation by treatment group. B) In Right figure, replacement of the black circle with a red double minus circle corrects the privileging of less salient outcomes and dissimilar representation by treatment group. However, the effect size distortion is not corrected. (Figure published with permission of UCLA Stroke Center.)
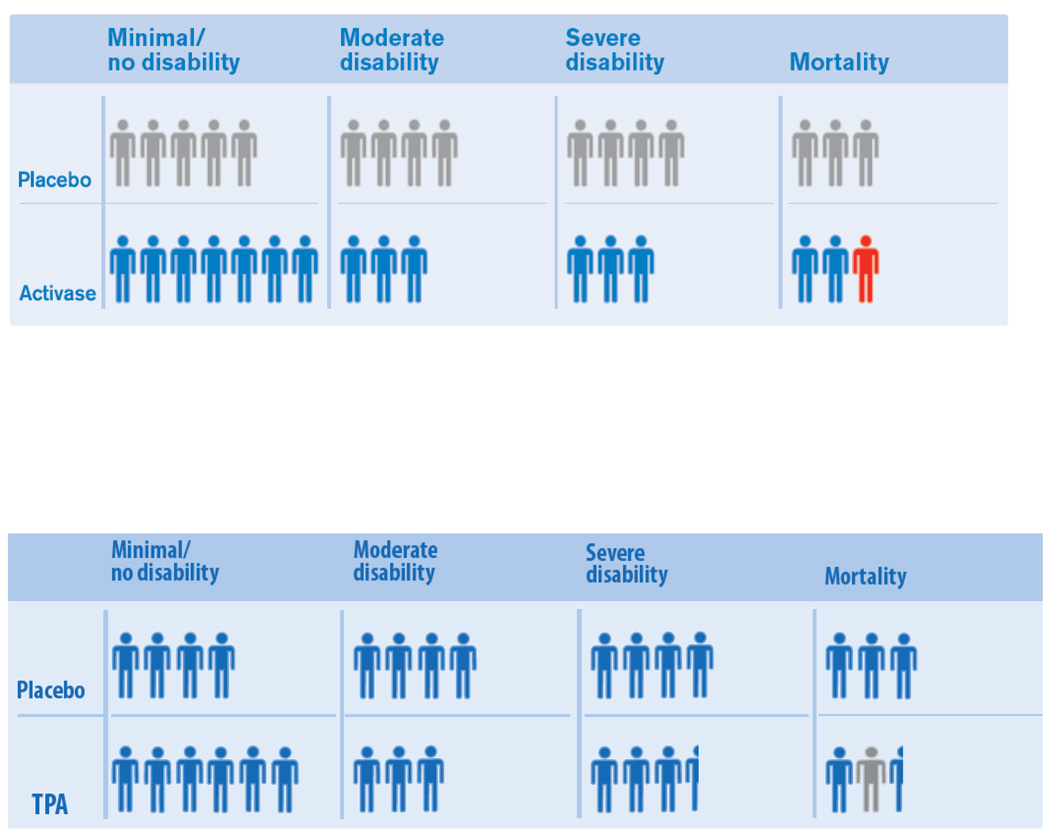
The other display, from Genentech, the US manufacturer of TPA, scored 4.0 on the quality rating scale.(Figure 2A)14 Defects included 1) distortion, 2) visual mis-privileging, 3) dissimilar representation by treatment group, and 4) no indication of inclusion of multiple specialties, methodologists, and lay individuals in figure generation.
Effect size distortion: Several graphic elements are not proportionate to the quantities they depict, including four cells with Tufte Lie Factors departing from 1.0 by 10% or more. For the TPA group, the rate of death is overestimated (LF of 1.10), while for the placebo group the rate of death is underestimated (LF of 0.89). Also, for the TPA group, the rates of moderate and for severely disabled outcomes are underestimated (LF of 0.89).
Visual mis-privileging: The figure hues give the greatest color emphasis to the single red person-figure (strong figure/ground psychophysical relationship), but the red color represents a less important short-term outcome (bleeding with early worsening) than the blue-gray colored final functional outcome, the endpoint that is of greatest importance to patients and families.
