Abstract
Background and Purpose
Although lower serum homocysteine concentration is associated with a reduced risk of stroke in epidemiologic studies, randomized controlled trials (RCTs) have yielded mixed findings regarding the effect of therapeutic homocysteine lowering on stroke prevention. We performed a meta-analysis of RCTs to assess the efficacy of folic acid supplementation in the prevention of stroke.
Methods
Salient trials were identified by formal literature search. Relative risk (RR) with 95% confidence interval (CI) was used as a measure of the association between folic acid supplementation and risk of stroke, pooling data across trials using a fixed-effects model.
Results
The search identified 13 RCTs of folic acid therapy to reduce homocysteine, enrolling 39,005 participants, in which stroke was reported as an outcome measure. Across all trials, folic acid supplementation was associated with a trend toward mild benefit that did not reach statistical significance in reducing the risk of stroke (RR 0.93, 95% CI 0.85-1.03; p=0.16). The RR for non-secondary prevention trials was 0.89 (95% CI 0.79-0.99; p=0.03). In stratified analyses, a greater beneficial effect was seen in the trials testing combination therapy of folic acid plus vitamins B6 and B12 (RR 0.83, 0.71-0.97; p=0.02) and in the trials which disproportionately enrolled male patients (men/women > 2, RR 0.84, 0.74-0.94; p=0.003).
Conclusions
Folic acid supplementation did not demonstrate a major effect in averting stroke. However, potential mild benefits in primary stroke prevention, especially when folate is combined with B vitamins and in male patients, merit further investigation.
Keywords: Homocysteine, Folic Acid, Stroke, Prevention, Meta-Analysis
Severe hyperhomocysteinemia, a feature of inborn errors of methionine metabolism, is associated with atherosclerosis.1 In experimental studies, homocysteine causes oxidative stress, enhances inflammatory process and damages vascular endothelium.2, 3 In epidemiological studies, elevated homocysteine is linked with ischemic heart disease and stroke.4-6 A meta-analysis of prospective observational studies showed a 25% lower homocysteine level was associated with 11% lower ischemic heart disease risk and 19% lower stroke risk.7 Folate and vitamin B12 are important regulators in the homocysteine metabolism and studies have shown inverse relationship between folate intake and homocysteine level.8, 9 Folic acid supplementation has also been associated with a reduction in carotid atherosclerosis progression.10, 11 These observations suggest that folic acid supplementation holds promise as a potential therapy for stroke prevention.
Multiple clinical trials have now been performed investigating folic acid for the prevention of cardiovascular and cerebrovascular outcomes in both primary and secondary prevention populations. At least two prior meta-analyses have pooled results of trials and reached contrasting conclusions, with one suggesting no benefit on cardiovascular endpoints and another indicating mild benefit in reducing stroke risk.12, 13 Also, these systematic reviews did not analyze gender effects even though some cohort studies found that higher folate intake was associated with reduced ischemic stroke risk in men but not women.14-16 Several large trials have been published in the interval since the most recent meta-analysis and offer more evidence on these issues.17-19 We therefore undertook an updated meta-analysis.
Materials and Methods
The study was performed in accordance with the recommendations of the Quality of Reporting of Meta-analysis (QUOROM) consensus group. 20
Search strategy: We searched MEDLINE (via Pubmed), Cochrane Central Register of Controlled Trials, and the clinical trial registry maintained at clinicaltrials.gov with the terms “homocysteine”, “folate”, “folic acid”, “vitamin B12”, “cobalamine”, “vitamin B6”, “pyridoxine”, and “multivitamin” crossed with the terms “cardiovascular disease”, “myocardial infarct”, “myocardial ischemia”, “coronary heart disease”, “angina”, “heart attack”, “stroke”, “cerebrovascular disease”, “cerebrovascular attack”, “brain attack”, “brain infarct”, “brain hemorrhage”, and “intracranial hemorrhage”. We restricted our search to human beings and clinical trials from January 1966 to May 2009. There were no language restrictions. We used the same search strategy to search abstracts in all American Heart Association (AHA) sponsored meetings, from January, 2003 to February, 2009, via the AHA Abstract Archive Tool (http://www.abstractsonline.com/arch/home.aspx?lookupkey=12345). We also reviewed the introduction and discussion sections of retrieved trials and of prior meta-analyses to identify additional trials.
Criteria for inclusion of a study were: (1) study design was a randomized controlled trial (RCT); (2) comparison of folic acid supplementation (with or without vitamins B6 and B12) with inactive or very low dose control; (3) intervention duration was at least 6 months; (4) total participants and the number of stroke events were reported by active treatment and control groups, respectively. Participants with any age or gender were included. Studies were excluded if (1) the control group received another active therapy that active treatment group did not receive; (2) the active group received another therapy in addition to folic acid and B vitamin that the control group did not receive.
All data from eligible trials were abstracted in duplicate by two investigators independently (ML and KSH) with a standard protocol. Discrepancies were resolved by discussion with a third investigator (JLS) and by referencing the original report.
