Summary
Recurrent Respiratory Papillomatosis (RRP) is a rare disease of the larynx caused by infection with human papillomaviruses (HPV) -6 or -11, associated with significant morbidity and on occasion mortality. Here we summarized our current understanding of the permissive adaptive and innate responses made by patients with RRP that support chronic HPV infection and prevent immune clearance of these viruses. Furthermore, we provide new evidence of TH2-like polarization in papillomas and blood of patients with RRP, restricted CD4 and CD8 Vβ repertoires, the effect of HPV-11 early protein E6 on T-cell alloreactivity, enriched Langerhans cell presence in papillomas, and evidence that natural killer (NK) cells are dysfunctional in RRP. We review the immunogenetics that regulate the dysfunctional responses made by patients with RRP in response to HPV infection of the upper airway. In addition, we are identifying T-cell epitopes on HPV-11 early proteins, in the context of HLA class II alleles enriched in RRP that should help generate a therapeutic vaccine. Taken together, RRP is a complex, multigene disease manifest as a tissue and HPV-specific, immune deficiency that prevents effective clearance and/or control of HPV-6 and -1 infection.
Introduction
Epidemiology of RRP
HPV infection causes RRP
Recurrent laryngeal papillomatosis (RRP) is a disease predominately caused by human papillomaviruses (HPV)-6 and -11 (1-4). These HPV types are part of a family of ubiquitous HPV viruses to which virtually all individuals have been exposed (5, 6). It is estimated that 5% of all individuals have evidence of HPV infection in the larynx (7), yet have no sign of clinical disease. HPV-6 and -11 cause benign lesions in the airway and skin, and are classified as “low risk” HPVs, compared to the “high risk” HPVs, 16 and 18, that cause the majority of cervical cancers (8). Additionally, HPV-11 may have a more aggressive clinical course and is associated with a younger age of diagnosis compared to those patients infected with HPV-6 (9, 10), although this is controversial(7).
Age, Gender, Incidence, and Prevalence, of Patients with RRP
The distribution of cases of RRP is bimodal, with an initial peak in childhood with a second peak in adulthood, ages 20-40. Children with RRP are most often diagnosed at 2-3 years of age (4, 11). In children, the male-to-female ratio is approximately equal(12) however, in adults, the male-to-female ratio is estimated to be 4:1 (12). Childhood onset for RRP is more common and more aggressive than in adults (12). Most children with RRP are the first born of young mothers and are from families with low economic status (13) while other studies have shown no association with socioeconomic status (14). Based on a survey of otolaryngologists in the United States, an estimate of the number of new cases of childhood-onset RRP has been reported as 1,500-2,500 each year(15). The incidence in the United States among children <14 year of age is estimated to be 4.3 per 105 (15) and among adults, 1.8/105 (16). In addition, the prevalence of juvenile RRP reported in two US centers (Seattle and Atlanta) was 1.69/105 and 2.59/105 respectively in the year 2000 without significant differences when stratified by sex or race (4). Extrapolation from these estimates to the United States (US) population suggested that 80-1500 incident cases and 700-3000 prevalent cases of juvenile RRP occurred in 1999 (4). An estimate of the annual incidence of pediatric RRP in Denmark was 0.4/105 for children less than 20 years of age (17) and 0.6/105 for children less than 14 years old(18). Since patients can require as many as 100 surgical procedures to maintain a patent airway (19-21), roughly 15,000 surgical procedures are performed each year for patients with at an estimated cost of greater than $100 million US dollars/year (16, 22).
Disease Severity of Patients with RRP
RRP can cause significant morbidity and on occasion mortality, secondary to the strategic location of papillomas in the airway, infrequent extension of disease into the trachea and lungs (21, 23) and more infrequent, malignant transformation (24, 25). Patients with RRP have a variable course of disease, with some cases never recurring after the first presentation, others having a mild disease that rarely recurs, and still others developing a severe disease with frequent recurrences of papillomas that require surgical removal as often as every 3-4 weeks.
Thus, in order to associate immunologic parameters with disease severity, we subdivided RRP into two categories, severe and mild-moderate disease, based on extent of disease at the time of surgery and the frequency of recurrence. At each surgery, the number of disease sites, the anatomic surface area of disease, and the extent of luminal obstruction is documented to yield a composite score as previously described (26). This composite score is divided by the number of days that had elapsed since the previous surgery to yield a growth rate which is a measurement of disease severity. The mean growth rate from multiple surgeries is used to define the overall severity score for an individual patient. An overall disease severity score of ≥ 0.06, or the presence of tracheal extension, is defined as severe disease. An overall severity score of < 0.06, and no tracheal extension is defined as mild-moderate disease (21, 26, 27). This scoring system has been used throughout all of the clinical studies at the Long Island Jewish Medical Center and was developed using a large cohort of patients (n >150) (26). There is clinical variability between patients, but only rarely observed within a given patient (1-3, 21). Because there is minimal variation in patients' scores over time, using the mean score for this large number of patients further improves the reliability of this variable (28).
RRP, an Immunologic Enigma
It is estimated that 5% of the general population without evidence of RRP has detectable HPV DNA in their larynx (7). Thus, it is unclear why a very small fraction of HPV-exposed individuals develop RRP (7), and why still fewer develop an unrelenting and severe course of disease. A central question that needs to be answered is: “How does the complex innate and adaptive immune response made by patients with RRP to infection with HPV-6 and -11 differ from that of individuals who are also infected with these HPVs but never develop RRP?”
It is clear that patients with RRP mount an immune response that is initially manifest by the production of measurable serum antibodies to these viruses (29-32). This documents immune recognition of these viruses by patients with RRP, which refutes the contention that these HPVs induce a “subliminal” immune response resulting in “clonal ignorance” that prevents an appropriate innate and adaptive immune response to these viruses. More likely, these patients establish “low level” tolerance (33) to the initial HPV-6 or -11 infection and respond to small amounts of HPV proteins expressed by HPV-infected keratinocytes, and because on their genetic predisposition, develop an exaggerated tolerogenic response to these viruses, rather than anti-HPV responses that effectively clear or contain them. Thus, understanding the mechanism(s) by which HPV-6 and -11 polarizes the immune response towards tolerance in RRP, as opposed to the development of cell-mediated immune clearance of these viruses, is critical in developing novel therapies that would prevent disease recurrence and/or reduce disease severity.
Early attempts at immune-based therapy have largely been centered around clinical trials of interferon alpha (IFN-α). These studies showed that disease-free intervals could be obtained on this therapy, but could not be sustained long-term (34, 35), and thus suggested that patients with RRP may not generate a sufficient IFN-α response to HPV infection. They also implicated a defect in innate immunity in RRP, given that plasmacytoid DCs are a major source of IFN-α (36). In addition, more recent studies have confirmed the short-term benefits of IFN-α therapy, yet this beneficial effect may be outweighed in patients infected with HPV-11 because they show poor long-term response to IFN-α, and they appear to have a higher incidence of malignant conversion following therapy, compared with those infected with HPV-6 (37, 38).
Immunologic Phenotype of Patients with RRP
Of interest, even in patients with RRP who have severe disease, peripheral blood immunophenotypes, including the frequency of CD4+ T-helper cells, CD8+ T-cytotoxic cells, B-cells, and NK-cells, are comparable to those of unaffected controls (28). In addition, patients with RRP have normal responses to other pathogens, in that they do not manifest any other chronic infections, and their cellular immune proliferative responses to mitogens were normal. In our clinical experience, only one RRP patient has had concomitant HIV-1 infection, and there have been no cases of coincidental cervical cancer (39). This suggests that the local HPV-specific immune responses that are permissive of chronic HPV-6/11 infection in the airway are likely “site specific” and thus, restricted in that anatomic site and are not common to other mucosal sites, such as the endocervix, where HPV-16 and -18 commonly cause disease. Thus, these immune defects in controling HPV6/11 infection in the airway are not likely to be transferred to other mucosal sites, since the incidence of chronic HPV infection in different epithelial sites in the same individual has not been described to our knowledge.
Model of the Polarized Immune Responses Made to HPV-6 and 11 in RRP
We hypothesized that an inhibitory cycle of immunocytes composed of several T-cell subpopulations, macrophages, and dendritic cells that produce inhibitory and regulatory cytokines and chemokines, together block effector TH1-like responses to HPV-6 and -11 in the upper airway in patients with RRP, see Figure 1. Furthermore, peptides from HPV early proteins E6 and E2, presented in the contex of select HLA class II molecules by professional APCs, induce and perpetuate memory TH2-like cells that express IL-4 and IL-10. This inhibitory cytokine milieu of IL-10 and IL-4, without counteracting IFN-γ (40, 41), likely polarizes resting macrophages (Mϕs) to become alternatively activated macrophages (AAMϕs) that express chemotactic, TH2-like, chemokines, CCL18 and CCL17 (42), and the immunosuppressive cytokine IL-10. This in turn, polarizes naïve T-cells to become regulatory T-cells, such as CD4+ CD25+ CD127low Foxp3+ Tregs, (43) and memory TH2-like, T-cells that express IL-10 and TGF-β. These cells would form a cycle of inhibitory immunocytes that when present together, inhibit HPV-specific, TH1-like T-cell function. Thus, HPV-infected keratinocytes would not be cleared, and papillomas would recur at a frequency dependent on a patient's HLA class II immune response genes and KIR gene haplotypes, see Immunogenetics of RRP and Innate Immunity of RRP below. This model is supported by evidence from our laboratory (28, 40-46), and that of others (47, 48).
