Abstract
The responses of the brain to infection, ischemia and trauma share remarkable similarities. These and other conditions of the CNS coordinate an innate immune response marked by activation of microglia, the macrophage-like cells of the nervous system. An important contributor to microglial activation is toll-like receptor 4 (TLR4), a pathogen-associated molecular pattern receptor known to initiate an inflammatory cascade in response to various CNS stimuli. The present review traces new efforts to characterize and control the contribution of TLR4 to inflammatory etiologies of the nervous system.
Keywords: Toll-like receptor, MD-2, glia, sepsis, endogenous ligand, neuroinflammation
I. Introduction
A toll-encoding gene was originally discovered for its role in dorsal-ventral axis development in Drosophila embryos (Anderson et al. 1985a, Anderson et al. 1985b). From its sequence the toll gene product was asserted to be a transmembrane receptor with a cytoplasmic domain similar to the interleukin-1 receptor and a large ectodomain characterized by leucine rich repeat (LRR) sequences (Hashimoto et al. 1988). A human analogue of Drosophila toll was identified and its signaling pathway suggested a role in the evolutionarily conserved host defense mechanism (Miyake et al. 1995). Plants, insects and vertebrates all use homologous mechanisms relying on toll recognition to coordinate an immune response (Medzhitov et al. 1997). Based on the discovery of additional toll genes, the toll-like family has grown to include 11 toll-like receptors (TLRs) in humans and 13 in mice (Gangloff et al. 2003). In vertebrates, TLRs recognize patterns characteristic to bacteria, fungi and viruses, collectively referred to as pathogen-associated molecular patterns (PAMPs). TLR4, for instance, recognizes cell wall components of gram-negative bacteria; other TLRs bind pathogenic or damage-associated molecules (Gangloff et al. 2003). These biological patterns are structurally diverse but well-conserved among pathogens, providing a molecular recognition tool to detect foreign invasion. TLRs were traditionally seen as discriminators of ‘self’ and ‘non-self,’ but current data suggests TLRs recognize a wide array of ligands, both exogenous and endogenous molecules of varying origins. Even within the tightly controlled blood-brain barrier, a myriad of TLR ligands have been reported. As such, the mechanism by which TLRs discern their ligands is a puzzling question whose answer lies in the fragile balance between immune signaling and neurotransmission in the CNS.
II. TLR4 signaling
TLRs and other PAMP receptors recognize molecular patterns. TLR4 is well known for its response to lipopolysaccharide (LPS), an outer cell wall component of gram-negative bacteria (Shimazu et al. 1999). Both in vivo and in vitro, TLR4 expression dictates LPS responsiveness (Lehnardt et al. 2003, Hoshino et al. 1999, Poltorak et al. 1998). To confer a signal TLR4 also requires its extracellular binding partner MD-2, or myeloid differentiation factor 2, which associates before ligand-induced signaling takes place (Nagai et al. 2002, Shimazu et al. 1999). In addition to LPS and its variants, a number of surprising exogenous and endogenous molecules have gained attention for their TLR4-binding properties. Exactly how TLR4 recognizes its many ligands is a longstanding question that has recently made progress due to breakthrough structural analyses of the TLR4-MD-2-ligand complexes (Park et al. 2009, Kim et al. 2007, Ohto et al. 2007).
Upon ligand binding, the TLR4-MD-2 complex may recruit another TLR4-MD-2 pair to form its homodimeric state. TLR4 agonists such as LPS are known to induce receptor aggregation, leading to homodimerization of the TLR4-MD-2 complex (Kobayashi et al. 2006, Prohinar et al. 2007). Several TLR4 inhibitors have been reported to disrupt homodimerization in the presence of agonists (Wong et al. 2009, Youn et al. 2006). Agonists induce homodimerization of the TLR4-MD2 complex, sending an intracellular signal through TLR4’s toll/interleukin-1 receptor (TIR) domain (Rittirsch et al., Kim et al. 2007, Gangloff et al. 2003). Exactly how the TIR domain coordinates this response is unclear, however it is known that the heteromeric assembly of TLR4’s TIR domains constitutes the initial step of signal transduction within the cell (Ohnishi et al. 2009).
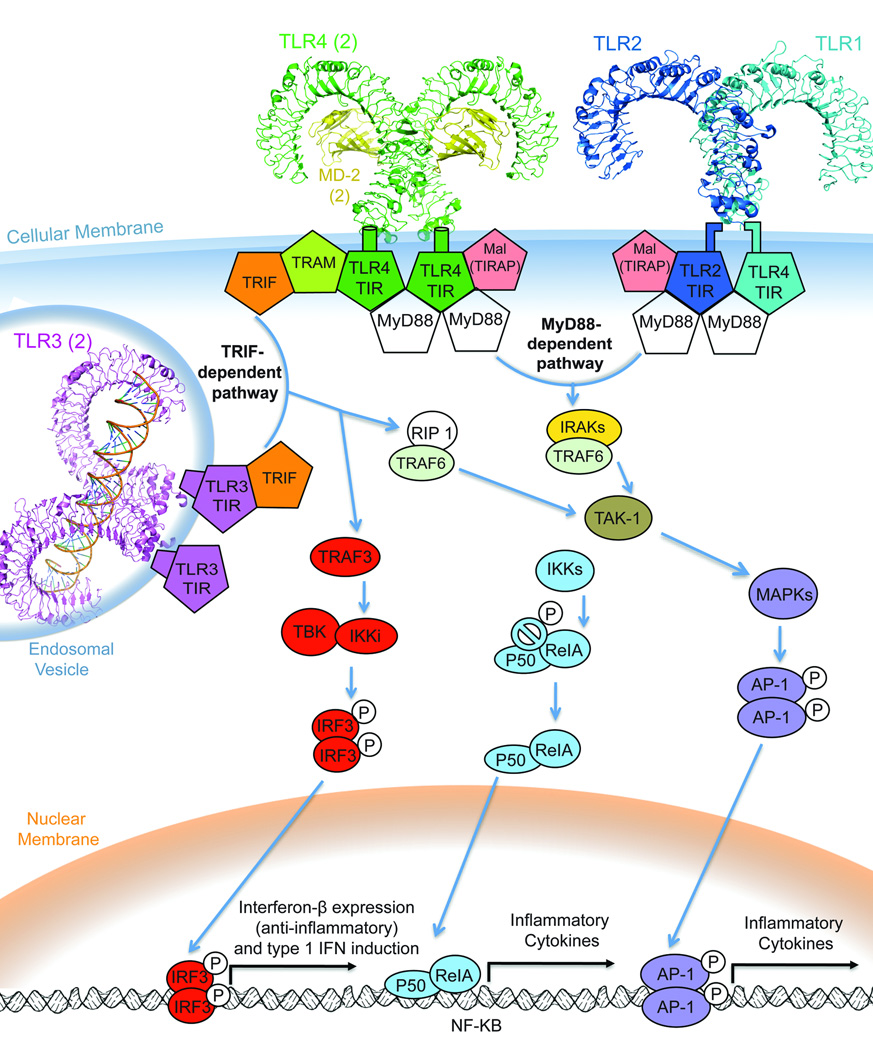
The activation signal diverges, following either of two inflammatory cascades, the MyD88 pathway to NF-κB activation or the TRIF pathway, for toll / IL-1 receptor-containing adaptor inducing IFN-β. All TLRs except TLR3 and 4 rely on the MyD88-dependant cascade (Akira et al. 2006). TLR3 signals solely through the TRIF adaptor. TLR4 is unique in that it can signal through the MyD88-dependent or TRIF-dependent cascades. It is unclear what criteria TLR4 uses to determine the downstream signaling adaptor and its subsequent pathway. How and when TLR4 signaling recruits MyD88 versus TRIF is the subject of much research. To coordinate the maximal inflammatory response, it has been suggested that TLR4 must signal through both pathways (Kawai & Akira 2007).
a. MyD88-dependant pathway
All TLRs except TLR3 and TLR4 use the MyD88 path exclusively (Akira et al. 2006). MyD88 is a cytosolic adaptor protein with a “death domain” and distal TIR domain similar to that of TLR4. The TLR4 pathway through MyD88 occurs via TIR-TIR association between TLR4 and MyD88, with the help of Mal, MyD88 adaptor-like protein (also known as TIRAP) (Horng et al. 2002). Mal is dispensable for TLR4 signaling, however its TIR domain is useful for recruiting MyD88 to the membrane for the crucial TIR-TIR association of MyD88 with TLR4 and MyD88 with TLR2 (Kagan & Medzhitov 2006). MyD88’s signal is conferred to the IRAK (interleukin-1 receptor-associated kinase) family of protein kinases through interaction of the MyD88 and IRAK4 death domains (Kawai & Akira 2007). This triggers a phosphorylation cascade activating NF-κB transcription factors. TAK1, a crucial ubiquitin-activated complex, sends the signal via a mitogen-activated protein kinase (MAPK) cascade and/or the complex involved in nuclear factor kappa-B (NF-κB) activation, the IKK (inhibitor of NF-κB) cascade. These paths induce NF-κB activation of the AP-1 (activator protein-1) and the RelA and P50 heterodimer, respectively. The AP-1 and RelA/P50 factors of NF-κB directly regulate pro-inflammatory cytokine transcription (Kawai & Akira 2007). NF-κB controls the expression of genes that regulate a broad range of biological processes in the central nervous system such as synaptic plasticity, neurogenesis, and differentiation (Sarnico et al. 2009). NF- κB is essential for neuron survival and its activation may protect neurons against oxidative stress or ischemic neurodegeneration (Sarnico et al. 2009, Lehnardt et al. 2003, Glezer et al. 2006). While NF-κB is associated with neuroprotective benefits, it can also contribute to inflammatory reactions and apoptotic cell death after brain injury and stroke (Caso et al. 2008, Caso et al. 2007, Sarnico et al. 2009). The MyD88-dependent signaling pathway is an important activator of NF-κB and the subsequent neuroregulatory effects of NF-κB signaling.
b. TRIF-dependant (MyD88-independent) pathway
A TRIF-dependant pathway is common to both TLR3 and TLR4. However, TLR4 signaling through TRIF requires the adaptor molecule TRAM while TLR3 does not (Rowe et al. 2006). Signaling through TRAM involves endocytosis of the TLR4 receptor complex (Tanimura et al. 2008). TRAM couples this endocytosis to the induction of IFN-β (Kagan et al. 2008). Studies suggest that TLR4 activates TRIF signaling from the endosome rather than the cell membrane (Tanimura et al. 2008, Kagan et al. 2008).
Downstream the TRIF adaptor molecule, TLR3 and MyD88-independent TLR4 signaling have identical pathways. The TRIF signal can recruit a TRAF3- or TRAF6-mediated adaptor molecule, diverging to different transcriptional effectors (Hacker et al. 2006). TRAF6 interacts with RIP to induce NF-κB activation through TAK1. TAK1 behaves the same as in the MyD88-dependent cascade, activating the NF-κBs ReIA/P50 and AP-1. The TRIF-dependant activation of NF-κB is aptly named “late phase” NF-κB activation, while the faster TLR4 route through MyD88 is the “early phase” NF-κB. TLR4 shares the “early phase” NF-κB pathway with all the TLRs except TLR3, which can only affect NF-κB through the RIP 1/TRAF6 “late phase” activation mechanism. The coordination of both “early” and “late” signaling is a capability unique to TLR4.
The TRIF-dependant signal through TRAF3 (as opposed to TRAF6) starts a TRIF-binding kinase (TBK1) inhibitor of NF-κB kinase (IKK) cascade terminating in IRF3 (interferon regulatory factor 3) dimerization and translocation into the nucleus (Poikonen et al.). IRF3 induces IFN-β synthesis, which regulates the cellular response to inflammation. IFN-β is both anti-inflammatory and anti-apoptotic, providing an endogenous mechanism to keep the innate immune system in check. Excretion of IFN-β coordinates the production of additional type-1 interferons, further suppressing the immune response.