Statistical analysis
Data were analyzed according to the intention-to-treat principle. The Cochrane Collaboration's Review Manager software package (RevMan 5) was used for the meta-analysis and R software was used for the meta-regression. Relative risk (RR) with 95% confidence interval (CI) was used as a measure of the association between folic acid supplementation and risk of stroke. Heterogeneity was assessed by p value of chi square statistics and I2, which describes the percentage of variability in the effect estimates that is due to heterogeneity rather than chance. 21, 22 Heterogeneity was considered significant if the p value of chi square statistics less than 0.05. We regarded I2 of less than 40% as minimal, 40-74% as modest, and more than 74% as considerable.23 We planned to pool data across trials using the fixed-effects model based on Mantel-Haenszel methods if considerable heterogeneity, p < 0.05 or I2 ≥ 75%, was not present. 24, 25 We also compared results obtained from a fixed-effect model with a random-effect model to address concerns about the influence of small-study effects on the results of a meta-analysis in which there is evidence of between-study heterogeneity.23 Publication bias was estimated visually by funnel plots displaying standard error as the measure of sample size and RR as the measure of treatment effect.26 We also did a sensitivity analysis to further explore the robustness of our results. To identify any study that may have exerted a disproportionate influence on the summery treatment effect, we removed each individual trial from the meta-analysis one at a time. For all analyses, p < 0.05 was considered statistically significant.
Results
The literature review identified 21 articles for detailed assessment, among which 7 were excluded for lack of data on stroke,11, 27-32 and 1 because it was derived from the same study population as another report (figure 1).33 Our final analysis included 13 RCTs, enrolling 39,005 individuals.17-19, 34-43 The study design characteristics are presented in table 1 and the baseline characteristics of the study participants and their homocysteine change at the end of the trial are presented in table 2. Of the 13 trials, eight reported adequate generation of the allocation sequence and adequate allocation concealment17-19, 37-40, 43 while ten reported adequate blinding of participants and outcome assessors. 17-19, 34, 37-40, 42, 43 Seven trials were done in regions without folic acid fortification, 18, 34-36, 39, 41, 43 three in regions with folic acid fortification, 17, 19, 38 and three were done in both fortified and non-fortified regions (i.e. partly fortified).37, 40, 42 All 13 trials included individuals with pre-existing conditions: stroke (1 trial),37 coronary heart disease (5 trials),18, 35, 36, 39, 43 manifest cardiovascular disease or multiple risk factors for atherosclerosis (2 trials) 17, 40, ESRD or advanced chronic kidney disease (4 trials),19, 38, 41, 42 and esophageal dysplasia (1 trial).34 Cerebrovascular events analyzed were combined nonfatal and fatal strokes in 10 trials; for 1 trial each, data was available only on fatal stroke; nonfatal and fatal ischemic stroke; and the composite of nonfatal and fatal stroke plus transient ischemic attack. Neuroimaging was explicitly mentioned as part of the stroke event ascertainment process in seven trials.17-19, 37, 39-41
Figure 1.
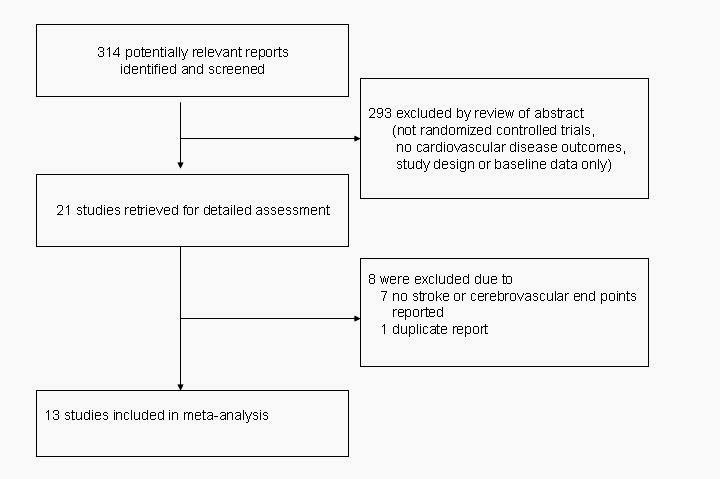
Flow of study selection process
Table 1.
Trial characteristics
| Allocation concealment | Blinding | Active treatment | Control | Duration (months) | Cerebrovascular Event Definition | Countries | Folic acid fortification | |
|---|---|---|---|---|---|---|---|---|
| Mark 199634 | Unclear | Double | Folic acid 0.8mg and vitamins B6 and B12 | Placebo | 72 | Fatal Stroke | China | No |
| Liem 200435 | Unclear | Open | Folic acid 5mg | Usual care | 12 | Fatal and Nonfatal Stroke | Netherlands | No |
| Toole 200437 | Yes | Double | Folic acid 2.5mg and vitamins B6 and B12 | Folic acid 0.02mg and vitamins B6 and B12 | 20 | Fatal and Nonfatal Ischemic Stroke | USA, Canada, Scotland | Partly (in USA and Canada but not Scotland) |
| Wrone 200438 | Yes | Double | Folic acid 5mg or 15mg and vitamins B6 and B12 | Folic acid 1mg and vitamins B6 and B12 | 24 | Fatal and Nonfatal Stroke | USA | Yes |
| Leim 200536 | Yes | Open | Folic acid 0.5mg | Usual care | 42 | Fatal and Nonfatal Stroke and Transient Ischemic Attack | Netherlands | No |
| Bonaa 200639 | Yes | Double | Folic acid 0.8mg and vitamin B12 with or without B6 | Placebo | 36 | Fatal and Nonfatal Stroke | Norway | No |
| Lonn 200640 | Yes | Double | Folic acid 2.5mg and vitamins B6 and B12 | Placebo | 60 | Fatal and Nonfatal Stroke | Canada, USA, Brazil, western Europe, Slovakia | Partly (in USA and Canada but not in other countries) |
| Righetti 200641 | Yes | Open | Daily or every other day folic acid 5mg and vitamins B6 and B12 | Vitamins B6 and B12 | 29 | Fatal and Nonfatal Stroke | Italy | No |
| Zoungas 200642 | Unclear | Double | Folic acid 15mg | Placebo | 43 | Fatal and Nonfatal Stroke | Australia and New Zealand | Partly |
| Jamison 200719 | Yes | Double | Folic acid 40mg and vitamins B6 and B12 | Placebo | 39 | Fatal and Nonfatal Stroke | USA | Yes |
| Albert 200817 | Yes | Double | Folic acid 2.5mg and vitamins B6 and B12 | Placebo | 88 | Fatal and Nonfatal Stroke | USA | Yes |
| Ebbing 200818 | Yes | Double | Folic acid 0.8mg and vitamin B12 with or without B6 | Placebo | 38 | Fatal and Nonfatal Stroke | Norway | No |
| Collins 200843 | Yes | Double | Folic acid 2mg and vitamin B12 | Placebo | 78 | Fatal and Nonfatal Stroke | UK | No |
Table 2.