Figure 1. A model of RRP Inhibitory Cycle.
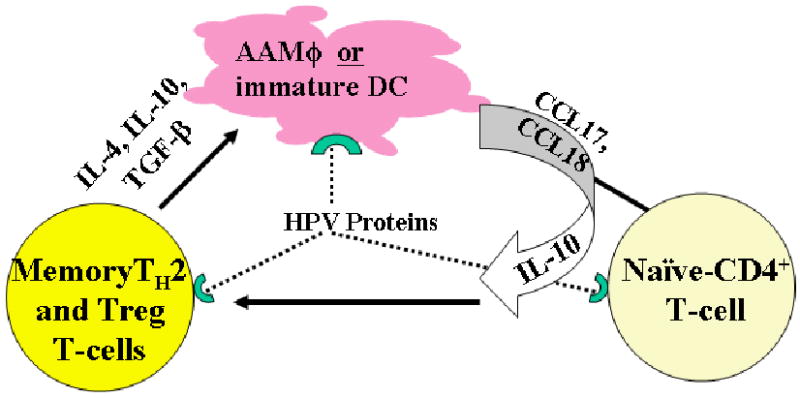
This model shows the inhibitory cycle of cells and mediators that are generated by HPV proteins in patients with RRP that are described in this review. Polarized macrophages (AAMΦ)/immature dendritic cells (iDCs), memory TH2-like T-cells and Tregs, generated from naïve T-cells that express TH2-like/T-regulatory cytokines and chemokines in response to HPV protein drive this cycle. Memory TH2-like T-cells express IL-4 and IL-10, and T-regulatory cells express IL-10 and TGFβ in response to HPV proteins are essential in the polarization of resting Mϕs to become AAMϕs that express CCL17, CCL18, and IL-10 which recruit and polarize naïve CD4+ T-cells to become Tregs and memory TH2-like T-cells. This establishes and perpetuates an inhibitory cycle by immunocytes that suppress effective anti-HPV TH1-like function
Not shown in Figure 1 is the role of innate immune cells, such as NK cells and their KIR gene products, that have been recently described (46). NK cells are present in papillomas, yet fail to clear keratinocytes with absent HLA class I expression (28). Our previous reports showed that HPV E7 likely suppresses HLA class I expression by blocking transporter associated with antigen presentation (TAP) function (20), yet NK recognition and/or cytolysis of these HLA class I deficient keratinocytes is impaired in RRP, see below.
HPV prevents an effective anti-viral T-cell response
Recent observations suggest that HPVs are part of the commensal microflora of human epithelia, held in check by a competent immune system, that can be activated under immunosuppressive conditions (5). However, some individuals infected with HPV-6 and -11, make immune responses that support chronic HPV infection. Indeed, the immunologic hallmark of HPV-induced diseases, including RRP, cervical cancer and skin warts, is the conspicuous absence of HPV-specific, cytotoxic T-lymphocytes (CTL), and helper type 1 CD4+ T-cells (TH1-like) that secrete inflammatory cytokines such as IFN-γ, IL-2, and TNF-α. While TH1-like T-cells are critical in generating effective anti-viral T-cell responses (49, 50), other T-cell populations suppress the inflammatory responses induced by TH1-like cells by expressing IL-4. These TH2-like and regulatory CD4+ T-cells can also express the immunoregulatory cytokines IL-10 and TGF-β, as do suppressor CD8+ T-cells (TC2-like cells) that also suppress TH1-like CD4+T-cells (50-54). The identification of these TH2-like and regulatory CD4+ T-cells and their function is discussed below.
The Adaptive TH1-/TH-2 Cytokine Balance in RRP
We have shown that RRP is a TH2-like disease characterized by the polarized expression of a TH2-like repertoire of cytokines by tumor-infiltrating lymphocytes (TILs) in papillomas, and by PBMC exposed to autologous papilloma tissues (40, 41).
Cytokine Expression by Papilloma cells and TILs
Previously we showed that IL-4 and IL-10 were dominantly expressed, compared to IFN-γ, in papillomas from patients with RRP. This imbalance correlated with disease severity (40). More recently we have shown a predominance of TH2-like chemokines in papillomas (44) and blood (42) of patients with RRP, see Innate Immunity and RRP below.
In PBMC from patients with RRP, greater concentrations of E6 protein were required for the expression of cytokine profiles, compared to controls. Specifically, a larger concentration of E6 proteins was needed to trigger TH1-like cytokines (IL-12, IL-18, IFN-γ). In contrast, there was no difference in the expression of IL-4 by patients and controls in response to E6 (41). Differences in IL-12, IL-18, and IFN-γ were highly significant, compared with differences observed in IL-10 expression by patients compared with controls (41). Thus, responses to E6 made by PBMC from patients with RRP were polarized away from a TH1-like cytokine (IL-12, IL-18, IFN-γ) repertoire, and biased towards a TH2-like/T-regulatory cytokine (IL-4/IL-10) profile (41). In ELISPOT experiments PBMC from patients with RRP exposed to E6 proteins induced T-cells, and many more non-T-cells that expressed IL-10 compared to controls. However, the number of IFN-γ secreting cells responding to E6 challenge was comparable between these patients and controls (41). These findings support the hypothesis that memory TH2-like T-cells and non-T-cells likely exist within an immunosuppressive cycle of immunocytes that support papilloma recurrence and chronic HPV infection, see model (Figure 1).
To determine if there were differences in the expression of TH1-/TH2-like, and T-regulatory cytokine repertoires in papillomas when T-cells were present, we performed semiquatitative, cytokine-specific, RT-PCR, see Table 1. The cytokine profiles expressed by two biopsies from different papillomas, one containing T-cells (CD3-δ mRNA positive*) and another without (CD3δ mRNA negative†), are shown in Table 1. Significant expression of the TH2-like (IL-4, IL-5, TGF-β) and T-regulatory cytokines (IL-10), with little expression of types I and II IFNs (IFN-α/β/ω, IFN-γ), IL-2, and IL-6, were identified in the papilloma-containing T-cells. In contrast, the papilloma biopsies devoid of T-cells showed more type I IFN (IFN-α) expression, no IL-4 or IL-10 expression, and less TGF-β. Thus, T-cells, but not keratinocytes, likely express TH2-like/T-regulatory cytokines in papillomas.
Table 1. Papillomas with TILs Express TH2 Cytokines.
Detection of TH1-like, TH2-like, and IL-10 expression in papillomas. Cytokine specific RT-PCR was performed as described in detail (40, 41) using mRNA obtained from papillomas containing or lacking tumor infiltrating lymphocytes (TILs containing T-cells identified by CD3δ expression). Cytokine mRNA were assayed by RT-PCR & semiquantitatively measured by densitometry of the PCR products on agarose (41). (-) no expression; (+) small mRNA expression; (++) moderate expression; (+++) significant expression.
| Papillomas | IL-2 | IL-4 | IL-5 | IL-6 | IL-10 | INF-α | IFN-γ | TGF-β |
|---|---|---|---|---|---|---|---|---|
| Containing TILs* | + | +++ | + | + | +++ | + | + | +++ |
| Lacking TILs† | - | - | - | + | - | ++ | - | + |
Peripheral Blood Mononuclear Cells (PBMC) from RRP Patients Show Increased Cytoplasmic IL-4 Expression
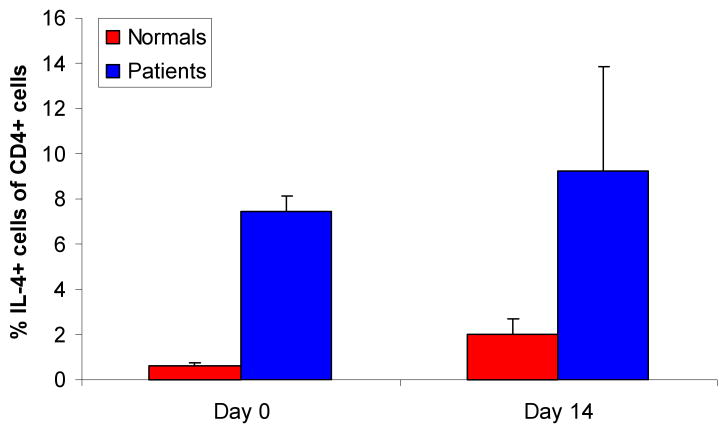
To determine which T-cell subclass(es) express IL-4 in the blood of patients with RRP, CD4+ and CD8+ T-cells from PBMC were stained for intracytoplasmic IL-4 expression using standard methods (55), and then analyzed by flow cytometry, see Figure 2. T-cells were studied immediately after surgical excision, or after 14 days in culture, as previously reported (40, 41). Interestingly, more resting CD4+ T-cells that constitutively expressed IL-4 were identified in the blood of RRP patients, in comparison to controls (Figure 2). In addition, culture of PBMC for 14 days had a minimal effect on the constitutive T-cell expression of IL-4, further showing an increased proportion of CD4+ T-cells that constitutively expressed IL-4 in patients with RRP as compared to controls (Figure 2). Intracytoplasmic expression of IFN-γ was also identified in PBMC from four patients and two controls, and was found to be comparable in both study populations (data not shown).
