Abstract
Although β-amyloid (Aβ) plaques and tau neurofibrillary tangles are hallmarks of Alzheimer’s disease (AD) neuropathology, loss of synapses is considered the best correlate of cognitive decline in AD, rather than plaques or tangles. How pathological Aβ and tau aggregation relate to each other and to alterations in synapses remains unclear. Since aberrant tau phosphorylation occurs in amyloid precursor protein (APP) Swedish mutant transgenic mice, and since neurofibrillary tangles develop in triple transgenic mice harboring mutations in APP, tau and presenilin 1, we utilized these well-characterized mouse models to explore the relation between Aβ and tau pathologies. We now report that pathological accumulation of Aβ and hyperphosphorylation of tau develop concomitantly within synaptic terminals.
Keywords: amyloid, tau, synapse, Alzheimer’s disease, neuropathology, electron microscopy, endosomes, microtubules, hippocampus
1. Introduction
AD neuropathology is traditionally characterized by the abnormal deposition of Aβ in extracellular plaques and tau in intracellular tangles. More recently, early intraneuronal accumulation of Aβ42, the most pathogenic Aβ species, has been described in AD (Alafuzoff et al., 2008; Cataldo et al., 2004; D'Andrea et al., 2001; Gouras et al., 2000; Ohyagi et al., 2005), Down syndrome (Busciglio et al., 2002; Cataldo et al., 2004; Gouras et al., 2000; Gyure et al., 2001; Mori et al., 2002), and transgenic AD mouse models (Lord et al., 2006; Oakley et al., 2006; Oddo et al., 2003; Sheng et al., 2003; Shie et al., 2003; Stokin et al., 2005; Takahashi et al., 2002; Van Broeck et al., 2008; Wirths et al., 2001; Zerbinatti et al., 2006). Further, transgenic AD mice develop physiological and behavioral abnormalities prior to plaques (Chapman et al., 1999; Holcomb et al., 1998; Moechars et al., 1999) but concomitant with intraneuronal Aβ peptide accumulation (Bayer and Wirths, 2008; Billings et al., 2005; Cruz et al., 2006; Echeverria et al., 2004; Knobloch et al., 2007; Lord et al., 2006; Oddo et al., 2003), supporting that intraneuronal Aβ peptides are involved in the initiation of AD pathogenesis (Gouras et al., 2005).
Evidence supports that Aβ accumulation precedes and promotes tau pathology. Crossbreeding of mutant amyloid precursor protein (APP) transgenic mice with (Lewis et al., 2001) or intracerebral injection of Aβ into tau mutant transgenic mice (Gotz et al., 2001) led to enhanced tau pathology. In human brains with early AD changes or Down syndrome, intraneuronal Aβ42 accumulation in CA1 pyramidal cell bodies preceded hyperphosphorylation of tau (Gouras et al., 2000; Gyure et al., 2001). In the 3xTg-AD mouse harboring mutations in APP, tau and presenilin, intraneuronal Aβ accumulation in cell bodies preceded tau hyperphosphorylation, and Aβ antibodies reduced both Aβ and tau pathologies (Oddo et al., 2004; 2003). Recent evidence that behavioral deficits in transgenic mouse models of AD can be attenuated by reduction in tau (Roberson et al., 2007) further underscores the relevance in elucidating the biological mechanism(s) linking Aβ and tau. Here we analyze the relation between Aβ42 and phosphorylated tau in two well established transgenic mouse models of AD and utilize the anatomy of the hippocampus to co-localize both early Aβ42 accumulation and tau phosphorylation to synapses.
2. Materials and Methods
2.1. Antibodies
Aβ42 antibody AB5078P (Chemicon, Temecula, CA) is a rabbit polyclonal antibody directed against the C-terminus of Aβ42 that was previously biochemically characterized (Kamal et al., 2001). The specificity of this Aβ42 antibody was additionally shown by absence of immunofluorescence in cultured neurons derived from well-established APP knockout mice (Zheng et al., 1995) compared to wild-type mice (Almeida et al., 2006). The well-established antibody AT8 (Endogen, Rockford, IL) detects tau phosphorylated at serine 202 and threonine 205. MC1 antibody recognizes a conformational tau epitope in paired helical filaments (Jicha et al., 1997). Tau antibody 12E8 detects tau phosphorylated at Ser 262 and 356 (Litersky et al., 1996). AT180 tau antibody (Endogen, Rockford, IL) is directed against the phosphorylated Thr231 residue.
2.2. Transgenic mouse brain tissue
All mouse experiments were performed in compliance with the institutional guidelines of the Institutional Animal Care and Use Committee of Weill Cornell Medical College in accordance with the National Institutes of Health guidelines. Brain sections from Tg2576 mice, wild-type mice, and heterozygous 3xTg-AD mice at 5 and 13 months of age, were analyzed with at least n=2–3 mice per group. Preparation of tissue sections from Tg2576 mice (Hsiao et al., 1996) and 3xTg-AD mice (Oddo et al., 2003) was similar to that described previously (Takahashi et al., 2002). Mice were anesthetized with sodium pentobarbital (150 mg/kg, i.p.) and perfused via the ascending aorta with 3.75% acrolein (Polyscience, Warrington, PA) and 2% paraformaldehyde in 0.1 M phosphate buffer (PB), pH 7.4. Vibratome-cut 40 µm tissue sections were kept in storage buffer composed of 30% sucrose and 30% ethylene glycol in PB at −20°C.
2.3. Human brain tissue
Vibratome-cut 40 µm tissue sections of human cortical brain biopsy tissue kept in storage buffer at −20°C was from neurologically normal controls (n=2; ages 44 and 54) and subjects with AD (n=2; ages 54 and 62) originally obtained from the Department of Pathology, Weill Medical College of Cornell University, as a result of neurosurgical procedures unrelated to this study (Takahashi et al., 2004; 2002).