Dissimilar representation by treatment group: The figure shows similar outcomes with an attractive blue color for TPA treated patients versus a dull gray color for placebo treated patients.
Participants in figure generation: The website on which the figure is displayed does not indicate who participated in figure generation. It is not clear if physicians from multiple specialties, stroke disease experts, health methodologists, or patients/lay individuals participated.
Figure 2.
A) Top figure from a pharmaceutical company show defects of effect size distortion, privileging less salient outcomes, and dissimilar representation by treatment group. B) In Bottom figure, recoloring of person-icons and basing graphic on actual, rather than imputed, disability measures reduces the effect size distortion and corrects the privileging of less salient outcomes and dissimilar representation by treatment group. (Figure published with permission of UCLA Stroke Center.)
A revised figure was produced that corrected the visual mis-privileging and dissimilar representation by treatment group, reduced the distortion of effect size, and partially corrected limited inclusiveness in stakeholder participation, with a resulting quality score of 7.75 .(Figure 2B). Only one element had a Tufte LF departing from 1.0 by 10% or more, an overestimate in the TPA group of the rate of severe disability (LF 1.11).
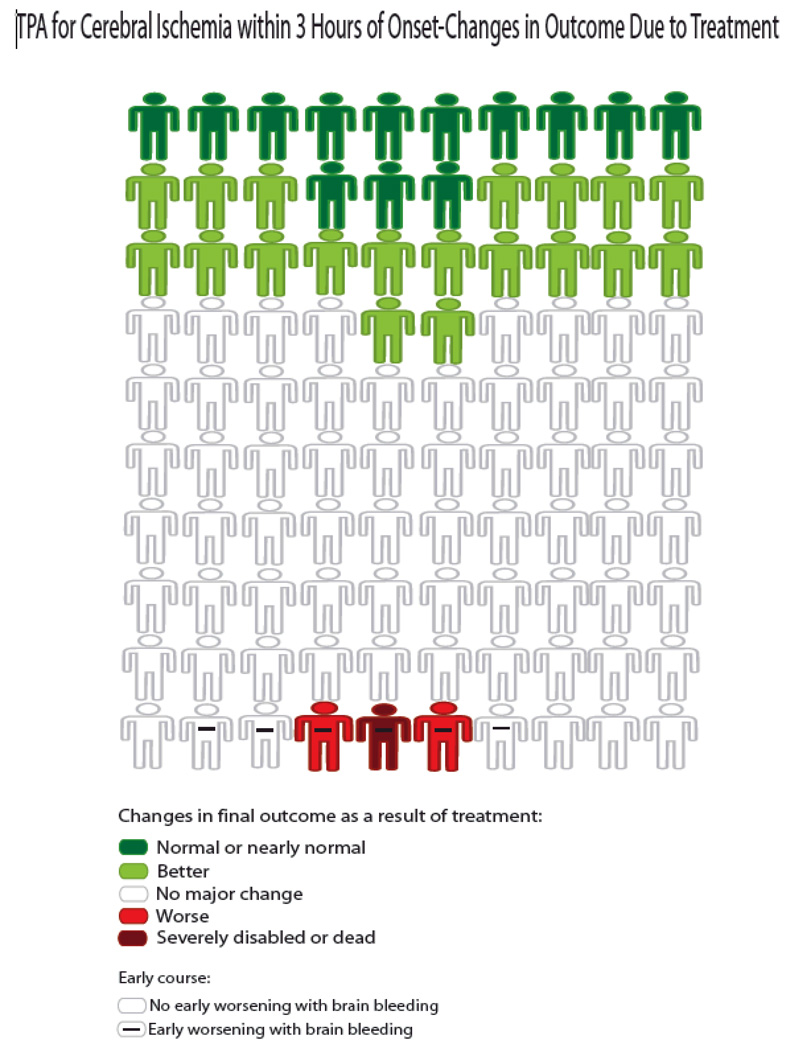
The literature review identified no existing choice consequence matrix graphic displays. Accordingly, our panel developed several. The version shown in Figure 3 was selected for general distribution as it complemented the all text decision aid that has been jointly developed and endorsed by the American Academy of Neurology, American College of Emergency Physicians, and the American Heart Association/American Stroke Association.15 The AAN-ACEP-AHA/ASA patient guide has no graphical depiction of benefits and risks, but the text does frame numeric statements of benefits and risks in a choice consequence manner, e.g. that 1 in 3 patients who receive TPA improve as a result and 6 of 100 have bleeding among whom 1 has death or serious disability as a result. The Figure 3 icon array graphically depicts the same information conveyed textually in the AAN-ACEP-AHA/ASA education sheet. The display scored 8.0 on the quality checklist and had no graphical element with a Tufte LF departing from 1.0 by 5% or more.