Figure 2. RRP Patients produce more IL4 than controls.
The percent of resting CD4+ T-cells in the blood of RRP patients and controls that constitutively express IL-4 is shown. PBMC from patients with RRP (n=4) and controls (n=2) were studied at day 0 (p<0.05), and some patients (n=2), and controls (n=2) were analyzed at day 14. Samples were processed for intracellular cytokine expression by standard methods.
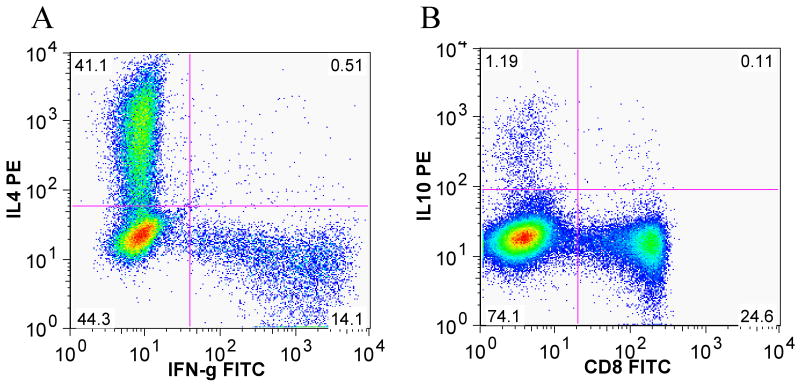
We have also identified the expression of IL-4 and IFN-γ in CD4+ T-cells isolated from papillomas (TILs) (Figure 3A), and identified IL-10 expression in PBMC from patients with RRP (Figure 3B). Clearly, CD4+ T-cells expressing IL-4 are enriched in TILs, and IL-10 expressing CD4+ T-cells are also present in the PBMC of patients with RRP (Figure 3B). However, expression of IFN-γ by CD4+ T-cells in TILs was reduced. These results suggest that patients with RRP, unlike controls, have increased numbers of circulatingCD4+ T-cells that constitutively express TH2-like cytokines that likely down-regulate TH1-like T-cell function (56).
Figure 3. TILs produce IL4 and IFN-γ, and PBMC from patients express IL-10.
A) TILs from a papilloma were for 13 days then stimulated with Phorbol-12-myristate-13-acetate/ionomycin (PMA/I). Distinct populations of CD4+ cells were evident, based on intracytoplasmic staining for IL-4 and INF-γ, at a ratio of 2.91 to 1 respectively. B) shows IL-10 intracytoplasmic staining of fresh PBMC obtained from a RRP patient and stimulated with PMA/I. Gating was performed on CD3+ cells. The frequency of IL-10 secreting CD4+ T-cells in PBMC is 1.19%.
Evidence of Oligoclonality of CD4+ and CD8+ T-cell Vβ Repertoires in TILs from Papillomas
To determine if there was a clonally restricted T-cell repertoire present in papillomas, and if there was trafficking of T-cells between the peripheral blood and papillomas, we used a multiplex PCR assay for Vβ, CDR3 length, to identify the T-cell receptor repertoires in α,β TCR bearing T-cells within TILs isolated from papillomas and from autologous PBMC of four patients with RRP. An assessment of oligoclonality in both the CD4+ and CD8+ T-cells obtained from papilloma tissue and the blood was performed, as previously described (57, 58) using 5′ fluorescein labeled primers for each of the TCR-Vβ families by a fluorescent DNA scanning method (59), and then analyzed by an ABI/DNA Sequencer, and are shown as fluorescence intensity.(60) (Figure 4).
Figure 4. Spectratype of T-cell populations from TILS and PBMC.
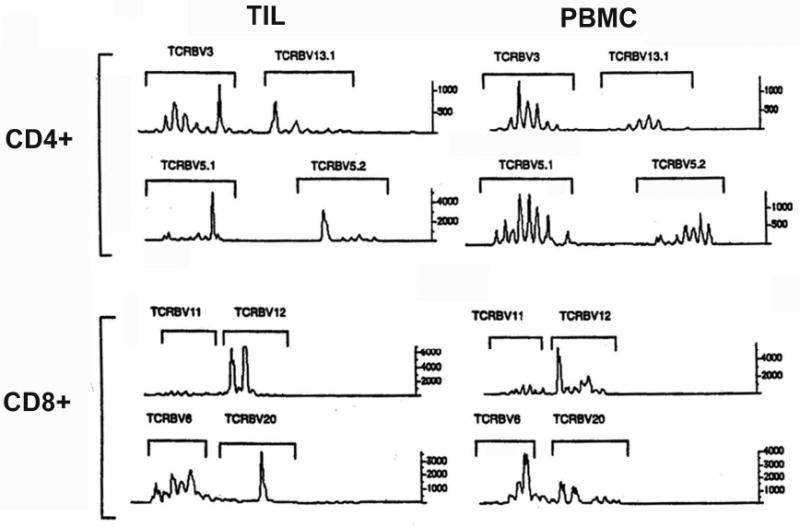
The TCR Vβ spectratypes of CD4+ T-cells in TILs and PBMC from a representative RRP patient (n=4) are shown. All patients showed oligo- and/or monoclonality in some T-cell families. CD8 data also showed oligo/monoclonality in some T-cell families (data not shown).
Oligoclonal, and in some instances monoclonal T-cell subpopulations were found in both CD4+ and CD8+ Vβ families in papillomas. A representative example of one of the spectratype experiments from a patient with severe RRP is shown in Figure 4 below. Of note, within the CD4+ TILs obtained from papillomas, there is evidence of dominant peaks (indicative of oligoclonality) in all four of the TCR-Vβ families shown. While this was characteristic of all four patients, there was no common individual TCR-Vβ family that was found to be oligoclonal for all four patients (data not shown). However, the CD4+ T-cells in PBMC from the same patient did not show oligoclonality. There is also evidence that the oligo/monoclonality of some TCR-Vβ families in TILs is derived from clones present in PBMC, specifically TCR-Vβ5.1 and TCR-Vβ5.2, see Figure 4. This CD4 Vβ repertoire enrichment is unexpected in that normal individuals do not show this restriction (59). This suggests that trafficking of CD4+ T-cell clones specific for HPV epitopes in papillomas likely occurs between the peripheral blood and papilloma tissue. Thus, the “bio-selected” T-cell clones in TILs that are enriched in papillomas, likely circulate in the peripheral blood. The CD4+ T-cells infiltrating into papillomas clearly have a highly restricted, oligoclonal T-cell repertoire, in stark contrast to the situation in these patients' PBMC.
Also shown in Figure 4 are representative analyses of the TCR repertoire of the CD8+ T-cell population in the TILs present in papillomas and in the peripheral blood of a severe patient with RRP. Of note, there is also evidence of dominant peaks (TCR-Vβ12 and TCR-Vβ6) in the PBMC and the TILs, although the pattern of this oligoclonality differs in the two T-cell populations. The existence of oligoclonality in the CD8+ subset of PBMC is not unexpected, since this finding is characteristic of the normal CD8+ memory repertoire in PBMC (59). However, it is again clear evidence that the repertoire of CD8+ TILs present in papillomas is distinct, albeit with selective overlap, from that found in PBMC from patients with RRP.
Altered Effector CD8+ T-cell Supopulations in RRP
We previously showed that two-week co-incubation of PBMC with autologous papilloma tissue, containing HPV proteins alters the ratio of CD8+ T-cell subpopulations in PBMC, as defined by their expression of the co-stimulatory molecule CD28 (40). CD8+28- T-cells were enriched in papillomas compared to PBMC, and predicted disease severity. This suggests that the repertoire of effector CD8+ T-cells and their function is likely altered by HPV. This functionally diverse, T-cell subpopulation (61-63) may represent immature HPV-specific, TC1-like CTLs, or they maybe TC2-like suppressor cells (50, 64) responding to HPV, and generated by the enriched IL-4 and IL-10 microenvironment present in papillomas that we identified (40, 65). In preliminary experiments we stained CD8+28- T-cells for granzyme B and found them to express this CT1-like enzyme (data not shown). Thus, these cells may be maturing CT1-like T-cells, however their cytolytic function remains to be determined.
Function Induced by Exposure to HPV-11, E6 Protein
To determine if alloreactive T-cell responses could be altered by pre-exposure of PBMC to E6 proteins, we pulsed PBMC with recombinant E6 (41), or BSA, prior to alloactivation in a mixed lymphocyte reaction (MLR). Alloactivated T-cells were then re-challenged five days later with the same HLA-class I, and class II mismatched stimulator PBMC in a cell mediated lympholysis (CML) assay. Allospecific CTL activity generated in CML assays was markedly reduced by PBMC exposure to E6, but not BSA, as measured by intercalation of the viability dye TO-PRO-3 iodide into the double-stranded DNA of PKH26-labeled HLA class I and II mismatched target cells, in a flow cytometric assay, Figure 5. CTL killing of HLA class I and II mismatched target cells, originally used as stimulators in the MLR was reduced from 30% in experiments with no added E6 or BSA, to 5% when PBMC were pre-exposed to E6. Thus, E6 can suppress alloreactive CTL function, suggesting that some HPV proteins may suppress HPV-specific CTL responses.
Figure 5. E6 inhibits alloreactive T-cell killing.