TLR4 activation ultimately induces the secretion of proinflammatory substances such as reactive oxide species (nitrous oxide, hydrogen peroxide, and superoxides), cytokines such as tumor necrosis factor-α (TNFα) and interleukins (Tsan & Gao 2004a, Bowie & O'Neill 2000, Blanco et al. 2005, Maier & Watkins 2003, Kagan et al. 2008). In contrast, TLR4 also affects IFN-β release, which counteracts inflammation (Kagan et al. 2008). Proinflammatory factors coordinate immune defense, repair and debris removal, but these factors can amplify out of control without regulation by anti-inflammatory substances. Neurons and oligodendroglia are especially fragile under inflammatory conditions (Lehnardt et al. 2003). Neurological stress provokes NF-κB induced release of reactive oxygen species (ROS), which in turn cause neuronal vulnerability (Hua et al. 2007, Keller et al. 1999). A comprehensive review of microglia-mediated inflammation and chronic excitation highlights TLR4’s contribution to neurotoxicity (Block et al. 2007).
The pro-inflammatory response is generally amplified by TLR signaling. Pro-inflammatory cytokines coordinate other immune cells, attracting them to the site of invasion or damage, amplifying it until the insult is eliminated or dampened by immune-suppressing feedback mechanisms. TRIF-mediated IFN-β release can counteract inflammation, whereas the MyD88-dependant path is pro-inflammatory. But when and why the IFN-β anti-inflammatory pathway is induced is not well understood. The criteria for TRIF versus MyD88 signaling are unknown, but common TLR4 ligands appear to utilize the same pathway or both pathways consistently.
III. TLR4 in the CNS
a. TLR4 expression and activation
The CNS was once thought to be an immune-privileged site, but researchers now recognize the role of immunity in the CNS. Microglia are the resident immune cells of the CNS, comprising about 12% of the cells in the brain and spinal cord (Lawson et al. 1990). It makes sense that innate immune receptors such as TLR4 would be expressed on the immune cells of the nervous system, the microglia. TLR4 is primarily expressed on glia, primarily microglia (Lehnardt et al. 2003); however TLR4 expression has been reported on other CNS cells including astrocytes, endothelial cells and neurons ((Jou et al. 2006)Tang et al. 2007). It has been disputed whether TLR4 is expressed on neurons under normal conditions (Lehnardt et al. 2003), but current studies leave little question as to whether TLR4 can be expressed on neurons in pathological environments (Tang et al. 2007, Tang et al. 2008).
The TLRs can regulate cellular development, in addition to their well-known immunological tasks. TLR4’s evolutionary precursor Toll was originally discovered for its role directing Drosophila development (Anderson et al. 1985b). In the vertebrate nervous system, microglial cells regulate neuronal development, differentiation and survival using immune mechanisms to elicit apoptosis or proliferation. Microglia enforce the programmed elimination of neurons throughout development (Marin-Teva et al. 2004, Wakselman et al. 2008) and they are necessary to elicit differentiation and migration of neural precursor cells (Aarum et al. 2003).
Microglia can also damage neighboring cells, through chronic overstimulation or prolonged inflammatory responses. Microglial inflammation can be erroneous, amplified and progressive (Block et al. 2007). Overstimulated microglia cause oxidative stress and damage to other CNS cells, most notably neurons (Block et al. 2007). Because microglial activation is widely controlled by pathogen-recognition receptors (PRRs), TLR4 is implicated in the microglia-mediated neurotoxicity that occurs in many brain pathologies. Although some reports suggest neuronal TLR4 is directly responsible for neuron death (Tang et al. 2008, Tang et al. 2007), the majority of studies investigate microglial expression of TLR4 and the subsequent neurotoxic effects of TLR4 signaling. Whether or not the TLR4 is activated on neurons or on microglia, it is widely accepted that the excreted products of TLR4 signaling alter neuronal functions. It is clear that TLR4’s detection system remains intact within the blood-brain barrier (Zhou et al. 2006); why this system can be both helpful and harmful is the subject of much research.
b. Sepsis: the classical TLR4-mediated syndrome
TLR4 is well known for its detrimental role in sepsis and endotoxemia, where LPS-induced TLR4 activation contributes to the systemic inflammation that characterizes these serious conditions. TLR4-mutant mice are resistant to LPS-induced inflammation and associated sepsis syndromes (Hoshino et al. 1999). However without an intact host defense system, LPS-hyporesponsive mice will die from gram-negative bacterial invasion (Poltorak et al. 1998). The innate immune response to LPS is similarly important for host defense by humans (Arbour et al. 2000, Hoshino et al. 1999). Inflammatory amplification in the case of sepsis shows the power of innate immunity to coordinate a disproportionate reaction to its trigger, initiating an inflammatory response so strong that viable cells are damaged. Proinflammatory cytokine responses signal the brain through neuronal and blood-borne routes, altering neural activity and proliferating the systemic immune response (Maier & Watkins 2003). If the TLR4 pathway is erroneously activated, or if a signal is amplified out of control, the cytokine response may have deleterious effects on the nervous system. TLR4-induced inflammatory signaling has the ability to instruct both necrosis and apoptosis in various CNS cell types. But TLR4 signaling can be beneficial, too. TLR4 has shown critical neuroprotective benefits in studies of stroke (Marsh et al. 2009), and amyloid-β clearance is diminished in TLR4-deficient mouse models of Alzheimer’s disease (Tahara et al. 2006). Altogether, innate immunity and the responses coordinated by PRRs are extremely powerful modulators of the CNS environment.
Sepsis and its associated inflammatory syndromes influence the nervous system through TLR4. In mouse models of bacterial sepsis, LPS administered peripherally induces a chronic proinflammatory response within the CNS. This inflammation requires the expression of TLR4 in the CNS and is independent of systemic cytokine levels (Chakravarty & Herkenham 2005). Further, LPS-induced mouse models of sepsis experienced progressive neurodegenerative effects analogous to Alzheimer’s or Parkinson disease. Mice lacking functional TLR4 expression in CNS were exempt from long-term progressive neuron loss. This example illustrates the paradoxical nature of TLR4 signaling: it is necessary for defense, yet it invokes a powerful cascade that can be toxic. The stakes are high in the CNS, where subtle modifications can tip TLR4 signaling over the neurotoxic edge.
c. Pathological implications of TLR4 signaling
Lehnardt et. al were the first to definitively illustrate the neurotoxic effects of TLR4 signaling. In mixed CNS cultures, LPS-induced neurodegeneration is microglia-dependant, manifesting in neuronal axon and dendrite loss. Similar cultures obtained from TLR4-deficient mice were resistant to neuronal insult from LPS, establishing TLR4 as a requirement for LPS-induced toxicity (Lehnardt et al. 2003). Oligodendroglia also exhibit damage upon LPS administration and subsequent TLR4 activation, but neurotoxicity prevails as the primary detriment to TLR4 activation. TLR4-induced neuron death occurs independent of organism species and on all neuronal subtypes (Lehnardt et al. 2003). Recent reports of TLR4 activation by endogenous ligands link TLR4 to autoimmunity as well as legitimate inflammation (Midwood et al. 2009). As the interactions of the immune and nervous systems gain attention, more and more ligands are reported to bind PRRs. The myriad of structurally diverse TLR4 ligands exemplifies the puzzling diversity and ambiguity of “pathogen-associated molecular patterns”. Based on the binding activity exhibited by TLR4, it is reasonable to implicate TLR4 signaling in several etiologies of the nervous system.
Evolution has produced pattern-like danger signals and highly conserved TLRs to recognize such pattern-associated pathogens and damage signals. The response coordinated by TLR4 is necessary to protect the CNS from foreign invasion. Microglial TLR4 activation also contributes to repair processes, improving remyelination and conferring cerebral tissue protection in the presence of neurotoxic compounds (Glezer et al. 2006). But at what point do the risks of aberrant TLR4 signaling outweigh the benefits conferred from damage repair and pathogen protection? We will investigate this question with respect to relevant diseases of the nervous system.
Because TLRs recognizes pathogenic and damage-associated molecules, TLR4 is intrinsically implied in pathologies of the nervous system. The neuroinflammatory origins of dementia were given attention as early as 1889. The 1927 Nobel Prize in Physiology or Medicine was awarded to Julius Wagner-Jauregg for his neuroinflammatory approach to dementia paralytica, whereby he discovered that infection with the malaria parasite mitigated the psychiatric and paralytic impairment associated with long-term syphilis infection (1965). But the work of Wagner-Jauregg diminished in the following years, most likely because a neurology-immunology link was intangible in the eyes of early 20th century physicians.
We now know that immunity remains intact within the nervous system, and immune cells are well represented by microglia in the brain. The peripheral lymphatic system itself is innervated and immune-to-brain communication is now well documented (Watkins & Maier 1999). This is underlined by the finding that neurotransmitters, cytokines and their respective receptors are both endogenous to the brain and immune system. The above discoveries substantiate Wagner-Jauregg’s prodigal connection between the brain and immunity. Nevertheless, only in the past 20 years have the interactions between the immune and nervous systems become the subject of intense research. Investigations into microglial signaling and TLR biology have rapidly expanded and eventually the fields have intersected, bringing TLRs into focus for important CNS diseases. Capable of both protective and pathological roles, TLR4 can be helpful or harmful under varying neurological conditions. When and why TLR4 initiates beneficial outcome is still largely unknown, but progress in the field suggests that this question will receive much attention, as TLR4 is a useful and druggable target.
i. Neurodegenerative Conditions and TLR4: Alzheimer’s Disease
Alzheimer’s is a progressive neurodegenerative disease marked by neuron loss, aggregation of amyloid beta peptide (Aβ) into plaques, and microglial activation and recruitment. Research suggests neuroinflammation is a major contributor to Alzheimer’s pathology, as Aβ plaques are closely associated with brain inflammation (Akiyama et al. 2000). Accordingly, microglia and astrocytes concentrate in and around Aβ plaques. An increase in complement components, pro-inflammatory factors and proteases suggests that the innate immune response is a crucial contributor to plaque-induced neurotoxicity (Akiyama et al. 2000). As such, TLR4 has been suggested as a mediator of Alzheimer disease (AD) and other neurodegenerative conditions (Keller et al. 1997, Tang et al. 2008, Hua et al. 2007, Zhao et al., Marta et al. 2009).
Aβ plaque deposits are the pathological hallmark of Alzheimer’s disease. Despite decades of research, the pathways through which neuritic plaques elicit neurodegeneration are poorly understood. An inflammatory mechanism appears to be responsible for local microglial activation and the subsequent pro-inflammatory sensitization and degradation of neurons in AD (Akiyama et al. 2000). The inflammatory nature of Alzheimer-related neurotoxicity is reinforced by data showing reduced risk of AD in patients taking acetaminophen, the anti-inflammatory agent (Stewart et al. 1997).
Paradoxically, TLR4 expression is also associated with increased uptake of Aβ peptide (Tahara et al. 2006). Under normal conditions, Aβ is removed before it accumulates as extracellular amyloid fibrils, suggesting that Aβ uptake by TLR4 is a beneficial mechanism (Akiyama et al. 2000). Inflammatory markers such as heat shock proteins are also associated with increased uptake and clearance of Aβ (Kakimura et al. 2002) and several heat shock proteins have been reported to activate TLR4 signaling (Hutchinson et al. 2009a, Lehnardt et al. 2008, Triantafilou & Triantafilou 2004). These inflammatory mechanisms may be necessary for normal Aβ clearance (Tahara et al. 2006), but heat shock proteins may also induce neurodegeneration through TLR4 signaling (Kakimura et al. 2002). It is unclear whether TLR4 favors Aβ uptake over neurotoxic inflammation, or if Aβ clearance and inflammatory reactions take place simultaneously or interdependently of TLR4 signaling. This important question stands to be answered, but the quantity of data favors TLR4’s harmful effects over its benefits. Many studies investigate microglial inflammation and TLR4 signaling in neurodegenerative pathologies such as AD.