Figure 3.
Decision matrix figure illustrating the benefits and risks of IV TPA in the under 3 hour window based upon data from the two NINDS-TPA trials. (Figure published with permission of UCLA Stroke Center.)
Additional choice consequence array options that were developed are shown in the supplemental figure.(Supplemental Figure A1, A2) Supplemental figure A2 is noteworthy as it is the figure that patient informants in the study generally preferred. Most stroke patients placed great emphasis upon the figure showing a clear depiction of final outcome, and considered the simultaneous depiction of intermediate events, such as early symptomatic hemorrhage or early recanalization, to be distracting and less desirable. However, because of concerns in the physician community, the early symptomatic hemorrhage depiction was retained in Figure 3.
Inter-rater agreement on the quality scale ratings for the 5 rated figures was substantial. The total scores for each of the 5 figures were 100% concordant, r=1.0. Across the 40 total individual line item ratings, interobserver agreement was 90% and kappa value was 0.71 (95% CI 0.45-0.98), indicating substantial agreement.
Discussion
Evidence describing the effectiveness and feasibility of patient decision aids is substantial.5–8 Trials indicate that decision aids are superior to standard counseling in improving patients’ knowledge and realistic expectations about the results of treatments and other procedure. Decision aids are particularly important for emergency decision-making. This study identified deficiencies in existing visual aids for acute stroke fibrinolysis decision-making, and developed corrected and new displays that convey the benefits and risks of therapy efficiently and more accurately to patients, family members, and practitioners.
The errors in existing graphics largely were in the direction to be expected given the competing interests of the stakeholders generating them. The AAEM graphic arose from a position statement process whose stated goal was to protect Emergency Physicians from medicolegal risk, not to facilitate patient decision-making.16 Accordingly, it is not surprising that the resulting graphic by color unduly visually highlighted therapy risks that might lead to medicolegal suits, and by element size underestimated the net benefit of therapy (if neither treatment arm is superior, the physician cannot be sued whatever treatment he or she pursued). The Genentech graphic arose from a for-profit pharmaceutical company. As a result, it is not surprising that the figure by color unduly visually emphasized as desirable outcomes in the treatment group that were not actually different from outcomes in the placebo group. Perhaps somewhat unexpected was that the Genentech figure by element size underestimated, rather than overestimated, the benefit of therapy. This underestimate may have reflected self-interested caution in advancing therapeutic claims, even when justified, in the face of skepticism from some members of the physician audience.
The elementary deficiencies identified in both graphics by the formal rating scale are not the only liabilities of these figures. An additional defect we noted in both the AAEM and Genentech figures was misapplication of global statistic. Both figures state that they depict “disability” outcomes. However, neither figure is actually based on just the measures of disability used in the two NINDS-TPA trials. While neither graphic is presented with an explanation of the derivation of the numeric values that the icon arrays represent (an additional, substantial weakness of the decision aids), internal evidence (AAEM) and personal communication (Genentech) indicates that both derived their underlying rates of disability numeric values by an application to individual patients of the global statistic test that was the primary outcome measure of the trials. In the NINDS Study, the effect of treatment in improving outcome was assessed on four different outcome scales measuring neurologic deficit (NIH Stroke Scale), instrumental activities of daily living (Barthel Index), and global disability (modified Rankin Scale and Glasgow Outcome Scale). An inherent defect of the global statistic is that the vector effect in populations that it assesses is not directly translatable into impact upon individual patients.17, 18 Despite this established, formal barrier, the AAEM and Genentech figure designers apparently simply averaged together the disability and the non-disability scales without regard for their divergent appearance in individual patients.