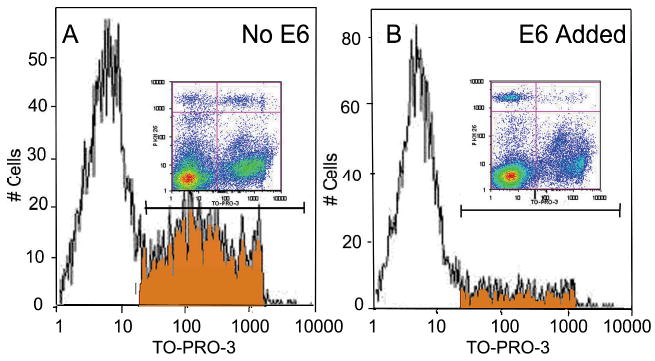
PBMC from a representative patient (n=3) with mild/moderate RRP were exposed to E6 or BSA for 20 hrs. prior to mixing with HLA class I and II mismatched and irradiated PBMC in a MLR at E:T ratio of 25:1. At day 6, patient's T-cells were re-challenged with PKH26 labeled PBMC from the same mismatched stimulator. Target cell death was detected using TO-PRO-3 iodide (TP3)(77, 96). Density plots (upper right quadrant = dead target cells) (upper left = live targets), and the histograms, gated on PKH26+ targets, shows a decreased number of dead target cells that incorporated TP3 with E6 exposure (5% killing), while maximum killing without E6 (data not shown) and BSA was 30%.
Regulatory T-cell (Treg) Expression in RRP
There are at least two major types of regulatory T-cells in humans: 1) natural Tregs (nTreg) (CD4+CD25+CD127low Foxp3+) that are generated in the thymus that traffic into tissues, and can suppress in a contact-dependent, cytokine-independent and antigen non-specific manner, and 2) adaptive or inducible Tregs (iTreg) that arise in the periphery and can become enriched in tissues. The latter likely consists of at least 3 subtypes: a) Type I Tregs (Tr1) (CD4+CD25-IL10+Foxp3low/-) which arise in a toleragenic environment via an IL-10 dependent process, b) Th3 cells which depend on IL-4 for functional differentiation and suppress by secretion of TGF-β and do not classically express Foxp3, and c) inducible Tregs which express Foxp3 but can be induced in the periphery. Unfortunately, there is no marker that can definitively differentiate between these various types of iTregs. (66-71)
We identified Tregs (CD4+CD25+CD127low Foxp3+) in papillomas from 17 patients with RRP and found two to seven fold enrichment of Tregs in papillomas compared to autologous blood from patients with RRP(43). The frequency of these Tregs in the blood of RRP patients was comparable to that found in the blood of controls. Thus, Tregs are enriched in papillomas and likely suppress TH1-like responses to HPV in papilloma tissues. Preliminary data from our laboratory shows that Tregs purified from papillomas can significantly inhibit anti-CD3/CD28 autologous CD4+ T-cell proliferation. Thus, Tregs, enriched in papillomas, likely participate in the immunosuppressive cycle of interactive cells that we hypothesize in the model shown in Figure 1. The Tregs we have observed in papillomas likely are a major component of the immunosuppressive cycle of immunoctyes that prevent effective anti-HPV-specific, TH1-like function in papillomas and perpetuate chronic HPV infection and the relentless recurrence of papillomas in this disease..
It is difficult to determine if the CD4+CD25+CD127low Foxp3+ T-cells in papillomas are indeed the naturally occurring Tregs or the FoxP3+ inducible supgroup of Tregs (iTreg) (70). iTregs that are CD4+ T-cells and differentiate into regulatory T-cells in an enriched TGF-β microenvironment, may in fact be the Tregs we have observed in papillomas and represent de novo generated, regulatory T-cells derived from CD4+ T-cell precursors, specific for HPV epitopes, that only after contact with HPV peptides within papillomas differentiate in to Tregs in the TH2-like/regulatory cytokine microenvironment present in papillomas. Such T_cells would acquire the regulatory T-cell phenotype marked by Foxp3 expression (70) as a result of this exposure.. In any case, the Tregs we have observed in papillomas likely play a major role in the immunosuppressive cycle of immunoctyes that prevent effective anti-HPV-specific, TH1-like function in papillomas and perpetuate chronic HPV infection and the relentless recurrence of papillomas in this disease..
Bias of Innate Immunity in RRP
Chemokine Repertoire Expression in RRP
The expression of chemokine repertoires in the blood of patients with RRP has been recently described (42, 45). The TH2-like chemokines CCL17, CCL18, and CCL22 were enriched in the serum of patients with RRP(42, 45), as measured by ELISA. In addition, using 12 matched pairs of papillomas and unaffected, autologous laryngeal tissues from patients with RRP, we observed that the TH1-like chemokines mRNA for CCL19 and CCL21 were down-regulated in papillomas, where as the mRNA for the TH2-like chemokine, CCL20, was markedly up-regulated (44). Both the papilloma and the unaffected adjacent tissues from the majority of these patients, also strongly express the TH2-like chemokine CCL18. Thus, there is a biased expression of TH2-like chemokines in papillomas consistent with the hypothesis that immature DCs and/or AAMΦ are present in papillomas and likely express these chemokines. Therefore, this polarized chemokine microenvironment attracts and maintains, in part, immunosuppressive immunocytes present in papillomas, as shown in Figure 1.
Professional Antigen Presenting Cells (Dendritic Cells and Langerhans Cells)
E6-specific T-cell memory has been reported to common in HPV-exposed, asymptomatic controls, but not in actively infected patients (6). Thus, the relatively unopposed IL-10 microenvironment present in papillomas (40, 41) could inhibit dendritic cell expression of the pro-inflammatory cytokines, IL-12 and IL-18, and thereby, prevent the development of anti-HPV, CTLs and CD4+ memory TH1-like cells, and in contrast, support the maintenance of memory TH2-like, and suppressor CD4+ T-cells, such as Tregs in RRP. HLA-class II-expressing dendritic cells (DCs), likely to be mainly Langerhans cells (LCs) because of their strategic position in the epidermis (72), were commonly found among keratinocytes within HPV-infected papillomas (28). The presence of these LCs in papillomas is shown in Figure 6. Of note, since LCs do not express IL-10 themselves, but can induce T-cells to do so by their expression of thymic stromal lymphopoietin (73), it is possible that some of these cells may be immature myeloid DCs that when kept immature, express IL-10, and only upon maturation express IL-12 (74). Thus, further study of whether DCs present in papillomas express IL-10, and are kept immature by HPV protein exposure is warranted.
Figure 6. Langerhans cells are present in papilloma tissues.
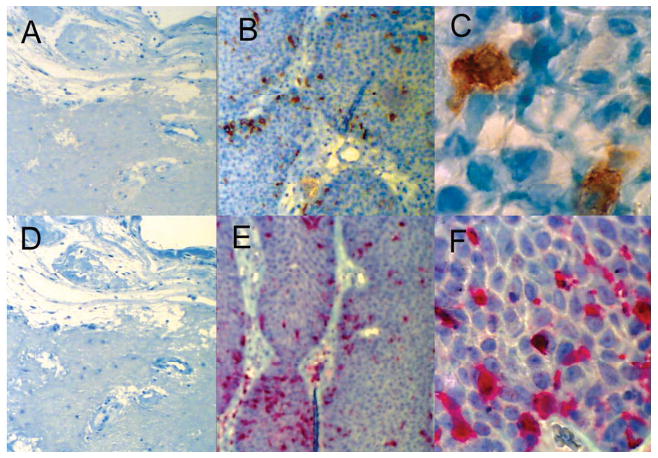
CD1a+ (brown) and S100+ (red) cells with Langerhans cell morphology in a laryngeal papilloma from a patient with RRP (B, C, E, F) and the absence of these cells in similarly stained control laryngeal tissue (A, D). Immunohistochemistry was performed on 8 μm sections of a paraffin embedded specimen using the Benchmark XT IHC/ISH Staining Module (Ventana Medical Systems, Tucson, AZ) utilizing a 3,3′diaminobenzidine substrate for CD1a (A, B, C) and Enhanced V-Red substrate for S100 (D, E, F).
Altered Frequency of Killer Cell Immunoglobulin-like Receptor (KIR) Gene Haplotypes in RRP
We recently showed that the frequencies of KIR gene haplotypes of patients with RRP differed significantly from those of controls (46). Specifically, the frequency of KIR haplotypes that lacked three activating KIR genes, KIR3DS1, KIR2DS1, KIR2DS5, predicted disease severity(46). These findings also suggested that these activating KIR genes might be required to remove HPV-infected keratinocytes devoid of HLA class I gene expression (28). Reduced or absent HLA class I expression should prevent ligation of inhibitory KIR molecules on NK cells, thereby releasing the inhibition of NK cytolysis that is normally dominant in NK cells. This would permit the ligation of activating KIRs by viral epitopes in, or outside of, the context of HLA class I molecules (46).