Several genetic mutations are known to induce amyloid deposition and subsequent AD symptoms, but the majority of AD cases are sporadic and genetically heterogeneous (Akiyama et al. 2000). Minoretti and coworkers investigated the contribution of TLR4 mutations to AD pathology, screening 277 Italian late-onset AD patients and 300 healthy patients for TLR4 polymorphisms. A TLR4 decreased function polymorphism was found to protect against AD (Minoretti et al. 2006). This common TLR4 polymorphism (Asp299Gly) was disproportionately represented in the control cohort, suggesting the mutation protects against late onset AD (Minoretti et al. 2006). The Asp299Gly mutation stunts TLR4 signaling and the associated inflammatory responses. Taken together, this study provides strong evidence that TLR4 signaling negatively contributes to late-stage AD onset (Minoretti et al. 2006). Further studies are needed to determine whether TLR4 signaling is consistently detrimental to AD onset. The benefits related to TLR4 Aβ uptake (Tahara et al. 2006) must be weighed against the neurotoxic effects of TLR4 signaling (Minoretti et al. 2006, Walter et al. 2007).
Another genetic study suggests aberrant TLR signaling contributes to AD neuroinflammation. Tan and coworkers constructed a Drosophila model of AD to express Aβ-42 in the fruit fly CNS. Toll gene activity through NF-κB signaling was identified as the key mediator of neurotoxic inflammation (Tan et al. 2008). The Toll gene is an evolutionary precursor to the toll-like receptors (e.g. TLR4, TLR6) found in vertebrates. In the fruit fly model of Aβ-42 mediated Alzheimer’s, Toll activity was correlated with shortened lifespan (Tan et al. 2008). Inhibition of Toll signaling lengthened lifespan in the same model. While Drosophila Toll bears similarity to TLR4 and other vertebrate TLRs, it ought to be noted that the Drosophila model simplifies a complex human disease. Nevertheless, the innate immune response is conserved through evolution and furthermore, induction of NF-κB is common to both TLR4 and Drosophila Toll. This study adds to a body of evidence suggesting toll-like receptors contribute to Alzheimer’s neuroinflammation and subsequent neurodegeneration.
TLR4 expression has been reported on both neurons and glia under normal conditions (Tang et al. 2008, Tang et al. 2007), although TLR4 is probably expressed more appreciably on microglia than other CNS cell types (Lehnardt et al. 2003). Tang and colleagues specifically investigated neuronal TLR4 expression and its effects in CNS pathologies (Tang et al. 2007, Tang et al. 2008). Murine neurons were found to increase TLR4 expression when exposed to Aβ peptide or the membrane peroxidation product, 4-hydroxynonenal (Tang et al. 2008). Aβ and 4-hydroxynonenal triggered neuronal apoptosis in wild-type murine cells, but neurons from TLR4-mutant mice displayed resistance to death under the same circumstance (Tang et al. 2008). Neuronal apoptosis was attributed to the TLR4-induced JNK signaling pathway, as a JNK inhibitor also protected against neurotoxicity in the presence of Aβ or 4-hydroxynonenal. Finally, levels of TLR4 were slightly decreased in tissue specimens from end-stage AD patients compared to aged-matched control subjects (Tang et al. 2008). The authors suggest that this finding results from the explicit loss of TLR4-expressing neurons due to TLR4-mediated neurotoxicity. These studies demonstrate that neuronal TLR4 expression may predispose neurons to apoptosis in the presence of Aβ and/or 4-hydroxynonenal.
Walter and colleagues further elucidated the role of TLR4 in Alzheimer-related neuroinflammation, focusing on the glial-mediated effects upon neurons. Their results indicate that aggregated Aβ induces inflammation through TLR4 activation in both microglia and macrophages. Microglia from wild-type mice had significantly increased levels of IL-6, TNFα and nitric oxide when compared with TLR4 loss-of-function mutants (Walter et al. 2007). In human embryonic kidney cells (HEK293), it was shown that TLR4’s accessory protein, MD-2, and the coreceptor CD-14 were also required to coordinate a response to aggregated Aβ. Furthermore, Aβ peptide was only recognized in its aggregated conformation; neither scrambled peptide nor non-aggregated peptide elicited an IL-8 response from the HEK293 cells. Finally, neurotoxicity was assessed using the supernatants of microglia exposed to aggregated Aβ peptide. The supernatants were added to primary murine neuronal cells and neurotoxicity assayed. Only 20% of neurons treated with wild-type Aβ-exposed microglial supernatant survived. Neurons incubated with supernatant from TLR4 mutants were much more likely to survive (70% viable) (Walter et al. 2007). TLR4 was also assessed in experimental AD, where APP-overexpressing mice possessed significantly elevated TLR4 mRNA when compared to their age-matched non-transgenic littermates. Finally, TLR4 expression was markedly increased in post-mortem brains of AD patients (Walter et al. 2007). This final finding stands in contrast to the Tang study, where TLR4 was slightly underexpressed in postmortem AD brains (Tang et al. 2008). The discrepancy could result from experimental differences in methodology, or simply from the small cohort size in both studies. Nevertheless, the data gathered by both Tang and Walters, and concurrent data (Tan et al. 2008, Minoretti et al. 2006) strongly suggest a function for TLR4 in Aβ-induced neurotoxicity.
In looking to the future we must be careful to remember the role TLR4 may play in clearance and uptake of Aβ (Tahara et al. 2006, Kakimura et al. 2002). Although an increasing volume of data favors TLR4-mediated neurotoxicity, TLR4 may also be essential to the uptake and phagocytic removal of Aβ plaques. In addition, it is unclear whether TLR4-mediated neurotoxic effects result from the neurons themselves (Tang et al. 2008), or from microglial signals (Walter et al. 2007). Until researchers can reconcile if and when TLR4 drives neurotoxicity over Aβ clearance, TLR4-targetting drugs will have limited clinical utility in Alzheimer’s treatment.
ii. Ischemic Stroke
It is increasingly clear that post-stroke neuroinflammation from TLR4 signaling worsens stroke outcome, as measured by infarct volumes, neurological function and inflammatory markers (Caso et al. 2007, Abate et al. 2009, Tasaki et al. 1997). Several models of cerebral ischemia have elucidated the role of TLR4 signaling in neuroinflammation and exacerbated stroke injury. Mice deficient in TLR4 have shown improved neurological and/or behavioral outcomes in various models of cerebral infarction (Hua et al. 2007, Cao et al. 2007).
TLR4 is well-known to confer immunological tolerance, giving a dampened response upon a second insult by immunogenic stimuli (usually LPS). Similar studies have demonstrated that preconditioning with LPS, the classical TLR4 ligand, protects against the cytotoxic damage elicited from ischemic stroke (Tasaki et al. 1997, Rosenzweig et al. 2004, Hickey et al. 2007). Initial LPS exposure signals through TLR4 to affect a cytotoxic TNFα response, but subsequent LPS-induced TLR4 activation is often dominated by IFN-β production (Hickey et al. 2007, Marsh et al. 2009). IFN-β is known to be anti-inflammatory and anti-apoptotic, and systemic administration of IFN-β reduced infarct volume in mouse and rabbit models of cerebral ischemia (Liu et al. 2002a, Veldhuis et al. 2003). The change from pro-inflammatory TNFα to the anti-inflammatory IFN-β production suggests TLR4 may switch predominant signaling pathways from MyD88 to TRIF/TRAM, responding more mildly to a second LPS exposure.
Marsh et al. demonstrated the change of pathways that LPS preconditioning induces from downstream TLR4 effectors. They measured IFN-β neuroprotection and its associated genetic expression. Based on evidence that IFN-β reduces ischemic brain damage (Liu et al. 2002a, Veldhuis et al. 2003), Marsh tested the underlying signaling mechanism by which IFN-β confers its neuroprotective benefits. RNA analysis of post-stroke genetic expression reveals upregulation of IFN-β and its transcriptional regulators in animals preconditioned with LPS (Marsh et al. 2009). Ischemic damage in LPS-pretreated animals is minimal compared to those without prior insult by LPS. This concurs with the finding that TNFα signaling is favored upon LPS pretreatment, but pretreated animals responded to infarction with IFN-β secretion which lends neuroprotective benefits. Control animals signal through the proinflammatory TNFα pathway, leading to worsened stroke outcome in comparison to animals conditioned with LPS prior to infarction. IFN-β does not play a role in the endogenous response of the brain to ischemia (Marsh et al. 2009). Microarray analysis revealed a novel genomic response to stroke in animals preconditioned with LPS. Pretreatment with LPS must change the cellular environment in such a way that subsequent TLR4 activation induces IFN-β neuroprotection, a beneficial change in response. LPS preconditioning appears to reprogram TLR4 signaling from MyD88-mediated proinflammatory factors to the TRIF/TRAM to pathway leading to IFN-β and subsequent type 1interferon secretion. TLR4-mediated IFN-β expression, if favored over TNFα proinflammatory signaling, has powerful neuroprotective benefits that could be exploited to minimize post-stroke neurodegeneration.
Heat-shock proteins (HSPs) released from damaged cells are another trigger of TLR4-induced neuroinflammation (Triantafilou & Triantafilou 2004, Lehnardt et al. 2008). Necrotic neurons death produces an immune response characterized by activation of the MyD88-mediated pathway (Pais et al. 2008). Similar to the response from primary LPS-activation of TLR4, HSP60 causes the proinflammatory activation of microglial TLR4, leading to nitrous oxide production and subsequent neurodegeneration in vitro and in vivo (Lehnardt et al. 2008). A vicious cycle ensues as neurons produce HSP60 in response to stress, in turn activating more and more TLR4 and its neurotoxic effectors.
The possibility of TLR4 changing from MyD88 to the TRIF-mediated pathway was not demonstrated in the above HSP60 study. However, uncontrolled proliferation of a toxic signal has no evolutionary support on which to stand. Lehnardt asserts that the ancient defense system of TLR recognition has evolved to recognize endogenous damage signals in addition to exogenous dangers. This conclusion is supported by the variety of endogenous ligands reported to bind TLR4 (Table 1). The role of TLR recognition is also complicated by its developmental functions in lower phylogenetic species, as is in the Toll gene product which determines neuronal differentiation patterns in Drosophila embryos (Anderson et al. 1985b). Clearly, more research is needed to determine when and why TLR4 affects infarctive stroke and other neuroinflammatory states.
Table 1.