2.4. Immunohistochemistry
Immunolabeling for light microscopy was performed as previously described (Milner et al., 1998; Takahashi et al., 2004). Free-floating sections were incubated in primary antibodies for 24 h at room temperature and then for 40–48 h at 4°C (Chemicon Aβ42 antibody, 1:150; AT8, AT180, 12E8, 1:500; MC1, 1:10). The sections were incubated in biotinylated horse anti-mouse immunoglobulin (IgG) secondary antibody (1:400, Vector Laboratories, Burlingame, CA) for 30 min, followed by the peroxidase-avidin complex (Vectastain ABC kit, Vector) for 30 min. The secondary antibody was diluted in 0.1 M Tris-saline (pH 7.6) containing 0.1% bovine serum albumin (BSA). The reaction product with the ABC kit was visualized after incubation of sections with 3, 3’-diaminobenzidine (Aldrich Chemical, Milwaukee, WI) and hydrogen peroxide. The sections were observed using a system consisting of a Nikon Eclipse E600 microscope (Morrell Instrument Co., Melville, NY) equipped with a computer-controlled LEP BioPoint motorized stage (Ludl Electronic Products, Hawthorne, NY), a DEI-750 video camera (Optronics, Goleta, CA), and a Dell Dimension 4300 computer (Dell, Round Rock, TX).
For immunofluorescence, free-floating sections were first incubated in primary antibodies (Chemicon Aβ42 antibody, 1:150; AT8, 1:500) for 24 h at room temperature and for 40–48 h at 4°C, followed by appropriate fluorescent secondary antibodies Alexa 488 goat anti-rabbit IgG (green) and/or Alexa 546 goat anti-mouse IgG (red) (1:500; Molecular Probes, Eugene, OR) for 1 h at 37°C. Nuclei were stained using 1 µg/ml Hoechst dye in PB (Bisbenzimide H33258, Sigma-Aldrich, St Louis, MO). Images were taken using either a Leica DM IRB microscope or an Olympus IX70 microscope with a Hamamatsu digital camera.
2.5. Immuno-electron microscopy
Immuno-EM localization was performed as previously described (Takahashi et al., 2002). Free-floating sections for dual-labeling immuno-EM with Aβ42 (1:50) and AT8 (1:500) antibodies were first processed for immunoperoxidase localization with AT8 antibodies as described above. Sections then were processed by the immunogold-silver method for localization of Aβ42 antibody. For this, sections were incubated with goat anti-rabbit IgG conjugated to 1 nm gold particles (AuroProbe One; Amersham Biosciences, Arlington Heights, IL) in 0.01% gelatin and 0.08% BSA in PBS, pH 7.4, for 2 hours at room temperature. Sections were rinsed in PBS, postfixed in 2% glutaraldehyde in PBS for 10 minutes, and rinsed in PBS and 0.2 M sodium citrate buffer (pH 7.4). Conjugated gold particles were enhanced by treatment with silver solution (Amersham Biosciences). Sections were fixed in 2% osmium tetroxide in PB, dehydrated, embedded in EMBed 812, sectioned (65- to 76-nm thick), and counterstained with uranyl acetate and Reynolds’ lead citrate. Final preparations were examined using a Philips CM10 electron microscope. Illustrations were generated from a high-resolution digital imaging CCD camera system (Advanced Microscopy Techniques, Danvers, MA) and processed using Adobe Photoshop 7.0 (Adobe System, Mountain View, CA). Aβ42 or AT8 immunolabeled profiles were classified as previously described (Takahashi et al., 2002). Somata were identified by the presence of a nucleus. Dendrites contained regular microtubule arrays and were usually postsynaptic to axon terminal profiles.
3. Results
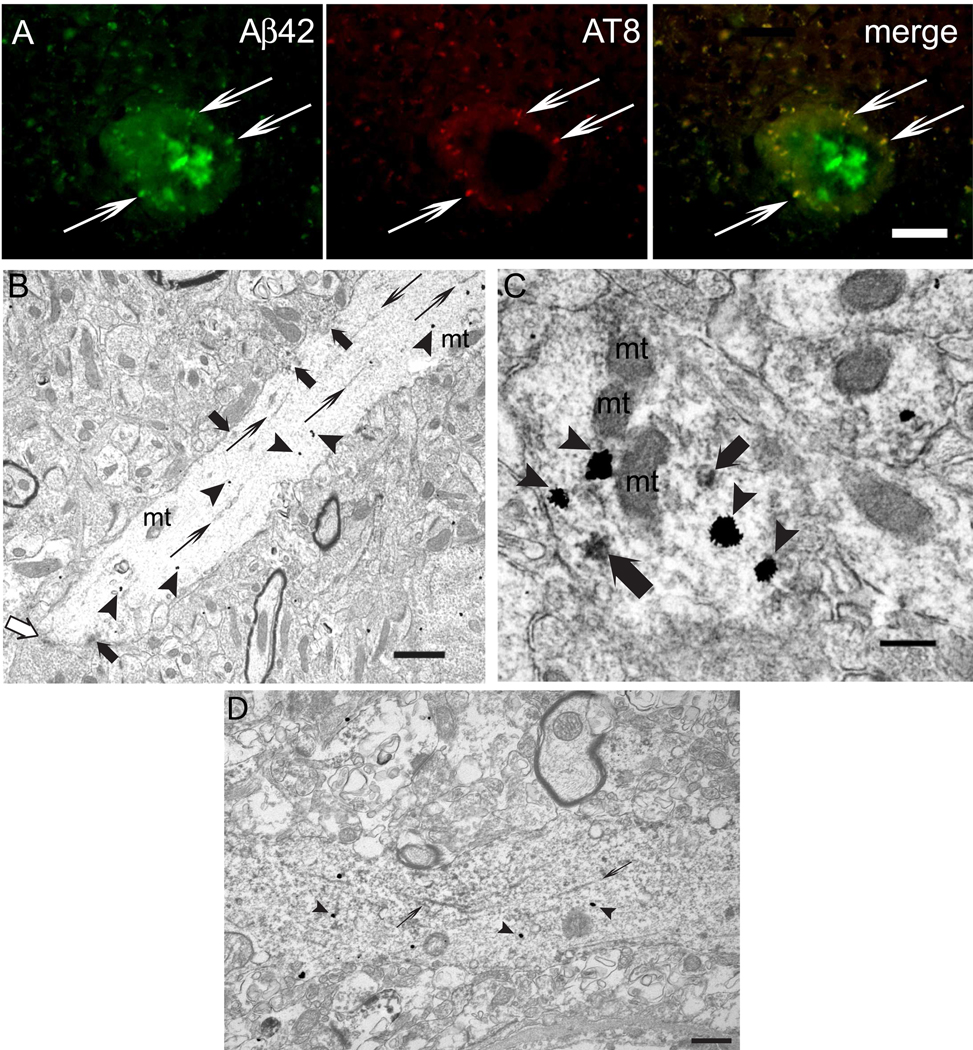
Since Aβ42 accumulates with aging especially in distal neurites of AD transgenic mouse brains (Takahashi et al., 2004; 2002), and since hyperphosphorylated tau localizes to dystrophic neurites around plaques in human subjects, as well as APP mutant transgenic mice (using antibodies AT8, PHF1, R27, R32 and Alz50) (Moechars et al., 1999; Otth et al., 2002; Sturchler-Pierrat et al., 1997), we investigated whether there was a relation between Aβ42 accumulation and hyperphosphorylated tau in dystrophic neurites of Tg2576 mice harboring Swedish mutant APP. Remarkably, hyperphosphorylated tau (using antibody AT8) co-localized with Aβ42 within dystrophic neurites around plaques (Fig. 1A). We next examined alterations in tau, considered normally to be an axonal protein, in relation to Aβ42 in dystrophic neurites around plaques in Tg2576 mice at an ultrastructural level, utilizing dual-labeling immuno-EM. Tau was hyperphosphorylated and mislocalized, near to sites of Aβ42 within dendritic profiles, including postsynaptic compartments (Fig. 1B,C). Hyperphosphorylated tau was evident on tubular-filamentous structures and in clusters associated with the microtubule network near to Aβ42 immuno-gold particles. Similar localization of hyperphosphorylated tau near sites of Aβ42 in neurites was also evident in human AD biopsy brain tissue (Fig. 1D).