Defective NK Function in RRP
To determine if NK cells obtained from the blood of patients with RRP can cause cytolysis of target cells, we performed a CD107a mobilization assay on NK cells from 12 patients with RRP and 13 controls. CD107a is a cell surface marker that is transiently expressed after release of cytolytic granules, and correlated with cytokine release and NK cytolysis (75, 76). The K562 cell line, devoid of HLA class I molecules (77), was used as NK target cells in this assay, Figure 7. A significant reduction of CD107a expression on NK cells was identified in patients with RRP, compared to controls (p=0.044). Thus, defective cellular innate responses are present in RRP, and manifest as the failure of NK cells to be activated by target cells that lack HLA class I molecules (28). Therefore papillomas containing keratinocytes which have been shown to lack HLA class I expression(78) would not be cleared by NK cells that are present in papillomas (46).
Figure 7. NK cells from patients have impaired function compared to controls.
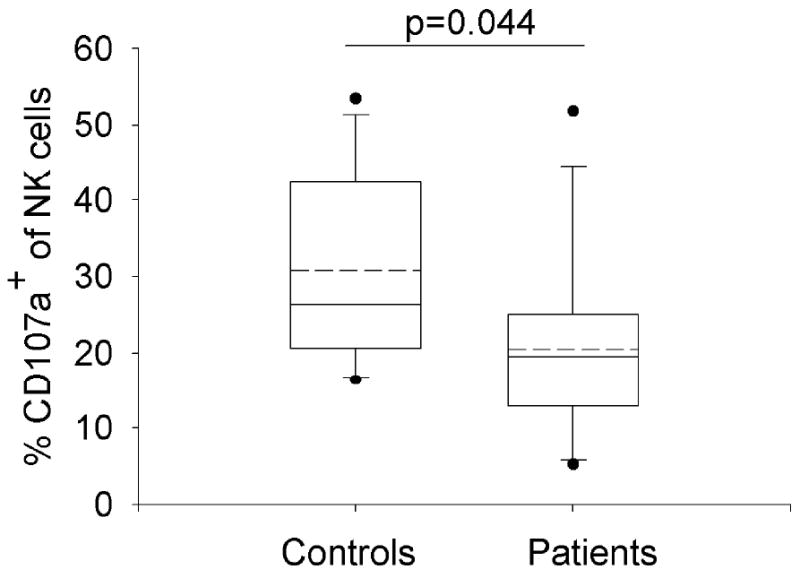
PBMC was cultured 4hrs in media alone or with the HLA class I devoid cell line, K562, at an effector:target ratio (E:T) of 5:1 in the presence of FITC-conjugated anti-CD107a and monensin(55). Following incubation, cells were stained with the amine-reactive live/dead fixable violet dead cell stain kit and CD3 APC, CD56 PE, CD14 and CD19 PerCP. Samples were analyzed by flow cytometry. Monocytes, B-cells, and dead cells were excluded from the analysis. Results are shown as the percentage of CD107a expression on CD3-56+ NK cells after subtraction of the spontaneous activity. Data are depicted by box plots (median and 75th/25th percentiles), whiskers (90th/10th percentiles); and outliers (outside the 95th and 5th percentiles) are shown. Dotted lines represent arithmetic means.
Immunogenetics of RRP
We hypothesize that RRP is a complex, multi-gene disease that polarizes adaptive and innate, HPV-specific immune responses that result in immune tolerance to these viruses and chronic infection. This disease appears to be governed by the enrichment of select HLA class II genes expressed by these patients (78-81), and as we also have recently reported, a significant difference in the frequencies of KIR gene haplotypes (46). In addition, select SNPs in the transporter associated with antigen presentation (TAP) gene have been identified (19, 82, 83). Lastly, the transcriptional profile in patients with RRP resembles genetic alterations seen in a variety of solid tumor malignancies, providing further support for this contention (44).
HLA and KIR Expression in RRP
To better understand the immune mechanism(s) that generate the suppressive microenvironment in papillomas, we have studied the genetic background of patients with RRP, and we have identified an enrichment of select of select HLA class II genes, specifically HLA-DRB1*0102, HLA-DRB1*0301, HLA-DQB1*0201 in this disease (78). We also found a significant difference in the frequencies of activating polymorphic KIR genes, specifically KIR3DS1, KIR2DS1, KIR2DS5, as described above (46). Taken together, the select HLA class II (40, 49, 50) and KIR haplotypes (46) in patients with RRP may explain the predisposition to impaired clearance of HPV-infected keratinocytes, and thereby prevent subsequent development of an effective adaptive response to these viruses. Further support for this hypothesis comes from the evidence that reduced IFN-γ expression associated with the expression of these HLA class II alleles (78).
Identification of Diseases Associated Genes
We have also identified differential expression of both immune and non-immune response genes in papillomas, compared to unaffected autologous laryngeal tissues from patients with RRP by gene array (44). Previous studies (84-87) examining individual prognostic indicators that participate in the development of RRP have recently been reviewed in detail (44). Using matched pairs of laryngeal tissues, we identified differences in both adaptive and innate immune response gene expression, as well as the altered expression of multiple non-immune gene pathways that are reminiscent of those expressed by a variety of solid tumor malignancies (44). In addition, our transcriptional analysis allowed for the confirmation of previous reports of disease-associated genes expressed in RRP (84-87) and the identification of new immune and non immune-associated genes previously unrecognized in RRP (44).
Towards Developing a Therapeutic Vaccine for RRP
While multiple studies have begun to characterize the immunologic nature of this complex, multi-gene disease, little is known about HPV-specific, innate signaling in RRP. Specifically, it is unknown what leads to polarization of innate and adaptive immune responses made by patients with RRP to HPV-6 and -11 which result in a selective tolerance to HPV. However, an attractive strategy to develop a therapeutic vaccine for RRP would be to reverse the TH2-like/Treg polarization that results from the interactive cycle of suppressor immunocytes. This polarization leads to the relatively unopposed IL-4 and IL-10 microenvironment in papillomas (40, 41), that is reminiscent of the microenvironment present in some malignant tumors (88, 89). The immune micromilieu in papillomas permits HPV to evade T-cell immune clearance and leads to papilloma regrowth. The reversal of the IL-4 and IL-10-induced immune suppression in papillomas would likely restore IL-12, IL-18, and IFN-γ expression, and thereby lead to CTL infiltration, maturation, and effector function that would cause papilloma regression, as described in malignant tumors (89).
Towards this end, we have begun to map T-cell epitopes within HPV-11 early proteins using the HLA class II alleles enriched in RRP (78), as labeled tetramers, to identify peptide-specific T-cells from patients with RRP, and ultimately determine their polarization by identifying their cytokine repertoires (90, 91). These studies are important to detect early protein epitopes that support TH2-like cytokine and chemokine expression. Such epitopes should be excluded from a therapeutic vaccine, while other epitopes should be included that: 1) support TH1-like function, 2) reduce Treg enrichment, and, 3) repolarize resting and AAMΦ to become classically activated macrophages (92, 93) in papillomas. Such a vaccine would be ideal in treating patients with RRP.
Conclusion
RRP is likely a disease that requires a repolarization of a complex repertoire of genes that coordinates HPV- and site-specific defects in the development of an effective immune response to HPV-6 and -11. RRP is in most cases is limited to the larynx and manifests as a local, tissue-specific disease, has trafficking of immunocytes from papillomas into the systemic circulation, thus allowing measurement of some altered immune responses in the peripheral blood. This complex repertoire selectively induces a failure of innate and adaptive immunity that should clear HPV-6 and 11-infected keratinocytes from the upper airway. Additionally patients with RRP appear to have normal responses to other pathogens, and their general immunophenotypes have been shown to be normal (28).
In this communication, we provide a model fro the inhibitory cycle of immunocytes that supports the development of RRP (Figure 1), we summarize previously reported findings in RRP, and we provide further evidence that the adaptive immune response in RRP is clearly polarized towards a TH2-like/T-regulatory phenotype. We present new evidence that there are more IL-4 expressing CD4+ T-cells in the blood of patients with RRP, compared to controls (Figure 2), and that papillomas which contain T-cells dominantly express TH2-like cytokines (Table 1 and Figure 3), whereas biopsies of papilloma tissue which have relatively few T-cells express a more balanced TH1-like/T regulatory cytokine repertoire (Table 1), thus, supporting our previous reports (40, 41). We provide new evidence that the CD4+ and CD8+ T-cell Vβ repertoires in papillomas are restricted (Figure 4), compared to autologous blood, and that some of these oligoclonal T-cells likely traffic between papillomas and the blood of these patients.
In addition, we provide evidence that HPV-11 early protein E6 can suppress T-cell alloreactivity (Figure 5). We also show an enrichment of Langerhans cells in papillomas, which likely direct the local immune response (Figure 6). For the first time we provide evidence that HLA class I specific, NK cytolysis in RRP patients is defective (Figure 7). The latter finding augments our previous report that there is a significant difference in the frequencies of activating KIR gene haplotypes in RRP(46), and supports the contention that defective innate function is a predisposing element in this disease. These findings require further investigation to understand how the innate responses to HPV polarize adaptive T-cell responses away from effective HPV clearance of HPV in respiratory tissues.