A variety of ligands are suggested to affect TLR4. Endogenous ligands are denoted with a star (*). It is important to note that endotoxin is a very common and potent contaminant in such studies. Especially where recombinant proteins are reported to activate TLR4, contamination is difficult to exclude.
| Putative TLR4 Interactor | Explanation | Report |
|---|---|---|
| lipopolysaccharide (LPS) and LPS derivatives (see Figure 2 for molecular structure) |
Outer cell wall component of gram- negative bacteria; potent initiator of TLR4 signaling. LPS structure varies with bacterial species. |
Structure-activity relationship of LPS and TLR4 (Park et al.2009), of LPS: (Rietschel et al. 1994). |
| curcumin | Polyphenol found in the plant Curcuma longa. Inhibits TLR4 by binding MD-2. |
(Youn et al. 2006) (Gradisar et al. 2007) |
| cinnamaldehyde (3-phenyl-2-propenal) |
Anti-inflammatory, inhibits ligand- induced TLR4 oligomerization and downstream signaling. |
(Youn et al. 2008) |
| ethanol | Appears to redistribute TLR4 complexes on the cellular membrane by preventing receptor association and/or dimerization in the lipid raft. |
(Szabo et al. 2007, Blanco et al. 2008, Blanco et al. 2005, Fernandez-Lizarbe et al. 2008) |
| E5564 (eritoran) |
LPS analogue clinically tested for sepsis; inhibits TLR4 signaling. |
(Yamada et al. 2005, Kim et al. 2007, Rossignol et al. 2004) |
| Opioids | Both opioid stereoisomers alter downstream TLR4 signaling. Opioid agonists (e.g. morphine) have different effects than antagonists (e.g. naloxone). |
(Hutchinson et al 2007, Hutchinson et al 2009b, Juni et al. 2007, Liu et al. 2000) |
| TAK-242 (Ethyl (6R)-6-[N-(2-chloro- 4-fluorophenyl) sulfamoyl] cyclohex-1-ene-1- carboxylate) |
Clinically tested cyclohexene derivative, selectively inhibits intracellular signaling by TLR4. |
(Ii et al. 2006, Sha et al. 2007, Takashima et al. 2009) |
| Paclitaxel (Taxol) |
Widely used cancer therapeutic, reported to inhibit MD-2, thereby knocking down TLR4 activity which correlated with drug efficacy. |
(Wang et al. 2009) |
| resveratrol (trans-3,5,4- trihydroxystilbene) |
Antioxidant reported to inhibit TLR4 signaling; found in the skin of grapes, it is known for anti-inflammatory and anti-carcinogenic effects. |
(Youn et al. 2005, Yusuf et al. 2009) |
| Statins | Statin drugs influence TLR4-mediated cytokine expression through a Rho- protein feedback mechanism. |
(Konat et al. 2008) |
| amyloid-β 42 peptide* | The peptide hallmark of Alzheimer’s pathogenesis, appears to activate TLR4 directly and also through signals from damaged neurons (e.g. 4- hydroxynonenal). |
(Liu et al. 2002b, Tang et al. 2008, Balistreri et al. 2007, Balistreri et al. 2009) |
| extracellular matrix proteins*
|
Negatively charged glycoproteins are reported to activate TLR4 signaling |
(Schaefer et al. 2005) (Smiley et al. 2001) (Okamura et al. 2001) (Midwood et al. 2009) |
| fatty acids* | Fatty acids are reported to regulate TLR4 receptor dimerization and recruitment into lipid rafts. |
(Weatherill et al. 2005, Wong et al. 2009) |
| heat shock proteins* (HSP) 60, 70, 90 |
Released from dead or dying cells. HSP 60 mediates neurodegeneration via TLR4 (Lehnardt et al. 2009). HSP 90 may influence TLR4 pain amplification (Hutchinson et al 2009b). LPS contamination is a common problem in HSP studies. |
HSP 60 (Lehnardt et al 2008); HSPs 70, 90 (Triantafilou & Triantafilou 2004, Hutchinson et al 2009b). Contamination, (Tsan & Gao 2004b) |
| polysaccharides* | Heparin sulfate and endogenous hyaluronic acid fragmentation products may activate dendritic cells and macrophages through TLR4. |
(Termeer et al. 2002) |
iii. Chronic Pain Pathologies
Researchers have found that pathological pain invokes an inflammatory response within the CNS. In cases where pathological pain persists beyond the resolution of tissue damage, it constitutes neuropathic pain. Pain management, especially pathological or neuropathic pain, is a significant public health issue. The National Institutes of Health reported that pain costs the USA more than $100 billion per year in medical expenses, lost wages and lost productivity (Department of Health and Human Services 1998). Pain research traditionally focused on neuronal mechanisms, but this work has yielded marginally effective therapeutics. New pain research shifts focus to investigate glial mechanisms. For a complete review on the roles of glia in chronic pain, see (Milligan & Watkins, 2009).
Microglia, the immunocompetent cells of the CNS, are important contributors to chronic pain pathologies (Milligan & Watkins 2009). The TLR4 receptor is one avenue through which microglia can be activated and primed for the pain response. TLR4 influences the CNS pain response, invoking the production of pro-inflammatory cytokines and reactive oxygen species (Tanga et al. 2005, Hutchinson et al. 2007, DeLeo et al. 2000). Recent studies link TLR4 to pain etiology in animal models and corresponding in vitro and in silico systems.
DeLeo and coworkers first explored this link based on the involvement of tumor necrosis factor (TNF) in painful neuropathy and other CNS disorders (DeLeo et al. 2000). Because TNF and other inflammatory-mediating cytokines are regulated by TLR4, the group hypothesized that microglial TLR4 influences hypersensitivity in models of neuropathy (Tanga et al. 2005). Using a standard L5 nerve transection procedure to induce chronic pain, they tested hypersensitivity in genetically altered (TLR4 knockout and point-mutant) mice and TLR4 antisense oligodeoxynucleotide treated rats. Both the mice and rats displayed attenuated behavioral hypersensitivity and decreased expression of proinflammatory cytokines relative to their respective controls. This work established a role for TLR4 in mice and rat models of neuropathic pain (Milligan & Watkins 2009).
A similar role for innate immunity in pain amplification is suggested from a recent clinical trial that found naltrexone useful in the treatment of fibromyalgia (Younger and Mackey 2009). Fibromyalgia is a common condition characterized primarily by diffuse chronic pain. Concurrent symptoms such as fatigue, sleep disturbance and cognitive impairment have led to the characterization of fibromyalgia as a CNS sensitivity disorder, but the molecular etiology of the condition is unknown. Naltrexone is a competitive antagonist of opioid receptors, and has been used for the clinical treatment of opioid dependence and reversal of opioid overdose. More recently, naltrexone has been used to suppress microglial signaling, thereby decreasing the production of proinflammatory cytokines and reactive oxygen species (Liu et al. 2000, Liu et al. 2002b). This mechanism is likely the source for decreased fibromyalgia symptoms in response to low doses of naltrexone, as measured by improved pain threshold testing and self-reported symptoms in a single-blind study (Younger & Mackey 2009).
Naltrexone must elicit its microglial effects through a mechanism distinct from the classical opioid receptors, as there is no evidence for abnormal endogenous opioid activity in fibromyalgia patients (Younger et al. 2009). In a follow-up to the above clinical trial, Younger and coworkers assessed fibromyalgia patients for opioid withdrawal symptoms upon opioid antagonism with 50 mg naltrexone. No withdrawal symptoms were reported, indicating that endogenous opioid activity is not dysregulated in fibromyalgia pathophysiology (Younger et al. 2009). Fibromyalgia must elicit hypersensitivity through a pathway distinct from the mu-opioid system, but that responds to low doses of naltrexone.
The presence of atypical opioid recognizing receptors is evidenced by opioid receptor knockout studies revealing hypersensitivity upon morphine administration (Juni et al. 2007). Evidence has accrued suggesting that opioids elicit their secondary effects through an atypical pathway characterized by inflammatory signaling due to glial activation (Hutchinson et al. 2007). LPS induces glial activation, and this activation can be ameliorated by naloxone administration (Wu et al. 2006). Based on the ability of naloxone to interfere with LPS signaling, the TLR4 pathway is implicated as a mediator of non-classical opioid responses (Hutchinson et al. 2009b, Liu et al. 2000).
We explored the involvement of TLR4 in opioid-induced microglial signaling due to opioid antagonists (i.e. naltrexone and naloxone) and opioid agonists (morphine, oxycodone and methadone etc.). In vitro TLR4 signaling was observed in response to clinically-relevant opioid agonists (Hutchinson et al. 2009b). Consistent with the ability of naltrexone to reduce chronic pain symptoms, opioid antagonists were shown to inhibit TLR4 signaling and the production of pro-inflammatory substances. TLR4 inhibition was associated with concomitant potentiation of morphine analgesia and attenuated the development of morphine tolerance, hyperalgesia and opioid withdrawal behaviors (Hutchinson et al. 2009b). Our data suggests opioid agonists and antagonists can affect downstream TLR4 signaling by respectively activating or inhibiting TLR4-mediated proinflammatory release.
The specificity of the TLR4-opioid interaction was addressed using TLR4 knockout mice to observe opioid response (Hutchinson et al. 2009b). Based on the negative side effects associated with TLR4 activation, it was hypothesized that TLR4 knockout mice would respond to lower doses of opioid agonist and display decreased development of tolerance, dependence and hyperalgesic behavior. These phenomena are observed in TLR4 knockout mice, as measured by analgesic response upon repeated morphine administration (Hutchinson et al. 2009b). TLR4 knockouts react to morphine with three-fold higher analgesia, and acute inhibition of TLR4 signaling can elicit the same response to a lesser degree (Slivka et al. 2009, Hutchinson et al. 2009b). Together this work strongly supports the role of TLR4 in opioid-induced glial dysregulation, leading to pain amplification and the development of tolerance.
Intriguingly, while classical opioid receptors respond only to the (−)-opioid stereoisomer, several studies report that glial cells respond to both opioid stereoisomers (e.g. (+)-morphine and (−)-morphine) (Wu et al. 2006, Hutchinson et al. 2008). The ability of opioid antagonists to inhibit TLR4 can therefore be exploited by administration of the unnatural (+)-opioid antagonist, as the (+)-opioid stereoisomers are inactive at classical opioid receptors (Hutchinson et al. 2009b, Wu et al. 2006). This strategy was successfully implemented to increase morphine potency by administration of (+)-naloxone, the naltrexone relative that was successfully used to treat fibromyalgia in preliminary studies (Younger & Mackey 2009, Hutchinson et al. 2008). The effectiveness of naltrexone in the treatment of chronic pain suggests a similar TLR4-mediated mechanism is at work in fibromyalgic pain amplification.
The ability of LPS to induce severe pain, and the ability of LPS inhibitors to relieve these painful effects, further links TLR4 to pain amplification (Maier et al. 1993, Mason 1993). But opioid-mediated hyperalgesia and tolerance may not function in the same way as LPS induction of hyperalgesia. Binding of opioids to TLR4 has not been directly observed, and so opioid-induced hyperalgesia and tolerance does not necessarily occur in the same manner as LPS, despite their phenotypic similarities. While TLR4 is necessary to induce neuropathic pain, TLR4 alone cannot coordinate a pain response to low-doses of LPS (Hutchinson et al. 2009a). These results are corroborated by an earlier finding that CD40 also perpetuates neuropathic pain in rat models of nerve injury (Cao et al. 2009, Hutchinson et al. 2009a). Interestingly, CD40 signaling was associated with production of spinal cord proinflammatory cytokines IL-1β, IL-6, IL-12 and TNFα, but not IL-6, which is upregulated upon induction of neuropathic pain in both CD40 knockouts and wild type mice. IL-6, TNFα and IL-1β are inducible by TLR4, suggesting a concomitant activation of CD40 and TLR4 may dually account for neuropathic inflammation.