Demographics and homocysteine at baseline and changes
| Preexisting condition | Patients | Age, years | Men, % | Previous stroke, % | DM, % | Baseline Hcy, μmol/L | Hcy reduction, % | |
|---|---|---|---|---|---|---|---|---|
| Mark 199634 | Esophageal dysplasia | 3318 | 54 | 44 | NR | NR | NR | NR |
| Liem 200435 | Acute MI | 283 | 59 | 70 | NR | 24 | NR | NR |
| Toole 200437 | Ischemic stroke | 3680 | 66.3±10.8 | 63 | 100 | 29 | 13.4 | 14.0 |
| Wrone 200438 | ESRD | 510 | 60.2±15.1 | 50 | NR | 45 | 33.0±20.4 | 20.0 |
| Leim 200536 | CHD | 593 | 65.2±9.8 | 78 | 7 | 9 | 12.1±4.3 | 21.7 |
| Bonaa 200639 | Acute MI | 2815 | 63.2 ±11.6 | 74 | 4 | 10 | 13.1±4.8 | 28.7 |
| Lonn 200640 | Vascular disease or multiple risk factors for atherosclerosis | 5522 | 68.9±6.9 | 71 | 9 | 40 | 12.2±1.6 | 24.8 |
| Righetti 200641 | ESRD | 88 | 64.5±1.8 | 56 | NR | 19 | 34.9±1.4 | 34.3 |
| Zoungas 200642 | ESRD | 315 | 56±13 | 68 | 9 | 23 | 27±13 | 10.0 |
| Jamison 200719 | ESRD and ACKD | 2056 | 65.8±11.7 | 98 | 15 | 55 | 22.4 (18.7-27.3) | 34.6 |
| Albert 200817 | CVD or multiple risk factors for atherosclerosis | 5442 | 62.8±8.8 | 0 | NR | 21 | 12.3 (9.6-15.5) | 18.5 |
| Ebbing 200818 | CHD and/or aortic valve stenosis | 2319 | 61.7±10.1 | 79 | 6 | 12 | 10.8±4.5 | 26 |
| Collins 200843 | MI | 12064 | 64±9 | 83 | 7 | 11 | 13.5±5 | 28 |
DM: diabetes mellitus; Hcy: homocysteine; MI: myocardial infarct; CVD: cardiovascular disease; CHD: coronary heart disease; ESRD: end-stage renal disease; ACKD: advanced chronic kidney disease; NR: not reported
Pooling all 13 trials, the effect of folic acid supplementation (with or without vitamins B6 and B12) on the occurrence of stroke was RR 0.93, 95% CI 0.85-1.03; p=0.16 (figure 2). The total events were 784 among 20415 participants (3.8%) in the active treatment group and 791 among 18590 participants (4.3%) in the control group. There was no substantial asymmetric appearance on funnel plot (Supplement Figure, online only). The estimate from a random-effect model (RR 0.92, 95% CI 0.82-1.03; p=0.16) was similar to the estimate from a fixed-effect model.
Figure 2.