In an attempt to generate an effective therapeutic vaccine for patients with RRP, we and others have studied the role of HPV early proteins in polarizing the adaptive immune responses of patients with RRP. Specifically, E6 and E2 have been shown to play an important role in HPV-specific, immune responses (6, 40, 41, 94, 95). We have begun to map T-cell epitopes within HPV early proteins to determine, in the context of select HLA class II alleles enriched in RRP (78), the repertoire of cytokines expressed by T-cells recognizing these peptides. These studies should help illuminate which HPV peptides may support or inhibit effective TH1-like responses to HPV-6 and -11 epitopes in the context of these and other HLA class II alleles expressed by patients with RRP. Ultimately, an effective therapeutic vaccine for RRP must provide long-term re-polarization of both the innate and adaptive immune responses made by patients with RRP to HPV-6 and 11 to reduce disease severity and potentially cure this chronic disease.
Much has been illuminated about the immunology associated with RRP, yet much remains unanswered about how different HPVs manipulate the immune responses of individuals who develop chronic HPV-induced diseases. If novel therapies are to be developed to treat RRP, and for that matter, other HPV-induced diseases, such as cervical cancer, a more complete understanding of the immunologic basis that explains why some HPV-infected individuals develop chronic disease when others do not, needs to be illuminated. This knowledge will be essential in successfully repolarizing the innate and adaptive local and systemic immune responses selective found in patients with HPV associated diseases.
Acknowledgments
Vincent Bonagura is supported a grant from the National Institute of Dental & Craniofacial Research ⁄ National Institutes of Health DE017227.
References
- 1.Mounts P, Shah KV, Kashima H. Viral etiology of juvenile- and adult-onset squamous papilloma of the larynx. Proc Natl Acad Sci U S A. 1982;79:5425–5429. doi: 10.1073/pnas.79.17.5425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gissmann L, Wolnik L, Ikenberg H, Koldovsky U, Schnurch HG, zur Hausen H. Human papillomavirus types 6 and 11 DNA sequences in genital and laryngeal papillomas and in some cervical cancers. Proc Natl Acad Sci U S A. 1983;80:560–563. doi: 10.1073/pnas.80.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holinger PH, Johnson KC, Anison GC. Papilloma of larynx: review of 109 cases with preliminary report of aureomycin therapy. Ann Otol Rhinol Laryngol. 1950;59:547–563. doi: 10.1177/000348945005900225. [DOI] [PubMed] [Google Scholar]
- 4.Armstrong LR, Preston EJ, Reichert M, et al. Incidence and prevalence of recurrent respiratory papillomatosis among children in Atlanta and Seattle. Clin Infect Dis. 2000;31:107–109. doi: 10.1086/313914. [DOI] [PubMed] [Google Scholar]
- 5.Broker TR, Jin G, Croom-Rivers A, et al. Dev Biol (Basel) Vol. 106. 2001. Viral latency--the papillomavirus model; pp. 443–451. discussion 452-443, 465-475. [PubMed] [Google Scholar]
- 6.Welters MJ, de Jong A, van den Eeden SJ, et al. Frequent display of human papillomavirus type 16 E6-specific memory t-Helper cells in the healthy population as witness of previous viral encounter. Cancer Res. 2003;63:636–641. [PubMed] [Google Scholar]
- 7.Abramson AL, Steinberg BM, Winkler B. Laryngeal papillomatosis: clinical, histopathologic and molecular studies. Laryngoscope. 1987;97:678–685. doi: 10.1288/00005537-198706000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Galani E, Christodoulou C. Human papilloma viruses and cancer in the post-vaccine era. Clin Microbiol Infect. 2009;15:977–981. doi: 10.1111/j.1469-0691.2009.03032.x. [DOI] [PubMed] [Google Scholar]
- 9.Buchinsky FJ, Donfack J, Derkay CS, et al. Age of child, more than HPV type, is associated with clinical course in recurrent respiratory papillomatosis. PLoS One. 2008;3:e2263. doi: 10.1371/journal.pone.0002263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carvalho CM, Huot L, Charlois AL, Khalfallah SA, Chapuis F, Froehlich P. Prognostic factors of recurrent respiratory papillomatosis from a registry of 72 patients. Acta Otolaryngol. 2009;129:462–470. doi: 10.1080/00016480902737986. [DOI] [PubMed] [Google Scholar]
- 11.Armstrong L, Jordan N, Millar A. Interleukin 10 (IL-10) regulation of tumour necrosis factor alpha (TNF-alpha) from human alveolar macrophages and peripheral blood monocytes. Thorax. 1996;51:143–149. doi: 10.1136/thx.51.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McClay JE. Recurrent Respiratory Papillomatosis. 2008. [Google Scholar]
- 13.Wiatrak BJ, Wiatrak DW, Broker TR, Lewis L. Recurrent respiratory papillomatosis: a longitudinal study comparing severity associated with human papilloma viral types 6 and 11 and other risk factors in a large pediatric population. Laryngoscope. 2004;114:1–23. doi: 10.1097/01.mlg.000148224.83491.0f. [DOI] [PubMed] [Google Scholar]
- 14.Leung R, Hawkes M, Campisi P. Severity of juvenile onset recurrent respiratory papillomatosis is not associated with socioeconomic status in a setting of universal health care. Int J Pediatr Otorhinolaryngol. 2007;71:965–972. doi: 10.1016/j.ijporl.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 15.Derkay CS. Task force on recurrent respiratory papillomas. A preliminary report. Arch Otolaryngol Head Neck Surg. 1995;121:1386–1391. doi: 10.1001/archotol.1995.01890120044008. [DOI] [PubMed] [Google Scholar]
- 16.Derkay CS, Wiatrak B. Recurrent respiratory papillomatosis: a review. Laryngoscope. 2008;118:1236–1247. doi: 10.1097/MLG.0b013e31816a7135. [DOI] [PubMed] [Google Scholar]
- 17.Lindeberg H, Elbrond O. Laryngeal papillomas: the epidemiology in a Danish subpopulation 1965-1984. Clin Otolaryngol. 1990;15:125–131. doi: 10.1111/j.1365-2273.1990.tb00444.x. [DOI] [PubMed] [Google Scholar]
- 18.Bomholt A. Juvenile laryngeal papillomatosis. An epidemiological study from the Copenhagen region. Acta Otolaryngol. 1988;105:367–371. doi: 10.3109/00016488809097020. [DOI] [PubMed] [Google Scholar]
- 19.Vambutas A, Bonagura VR, Reed EF, et al. Polymorphism of transporter associated with antigen presentation 1 as a potential determinant for severity of disease in recurrent respiratory papillomatosis caused by human papillomavirus types 6 and 11. J Infect Dis. 2004;189:871–879. doi: 10.1086/381764. [DOI] [PubMed] [Google Scholar]
- 20.Vambutas A, Bonagura VR, Steinberg BM. Altered expression of TAP-1 and major histocompatibility complex class I in laryngeal papillomatosis: correlation of TAP-1 with disease. Clin Diagn Lab Immunol. 2000;7:79–85. doi: 10.1128/cdli.7.1.79-85.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steinberg BM, Gallagher T, Stoler M, Abramson AL. Persistence and expression of human papillomavirus during interferon therapy. Arch Otolaryngol Head Neck Surg. 1988;114:27–32. doi: 10.1001/archotol.1988.01860130031010. [DOI] [PubMed] [Google Scholar]
- 22.McClay JE. Recurrent Respiratory Papillomatosis. [January 8, 2010];2008 In: http://emedicine.medscape.com/article/865758-overview (ed.) Updated: Oct 29, 2008.