Figure 1. Aβ42 accumulation and hyperphosphorylated tau localize to dystrophic neurites around plaques in Tg2576 mouse and human brain.
(A) Dystrophic neurites around amyloid plaques contained both Aβ42 peptides (antibody AB5078P) and hyperphosphorylated tau (antibody AT8). Thin arrows show examples of immunofluorescence co-localization. The core of the amyloid plaque was not labeled by AT8, and dystrophic neurites with Aβ42 labeling but no AT8 labeling were also present. (B) Dual immuno-EM with AT8 (immuno-peroxidase) and Aβ42 (gold particles) antibodies revealed AT8 labeling as filamentous structures (thin arrows) and clusters of peroxidase (thick arrows) in a distal dendrite/postsynaptic compartment accumulating Aβ42 peptides (arrowheads) in the brain of a 26-month-old Tg2576 mouse. A postsynaptic density is indicated by a white arrow. (C) Clusters of peroxidase markedly labeled by AT8 (thick arrows) in an Aβ42 peptide (arrowheads) accumulating synaptic compartment. (D) Localization of hyperphosphorylated tau (antibody AT8; immuno-peroxidase; thin arrows) near Aβ42 (immuno-gold; arrowheads) is evident in a neurite in human AD brain. Abbreviation: mt, mitochondrion. Scale bars, 25 µm (A); 1 µm (B); 250 µm (C): 500 nm (D).
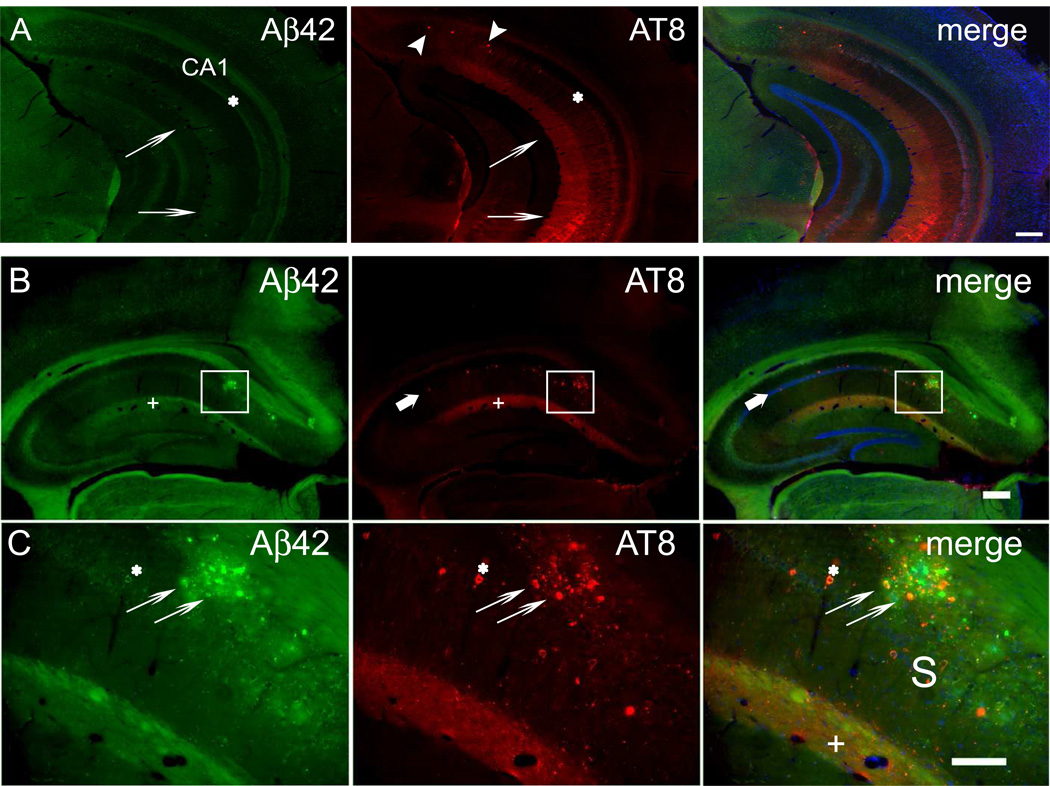
To further elucidate the relation between Aβ and tau, we next examined brain sections from 3xTg-AD mice, which develop neurofibrillary tangles as well as prominent early intraneuronal Aβ and subsequent plaques (LaFerla et al., 2007). Notably, prior to plaque formation, Aβ42 immunoreactivity in the CA1 region of the hippocampus of 5 month old 3xTg-AD mice was not only prominent in CA1 pyramidal neuron somata, but was also pronounced in the stratum lacunosum-moleculare (SLM) (Fig. 2A). The SLM contains the postsynaptic compartments of distal CA1 apical dendrites. In fact, band-like plaque deposition in the SLM of the CA1 region is often evident in images of the hippocampus in published studies on transgenic mouse models of AD. Remarkably, we noticed that hyperphosphorylated tau (AT8) was especially prominent in the SLM of CA1 in these 5-month-old 3xTg mice (Fig. 2A). A similar pattern of immunoreactivity in the SLM was also evident using additional phospho-tau specific antibodies (antibodies AT180 and 12E8; data not shown) and the abnormal tau conformational specific MC1 antibody (Supplementary Fig. 1). Such SLM staining is also evident, although not specifically commented on, in the original study characterizing tau phosphorylation in 12-month-old 3xTg mice using antibodies AT8, PHF1 and MC1 (Oddo et al., 2003). At 13 months of age, when 3xTg-AD mice deposit further plaques and intraneuronal Aβ42 declines in CA1 pyramidal somata, the SLM remained the most prominent site of both Aβ42 and hyperphosphorylated tau in the CA1 region, evident as the yellow staining (overlap of Aβ42, red, and AT8, green) of the SLM (Fig. 2B,C).