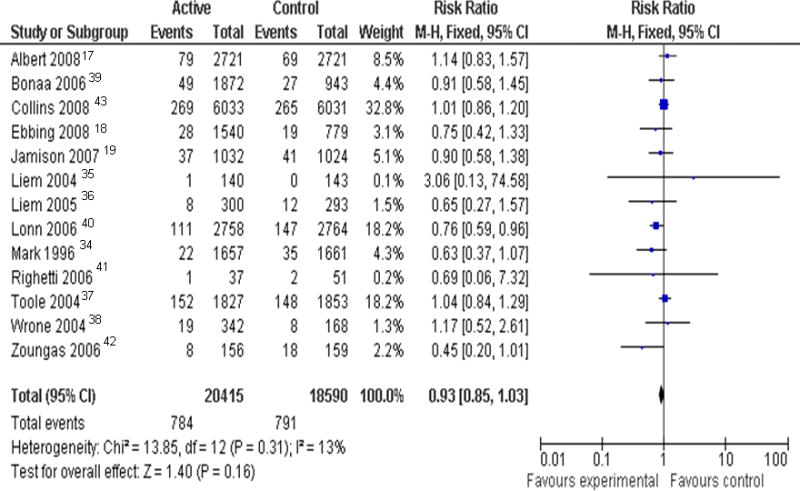
Relative risk (risk ratio) with 95% confidence interval (CI) estimates stroke (active treatment vs. control), by trial and pooled
Figure 3 shows the pooled RR for stroke stratified by stroke subtype, history of stroke, folic acid fortification, homocysteine reduction rate, duration of active treatment, daily dose of folic acid, treatment regimen, pre-existing conditions, and gender. The RR for non-secondary-prevention trials was 0.89 (95% CI 0.79-0.99; p=0.03). The RR for trials using folic acid plus vitamins B12 and B6 was 0.83 (95% CI 0.71-0.97; p=0.02) while trials using folic acid alone or folic acid plus vitamin B12 did not show any RR change. The RR for men predominant trials was 0.84 (95% CI 0.74-0.94; p=0.003) while women predominant did not show substantial RR change. Men predominant trials also differed from women predominant trials in having higher baseline homocysteine concentrations (13.8 μmol/L vs 12.3 μmol/L), greater reductions in homocysteine levels in the active treatment arm (27.5% vs 18.5%), and higher stroke event rates in control arms (4.3% vs 2.3%).
Figure 3.
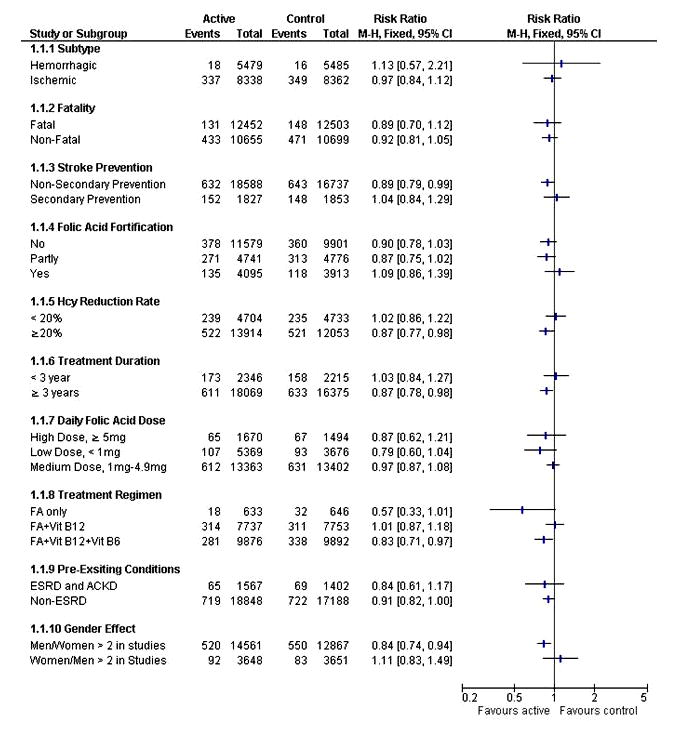
Effect of folic acid supplements on the risk of stroke in pre-specified subgroups. Hcy: homocysteine; FA: folic acid; ESRD: end-stage renal disease; ACKD: advanced chronic kidney disease
Reductions in stroke events were also found in the subgroup of trials with substantial achieved homocysteine reductions (≥ 20%) - RR 0.87, 95%CI 0.77-0.98; p=0.02, and in trials with longer treatment duration (≥ 3 years) - RR 0.87, 95% CI 0.78-0.98; p=0.02. However, meta-regression did not demonstrate a substantial linear relationship between degree of homocysteine reduction and stroke rate (p=0.73) or between length of treatment duration and stroke rate (p=0.77) (figure 4).
Figure 4.
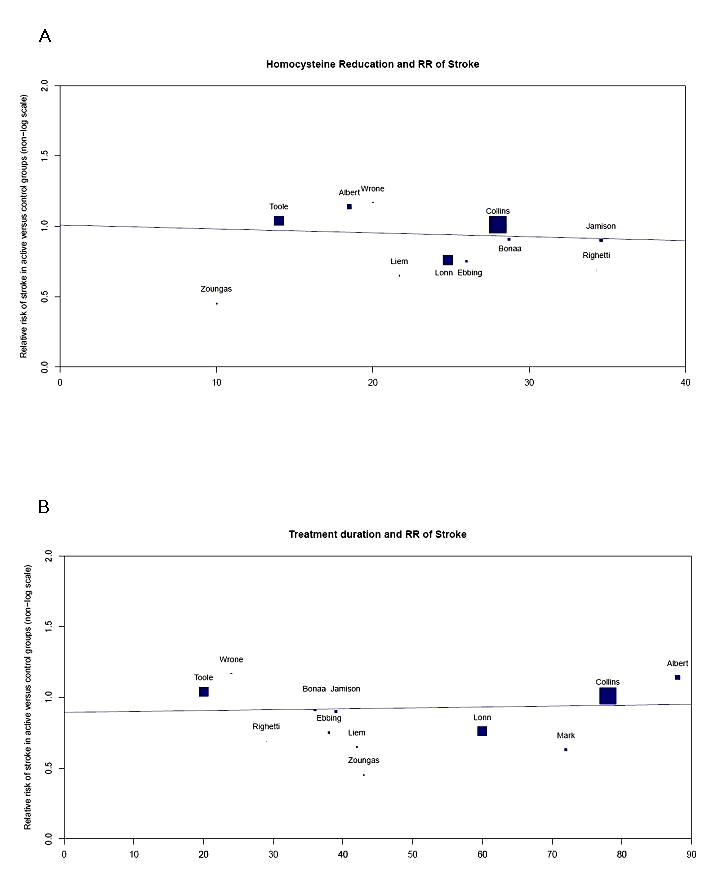
Relative risk (RR) of stroke
(A) RR of stroke in relation to percentage change in homocysteine concentrations (Mark 1996 and Liem 2004 were excluded due to no homocysteine concentrations recorded)
(B) RR of stroke in relation to active treatment duration (an outlier, Liem 2004, was excluded)
Discussion
Our meta-analysis, including 13 RCTs with over 39,000 participants, found a trend toward mild benefit that did not reach statistical significance of folic acid supplementation on the risk of stroke among high cardiovascular risk persons. This result contrasts with the prior meta-analysis conducted in 200713 which showed a positive result for stroke reduction. The main difference is the inclusion in our analysis of four large trials, encompassing 21,881 participants, that have been completed since 2007 which generally found neutral effects.