Recent evidence also suggests a secondary mediator is at work in TLR4-mediated pain enhancement, in addition to the factors involved in the TLR4 complex and signaling pathway. Heat-shock protein 90 (HSP90) was found to be critical for LPS-induced pain and sensitivity (Hutchinson et al. 2009a). This work indicates a mediator may be responsible for the antagonistic proinflammatory effects of opioids which occur, at least in part, through TLR4 (Hutchinson et al. 2009b). Clearly, more work is needed to determine the role of TLR4 in opioid-induced glial activation. Nevertheless, these studies define a relationship between activation of the TLR4 pathway and chronic pain pathologies. Interestingly, our later discussion of TLR4 ligands reveals many anti-inflammatory agents act to inhibit TLR4.
iv. Cancer
Resveratrol, a chemopreventive agent, has shown affinity for TLR4 and its downstream adaptor molecules of the MyD88-independent pathway (Youn et al. 2005, Yusuf et al. 2009). The widely used chemotherapeutic Paclitaxel is also reported to elicit TLR4 effects through MD-2 (Wang et al. 2009, Zimmer et al. 2008). These TLR4 compound interactions have implications for understanding aspects of cancer treatment and pain. However, such examples of beneficial TLR4 regulation must be tempered with the negative contribution of TLR4 to cancer-related inflammation.
Researchers have long worked to determine the relationship between chronic inflammation and cancer. Reactive oxygen species such as those produced upon TLR4 activation may be responsible, at least in part, for cancerous proliferation due to inflammation. Especially when cells are dividing rapidly, reactive oxygen species can promote carcinogenic genetic mutations (Marx 2004a). NF-κB is involved in important carcinogenic responses; it promotes cancer by inhibiting apoptosis and by encouraging cancerous cells to spread and proliferate in other parts of the body (Marx 2004b, Marx 2004a). Innate immune activation has profound influences on tumor growth, as evidenced by experiments reporting significant increase in tumor size in response to LPS injection (Marx 2004a). Although NF-κB is activated by TLR4, it is also influenced by a myriad of other signaling cascades. Why inflammation sometimes promotes cancer development and at other times keeps cancer in check remains unclear.
IV. TLR4 and MD-2 Structure and Ligand Activity
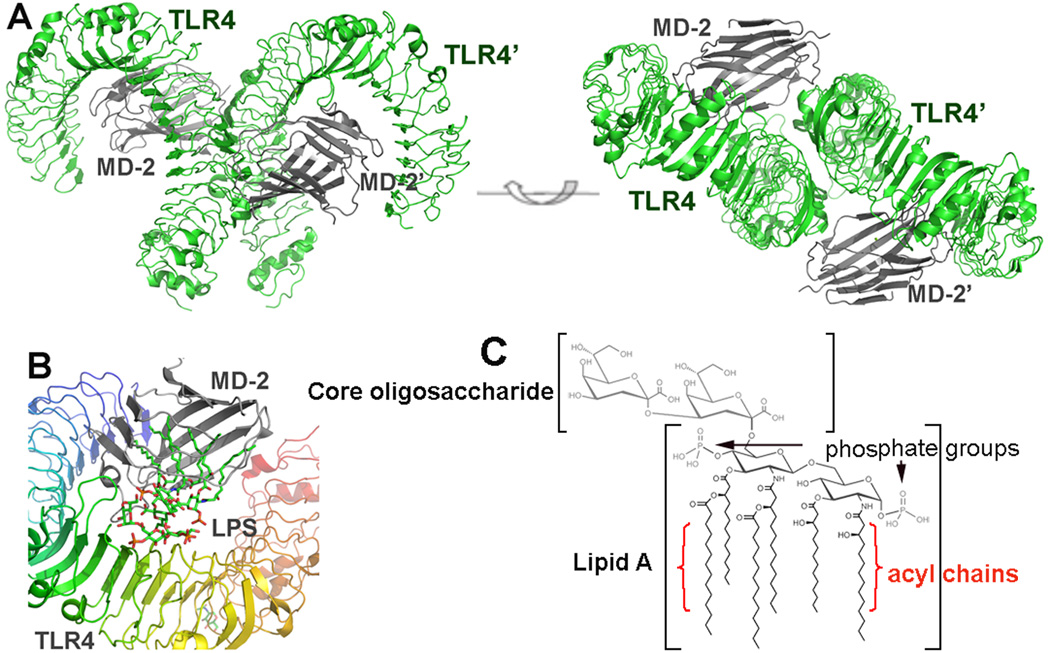
Three studies have been instrumental in establishing the structure-based function of TLR4 and its accessory protein MD-2. The first of these determined two crystal structures of human MD-2, one with and one without its ligand lipid IVa, an LPS derivative (Ohto et al. 2007). MD-2 takes a clamshell shape with a deep hydrophobic cleft flanked by two β-sheets. The structure with lipid IVa shows it sandwiched deep within the hydrophobic cleft of MD-2 (Ohto et al. 2007). Other ligands have since been shown to fit similarly inside MD-2 when complexed with TLR4 (Park et al. 2009, Kim et al. 2007).
The first high-resolution crystal structure of TLR4 itself showed murine TLR4 with its accessory protein MD-2 (Kim et al. 2007). Most recently, Park and colleagues established the basis for ligand recognition by the human TLR4 complex, showing that TLR4 agonist ligands cause two dimers of TLR4-MD-2 to associate, forming a multimeric complex (Figure 2). These multimers are associated with active TLR4 signaling, while a single unit of TLR4-MD-2 does not necessarily elicit a signal (Kim et al. 2007, Park et al. 2009).
Figure 2.
Overall structure of the TLR4/MD-2 complex. (A) Side and top view of an m-shaped receptor multimer composed of two copies of the TLR4/MD-2 complex arranged symmetrically. (B) Close-up view of the LPS binding site on the TLR4/MD-2 interface. LPS interacts with a large hydrophobic pocket in MD-2 and directly bridges the two components of the multimer. The primary interface between TLR4 and MD-2 is formed before binding LPS, and the dimerization interface is induced upon LPS binding. (C) Molecular structure of LPS.
The methods by which TLR4 recognizes its ligands have been studied through LPS structure-activity and TLR4 homodimerization studies (Rietschel et al. 1994, Park et al. 2009). There are many ligands with alleged TLR4 effects, however LPS contamination may be to blame in many of these cases. TLR4’s binding activity is perplexing nevertheless, as no obvious pattern describes the structures of TLR4 ligands. Further characterization of TLR4 ligands will be necessary to paint a more complete picture of “pattern” recognition receptors. Biophysical assays and direct evaluation of ligand binding, in particular, will help narrow the list of reported TLR4 ligands. This may in turn lead to a more concise repertoire of TLR-affecting agents, and clearer themes of TLR recognition. These themes will be crucial if we are to create more effective TLR-targeting therapeutic agents in the future.
V. Perspectives in Drug Discovery
Due to the pathological implications of aberrant TLR signaling, the ability to control TLR4 recognition and activation is a therapeutic topic of much interest. Given the ineffective therapeutics for Alzheimer’s, neuropathic pain and other central nervous system pathologies, drug candidates for these diseases are in urgent need. We believe that the traditional emphasis on neuronal targets is, at least in part, to blame for the void in effective pharmacotherapies to treat these conditions. Microglia are a dynamic, promising target in the aforementioned diseases, and TLR4 controls many microglia-specific responses known to dysregulate neuronal actions. TLR4 represents one of many receptors expressed primarily on microglia, and known to invoke the microglial proinflammatory response to a number of stimuli.
In addition, TLR4 is a feasible drug target due to its well-characterized structure and downstream signaling pathway. Two clinically relevant TLR4 inhibitors, TAK-242 and Eritoran, stand as justification that small molecules can target TLR4 with reasonably high affinity and specificity. Eritoran exploits LPS structural components; it is therefore large and probably blood-brain barrier impermeable, although this has not been tested to the best of our knowledge. Nevertheless the blood-brain barrier is compromised as a result of some CNS conditions, suggesting a possible venue for these molecules in the treatment of cerebral infarction and traumatic brain injury, for example.
Naloxone and naltrexone are also clinically relevant TLR4 effectors, but these are severely limited by short half-life. As interest in microglial targets grows, so too has the use of these opioid antagonists. Interestingly, naltrexone, the longer-acting of the two antagonists, has recently found a market in the treatment of severe alcoholism. Although cited for its ability to inhibit the reinforcing effects of endogenous opioids (i.e. endorphins), there exists a possibility that naltrexone also works to prevent the action of TLR4-mediated effects, as documented by several studies of TLR4 inhibition (Liu et al. 2000, Wu et al. 2006). The influence of alcohol on TLR4 signaling is reviewed extensively by Szabo and colleagues, who also suggest ethanol as a useful probe of TLR4 signaling with respect to lipid rafts, TLR4 complex association and receptor clustering (Szabo et al. 2007).
TLR4 is also special for its ability to control more than one inflammatory pathway, both the MyD88 and TRIF-mediated pathways. It is the only toll-like receptor known to signal through both the TRIF-dependent and MyD88-dependant pathways. TLR3 is the only other TLR with access to the TRIF/TRAM pathway, and TLR3 is an impractical target because it is expressed on endosomal vesicles (Yamamoto et al. 2003).
Within the TLR4 complex there are several well-studied interactions that give rise to feasible drug targets. Because both MD-2 and TLR4 are needed to coordinate a signal, inhibiting either one should theoretically inhibit downstream activity. For example, curcumin is suggested to inhibit TLR4 signaling by binding MD-2 (Gradisar et al. 2007). Another way to achieve TLR4 inhibition is to prevent the association of MD-2 and TLR4, a required interaction for signal transduction to occur. This has been achieved using rationally designed peptide inhibitors (Slivka et al. 2009). Finally, the homodimerization interface is an important regulatory site for TLR4 activity. By inhibiting the homodimerization between two TLR4-MD-2 molecules, drugs could feasibly stop activation without disrupting native inhibition mechanisms. In other words, the homodimerization interface described by Park and colleagues could be a more benign target to control TLR4 signaling, as it would allow ligands to occupy MD-2 or TLR4 without activating downstream signals through homodimerization.
TLR4 is a complex, dynamic target in structure-based drug design. Both agonists and antagonists have potential therapeutic applications for CNS conditions. The voids in our understanding of TLR4 recognition and subsequent intracellular signaling are balanced by recent advances in TLR4 and MD-2 structure/activity determination. Since the discovery of toll-like receptors, an unprecedented amount of progress has been made toward characterizing and controlling these ancient host defense mechanisms. Barring the challenges of blood-brain permeability, researchers are hot on the trail of a TLR4 antagonist for the treatment of the above pathologies. Future TLR4 studies will impact our understanding from basic cellular signaling to the treatment of important neurological diseases. The recent advances in TLR4 and microglial research stand as justification that even greater developments are yet to come.
Figure 1.
The signaling pathways of TLR4. MyD88-dependant signaling is common to most TLRs including TLRs 1,2,4 (shown) and 5,6,7,8,9,11 (not shown). TRIF-dependant signaling results from TLR4 and TLR3 activation only. Note that TLR3 resides in endosomal vesicles, shown here responding to engulfed foreign RNA. The pentagon shapes denotes proteins that interact through a TIR domain. The TRAM adaptor (lime green) is exclusive to TLR4 and coordinates the TRIF response through TLR4’s TIR domain. TRIF-dependant signaling primarily results in IFN-β production (red adaptors), but the TRIF pathway also induces “late stage” NF-κB activation through RIP 1 (white) and TRAF 6 (seafoam green). The MyD88-dependant cascade initiates “early stage” NF-κB activation through the IKKs (IKKs α,β,λ) and/or the MAPK pathway, leading to proinflammatory cytokine expression and subsequent amplification through additional immune pathways.
Acknowledgements
We thank the National Institutes of Health (DA026950, DA025740, NS067425, and DA029119) for support of this work. We are grateful to Dr. Catherine M. Joce and Leslie A. Morton for critical review of this manuscript.
Abbreviations used
- TLR
toll-like receptor
- LRR
leucine-rich repeat
- PAMP
pattern-associated molecular pattern
- LPS
lipopolysaccharide
- IL
interleukin
- TIR
toll / IL-1 receptor signaling domain
- TRIF
toll / IL-1 receptor-containing adaptor inducing IFN-β
- IFN-β
interferon (Vives-Pi et al.)