We created corrected versions of the figures. In these versions, visual mis-privileging and dissimilar representation by treatment group are removed and the breadth of stakeholders participating in figure creation increased. The corrected Genentech figure, though not the corrected AAEM figure, is based on an actual measure of individual patient disability (the modified Rankin Scale) rather than a misapplication of the global statistic. These corrected figures can be considered for use in patient counseling when a graphic that compares visual displays of outcomes with and without treatment is desired. A remaining drawback of these figures is that they visually depict the net effect of treatment at only one (AAEM) or three (Genentech) transitions in disability state, rather than all 6 transitions measured by the modified Rankin Scale or all 7 transitions recognized by the World Health Organization.19 As a result they substantially underestimate the net benefit conveyed by therapy.
We also created a choice consequence figure that scores well on the quality scale. Advantages of choice consequence displays compared with outcome comparison displays include focusing reader attention directly upon the outcomes affected by treatment selection and disambiguation of the beneficial and harmful effects of therapy. Disadvantages include showing only alterations in outcome, not the full array of outcomes that result from each treatment option. Choice consequence displays are frequently employed in formal standard gamble studies eliciting patient preferences about therapies and outcomes.20 The choice consequence figure we derived has the added advantage of being the visual correlate of the numeric text statement of treatment benefits and risks issued jointly by the American Academy of Neurology, American College of Emergency Physicians, and the American Heart Association/American Stroke Association, the leading US neurologic, emergency medicine, and stroke patient support societies.15
The choice consequence figure we derived does have a drawback of overemphasizing harms of TPA therapy. The rate of symptomatic intracerebral hemorrhage (SICH) shown is derived from the NINDS trials definition of SICH. This definition is now generally recognized to be overly inclusive, encompassing asymptomatic as well as truly symptomatic hemorrhages. The more modern definitions of SICH used in the ECASS 3 and SITS-MOST trials would provide patients with accurate understanding of risk, but we retained the NINDS definition rates because they are incorporated in the AAN-ACEP-AHA/ASA statement and are still most familiar to clinicians. In addition, while the graphic shows both beneficial and harmful effects of therapy upon final outcome, it displays only harmful short term effects of therapy. A balanced figure would show a beneficial short term outcome analogous to the displayed harmful SICH outcome, such as therapy related increase in the rate of early recanalization associated with early clinical improvement or in the rate of dramatic early recovery. We did generate and test such graphics, but the additional outcome made the figure too complex for rapid comprehension by some lay informants. Alternatively, a balanced figure can be created by removing all short term outcomes and showing only the effect of treatment upon final outcome, as shown in Supplemental Figure A2. This approach was favored by several lay informants, as it made the figure even more rapidly comprehensible and allowed them to focus on the outcome of greatest salience – final functional state. Practitioners who want to use the decision aid that appeared most supported by patients may prefer this supplemental figure.