Several subgroup analyses suggested potential benefit in particular settings, but must be regarded as hypothesis-generating given the multiplicity of analyses performed. Multiple analyses suggested that trials using treatment regimens of greater intensity or duration were of modest benefit, including trials in which the active treatment consisted of vitamins B6 and B12 in addition to folic acid, trials with substantial reductions in homocysteine levels in the active arm, and trials with longer treatment duration. However, meta-regression analyses were discordant, failing to show a linear relationship of homocysteine lowering or treatment duration to stroke prevention.
We also found that active treatment showed clinical benefit in trials in which men predominated among enrolled patients. This result is concordant with observational studies which showed higher folate intake is associated with reduced ischemic stroke risk in male US health professionals and male Finnish smokers,15, 16 but not female US nurses.14 Potential explanations for this gender interaction are that men have higher stroke event rates, increasing study power to detect treatment effects, and gender differences in the severity and treatment-responsiveness of hyperhomocysteinemia, both greater in men than women.
A mild benefit of folic acid supplementation was shown in trials in which stroke was not a qualifying event but not in secondary prevention trials. This observation raises the possibility that homocysteine lowering is beneficial at early stages of vascular disease elaboration, but less effective in the face of established, advanced disease. In a study with participants without diabetes or cardiovascular disease, B vitamin supplementation significantly reduced progression of early-stage subclinical atherosclerosis (carotid artery intima media thickness) but had no effect on progression of aortic or coronary artery calcification, markers of late-stage atherosclerosis.11
One explanation that has been advanced for neutral results in individual trials of folate therapy is that spread of fortification of the food supply with folic acid increased the background dietary intake of folate among all enrolled patients, diminishing the potential for the active treatment arm to exert a treatment effect. A physiologic study found that low-dose (0.4mg/d) folic acid treatment, comparable to daily intake and dietary fortification, improved vascular endothelial function and high-dose (5mg/d) folic acid treatment provided no additional benefit.44 Whereas plasma 5-methyltetrahydrofolate levels increased proportionately with treatment dose of folic acid, vascular tissue 5-methyltetrahydrofolate showed no further increment with high-dose compared with low-dose folic acid.44 These findings may partly explain the improvement in stroke mortality after folic acid fortification in Canada and the United States.45 Our meta-analysis found a trend toward treatment benefit in trials conducted only or partly in countries without background fortification of the food supply, but no evidence of benefit of pharmacologic folate therapy in trials performed wholly in countries with fortification.
Some studies reported that homocysteine reduces the concentration of high density lipoprotein (HDL) cholesterol in plasma by inhibiting the hepatic synthesis of apolipoprotein A1 (apoA-I), the main HDL apolipoprotein.46, 47 These studies not only explain the documented inverse correlation between the plasma concentrations of HDL cholesterol and homocysteine,48 but also raise the real possibility that a homocysteine-induced inhibition of apoA-I synthesis is the mechanism linking homocysteine to the development of atherosclerosis. A low concentration of HDL cholesterol has been shown in epidemiological studies to be predictive of stroke49 and treatment that increase the level of HDL cholesterol in plasma may related to regression of atherosclerosis.50 Since most participants in our meta-analysis were high cardiovascular risk persons and intensive lipid profile intervention was likely to be applied, the additional benefit for homocysteine lowering might be difficult to demonstrate. On the other hand, a meta-analysis of prospective observational studies did not adjust for HDL cholesterol levels and it is likely to overestimate the association of homocysteine and CVD.7
Some limitations need to be mentioned. Meta-analysis is retrospective research that can be constrained by comprehensiveness of searches, methodological rigor of the included studies, and publication bias. We tried to maximize study identification and minimize bias by developing the study protocol a priori, performing a thorough search of several databases, and using explicit criteria for study selection, data collection, and data analysis. An additional limitation of meta-analysis is that vary with respect to the characteristics of participants, duration and intensity of treatment, the type of cerebrovascular event identified and the expertise of stroke adjudicators, and other design features. However, formal testing did not identify any substantial resulting heterogeneity among trial findings.
In conclusion, meta-analysis of completed trials does not demonstrate a major benefit of folic acid supplementation in averting stroke. However, potential mild benefits were observed in primary stroke prevention, especially when folate is combined with B vitamins and in male patients. As folic acid supplementation is an inexpensive, safe, and widely applicable intervention, further investigation in these settings is warranted.