Figure 2. Aβ42 accumulation and tau hyperphosphorylation co-occur in the SLM of the CA1 region of hippocampus in 3xTg-AD mouse brain.
(A) Early Aβ42 accumulation and tau hyperphosphorylation in 5-month-old 3xTg-AD mouse brain prior to plaques and tangles. In the CA1 region of the hippocampus, Aβ42 is prominent in the pyramidal cell layer (asterisk) and in the SLM layer (thin arrows). AT8 antibody labeling of hyperphosphorylated tau is most pronounced in the SLM layer (thin arrows). A few pyramidal neurons with AT8 labeling (arrowheads) are evident in the subiculum at this age. (B) Section through the rostral hippocampus from a 13-month-old 3xTg-AD mouse labeled with Aβ42 and AT8 antibodies by immunofluorescence. Nuclei were labeled with Hoechst dye 33342 to distinguish the subiculum from the hippocampal CA1 region, where the pyramidal cell nuclei are clearly lined up. Amyloid plaques were apparent within the subiculum of the hippocampus (boxed area), but were also evident in the SLM of CA1. Both Aβ42 peptides and hyperphosphorylated tau were markedly increased in the SLM (plus signs) of CA1. Hyperphosphorylated tau (AT8) also labeled a few cell bodies within CA1 (thick arrows). (C) Higher magnification image of the subiculum from the squared area in (B) with prominent plaque pathology. Aβ42 and hyperphosphorylated tau co-localized in several pyramidal cell bodies (asterisks). Hyperphosphorylated tau and Aβ42 peptide co-localization was prominent in dystrophic neurites around a large amyloid plaque (thin arrows). Punctate co-localization of Aβ42 and hyperphosphorylated tau was also evident in the SLM (plus signs). Abbreviation: S, subiculum, SLM, stratum lacunosum-moleculare. Scale bars: 250 µm (A, B); 100 µm (C).
To determine whether Aβ42 and hyperphosphorylated tau within SLM of 3xTg-AD mice were intraneuronal and to investigate their potential subcellular relation, we again used dual-labeling immuno-EM. Remarkably, in the SLM, hyperphosphorylated tau specifically localized to CA1 dendritic profiles and their postsynaptic compartments, and in particular those demonstrating Aβ42 accumulation (Fig. 3A, B). In some Aβ42 accumulating neurites, AT8-positive filaments were tangled (Fig. 3C, inset).
Figure 3. Aβ42 accumulation and tau hyperphosphorylation localize near to each other within neurons of the hippocampus CA1 region in 3xTg-AD mouse brain using dual-labeling EM.
(A) Aβ42 gold particles (arrowheads) localized to a diffusely AT8-labeled postsynaptic compartment (thick black arrow) in the SLM of the CA1 region of hippocampus in a 13-month-old 3xTg-AD mouse. The postsynaptic compartment is recognizable by the postsynaptic density (thin arrow). (B) A dendrite/post-synaptic compartment with Aβ42 accumulation (arrowheads) revealed more localized tau hyperphosphorylation, including AT8-positive clusters (white thick arrows). Note also the synaptic compartments and distal neurites in A and B devoid of Aβ42 and AT8 labeling. Scale bars: 500 nm (A, B). (C) Image of a markedly AT8-positive apical dendrite (bottom, left inset). Higher magnification of the squared area in the inset demonstrated thick filaments immuno-labeled by antibody AT8 (thin arrows). Nearby Aβ42 peptide gold particles were associated with endosomal/smaller vesicles in the dendrite (arrowheads). AT8-labeled thick filaments were tangled (top, right inset). (D) Inset: Lower magnification image of pyramidal neuron cell somata in the CA1 region labeled with Aβ42 and AT8 antibodies. The higher magnification image of the squared area in the inset revealed Aβ42 immuno-gold particles especially in the outer membranes of multivesicular bodies (thick arrows). In contrast, AT8-labeled thick filaments (thin arrows) are present along microtubules throughout the cell body cytoplasm of the CA1 pyramidal neuron. Abbreviation: mt, mitochondrion, N, nucleus. Scale bars: 500 nm (A, B, C, D); 250 µm (inset C).
We quantified the number of identifiable dendritic terminals in a total area of 1406 µm2 in the Aβ42/AT8 dual-immuno-EM images of the SLM of 13 month-old 3xTg mouse brain. We stratified them as Aβ42-gold and AT8-peroxidase negative/negative, positive/negative, negative/positive and positive/positive. Of a total of 72 identifiable dendritic terminals, the majority (78%) were either −/− or +/+. Specifically, 48.6% were −/−, while 29.2% were +/+. Considerably fewer dendritic terminals were only positive for Aβ42 (13.9%) or only positive for AT8 (8.3%).