Our study has additional limitations. We focused upon visual decision aids. Numeric and verbal formats are also important means of conveying for presenting outcome information to decision-makers. Important work in this area in relation to stroke thrombolytic therapy has been performed.10, 15, 21, 22 The figure development process involved physicians and nurses from multiple specialties and stroke survivors, but not healthy individuals at risk for stroke or family members of stroke survivors. We searched Medline, but not other large bibliographic databases such as EMBASE, and the search was performed by only one experienced investigator, so we may not have identified all published figural aids, especially those published in non-English languages. This study assessed existing and developed new visuals aids assessed based on formal ratings scales and input from stakeholders, but did not test visual aid performance in a large group of patients or in the acute stroke settings. Future studies should analyze formally if patients and their surrogate decision-makers find the figures helpful in real time decision-making and how well the figures lead patients and proxies to reach decisions that accord with the patient’s underlying values.23
The benefit and risk display approaches developed in this study have general applicability to a variety of acute stroke treatments. In addition to aiding patient and clinician decision-making regarding IV TPA in the under 3 hour window, these graphic templates can be applied to facilitate treatment decisions regarding IV TPA in the 3–4.5 hour window, intra-arterial fibrinolysis under 6 hours, mechanical embolectomy under 8 hours, organized supportive care on a Stroke Unit, aspirin therapy within the first 48 hours, and hemicraniectomy for massive cerebral infarction.
The visual displays here presented are intended to support IV TPA decision-making and supplement, rather than replace, patient-practitioner discussions. In the brief time period available for brain resuscitation interventions in acute cerebral ischemia, they can help to convey the health benefits and risks of fibrinolytic stroke therapy efficiently and informatively to patients, family members, and clinicians.
Supplementary Material
Supplemental Figure. Decision matrix figures illustrating the benefits and risks of IV TPA in the under 3 hour window based upon data from the two NINDS-TPA trials. A1) Depicting only extreme changes in final outcome, not all changes in final outcome, appropriate for physicians who desiring a tool that uses only dichotomized outcome observations, not results from joint outcome table analysis. A2) Depicting both extreme and moderate changes in final outcome, but not changes in short term outcome. (Figure published with permission of UCLA Stroke Center.)
Acknowledgments
The authors are grateful to Joshua Emerson for graphic design consultation. This study was sponsored in part by NIH-NINDS Award P50 NS044378 and an American Heart Association PRT Health Outcomes Research Center Award.
Appendix Methods Text - Search Strategy
The Medline search for articles from January 1996 to June 2009 used the following combination of key words: <stroke OR cerebral ischemia OR brain ischemia OR cerebral infarct> AND <thrombolysis OR fibrinolysis OR acute treatment OR revascularization OR recanalization OR thrombolytic OR fibrinolytic OR TPA OR tissue plasminogen activator> AND <visual OR decision OR risks OR benefits OR consent OR graphical OR graphic OR guideline OR guide OR guidance OR aid OR number needed to treat OR framing OR presentation OR format>.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
Dr. Gadhia is an employee of the University of California, which holds a patent on retrieval device therapies for acute stroke.
Dr. Starkman is a site investigator in the NIH CLEAR-ER, IMS 2, IMS 3, and MR RESCUE multicenter clinical trials for which the UC Regents received payments based on the clinical trial contracts for the number of subjects enrolled, has served as a site investigator in a multicenter trials run by Vernalis, Paion, Lundbeck, and NTI for which the UC Regents received payments based on the clinical trial contracts for the number of subjects enrolled; is a site investigator in a multicenter registry run by Concentric for which the UC Regents received payments based on the clinical trial contracts for the number of subjects enrolled; received devices or study agent for use in NIH multicenter clinical trials from Concentric Medical, Genentech, Ekos Medical (all modest); administers stroke thrombolytic therapy in his practice (<5% of effort); is an employee of the University of California, which holds a patent on retriever devices for stroke; and is funded by NIH-NINDS Awards P50 NS044378 and U01 NS 44364.
Dr. Ovbiagele is a site investigator in multicenter trials sponsored by AGA Medical for which the UC Regents received payments based on the clinical trial contracts for the number of subjects enrolled; is a site investigator in the NIH CLEAR-ER, IMS 2, IMS 3, and MR RESCUE multicenter clinical trials for which the UC Regents received payments based on the clinical trial contracts for the number of subjects enrolled, administers stroke thrombolytic therapy in his practice (<5% of effort); and is an employee of the University of California, which holds a patent on retriever devices for stroke.