- Mal
MyD88 adaptor-like protein
- IRAK
interleukin-1 receptor-associated kinase
- MAPK
mitogen-activated protein kinase
- NF-κB
nuclear factor (Tian et al.)-B
- AP-1
activator protein-1
- TBK1
TRIF binding kinase-1
- IKK
inhibitor of NF-κB kinase
- IRF3
interferon regulatory factor 3
- ROS
reactive oxygen species
- NO
nitrous oxide
- TNFα
tumor necrosis factor (alpha)
- IR
interleukin receptor
- Aβ
amyloid-(Vives-Pi et al.) peptide
- AD
Alzheimer’s disease
References
- Nobel Lectures, Physiology or Medicine 1922–1941: Nobel lectures, including presentation speeches and laureates' biographies. Amsterdam: Elsevier Publishing Company; 1965. [Google Scholar]
- Aarum J, Sandberg K, Haeberlein SL, Persson MA. Migration and differentiation of neural precursor cells can be directed by microglia. Proc Natl Acad Sci U S A. 2003;100:15983–15988. doi: 10.1073/pnas.2237050100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abate W, Alghaithy AA, Parton J, Jones KP, Jackson SK. Surfactant lipids regulate LPS-induced interleukin-8 production in A549 lung epithelial cells by inhibiting translocation of TLR4 into lipid raft domains. J Lipid Res. 2009 doi: 10.1194/jlr.M000513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Akiyama H, Arai T, Kondo H, Tanno E, Haga C, Ikeda K. Cell mediators of inflammation in the Alzheimer disease brain. Alzheimer Dis Assoc Disord. 2000;14 Suppl 1:S47–S53. doi: 10.1097/00002093-200000001-00008. [DOI] [PubMed] [Google Scholar]
- Anderson KV, Bokla L, Nusslein-Volhard C. Establishment of dorsal-ventral polarity in the Drosophila embryo: the induction of polarity by the Toll gene product. Cell. 1985a;42:791–798. doi: 10.1016/0092-8674(85)90275-2. [DOI] [PubMed] [Google Scholar]
- Anderson KV, Jurgens G, Nusslein-Volhard C. Establishment of dorsal-ventral polarity in the Drosophila embryo: genetic studies on the role of the Toll gene product. Cell. 1985b;42:779–789. doi: 10.1016/0092-8674(85)90274-0. [DOI] [PubMed] [Google Scholar]
- Arbour NC, Lorenz E, Schutte BC, Zabner J, Kline JN, Jones M, Frees K, Watt JL, Schwartz DA. TLR4 mutations are associated with endotoxin hyporesponsiveness in humans. Nat Genet. 2000;25:187–191. doi: 10.1038/76048. [DOI] [PubMed] [Google Scholar]
- Balistreri CR, Candore G, Listi F, et al. Role of TLR4 polymorphisms in inflammatory responses: implications for unsuccessful aging. Ann N Y Acad Sci. 2007;1119:203–207. doi: 10.1196/annals.1404.003. [DOI] [PubMed] [Google Scholar]
- Balistreri CR, Colonna-Romano G, Lio D, Candore G, Caruso C. TLR4 polymorphisms and ageing: implications for the pathophysiology of age-related diseases. J Clin Immunol. 2009;29:406–415. doi: 10.1007/s10875-009-9297-5. [DOI] [PubMed] [Google Scholar]
- Blanco AM, Perez-Arago A, Fernandez-Lizarbe S, Guerri C. Ethanol mimics ligand-mediated activation and endocytosis of IL-1RI/TLR4 receptors via lipid rafts caveolae in astroglial cells. J Neurochem. 2008;106:625–639. doi: 10.1111/j.1471-4159.2008.05425.x. [DOI] [PubMed] [Google Scholar]
- Blanco AM, Valles SL, Pascual M, Guerri C. Involvement of TLR4/type I IL-1 receptor signaling in the induction of inflammatory mediators and cell death induced by ethanol in cultured astrocytes. J Immunol. 2005;175:6893–6899. doi: 10.4049/jimmunol.175.10.6893. [DOI] [PubMed] [Google Scholar]
- Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8:57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- Bowie A, O'Neill LA. The interleukin-1 receptor/Toll-like receptor superfamily: signal generators for pro-inflammatory interleukins and microbial products. J Leukoc Biol. 2000;67:508–514. doi: 10.1002/jlb.67.4.508. [DOI] [PubMed] [Google Scholar]
- Cao CX, Yang QW, Lv FL, Cui J, Fu HB, Wang JZ. Reduced cerebral ischemia-reperfusion injury in Toll-like receptor 4 deficient mice. Biochem Biophys Res Commun. 2007;353:509–514. doi: 10.1016/j.bbrc.2006.12.057. [DOI] [PubMed] [Google Scholar]
- Cao L, Palmer CD, Malon JT, De Leo JA. Critical role of microglial CD40 in the maintenance of mechanical hypersensitivity in a murine model of neuropathic pain. Eur J Immunol. 2009 doi: 10.1002/eji.200939657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caso JR, Pradillo JM, Hurtado O, Leza JC, Moro MA, Lizasoain I. Toll-like receptor 4 is involved in subacute stress-induced neuroinflammation and in the worsening of experimental stroke. Stroke. 2008;39:1314–1320. doi: 10.1161/STROKEAHA.107.498212. [DOI] [PubMed] [Google Scholar]
- Caso JR, Pradillo JM, Hurtado O, Lorenzo P, Moro MA, Lizasoain I. Toll-like receptor 4 is involved in brain damage and inflammation after experimental stroke. Circulation. 2007;115:1599–1608. doi: 10.1161/CIRCULATIONAHA.106.603431. [DOI] [PubMed] [Google Scholar]
- Chakravarty S, Herkenham M. Toll-like receptor 4 on nonhematopoietic cells sustains CNS inflammation during endotoxemia, independent of systemic cytokines. J Neurosci. 2005;25:1788–1796. doi: 10.1523/JNEUROSCI.4268-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLeo JA, Rutkowski MD, Stalder AK, Campbell IL. Transgenic expression of TNF by astrocytes increases mechanical allodynia in a mouse neuropathy model. Neuroreport. 2000;11:599–602. doi: 10.1097/00001756-200002280-00033. [DOI] [PubMed] [Google Scholar]
- Department of Health and Human Services, N. I. o. H. Nw directions in pain research. [web site] 1998 [Google Scholar]
- Fernandez-Lizarbe S, Pascual M, Gascon MS, Blanco A, Guerri C. Lipid rafts regulate ethanol-induced activation of TLR4 signaling in murine macrophages. Mol Immunol. 2008;45:2007–2016. doi: 10.1016/j.molimm.2007.10.025. [DOI] [PubMed] [Google Scholar]
- Gangloff M, Weber AN, Gibbard RJ, Gay NJ. Evolutionary relationships, but functional differences, between the Drosophila and human Toll-like receptor families. Biochem Soc Trans. 2003;31:659–663. doi: 10.1042/bst0310659. [DOI] [PubMed] [Google Scholar]
- Glezer I, Lapointe A, Rivest S. Innate immunity triggers oligodendrocyte progenitor reactivity and confines damages to brain injuries. FASEB J. 2006;20:750–752. doi: 10.1096/fj.05-5234fje. [DOI] [PubMed] [Google Scholar]
- Gradisar H, Keber MM, Pristovsek P, Jerala R. MD-2 as the target of curcumin in the inhibition of response to LPS. J Leukoc Biol. 2007;82:968–974. doi: 10.1189/jlb.1206727. [DOI] [PubMed] [Google Scholar]
- Hacker H, Redecke V, Blagoev B, et al. Specificity in Toll-like receptor signalling through distinct effector functions of TRAF3 and TRAF6. Nature. 2006;439:204–207. doi: 10.1038/nature04369. [DOI] [PubMed] [Google Scholar]
- Hashimoto C, Hudson KL, Anderson KV. The Toll gene of Drosophila, required for dorsal-ventral embryonic polarity, appears to encode a transmembrane protein. Cell. 1988;52:269–279. doi: 10.1016/0092-8674(88)90516-8. [DOI] [PubMed] [Google Scholar]
- Hickey EJ, You X, Kaimaktchiev V, Stenzel-Poore M, Ungerleider RM. Lipopolysaccharide preconditioning induces robust protection against brain injury resulting from deep hypothermic circulatory arrest. J Thorac Cardiovasc Surg. 2007;133:1588–1596. doi: 10.1016/j.jtcvs.2006.12.056. [DOI] [PubMed] [Google Scholar]
- Horng T, Barton GM, Flavell RA, Medzhitov R. The adaptor molecule TIRAP provides signalling specificity for Toll-like receptors. Nature. 2002;420:329–333. doi: 10.1038/nature01180. [DOI] [PubMed] [Google Scholar]
- Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol. 1999;162:3749–3752. [PubMed] [Google Scholar]
- Hua F, Ma J, Ha T, et al. Activation of Toll-like receptor 4 signaling contributes to hippocampal neuronal death following global cerebral ischemia/reperfusion. J Neuroimmunol. 2007;190:101–111. doi: 10.1016/j.jneuroim.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Bland ST, Johnson KW, Rice KC, Maier SF, Watkins LR. Opioid-induced glial activation: mechanisms of activation and implications for opioid analgesia, dependence, and reward. ScientificWorldJournal. 2007;7:98–111. doi: 10.1100/tsw.2007.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Ramos KM, Loram LC, et al. Evidence for a role of heat shock protein-90 (HSP90) in TLR4 mediated pain enhancement in rats. Neuroscience. 2009a doi: 10.1016/j.neuroscience.2009.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Zhang Y, Brown K, et al. Non-stereoselective reversal of neuropathic pain by naloxone and naltrexone: involvement of toll-like receptor 4 (TLR4) Eur J Neurosci. 2008;28:20–29. doi: 10.1111/j.1460-9568.2008.06321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Zhang Y, Shridhar M, et al. Evidence that opioids may have toll-like receptor 4 and MD-2 effects. Brain Behav Immun. 2009b doi: 10.1016/j.bbi.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ii M, Matsunaga N, Hazeki K, Nakamura K, Takashima K, Seya T, Hazeki O, Kitazaki T, Iizawa Y. A novel cyclohexene derivative, ethyl (6R)-6-[N-(2-Chloro-4-fluorophenyl)sulfamoyl]cyclohex-1-ene-1-carboxylate (TAK-242), selectively inhibits toll-like receptor 4-mediated cytokine production through suppression of intracellular signaling. Mol Pharmacol. 2006;69:1288–1295. doi: 10.1124/mol.105.019695. [DOI] [PubMed] [Google Scholar]
- Jou I, Lee JH, Park SY, Yoon HJ, Joe EH, Park EJ. Gangliosides trigger inflammatory responses via TLR4 in brain glia. Am J Pathol. 2006;168:1619–1630. doi: 10.2353/ajpath.2006.050924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juni A, Klein G, Pintar JE, Kest B. Nociception increases during opioid infusion in opioid receptor triple knock-out mice. Neuroscience. 2007;147:439–444. doi: 10.1016/j.neuroscience.2007.04.030. [DOI] [PubMed] [Google Scholar]
- Kagan JC, Medzhitov R. Phosphoinositide-mediated adaptor recruitment controls Toll-like receptor signaling. Cell. 2006;125:943–955. doi: 10.1016/j.cell.2006.03.047. [DOI] [PubMed] [Google Scholar]
- Kagan JC, Su T, Horng T, Chow A, Akira S, Medzhitov R. TRAM couples endocytosis of Toll-like receptor 4 to the induction of interferon-beta. Nat Immunol. 2008;9:361–368. doi: 10.1038/ni1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakimura J, Kitamura Y, Takata K, et al. Microglial activation and amyloid-beta clearance induced by exogenous heat-shock proteins. FASEB J. 2002;16:601–603. doi: 10.1096/fj.01-0530fje. [DOI] [PubMed] [Google Scholar]