- 23.Weiss MD, Kashima HK. Tracheal involvement in laryngeal papillomatosis. Laryngoscope. 1983;93:45–48. doi: 10.1288/00005537-198301000-00008. [DOI] [PubMed] [Google Scholar]
- 24.Lin HW, Richmon JD, Emerick KS, et al. Malignant transformation of a highly aggressive human papillomavirus type 11-associated recurrent respiratory papillomatosis. Am J Otolaryngol. 2009 doi: 10.1016/j.amjoto.2009.02.019. [DOI] [PubMed] [Google Scholar]
- 25.Blumin JH, Handler EB, Simpson CB, Osipov V, Merati AL. Dysplasia in adults with recurrent respiratory papillomatosis: incidence and risk factors. Ann Otol Rhinol Laryngol. 2009;118:481–485. doi: 10.1177/000348940911800704. [DOI] [PubMed] [Google Scholar]
- 26.Abramson AL, Shikowitz MJ, Mullooly VM, Steinberg BM, Amella CA, Rothstein HR. Clinical effects of photodynamic therapy on recurrent laryngeal papillomas. Arch Otolaryngol Head Neck Surg. 1992;118:25–29. doi: 10.1001/archotol.1992.01880010029011. [DOI] [PubMed] [Google Scholar]
- 27.Kashima HB, Leventhal B, Mounts P. Papilloma Study Group, Scoring system to assess severity and course in recurrent respiratory papillomatosis. In: Howley H, Booker T, editors. Papillomavirus: Molecular and Clinical Aspects. Alan R. Liss; New York: 1985. [Google Scholar]
- 28.Bonagura VR, Siegal FP, Abramson AL, et al. Enriched HLA-DQ3 phenotype and decreased class I major histocompatibility complex antigen expression in recurrent respiratory papillomatosis. Clin Diagn Lab Immunol. 1994;1:357–360. doi: 10.1128/cdli.1.3.357-360.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bonnez W, Kashima HK, Leventhal B, et al. Antibody response to human papillomavirus (HPV) type 11 in children with juvenile-onset recurrent respiratory papillomatosis (RRP) Virology. 1992;188:384–387. doi: 10.1016/0042-6822(92)90770-p. [DOI] [PubMed] [Google Scholar]
- 30.Sameshima A, Fujiyoshi T, Pholampaisathit S, et al. Demonstration of antibodies against human papillomavirus type-11 E6 and L2 proteins in patients with recurrent respiratory papillomatosis. Auris Nasus Larynx. 1997;24:185–191. doi: 10.1016/s0385-8146(96)00000-4. [DOI] [PubMed] [Google Scholar]
- 31.Tachezy R, Hamsikova E, Valvoda J, et al. Antibody response to a synthetic peptide derived from the human papillomavirus type 6/11 L2 protein in recurrent respiratory papillomatosis: correlation between Southern blot hybridization, polymerase chain reaction, and serology. J Med Virol. 1994;42:52–59. doi: 10.1002/jmv.1890420111. [DOI] [PubMed] [Google Scholar]
- 32.Thurmond LM, Brand CM, Leventhal BG, Finter NB, Johnston JM. Antibodies in patients with recurrent respiratory papillomatosis treated with lymphoblastoid interferon. J Lab Clin Med. 1991;118:232–240. [PubMed] [Google Scholar]
- 33.Faria AM, Weiner HL. Oral tolerance. Immunol Rev. 2005;206:232–259. doi: 10.1111/j.0105-2896.2005.00280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Healy GB, Gelber RD, Trowbridge AL, Grundfast KM, Ruben RJ, Price KN. Treatment of recurrent respiratory papillomatosis with human leukocyte interferon. Results of a multicenter randomized clinical trial. N Engl J Med. 1988;319:401–407. doi: 10.1056/NEJM198808183190704. [DOI] [PubMed] [Google Scholar]
- 35.Leventhal BG, Kashima HK, Weck PW, et al. Randomized surgical adjuvant trial of interferon alfa-n1 in recurrent papillomatosis. Arch Otolaryngol Head Neck Surg. 1988;114:1163–1169. doi: 10.1001/archotol.1988.01860220097032. [DOI] [PubMed] [Google Scholar]
- 36.Szabo G, Dolganiuc A. The role of plasmacytoid dendritic cell-derived IFN alpha in antiviral immunity. Crit Rev Immunol. 2008;28:61–94. doi: 10.1615/critrevimmunol.v28.i1.40. [DOI] [PubMed] [Google Scholar]
- 37.Gerein V, Rastorguev E, Gerein J, Jecker P, Pfister H. Use of interferon-alpha in recurrent respiratory papillomatosis: 20-year follow-up. Ann Otol Rhinol Laryngol. 2005;114:463–471. doi: 10.1177/000348940511400608. [DOI] [PubMed] [Google Scholar]
- 38.Szeps M, Dahlgren L, Aaltonen LM, et al. Human papillomavirus, viral load and proliferation rate in recurrent respiratory papillomatosis in response to alpha interferon treatment. J Gen Virol. 2005;86:1695–1702. doi: 10.1099/vir.0.80849-0. [DOI] [PubMed] [Google Scholar]
- 39.Abramson A. In: Personal Communication. Bonagura VR, editor. 2010. [Google Scholar]
- 40.Bonagura VR, Hatam L, DeVoti J, Zeng F, Steinberg BM. Recurrent respiratory papillomatosis: altered CD8(+) T-cell subsets and T(H)1/T(H)2 cytokine imbalance. Clin Immunol. 1999;93:302–311. doi: 10.1006/clim.1999.4784. [DOI] [PubMed] [Google Scholar]
- 41.DeVoti JA, Steinberg BM, Rosenthal DW, et al. Failure of gamma interferon but not interleukin-10 expression in response to human papillomavirus type 11 e6 protein in respiratory papillomatosis. Clin Diagn Lab Immunol. 2004;11:538–547. doi: 10.1128/CDLI.11.3.538-547.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosenthal DW, DeVoti JA, Schmidtmayerova H, Steinberg BM, Bonagura VR. Human Papillomavirus causes a TH2-like Chemokine Predominance in Recurrent Respiratory Papillomatotosis (RPR) Journal of Allergy and Clinical Immunology. 2008;121:S15. [Google Scholar]
- 43.Hatam LJ, Rosenthal DW, DeVoti JA, et al. CD4+Foxp3+CD127+low T-regulatory cells are increased in HPV infected Papillomas in patients with Recurrent Respiratory Papillomatosis(RRP) Journal of Allergy and Clinical Immunology. 2008;121:S211. [Google Scholar]
- 44.DeVoti JA, Rosenthal DW, Wu R, Abramson AL, Steinberg BM, Bonagura VR. Immune dysregulation and tumor-associated gene changes in recurrent respiratory papillomatosis: a paired microarray analysis. Mol Med. 2008;14:608–617. doi: 10.2119/2008-00060.DeVoti. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rosenthal DW, Schmidtmayerova H, Steinberg BM, et al. Recurrent Respiratory Papillomatosis (RRP): Disease Severity Associates with Enhanced TH2-like Dendritic Cell Chemokine (DC-CK1) Plasma Expression. Journal of Allergy and Clinical Immunology. 2005;115:S81. [Google Scholar]
- 46.Bonagura VR, Du Z, Ashouri E, et al. Activating killer cell immunoglobulin-like receptors 3DS1 and 2DS1 protect against developing the severe form of recurrent respiratory papillomatosis. Hum Immunol. 2009 doi: 10.1016/j.humimm.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dai WJ, Kohler G, Brombacher F. Both innate and acquired immunity to Listeria monocytogenes infection are increased in IL-10-deficient mice. J Immunol. 1997;158:2259–2267. [PubMed] [Google Scholar]
- 48.Wagner RD, Maroushek NM, Brown JF, Czuprynski CJ. Treatment with anti-interleukin-10 monoclonal antibody enhances early resistance to but impairs complete clearance of Listeria monocytogenes infection in mice. Infect Immun. 1994;62:2345–2353. doi: 10.1128/iai.62.6.2345-2353.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scott M, Nakagawa M, Moscicki AB. Cell-mediated immune response to human papillomavirus infection. Clin Diagn Lab Immunol. 2001;8:209–220. doi: 10.1128/CDLI.8.2.209-220.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sheu BC, Lin RH, Lien HC, Ho HN, Hsu SM, Huang SC. Predominant Th2/Tc2 polarity of tumor-infiltrating lymphocytes in human cervical cancer. J Immunol. 2001;167:2972–2978. doi: 10.4049/jimmunol.167.5.2972. [DOI] [PubMed] [Google Scholar]
- 51.Paul WE, Seder RA. Lymphocyte responses and cytokines. Cell. 1994;76:241–251. doi: 10.1016/0092-8674(94)90332-8. [DOI] [PubMed] [Google Scholar]
- 52.Picker LJ, Singh MK, Zdraveski Z, et al. Direct demonstration of cytokine synthesis heterogeneity among human memory/effector T cells by flow cytometry. Blood. 1995;86:1408–1419. [PubMed] [Google Scholar]
- 53.Mosmann TR, Sad S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol Today. 1996;17:138–146. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- 54.Prussin C. Cytokine flow cytometry: understanding cytokine biology at the single-cell level. J Clin Immunol. 1997;17:195–204. doi: 10.1023/a:1027350226435. [DOI] [PubMed] [Google Scholar]
- 55.Prussin C, Metcalfe DD. Detection of intracytoplasmic cytokine using flow cytometry and directly conjugated anti-cytokine antibodies. J Immunol Methods. 1995;188:117–128. doi: 10.1016/0022-1759(95)00209-x. [DOI] [PubMed] [Google Scholar]
- 56.Romagnani S. Th1 and Th2 in human diseases. Clin Immunol Immunopathol. 1996;80:225–235. doi: 10.1006/clin.1996.0118. [DOI] [PubMed] [Google Scholar]
- 57.Margolis D, Yassai M, Hletko A, McOlash L, Gorski J. Concurrent or sequential delta and beta TCR gene rearrangement during thymocyte development: individual thymi follow distinct pathways. J Immunol. 1997;159:529–533. [PubMed] [Google Scholar]
- 58.Maslanka K, Piatek T, Gorski J, Yassai M. Molecular analysis of T cell repertoires. Spectratypes generated by multiplex polymerase chain reaction and evaluated by radioactivity or fluorescence. Hum Immunol. 1995;44:28–34. doi: 10.1016/0198-8859(95)00056-a. [DOI] [PubMed] [Google Scholar]