Dr. Ali is a site investigator in the NIH CLEAR-ER, IMS 2, IMS 3, and MR RESCUE multicenter clinical trials for which the UC Regents received payments based on the clinical trial contracts for the number of subjects enrolled; has served as an unpaid site investigator in a multicenter trials run by Vernalis, Paion, Lundbeck, and NTI for which the UC Regents received payments based on the clinical trial contracts for the number of subjects enrolled; administers stroke thrombolytic therapy in his practice (<5% of effort); is an employee of the University of California, which holds a patent on retriever devices for stroke; and is funded by NIH-NINDS Awards P50 NS044378 and U01 NS 44364.
Dr. Liebeskind is a scientific consultant regarding trial design and conduct to CoAxia, Concentric Medical, Brainsgate (all modest); has served as an unpaid site investigator in a multicenter trials run by Vernalis, Paion, Lundbeck, and NTI for which the UC Regents received payments based on the clinical trial contracts for the number of subjects enrolled; is a site investigator in the NIH CLEAR-ER, IMS 2, IMS 3, and MR RESCUE multicenter clinical trials for which the UC Regents received payments based on the clinical trial contracts for the number of subjects enrolled, administers stroke thrombolytic therapy in his practice (υ% of effort); is an employee of the University of California, which holds a patent on retriever devices for stroke; and is funded by NIH-NINDS Awards P50 NS044378 and U01 NS 44364.
Dr. Saver is a scientific consultant regarding trial design and conduct to CoAxia, Concentric Medical, Talecris, Ferrer, BrainsGate, PhotoThera, and Cygnis (all modest); has received lecture honoraria from Ferrer and Boehringer Ingelheim (modest); received devices for use in an NIH multicenter clinical trial from Concentric Medical (modest); has declined consulting/honoraria monies from Genentech since 2002; is a site investigator in the NIH CLEAR-ER, IMS 2,and IMS 3 multicenter clinical trials for which the UC Regents received payments based on the clinical trial contracts for the number of subjects enrolled, has served as an unpaid site investigator in a multicenter trials run by Vernalis, Paion, Lundbeck, and NTI for which the UC Regents received payments based on the clinical trial contracts for the number of subjects enrolled; administers stroke thrombolytic therapy in his practice (<5% of effort); is an employee of the University of California, which holds a patent on retriever devices for stroke; and is funded by NIH-NINDS Awards P50 NS044378 and U01 NS 44364.
References
- 1.Lansberg MG, Schrooten M, Bluhmki E, Thijs VN, Saver JL. Treatment time-specific number needed to treat estimates for tissue plasminogen activator therapy in acute stroke based on shifts over the entire range of the modified Rankin Scale. Stroke. 2009;40:2079–2084. doi: 10.1161/STROKEAHA.108.540708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marler JR, Winters Jones P, Emr M. The National Institute of Neurological Disorders and Stroke: Proceedings of National Symposium on Rapid Identification and Treatment of Acute Stroke; Bethesda, MD: National Institute of Neurological Disorders and Stroke; 1997. [Google Scholar]
- 3.Ancker JS, Senathirajah Y, Kukafka R, Starren JB. Design features of graphs in health risk communication: a systematic review. J Am Med Inform Assoc. 2006;13:608–618. doi: 10.1197/jamia.M2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cosmides L, Tooby J. Are humans good intuitive statisticians after all? Rethinking some conclusions from the literature on judgment under uncertainty. Cognition. 1996;58:1–73. [Google Scholar]
- 5.International Patient Decision Aids Collaboration. IPDAS 2005: Criteria for judging the quality of patient decision aids [online] Available at: http://www.ipdas.ohri.ca/IPDAS_checklist.pdf.