- Kawai T, Akira S. Signaling to NF-kappaB by Toll-like receptors. Trends Mol Med. 2007;13:460–469. doi: 10.1016/j.molmed.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Keller JN, Hanni KB, Markesbery WR. 4-hydroxynonenal increases neuronal susceptibility to oxidative stress. J Neurosci Res. 1999;58:823–830. [PubMed] [Google Scholar]
- Keller JN, Pang Z, Geddes JW, Begley JG, Germeyer A, Waeg G, Mattson MP. Impairment of glucose and glutamate transport and induction of mitochondrial oxidative stress and dysfunction in synaptosomes by amyloid beta-peptide: role of the lipid peroxidation product 4-hydroxynonenal. J Neurochem. 1997;69:273–284. doi: 10.1046/j.1471-4159.1997.69010273.x. [DOI] [PubMed] [Google Scholar]
- Kim HM, Park BS, Kim JI, et al. Crystal structure of the TLR4-MD-2 complex with bound endotoxin antagonist Eritoran. Cell. 2007;130:906–917. doi: 10.1016/j.cell.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Saitoh S, Tanimura N, et al. Regulatory roles for MD-2 and TLR4 in ligand-induced receptor clustering. J Immunol. 2006;176:6211–6218. doi: 10.4049/jimmunol.176.10.6211. [DOI] [PubMed] [Google Scholar]
- Konat GW, Krasowska-Zoladek A, Kraszpulski M. Statins enhance toll-like receptor 4-mediated cytokine gene expression in astrocytes: implication of Rho proteins in negative feedback regulation. J Neurosci Res. 2008;86:603–609. doi: 10.1002/jnr.21509. [DOI] [PubMed] [Google Scholar]
- Lawson LJ, Perry VH, Dri P, Gordon S. Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience. 1990;39:151–170. doi: 10.1016/0306-4522(90)90229-w. [DOI] [PubMed] [Google Scholar]
- Lehnardt S, Massillon L, Follett P, Jensen FE, Ratan R, Rosenberg PA, Volpe JJ, Vartanian T. Activation of innate immunity in the CNS triggers neurodegeneration through a Toll-like receptor 4-dependent pathway. Proc Natl Acad Sci U S A. 2003;100:8514–8519. doi: 10.1073/pnas.1432609100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehnardt S, Schott E, Trimbuch T, Laubisch D, Krueger C, Wulczyn G, Nitsch R, Weber JR. A vicious cycle involving release of heat shock protein 60 from injured cells and activation of toll-like receptor 4 mediates neurodegeneration in the CNS. J Neurosci. 2008;28:2320–2331. doi: 10.1523/JNEUROSCI.4760-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Du L, Kong LY, Hudson PM, Wilson BC, Chang RC, Abel HH, Hong JS. Reduction by naloxone of lipopolysaccharide-induced neurotoxicity in mouse cortical neuron-glia co-cultures. Neuroscience. 2000;97:749–756. doi: 10.1016/s0306-4522(00)00057-9. [DOI] [PubMed] [Google Scholar]
- Liu H, Xin L, Chan BP, Teoh R, Tang BL, Tan YH. Interferon-beta administration confers a beneficial outcome in a rabbit model of thromboembolic cerebral ischemia. Neurosci Lett. 2002a;327:146–148. doi: 10.1016/s0304-3940(02)00371-3. [DOI] [PubMed] [Google Scholar]
- Liu Y, Qin L, Wilson BC, An L, Hong JS, Liu B. Inhibition by naloxone stereoisomers of beta-amyloid peptide (1–42)-induced superoxide production in microglia and degeneration of cortical and mesencephalic neurons. J Pharmacol Exp Ther. 2002b;302:1212–1219. doi: 10.1124/jpet.102.035956. [DOI] [PubMed] [Google Scholar]
- Maier SF, Watkins LR. Immune-to-central nervous system communication and its role in modulating pain and cognition: Implications for cancer and cancer treatment. Brain Behav Immun. 2003;17 Suppl 1:S125–S131. doi: 10.1016/s0889-1591(02)00079-x. [DOI] [PubMed] [Google Scholar]
- Maier SF, Wiertelak EP, Martin D, Watkins LR. Interleukin-1 mediates the behavioral hyperalgesia produced by lithium chloride and endotoxin. Brain Res. 1993;623:321–324. doi: 10.1016/0006-8993(93)91446-y. [DOI] [PubMed] [Google Scholar]
- Marin-Teva JL, Dusart I, Colin C, Gervais A, van Rooijen N, Mallat M. Microglia promote the death of developing Purkinje cells. Neuron. 2004;41:535–547. doi: 10.1016/s0896-6273(04)00069-8. [DOI] [PubMed] [Google Scholar]
- Marsh B, Stevens SL, Packard AE, Gopalan B, Hunter B, Leung PY, Harrington CA, Stenzel-Poore MP. Systemic lipopolysaccharide protects the brain from ischemic injury by reprogramming the response of the brain to stroke: a critical role for IRF3. J Neurosci. 2009;29:9839–9849. doi: 10.1523/JNEUROSCI.2496-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marta M, Meier UC, Lobell A. Regulation of autoimmune encephalomyelitis by toll-like receptors. Autoimmun Rev. 2009;8:506–509. doi: 10.1016/j.autrev.2009.01.006. [DOI] [PubMed] [Google Scholar]
- Marx J. Cancer research. Inflammation and cancer: the link grows stronger. Science. 2004a;306:966–968. doi: 10.1126/science.306.5698.966. [DOI] [PubMed] [Google Scholar]
- Marx J. Pain research. Why other people may not feel your pain. Science. 2004b;305:328. doi: 10.1126/science.305.5682.328. [DOI] [PubMed] [Google Scholar]
- Mason P. Lipopolysaccharide induces fever and decreases tail flick latency in awake rats. Neurosci Lett. 1993;154:134–136. doi: 10.1016/0304-3940(93)90189-r. [DOI] [PubMed] [Google Scholar]
- Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- Midwood K, Sacre S, Piccinini AM, et al. Tenascin-C is an endogenous activator of Toll-like receptor 4 that is essential for maintaining inflammation in arthritic joint disease. Nat Med. 2009;15:774–780. doi: 10.1038/nm.1987. [DOI] [PubMed] [Google Scholar]
- Milligan ED, Watkins LR. Pathological and protective roles of glia in chronic pain. Nature Reviews Neuroscience. 2009;10:23–26. doi: 10.1038/nrn2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minoretti P, Gazzaruso C, Vito CD, Emanuele E, Bianchi M, Coen E, Reino M, Geroldi D. Effect of the functional toll-like receptor 4 Asp299Gly polymorphism on susceptibility to late-onset Alzheimer's disease. Neurosci Lett. 2006;391:147–149. doi: 10.1016/j.neulet.2005.08.047. [DOI] [PubMed] [Google Scholar]
- Miyake K, Yamashita Y, Ogata M, Sudo T, Kimoto M. RP105, a novel B cell surface molecule implicated in B cell activation, is a member of the leucine-rich repeat protein family. J Immunol. 1995;154:3333–3340. [PubMed] [Google Scholar]
- Nagai Y, Akashi S, Nagafuku M, et al. Essential role of MD-2 in LPS responsiveness and TLR4 distribution. Nat Immunol. 2002;3:667–672. doi: 10.1038/ni809. [DOI] [PubMed] [Google Scholar]
- Ohnishi H, Tochio H, Kato Z, Orii KE, Li A, Kimura T, Hiroaki H, Kondo N, Shirakawa M. Structural basis for the multiple interactions of the MyD88 TIR domain in TLR4 signaling. Proc Natl Acad Sci U S A. 2009;106:10260–10265. doi: 10.1073/pnas.0812956106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohto U, Fukase K, Miyake K, Satow Y. Crystal structures of human MD-2 and its complex with antiendotoxic lipid IVa. Science. 2007;316:1632–1634. doi: 10.1126/science.1139111. [DOI] [PubMed] [Google Scholar]
- Okamura Y, Watari M, Jerud ES, Young DW, Ishizaka ST, Rose J, Chow JC, Strauss JF., 3rd The extra domain A of fibronectin activates Toll-like receptor 4. J Biol Chem. 2001;276:10229–10233. doi: 10.1074/jbc.M100099200. [DOI] [PubMed] [Google Scholar]
- Pais TF, Figueiredo C, Peixoto R, Braz MH, Chatterjee S. Necrotic neurons enhance microglial neurotoxicity through induction of glutaminase by a MyD88-dependent pathway. J Neuroinflammation. 2008;5:43. doi: 10.1186/1742-2094-5-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park BS, Song DH, Kim HM, Choi BS, Lee H, Lee JO. The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature. 2009;458:1191–1195. doi: 10.1038/nature07830. [DOI] [PubMed] [Google Scholar]
- Poikonen K, Lajunen T, Silvennoinen-Kassinen S, Leinonen M, Saikku P. Effects of CD14, TLR2, TLR4, LPB, and IL-6 gene polymorphisms on Chlamydia pneumoniae growth in human macrophages in vitro. Scand J Immunol. 2009;70:34–39. doi: 10.1111/j.1365-3083.2009.02267.x. [DOI] [PubMed] [Google Scholar]
- Poltorak A, He X, Smirnova I, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- Prohinar P, Re F, Widstrom R, Zhang D, Teghanemt A, Weiss JP, Gioannini TL. Specific high affinity interactions of monomeric endotoxin.protein complexes with Toll-like receptor 4 ectodomain. J Biol Chem. 2007;282:1010–1017. doi: 10.1074/jbc.M609400200. [DOI] [PubMed] [Google Scholar]
- Rietschel ET, Kirikae T, Schade FU, et al. Bacterial endotoxin: molecular relationships of structure to activity and function. FASEB J. 1994;8:217–225. doi: 10.1096/fasebj.8.2.8119492. [DOI] [PubMed] [Google Scholar]
- Rittirsch D, Flierl MA, Day DE, et al. Cross-talk between TLR4 and FcgammaReceptorIII (CD16) pathways. PLoS Pathog. 2009;5:e1000464. doi: 10.1371/journal.ppat.1000464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenzweig HL, Lessov NS, Henshall DC, Minami M, Simon RP, Stenzel-Poore MP. Endotoxin preconditioning prevents cellular inflammatory response during ischemic neuroprotection in mice. Stroke. 2004;35:2576–2581. doi: 10.1161/01.STR.0000143450.04438.ae. [DOI] [PubMed] [Google Scholar]
- Rossignol DP, Wasan KM, Choo E, Yau E, Wong N, Rose J, Moran J, Lynn M. Safety, pharmacokinetics, pharmacodynamics, and plasma lipoprotein distribution of eritoran (E5564) during continuous intravenous infusion into healthy volunteers. Antimicrob Agents Chemother. 2004;48:3233–3240. doi: 10.1128/AAC.48.9.3233-3240.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe DC, McGettrick AF, Latz E, et al. The myristoylation of TRIF-related adaptor molecule is essential for Toll-like receptor 4 signal transduction. Proc Natl Acad Sci U S A. 2006;103:6299–6304. doi: 10.1073/pnas.0510041103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarnico I, Lanzillotta A, Benarese M, Alghisi M, Baiguera C, Battistin L, Spano P, Pizzi M. NF-kappaB dimers in the regulation of neuronal survival. Int Rev Neurobiol. 2009;85:351–362. doi: 10.1016/S0074-7742(09)85024-1. [DOI] [PubMed] [Google Scholar]