- 59.Batliwalla F, Monteiro J, Serrano D, Gregersen PK. Oligoclonality of CD8+ T cells in health and disease: aging, infection, or immune regulation? Hum Immunol. 1996;48:68–76. doi: 10.1016/0198-8859(96)00077-8. [DOI] [PubMed] [Google Scholar]
- 60.Nishi T, Fujita T, Nishi-Takaoka C, et al. Cloning and expression of a novel variant of human interferon-gamma cDNA. J Biochem. 1985;97:153–159. doi: 10.1093/oxfordjournals.jbchem.a135039. [DOI] [PubMed] [Google Scholar]
- 61.Lum LG, Orcutt-Thordarson N, Seigneuret MC, Hansen JA. In vitro regulation of immunoglobulin synthesis by T-cell subpopulations defined by a new human T-cell antigen (9.3) Cell Immunol. 1982;72:122–129. doi: 10.1016/0008-8749(82)90289-1. [DOI] [PubMed] [Google Scholar]
- 62.Damle NK, Mohagheghpour N, Hansen JA, Engleman EG. Alloantigen-specific cytotoxic and suppressor T lymphocytes are derived from phenotypically distinct precursors. J Immunol. 1983;131:2296–2300. [PubMed] [Google Scholar]
- 63.Azuma M, Phillips JH, Lanier LL. CD28-T lymphocytes. Antigenic and functional properties. J Immunol. 1993;150:1147–1159. [PubMed] [Google Scholar]
- 64.Wirth S, van den Broek M, Frossard CP, et al. CD8(+) T cells secreting type 2 lymphokines are defective in protection against viral infection. Cell Immunol. 2000;202:13–22. doi: 10.1006/cimm.2000.1639. [DOI] [PubMed] [Google Scholar]
- 65.McHugh RS, Shevach EM. The role of suppressor T cells in regulation of immune responses. J Allergy Clin Immunol. 2002;110:693–702. doi: 10.1067/mai.2002.129339. [DOI] [PubMed] [Google Scholar]
- 66.Nandakumar S, Miller CW, Kumaraguru U. T regulatory cells: an overview and intervention techniques to modulate allergy outcome. Clin Mol Allergy. 2009;7:5. doi: 10.1186/1476-7961-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Curotto de Lafaille MA, Lafaille JJ. Natural and adaptive foxp3+ regulatory T cells: more of the same or a division of labor? Immunity. 2009;30:626–635. doi: 10.1016/j.immuni.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 68.Strauss L, Bergmann C, Szczepanski M, Gooding W, Johnson JT, Whiteside TL. A unique subset of CD4+CD25highFoxp3+ T cells secreting interleukin-10 and transforming growth factor-beta1 mediates suppression in the tumor microenvironment. Clin Cancer Res. 2007;13:4345–4354. doi: 10.1158/1078-0432.CCR-07-0472. [DOI] [PubMed] [Google Scholar]
- 69.Liu W, Putnam AL, Xu-Yu Z, et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203:1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bour-Jordan H, Bluestone JA. Regulating the regulators: costimulatory signals control the homeostasis and function of regulatory T cells. Immunol Rev. 2009;229:41–66. doi: 10.1111/j.1600-065X.2009.00775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhu J, Paul WE. Heterogeneity and plasticity of T helper cells. Cell Res. 20:4–12. doi: 10.1038/cr.2009.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mutyambizi K, Berger CL, Edelson RL. The balance between immunity and tolerance: the role of Langerhans cells. Cell Mol Life Sci. 2009;66:831–840. doi: 10.1007/s00018-008-8470-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ebner S, Nguyen VA, Forstner M, et al. Thymic stromal lymphopoietin converts human epidermal Langerhans cells into antigen-presenting cells that induce proallergic T cells. J Allergy Clin Immunol. 2007;119:982–990. doi: 10.1016/j.jaci.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 74.Banchereau J, Briere F, Caux C, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 75.Alter G, Malenfant JM, Altfeld M. CD107a as a functional marker for the identification of natural killer cell activity. J Immunol Methods. 2004;294:15–22. doi: 10.1016/j.jim.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 76.Rubio V, Stuge TB, Singh N, et al. Ex vivo identification, isolation and analysis of tumor-cytolytic T cells. Nat Med. 2003;9:1377–1382. doi: 10.1038/nm942. [DOI] [PubMed] [Google Scholar]
- 77.Hatam L, Schuval S, Bonagura VR. Flow cytometric analysis of natural killer cell function as a clinical assay. Cytometry. 1994;16:59–68. doi: 10.1002/cyto.990160109. [DOI] [PubMed] [Google Scholar]
- 78.Bonagura VR, Vambutas A, DeVoti JA, et al. HLA alleles, IFN-gamma responses to HPV-11 E6, and disease severity in patients with recurrent respiratory papillomatosis. Hum Immunol. 2004;65:773–782. doi: 10.1016/j.humimm.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 79.Gelder CM, Williams OM, Hart KW, et al. HLA class II polymorphisms and susceptibility to recurrent respiratory papillomatosis. J Virol. 2003;77:1927–1939. doi: 10.1128/JVI.77.3.1927-1939.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Comar M, Fabris A, Vatta S, Pelos G, Zocconi E, Campello C. HPV genotyping and HLA II analysis in a pedigree study of pediatric RRP: preliminary results. Int J Pediatr Otorhinolaryngol. 2006;70:1935–1939. doi: 10.1016/j.ijporl.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 81.Gregoire L, Reidy PM, Rabah R, Lancaster WD. HLA-DQ alleles in white and African American patients with juvenile-onset recurrent respiratory papillomatosis. Arch Otolaryngol Head Neck Surg. 2003;129:1221–1224. doi: 10.1001/archotol.129.11.1221. [DOI] [PubMed] [Google Scholar]
- 82.Artandi SE, Calame KL, Morrison SL, Bonagura VR. Monoclonal IgM rheumatoid factors bind IgG at a discontinuous epitope comprised of amino acid loops from heavy-chain constant-region domains 2 and 3. Proc Natl Acad Sci U S A. 1992;89:94–98. doi: 10.1073/pnas.89.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vambutas A, DeVoti J, Pinn W, Steinberg BM, Bonagura VR. Interaction of human papillomavirus type 11 E7 protein with TAP-1 results in the reduction of ATP-dependent peptide transport. Clin Immunol. 2001;101:94–99. doi: 10.1006/clim.2001.5094. [DOI] [PubMed] [Google Scholar]
- 84.Chong KT, Xiang L, Wang X, Jun EL, Xi LF, Schweinfurth JM. High level expression of human epithelial beta-defensins (hBD-1, 2 and 3) in papillomavirus induced lesions. Virol J. 2006;3:75. doi: 10.1186/1743-422X-3-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rahbar R, Vargas SO, Folkman J, et al. Role of vascular endothelial growth factor-A in recurrent respiratory papillomatosis. Ann Otol Rhinol Laryngol. 2005;114:289–295. doi: 10.1177/000348940511400407. [DOI] [PubMed] [Google Scholar]
- 86.Poetker DM, Sandler AD, Scott DL, Smith RJ, Bauman NM. Survivin expression in juvenile-onset recurrent respiratory papillomatosis. Ann Otol Rhinol Laryngol. 2002;111:957–961. doi: 10.1177/000348940211101101. [DOI] [PubMed] [Google Scholar]
- 87.Pham TT, Ongkeko WM, An Y, Yi ES. Protein expression of the tumor suppressors p16INK4A and p53 and disease progression in recurrent respiratory papillomatosis. Laryngoscope. 2007;117:253–257. doi: 10.1097/01.mlg.0000248241.95357.a6. [DOI] [PubMed] [Google Scholar]
- 88.Alleva DG, Burger CJ, Elgert KD. Tumor-induced regulation of suppressor macrophage nitric oxide and TNF-alpha production. Role of tumor-derived IL-10, TGF-beta, and prostaglandin E2. J Immunol. 1994;153:1674–1686. [PubMed] [Google Scholar]
- 89.Stolina M, Sharma S, Lin Y, et al. Specific inhibition of cyclooxygenase 2 restores antitumor reactivity by altering the balance of IL-10 and IL-12 synthesis. J Immunol. 2000;164:361–370. doi: 10.4049/jimmunol.164.1.361. [DOI] [PubMed] [Google Scholar]
- 90.Bonagura VR, James E, DeVoti JA, et al. Class II MHC Tetramer Guided Epitope Mapping (TGEM) of E6/E2-Specific T-Cells in Patients with Recurrent Respiratory Papillomatosis (RRP): Developing a Therapeutic Vaccine. J Allergy Clinical Immunology. 2008;121:S166. [Google Scholar]
- 91.James E, DeVoti JA, Rosenthal DW, et al. Human Papillomavirus (HPV)-Specific T-cells Recognizing Dominant E2/E6 Epitopes Elicit Reduced IFN-g in Patients with Recurrent Respiratory Papillomatosis (RRP) Journal of Allergy and Clinical Immunology. 2010;125:S. [Google Scholar]
- 92.Schebesch C, Kodelja V, Muller C, et al. Alternatively activated macrophages actively inhibit proliferation of peripheral blood lymphocytes and CD4+ T cells in vitro. Immunology. 1997;92:478–486. doi: 10.1046/j.1365-2567.1997.00371.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 94.Nakagawa M, Stites DP, Patel S, et al. Persistence of human papillomavirus type 16 infection is associated with lack of cytotoxic T lymphocyte response to the E6 antigens. J Infect Dis. 2000;182:595–598. doi: 10.1086/315706. [DOI] [PubMed] [Google Scholar]
- 95.Brandsma JL, Shlyankevich M, Zhang L, et al. Vaccination of rabbits with an adenovirus vector expressing the papillomavirus E2 protein leads to clearance of papillomas and infection. J Virol. 2004;78:116–123. doi: 10.1128/JVI.78.1.116-123.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lee-MacAry AE, Ross EL, Davies D, et al. Development of a novel flow cytometric cell-mediated cytotoxicity assay using the fluorophores PKH-26 and TO-PRO-3 iodide. J Immunol Methods. 2001;252:83–92. doi: 10.1016/s0022-1759(01)00336-2. [DOI] [PubMed] [Google Scholar]