Supplementary Material
Supplement Figure: Funnel plot of 13 trials included in the current meta-analysis. SE: standard error, RR: relative risk
Acknowledgments
Source of Funding: Meng Lee was supported by a grant from (CMRPG 660311, Taiwan) and Jeffrey L Saver was supported by NIH SPOTRIAS Center and AHA PRT Health Outcomes Center Awards.
JLS has received honoraria from Universities as a visiting professor; is an employee of the University of California, which holds a patent on retriever devices for stroke; is a scientific consultant regarding trial design and conduct to Concentric Medical, Talecris, and Ev3; is a site investigator in multicenter trials sponsored by Lundbeck for which the UC Regents received payments based on the clinical trial contracts for the number of subjects enrolled; is a site investigator in the NIH IMS 3 and CLEAR-ER multicenter clinical trials for which the UC Regents receive payments based on the clinical trial contracts for the number of subjects enrolled; has declined consulting/honoraria monies from Genentech since 2002; and is funded by NIH-NINDS Awards P50 NS044378 and U01 NS 44364.
Footnotes
The corresponding author (JLS) had full access to all the data in the study and had the final responsibility for the decision to submit for publication.
Conflict of interest: We declare that we have no conflict of interest.
References
- 1.McCully KS. Vascular pathology of homocysteinemia: Implications for the pathogenesis of arteriosclerosis. Am J Pathol. 1969;56:111–128. [PMC free article] [PubMed] [Google Scholar]
- 2.Curro M, Condello S, Caccamo D, Ientile R. Homocysteine-induced toxicity increases TG2 expression in neuro2a cells. Amino Acids. 2009;36:725–730. doi: 10.1007/s00726-008-0122-x. [DOI] [PubMed] [Google Scholar]
- 3.Welch GN, Loscalzo J. Homocysteine and atherothrombosis. N Engl J Med. 1998;338:1042–1050. doi: 10.1056/NEJM199804093381507. [DOI] [PubMed] [Google Scholar]
- 4.Ridker PM, Manson JE, Buring JE, Hennekens CH. Homocysteine and risk of cardiovascular disease among postmenopausal women. JAMA. 1999;281:1817–1821. doi: 10.1001/jama.281.19.1817. [DOI] [PubMed] [Google Scholar]
- 5.Khan U, Crossley C, Kalra L, Rudd A, Markus HS. Homocysteine and its relationship to stroke subtypes in a UK black population: The South London ethnicity and stroke study. Stroke. 2008;39:2943–2949. doi: 10.1161/STROKEAHA.107.513416. [DOI] [PubMed] [Google Scholar]
- 6.Iso H, Moriyama Y, Sato S, Kitamura A, Shimamoto T. Serum total homocysteine concentrations and risk of stroke and its subtypes in Japanese. Circulation. 2004;109:2766–2772. doi: 10.1161/01.CIR.0000131942.77635.2D. [DOI] [PubMed] [Google Scholar]
- 7.Homocysteine and risk of ischemic heart disease and stroke: A meta-analysis. JAMA. 2002;288:2015–2022. doi: 10.1001/jama.288.16.2015. [DOI] [PubMed] [Google Scholar]
- 8.Jacob RA, Wu MM, Henning SM, Swendseid ME. Homocysteine increases as folate decreases in plasma of healthy men during short-term dietary folate and methyl group restriction. J Nutr. 1994;124:1072–1080. doi: 10.1093/jn/124.7.1072. [DOI] [PubMed] [Google Scholar]
- 9.Dose-dependent effects of folic acid on blood concentrations of homocysteine: A meta-analysis of the randomized trials. Am J Clin Nutr. 2005;82:806–812. doi: 10.1093/ajcn/82.4.806. [DOI] [PubMed] [Google Scholar]
- 10.Till U, Rohl P, Jentsch A, Till H, Muller A, Riezler R. Decrease of carotid intima-media thickness in patients at risk to cerebral ischemia after supplementation with folic acid, vitamins B6 and B12. Atherosclerosis. 2005;181:131–135. doi: 10.1016/j.atherosclerosis.2004.12.043. [DOI] [PubMed] [Google Scholar]
- 11.Hodis HN, Mack WJ, Dustin L, Selzer RH. High-dose B vitamin supplementation and progression of subclinical atherosclerosis: A randomized controlled trial. Stroke. 2009;40:730–736. doi: 10.1161/STROKEAHA.108.526798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bazzano LA, Reynolds K, Holder KN, He J. Effect of folic acid supplementation on risk of cardiovascular diseases: A meta-analysis of randomized controlled trials. JAMA. 2006;296:2720–2726. doi: 10.1001/jama.296.22.2720. [DOI] [PubMed] [Google Scholar]
- 13.Wang X, Qin X, Demirtas H, Li J, Mao G, Huo Y, Sun N, Liu L, Xu X. Efficacy of folic acid supplementation in stroke prevention: A meta-analysis. Lancet. 2007;369:1876–1882. doi: 10.1016/S0140-6736(07)60854-X. [DOI] [PubMed] [Google Scholar]
- 14.Al-Delaimy WK, Rexrode KM, Hu FB, Albert CM, Manson JE. Folate intake and risk of stroke among women. Stroke. 2004;35:1259–1263. doi: 10.1161/01.STR.0000127813.12854.9c. [DOI] [PubMed] [Google Scholar]