- Schaefer L, Babelova A, Kiss E, et al. The matrix component biglycan is proinflammatory and signals through Toll-like receptors 4 and 2 in macrophages. J Clin Invest. 2005;115:2223–2233. doi: 10.1172/JCI23755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha T, Sunamoto M, Kitazaki T, Sato J, Ii M, Iizawa Y. Therapeutic effects of TAK-242, a novel selective Toll-like receptor 4 signal transduction inhibitor, in mouse endotoxin shock model. Eur J Pharmacol. 2007;571:231–239. doi: 10.1016/j.ejphar.2007.06.027. [DOI] [PubMed] [Google Scholar]
- Shimazu R, Akashi S, Ogata H, Nagai Y, Fukudome K, Miyake K, Kimoto M. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J Exp Med. 1999;189:1777–1782. doi: 10.1084/jem.189.11.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slivka PF, Shridhar M, Lee GI, et al. A peptide antagonist of the TLR4-MD2 interaction. Chembiochem. 2009;10:645–649. doi: 10.1002/cbic.200800769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smiley ST, King JA, Hancock WW. Fibrinogen stimulates macrophage chemokine secretion through toll-like receptor 4. J Immunol. 2001;167:2887–2894. doi: 10.4049/jimmunol.167.5.2887. [DOI] [PubMed] [Google Scholar]
- Stewart WF, Kawas C, Corrada M, Metter EJ. Risk of Alzheimer's disease and duration of NSAID use. Neurology. 1997;48:626–632. doi: 10.1212/wnl.48.3.626. [DOI] [PubMed] [Google Scholar]
- Szabo G, Dolganiuc A, Dai Q, Pruett SB. TLR4, ethanol, and lipid rafts: a new mechanism of ethanol action with implications for other receptor-mediated effects. J Immunol. 2007;178:1243–1249. doi: 10.4049/jimmunol.178.3.1243. [DOI] [PubMed] [Google Scholar]
- Tahara K, Kim HD, Jin JJ, Maxwell JA, Li L, Fukuchi K. Role of toll-like receptor signalling in Abeta uptake and clearance. Brain. 2006;129:3006–3019. doi: 10.1093/brain/awl249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashima K, Matsunaga N, Yoshimatsu M, et al. Analysis of binding site for the novel small-molecule TLR4 signal transduction inhibitor TAK-242 and its therapeutic effect on mouse sepsis model. Br J Pharmacol. 2009 doi: 10.1111/j.1476-5381.2009.00297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan L, Schedl P, Song HJ, Garza D, Konsolaki M. The Toll-->NFkappaB signaling pathway mediates the neuropathological effects of the human Alzheimer's Abeta42 polypeptide in Drosophila. PLoS One. 2008;3:e3966. doi: 10.1371/journal.pone.0003966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang SC, Arumugam TV, Xu X, et al. Pivotal role for neuronal Toll-like receptors in ischemic brain injury and functional deficits. Proc Natl Acad Sci U S A. 2007;104:13798–13803. doi: 10.1073/pnas.0702553104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang SC, Lathia JD, Selvaraj PK, et al. Toll-like receptor-4 mediates neuronal apoptosis induced by amyloid beta-peptide and the membrane lipid peroxidation product 4-hydroxynonenal. Exp Neurol. 2008;213:114–121. doi: 10.1016/j.expneurol.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanga FY, Nutile-McMenemy N, DeLeo JA. The CNS role of Toll-like receptor 4 in innate neuroimmunity and painful neuropathy. Proc Natl Acad Sci U S A. 2005;102:5856–5861. doi: 10.1073/pnas.0501634102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanimura N, Saitoh S, Matsumoto F, Akashi-Takamura S, Miyake K. Roles for LPS-dependent interaction and relocation of TLR4 and TRAM in TRIF-signaling. Biochem Biophys Res Commun. 2008;368:94–99. doi: 10.1016/j.bbrc.2008.01.061. [DOI] [PubMed] [Google Scholar]
- Tasaki K, Ruetzler CA, Ohtsuki T, Martin D, Nawashiro H, Hallenbeck JM. Lipopolysaccharide pre-treatment induces resistance against subsequent focal cerebral ischemic damage in spontaneously hypertensive rats. Brain Res. 1997;748:267–270. doi: 10.1016/s0006-8993(96)01383-2. [DOI] [PubMed] [Google Scholar]
- Termeer C, Benedix F, Sleeman J, et al. Oligosaccharides of Hyaluronan activate dendritic cells via toll-like receptor 4. J Exp Med. 2002;195:99–111. doi: 10.1084/jem.20001858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L, White JE, Lin HY, Haran VS, Sacco J, Chikkappa G, Davis FB, Davis PJ, Tsan MF. Induction of Mn SOD in human monocytes without inflammatory cytokine production by a mutant endotoxin. Am J Physiol. 1998;275:C740–C747. doi: 10.1152/ajpcell.1998.275.3.C740. [DOI] [PubMed] [Google Scholar]
- Triantafilou M, Triantafilou K. Heat-shock protein 70 and heat-shock protein 90 associate with Toll-like receptor 4 in response to bacterial lipopolysaccharide. Biochem Soc Trans. 2004;32:636–639. doi: 10.1042/BST0320636. [DOI] [PubMed] [Google Scholar]
- Tsan MF, Gao B. Cytokine function of heat shock proteins. Am J Physiol Cell Physiol. 2004a;286:C739–C744. doi: 10.1152/ajpcell.00364.2003. [DOI] [PubMed] [Google Scholar]
- Tsan MF, Gao B. Endogenous ligands of Toll-like receptors. J Leukoc Biol. 2004b;76:514–519. doi: 10.1189/jlb.0304127. [DOI] [PubMed] [Google Scholar]
- Veldhuis WB, Derksen JW, Floris S, et al. Interferon-beta blocks infiltration of inflammatory cells and reduces infarct volume after ischemic stroke in the rat. J Cereb Blood Flow Metab. 2003;23:1029–1039. doi: 10.1097/01.WCB.0000080703.47016.B6. [DOI] [PubMed] [Google Scholar]
- Vives-Pi M, Somoza N, Fernandez-Alvarez J, Vargas F, Caro P, Alba A, Gomis R, Labeta MO, Pujol-Borrell R. Evidence of expression of endotoxin receptors CD14, toll-like receptors TLR4 and TLR2 and associated molecule MD-2 and of sensitivity to endotoxin (LPS) in islet beta cells. Clin Exp Immunol. 2003;133:208–218. doi: 10.1046/j.1365-2249.2003.02211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakselman S, Bechade C, Roumier A, Bernard D, Triller A, Bessis A. Developmental neuronal death in hippocampus requires the microglial CD11b integrin and DAP12 immunoreceptor. J Neurosci. 2008;28:8138–8143. doi: 10.1523/JNEUROSCI.1006-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter S, Letiembre M, Liu Y, et al. Role of the toll-like receptor 4 in neuroinflammation in Alzheimer's disease. Cell Physiol Biochem. 2007;20:947–956. doi: 10.1159/000110455. [DOI] [PubMed] [Google Scholar]
- Wang AC, Su QB, Wu FX, Zhang XL, Liu PS. Role of TLR4 for paclitaxel chemotherapy in human epithelial ovarian cancer cells. Eur J Clin Invest. 2009;39:157–164. doi: 10.1111/j.1365-2362.2008.02070.x. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Maier SF. Implications of immune-to-brain communication for sickness and pain. Proc Natl Acad Sci U S A. 1999;96:7710–7713. doi: 10.1073/pnas.96.14.7710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weatherill AR, Lee JY, Zhao L, Lemay DG, Youn HS, Hwang DH. Saturated and polyunsaturated fatty acids reciprocally modulate dendritic cell functions mediated through TLR4. J Immunol. 2005;174:5390–5397. doi: 10.4049/jimmunol.174.9.5390. [DOI] [PubMed] [Google Scholar]
- Wong SW, Kwon MJ, Choi AM, Kim HP, Nakahira K, Hwang D. Fatty acids modulate toll-like receptor 4 activation through regulation of receptor dimerization and recruitment into lipid rafts in a ros-dependent manner. J Biol Chem. 2009 doi: 10.1074/jbc.M109.044065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu HE, Sun HS, Cheng CW, Terashvili M, Tseng LF. dextro-Naloxone or levo-naloxone reverses the attenuation of morphine antinociception induced by lipopolysaccharide in the mouse spinal cord via a non-opioid mechanism. Eur J Neurosci. 2006;24:2575–2580. doi: 10.1111/j.1460-9568.2006.05144.x. [DOI] [PubMed] [Google Scholar]
- Yamada M, Ichikawa T, Ii M, Sunamoto M, Itoh K, Tamura N, Kitazaki T. Discovery of novel and potent small-molecule inhibitors of NO and cytokine production as antisepsis agents: synthesis and biological activity of alkyl 6-(N-substituted sulfamoyl)cyclohex-1-ene-1-carboxylate. J Med Chem. 2005;48:7457–7467. doi: 10.1021/jm050623t. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Sato S, Hemmi H, et al. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 2003;301:640–643. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
- Youn HS, Lee JK, Choi YJ, Saitoh SI, Miyake K, Hwang DH, Lee JY. Cinnamaldehyde suppresses toll-like receptor 4 activation mediated through the inhibition of receptor oligomerization. Biochem Pharmacol. 2008;75:494–502. doi: 10.1016/j.bcp.2007.08.033. [DOI] [PubMed] [Google Scholar]
- Youn HS, Lee JY, Fitzgerald KA, Young HA, Akira S, Hwang DH. Specific inhibition of MyD88-independent signaling pathways of TLR3 and TLR4 by resveratrol: molecular targets are TBK1 and RIP1 in TRIF complex. J Immunol. 2005;175:3339–3346. doi: 10.4049/jimmunol.175.5.3339. [DOI] [PubMed] [Google Scholar]
- Youn HS, Saitoh SI, Miyake K, Hwang DH. Inhibition of homodimerization of Toll-like receptor 4 by curcumin. Biochem Pharmacol. 2006;72:62–69. doi: 10.1016/j.bcp.2006.03.022. [DOI] [PubMed] [Google Scholar]
- Younger J, Mackey S. Fibromyalgia symptoms are reduced by low-dose naltrexone: a pilot study. Pain Med. 2009;10:663–672. doi: 10.1111/j.1526-4637.2009.00613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younger JW, Zautra AJ, Cummins ET. Effects of naltrexone on pain sensitivity and mood in fibromyalgia: no evidence for endogenous opioid pathophysiology. PLoS One. 2009;4:e5180. doi: 10.1371/journal.pone.0005180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusuf N, Nasti TH, Meleth S, Elmets CA. Resveratrol enhances cell-mediated immune response to DMBA through TLR4 and prevents DMBA induced cutaneous carcinogenesis. Mol Carcinog. 2009;48:713–723. doi: 10.1002/mc.20517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, Beers DR, Henkel JS, Zhang W, Urushitani M, Julien JP, Appel SH. Extracellular mutant SOD1 induces microglial-mediated motoneuron injury. Glia. 58:231–243. doi: 10.1002/glia.20919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Lapointe BM, Clark SR, Zbytnuik L, Kubes P. A requirement for microglial TLR4 in leukocyte recruitment into brain in response to lipopolysaccharide. J Immunol. 2006;177:8103–8110. doi: 10.4049/jimmunol.177.11.8103. [DOI] [PubMed] [Google Scholar]
- Zimmer SM, Liu J, Clayton JL, Stephens DS, Snyder JP. Paclitaxel binding to human and murine MD-2. J Biol Chem. 2008;283:27916–27926. doi: 10.1074/jbc.M802826200. [DOI] [PMC free article] [PubMed] [Google Scholar]