We noted that dendritic terminals positive for AT8 were positive throughout the length of their course within the plane of the EM section. In contrast, Aβ42 gold localized to discrete locations within post-synaptic compartments. This discrete labeling pattern could lead to an underestimation of Aβ42 relative to AT8-labeled tau. There should not be false negatives for AT8, while false negatives are expected for Aβ if gold labeling is either above or below the plane of section in a given dendritic terminal. Additional factors resulting in underestimation of Aβ42 relative to AT8 include: (1) immuno-gold labeling identifies less antigen than immuno-peroxidase using our dual-immuno EM method. (2) Aβ42 antibodies have conformational specificity, so that not all Aβ species are detected. We previously showed that another Aβ42 end-specific antibody predominantly reacts to Aβ42 monomers (Takahashi et al., 2004). The Chemicon Aβ42 specific antibody similarly reacts preferentially to monomers on Western blot (data not shown).
Overall, the Aβ42/AT8 dual immuno-EM supports the conclusion of co-occurrence of Aβ and tau pathologies in dendritic terminals of the SLM. In contrast to labeling of dendrites, Aβ42 and AT8 did not co-occur in axons/pre-synaptic terminals in the SLM of 13-month-old 3xTg-AD mice.
Decline in the level of intracellular Aβ42 in neuron somata with plaque pathology (Gouras et al., 2000; Mori et al., 2002; Oddo et al., 2003) has been a critique regarding the importance of intraneuronal Aβ (Duyckaerts et al., 2008). It was proposed that intraneuronal Aβ42 declines from the emergence of extracellular Aβ plaques (LaFerla et al., 2007). Alternatively, peptide competition from abundant Aβ42 within plaques was hypothesized to lead to the erroneous appearance of reduced intraneuronal Aβ42 (Gouras et al., 2005). To test this latter possibility, we now co-processed CA1 hippocampal sections of 3xTg-AD mice with marked intraneuronal Aβ42 either with sections with or without plaques. Co-incubation with plaque-containing compared to non-plaque-containing sections had no influence on intraneuronal Aβ42 (Supplementary Fig. 2). This new data argue against peptide competition and confirm the age-related reduction of intraneuronal Aβ42 in cell bodies. Indeed, immuno-EM studies emphasized age-related intraneuronal Aβ42 accumulation within dystrophic neurites and synaptic compartments rather than in cell bodies of Tg2576 mice with aging (Takahashi et al., 2002). In contrast to Aβ42 accumulation, hyperphosphorylated tau (AT8) labeling is present throughout the cytoskeleton in vulnerable CA1 pyramidal neuron cell bodies and their apical dendrites by immuno-EM in older 3xTg-AD mice (Fig. 3D).
4. Discussion
The relationship between Aβ and tau is a central question in AD research, and synaptic alterations are the best correlate of cognitive dysfunction in AD (Coleman and Yao, 2003). Although plaques and tangles are not obviously linked to synapses, our work shows that Aβ and tau pathologies co-occur at synapses. Taking advantage of the welldefined anatomy of the hippocampus, we found that Aβ42 accumulation and the mislocalization and hyperphosphorylation of tau in the CA1 region occurred early and prominently in distal dendrites and postsynaptic compartments of pyramidal neuron apical dendrites in the SLM of 3xTg-AD mice. Ultrastructural analysis of the SLM was required to show that these events were in fact intracellular. Our data emphasize synapses not only as early sites of Aβ42 accumulation but now also link the initiation of tau pathology to Aβ42 accumulation in postsynaptic terminals.
We highlight that with age, intraneuronal Aβ42 decreases in cell bodies but not in dystrophic neurites and synapses. In contrast, tau phosphorylation is maintained throughout cell bodies and apical dendrites in 3xTg-AD mice. Our data provide new insights into the apparent anatomical dissociation of plaque and tangle pathologies in AD. Aβ42 accumulates progressively in distal processes whereas after initial increases, Aβ42 declines in cell bodies, with plaques developing preferentially in distal processes and synapses. In contrast, hyperphosphorylated tau develops throughout processes and cell bodies, with tangles eventually developing in neuron cell bodies and neuropil threads in neurites. We hypothesize that extracellular release of Aβ from degenerating neurites might up-regulate Aβ42 (Glabe, 2001) within adjacent synaptic compartments, leading to the synaptic spread of AD. Our present data emphasizes pathology in postsynaptic CA1 apical dendrites, because of the well-defined anatomy of the hippocampus, although post- and also pre-synaptic compartments with intraneuronal Aβ42 accumulation develop at other sites.
In summary, we show that tau alterations are spatially associated with intraneuronal Aβ accumulation, providing a link between plaques and tangles, the two neuropathologic hallmarks of AD. Aβ and tau pathologies develop in synapses, a key observation given the importance of synaptic alterations in cognitive dysfunction in AD. The spatial association of Aβ accumulation with tau pathology within neurites and synaptic compartments reinforces the importance of intraneuronal Aβ accumulation in AD.
Supplementary Material
Sections of 3xTg-AD mouse hippocampus show abnormal tau conformation with MC1 antibody already in 5-month-old 3xTg-AD compared to wild type mice (A, B). Notably, abnormal tau conformation immunoreactivity was not only prominent in CA1 pyramidal neuron somata, but was also pronounced in the SLM (plus sign). Progressively increased MC1 immunoreactivity was observed at 10 month of age. (C). No positive labeling was observed in 5 or 10 month-old wild-type mice (B, D). Insets: Higher magnification of the SLM. Abbreviation: SLM, stratum lacunosum-moleculare. Scale bar: 100 µm.
Sections of AD transgenic mouse CA1 hippocampus with high intraneuronal Aβ42 staining (5 month old 3xTg-AD mouse; A and C) were co-processed either with sections without plaques (2 month old Tg2576 mouse; B) or with high plaque burden (23 month old Tg2576 mouse, D). Co-incubation with the high plaque-containing section did not reduce levels of intraneuronal Aβ42 in CA1 pyramidal neurons of 3xTg-AD mouse (C) compared to co-incubation with the no plaque-containing section (A), arguing against peptide competition from Aβ42 within plaques as a cause of reduced intraneuronal Aβ42 immunoreactivity. Note also the age-related reduction of intraneuronal Aβ42 in the CA1 neurons in the section from the 23 month-old compared to the 2 month-old Tg2576 mouse. Scale bar: 100 µm.