- 6.Elwyn G, O'Connor A, Stacey D, et al. Developing a quality criteria framework for patient decision aids: online international Delphi consensus process. Bmj. 2006;333:417. doi: 10.1136/bmj.38926.629329.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lipkus IM. Numeric, verbal, and visual formats of conveying health risks: suggested best practices and future recommendations. Med Decis Making. 2007;27:696–713. doi: 10.1177/0272989X07307271. [DOI] [PubMed] [Google Scholar]
- 8.Tufte E. The Visual Display of Quantitative Information. 2nd ed. Cheshire, Connecticut: Graphics Press LLC; 2001. [Google Scholar]
- 9.NINDS rt-PA Stroke Group. Tissue plasminogen activator for acute ischemic stroke. New England Journal of Medicine. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 10.Saver JL. Number needed to treat estimates incorporating effects over the entire range of clinical outcomes: novel derivation method and application to thrombolytic therapy for acute stroke. Arch Neurol. 2004;61:1066–1070. doi: 10.1001/archneur.61.7.1066. [DOI] [PubMed] [Google Scholar]
- 11.Saver JL. Hemorrhage after thrombolytic therapy for stroke: the clinically relevant number needed to harm. Stroke. 2007;38:2279–2283. doi: 10.1161/STROKEAHA.107.487009. [DOI] [PubMed] [Google Scholar]
- 12.Anonymous. AAEM position statement educational tool: tPA for stroke – potential benefit, risk and alternatives. 2007 [Google Scholar]
- 13.The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. NEJM. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 14.Anonymous. Activase (t-PA) makes a positive clinical difference [online] Available at http://www.activase.com/about/index.jsp.
- 15.Anonymous. Tissue plasminogen activator (tPA): what you should know [online] Available at: http://www.giveme5forstroke.org/docs/tPA_Flyer.pdf.
- 16.McNamara R. AAEM comment (on position statement on the use of intravenous thrombolytic therapy in the treatment of stroke) [online] Available at: http://www.aaem.org/positionstatements/thrombolytictherapy.php.
- 17.Kasner SE. Clinical interpretation and use of stroke scales. Lancet Neurol. 2006;5:603–612. doi: 10.1016/S1474-4422(06)70495-1. [DOI] [PubMed] [Google Scholar]
- 18.Saver JL. Novel end point analytic techniques and interpreting shifts across the entire range of outcome scales in acute stroke trials. Stroke. 2007;38:3055–3062. doi: 10.1161/STROKEAHA.107.488536. [DOI] [PubMed] [Google Scholar]
- 19.World Health Organization. Geneva, Switzerland: World Health Organization; The Global Burden of Disease: 2004 Update. 2004
- 20.Lenert L, Kaplan RM. Validity and interpretation of preference-based measures of health-related quality of life. Med Care. 2000;38:II138–II50. doi: 10.1097/00005650-200009002-00021. [DOI] [PubMed] [Google Scholar]
- 21.Kent DM, Selker HP, Ruthazer R, Bluhmki E, Hacke W. he stroke-thrombolytic predictive instrument: a predictive instrument for intravenous thrombolysis in acute ischemic stroke. Stroke. 2006;37:2957–2962. doi: 10.1161/01.STR.0000249054.96644.c6. [DOI] [PubMed] [Google Scholar]
- 22.Demaerschalk BM. Thrombolytic therapy for acute ischemic stroke: the likelihood of being helped versus harmed. Stroke. 2007;38:2215–2216. doi: 10.1161/STROKEAHA.107.494112. [DOI] [PubMed] [Google Scholar]
- 23.Carling CL, Kristoffersen DT, Flottorp S, et al. The effect of alternative graphical displays used to present the benefits of antibiotics for sore throat on decisions about whether to seek treatment: a randomized trial. PLoS Med. 2009;6:e1000140. doi: 10.1371/journal.pmed.1000140. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure. Decision matrix figures illustrating the benefits and risks of IV TPA in the under 3 hour window based upon data from the two NINDS-TPA trials. A1) Depicting only extreme changes in final outcome, not all changes in final outcome, appropriate for physicians who desiring a tool that uses only dichotomized outcome observations, not results from joint outcome table analysis. A2) Depicting both extreme and moderate changes in final outcome, but not changes in short term outcome. (Figure published with permission of UCLA Stroke Center.)