- 15.He K, Merchant A, Rimm EB, Willett WC, Ascherio A. Folate, vitamin B6, and B12 intakes in relation to risk of stroke among men. Stroke. 2004;35:169–174. doi: 10.1161/01.STR.0000106762.55994.86. [DOI] [PubMed] [Google Scholar]
- 16.Larsson SC, Mannisto S, Virtanen MJ, Virtamo J. Folate, vitamin B6, vitamin B12, and methionine intakes and risk of stroke subtypes in male smokers. Am J Epidemiol. 2008;167:954–961. doi: 10.1093/aje/kwm395. [DOI] [PubMed] [Google Scholar]
- 17.Albert CM, Cook NR, Gaziano JM, Zaharris E, Manson JE. Effect of folic acid and B vitamins on risk of cardiovascular events and total mortality among women at high risk for cardiovascular disease: A randomized trial. JAMA. 2008;299:2027–2036. doi: 10.1001/jama.299.17.2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ebbing M, Bleie O, Ueland PM, Nordrehaug JE, Nilsen DW, Vollset SE, Refsum H, Pedersen EK, Nyagrd O. Mortality and cardiovascular events in patients treated with homocysteine-lowering B vitamins after coronary angiography: A randomized controlled trial. JAMA. 2008;300:795–804. doi: 10.1001/jama.300.7.795. [DOI] [PubMed] [Google Scholar]
- 19.Jamison RL, Hartigan P, Kaufman JS, Goldfarb DS, Warren SR, Guarino PD, Gaziano JM, Veterans Affairs Site Investigators Effect of homocysteine lowering on mortality and vascular disease in advanced chronic kidney disease and end-stage renal disease: A randomized controlled trial. JAMA. 2007;298:1163–1170. doi: 10.1001/jama.298.10.1163. [DOI] [PubMed] [Google Scholar]
- 20.Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta-analyses of randomised controlled trials: The QUOROM statement. Quality of reporting of meta-analyses. Lancet. 1999;354:1896–1900. doi: 10.1016/s0140-6736(99)04149-5. [DOI] [PubMed] [Google Scholar]
- 21.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 22.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions. Cochrane Collaboration and John Wiley & Sons Ltd.; 2008. [Google Scholar]
- 24.Greenland S, Robins JM. Estimation of a common effect parameter from sparse follow-up data. Biometrics. 1985;41:55–68. [PubMed] [Google Scholar]
- 25.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–748. [PubMed] [Google Scholar]
- 26.Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: Guidelines on choice of axis. J Clin Epidemiol. 2001;54:1046–1055. doi: 10.1016/s0895-4356(01)00377-8. [DOI] [PubMed] [Google Scholar]
- 27.Baker F, Picton D, Blackwood S, Brown MJ. Blind comparison of folic acid and placebo in patients with ischemic heart disease: An outcome trial. Circulation. 2002;106:A 3642. abstract. [Google Scholar]
- 28.Schnyder G, Roffi M, Flammer Y, Hess OM. Effect of homocysteine-lowering therapy with folic acid, vitamin B12, and vitamin B6 on clinical outcome after percutaneous coronary intervention: The Swiss Heart Study: A randomized controlled trial. JAMA. 2002;288:973–979. doi: 10.1001/jama.288.8.973. [DOI] [PubMed] [Google Scholar]
- 29.Righetti M, Ferrario GM, Milani S, Sessa A. Effects of folic acid treatment on homocysteine levels and vascular disease in hemodialysis patients. Med Sci Monit. 2003;9:PI37–42. [PubMed] [Google Scholar]
- 30.Lange H, Suryapranata H, De Luca G, Dambrink JHE. Folate therapy and in-stent restenosis after coronary stenting. N Engl J Med. 2004;350:2673–2681. doi: 10.1056/NEJMoa032845. [DOI] [PubMed] [Google Scholar]
- 31.Vianna AC, Mocelin AJ, Matsuo T, Matni AM. Uremic hyperhomocysteinemia: A randomized trial of folate treatment for the prevention of cardiovascular events. Hemodial Int. 2007;11:210–216. doi: 10.1111/j.1542-4758.2007.00171.x. [DOI] [PubMed] [Google Scholar]
- 32.Potena L, Grigioni F, Masetti M, Branzi A. Long-term effect of folic acid therapy in heart transplant recipients: Follow-up analysis of a randomized study. Transplantation. 2008;85:1146–1150. doi: 10.1097/TP.0b013e31816b2602. [DOI] [PubMed] [Google Scholar]
- 33.Liem A, Reynierse-Buitenwerf GH, Zwinderman AH, van Veldhuisen DJ. Secondary prevention with folic acid: Effects on clinical outcomes. J Am Coll Cardiol. 2003;41:2105–2113. doi: 10.1016/s0735-1097(03)00485-6. [DOI] [PubMed] [Google Scholar]
- 34.Mark SD, Wang W, Fraumeni JF, Jr, Li JY, Taylor PR, Wang GQ, Guo W, Dawsey SD, Li B, Blot WJ. Lowered risks of hypertension and cerebrovascular disease after vitamin/mineral supplementation: The Linxian Nutrition Intervention Trial. Am J Epidemiol. 1996;143:658–664. doi: 10.1093/oxfordjournals.aje.a008798. [DOI] [PubMed] [Google Scholar]