Acknowledgements
Supported by an Alzheimer’s Association Zenith award and National Institutes of Health grants NS045677, AG027140, AG028174 (GKG), and HL18974 (TAM). We thank Drs. Claudia Almeida, Jordi Magrane and Davide Tampellini for helpful discussions. We thank Drs. Frank LaFerla, University of California at Irvine, and Karen H. Ashe, University of Minnesota, for the 3xTg-AD and Tg2576 mice, respectively. We thank Dr. Peter Davies, Albert Einstein University School of Medicine for providing tau antibody MC1 and Dr. Peter Seubert, Elan Pharmaceuticals, for tau antibody 12E8.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement
All authors disclose that there are no actual or potential conflicts of interest including any financial, personal or other relationship with other people or organizations.
References
- Alafuzoff I, Pikkarainen M, Arzberger T, Thal DR, Al-Sarraj S, Bell J, Bodi I, Budka H, Capetillo-Zarate E, Ferrer I, Gelpi E, Gentleman S, Giaccone G, Kavantzas N, King A, Korkolopoulou P, Kovacs GG, Meyronet D, Monoranu C, Parchi P, Patsouris E, Roggendorf W, Stadelmann C, Streichenberger N, Tagliavini F, Kretzschmar H. Inter-laboratory comparison of neuropathological assessments of beta-amyloid protein: a study of the BrainNet Europe consortium. Acta Neuropathol. 2008;115:533–546. doi: 10.1007/s00401-008-0358-2. [DOI] [PubMed] [Google Scholar]
- Almeida CG, Takahashi RH, Gouras GK. Beta-amyloid accumulation impairs multivesicular body sorting by inhibiting the ubiquitin-proteasome system. J. Neurosci. 2006;26:4277–4288. doi: 10.1523/JNEUROSCI.5078-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer TA, Wirths O. Review on the APP/PS1KI mouse model: intraneuronal Abeta accumulation triggers axonopathy, neuron loss and working memory impairment. Genes. Brain Behav. 2008;7 Suppl 1:6–11. doi: 10.1111/j.1601-183X.2007.00372.x. [DOI] [PubMed] [Google Scholar]
- Billings LM, Oddo S, Green KN, McGaugh JL, LaFerla FM. Intraneuronal Abeta causes the onset of early Alzheimer's disease-related cognitive deficits in transgenic mice. Neuron. 2005;45:675–688. doi: 10.1016/j.neuron.2005.01.040. [DOI] [PubMed] [Google Scholar]
- Busciglio J, Pelsman A, Wong C, Pigino G, Yuan M, Mori H, Yankner BA. Altered metabolism of the amyloid beta precursor protein is associated with mitochondrial dysfunction in Down's syndrome. Neuron. 2002;33:677–688. doi: 10.1016/s0896-6273(02)00604-9. [DOI] [PubMed] [Google Scholar]
- Cataldo AM, Petanceska S, Terio NB, Peterhoff CM, Durham R, Mercken M, Mehta PD, Buxbaum J, Haroutunian V, Nixon RA. Abeta localization in abnormal endosomes: association with earliest Abeta elevations in AD and Down syndrome. Neurobiol. Aging. 2004;25:1263–1272. doi: 10.1016/j.neurobiolaging.2004.02.027. [DOI] [PubMed] [Google Scholar]
- Chapman PF, White GL, Jones MW, Cooper-Blacketer D, Marshall VJ, Irizarry M, Younkin L, Good MA, Bliss TV, Hyman BT, Younkin SG, Hsiao KK. Impaired synaptic plasticity and learning in aged amyloid precursor protein transgenic mice. Nat. Neurosci. 1999;2:271–276. doi: 10.1038/6374. [DOI] [PubMed] [Google Scholar]
- Coleman PD, Yao PJ. Synaptic slaughter in Alzheimer's disease. Neurobiol. Aging. 2003;24:1023–1027. doi: 10.1016/j.neurobiolaging.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Cruz JC, Kim D, Moy LY, Dobbin MM, Sun X, Bronson RT, Tsai LH. p25/cyclin-dependent kinase 5 induces production and intraneuronal accumulation of amyloid beta in vivo. J. Neurosci. 2006;26:10536–10541. doi: 10.1523/JNEUROSCI.3133-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Andrea MR, Nagele RG, Wang HY, Peterson PA, Lee DH. Evidence that neurones accumulating amyloid can undergo lysis to form amyloid plaques in Alzheimer's disease. Histopathology. 2001;38:120–134. doi: 10.1046/j.1365-2559.2001.01082.x. [DOI] [PubMed] [Google Scholar]
- Duyckaerts C, Potier MC, Delatour B. Alzheimer disease models and human neuropathology: similarities and differences. Acta Neuropathol. 2008;115:5–38. doi: 10.1007/s00401-007-0312-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echeverria V, Ducatenzeiler A, Dowd E, Janne J, Grant SM, Szyf M, Wandosell F, Avila J, Grimm H, Dunnett SB, Hartmann T, Alhonen L, Cuello AC. Altered mitogen-activated protein kinase signaling, tau hyperphosphorylation and mild spatial learning dysfunction in transgenic rats expressing the beta-amyloid peptide intracellularly in hippocampal and cortical neurons. Neuroscience. 2004;129:583–592. doi: 10.1016/j.neuroscience.2004.07.036. [DOI] [PubMed] [Google Scholar]
- Glabe C. Intracellular mechanisms of amyloid accumulation and pathogenesis in Alzheimer's disease. J. Mol. Neurosci. 2001;17:137–145. doi: 10.1385/JMN:17:2:137. [DOI] [PubMed] [Google Scholar]
- Gotz J, Chen F, van Dorpe J, Nitsch RM. Formation of neurofibrillary tangles in P301l tau transgenic mice induced by Abeta 42 fibrils. Science. 2001;293:1491–1495. doi: 10.1126/science.1062097. [DOI] [PubMed] [Google Scholar]
- Gouras GK, Almeida CG, Takahashi RH. Intraneuronal Abeta accumulation and origin of plaques in Alzheimer's disease. Neurobiol. Aging. 2005;26:1235–1244. doi: 10.1016/j.neurobiolaging.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Gouras GK, Tsai J, Naslund J, Vincent B, Edgar M, Checler F, Greenfield JP, Haroutunian V, Buxbaum JD, Xu H, Greengard P, Relkin NR. Intraneuronal Abeta42 accumulation in human brain. Am. J. Pathol. 2000;156:15–20. doi: 10.1016/s0002-9440(10)64700-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyure KA, Durham R, Stewart WF, Smialek JE, Troncoso JC. Intraneuronal abeta-amyloid precedes development of amyloid plaques in Down syndrome. Arch. Pathol. Lab. Med. 2001;125:489–492. doi: 10.5858/2001-125-0489-IAAPDO. [DOI] [PubMed] [Google Scholar]
- Holcomb L, Gordon MN, McGowan E, Yu X, Benkovic S, Jantzen P, Wright K, Saad I, Mueller R, Morgan D, Sanders S, Zehr C, O'Campo K, Hardy J, Prada CM, Eckman C, Younkin S, Hsiao K, Duff K. Accelerated Alzheimer-type phenotype in transgenic mice carrying both mutant amyloid precursor protein and presenilin 1 transgenes. Nat. Med. 1998;4:97–100. doi: 10.1038/nm0198-097. [DOI] [PubMed] [Google Scholar]
- Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- Jicha GA, Lane E, Vincent I, Otvos L, Jr, Hoffmann R, Davies P. A conformation- and phosphorylation-dependent antibody recognizing the paired helical filaments of Alzheimer's disease. J. Neurochem. 1997;69:2087–2095. doi: 10.1046/j.1471-4159.1997.69052087.x. [DOI] [PubMed] [Google Scholar]
- Kamal A, Almenar-Queralt A, LeBlanc JF, Roberts EA, Goldstein LS. Kinesin-mediated axonal transport of a membrane compartment containing beta-secretase and presenilin-1 requires APP. Nature. 2001;414:643–648. doi: 10.1038/414643a. [DOI] [PubMed] [Google Scholar]
- Knobloch M, Konietzko U, Krebs DC, Nitsch RM. Intracellular Abeta and cognitive deficits precede beta-amyloid deposition in transgenic arcAbeta mice. Neurobiol. Aging. 2007;28:1297–1306. doi: 10.1016/j.neurobiolaging.2006.06.019. [DOI] [PubMed] [Google Scholar]
- LaFerla FM, Green KN, Oddo S. Intracellular amyloid-beta in Alzheimer's disease. Nat. Rev. Neurosci. 2007;8:499–509. doi: 10.1038/nrn2168. [DOI] [PubMed] [Google Scholar]
- Lewis J, Dickson DW, Lin WL, Chisholm L, Corral A, Jones G, Yen SH, Sahara N, Skipper L, Yager D, Eckman C, Hardy J, Hutton M, McGowan E. Enhanced neurofibrillary degeneration in transgenic mice expressing mutant tau and APP. Science. 2001;293:1487–1491. doi: 10.1126/science.1058189. [DOI] [PubMed] [Google Scholar]
- Litersky JM, Johnson GV, Jakes R, Goedert M, Lee M, Seubert P. Tau protein is phosphorylated by cyclic AMP-dependent protein kinase and calcium/calmodulin-dependent protein kinase II within its microtubule-binding domains at Ser-262 and Ser-356. Biochem. J. 1996;316(Pt 2):655–660. doi: 10.1042/bj3160655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord A, Kalimo H, Eckman C, Zhang XQ, Lannfelt L, Nilsson LN. The Arctic Alzheimer mutation facilitates early intraneuronal Abeta aggregation and senile plaque formation in transgenic mice. Neurobiol. Aging. 2006;27:67–77. doi: 10.1016/j.neurobiolaging.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Milner TA, Lee A, Aicher SA, Rosin DL. Hippocampal alpha2a-adrenergic receptors are located predominantly presynaptically but are also found postsynaptically and in selective astrocytes. J. Comp. Neurol. 1998;395:310–327. [PubMed] [Google Scholar]
- Moechars D, Dewachter I, Lorent K, Reverse D, Baekelandt V, Naidu A, Tesseur I, Spittaels K, Haute CV, Checler F, Godaux E, Cordell B, Van Leuven F. Early phenotypic changes in transgenic mice that overexpress different mutants of amyloid precursor protein in brain. J. Biol. Chem. 1999;274:6483–6492. doi: 10.1074/jbc.274.10.6483. [DOI] [PubMed] [Google Scholar]
- Mori C, Spooner ET, Wisniewsk KE, Wisniewski TM, Yamaguch H, Saido TC, Tolan DR, Selkoe DJ, Lemere CA. Intraneuronal Abeta42 accumulation in Down syndrome brain. Amyloid. 2002;9:88–102. [PubMed] [Google Scholar]
- Oakley H, Cole SL, Logan S, Maus E, Shao P, Craft J, Guillozet-Bongaarts A, Ohno M, Disterhoft J, Van Eldik L, Berry R, Vassar R. Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer's disease mutations: potential factors in amyloid plaque formation. J. Neurosci. 2006;26:10129–10140. doi: 10.1523/JNEUROSCI.1202-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oddo S, Billings L, Kesslak JP, Cribbs DH, LaFerla FM. Abeta immunotherapy leads to clearance of early, but not late, hyperphosphorylated tau aggregates via the proteasome. Neuron. 2004;43:321–332. doi: 10.1016/j.neuron.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP, Akbari Y, LaFerla FM. Triple-transgenic model of Alzheimer's disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron. 2003;39:409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- Ohyagi Y, Asahara H, Chui DH, Tsuruta Y, Sakae N, Miyoshi K, Yamada T, Kikuchi H, Taniwaki T, Murai H, Ikezoe K, Furuya H, Kawarabayashi T, Shoji M, Checler F, Iwaki T, Makifuchi T, Takeda K, Kira J, Tabira T. Intracellular Abeta42 activates p53 promoter: a pathway to neurodegeneration in Alzheimer's disease. Faseb J. 2005;19:255–257. doi: 10.1096/fj.04-2637fje. [DOI] [PubMed] [Google Scholar]