- 35.Liem AH, van Boven AJ, Veeger NJ, Withagen AJ, van Veldhuisen DJ. Efficacy of folic acid when added to statin therapy in patients with hypercholesterolemia following acute myocardial infarction: A randomised pilot trial. Int J Cardiol. 2004;93:175–179. doi: 10.1016/j.ijcard.2003.02.001. [DOI] [PubMed] [Google Scholar]
- 36.Liem A, Reynierse-Buitenwerf GH, Zwinderman AH, Jukema JW, van Veldhuisen DJ. Secondary prevention with folic acid: Results of the Goes extension study. Heart. 2005;91:1213–1214. doi: 10.1136/hrt.2004.035030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Toole JF, Malinow MR, Chambless LE, Spence JD, Pettigrew LC, Howard VJ, Sides EG, Wang CH, Stampfer M. Lowering homocysteine in patients with ischemic stroke to prevent recurrent stroke, myocardial infarction, and death: The vitamin intervention for stroke prevention (VISP) randomized controlled trial. JAMA. 2004;291:565–575. doi: 10.1001/jama.291.5.565. [DOI] [PubMed] [Google Scholar]
- 38.Wrone EM, Hornberger JM, Zehnder JL, McCann LM, Coplon NS, Fortmann SP. Randomized trial of folic acid for prevention of cardiovascular events in end-stage renal disease. J Am Soc Nephrol. 2004;15:420–426. doi: 10.1097/01.asn.0000110181.64655.6c. [DOI] [PubMed] [Google Scholar]
- 39.Bonaa KH, Njolstad I, Ueland PM, Schirmer H, Tverdal A, Steigen T, Wang H. Homocysteine lowering and cardiovascular events after acute myocardial infarction. N Engl J Med. 2006;354:1578–1588. doi: 10.1056/NEJMoa055227. [DOI] [PubMed] [Google Scholar]
- 40.Lonn E, HOPE 2 investigators Homocysteine lowering with folic acid and B vitamins in vascular disease. N Engl J Med. 2006;354:1567–1577. doi: 10.1056/NEJMoa060900. [DOI] [PubMed] [Google Scholar]
- 41.Righetti M, Serbelloni P, Milani S, Ferrario G. Homocysteine-lowering vitamin B treatment decreases cardiovascular events in hemodialysis patients. Blood Purif. 2006;24:379–386. doi: 10.1159/000093680. [DOI] [PubMed] [Google Scholar]
- 42.Zoungas S, McGrath BP, Branley P, Kerr PG, Muske C, Walker R, McNeil JJ. Cardiovascular morbidity and mortality in the atherosclerosis and folic acid supplementation trial (ASFAST) in chronic renal failure: A multicenter, randomized, controlled trial. J Am Coll Cardiol. 2006;47:1108–1116. doi: 10.1016/j.jacc.2005.10.064. [DOI] [PubMed] [Google Scholar]
- 43.Collins R, Armitage J. Study of the effectiveness of additional reductions in cholesterol and homocysteine. [June 1, 2009];2008 doi: 10.1016/j.ahj.2007.06.034. http://directnews.americanheart.org/extras/pdfs/search_slides.pdf. [DOI] [PubMed]
- 44.Shirodaria C, Antoniades C, Lee J, Moat SJ. Global improvement of vascular function and redox state with low-dose folic acid: Implications for folate therapy in patients with coronary artery disease. Circulation. 2007;115:2262–2270. doi: 10.1161/CIRCULATIONAHA.106.679084. [DOI] [PubMed] [Google Scholar]
- 45.Yang Q, Botto LD, Erickson JD, Friedman JM. Improvement in stroke mortality in Canada and the United States, 1990 to 2002. Circulation. 2006;113:1335–1343. doi: 10.1161/CIRCULATIONAHA.105.570846. [DOI] [PubMed] [Google Scholar]
- 46.Mikael LG, Genest J, Jr, Rozen R. Elevated homocysteine reduces apolipoprotein A-I expression in hyperhomocysteinemic mice and in males with coronary artery disease. Circ Res. 2006;98:564–571. doi: 10.1161/01.RES.0000204825.66410.0b. [DOI] [PubMed] [Google Scholar]
- 47.Liao D, Tan H, Hui R, Wang H. Hyperhomocysteinemia decreases circulating high-density lipoprotein by inhibiting apolipoprotein A-I Protein synthesis and enhancing HDL cholesterol clearance. Circ Res. 2006;99:598–606. doi: 10.1161/01.RES.0000242559.42077.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qujeq D, Omran TS, Hosini L. Correlation between total homocysteine, low-density lipoprotein cholesterol and high-density lipoprotein cholesterol in the serum of patients with myocardial infarction. Clin Biochem. 2001;34:97–101. doi: 10.1016/s0009-9120(01)00187-4. [DOI] [PubMed] [Google Scholar]
- 49.Sanossian N, Saver JL, Navab M, Ovbiagele B. High-density lipoprotein cholesterol: An emerging target for stroke treatment. Stroke. 2007;38:1104–1109. doi: 10.1161/01.STR.0000258347.19449.0f. [DOI] [PubMed] [Google Scholar]
- 50.Nissen SE, Nicholls SJ, Sipahi I, Tuzcu EM, ASTEROID investigators Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: The ASTEROID trial. JAMA. 2006;295:1556–1565. doi: 10.1001/jama.295.13.jpc60002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement Figure: Funnel plot of 13 trials included in the current meta-analysis. SE: standard error, RR: relative risk