- Otth C, Concha II, Arendt T, Stieler J, Schliebs R, Gonzalez-Billault C, Maccioni RB. AbetaPP induces cdk5-dependent tau hyperphosphorylation in transgenic mice Tg2576. J. Alzheimers Dis. 2002;4:417–430. doi: 10.3233/jad-2002-4508. [DOI] [PubMed] [Google Scholar]
- Roberson ED, Scearce-Levie K, Palop JJ, Yan F, Cheng IH, Wu T, Gerstein H, Yu GQ, Mucke L. Reducing endogenous tau ameliorates amyloid beta-induced deficits in an Alzheimer's disease mouse model. Science. 2007;316:750–754. doi: 10.1126/science.1141736. [DOI] [PubMed] [Google Scholar]
- Sheng JG, Bora SH, Xu G, Borchelt DR, Price DL, Koliatsos VE. Lipopolysaccharide-induced-neuroinflammation increases intracellular accumulation of amyloid precursor protein and amyloid beta peptide in APPswe transgenic mice. Neurobiol. Dis. 2003;14:133–145. doi: 10.1016/s0969-9961(03)00069-x. [DOI] [PubMed] [Google Scholar]
- Shie FS, LeBoeuf RC, Jin LW. Early intraneuronal Abeta deposition in the hippocampus of APP transgenic mice. Neuroreport. 2003;14:123–129. doi: 10.1097/01.wnr.0000051151.87269.7d. [DOI] [PubMed] [Google Scholar]
- Stokin GB, Lillo C, Falzone TL, Brusch RG, Rockenstein E, Mount SL, Raman R, Davies P, Masliah E, Williams DS, Goldstein LS. Axonopathy and transport deficits early in the pathogenesis of Alzheimer's disease. Science. 2005;307:1282–1288. doi: 10.1126/science.1105681. [DOI] [PubMed] [Google Scholar]
- Sturchler-Pierrat C, Abramowski D, Duke M, Wiederhold KH, Mistl C, Rothacher S, Ledermann B, Burki K, Frey P, Paganetti PA, Waridel C, Calhoun ME, Jucker M, Probst A, Staufenbiel M, Sommer B. Two amyloid precursor protein transgenic mouse models with Alzheimer disease-like pathology. Proc. Natl. Acad. Sci. U. S. A. 1997;94:13287–13292. doi: 10.1073/pnas.94.24.13287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi RH, Almeida CG, Kearney PF, Yu F, Lin MT, Milner TA, Gouras GK. Oligomerization of Alzheimer's beta-amyloid within processes and synapses of cultured neurons and brain. J. Neurosci. 2004;24:3592–3599. doi: 10.1523/JNEUROSCI.5167-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi RH, Milner TA, Li F, Nam EE, Edgar MA, Yamaguchi H, Beal MF, Xu H, Greengard P, Gouras GK. Intraneuronal Alzheimer abeta42 accumulates in multivesicular bodies and is associated with synaptic pathology. Am. J. Pathol. 2002;161:1869–1879. doi: 10.1016/s0002-9440(10)64463-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Broeck B, Vanhoutte G, Pirici D, Van Dam D, Wils H, Cuijt I, Vennekens K, Zabielski M, Michalik A, Theuns J, De Deyn PP, Van der Linden A, Van Broeckhoven C, Kumar-Singh S. Intraneuronal amyloid beta and reduced brain volume in a novel APP T714I mouse model for Alzheimer's disease. Neurobiol. Aging. 2008;29:241–252. doi: 10.1016/j.neurobiolaging.2006.10.016. [DOI] [PubMed] [Google Scholar]
- Wirths O, Multhaup G, Czech C, Blanchard V, Moussaoui S, Tremp G, Pradier L, Beyreuther K, Bayer TA. Intraneuronal Abeta accumulation precedes plaque formation in beta-amyloid precursor protein and presenilin-1 double-transgenic mice. Neurosci. Lett. 2001;306:116–120. doi: 10.1016/s0304-3940(01)01876-6. [DOI] [PubMed] [Google Scholar]
- Zerbinatti CV, Wahrle SE, Kim H, Cam JA, Bales K, Paul SM, Holtzman DM, Bu G. Apolipoprotein E and low density lipoprotein receptor-related protein facilitate intraneuronal Abeta42 accumulation in amyloid model mice. J. Biol. Chem. 2006;281:36180–36186. doi: 10.1074/jbc.M604436200. [DOI] [PubMed] [Google Scholar]
- Zheng H, Jiang M, Trumbauer ME, Sirinathsinghji DJ, Hopkins R, Smith DW, Heavens RP, Dawson GR, Boyce S, Conner MW, Stevens KA, Slunt HH, Sisoda SS, Chen HY, Van der Ploeg LH. beta-Amyloid precursor protein-deficient mice show reactive gliosis and decreased locomotor activity. Cell. 1995;81:525–531. doi: 10.1016/0092-8674(95)90073-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sections of 3xTg-AD mouse hippocampus show abnormal tau conformation with MC1 antibody already in 5-month-old 3xTg-AD compared to wild type mice (A, B). Notably, abnormal tau conformation immunoreactivity was not only prominent in CA1 pyramidal neuron somata, but was also pronounced in the SLM (plus sign). Progressively increased MC1 immunoreactivity was observed at 10 month of age. (C). No positive labeling was observed in 5 or 10 month-old wild-type mice (B, D). Insets: Higher magnification of the SLM. Abbreviation: SLM, stratum lacunosum-moleculare. Scale bar: 100 µm.
Sections of AD transgenic mouse CA1 hippocampus with high intraneuronal Aβ42 staining (5 month old 3xTg-AD mouse; A and C) were co-processed either with sections without plaques (2 month old Tg2576 mouse; B) or with high plaque burden (23 month old Tg2576 mouse, D). Co-incubation with the high plaque-containing section did not reduce levels of intraneuronal Aβ42 in CA1 pyramidal neurons of 3xTg-AD mouse (C) compared to co-incubation with the no plaque-containing section (A), arguing against peptide competition from Aβ42 within plaques as a cause of reduced intraneuronal Aβ42 immunoreactivity. Note also the age-related reduction of intraneuronal Aβ42 in the CA1 neurons in the section from the 23 month-old compared to the 2 month-old Tg2576 mouse. Scale bar: 100 µm.